Abstract
The photosynthetic machinery and, in particular, the photosystem II (PSII) complex are susceptible to strong light, and the effects of strong light are referred to as photodamage or photoinhibition. In living organisms, photodamaged PSII is rapidly repaired and, as a result, the extent of photoinhibition represents a balance between rates of photodamage and the repair of PSII. In this study, we examined the roles of electron transport and ATP synthesis in these two processes by monitoring them separately and systematically in the cyanobacterium Synechocystis sp. PCC 6803. We found that the rate of photodamage, which was proportional to light intensity, was unaffected by inhibition of the electron transport in PSII, by acceleration of electron transport in PSI, and by inhibition of ATP synthesis. By contrast, the rate of repair was reduced upon inhibition of the synthesis of ATP either via PSI or PSII. Northern blotting and radiolabeling analysis with [35S]Met revealed that synthesis of the D1 protein was enhanced by the synthesis of ATP. Our observations suggest that ATP synthesis might regulate the repair of PSII, in particular, at the level of translation of the psbA genes for the precursor to the D1 protein, whereas neither electron transport nor the synthesis of ATP affects the extent of photodamage.
Light is essential for photosynthesis, but it can also be toxic to the photosynthetic machinery (Kok, 1956; Jones and Kok, 1966a, 1996b; for review, see Powles, 1984; Aro et al., 1993; Andersson and Aro, 2001; Adir et al., 2003). Exposure of photosynthetic organisms to strong light results in damage to the PSII complex (Mattoo et al., 1981, 1984; Kyle et al., 1984; Ohad et al., 1984). This process is referred to as photodamage or photoinhibition and has been studied extensively in vitro, for example, in isolated thylakoid membranes (for review, see Aro et al., 1993; Andersson and Aro, 2001; Adir et al., 2003). In intact cells of photosynthetic organisms, photoinhibition is a more complex phenomenon than it is in vitro because photodamaged PSII is rapidly repaired. The main feature of the repair process is the replacement of the D1 protein in the photodamaged PSII by newly synthesized D1 and reassembly of active PSII (Guenther and Melis, 1990; Kettunen et al., 1997; for review, see Mattoo et al., 1989; Aro et al., 1993; Andersson and Aro, 2001). Therefore, the extent of photoinhibition in vivo reflects the balance between the light-induced damage (photodamage) to PSII and the repair of the photodamaged PSII.
In a previous study in Synechocystis sp. PCC 6803 (hereafter Synechocystis; Allakhverdiev and Murata, 2004), we successfully measured photodamage and repair separately. The initial rate of photodamage to PSII was determined using lincomycin, an inhibitor of protein synthesis, whereas the initial rate of repair of PSII was measured immediately after the photodamage by very strong light. This technique allowed us to study the effect of environmental stress on the photodamage and repair of PSII. We found that the repair process was sensitive to various kinds of environmental stress, such as oxidative stress due to H2O2, salt stress due to NaCl, and cold stress, but that the photodamage was unaffected by these kinds of stress.
However, our understanding of the mechanisms that regulate the photodamage and repair of PSII is still far from complete. In particular, we still do not know how the photosynthetic transport of electrons and the photosynthetic synthesis of ATP regulate the photodamage and repair. Application of the technique to measure the photodamage and repair of PSII separately may allow us to elucidate explicitly the effect of electron transport and ATP synthesis on the photodamage and repair.
The effects of electron transport on photoinhibition have been studied using an inhibitor of electron transport in PSII, 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea (DCMU). Some research groups have examined the effects of DCMU on photoinhibition of PSII in vitro. Jegerschöld et al. (1990) and Kirilovsky et al. (1994) observed that DCMU did not affect the extent of photoinhibition in thylakoid membranes from spinach (Spinacia oleracea), suggesting that the mechanism of photoinhibition is not related to the electron transport in PSII or the overreduction of the primary electron acceptor, QA. In intact chloroplasts in which photodamage and repair both take place, Krause and Behrend (1986) and Barr et al. (1990) observed that DCMU appeared to accelerate photoinhibition of PSII (for review, see Krause, 1994).
Some research groups studied the effects of DCMU on photoinhibition in vivo. However, their results were controversial. Komenda and Masojidek (1998) observed that DCMU appeared to accelerate photoinhibition in Synechococcus PCC 7942. By contrast, Kyle et al. (1984) and Zer and Ohad (1995) observed that DCMU slowed down the photoinhibition in Chlamydomonas reinhardtii. However, in these studies the effect of DCMU was examined without separately measuring the photodamage and repair. This might be a reason why the results were different among research groups and organisms used.
The effects of electron transport on the degradation of D1 protein (one of the steps of repair) have been extensively studied using DCMU (for review, see Mattoo et al., 1986, 1989; Aro et al., 1993; Keren and Ohad, 1998; Melis, 1999; Andersson and Aro, 2001; Adir et al., 2003), and several research groups observed that DCMU inhibited the degradation of D1 protein in the photodamaged PSII in vivo and in vitro, namely in higher plants (Mattoo et al., 1981, 1984), cyanobacteria (Goloubinoff et al., 1988), C. reinhardtii (Zer and Ohad, 1995), a thermophilic cyanobacterium (Komenda and Masojidek, 1995), and isolated thylakoid membranes (Jegerschöld et al., 1990; Kuhn and Böger, 1990).
There have been several approaches to characterization of the roles of electron transport and ATP synthesis in the synthesis of the D1 protein. However, the results were also controversial. Mattoo et al. (1984) suggested that both electron transport and ATP synthesis are important in the D1 synthesis in Spirodela oligorrhiza. In Chlamydomonas, Trebitsh and Danon (2001) showed that the redox signal associated with electron transport in PSI, as well as reduction of the plastoquinone pool, activated the initiation of translation of the D1 protein. Studies in intact chloroplasts from spinach showed that the level of stromal ATP was correlated with the light-dependent synthesis of the D1 protein in intact chloroplasts (Kuroda et al., 1992). Kuroda et al. (1996) further demonstrated that electron transport via PSI was essential for the light-dependent translational elongation of the D1 protein. By contrast, Muhlbauer and Eichacker (1998) suggested that a proton gradient, formed as a result of electron transport, might be important for the light-dependent translational elongation of the D1 protein in intact chloroplasts from barley. Thus, it seems likely that electron transport and/or ATP synthesis are required for the light-induced synthesis of the D1 protein. However, the direct effects of electron transport and ATP synthesis on the repair of PSII remain to be investigated.
In this study, we investigated the effects of electron transport and the synthesis of ATP on the initial rates of photodamage and repair of PSII in Synechocystis. Our results revealed that the inhibition of electron transport in PSII and acceleration of electron transport in PSI had no effect on photodamage. By contrast, the synthesis of ATP, mediated by the electron transport, was essential for repair but not for direct avoidance of photodamage.
RESULTS
Inhibition of Electron Transport in PSII and of ATP Synthesis Both Enhanced the Extent of Photodamage to PSII
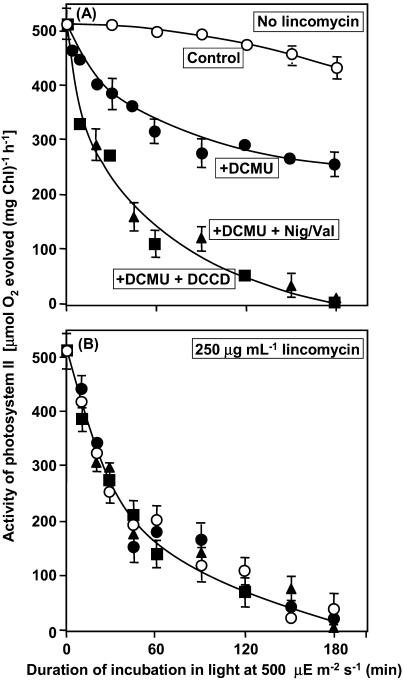
The photosynthetic transport of electrons is inhibited by DCMU on the acceptor side of PSII. Figure 1A shows the effects of this inhibitor on the extent of photodamage to PSII, as monitored in terms of the photosynthetic evolution of oxygen with 1,4-benzoquinone (BQ) as the artificial acceptor of electrons. The extent of photodamage was significantly enhanced by 20 μm DCMU, which was sufficient to inhibit completely the electron transport in PSII. In this experiment, DCMU was removed by washing cells twice, by centrifugation and resuspension, prior to measurements of electron transport activity (see “Materials and Methods”). This treatment completely removed DCMU and its inhibitory effects.
Figure 1.
Effects of DCMU, DCCD, and Nig/Val on the photodamage to PSII in Synechosystis in the absence of lincomycin (A) and in the presence of 250 μg mL−1 lincomycin (B). At designated times during incubation in light at 500 μE m−2 s−1 at 34°C, aliquots of each suspension of cells were withdrawn and PSII activity was measured in terms of the photosynthetic evolution of oxygen in the presence of 1.0 mm BQ, as the electron acceptor, after DCMU and lincomycin had been removed by washing as described in “Materials and Methods.” Each point and bar represent the average ± se of results from four independent experiments. ○, No addition (control); •, 20 μm DCMU; ▪, 20 μm DCMU plus 10 μm DCCD; ▴, 20 μm DCMU, 2 μm nigericin plus 2 μm valinomycin.
To investigate the contribution of ATP synthesis to the extent of photodamage, we used N,N-dicyclohexylcarbodiimide (DCCD), which is an inhibitor of ATP synthesis, and a combination of two ionophores, namely, nigericin and valinomycin (abbreviated as Nig/Val), which reverses the energization of thylakoid membranes (Dilley et al., 2001; Ke, 2001). The results in Figure 1A demonstrate that this inhibitor and these ionophores enhanced the photodamage to PSII that occurred in the presence of DCMU.
Next we examined the effects of DCMU, DCCD, and Nig/Val on the photodamage that occurred in the absence of repair, using 250 μg mL−1 lincomycin, an inhibitor of protein synthesis that blocks the repair of PSII (Tyystjärvi and Aro, 1996). The results in Figure 1B show that lincomycin enhanced the extent of photodamage and that further addition of DCMU, DCCD, or Nig/Val had no additional effect on the extent of photodamage. These observations suggested that inhibition of electron transport by DCMU and inhibition of ATP synthesis by DCCD or Nig/Val had no direct effect on photodamage. They also suggested that inhibition of electron transport and of ATP synthesis suppressed the repair of PSII, with resultant enhancement of the extent of photodamage.
Acceleration of Electron Transport in PSI and Inhibition of ATP Synthesis Affected the Extent of Photodamage to PSII
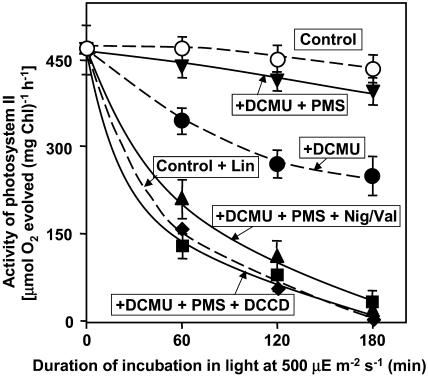
We examined the effects of the electron transport in PSI and the coupled synthesis of ATP using N-methyl phenazonium methosulfate (PMS), which mediates cyclic electron transport via PSI. In the presence of PMS, we detected no photodamage, even in the presence of DCMU (Fig. 2). The further addition of Nig/Val appeared to accelerate photodamage, as did lincomycin. DCCD had the same effects as Nig/Val. These observations suggested that the synthesis of ATP might be essential for the protection of PSII from the appearance of photodamage. It was of particular interest that the presence of 250 μg mL−1 lincomycin completely eliminated the protective effects of PMS (data not shown).
Figure 2.
Effects of DCMU, PMS, DCCD, and Nig/Val on the photodamage to PSII in Synechocystis. The experimental conditions were the same as described in the legend to Figure 1. ○, No addition (control); •, 20 μm DCMU; ▾, 20 μm DCMU and 20 μm PMS; ▪, 20 μm DCMU, 20 μm PMS, and 10 μm DCCD; ▴, 20 μm DCMU, 20 μm PMS, 2 μm nigericin, and 2 μm valinomycin; ♦, 250 μg mL−1 lincomycin. Each point and bar represent the average ± se of results from five independent experiments.
Photodamage to PSII Was Unaffected by Electron Transport and the Synthesis of ATP
To investigate details of the effects of electron transport and ATP synthesis on photodamage to PSII, we determined the initial rate of photodamage in the presence of lincomycin. The initial rate of photodamage was proportional to light intensity, as has been observed also in the leaves of higher plants and in Synechocystis cells that had been treated with lincomycin (Tyystjärvi and Aro, 1996; Lee et al., 2001; Allakhverdiev and Murata, 2004).
We next examined the initial rate of photodamage to PSII under various conditions, namely, in the presence of DCCD, DCMU, DCMU plus DCCD, DCMU plus PMS, DCMU plus PMS plus DCCD, and DCMU plus PMS plus Nig/Val. However, none of these conditions affected the initial rate of photodamage by light at 1,000 and 2,000 μE m−2 s−1 (Table I), and the initial rate of photodamage to PSII under these conditions was proportional to the light intensity. These observations suggested that photodamage to PSII in vivo might be independent of electron transport in PSII, the energized state of thylakoid membranes, and the synthesis of ATP.
Table I.
Effects of DCMU, PMS, DCCD, and Nig/Val on the initial rate of photodamage to PSII in the presence of lincomycin
Synechocystis cells, which had been grown in light at 70 μE m−2 s−1, were exposed to light at 1,000 or 2,000 μE m−2 s−1 and the initial rate of photodamage was determined from the time course (or by fitting the photodamage to a first-order reaction curve) in the presence of 250 μg mL−1 lincomycin under various conditions (Fig. 1B). Values are means ± sd of results from four independent experiments.
| Conditions
|
Initial Rate of Photodamage min−1
|
|
|---|---|---|
| 1,000 μE m−2 s−1 | 2,000 μE m−2 s−1 | |
| No addition (control) | 0.0090 ± 0.0007 | 0.0194 ± 0.0009 |
| DCCD (10 μm) | 0.0093 ± 0.0007 | 0.0191 ± 0.0008 |
| DCMU (20 μm) | 0.0087 ± 0.0006 | 0.0197 ± 0.0010 |
| DCMU (20 μm) | ||
| DCCD (10 μm) | 0.0103 ± 0.0011 | 0.0201 ± 0.0012 |
| DCMU (20 μm) | ||
| Nigericin (2 μm) plus valinomycin (2 μm) | 0.0096 ± 0.0010 | 0.0202 ± 0.0011 |
| DCMU (20 μm) | ||
| PMS (20 μm) | 0.0102 ± 0.0007 | 0.0193 ± 0.0010 |
| DCMU (20 μm) | ||
| PMS (20 μm) | ||
| DCCD (10 μm) | 0.0091 ± 0.0008 | 0.0187 ± 0.0009 |
| DCMU (20 μm) | ||
| PMS (20 μm) | ||
| Nigericin (2 μm) plus valinomycin (2 μm) | 0.0109 ± 0.0009 | 0.0207 ± 0.0008 |
Inhibition of Electron Transport in PSII and Inhibition of ATP Synthesis Depressed the Initial Rate of Repair of PSII
When Synechocystis cells are exposed to strong light at 2,500 μE m−2 s−1 for 65 min to reduce the activity of PSII to 90% of the original level, the activity is restored during exposure to weak light at various intensities (Allakhverdiev and Murata, 2004).
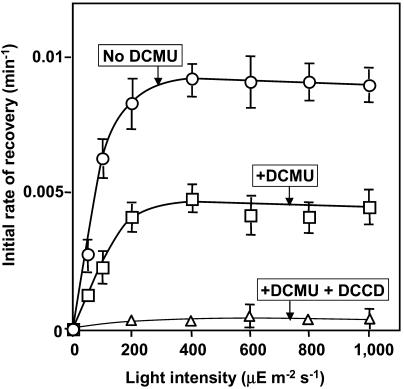
Figure 3 shows the effects of light intensity on the initial rate of recovery of PSII activity during the repair of PSII. The initial rate of recovery reached a maximum of 0.01 min−1 in light at an intensity of 300 μE m−2 s−1 and 50% of this level was achieved at approximately 70 μE m−2 s−1. These results suggested that a reaction might exist that limits the rate of the entire repair process and that the maximum turnover rate of this reaction was 0.01 min−1 under our experimental conditions.
Figure 3.
Effects of DCMU and DCCD on the initial rate of recovery of PSII activity after photodamage in Synechocystis. Cells were incubated for 65 min in light at 2,500 μE m−2 s−1 to induce 90% inactivation of PSII. Then cells were incubated in light at various intensities in the presence and absence of DCMU and DCCD. The initial rate of recovery was calculated from the time course of recovery experiments, as described in “Materials and Methods.” ○, No addition (control); □, in the presence of 20 μm DCMU; ▵, in the presence of 20 μm DCMU and 10 μm DCCD. Each point and bar represent the average ± se of results from six independent experiments.
Figure 3 also shows that inhibition by DCMU of the electron transport in PSII depressed the initial rate of recovery by 50% without changing the profile of dependence of light intensity, with this level being achieved at 300 μE m−2 s−1. This observation suggests that electron transport might be involved, in some way, in the repair of PSII and that inhibition of electron transport by DCMU might have repressed a reaction in the repair process. The further addition of DCCD completely abolished the capacity for repair. The addition of Nig/Val produced the same results as the addition of DCCD (data not shown). These observations suggested that ATP synthesis via PSI, which is assumed to occur in the presence of DCMU, might be important for the residual capacity for the repair of photodamaged PSII.
Acceleration of Electron Transport in PSI and Inhibition of ATP Synthesis Affected the Initial Rate of Repair of PSII
We examined the effects of DCMU, PMS, DCCD and Nig/Val on the initial rate of repair of PSII. The activity of PSII in Synechocystis cells that had been exposed to strong light at 2,500 μE m−2 s−1 for 65 min, to reduce the activity of PSII to 90% of the original level, was restored during exposure to weak light. Table II shows the effects of the various inhibitors and ionophores on the initial rate of recovery in light at 300 μE m−2 s−1 and 600 μE m−2 s−1. In the absence of inhibitors and ionophores, the initial rate yielded a “saturation-type” curve with a maximum rate of recovery of approximately 0.01 min−1 at 300 and 600 μE m−2 s−1. The initial rate of recovery was depressed by 50% by DCMU. However, the further addition of PMS significantly increased the rate of recovery. Recovery was suppressed completely by DCCD and Nig/Val. These observations suggested that the recovery of PSII in vivo might be accelerated by the electron transport in PSI, via the accelerated synthesis of ATP.
Table II.
Effects of DCMU, PMS, DCCD, and Nig/Val on the initial rate of recovery of PSII activity
Synechocystis cells, which had been grown in light at 70 μE m−2 s−1, were exposed to light at 2,000 μE m−2 s−1 for 75 min to induce 90% inactivation of PSII. Then cells were incubated in light at various intensities in the presence or absence of various reagents. The initial rate of recovery was calculated from the time course of recovery experiments (or by fitting the recovery to a first-order reaction curve). Values are means ± sd of results from four independent experiments.
| Conditions
|
Initial Rate of Recovery min−1
|
|
|---|---|---|
| 300 μE m−2 s−1 | 600 μE m−2 s−1 | |
| No addition (control) | 0.0089 ± 0.0014 | 0.0091 ± 0.0013 |
| DCCD (10 μm) | 0.0004 ± 0.0002 | 0.0007 ± 0.0004 |
| DCMU (20 μm) | 0.0047 ± 0.0008 | 0.0046 ± 0.0009 |
| DCMU(20 μm) | ||
| DCCD (10 μm) | 0.0005 ± 0.0001 | 0.0006 ± 0.0001 |
| DCMU (20 μm) | ||
| Nigericin (2 μm) plus valinomycin (2 μm) | 0.0003 ± 0.0001 | 0.0004 ± 0.0001 |
| DCMU (20 μm) | ||
| PMS (20 μm) | 0.0073 ± 0.0009 | 0.008 ± 0.0012 |
| DCMU (20 μm) | ||
| PMS (20 μm) | ||
| DCCD (10 μm) | 0.0006 ± 0.0001 | 0.0005 ± 0.0001 |
| DCMU (20 μm) | ||
| PMS (20 μm) | ||
| Nigericin (2 μm) plus valinomycin (2 μm) | 0.0005 ± 0.0001 | 0.0005 ± 0.0001 |
Changes in Intracellular Levels of ATP
We measured levels of ATP directly in Synechocystis cells and examined the effects of DCMU and other reagents on the intracellular levels of ATP. Table III shows that after incubation of Synechocystis cells in light at 500 μE m−2 s−1 for 30 min the level of ATP was about 70 μmol (mg Chl)−1. Inhibition of electron transport in PSII by DCMU reduced the level of ATP by approximately 20%. The further addition of PMS significantly increased the level of ATP. However, dissipation of the energization associated with thylakoid membranes by Nig/Val reduced the level of ATP to as little as 20% of the control level. Furthermore, DCCD also reduced the level of ATP to approximately 10% of the original level. Thus, the level of ATP was responsive to these various reagents. However, neither DCCD nor Nig/Val completely eliminated ATP in cells when PMS was present. Addition of DCCD or Nig/Val, in the presence of DCMU but in the absence of PMS, completely eliminated the intracellular accumulation of ATP.
Table III.
Effects of DCMU, PMS, DCCD, and Nig/Val on the intracellular levels of ATP in Synechocystis
Suspensions of cells at a Chl concentration of 3 μg mL−1 were exposed to light at 500 μE m−2 s−1 for 30 min with standard aeration at 34°C in the presence or absence of various reagents as indicated. The level of ATP was determined by luciferin/luciferase assay as described in “Materials and Methods.” Values are means ± sd of results from four independent experiments.
| Conditions | Concentration of ATP μmol ATP/mg Chl |
|---|---|
| No addition (control) | 71 ± 5.2 (100%) |
| DCCD (10 μm) | 7 ± 4.2 (9%) |
| DCMU (20 μm) | 58 ± 3.9 (82%) |
| DCMU (20 μm) | |
| DCCD (10 μm) | 0 ± 0 (0%) |
| DCMU (20 μm) | |
| Nigericin (2 μm) plus valinomycin (2 μm) | 0 ± 0 (0%) |
| DCMU (20 μm) | |
| PMS (20 μm) | 82 ± 4.3 (115%) |
| DCMU (20 μm) | |
| PMS (20 μm) | |
| DCCD (10 μm) | 9 ± 4.9 (12%) |
| DCMU (20 μm) | |
| PMS (20 μm) | |
| Nigericin (2 μm) plus valinomycin (2 μm) | 13 ± 5.2 (18%) |
Inhibition of Electron Transport in PSII and of ATP Synthesis Depressed the Levels of psbA Transcripts
The repair of PSII depends on the expression of the psbAII and psbAIII genes, which encode the precursor to the D1 protein (Mohamed et al., 1993). To clarify the mechanism responsible for the regulation of the repair of PSII, we examined the effects of DCMU on levels of psbA transcripts. In Synechocystis, the D1 protein is encoded by a small multigene family that consists of the psbAI, psbAII, and psbAIII genes (Jansson et al., 1987). However, psbAI is not expressed (Mohamed and Jansson, 1989), while psbAII and psbAIII, which are almost identical and encode only single form of D1, are both expressed (Mohamed and Jansson, 1989; Bouyoub et al., 1993; Mohamed et al., 1993) but at different levels. Approximately 95% of psbA transcripts originate from psbAII, while only 5% originate from psbAIII in Synechocystis cells that are grown under weak or strong light (Bouyoub et al., 1993; Mohamed et al., 1993). The reason for the differential expression of the psbA genes in Synechocystis is unknown.
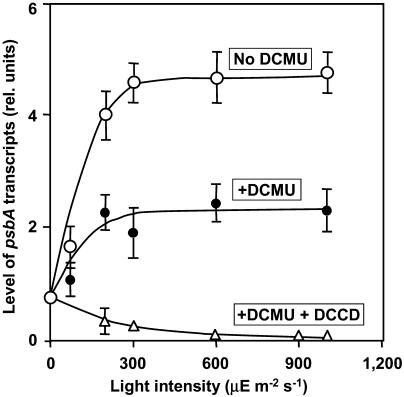
Northern-blotting analysis of psbA transcripts revealed that the level of psbA transcripts reached a maximum plateau value after illumination for approximately 10 min (data not shown). Figure 4 shows that levels of the mRNA were not very different over a range of light intensities from 200 to 1,000 μE m−2 s−1. Figure 4 also shows that DCMU depressed the level of psbA transcripts by approximately 50% at all light intensities examined. These results are consistent with those in previous reports (Alfonso et al., 1999; Sippola and Aro, 2000; Zhang et al., 2000; Trebitsh and Danon, 2001) and they suggest that inhibition of electron transport by DCMU blocked, in part, the synthesis of the psbA transcripts. The further addition of DCCD eliminated the synthesis of psbA transcripts, suggesting that the 50% level of psbA transcripts, observed in the presence of DCMU, depended on the ATP that is essential for transcription.
Figure 4.
Effects of DCMU and DCCD on the dependence on light intensity of the level of psbA transcripts in Synechocystis. Cells were incubated for 30 min in light at the indicated intensities in the presence and absence of DCMU and DCCD. A portion of each suspension of cells was withdrawn for extraction of RNA, which was subjected to northern-blotting analysis as described in “Materials and Methods.” ○, No addition (control); •, in the presence of 20 μm DCMU; ▵, in the presence of 20 μm DCMU and 10 μm DCCD. Each point and bar represent the average ± se of results from four independent experiments.
Effects of PMS, DCCD, and Nig/Val on Levels of psbA Transcripts
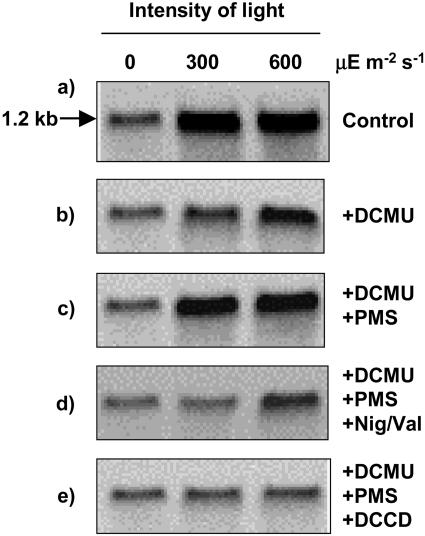
We monitored changes in levels of psbA mRNAs due to the presence of inhibitors and other reagents (Fig. 5). The presence of DCMU decreased the level of psbA transcripts, and PMS reversed this effect. The level of psbA transcripts was depressed by DCCD and by Nig/Val (Fig. 5; Table IV). However, neither Nig/Val nor DCCD completely eliminated the psbA transcripts.
Figure 5.
Effects of DCMU, PMS, DCCD, and Nig/Val on the level of psbA transcripts in Synechocystis. Cells were incubated for 30 min in darkness and in light at 300 μE m−2 s−1 and at 600 μE m−2 s−1 in the presence of 20 μm DCMU, 20 μm PMS, 10 μm DCCD, and 2 μm nigericin plus 2 μm valinomycin (Nig/Val) as indicated or in their absence (control). A portion of each suspension of cells was withdrawn for extraction of RNA, which was subjected to northern-blotting analysis as described in “Materials and Methods.” The arrow labeled 1.2 kb indicates the position of psbA transcripts.
Table IV.
Effects of DCMU, PMS, DCCD, and Nig/Val on the levels of psbA transcripts
Experimental conditions were the same as those described in the legend to Figure 5. The levels of transcripts were normalized by reference to levels of rRNA. Values are means ± sd of results from three independent experiments.
| Conditions
|
Levels of psbA Transcripts at 30 min (rel. units)
|
||
|---|---|---|---|
| 0 μE m−2 s−1 | 300 μE m−2 s−1 | 600 μE m−2 s−1 | |
| No addition (control) | 0.82 ± 0.07 | 4.56 ± 0.15 | 4.80 ± 0.14 |
| DCCD (10 μM) | 0.84 ± 0.07 | 0.32 ± 0.09 | 0 ± 0 |
| DCMU (20 μM) | 0.86 ± 0.08 | 1.86 ± 0.09 | 2.43 ± 0.07 |
| DCMU (20 μm) | |||
| DCCD (10 μm) | 0.87 ± 0.06 | 0.21 ± 0.09 | 0 ± 0 |
| DCMU (20 μm) | |||
| Nigericin (2 μm) plus valinomycin (2 μm) | 0.79 ± 0.12 | 0.68 ± 0.10 | 0 ± 0 |
| DCMU (20 μm) | |||
| PMS (20 μm) | 0.78 ± 0.11 | 4.14 ± 0.10 | 4.31 ± 0.15 |
| DCMU (20 μm) | |||
| PMS (20 μm) | |||
| DCCD (10 μm) | 0.84 ± 0.09 | 0.98 ± 0.11 | 1.83 ± 0.14 |
| DCMU (20 μm) | |||
| PMS (20 μm) | |||
| Nigericin (2 μm) plus valinomycin (2 μm) | 0.91 ± 0.13 | 1.21 ± 0.07 | 1.43 ± 0.11 |
Effects of Electron Transport and ATP Synthesis on the Synthesis of Proteins de Novo
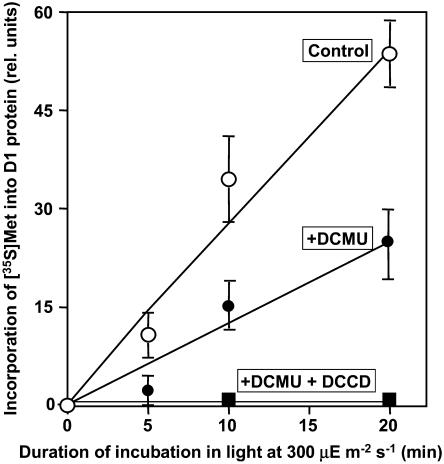
To identify the reaction(s) in the repair process that is inhibited by DCMU, we performed a labeling experiment with [35S]Met in light at 300 μE m−2 s−1 after the induction of photodamage. Incubation of cells in light at 2,500 μE m−2 s−1 decreased the activity of PSII to approximately 10% of the original level. During subsequent exposure of cells to light at 300 μE m−2 s−1, the activity of PSII returned to the original level. At this light intensity, the initial rate of recovery was maximal (Fig. 3). Figure 6 shows the time courses of incorporation of radiolabeled Met into the D1 protein. In the presence of DCMU, the synthesis of the D1 protein was depressed by approximately 50% as compared to that under control conditions. These observations indicated that the synthesis of the D1 protein de novo was inhibited to some extent by the inhibition of electron transport. The further addition of DCCD completely eliminated the synthesis of D1 protein, suggesting that conditions that allow the synthesis of ATP might be essential for the synthesis of the D1 protein, which is essential for the repair of PSII.
Figure 6.
Effects of DCMU and DCCD on the synthesis of the D1 protein de novo in Synechocystis. Cells were incubated at 34°C for 65 min in light at 2,500 μE m−2 s−1 to induce 90% inactivation of PSII. Cells were then incubated with 10 nm [35S]Met (>1,000 Ci mmol−1) for designated periods of time at 300 μE m−2 s−1 in the presence of 20 μm DCMU and 10 μm DCCD or in their absence (control) as indicated. At designated times, a portion of each suspension of cells was withdrawn for preparation of thylakoid membranes, which were subjected to PAGE as described in “Materials and Methods.” Proteins from thylakoid membranes corresponding to 0.8 μg of Chl were applied to each lane. Each point and bar represent the average ± se of results from four independent experiments.
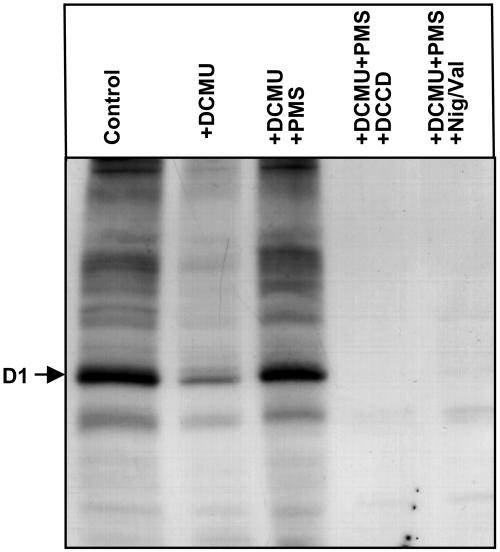
To examine the effects of DCMU, PMS, and ionophores on the synthesis of proteins de novo during the exposure of Synechocystis cells to light, we monitored incorporation of [35S]Met into the proteins of thylakoid membranes in light at 500 μE m−2 s−1 (Fig. 7). The synthesis of proteins during exposure of cells to light was markedly suppressed by DCMU. However, the further addition of PMS fully restored the synthesis of all proteins. Further addition of DCCD or Nig/Val completely abolished the synthesis of all proteins, including the D1 protein. These results suggested that exposure of cells to light in the presence of PMS induced the synthesis of proteins, including the D1 protein, even though electron transport in PSII was inhibited by the presence of DCMU and, moreover, that conditions that allowed the synthesis of ATP were essential for the synthesis of proteins, including the D1 protein, which is essential for the repair of PSII. It should be noted that protein synthesis was totally eliminated in the presence of DCCD or Nig/Val, in contrast to the synthesis of psbA transcripts, which occurred under the same conditions, albeit at a reduced rate.
Figure 7.
Effects of DCMU, PMS, DCCD, and Nig/Val on the synthesis of proteins de novo in Synechocystis. Cells were incubated with 10 nm [35S]Met (>1,000 Ci mmol−1) for 30 min in light at 300 μE m−2 s−1 in the presence of 20 μm DCMU, 20 μm PMS, 10 μm DCCD, and 2 μm nigericin plus 2 μm valinomycin (Nig/Val) as indicated, or in their absence (control). After incubation, a portion of each suspension of cells was withdrawn for preparation of thylakoid membranes, which were subjected to PAGE as described in “Materials and Methods.” Proteins from thylakoid membranes corresponding to 0.8 μg of Chl were applied to each lane. The arrow indicates the position of the D1 protein (molecular mass 32 kD). The results shown are representative of the results of four independent experiments, each of which gave similar results.
DISCUSSION
In this study, we demonstrated that the extent of photodamage in Synechocystis cells was enhanced by inhibition of electron transport in PSII by DCMU and also by inhibition of ATP synthesis by DCCD or Nig/Val (Figs. 1 and 2). We analyzed systematically the effects of inhibition of electron transport and of ATP synthesis on the rates of photodamage and repair of PSII by selecting suitable light conditions and by using lincomycin, an inhibitor of protein synthesis. We observed that the rate of photodamage, as determined in the presence of lincomycin, was proportional to light intensity (Table I), as observed previously in leaves of higher plants by Tyystjärvi and Aro (1996) and Lee et al. (2001). In this study, we demonstrated that this proportionality was unaffected by the inhibition of electron transport and by the acceleration of electron transport in PSI by PMS, and it was also unaffected by the inhibition of ATP synthesis by DCCD or Nig/Val (Table I). These findings suggest that the process of photodamage depends neither on the rate of electron transport nor on the level of ATP.
The extent of the repair of PSII after photodamage was diminished upon inhibition of the synthesis of ATP regardless of the type of electron transport, namely, that in PSI, which was accelerated by PMS, and that in PSII, which was inhibited by DCMU (Fig. 3; Table II). It is likely that an adequate intracellular level of ATP was essential for the repair. In fact, the rate of repair (Table II) was well correlated with the level of ATP (Table III). This is in agreement with the results of Mattoo et al. (1984) in which the light-driven synthesis of D1 protein depends on ATP in S. oligorrhiza. Muhlbauer and Eichacker (1998) reported that the stimulation by light of translation of elongation depends on the formation of a proton gradient across the thylakoid membrane. However, our observation that DCCD abolished the repair of PSII demonstrates that it is the level of ATP, rather than a proton gradient, that is essential for repair in Synechocystis.
The repair of PSII involves several steps (Aro et al., 1993), including the degradation of D1 protein, transcription of psbA genes, translation of psbA transcripts, incorporation of pre-D1 into PSII, processing of pre-D1, and assembly of the PSII dimer and the oxygen-evolving complex (Tyystjärvi et al., 2001). We examined the effects of electron transport and the synthesis of ATP on the transcription of psbA genes by northern-blotting analysis in Synechocystis (Figs. 4 and 5; Table IV). The results demonstrated that DCCD and Nig/Val depressed the level of psbA transcripts to a certain extent but did not completely eliminate them. By contrast, the incorporation of [35S]Met into D1 and other proteins was completely inhibited by DCCD and by Nig/Val (Figs. 6 and 7). These observations suggest that the translation of mRNAs might be the primary target of inhibition that results from a reduction in the intracellular level of ATP. Transcription is only partially affected by the level of ATP and is likely to be a secondary target.
Several research groups have suggested that translational elongation in chloroplasts of higher plants depends on light. For example, van Wijk and Eichacker (1996) observed that absence of light during translation leads to the increased accumulation of polysome-bound translational intermediate forms of D1, which indicates that light is required for efficient elongation of the D1 protein. The extent of incorporation of radiolabeled Met into the D1 protein and CP43 (Chl protein of 43 kD) decreased 3-fold in darkness, whereas the accumulation of the D2 reaction-center protein was unaffected by light. In addition, light was also required for the efficient incorporation of the D1 protein into the PSII core complex. In darkness, the newly synthesized D1 protein accumulated predominantly as unassembled protein or in PSII subcomplexes of less than 100 kD (van Wijk and Eichacker, 1996).
Various mechanisms for the induction by light of translational elongation of the D1 protein have been suggested in higher plants. For example, the stromal level of ATP might regulate the translational elongation of D1 (Kuroda et al., 1992); the electron transport in PSI and the subsequent reductive signal from PSI might activate translational elongation (Kuroda et al., 1996); the proton gradient formed by electron transport might promote translational elongation (Muhlbauer and Eichacker, 1998); and the redox signals transmitted via thiol groups and the cotranslational insertion of D1 into PSII might determine the rate of the translational elongation of D1 in light (Zhang et al., 2000).
Our findings in Synechocystis demonstrate that electron transport and the generation of ATP are essential not only for the synthesis of the D1 protein de novo in light but also for the total repair of photodamaged PSII. It seems likely that the requirement for ATP reflects the energy required for operation of the translational machinery. The addition of each amino acid to a polypeptide chain during translation requires at least one molecule of ATP for aminoacylation of the cognate tRNA and two molecules of GTP for binding of aminoacyl-tRNA to the ribosome and the subsequent translocation of the peptidyl tRNA (Gold, 1988). The tight correlation between the synthesis of ATP and the repair of PSII suggests that the synthesis of ATP might be the rate-limiting step in the complete repair of photodamaged PSII.
MATERIALS AND METHODS
Cyanobacterium and Culture Conditions
Synechocystis sp. PCC 6803 was kindly donated by Dr. J.G.K. Williams of DuPont de Nemours (Wilmington, DE). Cells were grown photoautotrophically in glass tubes (2.5 cm i.d. × 20 cm; 120 mL) at 34°C under constant illumination from incandescent lamps at 70 μE m−2 s−1 in BG-11 medium (Stanier et al., 1971) supplemented with 20 mm HEPES-NaOH, pH 7.5. Cultures were aerated with sterile air that contained 1% (v/v) CO2 (Ono and Murata, 1981).
Conditions for Photodamage
Cells from 3-d-old cultures at a chlorophyll (Chl) concentration of 3 μg mL−1 were incubated under strong light, as described below, to induce photodamage or under weak light (70 μE m−2 s−1) to induce repair in growth chambers at 34°C under the growth conditions described above. Small aliquots of cultures were withdrawn at designated times for measurements of PSII activity. Strong light, for induction of photodamage, was provided by two lamps (300 W; Toshiba, Tokyo) and attenuated to 250, 500, 1,000, 1,500, or 2,000 μE m−2 s−1 by passage through neutral density filters (PC-S380; 200 × 500 wide and 3 mm thick; Hoya Glass, Tokyo). The intensity of light was measured at the surface of glass tubes that contained suspensions of cells.
In some experiments, the synthesis of proteins was blocked by 250 μg mL−1 lincomycin (Sigma Chemical, St. Louis), which was added to the culture medium 10 min before the start of incubation. In some other experiments, the photosynthetic transport of electrons through PSII was inhibited by 20 μm DCMU (Sigma Chemical). For inhibition of the formation of a proton gradient and synthesis of ATP, we used a pair of uncouples, 2 μm nigericin (Sigma Chemical) and 2 μm valinomycin (Wako Chemicals, Osaka), or 10 μm DCCD (Wako Chemicals). For inhibition of the cyclic transport of electrons via PSI, 20 μm PMS (Wako Chemicals) was added during the incubation of cells. After incubation for designated times, 5 mL of the suspension of cells were withdrawn and diluted 10-fold with BG-11 medium. Cells were collected by centrifugation at 5,000g for 6 min at 34°C and resuspended in 50 mL of BG-11 medium. The centrifugation and resuspension were repeated once. Finally, cells were collected by centrifugation as above and suspended in BG-11 medium at a density of 3 μg Chl mL−1. This washing procedure completely removed DCMU and its effects on the activity of PSII in Synechocystis cells.
Measurement of Photosynthetic Activity
The activity of PSII in intact cells was measured by monitoring oxygen-evolving activity in the presence of 1.0 mm BQ, an artificial acceptor of electrons, with a Clark-type oxygen electrode (Hansatech Instruments, King's Lynn, UK). The sample, in a 3-mL cuvette, was illuminated by light from incandescent lamps that had been passed through a red optical filter (R-60; Toshiba, Tokyo) and an infrared-absorbing filter (HA-50; Hoya Glass). The intensity of light at the surface of the cuvette was 2,000 μE m−2 s−1. The initial rates of photodamage and recovery in the presence and absence of 250 μg/mL lincomycin, respectively, were calculated from the time courses of photodamage and recovery experiments and also by fitting the photodamage and recovery to a first-order reaction curve, as described previously (Allakhverdiev and Murata, 2004).
Measurement of Levels of ATP
A suspension of cells at a Chl concentration of 3 μg mL−1 was exposed to light at 500 μE m−2 s−1 for 30 min with standard aeration at 34°C in the absence or presence of various reagents, such as 20 μm DCMU, 20 μm PMS, 10 μm DCCD, and 2 μm nigericin plus 2 μm valinomycin. Then cells were immediately disrupted by vortex mixing for 30 s with glass beads (diameter, 0.1 mm; BioSpec Products, Bartlesville, OK), which was repeated a total of three times, with 30-s pauses. A portion of the homogenate (with a Chl concentration of 3 μg mL−1) was passed through a syringe-driven filter with 0.22-μm pores (Millipore, Bedford, MA) to separate the soluble fraction from the suspension. The resultant soluble fraction was subjected to assays for determination of levels of ATP. This assay was performed with a luminometer (MicroLumat LB 96 P, EG&G Berthold, Bad Wildbad, Germany) that had been designed for the measurement of bio- and chemiluminescence in 96-well microplates, using a luciferase-luciferin assay kit (ASR7921; Wako Pure Chemical Industries, Osaka). According to the protocol supplied with the kit, 100 μL of a solution of luciferase-luciferin in sterile distilled water were added to 200 μL of assay mixture and chemoluminescence was measured over 10 s. A standard curve was generated with ATP from Oriental Yeast (Tokyo).
Northern-Blotting Analysis
Total RNA was extracted from cells and northern-blotting analysis was performed as described previously (Los et al., 1997). An equal amount of RNA (4 μg) from each sample was loaded in individual wells of the gel and rRNA was visualized by staining with ethidium bromide. A 1.0-kb fragment of DNA that included the coding region of the psbA gene was amplified by the PCR with primers 5′-AACGACTCTCCAACAGCGCGAAA-3′ and 5′-CGTTCGTCATTACTTCAAAACCG-3′ and genomic DNA from Synechocystis as the template. The amplified fragment of DNA was ligated into the TA cloning vector pT7Blue-T (Novagen, Darmstadt, Germany). The plasmid was cleaved at the HincII and NcoI sites within the insert. The resultant 700-bp fragment of DNA was conjugated with alkaline phosphatase using an Alkphos Direct kit (Amersham Pharmacia Biotech, Piscataway, NJ) and the conjugate was used as the probe. After hybridization, blots were soaked in CDP-star solution (Amersham Pharmacia Biotech) and signals from hybridized mRNAs were detected with a digital camera system (LAS-1000; Fuji Photo Film, Tokyo).
Labeling of Proteins in Vivo
A suspension of cells, at a concentration corresponding to 5.00 ± 0.05 μg Chl mL−1, was supplemented with 10 nm [35S]Met (>1,000 Ci mmol−1; Amersham Pharmacia Biotech), as described previously (Nishiyama et al., 2001; Allakhverdiev et al., 2002). Then the suspension was incubated at 34°C for designated periods of time in light at 300 μE m−2 s−1. The labeling was terminated by the addition of nonradioactive Met to a final concentration of 1.0 mm and immediate cooling of samples on ice. Cells were collected by centrifugation at 5,000g for 6 min at 4°C, and thylakoid membranes were isolated from these cells as described previously (Allakhverdiev et al., 1999, 2000). Thylakoid membranes were solubilized by incubation for 5 min at 65°C in 60 mm Tris (pH adjusted to 6.8 with HCl) that contained 2% (w/v) SDS, 5% (v/v) 2-mercaptoethanol, and 10% (v/v) glycerol, and then proteins were separated by PAGE [12.5% (w/v) polyacrylamide] in the presence of 0.08% (w/v) SDS and 6 m urea, as described previously (Laemmli, 1970; Taguchi et al., 1993, 1995). Solubilized thylakoid membranes corresponding to 0.8 μg of Chl were loaded in each lane. Labeled proteins on the gel were visualized by exposure of the dried and fixed gel to x-ray film. Radioactivity of radiolabeled D1 was quantitated with the digital camera system.
Acknowledgments
The authors are grateful to Professor Kimiyuki Satoh for his generous gift of antibodies against the D1 protein. They also thank Professor Itzhak Ohad for helpful discussions and comments on the original manuscript. This work was supported, in part, by a Grant-in-Aid for Scientific Research (S; no. 13854002) from the Ministry of Education, Science and Culture, Japan, and by the Cooperative Research Program of the National Institute for Basic Biology on the Stress Tolerance of Plants and, in part, by the Japan Society for the Promotion of Science (Invitation Fellowship for Research in Japan to S.I.A.).
This work was supported, in part, by the Cooperative Research Program on the Stress Tolerance of Plants of the National Institute for Basic Biology, Japan.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.054478.
References
- Adir N, Zer H, Shochat S, Ohad I (2003) Photoinhibition: a historical perspective. Photosynth Res 76: 343–370 [DOI] [PubMed] [Google Scholar]
- Alfonso M, Perewoska I, Constant S, Kirilovsky D (1999) Redox control of psbA expression in cyanobacteria Synechocystis strains. J Photochem Photobiol B Biol 48: 104–113 [Google Scholar]
- Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657: 23–32 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Nishiyama Y, Miyairi S, Yamamoto H, Inagaki N, Kanesaki Y, Murata N (2002) Salt stress inhibits the repair of photodamaged photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiol 130: 1443–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev SI, Nishiyama Y, Suzuki I, Tasaka Y, Murata N (1999) Genetic engineering of the unsaturation of fatty acids in membrane lipids alters the tolerance of Synechocystis to salt stress. Proc Natl Acad Sci USA 96: 5862–5867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allakhverdiev SI, Sakamoto A, Nishiyama Y, Murata N (2000) Inactivation of photosystems I and II in response to osmotic stress in Synechococcus. Contribution of water channels. Plant Physiol 122: 1201–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B, Aro E-M (2001) Photodamage and D1 protein turnover in photosystem II. In E-M Aro, B Andersson, eds, Regulation of Photosynthesis, Vol 11. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 377–393
- Aro E-M, Virgin I, Andersson B (1993) Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143: 113–134 [DOI] [PubMed] [Google Scholar]
- Barr P, Young AJ, Britton G (1990) Photodestruction of pigments in higher plants by herbicide action. I. The effect of DCMU (diuron) on isolated chloroplasts. J Exp Bot 41: 123–134 [Google Scholar]
- Bouyoub A, Vernotte C, Astier C (1993) Functional analysis of the two homologous copies of the psbA gene in Synechocystis PCC 6714 and 6803. Plant Mol Biol 21: 249–258 [DOI] [PubMed] [Google Scholar]
- Dilley RA, Nishiyama Y, Gombos Z, Murata N (2001) Bioenergetic responses of Synechocystis 6803 fatty acid desaturase mutants at low temperatures. J Bioenerg Biomembr 33: 135–141 [DOI] [PubMed] [Google Scholar]
- Gold L (1988) Posttranscriptional regulatory mechanisms in Escherichia coli. Annu Rev Biochem 57: 199–233 [DOI] [PubMed] [Google Scholar]
- Goloubinoff P, Brusslan J, Golden SS, Haselkorn R, Edelman M (1988) Characterization of the photosystem II 32 kDa protein in Synechococcus PCC 7942. Plant Mol Biol 11: 441–447 [DOI] [PubMed] [Google Scholar]
- Guenther JE, Melis A (1990) The physiological significance of photosystem II heterogeneity in chloroplasts. Photosynth Res 23: 105–109 [DOI] [PubMed] [Google Scholar]
- Jansson C, Debus RJ, Osiewacz HD, Gurevitz M, McIntosh L (1987) Construction of an obligate photoheterotrophic mutant of the cyanobacterium Synechocystis 6803. Plant Physiol 85: 1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegerschöld C, Virgin I, Styring S (1990) Light-dependent degradation of the D1 protein in photosystem II is accelerated after inhibition of the water splitting reaction. Biochemistry 29: 6179–6186 [DOI] [PubMed] [Google Scholar]
- Jones LW, Kok B (1966. a) Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol 41: 1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Kok B (1966. b) Photoinhibition of chloroplast reactions. II. Multiple effects. Plant Physiol 41: 1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke B (2001) Proton transport and photophosphorylation. In B Ke, ed, Photosynthesis: Photobiochemistry and Photobiophysics, Vol 10. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 635–739
- Keren N, Ohad I (1998) State transition and photoinhibition. In J-D Rochaix, M Goldschmidt-Clermont, S Merchant, eds, The Molecular Biology of Chloroplasts and Mitochondria in Chlamydomonas, Vol 7. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 569–596
- Kettunen R, Pursiheimo S, Rintamaki E, van Wijk KJ, Aro E-M (1997) Transcriptional and translational adjustments of psbA gene expression in mature chloroplasts during photoinhibition and subsequent repair of photosystem II. Eur J Biochem 247: 441–448 [DOI] [PubMed] [Google Scholar]
- Kirilovsky D, Rutherford AW, Etienne A-L (1994) Influence of DCMU and ferricyanide on photodamage in photosystem II. Biochemistry 33: 3087–3095 [DOI] [PubMed] [Google Scholar]
- Kok B (1956) On the inhibition of photosynthesis by intense light. Biochim Biophys Acta 21: 234–244 [DOI] [PubMed] [Google Scholar]
- Komenda J, Masojidek J (1995) Functional and structural changes of the photosystem II complex induced by high irradiance in cyanobacterial cells. Eur J Biochem 233: 677–682 [DOI] [PubMed] [Google Scholar]
- Komenda J, Masojidek J (1998) The effect of photosystem II inhibitors DCMU and BNT on the high-light-induced D1 turnover in two cyanobacterial strains Synechocystis PCC 6803 and Synechococcus PCC 7942. Photosynth Res 57: 193–202 [Google Scholar]
- Krause GH (1994) The role of oxygen in photoinhibition of photosynthesis. In CH Foyer, PM Mullineaux, eds, Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. CRC Press, Boca Raton, FL, pp 43–77
- Krause GH, Behrend U (1986) ΔpH-dependent chlorophyll fluorescence quenching indicating a mechanism of protection against photoinhibition of chloroplasts. FEBS Lett 200: 298–302 [Google Scholar]
- Kuhn M, Böger P (1990) Studies on the light induced loss of the D1 protein in photosystem II membrane fragments. Photosynth Res 23: 291–296 [DOI] [PubMed] [Google Scholar]
- Kuroda H, Inagaki N, Satoh K (1992) The level of stromal ATP regulates translation of the D1 protein in isolated chloroplasts. Plant Cell Physiol 33: 33–39 [Google Scholar]
- Kuroda H, Kobashi K, Kaseyama H, Satoh K (1996) Possible involvement of a low redox potential component(s) downstream of photosystem I in the translational regulation of the D1 subunit of the photosystem II reaction center in isolated pea chloroplasts. Plant Cell Physiol 37: 754–761 [Google Scholar]
- Kyle DJ, Ohad I, Arntzen CJ (1984) Membrane proteins damage and repair: selective loss of a quinone protein function in chloroplast membranes. Proc Natl Acad Sci USA 81: 4070–4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- Lee HY, Hong YN, Chow WS (2001) Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in Capsicum annuum L. leaves. Planta 212: 332–342 [DOI] [PubMed] [Google Scholar]
- Los DA, Ray MK, Murata N (1997) Differences in the control of the temperature-dependent expression of four genes for desaturases in Synechocystis sp. PCC 6803. Mol Microbiol 25: 1167–1175 [DOI] [PubMed] [Google Scholar]
- Mattoo AK, Hoffman-Falk H, Marder JB, Edelman M (1984) Regulation of protein metabolism: coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci USA 81: 1380–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo AK, Marder JB, Edelman M (1989) Dynamics of the photosystem II reaction center. Cell 56: 241–246 [DOI] [PubMed] [Google Scholar]
- Mattoo AK, Marder JB, Gaba V, Edelman M (1986) Control of 32 kDa thylakoid protein degradation as a consequence of herbicide binding to its receptor. In G Akoyunoglou, H Senger, eds, Regulation of Chloroplast Differentiation, Plant Biology Series. Alan R. Liss, New York, pp 607–613
- Mattoo AK, Pick U, Hoffman-Falk H, Edelman M (1981) The rapidly metabolized 32,000-dalton polypeptide of the chloroplast is the “proteinaceous shield” regulating photosystem II electron transport and mediating diuron herbicide sensitivity. Proc Natl Acad Sci USA 78: 1572–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis A (1999) Photosystem II damage and repair cycle in chloroplasts: what modulates the rate of photodamage in vivo? Trends Plant Sci 4: 130–135 [DOI] [PubMed] [Google Scholar]
- Mohamed A, Jansson C (1989) Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol Biol 13: 693–700 [DOI] [PubMed] [Google Scholar]
- Mohamed A, Eriksson J, Osiewacz HD, Jansson C (1993) Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. Mol Gen Genet 238: 161–168 [DOI] [PubMed] [Google Scholar]
- Muhlbauer SK, Eichacker LA (1998) Light-dependent formation of the photosynthetic proton gradient regulates translation elongation in chloroplasts. J Biol Chem 273: 20935–20940 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20: 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohad I, Kyle DJ, Arntzen CJ (1984) Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptide in chloroplast membranes. J Cell Biol 99: 481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Murata N (1981) Chilling susceptibility of the blue-green alga Anacystis nidulans: effect of growth temperature. Plant Physiol 67: 176–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35: 15–44 [Google Scholar]
- Sippola K, Aro E-M (2000) Expression of psbA genes is regulated at multiple levels in the cyanobacterium Synechococcus sp. PCC 7942. Photochem Photobiol 71: 706–714 [DOI] [PubMed] [Google Scholar]
- Stanier RY, Kunisawa R, Mandel M, Cohen-Bazire G (1971) Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol Rev 35: 171–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi F, Yamamoto Y, Inagaki N, Satoh K (1993) Recognition signal for the C-terminal processing protease of D1 precursor protein in the photosystem II reaction center: an analysis using synthetic oligopeptides. FEBS Lett 326: 227–231 [DOI] [PubMed] [Google Scholar]
- Taguchi F, Yamamoto Y, Satoh K (1995) Recognition of the structure around the site of cleavage by the carboxy-terminal processing protease for D1 precursor protein of the photosystem II reaction center. J Biol Chem 270: 10711–10716 [DOI] [PubMed] [Google Scholar]
- Trebitsh T, Danon A (2001) Translation of chloroplast psbA mRNA is regulated by signals initiated by both photosystems II and I. Proc Natl Acad Sci USA 98: 12289–12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi E, Aro E-M (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93: 2213–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyystjärvi T, Herranen M, Aro E-M (2001) Regulation of translation elongation in cyanobacteria: membrane targeting of the ribosome nascent-chain complexes controls the synthesis of D1 protein. Mol Microbiol 40: 476–484 [DOI] [PubMed] [Google Scholar]
- van Wijk KJ, Eichacker L (1996) Light is required for efficient translation elongation and subsequent integration of the D1 protein into photosystem II. FEBS Lett 388: 89–93 [DOI] [PubMed] [Google Scholar]
- Zer H, Ohad I (1995) Photoinactivation of photosystem II induces changes in the photochemical reaction center II abolishing the regulatory role of the QB site in the D1 protein degradation. Eur J Biochem 231: 448–453 [DOI] [PubMed] [Google Scholar]
- Zhang L, Paakkarinen V, van Wijk KJ, Aro E-M (2000) Biogenesis of the chloroplast-encoded D1 protein: regulation of translation elongation, insertion, and assembly into photosystem II. Plant Cell 12: 1769–178111006346 [Google Scholar]