Abstract
When chilling-sensitive plants are chilled, root hydraulic conductance (Lo) declines precipitously; Lo also declines in chilling-tolerant plants, but it subsequently recovers, whereas in chilling-sensitive plants it does not. As a result, the chilling-sensitive plants dry out and may die. Using a chilling-sensitive and a chilling-tolerant maize genotype we investigated the effect of chilling on Lo, and its relationship to osmotic water permeability of isolated root cortex protoplasts, aquaporin gene expression, aquaporin abundance, and aquaporin phosphorylation, hydrogen peroxide (H2O2) accumulation in the roots and electrolyte leakage from the roots. Because chilling can cause H2O2 accumulation we also determined the effects of a short H2O2 treatment of the roots and examined the same parameters. We conclude from these studies that the recovery of Lo during chilling in the chilling-tolerant genotype is made possible by avoiding or repairing membrane damage and by a greater abundance and/or activity of aquaporins. The same changes in aquaporins take place in the chilling-sensitive genotype, but we postulate that membrane damage prevents the Lo recovery. It appears that the aquaporin response is necessary but not sufficient to respond to chilling injury. The plant must also be able to avoid the oxidative damage that accompanies chilling.
Many crop species such as maize (Zea mays) that originate in tropical regions are sensitive to low temperatures and grow poorly after exposure to a cold period (Miedema, 1982). Below 12°C to 14°C, maize seedlings are damaged as a result of leaf dehydration and an inhibition of photosynthesis. Such damage may result in the plant's death (Miedema, 1982). With the wide utilization in temperate climates of crops of tropical origin, the adverse effect of chilling is an important agricultural problem the physiological basis of which can be elucidated. Cold-tolerant species increase their root hydraulic conductance (Lo) during low temperature periods (Fennell and Markhart, 1998; Bigot and Boucaud, 2000), and the same phenomenon has been observed in a chilling-tolerant genotype of maize (Aroca et al., 2001b, 2003b). The water deficit in the aerial parts of a chilling-sensitive maize genotype is caused in part by low temperature inhibition of Lo (Aroca et al., 2001b, 2003b). The recovery of Lo in cold-tolerant plants may be associated with an increase in the activity or the abundance of aquaporins in the plasma membranes of root cells. On the other hand, the opposite may happen in cold-sensitive species: aquaporins may be down-regulated or inactivated (Aroca et al., 2001b; Sanders and Markhart, 2001;). Lee et al. (2004b) recently showed that in cucumber (Cucumis sativus), a cold sensitive species, a brief exposure to low temperature reduces root pressure, hydraulic conductivity, and active nutrient transport. These authors also postulated that changes in the activity of aquaporins underlie the changes in hydraulic conductivity.
Response to cold, and in some plants adaptation to cold, is a complex process that involves the additive properties of many gene products (Thomashow, 2001). In chilling-sensitive species, extensive oxidative damage occurs as a result of chilling, whereas chilling-tolerant plants have mechanisms to avoid and/or minimize such oxidative stress (Pinhero et al., 1997; Aroca et al., 2003a; Campos et al., 2003). One of the reactive oxygen species that accumulates in plant tissues during cold stress is hydrogen peroxide (H2O2; Kerdnaimongkol and Woodson, 1999; Kang et al., 2003). Recently, Ktitorova et al. (2002) suggested that the inhibitory effect of salt stress on Lo of wheat (Triticum aestivum) plants might be mediated by H2O2 produced in the roots during salt exposure. The inhibition of tomato Lo by the chemical disinfectant peracetic acid may also be mediated by H2O2 (Vines et al., 2003). In the same way, H2O2 generated during chilling could be the cause of the inhibition of Lo in chilling-sensitive species.
Aquaporins (or water channels proteins) are proteinaceous pores that facilitate passive water transport through membranes of living cells (Chrispeels and Agre, 1994). In plants, aquaporins are divided in four clades: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), nodulin-like intrinsic proteins (NIPs), similar to the Nod26 aquaporin found first in the symbiosome membrane of nitrogen-fixing nodules, and small intrinsic proteins (SIPs; Chaumont et al., 2001; Johanson et al., 2001). Aquaporins can be regulated translationally as well as posttranslationally. The expression of aquaporin genes responds to a number of abiotic stresses (Tyerman et al., 2002), and aquaporin activity can be regulated by phosphorylation at one or more sites (Johansson et al., 1998), divalent cations, and pH (Gerbeau et al., 2002; Tournaire-Roux et al., 2003). Aquaporins are also spatially and developmentally regulated and more than one isoform is present in any one membrane, suggesting the potential for redundancy and independent regulation (Tyerman et al., 2002). Moreover, it has recently been shown that the presence of one aquaporin in a membrane can synergistically influence the activity of other aquaporins (Fetter et al., 2004).
Radial water transport through the roots can follow both the apoplastic and the cell-to-cell path (see Steudle, 2000). In the first path, which predominates in transpiring plants, water travels primarily through the cell walls. The second path predominates in nontranspiring conditions and refers to water transport through the living cells. Recent studies that either made use of transgenic plants lacking one or more aquaporins (Siefritz et al., 2002; Javot et al., 2003) or used chemical blockers of aquaporin activity (Maggio and Joly, 1995) concluded that aquaporins may regulate whole root water transport (see Javot and Maurel, 2002).
In the research described here, the effect of chilling and exogenous H2O2 on Lo, osmotic water permeability of isolated root protoplasts, root aquaporin expression and phosphorylation, and root membrane injury was studied in two maize genotypes differing in chilling tolerance. We suggest on the basis of the results that the decrease in Lo during chilling in the sensitive genotype is due to oxidative damage of its root membranes and not to a decrease in aquaporin amount or phosphorylation.
RESULTS
The Effects of Chilling and H2O2 Treatment on Lo
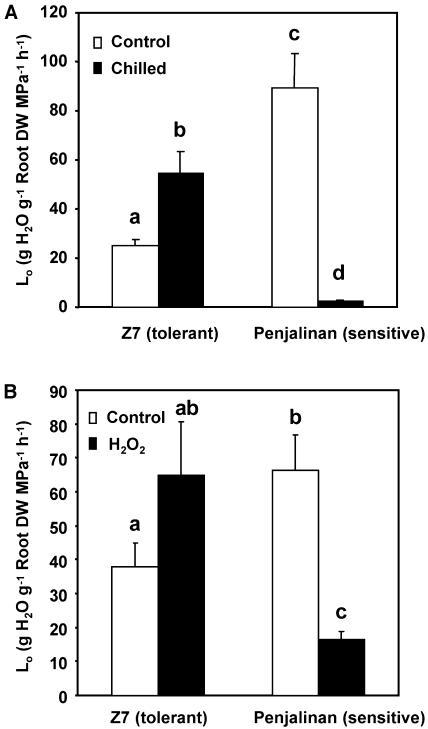
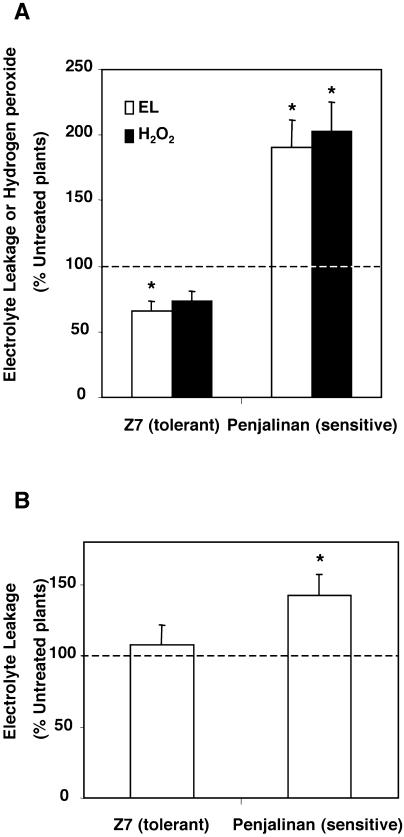
Initially we briefly confirmed the results of earlier studies (Aroca et al., 2001b, 2003b) showing that chilling (5°C) causes an immediate decline in the Lo of both chilling-sensitive (Penjalinan) and chilling-tolerant (Z7) maize genotypes, and that the Lo of the chilling-tolerant plants gradually recovers during the next 3 d, whereas the Lo of the chilling-sensitive plants continues to decline (data not shown). We chose 3 d of chilling as our reference point and the values obtained for the 2 genotypes after 3 d of chilling are shown in Figure 1A. The maize plants were cut at the base and the sap exudation was measured at room temperature during the subsequent 1-h or 3-h period for control and chilled plants, respectively (a longer time was needed for the chilled plants to get an accurate measurement). Measurements were made 2 h after the lights came on. The Lo of Penjalinan (sensitive) was nearly 4 times as large as the Lo of Z7 (tolerant) in the control plants not exposed to chilling, but after 3 d of chilling the Lo of Penjalinan was barely measurable and the Lo of Z7 had doubled.
Figure 1.
Lo determined in 11-d-old plants exuding at atmospheric pressure of two maize genotypes differing in chilling sensitivity (Z7, tolerant and Penjalinan, sensitive). A, Plants were kept at 25°C (control, white columns) or transferred for 3 d at 5°C (chilled, black columns; n = 9). B, Plants grown at 25°C (control, white columns) or after 1 h of exposure of the roots to 100 μm H2O2. (n = 37) by summing the values of three different and independent experiments. The columns represent mean ± se. The different letters represent significantly different values (P < 0.05, using the Student's t test).
Because chilling may cause H2O2 accumulation (Kerdnaimongkol and Woodson, 1999; Kang et al., 2003) we also measured the effect of a brief (1-h) exposure of the roots to 100 μm H2O2 (Fig. 1B). In this experiment, carried out 4 h after the lights came on, the Lo of untreated Penjalinan plants was twice as large as that of untreated Z7 plants. The difference with the previous experiment may be caused by diurnal changes in Lo (Henzler et al., 1999). After H2O2 treatment, the Lo of Penjalinan (sensitive) dropped dramatically (75%), but the Lo of Z7 was unchanged. (The apparent increase shown in Fig. 1B was not statistically significant). Thus, the response of the plants to a short oxidative stress resembled their response to a much longer chilling stress.
Osmotic Water Permeability of Isolated Root Protoplasts
Recent studies show that under certain conditions membrane water permeability of root cells correlates with whole Lo (Siefritz et al., 2002; Javot et al., 2003). In other situations a relationship between root hydraulic and cellular hydraulic conductance could not be demonstrated (Gunsé et al., 1997; Henzler et al., 1999).
To see if the two parameters are correlated in these plants and find out if changes in osmotic water permeability (Pos) underlie the changes on Lo, we measured Pos of isolated root protoplasts as described by Ramahaleo et al. (1999). The protoplasts were prepared 2 and 4 h after the lights came on for the chilling and the H2O2 experiments, respectively. The diameters of the protoplasts used ranged between 40 and 70 μm, corresponding to cortical cells (Roberts and Tester, 1995).
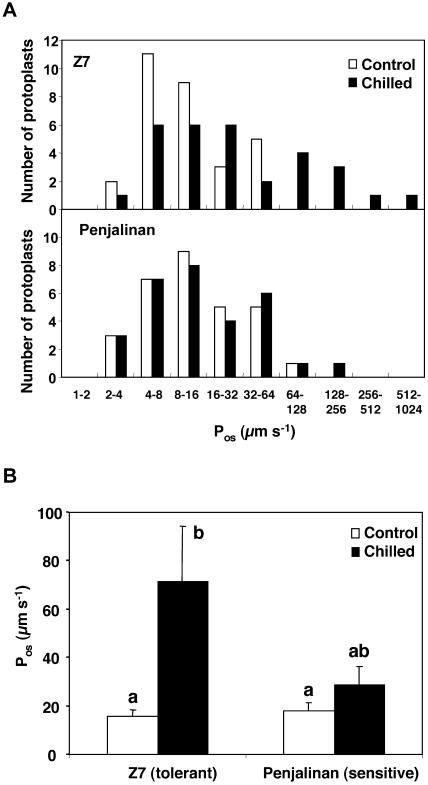
As has been found before (Ramahaleo et al., 1999; Morillon and Chrispeels, 2001; Siefritz et al., 2002), the Pos values of isolated protoplasts differ over a wide range, and Pos values are therefore shown as histograms showing the number of protoplasts in each interval (Fig. 2A). Mean Pos values are shown in Figure 2B. Clearly, chilling increased the distribution of Pos values of Z7 protoplasts as 9 out of 30 protoplasts examined had values greater than 64 μm s−1 (Fig. 2A). The mean Pos of Z7 protoplasts increased about 4 times after 3 d at 5°C (Fig. 2B). This result correlates with the increase of Z7 Lo caused by chilling (Fig. 1A). In contrast, the Pos of Penjalinan protoplasts did not change as a result of chilling (Fig. 2). Thus, the decrease of Penjalinan Lo by chilling cannot be explained by a decrease in cellular water permeability.
Figure 2.
Pos of root protoplasts isolated from 11-d-old plants of two maize genotypes differing in chilling sensitivity (Z7, tolerant and Penjalinan, sensitive). A, Histograms of the number of protoplasts included in each Pos range; plants were kept at 25°C (control, white columns) or transferred for to 3 d at 5°C (chilled, black columns). Z7, Top section and Penjalinan, bottom section. B, The columns represent mean ± se (n = 30) of each treatment. Otherwise as for Figure 1.
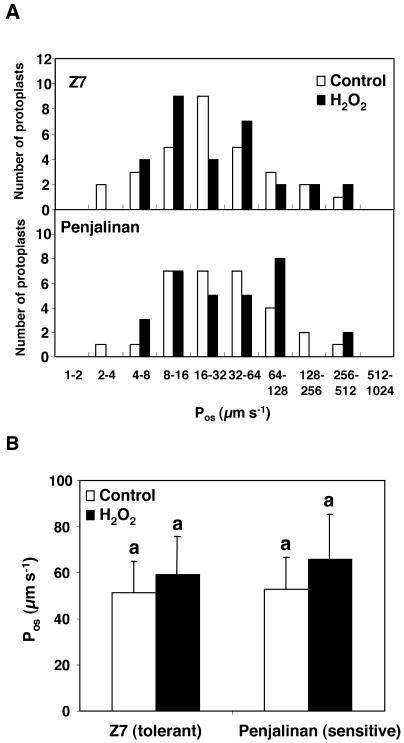
There was no effect of H2O2 on the Pos of protoplasts from either the sensitive or the tolerant genotype (Fig. 3), although the short (1-h) H2O2 treatment dramatically decreased the hydraulic conductance in the sensitive genotype. Thus, Pos did not correlate with Lo values in the sensitive genotype.
Figure 3.
Pos of isolated root protoplasts of 11-d-old plants of two maize genotypes differing in chilling sensitivity (Z7, tolerant and Penjalinan, sensitive). A, Histograms of the number of protoplasts included in each Pos range; plants that were grown at 25°C (control, white columns) and given a 1-h exposure of the roots to 100 μm H2O2 (black columns). Z7, Top and Penjalinan, bottom. B, The columns represent mean ± se (n = 30) of each treatment. Otherwise as for Figure 1.
Aquaporin Protein Abundance and Phosphorylation State
Some studies support the conclusion that aquaporins regulate root hydraulic properties (Javot and Maurel, 2002). We used antibodies against PIP1, PIP2, and phosphorylated PIP2 aquaporins to ascertain if aquaporin abundance and phosphorylation state were correlated with the Lo and Pos responses observed with chilling and H2O2 treatments. The sequences of the amino acids of maize PIP proteins and those of the peptides used to elicit the antibodies are shown in Figure 4. Based on amino acid sequence comparisons, the PIP1 antiserum is likely to recognize all the maize PIP1 aquaporins, and the PIP2 antiserum is likely to recognize ZmPIP2;1, 2;2, and 2;7, and possibly also ZmPIP2;3, 2;4, and 2;6 but not ZmPIP2;5.
Figure 4.
A and B, Multiple alignment of the carboxy-terminal region of the Zm PIP1 and Zm PIP2 proteins and the TaPIP1 and AtPIP2;3 proteins, respectively. The consensus amino acids are underlined. C, Alignment of the conserved phosphorylation site of the PIP2 proteins. The TaPIP1, AtPIP2;3, and TaPIP2 sequences shown correspond to the sequence of the peptide used to make the respective antibodies.
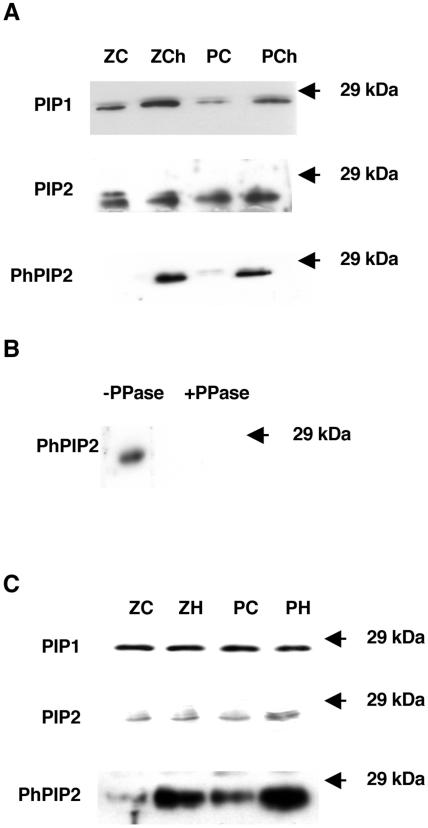
To quantify the results of the inmunoblots, the signal intensity of each band was normalized with the signal of the corresponding Coomassie Brillant Blue protein lane (Fig. 5). In some case, the anti-PIP2 antibody recognized two bands of a similar size (Fig. 6), and both bands were used for quantification. This double band phenomenon has been seen previously using a different PIP2 antibody (Hanba et al., 2004). These double bands could represent an as yet uncharacterized posttranslational modification.
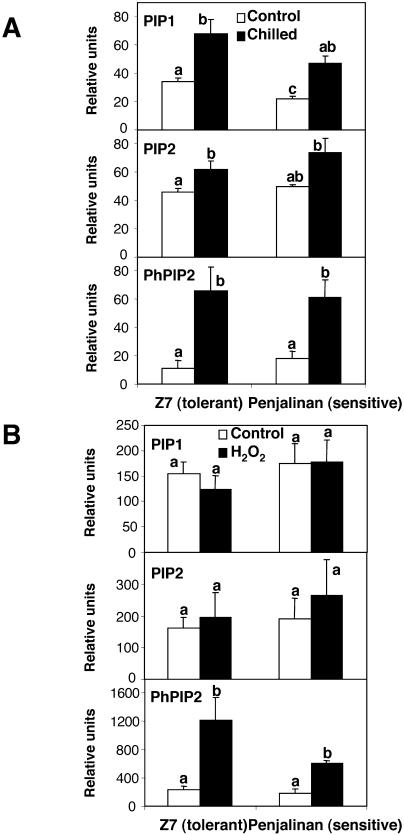
Figure 5.
Relative amount of PIP1, PIP2, and phosphorylated PIP2 (PhPIP2) proteins, quantifying the intensity of each band shown in Figure 6 relative to the intensity of the corresponding Coomassie Brilliant Blue band. Thus, the relative units are: (intensity of immunoblot band)/(intensity of Coomassie Brilliant Blue band) × 103. A and B correspond to the chilling and H2O2 experiments, respectively. The columns in the histogram represent the mean ± se (n = 3) for each treatment. Other details as in Figure 1.
Figure 6.
A, Immunoblot analysis of microsomal extracts for roots of 11-d-old plants of two maize genotypes differing in chilling sensitivity (Z7 (Z), tolerant and Penjalinan (P), sensitive) that have been grown at 25°C (C) or transferred for 3 d at 5°C (Ch). The blot was developed with antibodies against PIP1, PIP2, and phosphorylated PIP2 (PhPIP2) proteins (see “Materials and Methods” for details). B, Immunoblot analysis of microsomal extracts of roots; previous incubation of the nitrocelluse membrane was done with or without phosphatase (PPase). C, Immunoblot analysis of microsomal extracts for roots of 11-d-old plants of two maize genotypes differing in chilling sensitivity (Z7 (Z), tolerant and Penjalinan (P), sensitive) that have been grown at 25°C (C) or after 1 h of exposure of the roots to 100 μm of H2O2 (H). The blot was developed with antibodies against PIP1, PIP2, and phosphorylated PIP2 (PhPIP2) proteins (see “Materials and Methods” for details).
As a result of the chilling treatment, the abundance of PIP1 proteins as measured by immunoreactivity significantly increased in root tissues of both genotypes after 3 d at 5°C; the fold change was 2.0 and 2.1 for Z7 and Penjalinan, respectively (Fig. 5A), whereas the amount of PIP2 proteins detected with the PIP2 antiserum did not change in Penjalinan and increased by 1.4 factor in Z7 (Fig. 5A). However, the chilled values of the relative intensity of the PIP2 proteins were the same for both genotypes (Fig. 5A). Thus, the increase in aquaporins in the tolerant genotype may account for the increase in Pos and Lo, but the same is not true for the sensitive genotype. Since aquaporin activity can be activated by phosphorylation (Johansson et al., 1998), a specific antibody against phosphorylated PIP2 proteins was used. The antibody was designed against the most conserved sequence among all PIP2 proteins in diverse species with a possible phosphorylation site (Johansson et al., 1998), including maize PIP2 proteins (Fig. 4). The signal on the immunoblots for phosphorylated PIP2 increased dramatically after 3 d of chilling in both genotypes (Fig. 6A), with a fold change of 6.1 and 3.5 for Z7 and Penjalinan roots, respectively (Fig. 5A). These increases on the phosphorylation state of the PIP2 proteins were not due by the increase in the total amount of the PIP2 proteins. PIP2 proteins only increased after chilling treatment in Z7 by a factor of 1.4, while the increase factor of the phosphorylation state was 6.1 (Fig. 5A).
To confirm that this antibody only recognized phosphorylated PIP2, we incubated a nitrocellulose membrane strip for 24 h with phosphatase and another strip without, as described by Faye and Chrispeels (1985). No signal was detected from the strip incubated with phosphatase, indicating that the phosphatase had removed the epitope (Fig. 6B).
The brief (1-h) treatment with 100 μm H2O2 did not cause any significant change in the abundance of the PIP1 and PIP2 proteins (Figs. 5B and 6C). However, the phosphorylation state of the PIP2 proteins increased after the H2O2 treatment in the roots of both genotypes, with a fold change of 5.1 and 3.3 to Z7 and Penjalinan, respectively (Figs. 5B and 6C). Thus, the response of aquaporin proteins to chilling and H2O2 treatments was the same in both genotypes. Both treatments significantly increased the phosphorylation state of PIP2 proteins. Higher levels of PIP proteins and more phosphorylation may help explain the recovery of Z7 from chilling, but they are not sufficient to explain why the chilling-sensitive Penjalinan do not recover.
Aquaporin Gene Expression
We also analyzed the expression response of the maize aquaporin genes to chilling to determine if protein changes were related to mRNA levels. We performed an expression analysis of all the maize PIP1 and PIP2 aquaporins by northern blots using gene specific probes. Total root RNA was isolated at 0, 1, and 3 d after chilling. The mRNA level of each PIP gene after 1 and 3 d of chilling was expressed as the ratio to the value before chilling (Table I). The signals were normalized using the 18S rRNA signals on the blots.
Table I.
ZmPIP RNA expression
Comparison of the expression level of PIP genes in both genotypes was expressed as the ratio of the normalized signals (Penjalinan/Z7) at time 0. The RNA expression variation during cold stress was expressed as the ratio of the signal values obtained at time 0 and time 1 d or 3 d for both genotypes.
| Aquaporin
|
Expression Levels (Penjalinan/Z7) at 0 d
|
Fold Change
|
|||
|---|---|---|---|---|---|
| Penjalinan
|
Z7
|
||||
| 0/1 d | 0/3 d | 0/1 d | 0/3 d | ||
| ZmPIP1;1 | 2.14 | 0.88 | 0.20 | 0.40 | 0.17 |
| ZmPIP1;2 | 1.36 | 0.93 | 0.31 | 0.71 | 0.40 |
| ZmPIP1;3 | 1.44 | 0.55 | 0.16 | 0.54 | 0.29 |
| ZmPIP1;4 | 1.14 | 0.81 | 0.25 | 0.46 | 0.23 |
| ZmPIP1;5 | 0.78 | 0.74 | 0.32 | 0.38 | 0.26 |
| ZmPIP1;6 | 2.70 | 0.60 | 0.35 | 0.56 | 0.89 |
| ZmPIP2;1 | 1.08 | 0.88 | 0.20 | 0.79 | 0.40 |
| ZmPIP2;2 | 1.13 | 0.73 | 0.27 | 0.44 | 0.40 |
| ZmPIP2;3 | 0.99 | 0.94 | 0.14 | 0.47 | 0.42 |
| ZmPIP2;4 | 0.56 | 0.83 | 0.17 | 0.38 | 0.20 |
| ZmPIP2;5 | 0.80 | 0.73 | 0.32 | 0.64 | 0.59 |
| ZmPIP2;6 | 1.13 | 0.96 | 0.19 | 0.70 | 0.79 |
| ZmPIP2;7a | – | – | – | – | – |
Gene not expressed.
In control conditions (day 0), expression levels for most of the PIP genes were higher in Penjalinan than in Z7, except for the ZmPIP1;5, ZmPIP2;4, and ZmPIP2;5 mRNAs, where Z7 had the highest levels, and for ZmPIP2;1 and ZmPIP2;3 mRNAs, where no significant differences were found between genotypes (Table I). ZmPIP2;7 mRNA was not detected in any of the samples (Table I).
After 1 d of chilling, root mRNA levels of all 12 PIP genes decreased (Table I). After 3 d of chilling, mRNA levels of all PIP genes continued to decline, except that of the ZmPIP1;6 of Z7, which increased but did not recover its initial values (Table I). Thus, the increase of PIP1 proteins and the lack of change of the PIP2 proteins as a result of chilling are not related to the mRNA levels. This finding is not entirely unexpected since mRNA levels and protein levels are often unrelated because control of protein levels can be transcriptional or posttranscriptional. Unfortunately we were unable to ascertain whether the relative levels of specific aquaporins might change during stress.
Root Electrolyte Leakage and H2O2 Accumulation
One way to interpret the experiments presented so far is to conclude that increases in aquaporin levels and phosphorylation are necessary for the recovery of the chilling-tolerant maize genotype and do also occur in the chilling-sensitive genotype, but in this genotype they are not sufficient to permit recovery. The similarity of the responses to chilling and H2O2 treatments, the knowledge that both treatments can damage membranes (Pastori and Trippi, 1992; Alonso et al., 1997) and that chilling leads to H2O2 accumulation (Kerdnaimongkol and Woodson, 1999; Kang et al., 2003) prompted us to look at the effect of chilling on H2O2 accumulation and the effect of both treatments on electrolyte leakage (EL), an accepted measure of membrane damage (Pastori and Trippi, 1992; Capell and Dörffling, 1993; Alonso et al., 1997; Pinhero et al., 1997; Marrè et al., 1998; Campos et al., 2003).
EL was determined by calculating the amount of electrolytes that are released by roots during a 3-h period of incubation as a percentage of the total amount measured after a cycle of freezing and thawing. The values were expressed as a percentage of the values obtained for untreated plants, since the control values between the genotypes were very different (14.0 ± 1.1 and 10.7% ± 0.9% to Z7 and Penjalinan, respectively). After 3 d of chilling, EL from the roots had increased significantly in the chilling-sensitive Penjalinan but had decreased in Z7 (Fig. 7A, white columns). Treatment with H2O2 also caused an increase of EL from the roots of Penjalinan, but had no effect on Z7 (Fig. 7B). These results indicate that Penjalinan roots suffered from membrane injury during chilling or H2O2 treatment, while roots of Z7 did not.
Figure 7.
A, EL (white columns) and H2O2 levels (black columns) of 11-d-old plants of two maize genotypes differing in chilling sensitivity (Z7, tolerant and Penjalinan, sensitive) after transfer for 3 d at 5°C expressed as the percentage of the untreated plants. B, EL of 11-d-old plants of two maize genotypes differing in chilling sensitivity (Z7, tolerant and Penjalinan, sensitive) after 1 h of exposure of the roots to 100 μm of H2O2 expressed as the percentage of the untreated plants. The columns represent mean ± se (n = 11). An asterisk indicates significant difference from the control value of 100% (P < 0.05, using the Student's t test).
We then assayed the effect of chilling on root H2O2 levels of both genotypes. H2O2 levels were also expressed as the percentage of the level in untreated plants, since the control values between the genotypes were also very different (4.3 ± 0.6 and 1.9 ± 0.3 μmol g−1 root fresh weight to Z7 and Penjalinan, respectively). H2O2 levels increased 2-fold in Penjalinan roots after 3 d of chilling, while no significant changes were observed in Z7 (Fig. 7A, black columns). Thus, the chilling-tolerant genotype (Z7) has the capacity to avoid the generation of H2O2 or removes H2O2 formed during chilling, while the sensitive genotype has not. Therefore, the rise in EL observed in Penjalinan roots after chilling treatment may be caused by the accumulation of H2O2.
DISCUSSION
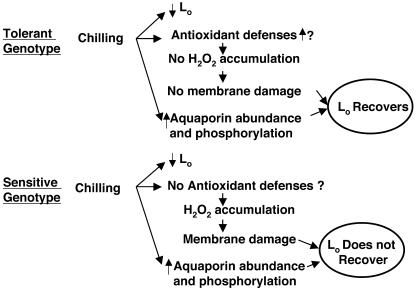
In this research, we tried to clarify the molecular basis of the difference in Lo after chilling of chilling-tolerant and -sensitive plants. We interpret our findings as follows. Chilling turns on an aquaporin response in both genotypes (higher levels of protein; more phosphorylation) and this response permits an increase in Pos and the recovery of Lo in the tolerant genotype; however, the sensitive genotype is unable to cope with the oxidative stress caused by chilling and the same aquaporin response does not permit recovery in the sensitive genotype. The tolerant genotype appears not to suffer membrane damage, possibly because it has the machinery to detoxify reactive oxygen species. This interpretation is summarized in Figure 8.
Figure 8.
Scheme summarizing the interpretation of the results. Chilling causes an initial decrease of Lo in both genotypes. After 3 d at 5°C, the tolerant genotype recovers its Lo thanks to the increase in aquaporin abundance and phosphorylation and to the maintenance of membrane integrity. On the contrary, the sensitive genotype does not recover its Lo because of membrane damage caused by oxidative stress. The tolerant genotype can cope with the oxidative stress, but the sensitive genotype cannot.
Relationship between Lo and Pos of Isolated Root Protoplasts
First we asked whether the Pos of root cells changed in the same direction as the Lo of the roots to see if changes in Pos could explain changes in Lo. There is no agreement in the literature as to whether cell hydraulic conductivities and Lo are related. In some studies they are (Siefritz et al., 2002), in others they are not (Gunsé et al., 1997; Henzler et al., 1999), and the correlation may depend on whether Lo is measured under osmotic or hydrostatic pressure (Javot et al., 2003). This is not unexpected since when Lo is measured, water can flow by the apoplastic and/or by the cell-to-cell paths. Under hydrostatic pressure, water will go predominantly via the apoplast, as occurs under high rates of transpiration. However, in fully developed roots, when the endodermis is well formed, water must cross the membranes of endodermal cells to enter the stele and the vascular system. On the other hand, under osmotic pressure water generally travels through the cell membranes, although Zimmermann and Steudle (1998) found some apoplastic water flow under osmotic conditions. Therefore, the two paths contribute to water transport at the same time, but their relative contribution depends on the environmental conditions and one of the two paths may predominate (for review, see Steudle, 2000; Javot and Maurel, 2002). Thus, Javot et al. (2003) only found a correlation between the cell hydraulic conductivities and Lo when root conductance was measured under osmotic pressure. However, Siefritz et al. (2002) also found a correlation when measuring root hydraulic conductivity under hydrostatic conditions using the pressure chamber.
We measured the Lo in plants that exuded xylem sap at atmospheric pressure. This approach measures osmotically-driven water transport only. In a previous paper (Aroca et al., 2001b) we showed that the response of Lo to chilling in both genotypes was the same under osmotic (plants exuding under atmospheric pressure) or hydrostatic (using a pressure chamber) conditions. Initially, the Lo decreased in both genotypes. In the sensitive genotypes the Lo remained at low value, but in the tolerant genotype the Lo recovered and even exceeded its initial value after 3 d at 5°C. The responses of Lo to chilling were remarkably similar to the responses to H2O2 treatment, but they differed between the two genotypes (dramatic inhibition in Penjalinan and no change or increase in Z7 for both treatments). In the tolerant genotype the changes in Pos were in the same direction as the changes in Lo, but in the sensitive genotype they were not: Lo decreased even though Pos remained the same.
As judged by the size of the protoplasts we used, they likely originated from cortical cells. Variability in hydraulic conductance of maize cortical cells was also shown in other studies (Zhu and Steudle, 1991; Gunsé et al., 1997), and the differences observed in Lo are often not reflected in the hydraulic conductance of cortical cells (Gunsé et al., 1997; Henzler et al., 1999). Gunsé et al. (1997) using root and cell pressure probes found that the decrease in root hydraulic conductivity of maize roots by Al3+ or acid pH only correlated with a decrease of cell hydraulic conductance of the first or fifth cell layers, respectively, while in other cell layers an increase was found. Henzler et al. (1999) found that the diurnal changes in Lo (a maximum at mid-day and minima in the morning and evening) in Lotus japonicus, measured under hydrostatic or osmotic pressures (pressure chamber and plants exuding at atmospheric pressure, respectively), did not correlate with changes in cell hydraulic conductivity of the cortical cells in any layer. These data suggest that endodermal and stelar cells may be responsible for the changes observed at the root level.
Therefore, the negative effect of chilling and H2O2 on Penjalinan Lo may be caused by a decrease of the hydraulic conductance of the endodermal or stellar cells. Maize roots possess an endodermis with a Casparian strip, which may act, when it is well developed, as an apoplastic water barrier, forcing the water to cross endodermal cell membranes (see Enstone et al., 2003). At the same time, the possible function of the exodermis as a water barrier during chilling has to be explored, since the exodermis can also inhibit the Lo (Zimmermann and Steudle, 1998). The development of the endodermis and exodermis are known to increase when plants are stressed (Enstone et al., 2003). Recent evidence obtained by Lee et al. (2004b) showed that after 1 d at 8°C the lignification status of the Casparian band of cucumber endodermal cells did not change as judged by the intensity of staining with KMnO4. However, the composition of the suberin and lignin domains of the Casparian band can influence Lo (Zimmermann et al., 2000). Obviously, more information is needed about the effect of chilling on endodermis and exodermis development.
For Pos measurements we used healthy protoplasts as judged for their cytoplasmic streaming; however, for Lo measurements a mixture of healthy and damaged cells may have contributed to the overall water transport measurements. This could be another reason for the lack of correlation between the decrease in Lo and the lack of change in Pos values after chilling or H2O2 treatment in Penjalinan plants. In fact Lee et al. (2002) found that in cucumber root tips exposed to 8°C from 15 min to 96 h contained damaged cortical cells as well as healthy looking cells.
Aquaporin Response to Chilling and H2O2
Since aquaporin gene expression is known to respond to stress (Tyerman et al., 2002), we asked whether aquaporin abundance might explain the changes in Lo. The mRNA levels of the 12 PIP genes decreased drastically after 3 d of chilling, but there were no differences between the two genotypes. Li et al. (2000) also found a decrease of the mRNA expression of a PIP1 aquaporin after 24 h of exposure at 4°C in rice roots. Similarly, Nogueira et al. (2003) found a decrease of mRNA expression of a putative PIP2 aquaporin in leaves of sugarcane exposed to 4°C during 48 h. Thus, the decrease of aquaporin mRNA expression in plant tissues may be a common effect of chilling.
Both genotypes responded to chilling and H2O2 by up-regulating root aquaporin levels and/or activity, as PIP1 protein content rose after chilling and PIP2 proteins increased their phosphorylation state after chilling and H2O2 treatment. Such results support the Lo response of Z7, which also increased after chilling. At the same time, these results confirm the previous studies where the aquaporin blocker HgCl2 caused more inhibition of Lo in Z7 chilled plants than in the control (Aroca et al., 2001b). Although the state of phosphorylation of PIP2 proteins increased after H2O2 exposure, the Lo of Z7 did not increase in contrast to what happened after the chilling treatment. This could be because other PIP2 proteins were phosphorylated and the response of Lo was different. Obviously, this idea would need to be tested. Although PIP1 proteins are often not active when tested in Xenopus oocytes and may not be active water channels, (Kammerloher et al., 1994; Biela et al., 1999; Chaumont et al., 2000), Fetter et al. (2004) found that PIP1 protein can be activated by small amounts of PIP2 in Xenopus oocytes expressing PIP1 and PIP2 proteins together. Thus, the activity of PIP1 proteins may depend on other factors to activate them. We cannot exclude that differences in Lo and Pos between Z7 and Penjalinan were partly due to the activity of specific isoforms in some root tissues. For instance, higher levels of ZmPIP1;6 and 2;6 RNA were detected after 3 d of chilling in Z7 in comparison to Penjalinan. Even if the overall level of PIP1 and PIP2 proteins was similar in both genotypes, the proportions of each expressed isoform may vary and modify Lo and Pos.
Recently, Ye et al. (2004) proposed that aquaporins of Chara corallina that are exposed to high osmotic pressures can undergo a mechanical deformation causing the closure of the pore. It is not likely that this type of regulation of aquaporin activity could explain the behavior of Lo and Pos of Penjalinan plants after chilling or H2O2 treatments, because the increase of the osmotic gradient potential between the nutrient solution and the exuded sap after chilling or H2O2 treatments in Penjalinan was only about 0.05 and 0.01 MPa, respectively (data not shown), far less than the 2 MPa used by Ye et al. (2004). At the same time, the inhibition of hydraulic conductance observed by Ye et al. (2004) in Chara by the higher osmotic pressure was about 50%. Here, we observed an inhibition in Penjalinan Lo of 97% and 75% caused by chilling and H2O2 treatments, respectively.
Aquaporin activity can be up-regulated by phosphorylation (Johansson et al., 1998) and phosphorylation in planta could be a measure of aquaporin activity, although such a correlation has not yet been established. The phosphorylation state of aquaporins can be ascertained with a specific antiserum. Recently, Guenther et al. (2003) developed a phosphospecific antibody against soybean nodulin 26 protein. We chose as epitope for the development of an antiserum the most conserved sequence among all PIP2 proteins with a possible phosphorylated Ser (Johansson et al., 1998). Interestingly, until now, this site has not been shown to be phosphorylated even if some indirect evidence obtained in Xenopus oocytes suggested the importance of this Ser residue in the regulation of the water channel activity of PIP2 proteins (Maurel et al., 1995; Johansson et al., 1998). Using this serum, we clearly demonstrated that this Ser residue was indeed phosphorylated in planta. The serum was used to ascertain how chilling and H2O2 influence the phosphorylation state of PIP2 proteins. Both chilling and H2O2 treatments increased the PIP2 phosphorylation state in both genotypes, suggesting that the activation of protein kinase(s) and/or inactivation of phosphatases may be a chilling and oxidative stress response (Neill et al., 2002; Viswanathan and Zhu, 2002). Although both genotypes responded similarly, Lo recovered only in the tolerant genotype, suggesting that this response is not sufficient for Lo recovery.
H2O2 and Membrane Injury
It is known that chilling can cause damage to root membranes, resulting in a decrease in their fluidity (Alonso et al., 1997). At the same time, exposure of plant tissues to H2O2 causes membrane damage as measured by EL (Pastori and Trippi, 1992; Marrè et al., 1998). Chilling and H2O2 treatments caused root membrane damage in Penjalinan plants, as indicated by the increase in EL. Roots of Z7 did not show an increase of EL and even decreased it after chilling. These results indicate that the root membranes of Penjalinan are more sensitive to chilling or H2O2 than those of Z7. Capell and Dörffling (1993) also found that after 3 d at 5°C Penjalinan leaves increased their EL, while those of Z7 almost did not change. The “leakiness” of the Penjalinan roots after chilling or H2O2 was not the cause of the change in Pos values. Indeed, for all Pos experiments we used healthy protoplasts as indicated by their cytoplasmic streaming.
The leaves of Z7 are more tolerant than those of Penjalinan to the oxidative stress that results from chilling (Aroca et al., 2001a, 2003a). Z7 plants coordinately enhance the activities of ascorbate peroxidase, glutathione reductase, and superoxide dismutase, reducing leaf necrosis caused by chilling, while in Penjalinan plants those enzyme activities do not increase and the plants develop leaf necrosis (Aroca et al., 2001a). Moreover, Penjalinan plants showed an increase of lipid peroxidation and a depletion of ascorbate contents after chilling (Aroca et al., 2003a). At the root level, Pinhero et al. (1997) found that maize chilling-tolerant genotypes had more antioxidant enzyme activities and different isozymes as compared to chilling-sensitive plants. Therefore, it is likely that Penjalinan roots have less antioxidant capacity than Z7 roots and are therefore more sensitive to H2O2 exposure than Z7. Pastori and Trippi (1992) also found that a drought-tolerant maize genotype developed less EL than a sensitive one after the exposure of the leaves to H2O2. Therefore, there could be a relationship between abiotic stress tolerance and oxidative stress tolerance, since oxidative stress may be common for all the stresses (for review, see Wang et al., 2003). Thus, Penjalinan membrane injury caused by chilling could be generated by an increase in the production of reactive oxygen species and/or by a decrease in the capacity to remove them, as has been found for leaves (Aroca et al., 2001a, 2003a). In fact, Penjalinan roots had twice the amount of H2O2 compared to control plants, indicating their inability to remove it.
It is known that H2O2 is essential for the formation of suberin lamallae (Razem and Bernards, 2002), and that suberin forms a hydrophobic barrier in both endodermis and exodermis (Enstone et al., 2003). Therefore, it is possible that Penjalinan roots developed more suberin lamellae during chilling, preventing the recovery of Lo. This explanation cannot be ruled out at the moment but would have to be substantiated by direct observation.
CONCLUSION
It appears that chilling stress is accompanied by an aquaporin response: the proteins increase in amount and in phosphorylation state. This response underlies the change in Lo and Pos of the protoplasts of the tolerant genotype during recovery from chilling stress. In the sensitive genotype this response is not sufficient to allow the Lo to recover from the initial decline. An antioxidant system that repairs or prevents membrane damage is also needed. We postulate that this is the weak link in the sensitive genotype. Thus, aquaporins would be necessary, but not sufficient to respond to chilling stress. This final conclusion has to be tested in future researches by manipulating the levels of aquaporins and/or of the reactive oxygen species.
MATERIALS AND METHODS
Plant Material and Experimental Design
Seeds of two maize (Zea mays) genotypes differing in chilling sensitivity (Z7, tolerant and Penjalinan, sensitive) were disinfected with a 0.02% HgCl2 solution, rinsed three times with distilled water, and then covered with wet filter paper and placed in petri dishes at 28°C for 4 d. After that period, the seedlings were grown hydroponically as described previously by Aroca et al. (2001b): 25°C, 16:8-h (light:dark) photoperiod with a photosynthetic photon flux density of 200 μmol m−2 s−1 and 60% relative humidity. Eleven-day-old plants were transferred to a cold room (5°C) during 3 d at constant light (50 μmol m−2 s−1 of photosynthetic photon flux density) and 80% relative humidity. The low light intensity was chosen to avoid photooxidative damage during chilling (Aroca et al., 2003a). Since during our chilling conditions (5°C) net growth of maize essentially stopped (Pérez de Juan et al., 1997) all the measurements were taken at the beginning of the chilling treatment (control plants) and after 3 d of chilling (chilled plants), except for the mRNA expression where samples after 1 d of chilling were taken as well, in order to have plants of the same size and stage of development. All the measurements and the collection of the samples were made 2 h after the lights had been turned on in control plants and at the same local time (9 am) for chilled plants.
For the peroxide experiments, 11-d-old plants grown hydroponically as described above for the chilling experiment were exposed to 100 μm of H2O2 in the nutrient solution during 1 h. The measurements were then made on treated or untreated plants 4 h after the lights had been turned on (11 am local time). The difference in time of measuring between chilling and H2O2 experiments could change the values of Lo, Pos, and aquaporins abundance since they change during the day (Henzler et al., 1999).
Root Hydraulic Conductance
Root hydraulic conductance (Lo) was measured as described previously by Aroca et al. (2001b, 2003b) in plants exuding at atmospheric pressure.
Pos of Root Protoplasts
Pos of isolated protoplasts from roots was determined using a transfer chamber as described by Ramahaleo et al. (1999). Root protoplasts were isolated as described by Ramahaleo et al. (1999), but 2 mm MgCl2 was added to the digestion solution. The root pieces were incubated in the digestion solution during 40 min. The solutions used during measurements contained 1 mm CaCl2, 2 mm MgCl2, 0.2% (w/v) bovine serum albumin (initial fractionation by cold alcohol precipitation, Fraction V, Sigma, St. Louis), and 10 mm Tris/MES, pH 5.5, with a sorbitol concentration of 0.4 or 0.2 m (iso- or hypoosmotic solutions, respectively). The osmolarity of the solutions was measured using a cryoscopic osmometer (Osmomat 030, Gonotec GmbH, Berlin).
RNA Extraction and Northern Blots
Roots were ground up in Trizol reagent (Roche Molecular Biochemicals) and total RNA extracted according to the manufacturer's recommendation. Total RNA samples (15 μg each) were fractionated by electrophoresis on a MOPS-formaldehyde-formamide 1.5% agarose gel, and were transferred on Nytran SuperCharge nylon membranes (Schleicher and Schuell, Dassel, Germany) using standard blotting techniques. The RNA was bound on the membrane and hybridized as described previously (Chaumont et al., 1998) except that yeast t-RNA was replaced by 200 mg mL−1 denatured herring sperm DNA. Specific PIP DNA fragments (142 nt to 362 nt) were amplified by PCR with the primers shown in Table II.
Table II.
Sequences of the PCR primers used to amplify the specific PIP fragments used as probes in the northern blots
| Name | Accession No. | Forward Primer | Reverse Primer | Fragment Position |
|---|---|---|---|---|
| ZmPIP1;1 | X82633 | PIP1;1-1 | PIP1;1-2 | 922–1,159 |
| 5′-TAAAGGAGCCGATGCTGCTG-3′ | 5′-GGATGAACTCTTAAAGCTTGAC-3′ | |||
| ZmPIP1;2 | AF131201 | PIP1;2-1 | PIP1;2-2 | 972–1,273 |
| 5′-GCGTCTTCCTGTGATGTCTTCT-3′ | 5′-AAATCAAGAAAACCCTGAATCG-3′ | |||
| ZmPIP1;3 | AF326487 | PIP1;3-1 | PIP1;3-2 | 1,023–1,283 |
| 5′-GTCTTCCTGTGGGGAGTGTCTT-3′ | 5′-GCTGATAGATAAACCCACGTTTCC-3′ | |||
| ZmPIP1;4 | AF326488 | PIP1;4-1 | PIP1;4-2 | 846–1,140 |
| 5′-CCATCATCTACAACCGGGATCA-3′ | 5′-ACACACAGCTCGGTACAGGAGT-3′ | |||
| ZmPIP1;5 | AF326489 | PIP1;5-1 | PIP1;5-2 | 957–1,243 |
| 5′-ATTACCAACAGCAACCATGCAG-3′ | 5′-CTTCACCGTACCAAAACCCAAG-3′ | |||
| ZmPIP1;6 | AF326490 | PIP1;6-1 | PIP1;6-1 | 1,103–1,280 |
| 5′-CTTGCCGTCATACACGGAGTTC-3′ | 5′-ATTGGGACACGTACAGCCACAT-3′ | |||
| ZmPIP2;1 | AF326491 | PIP2;1-1 | PIP2;1-2 | 872–1141 |
| 5′-CGGCCTTCTACCACCAGTACAT-3′ | 5′-CATGATTACATTGCAGGGGAAC-3′ | |||
| ZmPIP2;2 | AF326492 | PIP2;2-1 | PIP2;2-2 | 851–1,234 |
| 5′-CGTCTACAACAAGGACAAGCCA-3′ | 5′-CACACATGGTGATTGATGATTAC-3′ | |||
| ZmPIP2;3 | AF326493 | PIP2;3-1 | PIP2;3-2 | 926–1,101 |
| 5′-CTACCGGAGCAACGCCTAATAA-3′ | 5′-CCCGTCAAGGAAAGAAAGAAAA-3′ | |||
| ZmPIP2;4 | AF326494 | PIP2;4-3 | PIP2;4-4 | 936–1,168 |
| 5′-CTACCGGAGCAACGCCTAA-3′ | 5′-ATCGGATAAAAACTCACGCAAT-3′ | |||
| ZmPIP2;5 | AF130975 | PIP2;5-1 | PIP2;5-1 | 759–1,076 |
| 5′-GCGCTGCTGTCATCTACAACAA-3′ | 5′-GCAAGCAAAATGCAGTGGAAAT-3′ | |||
| ZmPIP2;6 | AF326495 | PIP2;6-1 | PIP2;6-2 | 887–1,182 |
| 5′-CGCTATACCACCAGATCGTCCT-3′ | 5′-AGTCACACTTGCTTTCCACACC-3′ | |||
| ZmPIP2;7 | AF326496 | PIP2;7-3 | PIP2;7-4 | 1–141 |
| 5′-GCACGAGCACACACATAGTAGC-3′ | 5′-CGGAGGAGGGTCATGGTAGTC-3′ |
The random-primer labeled probes were generated using a Rediprime II kit following the instructions of the manufacturer (Amersham Pharmacia Biotech, Piscataway, NJ). The unincorporated radioactive nucleotides were removed with a Micro-Spin chromatography column (Bio-Rad Laboratories, Hercules, CA). Hybridized membranes were washed under high stringency conditions (0.2× SSPE [sodium chloride monosodium phosphate EDTA], 0.1% SDS at 65°C for 20 min). The membranes were reprobed after boiling in 0.5% SDS and 0.1× SSPE and cooling to room temperature. Band intensities were determined by phosphorimaging using a Molecular Imager System (Bio-Rad Laboratories, Hercules, CA), corrected for background, and normalized to 18S rRNA from the same samples. Comparison of the expression level of each isoform in both genotypes was expressed as the ratio of the normalized signals (Penjalinan/Z7) at time 0. The RNA expression variation during cold stress was expressed as the ratio of the signal values obtained at time 0 and time 1 d or 3 d for both genotypes.
Antibodies to PIP1 and Antibodies to Phosphorylated PIP2
A peptide corresponding to the carboxy terminus of a PIP1 aquaporin (accession no. AF366564) and another one containing the phosphorylation site at the Ser-113 of the PIP2 (accession no. AF366565) from wheat (Triticum aestivum) were synthesized and the corresponding antibodies purchased from BioSource International (Hopkinton, MA). The sequences of these peptides are shown in Figure 4. The antibody used for the PIP2 aquaporin detection was made to the carboxy terminus of the AtPIP2;3 aquaporin from Arabidopsis (Arabidopsis thaliana; Daniels et al., 1994).
Preparation of Microsomes and Immunodetection
The microsomes from roots tissues were obtained as previously described by Daniels et al. (1994). For each gel, 15 μg of protein were loaded per lane in a 15% SDS-PAGE. The proteins were previously denatured by incubating them at 37°C during 30 min in presence of 1% (w/v) SDS and 100 mm ethanedithiol to separate the dimers of the aquaporins (Borgnia et al., 1999). The proteins were transferred to a nitrocellulose membrane during 1 h at 100 V. The membrane was blocked during 2 h at room temperature in 5% (w/v) nonfat milk in Tris-buffered-saline (TBS) with 0.05% Tween 20. After that, the membrane was incubated in TBS in presence of the corresponding antibody dilution (1:1,500 for PIP1 antibody or 1:500 for PIP2 and antibodies to phosphorylated PIP2) during 1 h at room temperature for PIP1 or overnight at 4°C for PIP2 and antibodies to phosphorylated PIP2. Goat anti-rabbit IgG coupled to horseradish peroxidase (Bio-Rad) was used as the secondary antibody (1:20,000 or 1:10,000 dilution for PIP1 or PIP2 and antibodies to phosphorylated PIP2, respectively). The signal was developed using a chemiluminescent substrate (West-Pico, Super Signal; Pierce, Rockford, IL).
To check the specificity of the antibodies to phosphorylated PIP2, one nitrocellulose membrane strip with phosphorylated protein sample was incubated during 24 h at 30°C with 2,000 units mL−1 of Lambda Protein Phosphatase (New England BioLabs, Beverly, MA), while another strip was incubated only with the phosphatase buffer before blotting with the antibody.
All the inmunoblots were repeated three times with samples from different sets of plants with the same results. The equal loading of the proteins in the different lines was confirmed by staining the gel with Coomassie Brilliant Blue. To quantify the inmunoblot signal, the intensity of each band was measured using Adobe PhotoShop 5.5 (Adobe Systems, Mountain View, CA), corrected for the background and normalized against the intensity of the corresponding Coomassie Brilliant Blue band.
Electrolyte Leakage
The whole root from 11 plants/treatment was placed in a tube containing 20 mL of distilled water and soaked during 3 h at room temperature; after that the conductivity of the solution was measured (Conductivity Meter, Wescan Instruments) and is referred to as C0. Then the tubes were placed at −80°C during 1 h and soaked again during 2 h at room temperature. The conductivity of the solution at this time is referred to as CT. The conductivity of distilled water before the root immersion was also measured and referred to as CW. The percentage of EL was calculated as follows: EL = [(C0 − CW)/(CT −CW)] × 100.
H2O2 in Root Tissues
The H2O2 of root tissues was measured as described by Aroca et al. (2003a), except that the potassium titanium oxalate was replaced by titanium chloride (TiCl4; Patterson et al., 1984).
Additional Note
While this paper was under review, Lee et al. (2004a) described the effect of low temperature exposure of cucumber roots on hydrogen peroxide accumulation. A brief H2O2 treatment of the roots causes a dramatic drop in the hydraulic conductivity of cortical cells and is the likely cause of the cold induced drop in hydraulic conductivity.
Sequence data from this article have been deposited with the EMBL/GenBank data libraries under accession numbers AF366564 and AF366565.
Acknowledgments
We thank Dr. Raphaël Morillon for his advice and help with the osmotic water permeability measurements and Prof. Peter Stamp (Eidgenossige Technische Hochschule, Zurich) for the gift of seeds.
This work was supported by the Ministerio de Educación y Ciencia (postdoctoral fellowship to R.A.) and by the Belgian Fund for Scientific Research and the Interuniversity Attraction Poles Programme-Belgian Science Policy (grants to F.C.).
This is the 70th research paper from the Chrispeels laboratory to be published in Plant Physiology.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.051045.
References
- Alonso A, Queiroz CS, Magalhães AC (1997) Chilling stress leads to increased cell membrane rigidity in roots of coffee (Coffea arabica L.) seedlings. Biochim Biophys Acta 1323: 75–84 [DOI] [PubMed] [Google Scholar]
- Aroca R, Irigoyen JJ, Sánchez-Díaz M (2001. a) Photosynthetic characteristics and protective mechanisms against oxidative stress during chilling and subsequent recovery in two maize varieties differing in chilling sensitivity. Plant Sci 161: 719–726 [Google Scholar]
- Aroca R, Irigoyen JJ, Sánchez-Díaz M (2003. a) Drought enhances maize chilling tolerance. II. Photosynthetic traits and protective mechanisms against oxidative stress. Physiol Plant 117: 540–549 [DOI] [PubMed] [Google Scholar]
- Aroca R, Tognoni F, Irigoyen JJ, Sánchez-Díaz M, Pardossi A (2001. b) Different root low temperature response of two maize genotypes differing in chilling sensitivity. Plant Physiol Biochem 39: 1067–1073 [Google Scholar]
- Aroca R, Vernieri P, Irigoyen JJ, Sánchez-Díaz M, Tognoni F, Pardossi A (2003. b) Involvement of abscisic acid in leaf and root of maize (Zea mays L.) in avoiding chilling-induced water stress. Plant Sci 165: 671–679 [Google Scholar]
- Biela A, Grote K, Otto B, Hoth S, Hedrich R, Kaldenhoff R (1999) The Nicotania tabacum plasma membrane aquaporin NtAQP1 is mercury-insensitive and permeable for glycerol. Plant J 18: 565–570 [DOI] [PubMed] [Google Scholar]
- Bigot J, Boucaud J (2000) Effects of Ca-signaling inhibitors on short-term cold-acclimation of hydraulic conductivity in roots of Brassica rapa plants. J Plant Physiol 157: 7–12 [Google Scholar]
- Borgnia MJ, Kozono D, Calamita G, Maloney PC, Agre P (1999) Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J Mol Biol 291: 1169–1179 [DOI] [PubMed] [Google Scholar]
- Campos PS, Quartin V, Ramalho JC, Nunes MA (2003) Electrolyte leakage and lipid degradation account for cold sensitivity in leaves of Coffea sp. plants. J Plant Physiol 160: 283–292 [DOI] [PubMed] [Google Scholar]
- Capell B, Dörffling K (1993) Genotype-specific differences in chilling tolerance of maize in relation to chilling-induced changes in water status and abscisic acid accumulation. Physiol Plant 88: 638–646 [DOI] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Herman EM, Chrispeels MJ (1998) Characterization of a maize tonoplast aquaporin expressed in zones of cell division and elongation. Plant Physiol 117: 1143–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Jung R, Chrispeels MJ (2000) Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. Plant Physiol 122: 1025–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R (2001) Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol 125: 1206–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels MJ, Agre P (1994) Aquaporins: water channel proteins of plants and animals cells. Trends Biochem Sci 19: 421–425 [DOI] [PubMed] [Google Scholar]
- Daniels MJ, Mirkov TE, Chrispeels MJ (1994) The plasma membrane of Arabidopsis thaliana contains a mercury-insensitive aquaporin that is a homolog of the tonoplast water channel protein TIP. Plant Physiol 106: 1325–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enstone DE, Peterson CA, Ma F (2003) Root endodermis and exodermis: structure, function, and responses to the environment. J Plant Growth Regul 21: 335–351 [Google Scholar]
- Faye L, Chrispeels MJ (1985) Characterization of N-linked oligosaccharides by affinobloting with concanavalin A-peroxidase and treatment of the blots with glycosidases. Anal Biochem 149: 218–224 [DOI] [PubMed] [Google Scholar]
- Fennell A, Markhart AH III (1998) Rapid acclimation of root hydraulic conductivity to low temperature. J Exp Bot 49: 879–884 [Google Scholar]
- Fetter K, Van Wilder V, Moshelion M, Chaumont F (2004) Interactions between plasma membrane aquaporins modulate their water channel activity. Plant Cell 16: 215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbeau P, Amodeo G, Henzler T, Santoni V, Ripoche P, Maurel C (2002) The water permeability of Arabidopsis plasma membrane is regulated by divalent cations and pH. Plant J 30: 71–81 [DOI] [PubMed] [Google Scholar]
- Guenther JF, Chanmanivone N, Galetovic MP, Wallace IS, Cobb JA, Roberts DM (2003) Phosphorylation of soybean nodulin 26 on serine 262 enhances water permeability and is regulated developmentally and by osmotic signals. Plant Cell 15: 981–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsé B, Poschenrieder C, Barceló J (1997) Water transport properties of roots and root cortical cells in proton- and Al-stressed maize varieties. Plant Physiol 113: 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, Terashima I, Katsuhara M (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol 45: 521–529 [DOI] [PubMed] [Google Scholar]
- Henzler T, Waterhouse RN, Smith AJ, Carvajal M, Cooke DT, Schäffner AR, Steudle E, Clarkson DT (1999) Diurnal variations in hydraulic conductivity and root pressure can be correlated with the expression of putative aquaporins in the roots of Lotus japonicus. Planta 210: 50–60 [DOI] [PubMed] [Google Scholar]
- Javot H, Lauvergeat V, Santoni V, Martin-Laurent F, Güçlü J, Vinh J, Heyes J, Frank KI, Schäffner AR, Bouchez D, et al (2003) Role of a single aquaporin isoform in root water uptake. Plant Cell 15: 509–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javot H, Maurel C (2002) The role of aquaporins in root water uptake. Ann Bot (Lond) 90: 301–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P (2001) The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol 126: 1358–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson I, Karlsson M, Shukla VK, Chrispeels MJ, Larsson C, Kjellbom P (1998) Water transport activity of the plasma membrane aquaporin PM28A is regulated by phosphorylation. Plant Cell 10: 451–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammerloher W, Fischer U, Piechottka GP, Schäffner AR (1994) Water channels in the plant plasma membrane cloned by immunoselection from a mammalian expression system. Plant J 6: 187–199 [DOI] [PubMed] [Google Scholar]
- Kang G, Wang C, Sun G, Wang Z (2003) Salicylic acid changes activities of H2O2-metabolizing enzymes and increase the chilling tolerance of banana seedlings. Environ Exp Bot 50: 9–15 [Google Scholar]
- Kerdnaimongkol K, Woodson WR (1999) Inhibition of catalase by antisense RNA increase susceptibility to oxidative stress and chilling injury in transgenic tomato plants. J Am Soc Hortic Sci 124: 330–336 [Google Scholar]
- Ktitorova IN, Skobeleva OV, Sharova EI, Ermakov EI (2002) Hydrogen peroxide appears to mediate a decrease in hydraulic conductivity in wheat roots under salt stress. Russ J Plant Physiol 49: 369–380 [Google Scholar]
- Lee SH, Singh AP, Chung GC (2004. a) Rapid accumulation of hydrogen peroxide in cucumber roots due to exposure to low temperature appears to mediate decreases in water transport. J Exp Bot 55: 1733–1741 [DOI] [PubMed] [Google Scholar]
- Lee SH, Singh AP, Chung GC, Ahn SJ, Noh EK, Steudle E (2004. b) Exposure of roots of cucumber (Cucumis sativus) to low temperature severely reduces root pressure, hydraulic conductivity and active transport of nutrients. Physiol Plant 120: 413–420 [DOI] [PubMed] [Google Scholar]
- Lee SH, Singh AP, Chung GC, Kim YS, Kong IB (2002) Chilling root temperature causes rapid ultrastructural changes in cortical cells of cucumber (Cucumis sativus L.) root tips. J Exp Bot 53: 2225–2237 [DOI] [PubMed] [Google Scholar]
- Li L-G, Li S-F, Tao Y, Kitagawa Y (2000) Molecular cloning of a novel water channel from rice: its products expression in Xenopus oocytes and involvement in chilling tolerance. Plant Sci 154: 43–51 [DOI] [PubMed] [Google Scholar]
- Maggio A, Joly RJ (1995) Effects of mercuric chloride on the hydraulic conductivity of tomato root systems: evidence for a channel-mediated pathway. Plant Physiol 109: 331–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrè MT, Amicucci E, Zingarelli L, Albergoni F, Marrè E (1998) The respiratory burst and electrolyte leakage induced by sulfhydril blockers in Egeria densa leaves are associated with H2O2 production and are dependent on Ca2+ influx. Plant Physiol 118: 1379–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurel C, Kado RT, Guern J, Chrispeels MJ (1995) Phosphorylation regulates the water channel activity of the seed-specific aquaporin α-TIP. EMBO J 14: 3028–3035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedema P (1982) The effects of low temperature on Zea mays. Adv Agron 35: 93–128 [Google Scholar]
- Morillon R, Chrispeels MJ (2001) The role of ABA and the transpiration stream in the regulation of the osmotic water permeability of leaf cells. Proc Natl Acad Sci USA 98: 14138–14143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signaling. Curr Opin Plant Biol 5: 388–395 [DOI] [PubMed] [Google Scholar]
- Nogueira FTS, De Rosa VE Jr, Menossi M, Ulian EC, Arruda P (2003) RNA expression profiles and data mining of sugarcane response to low temperature. Plant Physiol 132: 1811–1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastori GM, Trippi VS (1992) Oxidative stress induces high-rate of glutathione-reductase synthesis in a drought-resistant maize strain. Plant Cell Physiol 33: 957–961 [Google Scholar]
- Patterson BD, MacRae EA, Ferguson IB (1984) Estimation of hydrogen peroxide in plants extracts using titanium (IV). Anal Biochem 139: 487–492 [DOI] [PubMed] [Google Scholar]
- Pérez de Juan J, Irigoyen JJ, Sánchez-Díaz M (1997) Chilling of drought-hardened and non-hardened plants of different chilling-sensitive maize lines. Changes in water relations and ABA contents. Plant Sci 122: 71–79 [Google Scholar]
- Pinhero RG, Rao MV, Paliyath G, Murr DP, Fletcher RA (1997) Changes in activities of antioxidant enzymes and their relationship to genetic and paclobutrazol-induced chilling tolerance of maize seedlings. Plant Physiol 114: 695–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramahaleo T, Morillon R, Alexandre J, Lassalles J-P (1999) Osmotic water permeability of isolated protoplasts. Modifications during development. Plant Physiol 119: 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razem FA, Bernards MA (2002) Hydrogen peroxide is required for poly(phenolic) domain formation during wound-induced suberization. J Agric Food Chem 50: 1009–1015 [DOI] [PubMed] [Google Scholar]
- Roberts SK, Tester M (1995) Inward and outward K+-selective currents in the plasma membrane of protoplasts from maize root cortex and stele. Plant J 8: 811–825 [Google Scholar]
- Sanders PL, Markhart AH (2001) Root system functions during chilling temperatures: injury and acclimation. In S Basra, ed, Crop Responses and Adaptations to Temperature Stress. Food Products Press, New York, pp 77–108
- Siefritz F, Tyree MT, Lovisolo C, Schubert A, Kaldenhoff R (2002) PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. Plant Cell 14: 869–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steudle E (2000) water uptake by plant roots: an integration of views. Plant Soil 226: 45–56 [Google Scholar]
- Thomashow MF (2001) So what's new in the field of plant cold acclimation? Lots! Plant Physiol 125: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournaire-Roux C, Sutka M, Javot H, Gout E, Gerbeau P, Luu D-T, Bligny R, Maurel C (2003) Cytosolic pH regulates root water transport during anoxic stress through gating of aquaporins. Nature 425: 393–397 [DOI] [PubMed] [Google Scholar]
- Tyerman SD, Niemietz CM, Brameley H (2002) Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 25: 173–194 [DOI] [PubMed] [Google Scholar]
- Vines JRL, Jenkins PD, Foyer CH, French MS, Scott IM (2003) Physiological effects of peracetic acid on hydroponic tomato plants. Ann Appl Biol 143: 153–159 [Google Scholar]
- Viswanathan C, Zhu J-K (2002) Molecular genetic analysis of cold-regulated gene transcription. Philos Trans R Soc Lond B Biol Sci 357: 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Vinocur B, Altman A (2003) Plant response to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. Planta 218: 1–14 [DOI] [PubMed] [Google Scholar]
- Ye Q, Wiera B, Steudle E (2004) A cohesion/tension mechanism explains the gating of water channels (aquaporins) in Chara internodes by high concentration. J Exp Bot 55: 449–461 [DOI] [PubMed] [Google Scholar]
- Zhu GL, Steudle E (1991) Water transport across maize roots. Plant Physiol 95: 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann HM, Hartmann K, Schreiber L, Steudle E (2000) Chemical composition of apoplastic transport barries in relation to radial hydraulic conductivity of corn roots (Zea mays L.). Planta 210: 302–311 [DOI] [PubMed] [Google Scholar]
- Zimmermann HM, Steudle E (1998) Apoplastic transport across young maize roots: effect of the exodermis. Planta 206: 7–19 [Google Scholar]