Abstract
While callose is a well-known permeability barrier and leak sealant in plant cells, it is largely unknown whether this cell wall polymer can also serve as a load-bearing structure. Since callose occurs in exceptionally large amounts in pollen, we assessed its role for resisting tension and compression stress in this cell. The effect of callose digestion in Solanum chacoense and Lilium orientalis pollen grains demonstrated that, depending on the species, this cell wall polymer represents a major stress-bearing structure at the aperture area of germinating grains. In the pollen tube, it is involved in cell wall resistance to circumferential tension stress, and despite its absence at the growing apex, callose is indirectly involved in the establishment of tension stress resistance in this area. To investigate whether or not callose is able to provide mechanical resistance against compression stress, we subjected pollen tubes to local deformation by microindentation. The data revealed that lowering the amount of callose resulted in reduced cellular stiffness and increased viscoelasticity, thus indicating clearly that callose is able to resist compression stress. Whether this function is relevant for pollen tube mechanics, however, is unclear, as stiffened growth medium caused a decrease in callose deposition. Together, our data provide clear evidence for the capacity of cell wall callose to resist tension and compression stress, thus demonstrating that this amorphous cell wall substance can have a mechanical role in growing plant cells.
β-1,3-Glucan (callose) is one of the most dynamic components of the plant cell wall. It is known to be synthesized and deposited at the outer surface of the plasma membrane by callose synthases that are localized in the membrane (Carpita and Gibeaut, 1993). The synthesis of this amorphous polymer is an important part of plant cell responses to pathogen attacks as well as physical and chemical stresses (Currier, 1957; Esau and Cronshaw, 1967; Coffey, 1976; Delmer and Amor, 1995). While injured plant cells use the polymer as a leak sealant, in certain plant cell types callose is produced during normal development (Esau, 1948; Currier, 1957; Heslop-Harrison, 1964; Scott et al., 1967; Morrison and O'Brien, 1976; Waterkeyn, 1981; Stone and Clarke, 1992). From the location of these callose deposits, it has been concluded that the polymer acts as a permeability barrier, as in pollen mother cell walls (Heslop-Harrison, 1964) and muskmelon endosperm envelopes (Yim and Bradford, 1998); as a matrix for deposition of other cell wall materials, as in developing cell plates and sieve-plate pores; and as a sealing or plugging material at the plasma membrane of pit fields, plasmodesmata, and sieve-plate pores (Eschrich, 1975). Despite the widespread occurrence of callose, its functions other than as a leak-sealing cement or permeability barrier are not well understood (Stone and Clarke, 1992). One of the few suggestions of callose playing a role in cell wall mechanics was made based on circumstantial evidence in cottonseed hairs (Maltby et al., 1979). The abundance of callose in pollen grains and pollen tubes and their interesting growth behavior suggest that callose might have a structural function in these cells (Geitmann, 1999). While indirect evidence has been described, the mechanical aspect of callose in pollen has not previously been assessed by any kind of quantitative approach.
In recent years, it has been increasingly obvious that, for the functional analysis of structural cell components, knowledge of their mechanical characteristics is pivotal. Both in vitro and in vivo approaches have been applied by physicists and biologists to investigate various structural molecules, such as the cytoskeletal elements actin and microtubules (Dogterom and Yurke, 1997; Ingber, 2003). These studies used, in particular, mammalian cells such as fibroblasts and erythrocytes (Discher et al., 1988; Sokabe et al., 1991; Nash and Gratzer, 1993; Knowles et al., 1994; Yuan et al., 1995). The physical properties of the plant cell wall components have been studied intensively, but mostly at the tissue level (Showalter, 1993; Edelmann, 1995; Nolte and Schopfer, 1997; Buntemeyer et al., 1998) or in dead cells (Wimmer et al., 1997). Callose is primarily functional in living plant cells and, therefore, to assess its mechanical function, an in vivo system is essential. Here we investigated the role of callose for cell wall resistance to tension and compression stresses on the cellular level.
Our model system is pollen tube formation and growth. In addition to producing abundant amounts of callose during normal cell development (Geitmann, 1999), these cells grow individually and are thus readily manipulated for mechanical studies in vitro. In flowering plants, pollen grains germinate on a receptive stigma and send pollen tubes down the style toward the ovules with impressive growth rates, thus allowing the delivery of the sperm cells to perform fertilization. The pollen tube could therefore be compared to a tunnel transport system for the male gametes. The pollen tube cell wall is generally composed of two or three layers: an outer pecto-cellulosic sheath and an inner callosic lining (Heslop-Harrison, 1987; Steer and Steer, 1989). It has to resist both tension stress and compression stress: Tension stress in the cell wall is created at the growing hemisphere-shaped apex of the tube and at the pollen grain aperture since cell growth is governed by a tightly controlled equilibrium between inner driving forces, the expansion of the existing cell wall, and the continuous insertion of new cell wall material (Taylor, 1997). While there is an ongoing discussion about the nature of growth-driving forces in apically growing cells (Money, 1997, 2001; Harold, 2002), there seems to be no doubt that the key factor counteracting these forces is the apical, expanding part of the cell wall. It must be able to continuously yield to the growth-propulsing forces, while at the same time it needs to provide enough resistance to prevent bursting. Tension forces are also established in the cylindrical region of the tubular cell due to the action of turgor pressure. The mechanics of thin-wall pressure vessels dictates that the turgor-induced circumferential stress in the cylindrical part is twice as high as the tension stress in the wall of the apical hemisphere (Green, 1962; Lockhart, 1965). Therefore, maintenance of the cylindrical shape is certain to require tension-withstanding properties in the distal cell wall. At the same time, in the in planta situation, compression stress is presumably exerted on the cylindrical cell by the surrounding tissue, thus adding additional requirements to its structural characteristics.
To understand the mechanical properties of callose, we analyzed which role it plays in the cell wall's capacity to resist the different types of stresses present in the growing pollen tube. To do so, we related structure and function combining two approaches: (1) localization of callose in pollen grains and tubes in two different species; and (2) analysis of the effect of enzymatic callose degeneration on germination and growth behavior (tension stress) and resistance to lateral deformation (compression stress).
RESULTS
Callose Digestion Affects Pollen Germination
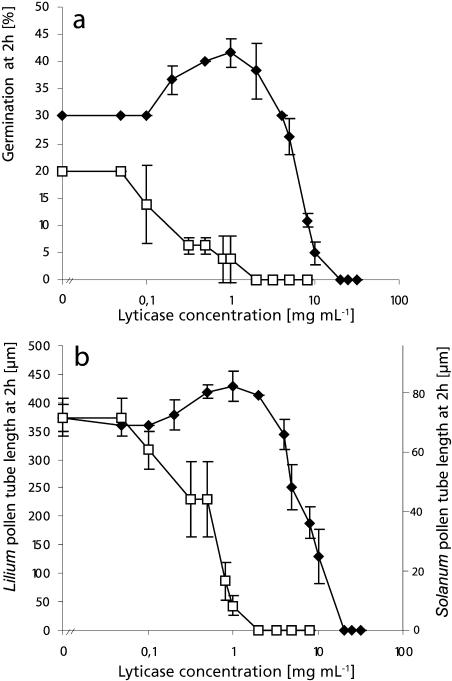
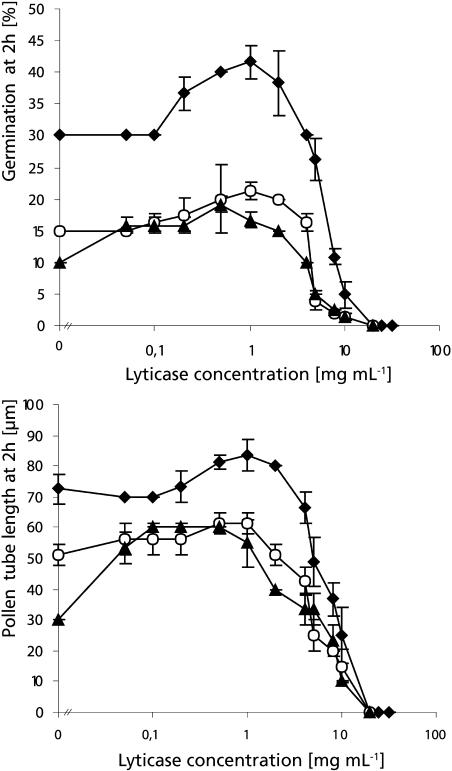
To establish whether callose is a structural factor in the resistance of the cell wall to cytoplasmic germination driving forces, we tested whether lowering its amount influenced germination behavior and pollen tube growth rate in Solanum chacoense and Lilium orientalis. To do so, we used the enzyme lyticase, which specifically hydrolyzes callose. Figure 1 reveals that high lyticase concentrations reduced the percentage of pollen germination and also resulted in a shorter pollen tube length after 2 h. The sensitivity to the enzyme differed between the two species, however. The lyticase concentration that was necessary to completely inhibit germination of Solanum pollen grains was 10-fold higher than the one effective in Lilium. Surprisingly, only in Solanum pollen was the inhibitory effect attributable to visible bursting (95% of pollen grains burst upon addition of 20 mg mL−1 lyticase), whereas Lilium pollen grains seemed to have unaltered morphology at inhibitory enzyme concentrations.
Figure 1.
Effect of lyticase on germination percentage and pollen tube length. Germination percentage (A) and pollen tube length (B) at 2 h in Solanum (♦) and Lilium (□) pollen grown in liquid medium are plotted against lyticase concentration. High enzyme concentrations were inhibitory for both species, but Lilium had a 10-fold higher sensitivity compared to Solanum. Only germination in Solanum was stimulated at moderate enzyme concentrations.
Moderate enzyme concentrations caused stimulation of both germination percentage (Fig. 1A) and pollen tube length at 2 h (Fig. 1B) in Solanum pollen. The highest increase in pollen germination (35%) and pollen tube length (15%) was accomplished by addition of lyticase at 1 mg mL−1. In Lilium, on the other hand, no stimulatory effect was observed at 2 h for any of the enzyme concentrations tested. We hypothesized that the increase in tube length observed in Solanum pollen that had germinated in the presence of the enzyme could be due to either of two factors or a combination of both: an earlier onset of the germination process and/or an increase in pollen tube growth rate. To distinguish between these two variables, we applied stimulating concentrations of the enzyme 30 min after Solanum pollen had germinated under control conditions. After additional 90-min incubation in the presence of 2 or 1 mg mL−1 lyticase, pollen tubes treated with the enzyme had a length of 159 ± 3 μm, which was not significantly different from the control cells treated with denatured enzyme, which had a length of 160 ± 3 μm. This indicates that the stimulating effect on pollen tube length was due to an acceleration of the onset of germination but not to a change in pollen tube growth rate.
Solanum and Lilium Pollen Grains Show Different Patterns of Callose Distribution
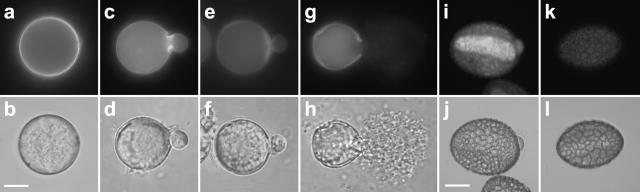
Since the lyticase effect on germination differed between Solanum and Lilium pollen, we investigated whether this was due to the distribution of the polymer in each of the species. As expected, fluorescent label with decolorized aniline blue revealed significant differences in the callose patterns of the two species. In ungerminated Solanum pollen grains (Fig. 2, A and B), callose label was present uniformly in the cell wall. Shortly prior to the onset of germination, one of the three apertures was strongly labeled for callose and later featured an accumulation at the base of the emerging pollen tube (Fig. 2, C and D). Lilium pollen grains showed weak overall label and some callose accumulation at the entire colpus area. No significant accumulation was observed at the base of the emerging tube, however (Fig. 2, I and J). This might indicate that callose at the aperture plays a less important role in Lilium germination compared to Solanum.
Figure 2.
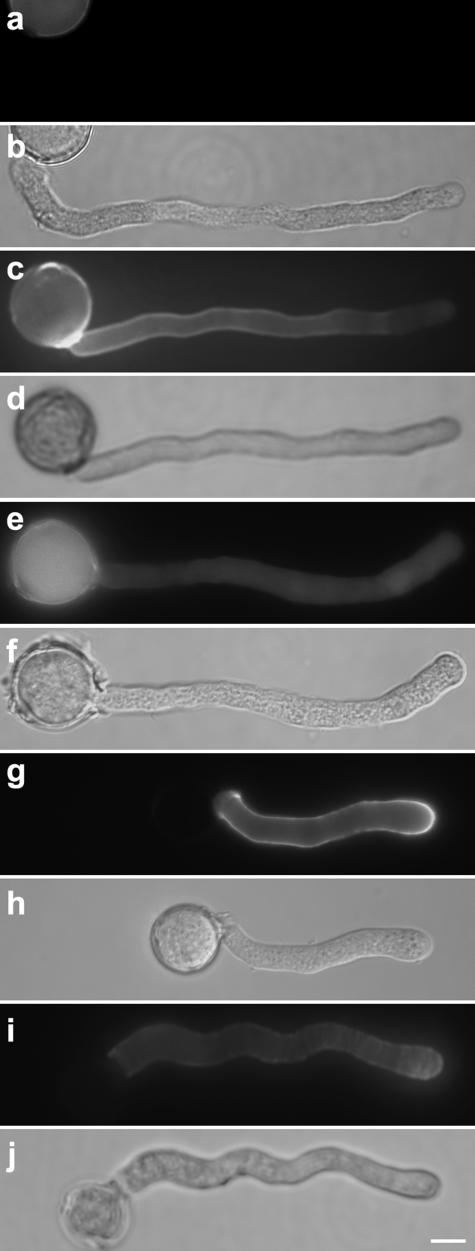
Effect of lyticase on the abundance of callose in Solanum and Lilium pollen grains observed after labeling with decolorized aniline blue and corresponding differential interference contrast (DIC) images. A and B, Solanum pollen at the beginning of imbibition in liquid control medium. Callose is distributed evenly around the grain. C and D, Solanum pollen after 30 min of imbibition in liquid control medium. Callose accumulations are visible at the base of the emerging pollen tube. E and F, Solanum pollen grain germinating in solidified medium shows significantly weaker callose label. G and H, Solanum pollen after 30 min of imbibition in medium containing 8 mg mL−1 lyticase. The pollen grain has burst and callose label at the functional aperture is weak. I and J, Lilium pollen after 45 min of imbibition in control medium. Callose label is present in the colpus, but no accumulation is visible at the base of the emerging pollen tube. K and L, Lilium pollen after 45 min of imbibition in 0.5 mg mL−1 lyticase. Callose label is very weak; the pollen grain remains intact. Bars = 10 μm (A–H); 30 μm (I–L).
To confirm the effect of lyticase on the amount of callose in the Solanum and Lilium pollen grain cell wall, we applied enzyme concentrations that reduced pollen germination to approximately one-third (8 mg mL−1 for Solanum and 0.5 mg mL−1 for Lilium). This treatment resulted in a significant loss of callose label in both Lilium and Solanum pollen grains while causing bursting in Solanum grains only (Fig. 2, G, H, K, and L).
Cell Wall Thickness Influences the Stability of Pollen Grain Architecture
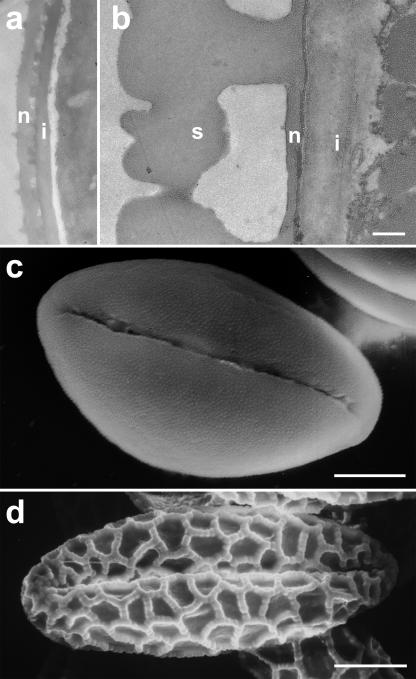
Our data showed that exposure to lyticase at high concentrations inhibited germination in both Solanum and Lilium pollen, but only in the former was bursting induced. This raised the question as to whether pollen grain architecture or other cell wall components might withstand the turgor pressure in Lilium but not in Solanum once callose was digested. To analyze the effect of pollen grain architecture, we compared the cell wall thickness and exine ornamentation in both species. Transmission electron microscopy revealed that the cell wall of Lilium pollen grains was 3.6 μm thick, whereas that of Solanum pollen grains had an average thickness of 0.5 μm. In particular, the intine and the sexine were considerably thicker in Lilium pollen grains, whereas the nexine thickness was comparable between the two species (Fig. 3, A and B). Furthermore, scanning electron microscopy showed that the Lilium sexine had a coarse reticulate structure, whereas the surface of Solanum pollen grains was characterized by fine scabrate surface ornamentation (Fig. 3, C and D). These observations are consistent with, but not conclusive for, Lilium pollen grain walls having higher structural resistance against internal turgor forces, thus providing a mechanical fortification against tension stress.
Figure 3.
Electron micrographs of the pollen grain cell wall structure. Transmission electron micrographs reveal that Solanum pollen grains (A) have a considerably thinner intine (i) than Lilium grains (B). While nexine (n) thickness is comparable, the sexine (s) of Lilium is extremely thick. Scanning electron micrographs show also that the Lilium sexine is structured in a coarse reticulate pattern (D), whereas Solanum is ornamented by very small scabrate structures (C). Bars = 0.5 μm (A and B); 5 μm (C); 20 μm (D).
Solanum and Lilium Pollen Grains Show Different Patterns of Cellulose and Pectin Distributions in the Pollen Grain Aperture
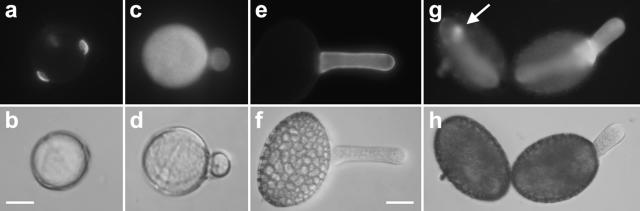
To investigate whether other cell wall components were responsible for preventing Lilium but not Solanum pollen grains from bursting upon callose digestion, we examined the distribution of other major cell wall polysaccharides in both species. Immunolabel for pectins revealed that, in Solanum pollen grains, acidic pectins (labeled with monoclonal antibody JIM5) were present at the apertures (Fig. 4, A and B), whereas methyl-esterified pectins (labeled with monoclonal antibody JIM7) were absent from the pollen grain (data not shown, since no label visible; compare with Fig. 7G). Calcofluor label for cellulose was rather weak and evenly distributed in Solanum pollen grains (Fig. 4, C and D). Callose and acidic pectins therefore seemed to be the main cell wall components at the Solanum pollen grain apertures. In Lilium pollen grains, on the other hand, label for methyl-esterified pectins was absent (data not shown) and that for acid pectins was extremely weak in the entire grain (Fig. 4, E and F). Calcofluor label revealed weak label all around the grain, but a considerable accumulation of cellulose at the site of outgrowth of a future pollen tube and at the base of the tube after germination had occurred (Fig. 4, G and H).
Figure 4.
Fluorescent label for acidic pectins (monoclonal antibody JIM5) and cellulose (calcofluor white) in germinating pollen grains and corresponding DIC images. A and B, Solanum pollen grain showing intensive label for acidic pectins at all three apertures. C and D, Germinating Solanum pollen grain showing weak and evenly distributed label for cellulose. E and F, Lilium pollen grain showing very weak label for acidic pectins. The prominent label in the pollen tube serves as a control for the success of the label technique. G and H, Ungerminated and germinated Lilium pollen grains showing weak cellulose label around the grain. The ungerminated grain shows a spot of denser label (arrow) that might be the site of an emerging pollen tube. The germinated grain shows a considerable accumulation of cellulose at the base of the newly formed pollen tube. Bars = 10 μm (A–D); 30 μm (E–H).
Figure 7.
Callose and pectin content in pollen tubes. A–F, Solanum pollen tubes labeled for callose with decolorized aniline blue and corresponding DIC images. Tubes grown in solidified medium (A and B) showed reduced abundance of callose compared to the control tubes grown in liquid medium (C and D). The presence of 1 mg mL−1 lyticase in liquid medium reduced the abundance of callose in the pollen tube (E) and caused an increase of the pollen tube diameter (F) compared to the control tubes (C and D). G to J, Solanum pollen tubes labeled for methyl-esterified pectins with monoclonal antibody JIM7 and corresponding DIC images. Pollen tubes grown in the presence of 1 mg mL−1 lyticase (I and J) showed significantly weaker label than the control cells (G and H). The distribution pattern remained the same, however, with a higher concentration of methyl-esterified pectin at the pollen tube apex. Bar = 10 μm.
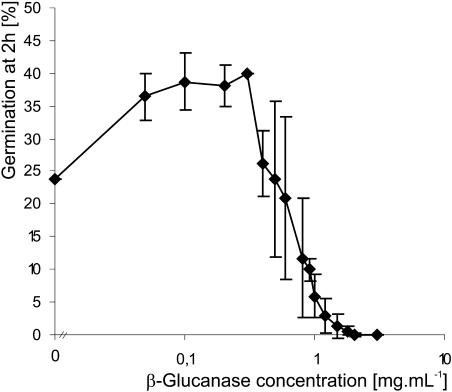
The presence of cellulose, in addition to callose, at the aperture of Lilium pollen grains might therefore provide an explanation for the absence of bursting after callose digestion in these grains. To test this hypothesis, we added β-glucanase, an enzyme that digests both callose and cellulose, as well as cellulase, an enzyme that is specific for cellulose, to Lilium pollen. We observed that at 3 mg mL−1 β-glucanase inhibited germination of Lilium by causing bursting of 95% of pollen grains. Furthermore, moderate amounts of β-glucanase significantly stimulated the percentage of germination in Lilium (Fig. 5), whereas this effect was never observed when cellulase and lyticase were added separately as summarized in Table I. These results clearly show that either component, callose or cellulose, at the Lilium aperture is sufficient to prevent bursting of pollen grains, and both need to be softened to stimulate germination.
Figure 5.
Effect of β-glucanase on Lilium pollen germination. Moderate concentrations of the enzyme are able to stimulate the germination percentage.
Table I.
Effect of cell wall-digesting enzymes lyticase, cellulase, and β-glucanase on germination percentage and pollen grain morphology in Lilium
Addition of moderate amounts of β-glucanase stimulated germination percentage and higher concentrations caused bursting, whereas neither of these effects was observed after addition of cellulase or lyticase. Relative increase (RI) is calculated as RI = (GO − GC)/GC, with GO germination at optimal enzyme concentration and GC germination at zero enzyme concentration (control).
| Enzyme
|
Stimulation of Germination
|
Inhibition of Germination
|
||
|---|---|---|---|---|
| Relative Increase Compared with Untreated Control | Optimal Enzyme Concentration | Enzyme Concentration Achieving Complete Inhibition | Pollen Grain Morphology | |
| % | mg mL−1 | mg mL−1 | ||
| Lyticase | 0 | n/a | 2 | Intact |
| Cellulase | 0 | n/a | 50 | Intact |
| β-Glucanase | 70 | 0.05 | 2 | Burst |
The Lyticase Effect Depends on the Stiffness of the Medium
Previous studies using pectinase have shown that the stiffness of the growth medium affects the abundance of pectin polymers in the cell wall of pollen grains and tubes (Parre and Geitmann, 2004). This finding has implications for the comparison between the in vitro and in vivo situations. Since in Solanum in vivo pollen tube growth takes place within the solid style of the receptive flower, we wanted to investigate whether the stiffness of the medium influenced the pollen response to lyticase. As shown earlier (Parre and Geitmann, 2004), solidification of the medium reduced the germination percentage in this species (Fig. 6).
Figure 6.
Effect of various lyticase concentrations and medium stiffness on Solanum pollen germination and pollen tube length. Germination percentage and pollen tube length were assessed after 2 h in liquid medium (♦), media solidified with 20 mg mL−1 (○), and 40 mg mL−1 (▴) agarose. Both were reduced in stiffer media, but moderate amounts of lyticase nevertheless had a significant stimulating effect. However, optimal enzyme concentrations and relative stimulation in stiff media had a tendency to differ from those observed in liquid medium, as summarized in Table II.
Lyticase had a stimulatory effect in medium containing either 20 or 40 mg mL−1 agarose, but, interestingly, the relative stimulation of the germination percentage was greater in solidified medium compared to the liquid control. Furthermore, the enzyme concentration necessary to accomplish this was 2- to 5-fold lower compared to liquid media, as summarized in Table II. Optimal concentrations for the stimulation of pollen tube length at 2 h were also shifted, with the highest increase of tube length reaching 100% in agarose-complemented media compared to liquid medium in which a maximal increase in length of 14% was achieved. Similarly to germination, lyticase concentrations necessary to increase pollen tube length at 2 h were 2- to 5-fold lower in solidified than in liquid medium (Table II).
Table II.
Optimal lyticase concentrations that result in stimulation of Solanum pollen germination and tube length at 2 h in liquid and solidified media
Relative increase was calculated as RI = (GO − GC)/GC, with GO germination percentage or pollen tube length, respectively, at optimal enzyme concentration and GC germination percentage or pollen tube length, respectively, at zero enzyme concentration (control).
| Medium Stiffness
|
Maximal Germination at 2 h
|
Maximal Pollen Tube Length at 2 h
|
||
|---|---|---|---|---|
| Relative Increase Compared with Untreated Control | Optimal Lyticase Concentration | Relative Increase Compared with Untreated Control | Optimal Lyticase Concentration | |
| % | mg mL−1 | % | mg mL−1 | |
| Liquid | 36 | 1 | 14 | 1 |
| 20 mg mL−1 agarose | 41 | 1 | 19 | 0.5–1 |
| 40 mg mL−1 agarose | 91 | 0.5 | 100 | 0.2–0.5 |
The Amount of Callosic Cell Wall Is Affected by the Stiffness of the Growth Medium
The effect of medium stiffness on pollen sensitivity toward lyticase treatment suggests that the physical properties of the environment in which the pollen germinates might affect the force equilibrium governing pollen germination and growth by altering cell wall thickness. To investigate whether the abundance of callose in the pollen grain indeed differed between liquid and solidified media, we compared the fluorescence intensity of callose label. Pollen grains were germinated either in liquid medium or in medium solidified by the addition of Gel-Gro to allow for resuspension of pollen prior to label. Label with decolorized aniline blue revealed that the amount of callose was significantly reduced in pollen grains grown in solidified medium, although the distribution pattern remained the same, featuring an accumulation of callose at the functional aperture (Fig. 2, E and F). Furthermore, we observed that, in stiff medium, pollen tubes as well showed a reduction of callose content (Fig. 7, A and B), while the distribution remained the same, featuring the characteristic absence of callose at the tube apex as observed in control pollen tubes (Fig. 7, C and D).
Lyticase Affects the Pollen Tube Diameter
It is well known, and our data confirm, that callose is absent from the growing pollen tube apex, whereas significant amounts of the polymer are present in the distal part of the cell. We wanted to investigate whether in these distal parts callose plays a role in the resistance to circumferential tension stress in the cell wall created by the internal turgor pressure. To do so, we assessed whether the pollen tube diameter changes in the presence of various concentrations of lyticase. At 0.1 mg mL−1 and above, lyticase caused an increase in pollen tube diameter both at the apex and the distal regions compared to control pollen tubes, as summarized in Table III. Decolorized aniline blue label for callose confirmed that, after addition of 1 mg mL−1 lyticase, the amount of cell wall callose in the pollen tube wall was reduced compared with the control sample (Fig. 7, C–F).
Table III.
Effect of lyticase on pollen tube diameter at the apex and at distal locations of Solanum pollen tubes
The presence of 0.1 mg mL−1 lyticase and above caused a significant increase in diameter at both locations.
| Lyticase Concentration | Distal Diameter | Apical Diameter |
|---|---|---|
| mg mL−1 | μm | μm |
| 0 | 6.96 ± 0.56 | 6.08 ± 0.42 |
| 0.1 | 7.73 ± 0.50 | 6.83 ± 0.85 |
| 1 | 8.19 ± 0.42 | 7.28 ± 0.61 |
Callose Digestion Has an Indirect Effect on Pectin Distribution
Since the pollen tube apex showed an increase in diameter in the presence of lyticase even though no visible amounts of callose are present in the pollen tube tip, we suspected that other cell wall components were affected by the continuous digestion of callose. To confirm this, we labeled cellulose and pectins in pollen tubes treated with lyticase and compared fluorescence intensity and localization with that of control pollen tubes. Calcofluor white label for cellulose revealed that the amount and distribution of cellulose remained the same after callose digestion by lyticase at 1 mg mL−1 (data not shown). Similarly, immunolabel with JIM5 for acidic pectins was comparable between treated and untreated pollen tubes. Surprisingly, immunolabel with JIM7 revealed that the amount of methyl-esterified pectins was somewhat reduced in pollen tubes treated with 1 mg mL−1, although the distribution pattern remained the same with methyl-esterified pectin present mainly at the pollen tube apex (Fig. 7, G–J). Since alteration of the pectin contents caused apical swelling in pollen tubes (Parre and Geitmann, 2004), the observed indirect effect of lyticase on the abundance of methyl-esterified pectins most likely provides the explanation for the increased apical diameter visible after application of 1 mg mL−1 lyticase.
Callose Plays a Role in the Resistance to Compression Stress
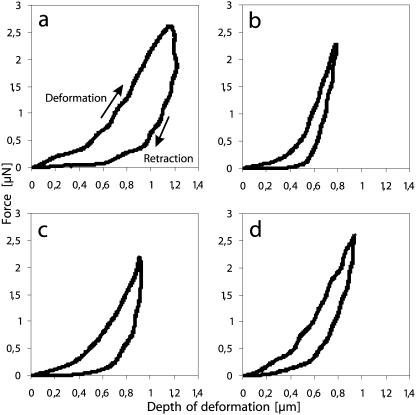
To investigate whether callose provides compression stress resistance on the individual cell level, we assessed local cellular stiffness and viscoelasticity with microindentation, a technique that has proven useful for the assessment of pollen tube cytomechanics (Geitmann et al., 2004; Geitmann and Parre, 2004; Parre and Geitmann, 2004). Local deformations were performed at two positions of the tube, at the growing apex and at a distal position around 30 μm from the apex, where visible amounts of callose were observed. As shown earlier (Geitmann and Parre, 2004), normally growing Solanum pollen tubes were characterized by a difference in stiffness and in the degree of viscoelasticity between the growing apex and the distal region, with the apex being more viscoelastic and having a stiffness 85% ± 5% of that of the distal region in an individual tube. For lyticase treatment, we used 1 mg mL−1, since this concentration caused an increase in pollen tube diameter and a significant reduction in aniline blue fluorescence label intensity. We observed that the distal stiffness of pollen tubes that were germinated in the presence of 1 mg mL−1 lyticase decreased to 2.33 ± 0.25 μN μm−1 (Fig. 8A), compared with control values of 4.97 ± 0.17 μN μm−1 (Fig. 8B), whereas apical stiffness (4.02 ± 0.38 μN μm−1; Fig. 8C) remained virtually identical to that of untreated pollen tubes (4.06 ± 0.20 μN μm−1; Fig. 8D). As a consequence, the ratio between apex and distal local stiffness in individual tubes increased to 177% ± 14% (n = 6).
Figure 8.
Force deformation graphs for microindentation experiments. Local deformations were performed at the apex and at a distal location 30 μm behind the tip of growing Solanum pollen tubes. The top curve represents the deforming and the bottom curve the retracting movement of the stylus. The slope of the linear part of the deforming curve indicates the stiffness, whereas the surface area between the two curves expresses the delay upon retraction of the deforming stylus (hysteresis), which is an indication for the dissipated energy and thus the viscoelasticity of the deformed object. The deformation profiles at the distal pollen tube location show that the presence of 1 mg mL−1 lyticase caused a dramatic decrease in stiffness and an increase in viscoelasticity (A) compared to the control (B). At the apex, on the other hand, neither of these parameters was significantly affected by lyticase (C) if compared to the control situation (D).
Interestingly, contrary to the control samples, the deformation at the distal area of lyticase-treated pollen tubes also generally revealed high hysteresis upon retraction of the stylus, thus indicating an increase in the overall cellular viscoelasticity. Figure 8, A to D, illustrates the differences in the force-distance profiles concerning stiffness and viscosity for both the apex and distal regions of the tube.
DISCUSSION
Cell Wall Resistance to Tension Stress at the Pollen Grain Aperture Depends on Callose
The pollen grain aperture has several interesting functions. It provides routes for transfer of water and other substances and allows for harmomegathy, the process by which pollen grains change in shape to accommodate variations in the volume of the cytoplasm caused by changing hydration. From a mechanical point of view, the most interesting function is the emergence of the pollen tube. During this process, the aperture covering the cell wall has to yield to germination driving forces to allow pollen tube emergence. At the same time, it has to withstand these same forces to avoid bursting of the cell at this weakened location in the otherwise rigid shell protecting the pollen grain protoplast (Roggen and Stanley, 1969; El-Ghazaly, 1999; Li et al., 1999; Johnson and McCormick, 2001; Ressayre et al., 2002). The presence of callose in the pollen grain and, in particular, at its aperture therefore provides an excellent experimental system to test whether or not callose is able to resist tension forces in living plant cells.
Previous studies using β-1,3-glucanase, an enzyme-digesting callose, revealed a stimulatory effect on pollen germination in Pyrus communis at moderate concentrations (Roggen and Stanley, 1969). This was a first indication for callose playing a role in controlling the equilibrium between the germinating driving forces and the resisting cell wall. In turn, this would imply that callose has the capability of resisting tension stress in the cell wall, thus attributing to it a mechanical function that has not been previously described. Our data corroborate the findings by Roggen and Stanley (1969) and provide more detailed information by relating the mechanical functioning of callose to its distinct distribution patterns in two species, S. chacoense and L. orientalis. Germination of Solanum pollen grains was stimulated by lyticase, and higher enzyme concentrations caused bursting of the grains at the aperture. It seems, therefore, that slight softening of the cell wall allowed the driving forces to act earlier and more effectively, causing a higher germination percentage and earlier onset of the process. On the other hand, digesting the callosic cell wall too much destroyed the load-bearing structure, resulting in bursting, as the internal turgor pressure exceeded the cell wall resistance. Both findings are consistent with the fact that callose is abundant at the functional aperture in pollen grains of Solanum, whereas most other major cell wall components were present only in small amounts at this cellular location. Similar compositions of aperture cell walls have been described for other species (Geitmann et al., 1995; Aouali et al., 2001; Suarez-Cervera et al., 2002). The fact that lyticase treatment was sufficient to induce bursting in Solanum indicated that acidic pectins, the most abundant component next to callose, were not able to withstand the cellular turgor once callose was digested. Callose does, therefore, seem to be the main tension stress-bearing structure in the aperture cell wall of Solanum pollen grains. Further support for this concept was provided by the observation that Solanum pollen grains germinating in stiffened medium showed a shift in the sensitivity toward lyticase, which was caused by a reduced amount of callose in the functional aperture. This is consistent with the concept of the stiff medium counteracting the cellular turgor, thus reducing the tension stress in the aperture cell wall, which in turn needs less callose.
Pollen grains of Lilium, on the other hand, lacked prominent callose accumulations at the site of the emerging pollen tube. Consistent with this, germination in this species was not stimulated by any of the lyticase concentrations tested. Moreover, Lilium pollen grains remained morphologically intact when treated with high lyticase concentrations, even though germination was inhibited. We cannot exclude that the thicker, coarser overall cell wall architecture provided mechanical fortification against turgor forces in pollen grains of this species. However, the observed lower amount of callose at the site of pollen tube emergence is consistent with it playing a less important role in aperture cell wall mechanics, thus making the grain less prone to bursting upon lyticase treatment. The abundance of cellulose in Lilium pollen grain apertures suggests that the task of resisting turgor forces is shared by both callose and cellulose in this species. This is corroborated by the fact that only simultaneous digestion of both polymers by β-glucanase was able to stimulate germination, whereas neither lyticase nor cellulase alone could achieve this effect.
What remains puzzling is the fact that higher concentrations of lyticase were nevertheless able to inhibit germination in Lilium. Since the grains did not burst in the presence of the enzyme, it is unclear which mechanism led to inhibition in this species. It might be that the development of a callose deposit at the aperture is critical for pollen germination by controlling water influx, and continuous digestion of the polysaccharide disturbs the regulation mechanism of the process.
Callose Is Indirectly Involved in Tension Stress Resistance at the Growing Pollen Tube Apex
As has been observed in numerous species (Roggen and Stanley, 1969; Li et al., 1994, 1997, 2002; Derksen, 1996; Ferguson et al., 1998; Hasegawa et al., 1998; Holdaway-Clarke and Hepler, 2003), in Solanum pollen tubes callose was detected only starting approximately 20 to 30 μm behind the growing apex. This suggests that callose is unlikely to be involved directly in the pollen tube elongation process (Roggen and Stanley, 1969). While moderate pectin digestion resulted in an increase in pollen tube growth rate (Parre and Geitmann, 2004), no growth stimulation occurred when 30-min-old pollen tubes were treated with lyticase. This confirms that the increased pollen tube length observed in samples with continuous presence of lyticase was primarily the consequence of an accelerated germination resulting from the enzyme dissolving the callose inside the pore intine and initial pollen tube (Roggen and Stanley, 1969). Lyticase was not completely without effect on the pollen tube apex, however, since, surprisingly, a significant increase in apical diameter was observed when pollen was germinated in the presence of lyticase. Consistent with this, application of lyticase on older pollen tubes caused apical swelling (E. Parre, unpublished data). Unless callose was present in the pollen tube apex in invisible amounts, this finding suggests an indirect effect of callose digestion on the deposition and/or configuration of other cell wall polymers.
The labeling pattern for the two other main cell wall components, cellulose and pectin, showed clearly that cellulose distribution and abundance was not affected in the presence of lyticase. However, the amount of methyl-esterified pectin deposition was reduced after callose digestion. Earlier studies have shown that a reduction in the amount of pectins in the cell wall caused apical swelling in pollen tubes (Parre and Geitmann, 2004), thus being consistent with the data presented here. This intriguing result suggests that, although absent from the growing apex, callose seems to play an indirect role in the turgor/cell wall equilibrium involved in apical expansion by interacting with the deposition of methyl-esterified pectin.
Callose Is a Crucial Factor in Cell Wall Resistance to Circumferential Tension Stress
For geometric reasons, the circumferential tension stress in the cell wall of the cylindrical part of the tube is twice as high as that in the wall of the dome-shaped apex (Green, 1962; Lockhart, 1965). Yet, in normally growing pollen tubes, it is the apex that yields to allow expansion, whereas the cylinder remains stable (Derksen, 1996; Geitmann and Cresti, 1998; Hepler et al., 2001). The distal cell wall must therefore be strong enough to resist considerable tension forces created by the internal pressure. Our previous experiments have shown that application of pectinase caused the swelling of the apical part of the pollen tube, whereas the diameter of the distal tube is maintained, thus indicating that pectin is not a major structural factor in creating this resistance to tension stress in the cylindrical part (Parre and Geitmann, 2004).
The presence of callose in the distal regions of the pollen tube suggested that this polymer might possibly be a load-bearing component. The observed increase of cellular diameter in pollen tubes grown in the presence of lyticase confirms this hypothesis. The fact that bursting was never observed in the distal part of the tube indicates, however, that callose is not the only cell wall component resistant to circumferential tension stress. Cellulose is likely to play an important role as well.
Callose Is Able to Resist Compression Stress But Does Not Necessarily Have That Function in Pollen Tubes
Microindentation studies have shown that normally growing pollen tubes of S. chacoense and Papaver rhoeas are characterized by differences in the mechanical parameters of the cell between the growing apex and the cylindrical distal part of the cell (Geitmann and Parre, 2004; Parre and Geitmann, 2004). The apex is generally less stiff than the distal part of the same tube, with the apical stiffness amounting to 85% of the value for the distal region in Solanum (Parre and Geitmann, 2004). Furthermore, the distal region reacts almost completely elastic to deformation, whereas the apex shows viscoelastic behavior.
The data presented here show clearly that digesting the callosic cell wall was sufficient to dramatically reduce the cellular stiffness and increase the cellular viscoelasticity in the distal part of Solanum pollen tubes. On the other hand, no significant change in cellular mechanical parameters was observed in the apex of lyticase-treated pollen tubes, thus confirming that callose does not play an important role in the cell wall mechanics of this part of the cell.
These results provide clear evidence that callose has load-bearing capacities and thus could theoretically have a stabilizing function in the mature cylindrical part of the pollen tube. One would presume that in planta pollen tubes have to exert mechanical resistance forces to withstand the compression stress exerted by the surrounding transmitting tissue. Of all tip-growing cell types, this function would seem to be particularly important in the pollen tube, since it serves as a delivery tunnel for the male gametes on their way from the pollen grain to the ovule and, therefore, must not collapse before their passage has taken place. It was therefore puzzling to observe that pollen tubes grown in stiffened medium revealed an increase in sensitivity to lyticase due to a reduced amount of callose in their cell walls, as confirmed by fluorescence label. Since the stiffened medium should be expected to exert lateral deformation forces on the pollen tubes, this finding is not consistent with a compression load-bearing function of callose in pollen tubes. On the other hand, this corroborates earlier observations that revealed that cellular stiffness at pollen tube locations featuring callosic plugs was not higher than that at locations in adjacent turgescent parts of the cell (A. Geitmann, unpublished data). Only in distal parts of pollen tubes that had lost turgor pressure were callosic plugs stiffer than the adjacent parts of the cell. This indicated that the hydroskeleton established by the turgor pressure is an important, if not the only, factor in compression resistance.
In summary, the most important structural function of callose in pollen seems to be the control of the cell wall-turgor equilibrium at the functional pollen grain aperture and the resistance toward circumferential tension stress in the distal pollen tube cell wall. Our results therefore show conclusively that callose does not only have a function as a leak sealant or as a layer controlling water permeability, but also it is able to resist tension stress, and it is used in that function, albeit perhaps not exclusively, in pollen grains and pollen tubes.
We showed that callose is also able to resist compression stress, but this function does not seem to play a role in pollen tubes. Whether or not other cell wall or cytoplasmic components are involved in the resistance to lateral deformation stress in these cells, or whether the hydrostatic pressure established by the turgor is the only structural feature, remains to be elucidated.
MATERIALS AND METHODS
Pollen Tube Growth
Solanum chacoense plants were grown in the Montreal Botanical Garden greenhouses, and Lilium orientalis was obtained from a local flower shop. Pollen was collected after dehiscence, dehydrated, and stored at −20°C. On the day of use, pollen was rehydrated and cultivated in drops of liquid or solidified media. The latter was obtained by addition of low-melting agarose (Agarose Type I-B Low EEO; Sigma, St. Louis) or Gel-Gro (gellan gum; gel strength twice as high as that of agarose; ICN Biomedical, Aurora, OH). The growth medium (GM) contained 100 μg mL−1 H3BO3, 300 μg mL−1 Ca(NO3)2 × H2O, 100 μg mL−1 KNO3, 200 μg mL−1 MgSO4 × 7H2O, and 50 μg mL−1 Suc (Brewbaker and Kwack, 1963) for Solanum pollen and 0.29 μg mL−1 MES, 0.01 μg mL−1 H3BO3, 0.0147 μg mL−1 CaCl2, and 50 μg mL−1 Suc for lily pollen grains. Various concentrations of lyticase (332 units mg−1 solid; Sigma) were added at the start of imbibition (to assess the effect on germination) or after 30 min of imbibition (to assess the effect on elongation). Cellulase (6.3 units mg−1 solid; Sigma) and β-glucanase (1 unit mg−1 solid; Sigma) were added at the beginning of imbibition. Controls were performed by adding enzyme that had been denatured by boiling for 10 min. Percentage of germination and pollen tube length were assessed in the light microscope after 2 h of incubation on microscope slides. The results are mean values from eight repetitions of the experiment.
Fluorescence Label
For fluorescence microscopy, pollen tubes were fixed after 2 h of germination in 3% freshly prepared formaldehyde in PIPES buffer (1 mm EGTA, 0.5 mm MgCl2, 50 mm PIPES) for 30 min. To allow for quantitative comparison of fluorescence intensity, pollen tubes grown in solidified medium (Gel-Gro) were resuspended by adding 0.1 m citrate buffer (55 mm citric acid, 125 mm sodium citrate, pH 6) at 30°C, to assure that the gel did not limit the access of fluorochrome to the cells (liquid medium controls were treated similarly). Decolorized aniline blue and calcofluor white staining (for callose and cellulose, respectively) were carried out on fixed pollen tubes. After two washes, cells were incubated for 15 min with the staining agent (0.1% aniline blue in 0.15 m K2HPO4; 0.1% calcofluor white in double distilled water), mounted immediately, and observed at UV light excitation.
For immunofluorescence, fixed cells were incubated with monoclonal antibodies JIM5 and JIM7 (generously provided by Dr. Paul Knox, Leeds, UK, and Keith Roberts, John Innes Centre, Norwich, UK), diluted 1:50 in phosphate-buffered saline followed by an incubation with goat anti-rat IgG-AlexaFluor 594 (diluted 1:100 in phosphate-buffered saline) overnight at 4°C. JIM5 and JIM7 recognize homogalacturonans with low and high degrees of esterification, respectively (VandenBosch et al., 1989; Knox et al., 1990). Tubes were mounted and observed in the fluorescence microscope using a Texas red filter set. Controls were performed by omitting incubation with the primary or the secondary antibody.
Bright-Field and Fluorescence Microscopy
Specimens labeled for cell wall components were observed in the fluorescence microscope (Nikon TE2000; Tokyo) equipped with a Roper fx-cooled CCD camera (Roper Scientific, Tucson, AZ). Exposure times of images that had to be compared for fluorescence intensity were identical. These images were not manipulated for contrast or brightness before reproduction.
Transmission Electron Microscopy
Samples for transmission electron microscopy were fixed in 2% formaldehyde and 2.5% glutaraldehyde solution in 0.05 m phosphate buffer, pH 7.2, for 2 h at room temperature. They were washed in 0.05 m phosphate buffer, pH 7.2, and postfixed with 1% osmium tetroxide in the same buffer for 2 h. This was followed by dehydration in acetone and embedding in Spurr's resin. Ultrathin sections were cut with a Reichert OM U2 ultramicrotome, collected on formvar-coated copper grids, and stained with 2% uranyl acetate and lead citrate (Reynolds). Specimens were observed with a JEOL 100-S transmission electron microscope (JEOL, Tokyo) operated at 80 kV.
Scanning Electron Microscopy
Unfixed dehydrated Solanum and Lilium pollen grains were coated with gold using a Hummer II sputter coater (Technics, Alexandria, VA) and observed with a JEOL JSM 35 scanning electron microscope operated at 15 kV.
Microindentation
Hydrated pollen was grown on coverslips coated with poly-l-Lys or stigmatic exudate and covered with liquid GM. After germination had occurred, coverslips were submerged in the GM containing the experimental chamber of the microindenter that was mounted on a Nikon TE2000 inverted microscope. The design and principles of operation of the microindenter have been described previously (Petersen et al., 1982; Elson et al., 1983). Briefly, the bending of a horizontal glass beam gauges the resistance to cellular deformation. A vertical glass stylus (tip diameter 10 μm) is mounted at the end of a 23-mm-long horizontal Vycor glass beam. The other end of the beam is mounted on a linear piezoelectric motor, which moves vertically according to a programmed waveform. In the experiments reported in this article, the motor was programmed to execute a single triangular waveform with a velocity of 14 μm s−1 and a total amplitude of 7 μm. Optical sensors monitor the vertical positions of the stylus and the motor. The extent to which the beam is bent is proportional to the force exerted on the tip by the cell and is determined by comparing tip displacements in the presence and absence of cell contact. The force exerted by the cell on the stylus is determined with the help of the force constant of the beam, which is obtained by prior calibration. The stiffness is obtained by calculating the slope of the linear part of the graph obtained by plotting force against depth of deformation.
Acknowledgments
The generous gift of monoclonal antibodies JIM5 and JIM7 from Keith Roberts, John Innes Centre, Norwich, UK, and Paul Knox, Leeds University, Leeds, UK, is gratefully acknowledged.
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada, the Fonds Québecois de la Recherche sur la Nature et les Technologies, and the Canadian Foundation for Innovation to A.G.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.050773.
References
- Aouali N, Laporte P, Clement C (2001) Pectin secretion and distribution in the anther during pollen development in Lilium. Planta 213: 71–79 [DOI] [PubMed] [Google Scholar]
- Brewbaker J, Kwack B (1963) The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot 50: 859–865 [Google Scholar]
- Buntemeyer K, Luthen H, Bottger M (1998) Auxin-induced changes in cell wall extensibility of maize roots. Planta 204: 515–519 [Google Scholar]
- Carpita N, Gibeaut D (1993) Structural models of primary cell walls in flowering plants: consistency of molecular structure with the physical properties of the wall during growth. Plant J 3: 1–30 [DOI] [PubMed] [Google Scholar]
- Coffey MD (1976) Flax rust resistance involving the K gene: an ultrastructural survey. Can J Bot 54: 1443–1457 [Google Scholar]
- Currier HB (1957) Callose substance in plant cells. Am J Bot 44: 478–488 [Google Scholar]
- Delmer DP, Amor Y (1995) Cellulose biosynthesis. Plant Cell 7: 987–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen J (1996) Pollen tubes: a model system for plant cell growth. Bot Acta 109: 341–345 [Google Scholar]
- Discher DE, Boal DH, Boey SK (1988) Simulations of the erythrocyte cytoskeleton at large deformation. II. Micropipette aspiration. Biophys J 75: 1584–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogterom M, Yurke B (1997) Measurement of the force-velocity relation for growing microtubules. Science 278: 856–860 [DOI] [PubMed] [Google Scholar]
- Edelmann HG (1995) Wall extensibility during hypocotyl growth—a hypothesis to explain elastic-induced wall loosening. Physiol Plant 95: 296–303 [Google Scholar]
- El-Ghazaly G (1999) Development and substructures of pollen grains wall. In M Cresti, G Cai, A Moscatelli, eds, Fertilization in Higher Plants. Springer-Verlag, Berlin, pp 175–200
- Elson EL, Daily BB, McConnaughey WB, Pasternak C, Petersen NO (1983) Measurement of forces which determine the shapes of adherent cells in culture. In T-Y Liu, S Sakakibara, A Schechter, K Yagi, H Yajima, KT Yasunobu, eds, Frontiers in Biochemical and Biophysical Studies of Proteins and Membranes. Elsevier, New York, pp 399–411
- Esau K (1948) Phloem structure in the grapevine, and its seasonal changes. Hilgardia 18: 217–296 [Google Scholar]
- Esau K, Cronshaw J (1967) Relation of tobacco mosaic virus to the host cells. J Cell Biol 33: 665–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschrich W (1975) Sealing systems in phloem. In MH Zimmermann, JA Milburn, eds, Encyclopedia of Plant Physiology. Springer-Verlag, Berlin, pp 39–56
- Ferguson C, Teeri TT, Siikaaho M, Read SM, Bacic A (1998) Location of cellulose and callose in pollen tubes and grains of Nicotiana tabacum. Planta 206: 452–460 [Google Scholar]
- Geitmann A (1999) The rheological properties of the pollen tube cell wall. In M Cresti, G Cai, A Moscatelli, eds, Fertilization in Higher Plants. Springer-Verlag, Berlin, pp 283–297
- Geitmann A, Cresti M (1998) Ca2+ channels control the rapid expansions in pulsating growth of Petunia hybrida pollen tubes. J Plant Physiol 152: 439–447 [Google Scholar]
- Geitmann A, Hudak J, Vennigerholz F, Walles B (1995) Immunogold localization of pectin and callose in pollen grains and pollen tubes of Brugmansia suaveolens—implication for the self-incompatibility reaction. J Plant Physiol 147: 225–235 [Google Scholar]
- Geitmann A, McConnaughey W, Lang-Pauluzzi I, Franklin-Tong VE, Emons AMC (2004) Cytomechanical properties of Papaver pollen tubes are altered after self-incompatibility challenge. Biophys J 86: 3314–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geitmann A, Parre E (2004) The local cytomechanical properties of growing pollen tubes correspond to the axial distribution of structural cellular elements. Sex Plant Reprod 17: 9–16 [Google Scholar]
- Green PB (1962) Mechanism for plant cellular morphogenesis. Science 138: 1404–1405 [DOI] [PubMed] [Google Scholar]
- Harold FM (2002) Force and compliance: rethinking morphogenesis in walled cells. Fungal Genet Biol 37: 271–282 [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Nakamura S, Kakizoe S, Sato M, Nakamura N (1998) Immonocytochemical and chemical analyses of Golgi vesicles isolated from the germinated pollen of Camellia japonica. J Plant Res 111: 421–429 [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17: 159–187 [DOI] [PubMed] [Google Scholar]
- Heslop-Harrison J (1964) Cell walls, cell membranes and protoplasmic connections during meiosis and pollen development. In H Linskens, ed, Pollen Physiology and Fertilization. North-Holland, Amsterdam, pp 29–47
- Heslop-Harrison J (1987) Pollen germination and pollen-tube growth. Int Rev Cytol 107: 1–78 [Google Scholar]
- Holdaway-Clarke TL, Hepler PK (2003) Control of pollen tube growth: role of ion gradients and fluxes. New Phytol 159: 539–563 [DOI] [PubMed] [Google Scholar]
- Ingber DE (2003) Tensegrity. II. How structural networks influence cellular information processing networks. J Cell Sci 116: 1397–1406 [DOI] [PubMed] [Google Scholar]
- Johnson SA, McCormick S (2001) Pollen germinates precociously in the anthers of raring-to-go, an Arabidopsis gametophytic mutant. Plant Physiol 126: 685–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles DW, Chasis JA, Evans EA, Mohandas N (1994) Cooperative action between band 3 and glycophorin A in human erythrocytes—immobilization of band 3 induced by antibodies to glycophorin A. Biophys J 66: 1726–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J, Linstead P, King J, Cooper C, Roberts K (1990) Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521 [DOI] [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, Yang Z (1999) Control of pollen tube growth by a rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11: 1731–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mareck A, Faleri C, Moscatelli A, Liu Q, Cresti M (2002) Detection and localization of pectin methylesterase isoforms in pollen tubes of Nicotiana tabacum L. Planta 214: 734–740 [DOI] [PubMed] [Google Scholar]
- Li Y, Moscatelli A, Cai G, Cresti M (1997) Functional interactions among cytoskeleton membranes and cell wall in pollen tube of flowering plants. Int Rev Cytol 176: 133–199 [DOI] [PubMed] [Google Scholar]
- Li YQ, Chen F, Linskens HF, Cresti M (1994) Distribution of unesterified and esterified pectins in cell walls of pollen tubes of flowering plants. Sex Plant Reprod 7: 145–152 [Google Scholar]
- Lockhart JA (1965) An analysis of irreversible plant cell elongation. J Theor Biol 8: 264–275 [DOI] [PubMed] [Google Scholar]
- Maltby D, Carpita NC, Montezinos D, Kulow C, Delmer DP (1979) β-1,3-Glucan in developing cotton fibers. Plant Physiol 63: 1158–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Money NP (1997) Wishful thinking of turgor revisited: the mechanics of fungal growth. Fungal Genet Biol 21: 173–187 [Google Scholar]
- Money NP (2001) Biomechanics of invasive hyphal growth. In RJ Howard, NAR Gow, eds, Mycota VIII: Biology of the Fungal Cell. Springer-Verlag, New York, pp 3–17
- Morrison IN, O'Brien TP (1976) Cytokinesis in the developing wheat grain: division with and without a phragmoplast. Planta 130: 57–67 [DOI] [PubMed] [Google Scholar]
- Nash GB, Gratzer WB (1993) Structural determinants of the rigidity of the red cell membrane. Biorheology 30: 397–407 [DOI] [PubMed] [Google Scholar]
- Nolte T, Schopfer P (1997) Viscoelastic versus plastic cell wall extensibility in growing seedling organs—a contribution to avoid some misconceptions. J Exp Bot 48: 2103–2107 [Google Scholar]
- Parre E, Geitmann A (2004) Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta: doi 10.1007/s00425-004-1368-5 [DOI] [PubMed]
- Petersen NO, McConnaughey WB, Elson EL (1982) Dependence of locally measured cellular deformability on position on the cell, temperature, and cytochalasin B. Proc Natl Acad Sci USA 79: 5327–5331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressayre A, Raquin C, Mignot A, Godelle B, Gouyon PH (2002) Correlated variation in microtubule distribution, callose deposition during male post-meiotic cytokinesis, and pollen aperture number across Nicotiana species (Solanaceae). Am J Bot 89: 393–400 [DOI] [PubMed] [Google Scholar]
- Roggen HS, Stanley RG (1969) Cell wall hydrolysing enzymes in wall formation as measured by pollen tube extension. Planta 84: 295–303 [DOI] [PubMed] [Google Scholar]
- Scott P, Miller LW, Webster BD, Leopold AC (1967) Structural changes during bean leaf abscission. Am J Bot 54: 730–734 [Google Scholar]
- Showalter AM (1993) Structure and function of plant cell wall proteins. Plant Cell 5: 9–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokabe M, Sachs F, Jing Z (1991) Quantitative video microscopy of patch clamped membranes—stress, strain, capacitance and stretch channel activation. Biophys J 59: 722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steer M, Steer J (1989) Pollen tube tip growth. New Phytol 111: 323–358 [DOI] [PubMed] [Google Scholar]
- Stone BA, Clarke AE (1992) Chemistry and Biology of (1-3)-β-Glucans. La Trobe University Press, Victoria, Australia
- Suarez-Cervera M, Arcalis E, Le Thomas A, Seoane-Camba J (2002) Pectin distribution pattern in the apertural intine of Euphorbia peplus L. (Euphorbiaceae) pollen. Sex Plant Reprod 14: 291–298 [Google Scholar]
- Taylor LP (1997) Pollen germination and tube growth. Annu Rev Plant Physiol Plant Mol Biol 48: 461–491 [DOI] [PubMed] [Google Scholar]
- VandenBosch K, Bradley D, Knox J, Perotto S, Butcher G, Brewin N (1989) Common components of the infection thread matrix and the intercellular space identified by immunocytochemical analysis of pea nodules and uninfected roots. EMBO J 8: 335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterkeyn L (1981) Cytochemical localization and function of the 3-linked glucan callose in the developing cotton fibre cell wall. Protoplasma 106: 49–67 [Google Scholar]
- Wimmer R, Lucas BN, Tsui TY, Oliver WC (1997) Longitudinal hardness and Youngs modulus of spruce tracheid secondary walls using nanoindentation technique. Wood Sci Technol 31: 131–141 [Google Scholar]
- Yim KO, Bradford KJ (1998) Callose deposition is responsible for apoplastic semipermeability of the endosperm envelope of muskmelon seeds. Plant Physiol 118: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Bunyaratvej A, Fucharoen S, Fung C, Shinar E, Schrier SL (1995) The instability of the membrane skeleton in thalassemic red blood cells. Blood 86: 3945–3950 [PubMed] [Google Scholar]