Summary
Background
Identifying interventions to increase men’s uptake of HIV testing in sub-Saharan Africa is essential for the success of combination HIV prevention. HIV self-testing is an emerging approach with high acceptability, but limited evidence exists on optimal strategies for distributing self-tests. We explored a novel approach of providing multiple self-tests to women at high risk of HIV acquisition in order to promote partner HIV testing and facilitate safer sexual decision-making.
Methods
HIV-uninfected women aged 18–39 years were recruited at two sites in Kisumu, Kenya: a health facility with antenatal (ANC) and postpartum (PPC) clinics, and a drop-in center for female sex workers (FSW). Following informed consent and instructions on using the OraQuick Rapid HIV 1/2 Test, index participants (IPs) enrolled at the health facility and drop-in center received 3 and 5 self-tests, respectively. Structured interviews were conducted with IPs at enrollment and over 3 months to determine how self-tests were used. Key outcomes included the number of self-tests distributed by IPs, the proportion of IPs whose sexual partners used a self-test, couples testing, and sexual behavior following self-testing.
Findings
Between January 14, 2015 and March 13, 2015, 280 IPs were enrolled (61 ANC, 117 PPC, 102 FSW). Follow-up interviews were completed with 265 IPs (96%). Most IPs with a primary sexual partner distributed a self-test to that partner (53/58, 91% ANC; 91/106, 86% PPC; 64/85, 75% FSW). A vast majority of FSW IPs also distributed ≥1 self-tests to commercial sex clients (82/101, 81%). Among self-tests distributed to and used by IPs’ primary sexual partners, couples testing occurred frequently (27/53, 51% ANC; 62/91, 68% PPC; and 53/64; 83% FSW). Among self-tests distributed to and used by IPs’ sexual partners, an HIV-positive result was obtained for 3.8% (2/53), 2.2% (2/91), and 13.8% (41/298) of the tests in the ANC, PPC, and FSW participant groups, respectively. Sexual intercourse was significantly less likely after a sexual partner tested HIV-positive versus HIV-negative (18% vs. 62%, p<0.0001), while condom use was significantly more likely (100% vs. 44%, p=0.0018). Of 265 IPs with follow-up data, four reported intimate partner violence as a result of self-test distribution. No other adverse events were reported.
Interpretation
Provision of multiple HIV self-tests to women at high risk of HIV infection was successful in promoting HIV testing among their sexual partners and in facilitating safer sexual decisions. This novel strategy warrants further consideration as countries develop self-testing policies and programs.
Introduction
About half the HIV-infected individuals in sub-Saharan Africa (SSA) are unaware of their serostatus.1 Increasing the uptake of HIV testing among these individuals is essential for ensuring the success of HIV treatment as prevention and meeting the UNAIDS “90-90-90” targets. Although wider availability of facility-based HIV testing and counseling (HTC) services and community-based HTC strategies have helped increase testing coverage,2,3 achieving high levels of testing coverage has proven to be challenging in many countries. Men in particular are far less likely to test than women.1 There is also a dearth of HTC interventions targeted to key populations such as female sex workers (FSW), who can benefit from regular repeat testing.3 Even less common has been the occurrence of couples testing, which can have larger health impacts through improved sexual decision-making and higher antiretroviral therapy (ART) use to prevent HIV transmission.4,5
HIV self-testing (HIVST) is a promising approach that has the potential to substantially increase access to testing for individuals and couples in a manner that is confidential and empowering for users.6,7 Rapid testing technologies include simple-to-use oral HIV tests that offer high sensitivity and specificity, ideal for HIVST strategies. Prior studies have documented high interest in and acceptability of HIVST among a wide range of populations, including those with lower likelihood of testing in other HTC strategies or higher risks of being HIV-infected.6,8,9 Some countries, such as Kenya, have already included regulated HIVST in their national HTC guidelines and several are considering scaling-up the availability of self-tests.10,11
Although HIVST can be a good alternative for those not engaged in regular repeat testing, little is known about optimal distribution strategies for facilitating self-test use by hard-to-reach and high risk individuals. One such strategy is ‘secondary distribution’ of HIV self-tests, whereby an individual who is given multiple self-tests can distribute them to sexual partners or to others in their social network. In addition to promoting HIV testing, secondary distribution of self-tests also has potential to facilitate point-of-sex testing, which can lead to results disclosure and safer, more informed sexual decisions. To date there are no data on the feasibility of secondary distribution of self-tests in SSA, although a few qualitative studies conducted among men who have sex with men (MSM) in the United States have shown that this strategy may help increase individuals’ knowledge of their partner’s status and promote safer sexual decisions.12
We explored whether secondary distribution of self-tests can promote HIV testing among partners of pregnant women, postpartum women, and FSW in a high HIV prevalence setting in Kenya. We also assessed whether secondary distribution of self-tests led to couples testing and more informed, safer sexual decisions by women.
Methods
Study design and participants
The study was conducted at several sites in the Kenyan city of Kisumu, where adult HIV prevalence is 20.6% among women and 17.8% among men).13 The study explored secondary distribution of self-tests by several different populations of HIV-uninfected women. Women seeking antenatal care (ANC) or post-partum care (PPC) were recruited since there is a high risk of HIV transmission in these populations and male partner testing can help reduce this risk.14–16 In addition, female sex workers (FSW) were recruited since promoting HIV testing among their sexual partners (including commercial sex clients) can help to lower the HIV infection risk faced by FSW.17,18
Women receiving ANC and PPC were recruited from a government health facility where nurses gave each woman a referral coupon to bring to study staff if she was interested in participating. Women were screened for these eligibility criteria based on clinic records and self-reported information: 18–39 years of age, HIV-uninfected at most recent HIV test (based on clinic records), gestational age ≤5 months (ANC) or child age greater than 6 weeks and less than 6 months (PPC), having at least one sexual partner. Women who stated they believed violence would result from distributing a self-test to their sexual partners were excluded.
FSW were recruited from a drop-in center that provides HIV prevention, care and treatment services to key populations including FSW, MSM, and people who inject drugs. Using the center’s records of HIV-negative FSW (based on having tested at the drop-in center in the past 3 months), a random sample of FSW was selected to participate in the study. FSW peer educators at the center who had undergone human subjects training contacted and referred the sampled FSW to study staff. The FSW who were randomly selected had the option of declining to participate in the study. Those who met with study staff were screened for these eligibility criteria: age 18–39 years of age and HIV-uninfected.
The study received approval from the Office of Human Research Ethics at the University of North Carolina at Chapel Hill and the Ethical Review Committee at the Kenya Medical Research Institute. Eligible women who wished to participate provided written informed consent in their preferred language as well as their phone number and locator information.
Procedures
Following informed consent by women who met eligibility criteria, trained study staff instructed the women – index participants (IPs) – on how to use oral fluid based rapid HIV tests (OraQuick Rapid HIV-1/2 antibody tests, OraSure Technologies) and gave them multiple test kits. ANC/PPC IPs were given 3 test kits each and FSW IPs were given 5 test kits each. Study staff demonstrated how to use the tests in order to facilitate correct use by IPs and increase IPs’ capability to correctly explain this to other individuals. Specifically, study staff explained self-testing procedures, including opening the test kit, collecting an oral fluid sample, and waiting 20 minutes to read results. They also informed IPs about the window period during which antibodies to HIV cannot be detected and gave them one-page instruction sheets to accompany each test kit. In addition, study staff counseled IPs to use discretion when introducing self-tests to sexual partners by assessing likely reactions of their partner(s) as well as the risk of intimate partner violence (IPV). A 24-hour telephone hotline was established for IPs or other self-test users to call for receiving further advice on using the tests, reporting IPV, or receiving referral for HIV confirmatory testing, treatment, or IPV services. At enrollment, IPs also responded to a baseline questionnaire about demographic characteristics, prior HIV testing behavior, and sexual behavior.
Follow-up questionnaires were administered to IPs within 3 months after enrollment; these typically occurred at the health facility or drop-in center, but in some cases IPs were visited at home if that was more convenient to them. These questionnaires obtained information from IPs on how they used the self-tests as well as reactions and behaviors following secondary distribution of self-tests. All questionnaires were administered by study staff in face-to-face interviews and in a private space. Data were recorded using an open-source software package (Open Data Kit) installed on Android tablet devices. IPs were considered lost to follow-up if study staff were unable to contact them at 3 months after at least three attempts.
Outcomes
Key outcomes assessed included the number of self-tests distributed by IPs to other individuals, the proportion of IPs in each group who distributed a self-test to their primary sexual partner, and among FSW IPs, the proportion who distributed ≥1 self-test to their commercial sex clients. The proportion of IPs who reported offering self-tests to ≥1 individuals who declined to accept the tests was also calculated.
For each self-test distributed to and used by a male sexual partner, couples testing was defined as having occurred if the IP reported testing together with the partner. Additionally, we summarized the proportion of all self-tests distributed that were reported to have an HIV-negative, HIV-positive, or unknown result. In cases where an HIV-positive test result was reported, IPs were asked if the individual sought confirmatory testing at a clinic and whether they had sought HIV care at the time of the follow-up interview.
Sexual decision-making following self-test distribution to a sexual partner was examined by assessing whether the IP reported having sexual intercourse with the partner since distribution of the self-test, and whether a condom was used when sexual intercourse did occur. IPV was assessed using questions adapted from the Kenya Demographic and Health Survey19 that inquired whether IPs had experienced physical, emotional, or sexual violence from their primary sexual partner (for ANC/PPC IPs) and from any sexual partner (for FSW IPs). IPV assessments in follow-up interviews were categorized by whether or not IPV resulted from self-test distribution.
Statistical Analysis
For all outcomes, we performed descriptive analyses of the outcomes for IPs in the three participant groups who were not lost to follow-up. For analyses of couples testing and of reported HIV test result for those receiving self-tests from IPs, we focused on self-tests distributed by IPs to sexual partners. To understand how sexual behavior was influenced by distribution of self-tests to sexual partners, we conducted Fisher’s exact tests comparing IPs’ reports of sexual intercourse and condom use (conditional upon intercourse) with sexual partners by HIV self-test result. All analyses were performed using Stata 14.1 (StataCorp).
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between January 14, 2015 and March 13, 2015, 280 IPs were enrolled (61 ANC, 117 PPC, 102 FSW). At the facility where ANC and PPC participants were recruited, clinic staff referred 78 women seeking ANC and of these, 12 (15%) declined to participate in the study and 5 (6%) were determined to be ineligible upon further screening. Clinic staff referred 148 women seeking PPC, among whom 14 (9%) declined to participate and 17 (11%) were determined to be ineligible. A total of 143 FSW were randomly selected for tracing by peer educators. Of these, 21 (15%) could not be located, 12 (8%) declined study participation, and 9 (6%) were referred to study staff but did not meet study inclusion criteria. Among the 280 enrolled IPs, 265 (94.6%) completed a follow-up interview by May 30, 2015, 3 (1%) withdrew from the study and were excluded from the analyses, and 12 (4.2%) were lost to follow-up.
Over half the ANC and PPC IPs were between 18–24 years of age whereas nearly half the FSW IPs were between 25–29 years of age (table 1). The majority of IPs had completed primary or some secondary education. However, compared to ANC and PPC IPs, a smaller proportion of FSW IPs had completed secondary education. Nearly all ANC or PPC IPs were married. In contrast only 10 (10%) of 101 FSW IPs were married, though 85 (84%) reported having a primary sexual partner. Almost all IPs had been tested for HIV in the past 12 months. IPs in all three participant groups reported high rates of IPV in the past year, ranging from 27%–46%.
Table 1.
Baseline characteristics of index participants
| Participant group
|
|||
|---|---|---|---|
| ANC | PPC | FSW | |
| Number of index participants enrolled, n* | 60 | 116 | 101 |
| Age in years | |||
| 18–24 | 31 (52) | 61 (53) | 22 (22) |
| 25–29 | 24 (40) | 42 (36) | 46 (46) |
| >30 | 5 (8) | 13 (11) | 33 (33) |
| Ethnic group | |||
| Luo | 40 (67) | 84 (72) | 79 (78) |
| Luhya | 10 (17) | 18 (16) | 12 (12) |
| Other | 10 (17) | 14 (12) | 10 (10) |
| Highest level of education | |||
| Some primary or completed primary | 11 (19) | 38 (33) | 59 (59) |
| Some secondary | 27 (46) | 45 (39) | 34 (34) |
| Secondary or higher | 21 (36) | 32 (28) | 7 (7) |
| Marital status | |||
| Married | 51 (85) | 109 (94) | 10 (10) |
| Not married but living together | 2 (3) | 0 (0) | 9 (9) |
| Separated of widowed | 1 (2) | 0 (0) | 31 (31) |
| Never married | 6 (10) | 7 (6) | 51 (50) |
| Has a primary sexual partner | 60 (100) | 116 (100) | 85 (84) |
| Used a condom during last sexual encounter | 3 (5) | 23 (20) | 73 (72) |
| Had an HIV test in past 12 months | 59 (98) | 113 (97) | 101 (100) |
| Primary partner had an HIV test in past 12 months** | |||
| No | 3 (5) | 17 (15) | 9 (11) |
| Yes | 49 (82) | 74 (64) | 58 (70) |
| Don’t know | 8 (13) | 25 (22) | 16 (19) |
| Experienced IPV in past 12 months | 16 (27) | 53 (46) | 44 (44) |
| Heard of HIV self-testing prior to study | 5 (8) | 21 (18) | 18 (18) |
Data are n (%) unless otherwise specified.
Abbreviations: ANC, antenatal care; PPC, postpartum care; FSW, female sex workers; IPV, intimate partner violence.
Excludes 3 index participants who withdrew from study after enrollment.
Among index participants who report having a primary sexual partner.
The majority of IPs in all participant groups reported using at least one self-test themselves (table 2). They also distributed self-tests to other individuals, as the 265 IPs with follow-up data reported distributing a total of 709 self-tests. ANC and PPC IPs distributed a mean of 2.14 and 1.95 self-tests to other individuals, respectively, while FSW IPs distributed a mean of 3.69 self-tests (figure 1). As shown in Table 2, all but one IP reported distributing one or more self-tests to other individuals and about 90% of ANC and PPC IPs distributed two or three self-tests, while all FSW distributed three or more self-tests.
Table 2.
Test use and distribution by index participants
| Participant group
|
|||
|---|---|---|---|
| ANC | PPC | FSW | |
| Number of IPs completing a follow-up interview, n | 58 | 106 | 101 |
| Number of self-tests used by IPs | |||
| 0 | 22 (38) | 25 (24) | 29 (29) |
| 1 | 36 (62) | 78 (74) | 66 (65) |
| 2 | 0 (0) | 3 (3) | 6 (6) |
| Number of self-tests distributed by IPs to other individuals | |||
| 0 | 1 (2) | 0 (0) | 0 (0) |
| 1 | 4 (7) | 12 (11) | 1 (1) |
| 2 | 41 (71) | 87 (82) | 8 (8) |
| 3 | 12 (21) | 7 (7) | 24 (24) |
| 4 | – | – | 57 (56) |
| 5 | – | – | 11 (11) |
| IPs who distributed ≥1 self-tests to a: | |||
| Primary sexual partner** | 53 (91) | 91 (86) | 64 (75) |
| Non-primary sexual partner*** | – | – | 17 (17) |
| Commercial sex client | – | – | 82 (81) |
| Female friend | 42 (72) | 84 (79) | 32 (32) |
| Male friend | 19 (33) | 18 (17) | 19 (19) |
| Offered ≥1 self-test but received a refusal | 14 (24) | 20 (19) | 20 (20) |
| Number of individuals who refused a self-test, mean (SD) | 0.50 (0.98) | 0.27 (0.54) | 0.25 (0.54) |
| Would recommend self-testing to a friend | 57 (98) | 104 (98) | 101 (100) |
| Belief about accuracy of self-tests | |||
| Very accurate | 48 (83) | 78 (74) | 97 (96) |
| Somewhat accurate | 6 (10) | 22 (21) | 4 (4) |
| Neutral | 1 (2) | 4 (4) | 0 (0) |
| Don’t know | 3 (5) | 2 (2) | 0 (0) |
| Reported IPV due to self-test distribution | 0 (0) | 2 (2) | 2 (2) |
Data are n (%) unless otherwise specified.
Abbreviations: ANC, antenatal care; PPC, postpartum care; FSW, female sex workers; IPV, intimate partner violence, IP, Index participant.
Except where otherwise noted.
Percentages based on number of IPs who reported having a primary sexual partner at baseline.
Defined as sexual partners who were neither primary nor commercial sex clients.
Figure 1. Number of self-tests used by index participants or distributed to other individuals, by participant group.
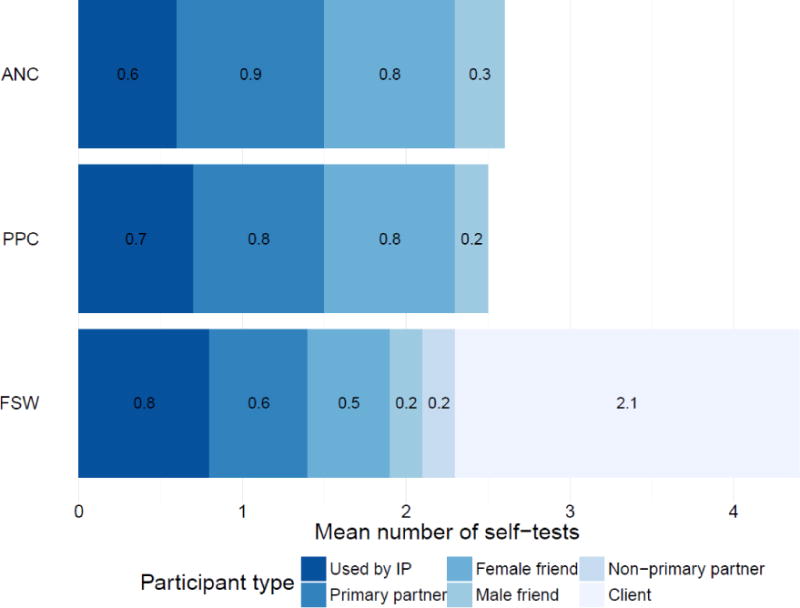
Abbreviations: ANC, antenatal care; PPC, postpartum care; FSW, female sex workers; IP, index participant.
Nearly all IPs with a primary sexual partner at baseline reported distributing a self-test to that partner (53/58, 91% ANC; 91/106, 86% PPC; 64/85, 75% FSW). FSW IPs were very likely to distribute self-tests to other sexual partners as well, with over 80% of them distributing one or more self-tests to commercial sex clients. IPs also distributed self-tests to individuals who were not their sexual partners. About 75% of ANC and PPC IPs distributed one or more self-tests to a female friend, whereas about 30% of the 101 FSW IPs distributed one or more self-tests to a female friend.
IPs did not always succeed in distributing self-tests to individuals in their social and sexual networks. In the three participant groups, about 20% of IPs reported offering a self-test to at least one individual who declined to accept it (table 2). In addition, of the 265 IPs with follow-up data, very few (4 in total) reported IPV during the 3-month follow-up period as a result of use or distribution of self-tests. Three reported physical violence following self-testing with their partner, while one reported leaving her home temporarily due to verbal abuse from her partner. No other adverse events were reported.
Of the 445 self-tests that IPs distributed to male sexual partners, 442 (99%) were reported to be used by those partners (table 3). IPs almost always reported being present when the sexual partner used the self-test and couples testing occurred frequently when self-tests were distributed to primary sexual partners. For the 53 self-tests that ANC IPs reported distributing to and being used by their primary sexual partner, couples testing occurred in 27 (51%) of the cases. For PPC IPs, couples testing occurred with 62 (68%) of the 91 primary sexual partners who accepted self-tests. For FSW, couples testing occurred with the vast majority of primary sexual partners who accepted self-tests (53/64, 83%) but less frequently with other sexual partners (including clients) who accepted self-tests (39/234, 17%). Overall, when including the few IPs who did not distribute a self-test to their primary sexual partner, couples testing with the primary sexual partner ranged from 47%–62% in the three participant groups (figure 2). Among male sexual partners who received self-tests from IPs, acceptability indicators for self-testing as reported by IPs were high. A large majority of these partners were reported to consider self-tests somewhat or very easy to use.
Table 3.
Characteristics of sexual partners who received self-tests from index participants
| Participant group
|
|||
|---|---|---|---|
| ANC | PPC | FSW | |
| Self-tests distributed by IPs to male sexual partners, n | 53 | 91 | 301 |
| Self-tests distributed to and used by male sexual partners, n | 53 | 91 | 298 |
| Relationship to IP of sexual partner who used self-test | |||
| Primary sexual partner* | 53 (100) | 91 (100) | 64 (21) |
| Non-primary sexual partner | – | – | 23 (8) |
| Commercial sex client | – | – | 211 (71) |
| IP reported being present when sexual partner used self-test | 53 (100) | 88 (97) | 248 (83) |
| IP reported couples testing with primary sexual partner** | 27 (51) | 62 (68) | 53 (83) |
| IP reported couples testing with non-primary sexual partner or commercial sex client*** | – | – | 39 (17) |
| Time between enrollment and use of self-test | |||
| Within one week | 46 (87) | 76 (84) | 193 (65) |
| 1–2 weeks | 4 (8) | 10 (11) | 62 (21) |
| >2 weeks | 3 (6) | 5 (5) | 23 (8) |
| Don’t know | 0 (0) | 0 (0) | 20 (7) |
| Location where self-test was used | |||
| Own home | 50 (94) | 88 (97) | 39 (13) |
| Recipient home | 3 (6) | 2 (2) | 101 (34) |
| Guesthouse/bar/club | 0 (0) | 0 (0) | 150 (50) |
| Don’t know | 0 (0) | 1 (1) | 8 (3) |
| Perception of self-test for individual who received self-test from IP | |||
| Very positive | 40 (75) | 58 (64) | 264 (89) |
| Somewhat positive | 9 (17) | 25 (27) | 16 (5) |
| Neutral | 0 (0) | 0 (0) | 2 (1) |
| Somewhat negative | 4 (8) | 7 (8) | 5 (2) |
| Very negative | 0 (0) | 0 (0) | 1 (0) |
| Don’t know | 0 (0) | 1 (1) | 10 (3) |
| Ease of use of self-test for individual who received self-test from IP | |||
| Very easy | 32 (60) | 54 (59) | 171 (57) |
| Somewhat easy | 13 (25) | 21 (23) | 43 (14) |
| Neutral | 2 (4) | 9 (10) | 8 (3) |
| Somewhat difficult | 6 (11) | 7 (8) | 51 (17) |
| Very difficult | 0 (0) | 0 (0) | 25 (8) |
Data are n (%) unless otherwise specified.
Abbreviations: ANC, antenatal care; PPC, postpartum care; FSW, female sex workers; IPV, intimate partner violence, IP, index participant.
2 primary partners in ANC group and 3 in PPC group each used 2 self-tests, these duplicate tests were not included in this table.
Among self-tests distributed to and used by a primary sexual partner.
Among self-tests distributed to and used by a non-primary sexual partner or commercial sex client.
Figure 2. Proportion of index participants who distributed self-tests to and undertook couples testing with their primary sexual partners.
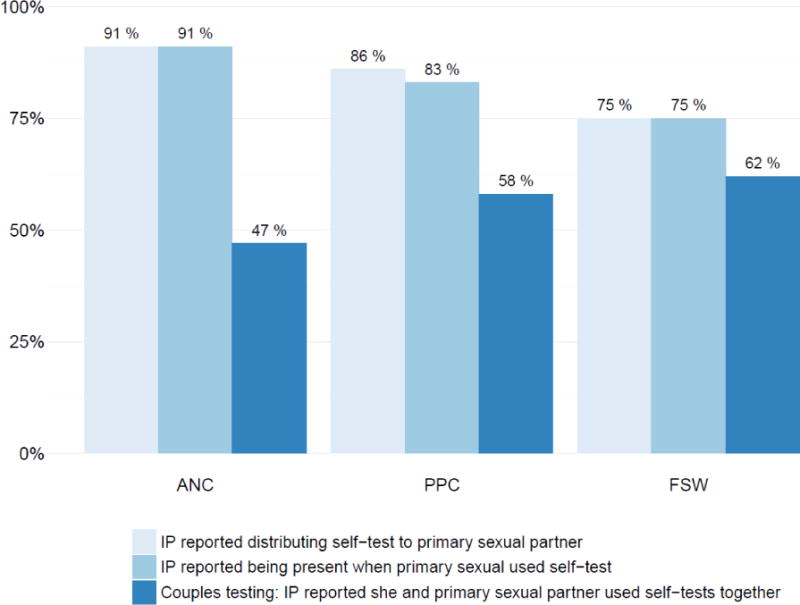
Abbreviations: ANC, antenatal care; PPC, postpartum care; FSW, female sex workers; IP, index participant.
Among IPs who have a primary sexual partner and completed a follow-up interview (n=58 ANC; n=106 PPC; n=85 FSW).
The proportion of male sexual partners who accepted self-tests who obtained an HIV-positive result ranged from 2.2% (PPC) to 13.8% (FSW) (table 4). Relatively few primary sexual partners were reported obtain an HIV-positive result. In the FSW group, however, 37 (90%) of the 41 sexual partners who obtained an HIV-positive result were commercial sex clients. Neither of the 2 HIV-positive sexual partners of ANC IPs were reported to have sought confirmatory testing or linked to HIV care within the 3-month follow-up period. However, both of the HIV-positive individuals identified by PPC IPs were reported to have sought confirmatory testing and linked to care within the same period. Of the 40 HIV-positive individuals identified by FSW IPs who did not already know their HIV status, 26 (65%) had sought confirmatory testing within the 3-month follow-up period and 23 (58%) were reported to have linked to care. In 11 cases (28%), FSW IPs did not know whether the individual had sought confirmatory testing and in 14 cases (35%) linkage to care was not known.
Table 4.
Self-test results, confirmatory testing, and linkage to care of sexual partners
| Participant group
|
|||
|---|---|---|---|
| ANC | PPC | FSW | |
| Sexual partners tested using self-tests, n | 53 | 91 | 298 |
| HIV self-test results of sexual partners | |||
| HIV-negative | 51 (96.2) | 87 (95.6) | 242 (81.2) |
| HIV-positive | 2 (3.8) | 2 (2.2) | 41 (13.8) |
| Don’t know | 0 (0) | 2 (2.2) | 15 (5) |
| Relationship to IP of sexual partner who used self-test (HIV-positive only) | |||
| Primary partner | 2 (100) | 2 (100) | 2 (5) |
| Non-primary sexual partner | – | – | 2 (5) |
| Commercial sex client | – | – | 37 (90) |
| Went for confirmatory testing after receiving HIV-positive HIVST result?* | |||
| Yes | 0 (0) | 2 (100) | 26 (65) |
| No | 2 (100) | 0 (0) | 3 (8) |
| Don’t know | 0 (0) | 0 (0) | 11 (28) |
| Enrolled in HIV care after receiving HIV-positive HIVST result?* | |||
| Yes | 0 (0) | 2 (100) | 23 (58) |
| No | 2 (100) | 0 (0) | 3 (8) |
| Don’t know | 0 (0) | 0 (0) | 14 (35) |
Data are n (%) unless otherwise specified.
Abbreviations: ANC, antenatal care; PPC, postpartum care; FSW, female sex workers; IP, index participant.
Confirmatory testing and linkage to care by sexual partners are based on IPs’ reports. One HIV-positive sexual partner of an FSW IP was excluded because he was reported to already know his HIV status.
Sexual intercourse was significantly less likely when IPs’ sexual partners tested HIV-positive versus HIV-negative (18% vs. 62%, p<0.0001) (table 5). Similarly, condom use among those who reported sexual intercourse was significantly higher after a sexual partner tested HIV-positive versus HIV-negative (100% vs. 44%, p=0.0018). Such sexual decision-making based on the result of the partner’s self-test result was evident in ANC and PPC IPs as well as FSW IPs.
Table 5.
Sexual decision making of index participants by sexual partner HIV self-testing status
| Participant group
|
||||
|---|---|---|---|---|
| ANC | PPC | FSW | Total | |
| IP had sexual intercourse with sexual partner after he used a self-test | ||||
| HIV-negative self-test result | 38 (75) | 66 (76) | 131 (54) | 235 (62) |
| HIV-positive self-test result | 0 (0) | 1 (50) | 7 (17) | 8 (18) |
| P-value** | p<0.0001 | |||
| Condom used during last sexual intercourse with sexual partner* | ||||
| HIV-negative self-test result | 1 (3) | 12 (18) | 91 (69) | 104 (44) |
| HIV-positive self-test result | – | 1 (100) | 7 (100) | 8 (100) |
| P-value** | p=0.0018 | |||
Data are n (%) unless otherwise specified.
Abbreviations: ANC, antenatal care; PPC, postpartum care; FSW, female sex workers; IP, index participant.
Among IPs who reported sexual intercourse with sexual partner after he used a self-test.
P-value from Fisher’s exact test comparing means for IPs whose sexual partners obtained an HIV-negative and HIV-positive self-test result.
Discussion
Provision of multiple self-tests to women for secondary distribution seems to be a safe, viable, and promising strategy for promoting HIV testing among male sexual partners and facilitating safer sexual decisions. A high proportion of pregnant and postpartum women as well as FSW were able to distribute self-tests to other individuals, particularly their sexual partners. This strategy also resulted in high levels of couples testing. Among FSW in particular, secondary distribution resulted in HIV testing among high risk male clients, with a high proportion of individuals obtaining an HIV-positive result. Another important finding was that secondary distribution of self-tests led to significant changes in sexual behavior based on test results, a further indication of the significant HIV prevention potential of this strategy.
While a growing number of studies have documented the acceptability, accuracy, and safety of self-testing among individuals who are provided with self-tests directly (“primary distribution”),6,9,20,21 to our knowledge this is the first study to assess the feasibility of secondary distribution of self-tests in SSA. One pilot study in the United States has explored this strategy among MSM12,22 and a recent community-based study in Malawi included an option for couples to receive multiple self-tests in their homes but did not directly assess secondary distribution as a strategy.21,23 In this study, secondary distribution of self-tests by women who received multiple self-tests was extremely high, with most women distributing all of the tests provided to them.
Approximately 90% of pregnant and postpartum women reported that their primary sexual partner used a self-test, and a majority of women reported testing together with their partner. Promoting HIV testing among men in these settings would not only carry prevention benefits for their female sexual partners, but could also be beneficial for their own health through earlier initiation of ART for those who are HIV-positive.24 The high levels of male partner testing and couples testing achieved in this study are noteworthy since accessing male partners at clinics has proven challenging and provider-initiated strategies such as the provision of written invitations for partners or conducting home visits have either had limited success or are likely to require significant financial and human resources for implementation.25–27 These results suggest that provision of multiple self-tests to women attending antenatal and postpartum clinics should receive further consideration.
Among FSW who participated in the study, a key finding is that about one out of every six self-tests they distributed to a commercial sex client were reported to have an HIV-positive result. This proportion was comparable to HIV prevalence among adult males in the study region (17.8%),13 although it is surprising that an even higher proportion of clients did not test HIV-positive given their higher risk profiles. Underreporting of HIV-positive results could be one explanation, as could the age distribution among clients and the possibility that FSW avoided self-test distribution and used condoms with their highest-risk clients. Overall, this finding suggests that providing FSW with multiple self-tests can be a highly efficient way to identify HIV-infected individuals. More generally, an appealing aspect of the strategy is that it promotes testing by utilizing existing sexual and social networks of FSW.
Another important finding is the potential for the secondary distribution intervention to facilitate more informed sexual decisions when used as a point-of-sex decision-making tool. FSW in particular were less likely to report having sexual intercourse, and when they did have sexual intercourse they were more likely to use condoms, with men who used a self-test and obtained an HIV-positive result as compared to an HIV-negative result. Considering the structural barriers to condom use among FSW and economic incentives favoring condomless sex,18,28 this is an important result from an HIV prevention standpoint and it suggests that access to multiple self-tests may empower FSW to protect themselves from HIV. These results are also consistent with pilot studies conducted among MSM in the United States, in which MSM reported using self-tests to screen partners and make informed sexual decisions.12,29 Such use of self-tests may nonetheless pose risks due to the window period of many existing tests. However, it may also result in a net benefit if those given multiple self-tests engage in unsafe sexual behavior in the absence of self-tests being available. Additional qualitative research and mathematical modeling is needed to explore implications of wider use of self-tests for sexual decision-making.
Reported IPV resulting from self-test distribution was extremely rare in this study, a promising finding considering the high proportion of IPs who reported prior IPV at baseline. This could be due to women using discretion when choosing individuals to whom they offered self-tests. IPs also reported that acceptability of self-tests by individuals who received self-tests from them was very high. Compared to rates of IPV reported in studies of counselor-administered couples testing and studies that sought to promote male partner testing, the IPV rates in our study were lower.25 Nonetheless, it is important to further monitor IPV in future implementation secondary distribution interventions and have systems in place to support women who experience IPV.
In Kenya and other countries, persons receiving a positive self-test result are recommended to seek confirmatory testing for diagnosis of HIV.11 While promotion of linkage to care was not an objective of the study, all self-test kits included information about the importance of confirmatory testing and included a phone hotline that users could call. We estimate that over 50% those who obtained an HIV-positive result sought confirmatory testing and linked to care. Linkage to care was only measured during the 3 month study follow-up period. Since individuals who received a self-test from IPs soon after study enrollment in the study had more time to link to care than who received a self-test several weeks after enrollment, our estimate of linkage may be an underestimate. Nonetheless, these estimates are comparable to those from a large-scale self-testing study in Malawi that actively promoted linkage to care21 and are on par with estimates from many facility- and community-based HTC strategies in SSA,3,30 although lower than estimates from community-based interventions that facilitated linkage to care.3
An inherent limitation of many studies of unsupervised self-testing is that it is challenging to verify usage of self-tests and accuracy in the hands of the user. In this study, all information was obtained through IPs’ reports, and thus we were unable to verify self-test distribution, usage, or test results. However, all IPs were told there was no obligation to distribute self-tests and reporting bias is likely to have been minimal. The fact that many IPs reported not distributing all the self-tests given to them supports the notion of limited reporting bias. Moreover, bias in reporting HIV test results for the individuals who accepted self-tests from IPs would most likely be an underestimate of the proportion of HIV-positive persons identified. Generalizability of our results may be limited by the selection of participants into the study, which included women who were actively engaged in health services and were interested in distributing them in their sexual and social networks. From an implementation perspective, however, providing multiple self-tests to women who are easily accessed can be a feasible and efficient way to implement the intervention and reach male partners. The study also did not assess the feasibility of secondary distribution by HIV-infected women or by men, or the implications of providing more than 3 or 5 self-tests. These should be among the priorities for future research.
Other limitations include not inquiring about prior knowledge of HIV status among men who accepted self-tests from IPs. Future research would benefit not only from acquiring more information on testing history of sexual partners who accept a self-test, but also from engaging with men directly to solicit additional information on perceptions around secondary distribution by women. Moreover, although our results suggest that secondary distribution of self-tests is an promising strategy for promoting male partner testing and also identifying HIV-infected persons, further research is necessary to assess the effectiveness and cost-effectiveness of this strategy.
In summary, secondary distribution of self-tests by women is a novel strategy that has the potential to accomplish multiple HIV prevention objectives, including male partner HIV testing, couples testing, and safer sexual decision-making. It may be a particularly effective strategy among key populations such as FSW, since it identified many HIV-infected partners and led to reduced sexual risk behaviors. Further implementation and evaluation of this strategy is warranted as countries develop HIVST policies and consider how these technologies can be used to prevent new HIV infections.
Research in Context.
Evidence before this study
We searched PubMed for studies published until March 1, 2016 with the terms “HIV” AND (“self-test*” OR “home test”). Several studies have documented high acceptability, accuracy and safety of HIV self-testing in sub-Saharan Africa among individuals who directly receive a self-test. Community-based studies in Malawi have shown high uptake of self-testing, as well as linkage to HIV care that is comparable to what is found in other testing strategies. However, little is known about optimal distribution strategies for facilitating self-test use by high-risk, hard-to-reach individuals and for identifying HIV-infected persons. As opposed to “primary distribution” strategies that directly reach these individuals, “secondary distribution” is a strategy in which an easier-to-reach individuals are given multiple self-tests for distribution to harder-to-reach individuals such as sexual partners or others in their social network. One pilot study involving a high risk sample of men who have sex with men in the U.S. explored the feasibility of secondary distribution of HIV self-tests. The study had 27 participants and documented high acceptability and demonstrated potential for use of self-tests to screen sexual partners. No studies in sub-Saharan Africa have explored secondary distribution of self-tests by pregnant and postpartum women or other key populations such as female sex workers.
Added value of this study
To our knowledge, this is the first assessment of the potential for secondary distribution of HIV self-tests by multiple populations of women to promote HIV testing among male sexual partners and among couples. This is also the first study to demonstrate that secondary distribution of self-tests by female sex workers can result in HIV testing among their high-risk commercial sex clients and that this strategy can identify a high proportion of HIV-infected individuals. Another important contribution of this study is its identification of a significant potential for a secondary distribution strategy to facilitate ‘point-of-sex’ HIV testing and lead to safer, more informed sexual decision making. The study shows that secondary distribution of self-tests is a safe and efficient way to promote HIV testing among hard-to-reach individuals and also achieve other HIV prevention objectives.
Implications of all the available evidence
These findings suggest that secondary distribution of HIV self-tests is a promising strategy for increasing HIV testing among populations that are traditionally hard to reach, including male partners of pregnant and postpartum women and clients of female sex workers. It may also facilitate safer sexual decisions by removing some barriers to mutual disclosure of HIV status. Further implementation and evaluation of this strategy is warranted as countries are developing HIV self-testing policies and considering how these technologies can be used to prevent new HIV infections. Along with other community-based HIV testing strategies implemented recently, secondary distribution of self-tests can be part of a multi-pronged approach to achieving the first of the UNAIDS “90-90-90” targets.
Acknowledgments
This study was funded by the Bill and Melinda Gates Foundation (grant no. OPP1069673). We thank Kate Murray and Immaculate Akello for assistance in implementing the study, the research assistants who conducted enrollment and follow-up, staff at the health facility and drop-in center, as well as all study participants.
Footnotes
Contributors
HT and KA designed the study. HT, KA, and EO supervised the data collection. SHM and HT analyzed the data. HT, SHM, and SNM wrote the first draft. All authors reviewed the manuscript and approved the final draft.
Declaration of interests
We declare no competing interests.
References
- 1.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [Google Scholar]
- 2.Suthar AB, Ford N, Bachanas PJ, et al. Towards universal voluntary HIV testing and counselling: a systematic review and meta-analysis of community-based approaches. PLoS Med. 2013;10(8):e1001496. doi: 10.1371/journal.pmed.1001496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77–85. doi: 10.1038/nature16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37(5):1620–6. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Guidance on couples HIV testing and counselling including antiretroviral therapy for treatment and prevention in serodiscordant couples: recommendations for a public health approach. Geneva: World Health Organization; 2012. [PubMed] [Google Scholar]
- 6.Napierala Mavedzenge S, Baggaley R, Corbett EL. A review of self-testing for HIV: research and policy priorities in a new era of HIV prevention. Clin Infect Dis. 2013;57(1):126–38. doi: 10.1093/cid/cit156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson C, Baggaley R, Forsythe S, et al. Realizing the potential for HIV self-testing. AIDS Behav. 2014;18(Suppl 4):S391–5. doi: 10.1007/s10461-014-0832-x. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and Acceptability on HIV Self-testing Among Key Populations: A Literature Review. AIDS Behav. 2015;19(11):1949–65. doi: 10.1007/s10461-015-1097-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choko AT, Desmond N, Webb EL, et al. The Uptake and Accuracy of Oral Kits for HIV Self-Testing in High HIV Prevalence Setting: A Cross-Sectional Feasibility Study in Blantyre, Malawi. PLoS medicine. 2011;8(10):e1001102. doi: 10.1371/journal.pmed.1001102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Consolidated guidelines on HIV testing services. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 11.National AIDS and STI Control Programme. Guidelines for HIV Testing Services in Kenya. Nairobi: National AIDS Control Programme; 2015. [Google Scholar]
- 12.Carballo-Dieguez A, Frasca T, Balan I, Ibitoye M, Dolezal C. Use of a rapid HIV home test prevents HIV exposure in a high risk sample of men who have sex with men. AIDS and behavior. 2012;16(7):1753–60. doi: 10.1007/s10461-012-0274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National AIDS Control Council. Kenya HIV County Profiles. Nairobi: National AIDS Control Council; 2014. [Google Scholar]
- 14.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1-serodiscordant couples. AIDS. 2011;25(15):1887–95. doi: 10.1097/QAD.0b013e32834a9338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunkle KL, Stephenson R, Karita E, et al. New heterosexually transmitted HIV infections in married or cohabiting couples in urban Zambia and Rwanda: an analysis of survey and clinical data. Lancet. 2008;371(9631):2183–91. doi: 10.1016/S0140-6736(08)60953-8. [DOI] [PubMed] [Google Scholar]
- 16.Moodley D, Esterhuizen T, Reddy L, et al. Incident HIV infection in pregnant and lactating women and its effect on mother-to-child transmission in South Africa. J Infect Dis. 2011;203(9):1231–4. doi: 10.1093/infdis/jir017. [DOI] [PubMed] [Google Scholar]
- 17.Bekker LG, Johnson L, Cowan F, et al. Combination HIV prevention for female sex workers: what is the evidence? Lancet. 2015;385(9962):72–87. doi: 10.1016/S0140-6736(14)60974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shannon K, Strathdee SA, Goldenberg SM, et al. Global epidemiology of HIV among female sex workers: influence of structural determinants. Lancet. 2015;385(9962):55–71. doi: 10.1016/S0140-6736(14)60931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kenya National Bureau of Statistics. Kenya Demographic and Health Survey 2008–09. Nairobi: Kenya National Bureau of Statistics; 2010. [Google Scholar]
- 20.Pant Pai N, Sharma J, Shivkumar S, et al. Supervised and unsupervised self-testing for HIV in high- and low-risk populations: a systematic review. PLoS Med. 2013;10(4):e1001414. doi: 10.1371/journal.pmed.1001414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choko AT, MacPherson P, Webb EL, et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015;12(9):e1001873. doi: 10.1371/journal.pmed.1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carballo-Dieguez A, Frasca T, Dolezal C, Balan I. Will Gay and Bisexually Active Men at High Risk of Infection Use Over-the-Counter Rapid HIV Tests to Screen Sexual Partners? Journal of sex research. 2012 doi: 10.1080/00224499.2011.647117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumwenda M, Munthali A, Phiri M, et al. Factors Shaping Initial Decision-Making to Self-test Amongst Cohabiting Couples in Urban Blantyre, Malawi. AIDS Behav. 2014 doi: 10.1007/s10461-014-0817-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Insight Start Study Group. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osoti AO, John-Stewart G, Kiarie J, et al. Home visits during pregnancy enhance male partner HIV counselling and testing in Kenya: a randomized clinical trial. AIDS. 2014;28(1):95–103. doi: 10.1097/QAD.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg NE, Mtande TK, Saidi F, et al. Recruiting male partners for couple HIV testing and counselling in Malawi’s option B+ programme: an unblinded randomised controlled trial. The lancet HIV. 2015;2(11):e483–91. doi: 10.1016/S2352-3018(15)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brusamento S, Ghanotakis E, Tudor Car L, van-Velthoven MH, Majeed A, Car J. Male involvement for increasing the effectiveness of prevention of mother-to-child HIV transmission (PMTCT) programmes. Cochrane Database Syst Rev. 2012;10:CD009468. doi: 10.1002/14651858.CD009468.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jakubowski A, Omanga E, Agot K, Thirumurthy H. Large price premiums for unprotected sex among female sex workers in Kenya: a potential challenge for behavioral HIV prevention interventions. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2015 doi: 10.1097/QAI.0000000000000929. in press. [DOI] [PubMed] [Google Scholar]
- 29.Balan IC, Carballo-Dieguez A, Frasca T, Dolezal C, Ibitoye M. The Impact of Rapid HIV Home Test Use with Sexual Partners on Subsequent Sexual Behavior Among Men Who have Sex with Men. AIDS and behavbior. 2013 doi: 10.1007/s10461-013-0497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8(7):e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]


