Abstract
Proteins that fail to fold in the endoplasmic reticulum (ER) or cannot find a pattern for assembly are often disposed of by a process named ER-associated degradation (ERAD), which involves transport of the substrate protein across the ER membrane (dislocation) followed by rapid proteasome-mediated proteolysis. Different ERAD substrates have been shown to be ubiquitinated during or soon after dislocation, and an active ubiquitination machinery has been found to be required for the dislocation of certain defective proteins. We have previously shown that, when expressed in tobacco (Nicotiana tabacum) protoplasts, the A chain of the heterodimeric toxin ricin is degraded by a pathway that closely resembles ERAD but is characterized by an unusual uncoupling between the dislocation and the degradation steps. Since lysine (Lys) residues are a major target for ubiquitination, we have investigated the effects of changing the Lys content on the retrotranslocation and degradation of ricin A chain in tobacco protoplasts. Here we show that modulating the number of Lys residues does not affect recognition events within the ER lumen nor the transport of the protein from this compartment to the cytosol. Rather, the introduced modifications have a clear impact on the degradation of the dislocated protein. While the substitution of the two Lys residues present in ricin A chain with arginine slowed down degradation, the introduction of four extra lysyl residues had an opposite effect and converted the ricin A chain to a standard ERAD substrate that is disposed via a process in which dislocation and degradation steps are tightly coupled.
Proteins that enter the secretory pathway interact with a variety of endoplasmic reticulum (ER) chaperones and enzymes in a process that is believed to promote protein folding and assembly and to allow onward transport only when structural maturation has been successfully accomplished. In general, proteins that fail to fold or that cannot find a partner for assembly are eventually degraded by proteasomes (Lord et al., 2000; Tsai et al., 2002). Since proteasomes reside in the cytosol (Rivett, 1998), soluble proteins must be extracted from the ER lumen and at least partially retrotranslocated (dislocated) to the cytosol before degradation can begin. Similarly, lumenal and transmembrane domain(s) of membrane proteins must be extracted to become accessible to the degradation machinery. This degradative pathway is often referred to as ER-associated degradation (ERAD) and has been extensively studied in mammals and yeast (Saccharomyces cerevisiae). According to a widely accepted model, the ERAD pathway can be divided into four stages: (1) recognition of the substrate and targeting to the retrotranslocation machinery; (2) transport across the ER membrane; (3) release of the substrate from the membrane; and (4) degradation in the cytosol (Tsai et al., 2002). A pathway closely resembling ERAD also operates in plant cells, but very little is known about the mechanisms of substrate recognition, dislocation, and degradation in plants (Di Cola et al., 2001; Brandizzi et al., 2003; Kostova and Wolf, 2003).
In eukaryotic cells, ubiquitination plays a central role in regulated proteolysis. Ubiquitin is a highly conserved protein that is ligated to the substrate through an isopeptide bond between the C terminus of ubiquitin and a Lys residue of the target protein. Initially, ubiquitin is activated by an activating enzyme (E1) and then ligated to the substrate by the concerted action of a ubiquitin-conjugating enzyme (E2) and a ubiquitin-protein ligase (E3; Bachmair et al., 2001; Vierstra, 2003). The addition of many ubiquitin molecules, normally in the form of polymeric chains, constitutes the principal signal for targeting to the 26S proteasome (Pickart, 2000). Ubiquitination has been shown to be required for degradation of many ERAD substrates, but the role of this modification is not restricted to the last degradative phase of the disposal process. Rather, this modification appears to play an important role in the ER-to-cytosol transport of the misfolded protein, which often accumulates in the ER in the absence of fully active ubiquitination machinery (Biederer et al., 1997; Bordallo et al., 1998; de Virgilio et al., 1998; Kikkert et al., 2001; Jarosch et al., 2002). While these conclusions were based on results obtained in mammalian and yeast cells, the role of ubiquitination in the plant ERAD pathway still remains to be established. Here we have taken advantage of the low Lys content that characterizes the ricin A chain to investigate whether the presence of internal Lys residues that could act as attachment sites for polyubiquitin chains is required for the transport and/or degradation of an ERAD substrate in plant cells.
Ricin is a heterodimeric ribosome-inactivating protein that accumulates in castor bean (Ricinus communis) endosperm cells during seed development. The mature toxin comprises a catalytic A chain (RTA) and a Gal-binding B chain (RTB) linked by a single disulfide bond. However, when it is synthesized in castor bean endosperm, it is made as a glycosylated precursor, preproricin (Lamb et al., 1985), in which mature RTA is preceded by an N-terminal signal peptide for insertion in the ER and by a propeptide of unknown function. In this preproricin precursor, RTA and RTB are also connected by an internal propeptide that is removed in the vacuole and contains essential information for targeting to this organelle (Frigerio et al., 1998, 2001). We have previously shown that, when the ricin precursor is expressed in tobacco (Nicotiana tabacum) protoplasts, correctly processed ricin accumulated in the vacuole (Frigerio et al., 1998). Conversely, ER-targeted RTA, when expressed in the same system in the absence of RTB, was subjected to a process that closely resembled ERAD (Frigerio et al., 1998; Di Cola et al., 2001). These results, together with the more recalcitrant nature of plant ribosomes to the action of RTA (Taylor et al., 1994), established tobacco protoplasts as a convenient expression system in which to biochemically study the details of RTA dislocation.
Ricin is one of a number of toxins that exploit the ERAD pathway to reach their cytosolic substrates after being internalized by mammalian cells and transported in a retrograde fashion from the cell surface to the ER (for review, see Lord and Roberts, 1998). Within the ER, these proteins may be reduced and recognized as misfolded by the quality control system and hence directed to the ERAD pathway. Since the accumulation in the cytosol of unfolded proteins and of hydrophobic integral membrane proteins can pose a serious aggregation risk to the cell, standard ERAD substrates do not accumulate in the cytosol unless proteasomes are inhibited (Hughes et al., 1997; Yu et al., 1997; de Virgilio et al., 1998; Mancini et al., 2000) or the degradation system is saturated due to substrate overexpression (Johnston et al., 1998). This tight coupling between dislocation and degradation that characterizes known ERAD substrates implies that, to kill their target cells, the dislocated subunits of protein toxins (or at least a fraction of these subunits) must be able to leave the ERAD pathway during or shortly after dislocation and then refold to avoid immediate destruction by cytosolic proteasomes. Once in the cytosol, RTA inactivates ribosomes by removing a specific adenine from a conserved loop that constitutes the EF-2 binding site in the 28S rRNA of 80S ribosomes (Endo et al., 1987).
Like the other toxins that retrotranslocate from the mammalian ER, RTA (but not RTB) is characterized by a very low Lys content. It has been proposed that, since Lys residues can serve as attachment sites for polyubiquitin chains, a low Lys content may help these toxins avoid proteasome-mediated degradation in intoxicated cells (Hazes and Read, 1997). Consistent with this hypothesis, the introduction of additional Lys residues into the RTA primary sequence has been shown to decrease the cytotoxicity of reconstituted RTA-RTB heterodimers toward mammalian cells, an effect that could be countered by treatment with proteasome inhibitors (Deeks et al., 2002). These results demonstrated that additional Lys residues allowed a more efficient targeting of RTA to the proteasome for degradation. Interestingly, elimination of the two endogenous Lys residues present in RTA failed to enhance toxin potency, suggesting that attachment of ubiquitin to the naturally occurring lysyl residues does not normally occur during intoxication of mammalian cells and supporting the notion that toxin degradation in mammalian cytosol is ubiquitin independent (Deeks et al., 2002). However, the vanishingly small amount of toxin that reaches the ER of mammalian cells did not permit direct biochemical analysis of the fate of endocytosed ricin molecules and of the consequences that changes in Lys content may have had on different steps of the ERAD pathway. Furthermore, since the delivery of ricin to the ER of mammalian cells via endocytosis is very different from the biosynthesis of ricin in the early secretory pathway of the plant cell, it should not be assumed that the role of Lys residues on the dislocation and degradation of RTA will be the same in both systems.
To begin to characterize the ERAD pathway in plant cells, we have directly investigated the effect of changes in Lys content on the dislocation and degradation of RTA expressed in plant protoplasts. Our results show that, although the number of lysyl residues has no effect on ER-to-cytosol transport of RTA, the accumulation of active toxin in the plant cytosol is exquisitely sensitive to Lys content, more so than in mammalian cytosol. The potential implications for the mechanism of misfolded protein dislocation and degradation in plant cells are discussed.
RESULTS
The Number of Lysyl Residues Modulates the Kinetics of RTA Degradation
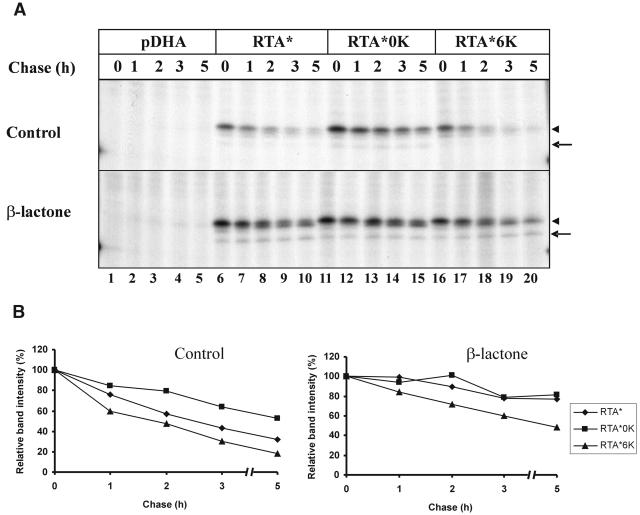
To assess whether the Lys content could modulate the degradation kinetics of an ERAD substrate in plant cells, we produced two mutated forms of RTA either lacking Lys residues or containing four extra ones. To produce Lys-free RTA, site-directed mutagenesis was used to substitute Arg for the two endogenous Lys residues present in the RTA sequence (K4 and K239). In the second mutant, four residues of RTA (V38, E67, N132, and S228) were substituted for Lys, thus bringing the total number of Lys residues to six (Deeks et al., 2002). These residues were selected because they are located in exposed surface loops in the crystal structure of pokeweed antiviral protein (Monzingo et al., 1993), a type I ribosome-inactivating protein structurally homologous to RTA but characterized by a high Lys content. Since RTA expression was known to be toxic to plant cells (Frigerio et al., 1998), and since this toxicity may somehow affect degradation kinetics, all these changes were introduced in the background of an RTA active site mutant in which E177 was changed to Asp. This mutant (RTA*) is characterized by a 70-fold reduction in catalytic activity (Chaddock and Roberts, 1993). Previous expression of an ER-targeted RTA allowed visualization of a glycosylated RTA in the cytosol of tobacco protoplasts (Di Cola et al., 2001). RTA* and the two mutants with an altered number of Lys residues (RTA*0K and RTA*6K) were transiently expressed with the ricin ER signal peptide in tobacco leaf protoplasts to produce glycosylated products. It was clear from pulse-chase and immunoprecipitation analyses that, while RTA*6K was degraded at a slightly faster rate than the wild-type protein, substitution of the two naturally occurring lysyl residues present in native RTA with Arg residues had a stabilizing effect (Fig. 1, A, top, and B, left). Since these mutations do not affect the catalytic activity of RTA (Deeks et al., 2002), it was unlikely that changes in the tertiary structure were responsible for the observed differences in the degradation rate. Rather, these data suggested that the overall stability of ER-targeted RTA was sensitive to the Lys content of the protein, likely because this affects the efficiency of RTA ubiquitination.
Figure 1.
The number of lysyl residues modulates the kinetics of RTA degradation. Tobacco protoplasts transfected with empty vector (pDHA) or plasmids encoding RTA*, RTA*0K, or RTA*6K were pulsed for 1 h with radiolabeled amino acids and chased for the times indicated either without protease inhibitor or in the presence of 80 μm β-lactone. A, Polypeptides immunoselected with anti-RTA antiserum were analyzed by SDS-PAGE and fluorography. Arrowheads, Position of trimmed RTA; arrows, position of deglycosylated RTA. B, The intensity of the RTA bands from the gels shown in A and from a second analogous experiment were quantified as described in “Materials and Methods” and plotted as a percentage of the signal at 0 h of chase.
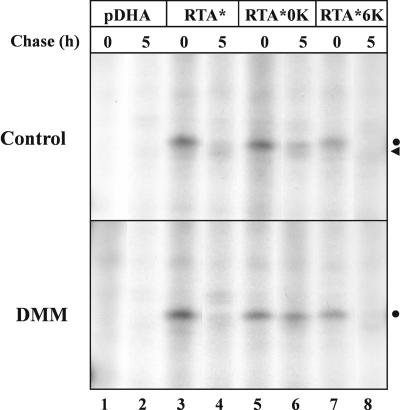
All forms of RTA* underwent processing during the chase and were converted into faint, slightly faster migrating species (Fig. 1A, arrowhead). We have previously shown that this is due to the action of mannosidase(s) acting on the RTA glycan chain (Di Cola et al., 2001). Since mannosidase action has been implicated in the degradation of different ERAD substrates (Frigerio and Lord, 2000; Braakman, 2001), we analyzed the effect of the mannosidase inhibitor 1-deoxymannojirimycin (DMM) on the degradation of RTA* and of the two Lys mutants. The results in Figure 2 show that a large fraction of RTA* and RTA*6K was degraded also in the presence of the mannose inhibitor, clearly indicating that mannosidase trimming does not play an essential role in RTA recognition as an ERAD substrate in plant cells. In accordance with the results shown in Figure 1, RTA*0K was more stable than the two other RTA forms, and this difference was maintained in the presence of DMM.
Figure 2.
Mannose trimming is not required for the degradation of RTA. Tobacco protoplasts transfected with empty vector (pDHA) or plasmids encoding RTA*, RTA*0K, or RTA*6K were pulsed for 1 h with radiolabeled amino acids and chased for 5 h either in the absence (control) or in the presence of 5 mm DMM. Polypeptides immunoselected with anti-RTA antiserum were analyzed by SDS-PAGE and fluorography. Dot, Untrimmed RTA; arrowhead, trimmed RTA.
We have previously shown that degradation of RTA can be slowed down by treatment with the specific proteasome inhibitor clasto-lactacystin-β-lactone (hereafter described as β-lactone). To study the role of cytosolic proteasomes in the turnover of RTA*0K and RTA*6K, we examined the effect of this inhibitor on the degradation of these mutants. In the presence of β-lactone, the degradation of RTA* was retarded and the clear difference in degradation kinetics that distinguished RTA* and RTA*0K synthesized in untreated protoplasts was virtually abolished (Fig. 1, A, lanes 6–15, and B), confirming that the two Lys residues normally target RTA to proteasomal degradation. A stabilizing effect was also evident in the case of RTA*6K, but this mutant was still degraded faster than the two other proteins (Fig. 1, A, lanes 16–20, and B), suggesting that addition of extra Lys residues converted RTA to a preferential substrate for any residual proteasomal activity. The small stabilizing effect of β-lactone on RTA*0K indicated that either this Lys-free form of RTA* was also eventually degraded by proteasomes, presumably in a ubiquitin-independent manner, or that N-terminal ubiquitination (Breitschopf et al., 1998) was very effective in this mutant.
The proteasome inhibitor also caused the accumulation of a faster migrating band (Fig. 1A, arrow) corresponding in migration to what we have previously shown to be a cytosolic deglycosylated RTA intermediate (Di Cola et al., 2001). Importantly, in the case of RTA*0K, the same degradation intermediate was also apparent in the absence of the proteasome inhibitor (Fig. 1A, top arrow, lanes 11–15). This suggested that the presence of Lys residues was not required for recognition by the deglycosylating enzyme(s), and that substitution of the two Lys residues with Arg affected the degradation process at a step downstream of substrate deglycosylation.
Dislocation of RTA Is Not Dependent on the Presence of Lysyl Residues
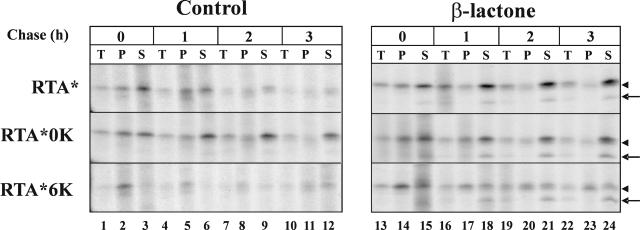
Lys content could, in principle, modulate RTA degradation by affecting the rate of dislocation and/or the stability of the dislocated protein. To examine these possibilities, protoplasts expressing RTA* or the two mutants were pulse labeled and chased for different times in the absence or in the presence of β-lactone. Protoplasts were then fractionated into cytosolic (S, soluble) and microsomal (P, pellet) fractions (Fig. 3). In the absence of β-lactone, a significant amount of RTA* and RTA*0K was recovered in the soluble fraction at the end of the pulse (lane 3). Conversely, the vast majority of RTA*6K was recovered in the microsomal fraction (lane 2). At the end of the 3-h chase, little protein was recovered in the microsomal fraction both in the case of RTA* and in the case of the mutated proteins (lane 11), but while RTA*0K stably accumulated in the cytosol during the chase, only a small amount of RTA* and RTA*6K could be recovered, in the form of a smeared band, in the cytosolic fraction (lane 12). Thus, for the RTA*0K mutant, the increased stability cannot be ascribed to a defect in retrotranslocation to the cytosol but to inefficient targeting to the degradation machinery after dislocation has occurred. In addition, the very low level of RTA*6K recovered in the soluble fraction even at the end of the pulse (lane 3) suggested that four additional Lys residues rendered the dislocated/ing protein a better substrate for proteasomal degradation. This interpretation was confirmed by the results obtained when β-lactone was included in the protoplast incubation medium (lanes 13–24). Under these conditions, glycosylated RTA*6K could be recovered in the soluble fraction at the end of the pulse and throughout the chase (lanes 15, 18, 21, and 24), showing that when proteasomes are active, RTA*6K is indeed rapidly dislocated and targeted to proteasomes for destruction. β-Lactone stimulated the accumulation of RTA* in the cytosol during the chase but had little effect in the case of RTA*0K. In all cases, a polypeptide having the expected mobility of a deglycosylated degradation intermediate was recovered from the soluble fraction (Fig. 3, arrows). Interestingly, the kinetic analysis shown in Figure 3 revealed that untrimmed forms of the proteins accumulated in the cytosol at early chase points (lane 15), while trimmed forms were generated within the ER, becoming evident in the cytosol fractions during the chase (lanes 18, 21, and 24; arrowheads). This confirmed that recognition events within the ER are not mediated by a specific structure presented by RTA N-linked glycan chains.
Figure 3.
Subcellular fractionation of RTA-expressing protoplasts. Tobacco protoplasts transfected with plasmids encoding RTA*, RTA*0K, or RTA*6K were pulsed for 1 h with radiolabeled amino acids and chased for the times indicated either without protease inhibitor or in the presence of 80 μm β-lactone. Protoplasts were homogenized in the absence of detergent, and an aliquot of the homogenate was saved (total protoplast homogenate), while the rest was fractionated into microsomal and soluble fractions. Polypeptides immunoselected with anti-RTA antiserum from total protoplast homogenate (T), from the microsomal pellet (P), or from the soluble fraction (S) were analyzed by SDS-PAGE and fluorography. Arrowheads, Position of trimmed RTA; arrows, position of deglycosylated RTA.
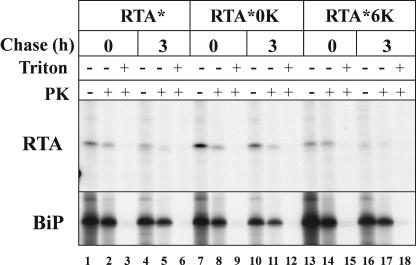
Since fractionation analysis does not allow us to distinguish between lumenal RTA and RTA bound to the surface of the microsomes, the effect of changes in Lys content on the dislocation of RTA was also examined using a protease protection assay (Fig. 4, top). At the end of a pulse, a fraction of RTA* and RTA*0K was susceptible to protease digestion (compare lane 1 with lane 2 and lane 7 with lane 8), confirming the observation that these proteins accumulate in the cytosol during the pulse. Conversely, RTA*6K was almost quantitatively protected by protease attack, indicating that the protein was located within the lumen of the microsomes and not associated to their external surfaces (compare lane 13 with lane 14). Similar results were obtained at the end of the chase (compare lanes 4, 10, and 16 with lanes 5, 11, and 17, respectively). Both at the end of the pulse (lanes 2, 8, and 14) and at the end of the chase (lanes 5, 11, and 17), the total amount of protein protected from protease attack was similar for all three proteins. Assuming a similar rate of synthesis for the three RTA forms, this would be in agreement with our conclusion that the dislocation process of RTA was insensitive to changes in Lys content.
Figure 4.
Modulating Lys content does not affect RTA dislocation. Tobacco protoplasts transfected with plasmids encoding RTA*, RTA*0K, or RTA*6K were pulsed for 1 h with radiolabeled amino acids and chased for the times indicated. After homogenization, the samples were split into three identical aliquots and incubated either in the absence or in the presence of proteinase K (PK) and detergent (Triton), as indicated. Proteins were immunoselected using anti-RTA or anti-BiP antisera, and analyzed by SDS-PAGE and fluorography.
RTA can be highly resistant to treatment with proteases (Walker et al., 1996) and, under the relatively harsh conditions of the assay, a fraction of the lumenal chaperone BiP was degraded (Fig. 4, bottom). However, the almost complete recovery of RTA*6K in the absence of detergent (compare lane 13 with lane 14), and its virtually complete degradation in the presence of detergent (lane 15), confirmed that the conditions of the assay were appropriate to reliably determine the topological state of RTA.
Taken together, these data allow us to conclude that changes in Lys content affect the degradation of dislocated RTA, but not the ER-to-cytosol transport of this protein. In addition, the accumulation of RTA*0K in the cytosol indicates that ubiquitination of Lys residues was not crucial for ER-to-cytosol transport of RTA in plant cells.
To gain insight into the mechanism of degradation of dislocated RTA, we investigated whether this protein is targeted for destruction via the N-end rule pathway. The metabolic stability of a protein degraded by this pathway depends on the N-terminal residue. This can be either a stabilizing or a destabilizing amino acid. Signal peptide cleavage site prediction algorithms (Bendtsen et al., 2004) and experimental evidence (Ferrini et al., 1995) reveal that the RTA signal peptide cleavage occurs after G26 (the numbering refers to the position in the ricin precursor). This exposes an N-terminal Trp, a residue classified as destabilizing in bacteria, yeast, and mammalian cells. This residue was therefore mutated to Val, a stabilizing amino acid (Varshavsky, 1996; Worley et al., 1998) that was not predicted to change the site of signal peptide cleavage. RTA and RTAW27V were transiently expressed in tobacco protoplasts and the stabilities of the two proteins were compared by a pulse-chase experiment. Our data show that the introduced mutation did not afford enhanced stabilization of RTA (Fig. 5).
Figure 5.
Degradation of RTA is not dependent on the presence of an N-terminal Trp residue. Tobacco protoplasts transfected with empty vector (pDHA) or a plasmid encoding either RTA or RTAW27V were pulsed for 1 h with radiolabeled amino acids and chased for the times indicated. Polypeptides immunoselected with anti-RTA antiserum were analyzed by SDS-PAGE and fluorography.
The Number of Lysyl Residues Modulates the Toxicity of Endogenously Synthesized RTA
The results so far have shown that variations in the number of Lys residues did not markedly affect the rate of RTA transport to the cytosol but did modulate the overall rate of RTA degradation. Since tobacco ribosomes are sensitive to the action of RTA (Taylor et al., 1994; Frigerio et al., 1998), differences in the levels of cytosolic RTA should result in a difference in toxicity toward the expressing cells. To examine whether this was the case, we compared the toxic effect associated with the expression of native RTA, RTA0K, and RTA6K (i.e. with functional active sites). Since a variable fraction of protoplasts is transfected in each experiment, the toxic effect must be monitored by examining the level of synthesis of a coexpressed reporter. This was achieved by quantifying immunoprecipitates of the reporter made in cells that coexpressed the RTA proteins relative to cells that did not. Plasmids coding for different RTA variants were therefore cotransfected with a plasmid encoding the bean storage protein phaseolin as a protein synthesis reporter. While the Lys-free RTA0K mutant was markedly more toxic than the wild-type protein, coexpression of the RTA6K protein had a minor effect on protein synthesis (Fig. 6A). This confirmed that the toxicity associated with the expression of the three RTA variants correlated with their overall stability. When mutants bearing one residual Lys residue (either K4 or K239) were similarly analyzed, no differences in toxicity were evident with respect to the wild-type protein (data not shown). In addition, the W27V mutation to alter the N-terminal residue did not alter the toxicity associated with its expression in tobacco protoplasts (Fig. 6B). This result, together with that shown in Figure 5, confirms that the instability of dislocated RTA is not related to the presence of a Trp residue at the N terminus of the protein.
Figure 6.
The number of lysyl residues modulates the toxicity of endogenously synthesized RTA. Tobacco protoplasts were cotransfected with a plasmid encoding phaseolin and either empty vector (pDHA) or a plasmid encoding wild-type or mutated RTA. After overnight recovery, protoplasts were pulsed for 1 h with radiolabeled amino acids, and proteins were immunoselected with anti-phaseolin antiserum. The intensity of the phaseolin band was quantified and plotted as a percentage of the intensity in samples cotransfected with the pDHA vector. Vertical bars indicate sd. A, Effect of wild-type RTA, RTA0K, and RTA6K expression on the synthesis of the phaseolin reporter. This represents an average of three experiments performed in triplicate. B, Effect of wild-type RTA and RTAW27V expression on the synthesis of the phaseolin reporter. This represents an average of two experiments performed in triplicate.
DISCUSSION
In this article, we demonstrate that changes in Lys content have an impact on the overall stability of ER-targeted RTA expressed in tobacco protoplasts. Although we were not able to directly detect ubiquitinated forms of RTA (data not shown), it is likely that these changes in Lys content affected the ability of RTA to act as a substrate for the ubiquitination machinery. The incomplete effect of proteasome inhibitors in plant cells, the relatively low expression levels that can be obtained in transfected protoplasts (and in transgenic plants), and the transient and reversible nature of protein ubiquitination may have contributed to preclude the accumulation of ubiquitinated forms of RTA to a level appropriate for detection.
Our results are summarized in Figure 7 and have several implications for the mechanisms of substrate recognition, dislocation, and degradation of misfolded secretory proteins in plant cells. Available information indicates that the ER exploits a set of distinct recognition mechanisms to identify structurally defective proteins. While ER-located mannosidases have been suggested to play a key role in the degradation of certain yeast and mammalian glycoprotein substrates (for review, see Frigerio and Lord, 2000; Braakman, 2001), a similar mechanism does not seem to operate in the case of wild-type RTA and of the Lys mutants described in this article. These proteins are clearly degraded even when mannosidase action is inhibited or when the glycans have been extensively modified.
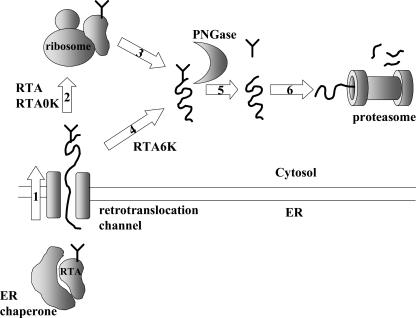
Figure 7.
A proposed model for the degradation of RTA in tobacco protoplasts. A specific feature exposed on the surface of RTA is recognized in the ER and the protein is retrotranslocated to the cytosol (1). These steps occur at a similar rate and with similar efficiencies in the case of RTA, RTA0K, and RTA6K. Once in the cytosol, most RTA and RTA0K refold (2), possibly with the help of ribosomes (Argent et al., 2000), while most RTA6K is rapidly targeted to proteasomal degradation on a pathway that may not involve a refolding step (4–6). Refolded RTA and RTA0K are also eventually targeted for degradation (3, 5, 6), although at different rates. Deglycosylation of RTA and RTA0K may require partial unfolding of the substrate protein (3). A fraction of the dislocated proteins is deglycosylated before degradation (5), although the size of this fraction may depend on Lys content. The rest may be degraded without prior deglycosylation (not shown).
Our results also indicate that specific features present in the polypeptide chain, rather than overall stability, allow the recognition of RTA as a misfolded protein in the ER. This is suggested by the observation that, while RTA6K is 7°C more thermally stable than the wild-type protein (Deeks et al., 2002), RTA* and RTA*6K are dislocated with similar kinetics (Figs. 3 and 4). Thus, significant changes in the overall structural stability of this protein do not necessarily impact on its recognition by the ER quality control system. Following recognition, RTA must be retrotranslocated from the ER to the cytosol. Here we demonstrate that the kinetics and efficiency of dislocation are not significantly altered by mutating the two Lys residues present in wild-type RTA. Although it is still possible that retrotranslocation of RTA depends on N-terminal ubiquitination (Breitschopf et al., 1998; Bloom et al., 2003), the absence of any evident effect on the retrotranslocation of the RTA-Lys mutants demonstrates that ubiquitination of internal Lys residues is not a strict requirement for the ER-to-cytosol transport of proteins in plant cells.
While changes in Lys content did not affect the dislocation of RTA, they did have an impact on the ultimate fate of the proteins in the cytosol. Our results show that mutation of the two native Lys residues significantly stabilizes RTA in the cytosol of tobacco protoplasts. The transient accumulation of RTA*0K and wild-type RTA* indicates that ubiquitination occurring at the N-terminal amino group or at two internal lysyl residues is not sufficient to guarantee the prompt destruction of the retrotranslocated toxin. Conversely, the introduction of four additional Lys residues led to rapid degradation, with the protein being detectable in the cytosol only when proteasomes were inhibited (Fig. 3). It is likely that addition of extra Lys residues converts RTA* into a more standard ubiquitination substrate, allowing tight coupling between retrotranslocation and degradation.
Our results further indicate that RTA stability is not affected by mutations that may be expected to influence the half-life of a substrate degraded via the N-end rule pathway. Future studies are required to identify the degron(s) present in the RTA polypeptide and the enzymes involved in the recognition of this protein as a substrate for proteasomal degradation.
The toxic effects associated with the expression of the catalytically active counterparts correlated with the ability of the different proteins to transiently accumulate in the cytosol (Fig. 6A). Since RTA and RTA0K produced in Escherichia coli have indistinguishable catalytic activities (Deeks et al., 2002), the higher toxicity of RTA0K is likely to reflect higher steady-state levels of the protein in the protoplast cytosol, which in turn must be determined by a longer cytosolic half-life.
The fate of the retrotranslocated Lys-deficient RTA0K in plant and mammalian cytosol is clearly not equivalent. RTA0K in plant cells is stable and more toxic than wild-type RTA (Figs. 1 and 6), whereas the same protein in mammalian cells was indistinguishable from wild-type RTA (Deeks et al., 2002). This latter observation raises the possibility that the endogenous lysyl residues have been maintained during evolution because their presence during ricin uptake into the cells of predators and foragers does not target RTA for degradation and mitigate toxin potency. Indeed, these residues may well have been maintained because they also have a specific and contrary role in planta. Errors in mRNA synthesis, processing, translation, and inefficient protein folding can lead to the generation of aberrant proteins, and it has been shown that, even under normal conditions, a large fraction of newly synthesized proteins is rapidly turned over by proteasomes (Yewdell et al., 1996; Schubert et al., 2000). It is therefore tempting to speculate that the two Lys residues have been maintained to guarantee rapid clearance of any nascent RTA that might be produced by premature termination of preproricin mRNA translation. Such RTA polypeptides would clearly have the capacity to be dislocated to the cytosol and would, if not rapidly destroyed, intoxicate the castor bean endosperm cell that contains ribosomes sensitive to ricin action (Hartley et al., 1996).
The slow degradation of dislocated RTA*0K was accompanied by the accumulation of a polypeptide comigrating with what we have previously shown to be a cytosolic deglycosylated intermediate. It is likely that this intermediate is generated by the action of a cytosolic peptide: N-glycanase (for review, see Suzuki et al., 2002), which may act in concert with the proteasome itself in the degradation of dislocated proteins. For other glycosylated ERAD substrates, it is only when proteasomes are defective or inhibited that analogous intermediates are evident. This raises the possibility that the deglycosylated species is an artifact of proteasome inhibition and that, under normal conditions, glycan removal follows, rather than precedes, proteolysis. Our results clearly indicate that this is not the case. Here, under conditions in which proteasomes are fully active, a protein completely devoid of Lys residues can accumulate in deglycosylated form in the cytosol. The cytosolic accumulation of deglycosylated RTA*0K under normal cellular conditions suggests that the absence of internal Lys residues somehow hampers subsequent targeting of the deglycosylated protein to the proteasome and highlights the role of ubiquitination in linking the deglycosylation and degradation steps of the ERAD pathway.
MATERIALS AND METHODS
Recombinant DNA
All DNA constructs were generated in the expression vector pDHA (Tabe et al., 1995). pRTA and phaseolin expression constructs (pDHET343F) have already been described (Pedrazzini et al., 1997; Frigerio et al., 1998). The pRTA construct was used as the initial template to generate pRTAK4R, pRTAK239R, pRTA0K, and pRTAW27V using the QuickChange method (Stratagene, La Jolla, CA) and the following mutagenic primers: 5′-GATAACAACATATTCCCCAGACAATACCCAATTATAAAC-3′, 5′-CAAAGACGTAATGGTTCCAGATTCAGTGTGTACGATGTG-3′, and 5′-GGATCCACCTCAGGGGTGTCTTTCACATTA-3′ (changes with respect to the wild-type sequence are underlined).
Plasmid pRTA6K was obtained by digesting a construct coding for an RTA mutant containing four extra Lys residues (Deeks et al., 2002), with BanI and AseI, and ligating the resulting BanI-AseI fragment into BanI-AseI-restricted pRTA. The active site mutant set in which Glu-177 has been substituted by Asp was originated by site-directed mutagenesis as described (Chaddock and Roberts, 1993) using RTA, RTA0K, and RTA6K as templates.
Transformation of Protoplasts and Pulse-Chase Experiments
Protoplasts were prepared from axenic leaves (4–7 cm long) of tobacco (Nicotiana tabacum) cv Petit Havana SR1. Protoplasts were subjected to polyethylene glycol-mediated transfection as described (Ceriotti et al., 2003). Cells were radiolabeled with Pro-Mix (Amersham Biosciences, Buckinghamshire, UK) and chase was performed exactly as described (Frigerio et al., 1998). In some experiments, before radioactive labeling, protoplasts were incubated for 1 h at 25°C in K3 medium supplemented with 5 mm DMM (Sigma, St. Louis; 200 mm stock in water). When indicated, β-lactone (Calbiochem, San Diego; 20 mm stock in dimethyl sulfoxide) was added, at 80 μm final concentration, at the beginning of the labeling period. At the desired time points, 3 volumes of W5 medium (Ceriotti et al., 2003) were added and protoplasts were pelleted by centrifugation at 60g for 5 min. Cells were frozen in liquid nitrogen and stored at −80°C.
Protoplast Fractionation
Protoplast pellets (from 500,000 cells) were resuspended in 170 μL Suc buffer (100 mm Tris-HCl, pH 7.6, 10 mm KCl, 1 mm EDTA, 12% [w/w] Suc) supplemented with Complete protease inhibitor cocktail (Roche Diagnostics, Basel) and homogenized by pipetting 50 times with a Gilson-type micropipette thought a 200-μL tip. Intact cells and debris were removed by centrifugation for 5 min at 500g. Thirty microliters of the supernatants (total protoplast homogenate) were saved and directly used for immunoprecipitation. The rest was loaded on top of a 17% (w/w) Suc pad and centrifuged at 100,000g for 30 min at 4°C. Pellets (microsomes) and supernatants (soluble proteins) were diluted in protoplast homogenization buffer and used for immunoprecipitation.
Protease Protection Assay
Protoplast pellets (500,000 cells) were homogenized in 12% Suc buffer as described above, omitting protease inhibitors, and debris was removed by spinning at 500g for 5 min. Supernatants were divided into 3 aliquots and incubated for 30 min at 25°C with either buffer (as control) or proteinase K (Calbiochem; 5 mg/mL stock in 50 mm Tris-Cl, pH 8, 1 mm CaCl2) at the final concentration of 75 μg/mL in the presence or absence of 1% Triton X-100. Phenylmethylsulfonyl fluoride was added at 20 mm final concentration to inhibit proteinase K before immunoprecipitation.
Preparation of Protein Extracts and Immunoprecipitation
Frozen samples were homogenized by adding 300 μL of protoplast homogenization buffer (Ceriotti et al., 2003) supplemented with Complete protease inhibitor mixture (Roche Diagnostics). Homogenates were used for immunoprecipitation with polyclonal rabbit anti-RTA, anti-BiP (Pedrazzini et al., 1997) or antiphaseolin antisera (a kind gift from R. Bollini, National Research Council, Milan). Immunoselected polypeptides were analyzed by 15% SDS-PAGE. Gels were treated with Amplify (Amersham) and radioactive polypeptides were revealed by fluorography. Band intensity was determined using TotalLab software (Nonlinear Dynamics, Newcastle upon Tyne, UK).
Toxicity Measurement
Triplicate aliquots of tobacco SR1 protoplasts were cotransfected with a toxin-encoding plasmid or with empty vector (pDHA) together with the phaseolin-encoding construct pDHAT343F. After overnight recovery, protoplasts were pulse labeled for 1 h. Polypeptides immunoselected with antiphaseolin antiserum were separated on SDS-PAGE and toxicity of the various constructs was expressed as a percentage reduction in phaseolin band intensity with respect to protoplasts that were cotransfected with the empty vector.
Acknowledgments
We thank Alessandro Occhialini and Pietro Della Cristina for assistance during the late stages of this work, and Serena Fabbrini for a critical reading of the manuscript.
This work was supported by U.K. Biotechnology and Biological Sciences Research Council (grant no. C08612 to L.M.R. and J.M.L.) and by Fondo per gli Investimenti della Ricerca di Base (project no. RBNE01TYZF to A.C.).
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.055434.
References
- Argent RH, Parrott AM, Day PJ, Roberts LM, Stockley PG, Lord JM, Radford SE (2000) Ribosome-mediated folding of partially unfolded ricin A-chain. J Biol Chem 275: 9263–9269 [DOI] [PubMed] [Google Scholar]
- Bachmair A, Novatchkova M, Potuschak T, Eisenhaber F (2001) Ubiquitinylation in plants: a post-genomic look at a post-translational modification. Trends Plant Sci 6: 463–470 [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340: 783–795 [DOI] [PubMed] [Google Scholar]
- Biederer T, Volkwein C, Sommer T (1997) Role of Cue1p in ubiquitination and degradation at the ER surface. Science 278: 1806–1809 [DOI] [PubMed] [Google Scholar]
- Bloom J, Amador V, Bartolini F, DeMartino G, Pagano M (2003) Proteasome-mediated degradation of p21 via N-terminal ubiquitinylation. Cell 115: 71–82 [DOI] [PubMed] [Google Scholar]
- Bordallo J, Plemper RK, Finger A, Wolf DH (1998) Der3p/Hrd1p is required for endoplasmic reticulum-associated degradation of misfolded lumenal and integral membrane proteins. Mol Biol Cell 9: 209–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I (2001) A novel lectin in the secretory pathway. An elegant mechanism for glycoprotein elimination. EMBO Rep 2: 666–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Hanton S, DaSilva LL, Boevink P, Evans D, Oparka K, Denecke J, Hawes C (2003) ER quality control can lead to retrograde transport from the ER lumen to the cytosol and the nucleoplasm in plants. Plant J 34: 269–281 [DOI] [PubMed] [Google Scholar]
- Breitschopf K, Bengal E, Ziv T, Admon A, Ciechanover A (1998) A novel site for ubiquitination: The N-terminal residue, and not internal lysines of MyoD, is essential for conjugation and degradation of the protein. EMBO J 17: 5964–5973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriotti A, Vitale A, Paris N, Frigerio L, Neuhaus J-M, Hillmer S, Robinson DG (2003) Plant cell biology. In J Davey, JM Lord, eds, Essential Cell Biology, Vol 1. Oxford Univesity Press, Oxford, pp 133–161
- Chaddock JA, Roberts LM (1993) Mutagenesis and kinetic analysis of the active site Glu177 of ricin A chain. Protein Eng 6: 425–431 [DOI] [PubMed] [Google Scholar]
- Deeks ED, Cook JP, Day PJ, Smith DC, Roberts LM, Lord JM (2002) The low lysine content of ricin A chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol. Biochemistry 41: 3405–3413 [DOI] [PubMed] [Google Scholar]
- de Virgilio M, Weninger H, Ivessa NE (1998) Ubiquitination is required for the retrotranslocation of a short-lived luminal endoplasmic reticulum glycoprotein to the cytosol for degradation by the proteasome. J Biol Chem 273: 9734–9743 [DOI] [PubMed] [Google Scholar]
- Di Cola A, Frigerio L, Lord JM, Ceriotti A, Roberts LM (2001) Ricin A chain without its partner B chain is degraded after retrotranslocation from the endoplasmic reticulum to the cytosol in plant cells. Proc Natl Acad Sci USA 98: 14726–14731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo Y, Mitsui K, Motizuki M, Tsurugi K (1987) The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes. The site and the characteristics of the modification in 28S ribosomal RNA caused by the toxins. J Biol Chem 262: 5908–5912 [PubMed] [Google Scholar]
- Ferrini JB, Martin M, Taupiac MP, Beaumelle B (1995) Expression of functional ricin B chain using the baculovirus system. Eur J Biochem 233: 772–777 [DOI] [PubMed] [Google Scholar]
- Frigerio L, Jolliffe NA, Di Cola A, Hernàndez Felipe D, Paris N, Neuhaus J-M, Lord JM, Ceriotti A, Roberts LM (2001) The internal propeptide of the ricin precursor carries a sequence-specific determinant for vacuolar sorting. Plant Physiol 126: 167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigerio L, Lord JM (2000) Glycoprotein degradation: Do sugars hold the key? Curr Biol 10: R674–R677 [DOI] [PubMed] [Google Scholar]
- Frigerio L, Vitale A, Lord JM, Ceriotti A, Roberts LM (1998) Free ricin A chain, proricin, and native toxin have different cellular fates when expressed in tobacco protoplasts. J Biol Chem 273: 14194–14199 [DOI] [PubMed] [Google Scholar]
- Hartley MR, Chaddock JA, Bonness MS (1996) The structure and function of ribosome-inactivating proteins. Trends Plant Sci 1: 254–268 [Google Scholar]
- Hazes B, Read RJ (1997) Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36: 11051–11054 [DOI] [PubMed] [Google Scholar]
- Hughes EA, Hammond C, Cresswell P (1997) Misfolded major histocompatibility complex class I heavy chains are translocated into the cytoplasm and degraded by the proteasome. Proc Natl Acad Sci USA 94: 1896–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosch E, Taxis C, Volkwein C, Bordallo J, Finley D, Wolf DH, Sommer T (2002) Protein dislocation from the ER requires polyubiquitination and the AAA-ATPase Cdc48. Nat Cell Biol 4: 134–139 [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR (1998) Aggresomes: a cellular resonse to misfolded proteins. J Cell Biol 143: 1883–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikkert M, Hassink G, Barel M, Hirsch C, van der Wal FJ, Wierz E (2001) Ubiquitination is essential for human cytomegalovirus US11-mediated dislocation of MHC class I molecules from the endoplasmic reticulum to the cytosol. Biochem J 358: 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostova Z, Wolf DH (2003) Waste disposal in plants: where and how? Trends Plant Sci 8: 461–462 [DOI] [PubMed] [Google Scholar]
- Lamb FI, Roberts LM, Lord JM (1985) Nucleotide sequence of cloned cDNA coding for preproricin. Eur J Biochem 148: 265–270 [DOI] [PubMed] [Google Scholar]
- Lord JM, Davey J, Frigerio L, Roberts LM (2000) Endoplasmic reticulum-associated protein degradation. Semin Cell Dev Biol 11: 159–164 [DOI] [PubMed] [Google Scholar]
- Lord JM, Roberts LM (1998) Toxin entry: retrograde transport through the secretory pathway. J Cell Biol 140: 733–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini R, Fagioli C, Fra A, Maggioni C, Sitia R (2000) Degradation of unassembled soluble Ig subunits by cytosolic proteasomes: evidence that retrotranslocation and degradation are coupled events. FASEB J 14: 769–778 [DOI] [PubMed] [Google Scholar]
- Monzingo AF, Collins EJ, Ernst SR, Irvin JD, Robertus JD (1993) The 2.5 Å structure of pokeweed antiviral protein. J Mol Biol 233: 705–715 [DOI] [PubMed] [Google Scholar]
- Pedrazzini E, Giovinazzo G, Bielli A, de Virgilio M, Frigerio L, Pesca M, Faoro F, Bollini R, Ceriotti A, Vitale A (1997) Protein quality control along the route to the plant vacuole. Plant Cell 9: 1869–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickart CM (2000) Ubiquitin in chains. Trends Biochem Sci 25: 544–548 [DOI] [PubMed] [Google Scholar]
- Rivett AJ (1998) Intracellular distribution of proteasomes. Curr Opin Immunol 10: 110–114 [DOI] [PubMed] [Google Scholar]
- Schubert U, Anton LC, Gibbs J, Norbury CC, Yewdell JW, Bennink JR (2000) Rapid degradation of a large fraction of newly synthesised proteins by proteasomes. Nature 404: 770–774 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Park H, Lennarz WJ (2002) Cytoplasmic peptide:N-glycanase (PNGase) in eukaryotic cells: occurrence, primary structure, and potential functions. FASEB J 16: 635–641 [DOI] [PubMed] [Google Scholar]
- Tabe LM, Wardley-Richardson T, Ceriotti A, Aryan A, McNabb W, Moore A, Higgins TJV (1995) A biotechnological approach to improving the nutritive value of alfalfa. J Anim Sci 73: 2752–2759 [DOI] [PubMed] [Google Scholar]
- Taylor S, Massiah A, Lomonossoff G, Roberts LM, Lord JM, Hartley M (1994) Correlation between the activities of five ribosome-inactivating proteins in depurination of tobacco ribosomes and inhibition of tobacco mosaic virus infection. Plant J 5: 827–835 [DOI] [PubMed] [Google Scholar]
- Tsai B, Ye Y, Rapoport TA (2002) Retro-translocation of proteins from the endoplasmic reticulum into the cytosol. Nat Rev Mol Cell Biol 3: 246–255 [DOI] [PubMed] [Google Scholar]
- Varshavsky A (1996) The N-end rule: functions, mysteries, uses. Proc Natl Acad Sci USA 93: 12142–12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD (2003) The ubiquitin/26S proteasome pathway, the complex last chapter in the life of many plant proteins. Trends Plant Sci 8: 135–142 [DOI] [PubMed] [Google Scholar]
- Walker D, Chaddock AM, Chaddock JA, Roberts LM, Lord JM, Robinson C (1996) Ricin A chain fused to a chloroplast-targeting signal is unfolded on the chloroplast surface prior to import across the envelope membranes. J Biol Chem 271: 4082–4085 [DOI] [PubMed] [Google Scholar]
- Worley CK, Ling R, Callis J (1998) Engineering in vivo instability of firefly luciferase and Escherichia coli β-glucuronidase in higher plants using recognition elements from the ubiquitin pathway. Plant Mol Biol 37: 337–347 [DOI] [PubMed] [Google Scholar]
- Yewdell JW, Anton LC, Bennink JR (1996) Defective ribosomal products (DRiPs): a major source of antigenic peptides for MHC class I molecules? J Immunol 157: 1823–1826 [PubMed] [Google Scholar]
- Yu H, Kaung G, Kobayashi S, Kopito RR (1997) Cytosolic degradation of T-cell receptor α chains by the proteasome. J Biol Chem 272: 20800–20804 [DOI] [PubMed] [Google Scholar]