Abstract
The global epidemic of obesity continues unabated with sequelae of diabetes and metabolic syndrome. This review reflects the dramatic increase in research on the role of increased expression of heme oxygenase (HO)-1/HO-2, biliverdin reductase, and HO activity on vascular disease. The HO system engages with other systems to mitigate the deleterious effects of oxidative stress in obesity and cardiovascular disease (CVD). Recent reports indicate that HO-1/HO-2 protein expression and HO activity have several important roles in hemostasis and ROS-dependent perturbations associated with metabolic syndrome. HO-1 protects tissue during inflammatory stress in obesity through the degradation of pro-oxidant heme and the production of carbon monoxide (CO) and bilirubin, both of which have anti-inflammatory and anti-apoptotic properties. In contrast, repression of HO-1 is associated with increases of cellular heme and inflammatory conditions including hypertension, stroke and atherosclerosis. HO-1 is a major focus in the development of potential therapeutic strategies to reverse the clinical complications of obesity and metabolic syndrome.
Obesity and Metabolic Syndrome
Obesity and metabolic syndrome have earned the name “the silent disease” because their adverse effects are insidious. Although obesity and metabolic syndrome are theoretically treatable with modern medical and lifestyle management, this is not a trivial undertaking. While a discussion about the long-term success of different therapeutic strategies addressing obesity and metabolic syndrome is beyond the scope of this introduction, it is fair to say that our national and regional battle with this “silent disease” is not going well. Obesity and metabolic syndrome are statistically on the rise in the USA in general and in West Virginia and Mississippi in particular (http://www.cdc.gov/obesity/data/adult.html). Obesity is major risk factor for vascular dysfunction, insulin resistance and vascular diseases including obesity/diabetes and hypertension1–3, while increasing inflammatory cytokines and insulin resistance 4,5. These consequences of obesity-mediated adipocyte dysfunction may be far-reaching, as changes in adipocyte-derived paracrine factors including adipokines and cytokines may impact the function of other organs and in particular the vasculature 6–8.
Several studies have demonstrated that increases in reactive oxygen species (ROS) in vitro and in vivo, 9–11 as well as increases in heme, induce the differentiation of pre-adipocytes and increase adipogenesis in vitro and in vivo 10,12–14. Interestingly, despite this increased ROS production, HO-1 expression is not increased, further contributing to the development of obesity and metabolic syndrome 13.
Obesity leads to the activation of nicotinamide adenine dinucleotide phosphate-oxidase (NADPH oxidase) and the angiotensin II system, resulting in the development of diabetes, hypertension and CVD in part due to impairment of adipocyte function 1,15,16. Obesity-mediated development of hyperglycemia has a direct effect on HO-1 suppression 17–19, and its inactivation by peroxynitrite 11,20,21 increases cellular heme levels 11,20,22. Heme, a pro-oxidant, acts together with increased ROS to produce vascular and adipocyte dysfunction 14. This review will examine the role of HO-1 and of the heme degradation products biliverdin/bilirubin and CO in mitigating obesity, as well as their role in the regulation of oxidative stress and cell survival. We discuss these findings in the context of the identification of potential future therapeutic approaches to prevent the development of obesity, diabetes and the detrimental effects associated with the progression of atherosclerotic disease 23,24 (Figure 1).
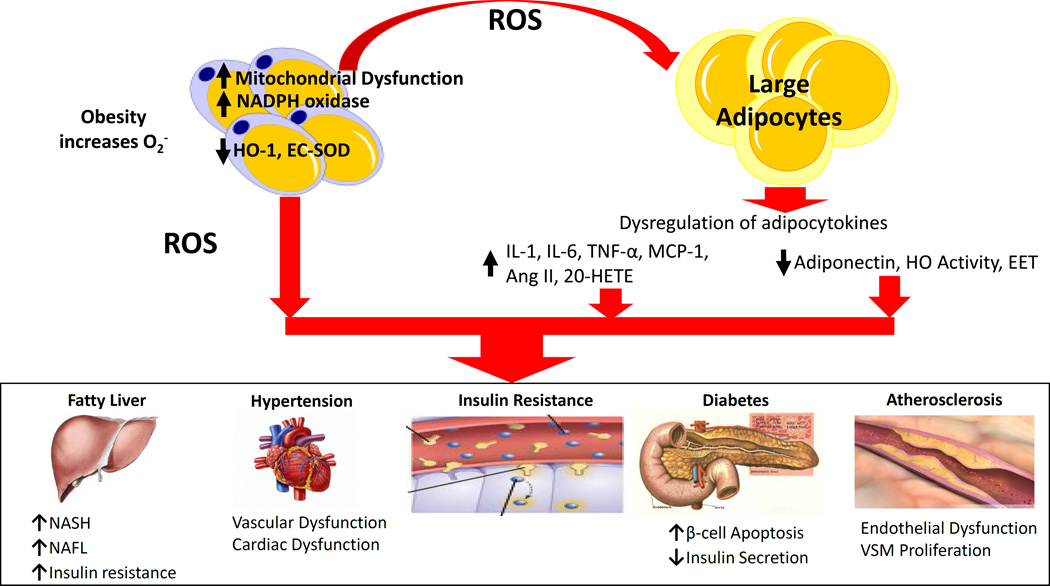
Figure 1.
Obesity increases risk for cardiovascular disease. Obesity leads to an increase in ROS within adipocytes, accomplished by increasing NADPH oxidase activity, mitochondrial ROS production, and heme levels while repressing antioxidative enzymes such as HO-1 and SOD. This increase in adipocyte ROS and heme leads to increased adipocyte differentiation, maturation, resulting in increased production of proinflammatory compounds such as cytokines and decreased production of antioxidative compounds and compounds preventing adipocyte growth and differentiation. The consequences of obesity-mediated adipocyte dysfunction may lead to vascular dysfunction which is a prelude to vascular disease and hypertension.
Heme Oxygenase
HO exists in two forms, HO-1, the inducible form, and HO-2, the constitutive form. Both isozymes degrade heme in an identical stereospecific manner to biliverdin with the concurrent release of CO and iron 25. In mammals, biliverdin is rapidly reduced by biliverdin reductase to bilirubin 26,27. HO-1 and HO-2 are alike in terms of mechanism, cofactor and substrate requirements, as well as their susceptibility to inhibition by synthetic metalloporphyrins in which the central iron atom is replaced by other elements including tin, zinc, cobalt and chromium (reviewed in 14). HO-1 can be induced by an extraordinarily wide variety of drugs and chemical agents including statins, aspirin, niacin, certain prostaglandins, eicosanoids such as epoxyeicosatrienoic (EETs) and free and complexed metals 25. Iron, bilirubin and CO, the three degradation products of the HO reaction, may have important regulatory functions in cells. Iron is, of course, an essential requirement for the synthesis of hemoglobin and ferritin, and HO-1-deficient mice are known to develop anemia. Reduced stress defense in HO-1 deficient cells is well described 28. HO-1 deficiency accelerates formation of arterial thrombosis29 that is rescued by inhaled CO 30. The discovery that HO-1 is a novel target for modulation of the inflammatory response 31,32 and for diminishing fibrosis 33 increases interest in HO-1 signaling pathways. Yachie et al., 34 reported that a child with congenital HO-1 deficiency, or mice deficient in HO-1 displayed severe growth retardation in addition to microcytic hypochronic anemia, renal endothelial cell injury34–37. Similarly, HO-2 deficiency increased oxygen toxicity and iron accumulation in lung 38 and in response, diabetes-induced renal injury 39, an increase in adiposity and systolic blood pressure 40 and impaired vasodilation to acetylcholine 41. The constitutive nature of HO-2, however, makes it less attractive as a drug target.
The first therapeutic approach to regulate HO activity began by devising a means to downregulate HO activity by competitive inhibition. This was recognized early 42,43 and achieved clinical fruition in neonatology when a safe, rapidly acting and effective method for transiently blocking bilirubin production in newborns was developed 43,44. The development of inhibitors of HO activity having a pharmacological profile permitting their use in infants provided the first demonstration of the potential clinical usefulness of agents that can downregulate HO. However, when selecting an inducer or an inhibitor of HO activity, one must consider the mechanism of induction/inhibition and duration of response. Rapid response of HO-1 mRNA by inducers such as cobalt protoporphyrin IX dichloride (CoPP) do not reflect an immediate increase in HO activity 45, suggesting that an increase of HO-1 mRNA is meaningless unless HO activity is also determined. Induction of HO-1 by heme or CoPP has a differential effect on the timing of an increase in HO activity, with heme resulting in a maximum increase in HO activity at 16 hr, whereas with CoPP maximal HO activity is attained at 3 days and lasts for extended periods of time up to 14 days 25. In metabolic syndrome and vascular disease, elevation of inducible nitric oxide synthase (iNOS) and peroxynitrite resulted in inactivation of HO-1 protein 20,21 and decreased HO activity. These considerations regarding the time frame of induction or inhibition of HO activity, not just HO-1 expression levels, must be taken into account in the development of new therapeutic strategies targeting HO-1 for the treatment of obesity-hypertension and metabolic syndrome as has been suggested in numerous publications. As an alternative strategy to drugs, gene therapy has been identified as a long lasting (1 year) and effective way to induce HO-1 expression to prevent against CVD 20,46–51.
Both biliverdin and bilirubin are potent antioxidants and may exert cellular protective effects against injurious stimuli in vivo and in vitro 52,53. CO has been identified as a second messenger in the central nervous system (CNS) 54 and suppresses endothelial cell apoptosis through activation of p38 MAPK 55,56. The gas has a multitude of functions in biology and medicine which are described in series of articles 57–59 and include acting as a vasodilator via stimulation of soluble guanylate cyclase (sGC).
Biological Action of Bilirubin/Biliverdin
The biologic actions of HO-1-derived bilirubin may be especially relevant to the prevention of oxidant-mediated cell death 10,11,60 (Figure 2). Bilirubin, at a low concentration, scavenges ROS, thereby reducing oxidant-mediated cellular damage and attenuating oxidant stress in vivo 52. The roles of biliverdin and bilirubin in counteracting oxidative and nitrosative stress have been extensively reviewed 32. Administration of the degradation products of heme confers protection against various types of pathological injuries. Biliverdin therapy protects the liver of rats from ischemia and reperfusion injury, while bilirubin was reported to increase tolerance to islet allograft by both its anti-inflammatory and anti-oxidative properties61. Bilirubin increased tolerance to islet allograft by both its anti-inflammatory and anti-oxidative properties 62,63. Additionally, biliverdin reductase acts as transcriptional factor and increases HO-1/HO-2 levels and HO activity 26 and protects against glucose intolerance (reviewed in 64) through a mechanism involving activation of phosphatidylinositol 3-kinase (P13K) 65. Unconjugated bilirubin mediated HO-1-induced vascular benefit in diabetes 66 and administration of mesobiliverdin analogues of biliverdin to the pancreas lowered blood glucose and increased insulin function 67.
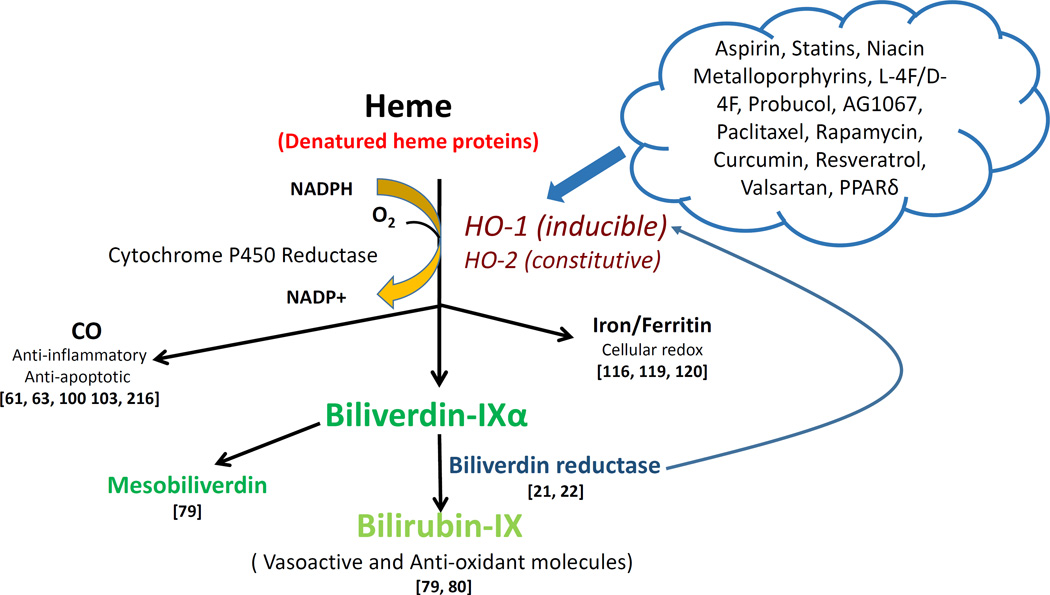
Figure 2.
Drug actions in the heme degradation pathway. HO-1 (inducible) and HO-2 (constitutive) cleave free heme or denatured heme proteins to generate CO, ferritin, and biliverdin, which is subsequently converted to bilirubin by biliverdin reductase. CO has both anti-inflammatory and anti-apoptotic properties 55,57,57,59,171 Ferritin is essential for cellular redox reactions 99,100,102 Serum bilirubin levels are positively linked with a decreased risk of CVD and protection against diabetes and vascular dysfunction 67,68. Drugs focused on the heme degradation pathway predominantly induce HO-1 activity, possibly by interacting with the gene promoter. Biliverdin reductase functions via a direct increase of HO-1 or an increase in bilirubin levels to promote a reduction in oxidative species 26,27. And mesobiliverdin enhances β-cell function in the pancreas through its antioxidant properties 67 This pathway provides the basis for multiple pharmaceutical and genetic agents that can protect against CVD by increasing HO-1 expression.
The potential beneficial and protective role of bilirubin in obesity and metabolic syndrome as well as the resulting cardiovascular disease has been highlighted in recent years. First, bilirubin was reported to increase insulin sensitivity and ameliorated obesity in leptin-receptor deficient and suppression of chronic inflammation 68. Bilirubin also displays cytoprotective properties in the cardiovascular system 69,70, as elevated serum bilirubin levels are associated with a decreased risk for coronary artery disease in humans (reviewed in 71,72). Additional studies have demonstrated that both free and albumin-bound bilirubin can inhibit the oxidation of low density lipoproteins (LDL) 73. The roles of bilirubin and biliverdin reductase in obesity and CVD have been recently reviewed 64,72.
Biological Action of Carbon Monoxide
The function of CO is unclear. It is generally accepted that CO is toxic to cells, but less is known about pico-levels of CO in physiological function (reviewed in 74). A dose-dependent toxic effect of CO on BSC-1 cells established that this was due to stimulation of iNOS and production of nitrate NOO−, which is known to cause cellular injury 75,76. Although CO, like NO, functions as a vasorelaxant via stimulation of sGC; the relative potency of CO is miniscule compared to NO, however, the potency of CO mediated increase in cGMP levels may be increased by other agents, 77 including the benzyloid derivative such as YC-1, which increases CO-mediated cGMP formation to a level similar to that of NO. HO-1-derived CO was shown to relax vascular tissue of aorta 78, hepatic vein, piglet mesenteric artery, pial arterioles 79, and pulmonary artery.
While the vascular effects of CO have been established for some time now, the regulation of metabolism by CO is an emerging area. Chronic CO treatment through the use of a CO-releasing molecule provide vascular protection 80,81. CORM-A1-derived CO given to mice fed a high fat diet resulted in decreased levels of fasting blood glucose, insulin and body fat and increased oxygen consumption and heat production compared to controls 82. Chronic CORM-A1 treatment was found to reverse obesity in mice chronically fed a high fat diet through similar changes in adipocyte phenotype as well as significant reductions in pro-inflammatory adipokines such as high mobility group box (HMGB) protein-183.
HO-1 and HO-2-derived CO increases insulin secretion, and this function involves Ca+ signaling, both of which are prevented by inhibition of HO activity 84. This may also be due to a CO-mediated anti-inflammatory effect 59,85. The beneficial effects of chronic exposure to CO as a direct result of heme degradation have been recently reviewed 57,57,58,86.
Biological Action of Ferritin
Heme metabolism results in the liberation of iron, and tissues rely on storage proteins to regulate "free" levels of this metal. Free iron is well known to lead to the generation of ROS, potentially leading to damage of various cellular components. Iron can become integrated into the phospholipid bilayer, and act to oxidize cell membrane constituents and/or participate in reactions leading to the generation of ROS 87,88. The link between iron, metabolic syndrome and Alzheimer’s disease has been recently reviewed 89. Plasma iron is bound to transferrin, which can transfer iron to the intracellular milieu of endothelial cells via cell surface receptor binding. Ferritin is a high capacity, low affinity protein responsible for binding most intracellular iron, and its synthesis is rapidly upregulated when free iron is present 90. Under normal conditions, very minute amounts of iron exist in the free metal form; thus the concentration of ferritin is an efficient indicator of intracellular iron levels 91. The most abundant source of iron is heme, which can release iron during metabolism by HO or via oxidative degradation 92. While this transition metal is essential to biological processes, iron can be extremely toxic if intracellular concentrations are not tightly regulated; thus, maintenance of cellular homeostasis relies on upregulation of ferritin when HO-1 transcription is increased 93–95. Iron-mediated oxidative damage increases heme with a concomitant increase in ferritin 96–98. A cytoprotective effect of ferritin is related to the ferritin H chain, which controls the pro-oxidant effect of iron released during heme degradation by HO-1 99,100. Induction of HO-1 is associated with an increase of ferritin synthesis and the subsequent chelation of iron by ferritin to prevent iron toxicity 94,96. Recently, H-ferritin feroxidate was shown to induce cytoprotection in sickle cell mice. Mice lacking ferritin are more sensitive to doxorubicin induced cellular injury 101. A clear relationship exists between HO-1 and an increase in ferritin and the prevention of inflammation involving janus kinase (JNK) activation 99. HO-1 and hemoglobin scavenger receptors play a role as anti-inflammatory agents in human coronary stable and unstable plaques resulting in an atheroprotection effect 102.
Impact of Heme on Adipocyte Differentiation-Adipogenesis and Obesity
The discovery by London’s group in 1981 that heme is essential for the increase in pre-adipocyte differentiation and adipogenesis 12 was followed by the elegant work of the Burris group showing that synthesis and an increase of heme is associated with recruitment of REV-ERB ligands and an increase in adipogenesis 13. This suggests that heme metabolism plays an important role in adiposity and the discovery of new targets in HO-1 signaling may prove useful for the treatment of metabolic disease (Figure 3). More recently, the heme-mediated increase of 3T3- cell differentiation was shown to be dependent on suppression of sirtuin 1 (SIRT1) 103. The effect of heme on cell differentiation was not limited to 3T3 cells as adipocyte as heme is indispensable for hematopoietic stem cells differentiation to myeloid and erythroid cell linages 104,105. While the decrease of heme by the increase in heme degradation by HO-1 decreases adipogenesis, it increases osteoblast differentiation 106,107. These unique findings opened a new avenue of investigation on the effect of heme and HO-1 expression on metabolic diseases. Similar to HO-1 overexpression, EET was shown to increase osteoblast differentiation but to decrease adipocyte differentiation 107–110. This finding was strengthened by the observation that administration of CoPP both affected adipocyte differentiation in adult rats and resulted in prompt weight loss, without a decrease in food consumption as measured by pair fed analysis 111. The beneficial effect of CoPP to increase HO-1 levels and reduce adiposity is shared by other pharmacological agents including hemin, Apo-A1 mimetic peptide L-4F and D-4F, EET and peroxisome proliferators-activated receptors alpha. HO-1 expression is also transcriptionally regulated by PPARα and PPARγ, indicating a mechanism of antiinflammatory and antiproliferative action of PPAR ligands involved upregulation of HO-1112. Hemin, EET, and L-4F are also associated with a decrease in visceral subcutaneous fat and an increase in insulin sensitivity 24,112–115, as well as a decrease in the number of large adipocytes (differentiated adipocytes) and an increase in the number of smaller healthy adipocytes 51,109,110,116. CoPP or an analogue may have cardiovascular benefit as CoPP-mediated induction of HO-1 attenuates inflammatory markers and hypertension 117 and reduces glomerular injury 118 and obstructive nephropathy 119. In addition, CoPP induction of HO-1 protects skeletal muscle and ameliorates high fat diet induced liver injury 120–122 in an SIRT-1-dependent manner. Other reports establish that induction of HO-1 by CoPP decreased circulating free fatty acids and C-reactive protein and increased adiponectin through the activation of the AMPK-P13K- eNOS pathway 51,113,115,116, toll-like receptor 4 signaling 123, and adiponectin- downstream signaling pAMPK-AKT and peNOS pathways 14,115 . CoPP-mediated induction of HO-1 provided renal and vascular protection6,124,125. It should be emphasized that HO-1 does not directly increase adiponectin per se; the HO-1-mediated antioxidant mechanism and decreases in heme associated with an increase in the levels of glutathione and superoxide dismutase decreased levels of ROS results in increased levels of adiponectin 10,24,113,114,126,127.
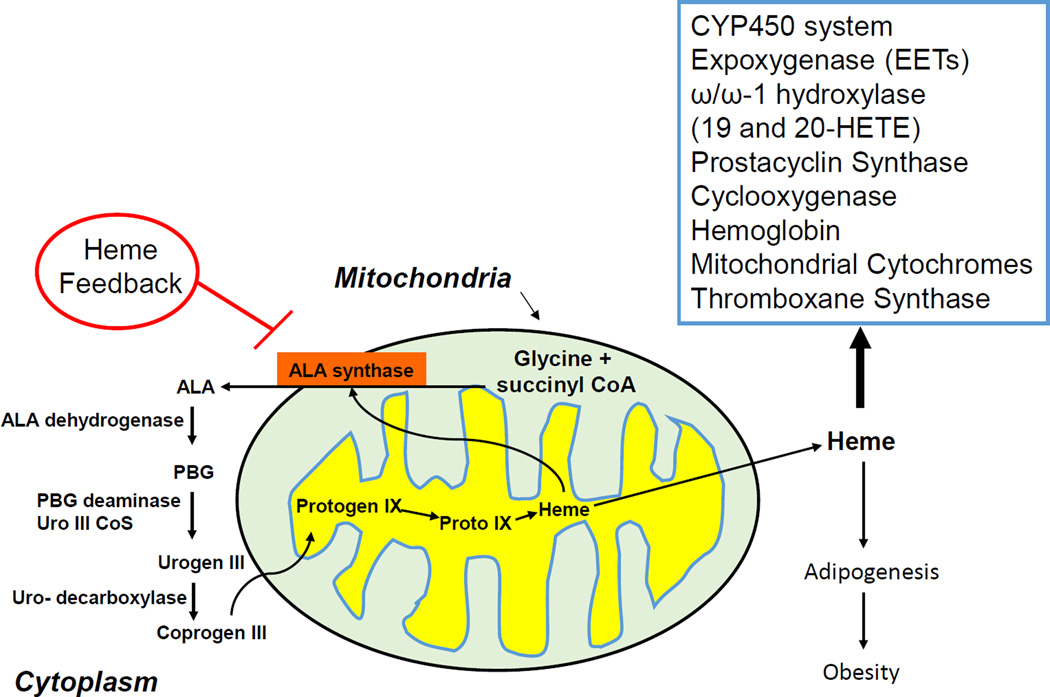
Figure 3.
Heme synthethic pathway. The rate-limiting synthetic enzymes are believed to be ALA synthase and, in part, porphobilinogen deaminase (PBGD). Both enzymes exist in adipocytes and in erythroid and non-erythroid forms. In non-erythroid cells such as liver, kidney, heart, ALA synthase essentially plays a housekeeping role, maintaining intracellular heme levels. High levels of heme thus repress the synthesis of ALA synthase while stimulating heme degradation through the induction of HO-1 12,228. In the origin of hematopoietic-derived cells such as adipocyte and erythroid cells, heme is essential for cellular proliferation and differentiation 12,14,14,228 and increase ALA synthase mRNA levels and enzyme activity. Further, excess heme enhances the synthesis of globin mRNA 14 An iron-binding element has been located on the 5’ untranslated region of the erythroid type cells ALA synthase, so it is possible that the enzyme is actually regulated by intracellular levels of iron. Thus, an increase in heme may induce HO, increasing the levels of free iron which in turn stimulate the formation of adipocyte ALA synthase mRNA and adipocyte during differentiations.
HO, Inflammation and Vascular Disease
The protective role of HO-1 and CO on inflammation occurs in many different disease models including ethanol-induced liver cell death 128. Resveratrol both in vitro and in vivo upregulates HO-1 expression, NAD(P)H; quinone oxidoreductase 1, gamma glutamylcysteine synthetase via activation of nuclear factor (erythroid-derived)-like 2 (Nrf2) target genes. Resveratrol administered to mice fed a high fat diet attenuated oxidative stress and improved acetylcholine-induced vasodilation. The beneficial effects of resveratrol were attenuated in Nrf(−/−) mice fed a high-fat diet, indicating that the endothelial protective function of resveratrol is mediated by the activation of Nrf2 129. Resveratrol as well as other natural HO-1 inducers are reported to prevent CVD 130. The beneficial effect of HO-1 induction along with a subsequent increase in adiponectin and EET production is not limited to obesity. The HO-1-TTP signaling pathway has been shown to be effective in: i) treatment of inflammatory diseases 131; ii) induction of mitochondrial biogenesis 132; iii) preservation of cardiac function 133; iv) down regulation of inflammatory response to osteoarthritis 134; v) decreasing LPS-induced vascular inflammation triggered by bacterial infection; vi) suppression of macrophages migration 135 and v) decreasing contact hypersensitivity 136 and regulators of pregnancy and preeclampsia, meaing it may serve as a valuable therapeutic tool in the management of a momentous health problem 137. Several studies have indicated that the absence of HO-1 exacerbated ventricle dilation in hypoxia 138, atherosclerosis and vascular remodeling 138. CoPP-mediated vascular and cardioprotection is well described 139,140 and includes the effect of CO releasing molecules that induce cardioprotection 141. In HO-1 deficient mice there is a reduction of the proliferative response to vascular injury, while an increase of HO-1 expression inhibited lesion formation 30,
Taken together, these results demonstrate that HO-1 levels determine atherosclerotic lesion progression 142 and that the induction of the HO-1 pathway provides an important adaptive mechanism to reduce the severity of vascular dysfunction, thus representing a potential therapeutic target for vascular diseases. Li et al 143 discovered a previously unrecognized EET pathway to affect or increase hematopoietic stem cell transplant associated with activation of transcriptional factor AP-1. Since AP-1 is present in humans in the HO-1 promoter; this pathway activates HO-1 gene expression 144,145. HO-1 expression is essential for stem cell differentiation to the osteoblast lineage, consistent with the role of heme and HO-1 in differentiation of hematopoietic stem cell 104–107. HETE and EET modulate adherent stromal stem cells differentiation 146.
Impact of HO-1 /HO-2 on Hypertension
The biological action of HO-1 and HO-2 gene expression suggests their capacity to participate in the regulation of renal function and blood pressure 147–152. HO-2 deficiency contributes to a diabetes-mediated increase in superoxide anion and renal dysfunction 39. Inhibitors of HO activity exacerbate salt-sensitive hypertension in Dahl salt-sensitive rats via inhibition of the pressure-natriuretic response 153. Inhibition of HO activity can blunt pressure-natriuresis via two mechanisms, with the first being a decrease in renal blood flow, implying that the renal HO system supports renal circulation via formation of CO 154–156. This hypothesis is supported by the observation that upregulation of HO-1 expression by both CoPP 157 and SnCl2 increases mesenteric artery relaxation in spontaneously hypertensive rats (SHR) and decreases the CYP4A-mediated generation of vasoconstrictors by 20-HETE, and that HO-1-derived CO counterbalances 20-HETE mediated vasoconstriction 158. HO-1 can also regulate renal tubular function by regulating ROS production in renal tubules and by regulation of renal sodium transporters such as the NKCC2 channel of the thick ascending loop of Henle159.
HO and Regulation of Lipid-mediators Signaling in Hypertension and Obesity
The cytochrome P450 (CYP) monooxygenases/epoxygenases family is responsible for formation of 20-HETE and EETs 160,161. Upon formation, EETs are subjected to rapid hydrolysis by epoxide hydrolases and ROS (preventable by HO-1 induction) to their respective dihydroxyepoxytrienoic acids (DHETs), as well as to esterification primarily to glycerophospholipids. Vasodilatory, anti-inflammatory and anti-apoptotic actions of EETs are well established and it is well documented that sEH inhibition significantly increases cellular and circulating EET levels 162,163. An effect of HETE and EET on stromal adherent stem cells differentiation (i.e., mesenchymal stem cells), was first reported in 1991 146. In contrast EET increases adipocyte proliferation but may inhibit differentiation and hypertrophy in vitro and in vivo 40,109,110,116,164 by reprogramming adipocyte phenotype to increase uncoupling proteins (UCP1) and (UCP2) expression and decrease PPARγ and mesoderm-specific transcript (MEST) 40,109,110,116,164,165. UCP1 and UCP2 are expressed in adipose tissue that plays an important role in the control of energy expenditure by uncoupling respiration from ATP synthesis, thereby dissipating energy as heat and affecting energy metabolism efficiency 166. Thus, EET and HO-1 appear to form a module that serves as a molecular "switch" to genetically reprogram the adipocyte phenotype to express lower levels of MEST and prevent hypoadiponectinemia 109,110,116. EETs is the first lipid-mediator derived from lipid metabolism via the CYP system shown to regulate insulin sensitivity and abate obesity associated adipose tissue and vascular dysfunction; an effect which is reversed by the inhibition of HO activity 165.
The mechanism by which EET increases HO-1 could be related to an EET-mediated decrease in Bach1, which is a known supressor of HO-1 gene expression. It is also possible that EETs act as a transcriptional regulator of the HO-1 promoter through glucocorticoid and AP-1 binding sites which are present on the human promoter 144. These binding sites can activate HO-1 gene expression 144 and subsequently increase HO activity. Recently, a novel EET isomer, 11,12-EET, was reported to promote hematopoietic stem cell transplant by activating a unique activation protein 1 and activation of P13K pathway 143. This novel pathway of EET activation of HO-1 may lead to clinical application for EETs and drug development.
It is clear that the pleiotropic effect of the HO system and its subsequent signalling mechanisms leads to increases in EETs, adiponectin and NO bioavailability. Activation of EETs can also increase HO activity as outlined above. The antioxidant action of HO metabolites is associated with expansion of small adipocytes which are associated with increased adiponectin and downstream signals that include phosphorylated liver kinase B1 (pLKB1), pAMPK, phosphorylated endothelial nitric oxide synthase (peNOS) and an increase in NO bioavailability 108,110,113,165,167. Upregulation of these pathways is associated with improvement of vascular function and attenuation of hypertension. It is evident that the pleiotropic effect of the HO system and signalling mechanism 11,51,71,165,168 and increase in biliverdin leads to increases in the protection of EET from degradation by ROS and adiponectin. These results establish the interdependence of four protective pathways, namely HO, EETs, bilirubin and adiponectin, all of which are affected by HO activity and result in the prevention of obesity, hypertension and insulin resistance. Activation of these pathways also protects the vasculature from injury which is known to increase organ dysfunction and vascular diseases (Figure 1 and 4).
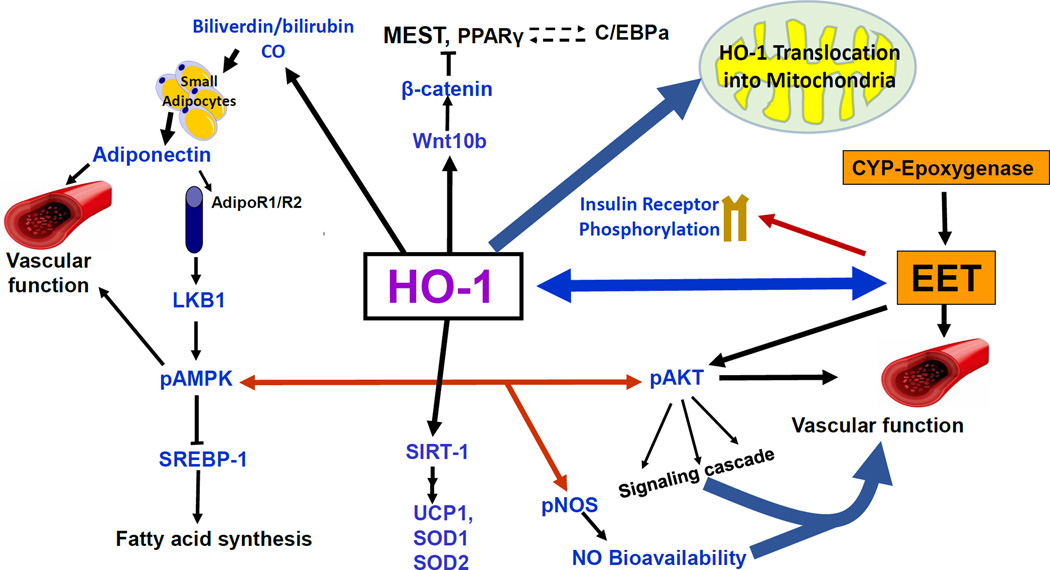
Figure 4.
Schematic representation of the potential mechanism of HO-1 signaling pathways. HO-1 signaling pathways act to improve vascular function and attenuate adiposity adipocyte differentiation. Some of these signaling targets are insulin receptors, adiponectin, via an increase in small adipocytes, EET, SIRT-1, Wnt10b, and β-catenin. The decrease in ROS as a result of an increase of HO-1 and HO-1 derived biliverdin/bilirubin provides stability to EET, leading to an enhancement of insulin sensitivity and an increase in vascular function. HO-1 also translocates into the mitochondria, increasing mitochondrial biogenesis and transport carriers and decreasing mitochondrial ROS 185,189,190.
HO-1/HO-2 and Health Impact
Obesity is a critical risk factor for endothelial dysfunction and the subsequent development of diabetes mellitus and vascular diseases including hypertension. Abdominal obesity is associated with insulin resistance and the pathogenesis of T2DM and hypertension, contributes to high serum levels of LDL and triglycerides but low serum levels of HDL, and leads to the development of atherosclerotic CVD 169–171. HO-1-mediated decreases ROS and of LDL were reported in a number of diabetes models 113,172. Leptin-deficient mice or mice fed a high-fat diet exhibit a metabolic syndrome-like phenotype which includes an increase in LDL which is amenable to rescue by increases in HO-1 and adiponectin 113. Chronic HO-1 induction increased oxygen consumption, and lowered body weight in obese melanocortin-4 receptor deficient mice with an improvement in vascular function 86,173. Induction of HO-1 by several inducers such L-4F, CoPP, heme, or by gene transfer 4,113,114,172,174–176 is associated with an increased number of healthy adipocytes, a concomitant increase of plasma adiponectin levels, improved insulin sensitivity and a decrease in inflammatory adipokines and blood pressure 20,24,115,172,174,175,177. This effect of HO-1 induction on adipocyte morphology was confirmed in Zucker diabetic rats 175 and extended to ob/ob diabetic mice, where increased levels of HO-1 and HO activity prevented weight gain and decreased visceral and subcutaneous fat levels. Additional studies have reported that upregulation of HO-1 decreases adipogenesis in mesenchymal stem cells (MSCs) and increases adiponectin levels in culture media, which is reversed by the inhibition of HO activity 4,114. These studies confirm the existence of an HO-1-adiponectin-EET regulatory module that can be manipulated to ameliorate the deleterious effects of obesity, diabetes and metabolic syndrome. This offers a portal into the therapeutic benefits of the upregulation of HO-1 and increase in HO activity. Chronic HO-1 induction also increases oxygen consumption, which is another mechanism by which HO-1 induction lowers body weight. This effect on oxygen consumption is independent of the melanocortin-4 system as chronic treatment of obese melanocortin-4 receptor deficient mice results in the attenuation of obesity and type II diabetes 86,173. The effect of HO-1 induction of oxygen consumption is likely mediated through increases in CO release as chronic treatment with a CORM increases oxygen consumption and attenuates obesity in mice fed a high fat diet 82.
While the general induction of HO-1 has been reported to result in improved insulin sensitivity, downregulation of the peripheral endocannabinoid system, and a reduction in adipose tissue volume and adipose tissue remodeling, there have been some sex dependent differences reported 113,115. Adipocyte HO-1 induction by CoPP, L-4F or VECAD-HO-1 attenuated metabolic syndrome in both obese male and female mice although the rate of weight gain was slowed only in obese male. 113,114,178. These results emphasize that gender differences must be considered in the development of therapeutic approaches targeting induction of HO-1 for the treatment of obesity and diabetes 113.
Another approach which may be beneficial for the treatment of obesity is adipose-specific induction of HO-1. Recently, it was reported that induction of HO-1 in adipocytes was able to reverse the detrimental metabolic consequences of obesity, including insulin resistance and dyslipidemia as well as decreasing blood pressure in a mouse model of obesity 113. These studies further highlight the protective cardiovascular role of the HO-1-adiponectin axis in hypertensive animals 172. While adipose specific targeting of the HO-1 gene was successful in attenuating adiposity, vascular dysfunction and hypertension in mice fed a high-fat diet, others reported that HO-1 overexpression in adipocytes does not protect against a high-fat diet induced obesity and the development of insulin resistance 179 These differences in phenotypes reported between these two studies are not clear, although the specific activity of HO in the adipose tissue of the transgenic model was not reported. The importance of measuring not only HO-1 protein levels but also HO activity was highlighted in a study in ZDF 20,21. In this study, the onset of diabetes coincided with an increase of HO-1 protein and a paradoxal decrease in HO activity 20, most likely due to an increase of peroxynitrite 20,21 which results in an inactivation of HO activity.
Recently, HO-1 overexpression has been shown to ameliorate the development of non-alcoholic fatty liver disease in obese leptin deficient mice via a decrease in hepatic heme 22. In addition, HO-1 induction decreased hepatic lipid droplet size, fatty acid synthase levels, PPARα and glucose transporter 1 and all of these beneficial effects were reversed by inhibition of HO activity indicating that low levels of HO-1 and HO activity exacerbate the development of obesity induced fatty liver 22. As in 3T3 adipocyte cells, an increase in heme increases adipogenesis 12,13 without upregulation of HO-1 13; however, chronic induction of HO-1 decreases adipocyte heme which in turn decreases adipogenesis 110. This decrease in adipogenesis is also associated with an increase in the levels of CYP-epoxygenase-derived EETs and adiponectin 164.
A number of clinical studies have examined the relationship between HO-1 and obesity. CD163 expression was upregulated in human adipose tissue and soluble CD163 concentration was elevated in obese (BMI > 40 Kg m−2) compared to lean subjects (BMI < 30 Kg M−2). The HO-1 gene was upregulated in adipose tissue and expressed predominantly in macrophages 180,181 and in fat tissues 164. Similarly, diminished upregulation of visceral adipose HO-1 correlates with waist-to-hip ratio and insulin resistance 182. Visceral adipose tissue expression of HMOX1 negatively correlated with insulin resistance 182. Morbid obesity is associated with thrombophilia. Adipocytes obtained from obese patients have increased HO activity as nonsmoking bariatric patients increased COHb concentrations, indicative of HO-1 upregulation 183. Assessment of HO activity by measuring CO production may yield conflicting result unless adequate steps are taken to differentiate between HO-dependent and HO-independent CO generation independent 74,92,184. Rodgers et al 184, described increased CO formation in an HO-independent fashion as a result of photo-oxidation most commonly observed via the peroxidation of lipids along with the auto-oxidation of organic molecules such as phenols and flavenoids as a result of severe stress 184. These differences in HO dependent versus HO independent CO generation should be carefully taken into consideration when interpreting the results of studies in which CO production is measured as an index of HO activity.
Therapeutic Potential of HO-1 and Signaling Pathways
HO-1, as the only enzyme that degrades the pro-oxidant heme and generates antioxidant products, may be a beneficial target to limit the pathogenesis of obesity and diabetes and their complications (Figure 5). In diabetes, increased levels of HO-1 provide vascular cytoprotection against oxidative stress via mechanism(s) that involve improvements in mitochondrial biogenesis and function 127,185. Cardiac mitochondrial damage, such as that seen in type 1 diabetes mellitus (TD1M), is the result of a decrease in glutathione levels which negatively impact the mitochondrial respiration system 186. A deficiency in the deoxynucleotide carrier has been associated with abnormal brain growth, and a deficiency in carnitine-acylcarnitine linked to muscle weakness and cardiomyopathy in diabetes. Diabetic complications have also been related to abnormalities in mitochondrial function 186,187 as well as increased endothelial cell death and detachment 188. Furthermore, translocation of HO-1 into mitochondria 185,189,190 suggests that targeting of HO-1 specifically may prove to be essential in the modulation of the redox state in favor of antioxidants, enhancing mitochondrial transport of substrates and metabolites and restoring mitochondrial function in diabetes 185. Induction of HO-1 has been reported to result in the restoration of six mitochondrial carriers, i.e., carnitine, citrate, phosphate, deoxynucleotide, ATP and dicarboxylate in diabetes 185. An increase in AKT phosphorylation is also critical to cell survival in diabetes 191. The alteration in mitochondrial function in vitro and in vivo has been correlated with the levels of activation of AKT and the BcL-2 family of proteins 192,193. A decrease in BcL-2 family members has been suggested to contribute to apoptosis and the translocation of cytochrome c from the mitochondria to cytosol 191,192,194. Activation of AKT has been reported to augment ATP synthesis 195 and promote association of hexokinase with the voltage-dependent anion channel (VDAC) channel and, in so doing, results in VDAC closure which blocks release of cytochrome c. The lack of HO-1 or HO-2 results in increased apoptotic cell death 19,38,196. While these results suggest that increases in mitochondrial HO-1 may favorably modulate the balance between pro-and anti-apoptotic mechanisms, the clinical applicability of targeting either HO-1 or its metabolites, bilirubin and CO, specifically to the mitochondria has not been tested as therapeutic approach for the treatment of diabetes, although pre-clinical results support such a clinical application 197.
Figure 5.
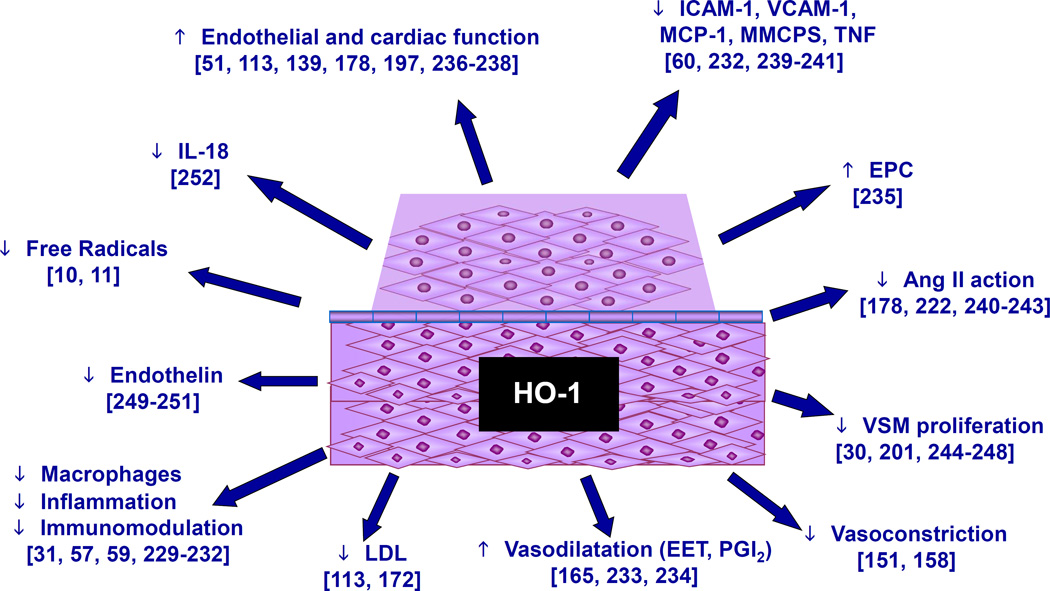
Pleiotropic effect of HO-1 on obesity and CVD. HO-1 has a diverse range of actions on blood vessel endothelial tissue, decreasing inflammation 31,57,59,229–232 and vasoconstriction 151,158 while increasing vasodilation 165,233,234, endothelial progenitor cells 235 and endothelial and cardiac cell function 51,113,139,178,197,236–238. As seen on the scheme, HO-1, bilirubin, and CO decrease pro-inflammatory molecules 60,232,239–241, angiotensin II 178,222,240–243, free radicals 10,11, VSM proliferation 30,201,244–248, LDL 113,172, endothelin 249–251, and IL-18 252. The overall effect is the amelioration of cardiovascular disease and protection against the development of a disease state.
It is of interest that beta cell destruction caused by elevated intracellular levels of ROS, including superoxide radicals, hydrogen peroxide and nitric oxide, is a process that occurs through both apoptotic and necrotic mechanisms 198. T cell-mediated infiltration of the pancreas leads to ROS generation and proinflammatory cytokines. The HO system regulates T cell proliferation and immune response 199,200. CD4+ T cells express HO-1 in response to CoPP, and that the lack of HO-1 modulates T cell proliferation and maturation 201,202. An increase in HO-1 activity resulted in a decrease in infiltrated CD11c+ dendritic cells, and suggested that induction of HO-1 and increased HO activity can prevent the development and/or moderate the diabetic state 14. HO-1 upregulation has proven capable of providing cytoprotection to pancreatic beta cells in vivo 203,204. An increase in HO-1 levels has a salutary effect, modulating the pancreas phenotype and rendering beta cells resistant to oxidant stress and, hence, preventing the development of type 1 diabetes.
A protective effect is also seen in diabetes where insulin induces HO-1 through the pI3K/Akt pathway and the Nrf2 transcription factor in renal cells 205. HO-2 deficiency in diabetic HO-2 knockout mice caused major renal morphological injury and impaired renal function that was rescued by upregulation of HO-1 in the STZ animal model of diabetes 39.
The significant role that HO-1 plays in obesity/diabetes stems from the presence of binding sites for several transcriptional factors including CRE B, OKT1, STATS and glucocorticoid-response elements that are expressed on the human HO-1 promoter 144,145. Targeting HO-1 or the products of heme degradation stems from the finding that induction of HO-1 increases oxygen consumption, heat production and lowers body weight 173. While upregulation of HO-1 reduces body weight in obese animals, it also is associated with a decrease in the levels of adipokines including TNF, IL-6, MCP-1 and an increase of adipocyte secretion of adiponectin. Adiponectin is secreted only by adipose tissue and is associated with decreased body weight 206–209, suggesting that the HO-1-mediated increase bilirubin and the number of smaller healthy adipocyte capable of secreting adiponectin is related to the effect of adiponectin on body weight loss and attenuation of inflammatory process 206–209.
HO-1 Genetic Polymorphism and its Impact on Atherosclerosis and CVD
Genetic polymorphism of the HO-1 gene indicates the potential importance of HO-1 in the pathogenesis of cardiovascular and pulmonary diseases 210. The larger the size of the (GT)n repeats in the HO-1 gene promoter, the greater the chance of reducing HO-1 indelibility by ROS in cigarette smoke and reducing bilirubin, resulting in the development of emphysema 210. Patients with short (<25 GT) dinucleotide repeats in the HO-1 gene promoter on either allele had restenosis significantly less often that patients with longer (≥25 GT) dinucleotide repeats 211. Diabetic patients who have Gilbert syndrome, elevated levels of bilirubin, have a lower rate of vascular complications, compared to normal bilirubin levels seen in diabetes 212. It is believed that shorter (GT)n repeats, compared to longer (GT)n repeats, have a higher transcriptional activity and thus higher HO-1 expression levels. In an Asian population, type-2 diabetics carrying longer (≥32) (GT)n repeats had higher oxidative stress and increased susceptibility to the development of coronary artery disease and atherosclerosis 213. Patients with significant risk factors (hyperlipidemia, diabetes, and smoking) for coronary artery disease and who possessed shorter (<27) (GT)n repeats were associated with less disease 214. A cohort study in patients to evaluate HO-1 gene promoter polymorphisms and the risk for restenosis after percutaneous transluminal angioplasty found that patients with short (<25 GT) dinucleotide repeats in the HO-1 gene promoter on either allele had restenosis significantly less often that patients with longer (≥25 GT) dinucleotide repeats 215. These data imply that up-regulation of HO-1, associated with shorter dinucleotide repeats, may be protective after balloon angioplasty. However, not all studies support the clinical effect of genetic polymorphism, for example, in a study of 1807 patients with coronary artery disease, no clinically relevant association of a HO-promoter polymorphism and ischemic events after coronary stenting was reported 216. In support of this finding, no evidence of a protective effect for short alleles, i.e., low (GT)n repeat, for graft or recipient survival in clinical renal transplant was seen 217. In addition to (GT)n dinucleotide-length polymorphism, a single nucleotide polymorphism in the HO-1 promoter, T(-413)A, correlated with a reduced incidence of ischemic heart disease 218. In a study of 3,104 patients with vascular disease, restenosis after percutaneous coronary intervention was associated with angiotensin II-type l receptor 116 A/C polymorphism but was not associated with polymorphism of HO-1 219. These studies both advocate and/or contradict the role of the HO-1 gene in genetic polymorphism and atherosclerotic processes. Recently, in an elegant well designed study to investigate the role of genetic polymorphism of HO-1 in CVD, the authors focused on the incidence of stroke, MI and vascular death of patients registered between 1995 and 2010. In more than 800 patients aged between 45–84 years 220, there was an association between the HO-1 variable number tandem repeat polymorphism and CVD confined to subjects with a high number of repeats on both HO-1 alleles, providing evidence of atherogenesis and decreased antioxidant defense system in vascular high risk subjects. These studies support an important role of HO-1 gene regulation in the atherosclerotic disease processes.
A new approach would be to examine whether increasing HO-1 expression via either pharmaceutical or genetic agents has the potential to correct for the GT repeat leading to low expression, or whether introducing anti-oxidative agents may correct for the increased oxidative stress caused by low HO-1 expression. Genetic testing may also have a role in identifying patients with HO-1 polymorphisms for which HO-1 based therapies could have a corrective effect.
Concluding Remarks
The pharmaceutical industry may be interested in finding novel drugs with the potential to attenuate adiposity and reprogram adipocyte stem cells to new phenotypes to express HO-1 or HO-1 downstream targets (see Outstanding Questions). A potential inducer of bilirubin and HO-1 is the peptide derived from the human biliverdin reductase protein 221. This peptide, as well as the L-4F (ApoA-1 mimetic) peptide, could have a powerful effect on the induction of HO-1, with a reduction of fatty liver insulin resistance and adiposity. Selective expression of the HO-1 gene has also been identified by numerous sources as a viable method of stimulating HO-1 gene expression 10,47,141,152,178,222–224. Another novel dimension of targeting the bilirubin/biliverdin pathway includes the development of meso-biliverdin as an anti-obesity and anti-vascular disease agent 67,225, as well as the development of a CO-releasing molecule for preconditioning the heart in MI or in the protection of pancreatic function 67. Niacin has additionally been shown to induce the HO-1 gene to counter cardiovascular disease 226 and may have a beneficial effect on obesity-induced hypertension. Further, certain statins have activated the HO-1 promoter in mouse pre-adipocytes, preventing adipocyte differentiation, suggesting this class of drugs can combat adiposity and adipogenesis 227. Additionally, analogs of EET targeting β-catenin/SIRT1 will represent novel small molecules enhancing bilirubin downstream signaling to prevent pre-adipocyte full differentiation and inflammation and protect against obesity and diabetes.
Outstanding Questions.
Is obesity is the hallmark of vascular disease?
Can drugs correct HO-1 genetic polymorphisms resulting in low expression?
Do HO-1 inducing drugs affect people uniformly?
Can genetic testing identify individuals that would be helped by HO-1 inducing drugs?
Are there side effects to HO-1 inducing drugs?
The wide spectrum of inducers of HO-1 highlights the pivotal role this enzyme plays in providing protection against metabolic insults in humans. This offers an obvious target for designing compounds with clinical application in multiple human disease states.
Trend Box.
Obesity is a major risk factor in the development of diabetes, hypertension, fatty liver and CVD.
HO-1/HO-2 catalyzes the breakdown of heme, a potentially harmful pro-oxidant, into potent anti-oxidants such as biliverdin/bilirubin and CO, with an anti-inflammatory effect.
This is the first review to discuss translational research that summarizes human genetic polymorphism of HO-1 and the effectiveness of bilirubin to ameliorate CVD.
This review uncovers a mechanistic link between obesity and the vascular system and provides a conceptual basis for developing new drugs for the management of metabolic syndrome.
We provide a conceptual basis for the development of new therapeutic strategies that target HO-1 and biliverdin to ameliorate obesity, adipocyte (fat stem cell) dysfunction and, vascular dysfunction associated with the metabolic syndrome.
ACKNOWLEDGEMENTS
This work was supported by NIH grants HL55601 and HL34300 (NGA). We thank Mrs. Jennifer Brown for her outstanding assistance in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 3.Fontana L, et al. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 4.Li M, et al. Treatment of obese diabetic mice with a heme oxygenase inducer reduces visceral and subcutaneous adiposity, increases adiponectin levels, and improves insulin sensitivity and glucose tolerance. Diabetes. 2008;57:1526–1535. doi: 10.2337/db07-1764. [DOI] [PubMed] [Google Scholar]
- 5.Briggs DB, et al. Disulfide-dependent self-assembly of adiponectin octadecamers from trimers and presence of stable octadecameric adiponectin lacking disulfide bonds in vitro. Biochemistry (Mosc) 2009;48:12345–12357. doi: 10.1021/bi9015555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51:8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- 7.Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high fat diet hypertensive rats. Clin. Sci. (Lond) 2010;118:291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang MH, et al. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension. 2003;42:594–599. doi: 10.1161/01.HYP.0000090123.55365.BA. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y, et al. The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species. J. Biol. Chem. 2005;280:4617–4626. doi: 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- 10.Abraham NG, et al. Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells. Circ. Res. 2003;93:507–514. doi: 10.1161/01.RES.0000091828.36599.34. [DOI] [PubMed] [Google Scholar]
- 11.Abraham NG, et al. Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes. Am. J Physiol Heart Circ. Physiol. 2004;287:H2468–H2477. doi: 10.1152/ajpheart.01187.2003. [DOI] [PubMed] [Google Scholar]
- 12.Chen JJ, London IM. Hemin enhances the differentiation of mouse 3T3 cells to adipocytes. Cell. 1981;26:117–122. doi: 10.1016/0092-8674(81)90039-8. [DOI] [PubMed] [Google Scholar]
- 13.Kumar N, et al. Regulation of adipogenesis by natural and synthetic REVERB ligands. Endocrinology. 2010;151:3015–3025. doi: 10.1210/en.2009-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 15.Cassis LA, et al. Local adipose tissue renin-angiotensin system. Curr. Hypertens. Rep. 2008;10:93–98. doi: 10.1007/s11906-008-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boustany CM, et al. Activation of the systemic and adipose renin-angiotensin system in rats with diet-induced obesity and hypertension. Am. J Physiol Regul. Integr. Comp Physiol. 2004;287:R943–R949. doi: 10.1152/ajpregu.00265.2004. [DOI] [PubMed] [Google Scholar]
- 17.Chang SH, et al. Glucose deprivation induces heme oxygenase-1 gene expression by a pathway independent of the unfolded protein response. J. Biol. Chem. 2002;277:1933–1940. doi: 10.1074/jbc.M108921200. [DOI] [PubMed] [Google Scholar]
- 18.Chang SH, et al. Haem oxygenase 1 gene induction by glucose deprivation is mediated by reactive oxygen species via the mitochondrial electron-transport chain. Biochem. J. 2003;371:877–885. doi: 10.1042/BJ20021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan S, et al. Heme oxygenase-1 prevents superoxide anion-associated endothelial cell sloughing in diabetic rats. Biochem. Biophys. Res. Commun. 2004;315:509–516. doi: 10.1016/j.bbrc.2004.01.086. [DOI] [PubMed] [Google Scholar]
- 20.Kruger AL, et al. Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol. Exp. Ther. 2006;319:1144–1152. doi: 10.1124/jpet.106.107482. [DOI] [PubMed] [Google Scholar]
- 21.Kinobe R, et al. Peroxynitrite-mediated inactivation of heme oxygenases. BMC. Pharmacol. 2004;4:26. doi: 10.1186/1471-2210-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hinds TD, Jr, et al. Increased HO-1 levels ameliorate fatty liver development through a reduction of heme and recruitment of FGF21. Obesity. (Silver. Spring) 2014;22:705–712. doi: 10.1002/oby.20559. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Abraham NG, et al. Heme oxygenase: a target gene for anti-diabetic and obesity. Curr. Pharm. Des. 2008;14:412–421. doi: 10.2174/138161208783597371. [DOI] [PubMed] [Google Scholar]
- 24.Ndisang JF. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators. Inflamm. 2010;2010:359732. doi: 10.1155/2010/359732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abraham NG, et al. The biological significance and physiological role of heme oxygenase. Cell. Physiol. Biochem. 1996;6:129–168. [Google Scholar]
- 26.Kapitulnik J, Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol. Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Ahmad Z, et al. Human biliverdin reductase is a leucine zipper-like DNA-binding protein and functions in transcriptional activation of heme oxygenase-1 by oxidative stress. J. Biol. Chem. 2002;277:9226–9232. doi: 10.1074/jbc.M108239200. [DOI] [PubMed] [Google Scholar]
- 28.Poss KD, Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. U. S. A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.True AL, et al. Heme Oxygenase-1 Deficiency Accelerates Formation of Arterial Thrombosis Through Oxidative Damage to the Endothelium, Which Is Rescued by Inhaled Carbon Monoxide. Circ. Res. 2007 doi: 10.1161/CIRCRESAHA.107.158998. [DOI] [PubMed] [Google Scholar]
- 30.Duckers HJ, et al. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat. Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 31.Willis D, et al. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med 2. 1996;2:87–90. doi: 10.1038/nm0196-87. [DOI] [PubMed] [Google Scholar]
- 32.Morse D, Choi AM. Heme oxygenase-1: from bench to bedside. Am. J. Respir. Crit Care Med. 2005;172:660–670. doi: 10.1164/rccm.200404-465SO. [DOI] [PubMed] [Google Scholar]
- 33.Lundvig DM, et al. Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly? Front Pharmacol. 2012;3:81. doi: 10.3389/fphar.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yachie A, et al. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kie JH, et al. Heme oxygenase-1 deficiency promotes epithelial-mesenchymal transition and renal fibrosis. J. Am. Soc. Nephrol. 2008;19:1681–1691. doi: 10.1681/ASN.2007101099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kapturczak MH, et al. Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004;165:1045–1053. doi: 10.1016/S0002-9440(10)63365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asterholm IW, Scherer PE. Enhanced metabolic flexibility associated with elevated adiponectin levels. Am. J Pathol. 2010;176:1364–1376. doi: 10.2353/ajpath.2010.090647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dennery PA, et al. Oxygen toxicity and iron accumulation in the lungs of mice lacking heme oxygenase-2. J. Clin. Invest. 1998;101:1001–1011. doi: 10.1172/JCI448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman AI, et al. Heme oxygenase-2 deficiency contributes to diabetes-mediated increase in superoxide anion and renal dysfunction. J. Am. Soc. Nephrol. 2006;17:1073–1081. doi: 10.1681/ASN.2004121082. [DOI] [PubMed] [Google Scholar]
- 40.Sodhi K, et al. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J. Pharmacol. Exp. Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts CK, et al. Oxidative stress and dysregulation of NAD(P)H oxidase and antioxidant enzymes in diet-induced metabolic syndrome. Metabolism. 2006;55:928–934. doi: 10.1016/j.metabol.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 42.Kappas A, Drummond G. Control of heme metabolism with synthetic metalloporphyrins. J. Clin. Invest. 1986;77:335–339. doi: 10.1172/JCI112309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kappas A. A method for interdicting the development of severe jaundice in newborns by inhibiting the production of bilirubin. Pediatrics. 2004;113:119–123. doi: 10.1542/peds.113.1.119. [DOI] [PubMed] [Google Scholar]
- 44.Drummond GS, Kappas A. Chemoprevention of neonatal jaundice: potency of tin-protoporphyrin in an animal model. Science. 1982;217:1250–1252. doi: 10.1126/science.6896768. [DOI] [PubMed] [Google Scholar]
- 45.Lin JH, et al. Regulation of heme oxygenase gene expression by cobalt in rat liver and kidney. Eur. J Biochem. 1990;192:577–582. doi: 10.1111/j.1432-1033.1990.tb19263.x. [DOI] [PubMed] [Google Scholar]
- 46.Jazwa A, et al. HIF-regulated HO-1 gene transfer improves the post-ischemic limb recovery and diminishes TLR-triggered immune responses - Effects modified by concomitant VEGF overexpression. Vascul. Pharmacol. 2015;71:127–138. doi: 10.1016/j.vph.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, et al. Gene transfer as a strategy to achieve permanent cardioprotection II: rAAV-mediated gene therapy with heme oxygenase-1 limits infarct size 1 year later without adverse functional consequences. Basic Res. Cardiol. 2011;106:1367–1377. doi: 10.1007/s00395-011-0208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du D, et al. Adenovirus-mediated heme oxygenase transfer inhibits graft arteriosclerosis in rat aortic transplants. Transplant. Proc. 2007;39:3446–3448. doi: 10.1016/j.transproceed.2007.03.114. [DOI] [PubMed] [Google Scholar]
- 49.Liu X, et al. Preemptive heme oxygenase-1 gene delivery reveals reduced mortality and preservation of left ventricular function 1 yr after acute myocardial infarction. Am. J. Physiol Heart Circ. Physiol. 2007;293:H48–H59. doi: 10.1152/ajpheart.00741.2006. [DOI] [PubMed] [Google Scholar]
- 50.Chen W, et al. CYP2J2 and EETs protect against lung ischemia/reperfusion injury via anti-inflammatory effects in vivo and in vitro. Cell Physiol Biochem. 2015;35:2043–2054. doi: 10.1159/000374011. [DOI] [PubMed] [Google Scholar]
- 51.Cao J, et al. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension. 2012;60:467–475. doi: 10.1161/HYPERTENSIONAHA.112.193805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stocker R, et al. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 53.Hopkins PN, et al. Higher serum bilirubin is associated with decreased risk for early familial coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 1996;16:250–255. doi: 10.1161/01.atv.16.2.250. [DOI] [PubMed] [Google Scholar]
- 54.Verma A, et al. Carbon monoxide: a putative neural messenger. Science. 1993;259:381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- 55.Otterbein LE, Choi AM. Heme oxygenase: colors of defense against cellular stress. Am. J. Physiol Lung Cell Mol. Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 56.Zhan Y, et al. Role of JNK, p38, and ERK in platelet-derived growth factor-induced vascular proliferation, migration, and gene expression. Arterioscler. Thromb. Vasc. Biol. 2003;23:795–801. doi: 10.1161/01.ATV.0000066132.32063.F2. [DOI] [PubMed] [Google Scholar]
- 57.Otterbein LE, et al. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 58.Kim HP, et al. CO as a cellular signaling molecule. Annu. Rev. Pharmacol. Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 59.Ryter SW, et al. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 60.Kushida T, et al. TNF-alpha-mediated cell death is attenuated by retrovirus delivery of human heme oxygenase-1 gene into human microvessel endothelial cells. Transplant. Proc. 2002;34:2973–2978. doi: 10.1016/s0041-1345(02)03506-6. [DOI] [PubMed] [Google Scholar]
- 61.Fondevila C, et al. Biliverdin therapy protects rat livers from ischemia and reperfusion injury. Hepatology. 2004;40:1333–1341. doi: 10.1002/hep.20480. [DOI] [PubMed] [Google Scholar]
- 62.Lee SS, et al. Hemeoxygenase-1, carbon monoxide, and bilirubin induce tolerance in recipients toward islet allografts by modulating T regulatory cells. FASEB J. 2007 doi: 10.1096/fj.07-8472com. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, et al. Bilirubin can induce tolerance to islet allografts. Endocrinology. 2006;147:762–768. doi: 10.1210/en.2005-0632. [DOI] [PubMed] [Google Scholar]
- 64.O’Brien L, et al. Biliverdin reductase isozymes in metabolism. Trends Endocrinol. Metab. 2015;26:212–220. doi: 10.1016/j.tem.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ding B, et al. The coordinated increased expression of biliverdin reductase and heme oxygenase-2 promotes cardiomyocyte survival: a reductase-based peptide counters beta-adrenergic receptor ligand-mediated cardiac dysfunction. FASEB J. 2011;25:301–313. doi: 10.1096/fj.10-166454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J, et al. Unconjugated bilirubin mediates heme oxygenase-1-induced vascular benefits in diabetic mice. Diabetes. 2014 doi: 10.2337/db14-1391. [DOI] [PubMed] [Google Scholar]
- 67.Ito T, et al. Mesobiliverdin IXalpha Enhances Rat Pancreatic Islet Yield and Function. Front Pharmacol. 2013;4:50. doi: 10.3389/fphar.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dong H, et al. Bilirubin increases insulin sensitivity in leptin-receptor deficient and diet-induced obese mice through suppression of ER stress and chronic inflammation. Endocrinology. 2014;155:818–828. doi: 10.1210/en.2013-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark JE, et al. Heme oxygenase-1-derived bilirubin ameliorates postischemic myocardial dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H643–H651. doi: 10.1152/ajpheart.2000.278.2.H643. [DOI] [PubMed] [Google Scholar]
- 70.Hill-Kapturczak N, et al. Heme oxygenase and the kidney. DNA Cell Biol. 2002;21:307–321. doi: 10.1089/104454902753759726. [DOI] [PubMed] [Google Scholar]
- 71.Abraham NG, Kappas A. Heme oxygenase and the cardiovascular-renal system. Free Radic. Biol. Med. 2005;39:1–25. doi: 10.1016/j.freeradbiomed.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Vitek L. The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol. 2012;3:55. doi: 10.3389/fphar.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Neuzil J, Stocker R. Free and albumin-bound bilirubin are efficient co-antioxidants for alpha-tocopherol, inhibiting plasma and low density lipoprotein lipid peroxidation. J. Biol. Chem. 1994;269:16712–16719. [PubMed] [Google Scholar]
- 74.Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 2005;57:585–630. doi: 10.1124/pr.57.4.3. [DOI] [PubMed] [Google Scholar]
- 75.Oury TD, et al. Cold-induced brain edema in mice. Involvement of extracellular superoxide dismutase and nitric oxide. J. Biol. Chem. 1993;268:15394–15398. [PubMed] [Google Scholar]
- 76.Lipton SA, et al. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- 77.Friebe A, Koesling D. Mechanism of YC-1-induced activation of soluble guanylyl cylase. (53 edn) 1998:123–127. doi: 10.1124/mol.53.1.123. [DOI] [PubMed] [Google Scholar]
- 78.Furchgott RF, Jothianandan D. Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light. Blood Vessels. 1991;28:52–61. doi: 10.1159/000158843. [DOI] [PubMed] [Google Scholar]
- 79.Leffler CW, et al. Carbon monoxide and cerebral microvascular tone in newborn pigs. Am. J. Physiol. 1999;276:H1641–H1646. doi: 10.1152/ajpheart.1999.276.5.H1641. [DOI] [PubMed] [Google Scholar]
- 80.Czibik G, et al. Heme oxygenase-1: an emerging therapeutic target to curb cardiac pathology. Basic Res. Cardiol. 2014;109:450. doi: 10.1007/s00395-014-0450-9. [DOI] [PubMed] [Google Scholar]
- 81.Motterlini R, Foresti R. Heme oxygenase-1 as a target for drug discovery. Antioxid. Redox. Signal. 2014;20:1810–1826. doi: 10.1089/ars.2013.5658. [DOI] [PubMed] [Google Scholar]
- 82.Hosick PA, et al. Chronic carbon monoxide treatment attenuates development of obesity and remodels adipocytes in mice fed a high-fat diet. Int. J. Obes. (Lond) 2014;38:132–139. doi: 10.1038/ijo.2013.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hosick PA, et al. Chronic treatment with a carbon monoxide releasing molecular revereses dietary induced obesity in mice. Adipocyte. 2016;5:1–10. doi: 10.1080/21623945.2015.1038443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lundquist I, et al. Carbon monoxide stimulates insulin release and propagates Ca2+ signals between pancreatic beta-cells. Am. J. Physiol Endocrinol. Metab. 2003;285:E1055–E1063. doi: 10.1152/ajpendo.00498.2002. [DOI] [PubMed] [Google Scholar]
- 85.Brouard S, et al. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J. Biol. Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- 86.Hosick PA, Stec DE. Heme oxygenase, a novel target for the treatment of hypertension and obesity? Am. J. Physiol Regul. Integr. Comp Physiol. 2012;302:R207–R214. doi: 10.1152/ajpregu.00517.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Balla G, et al. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab. Invest. 1991;64:648–654. [PubMed] [Google Scholar]
- 88.Valko M, et al. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005;12:1161–1208. doi: 10.2174/0929867053764635. [DOI] [PubMed] [Google Scholar]
- 89.Grunblatt E, et al. The link between iron, metabolic syndrome, and Alzheimer's disease. J. Neural Transm. 2011;118:371–379. doi: 10.1007/s00702-010-0426-3. [DOI] [PubMed] [Google Scholar]
- 90.Ferris CD, et al. Haem oxygenase-1 prevents cell death by regulating cellular iron. Nat. Cell Biol. 1999;1:152–157. doi: 10.1038/11072. [DOI] [PubMed] [Google Scholar]
- 91.Ponka P, et al. Function and regulation of transferrin and ferritin. Semin. Hematol. 1998;35:35–54. [PubMed] [Google Scholar]
- 92.Nagababu E, Rifkind JM. Heme degradation by reactive oxygen species. Antioxid. Redox. Signal. 2004;6:967–978. doi: 10.1089/ars.2004.6.967. [DOI] [PubMed] [Google Scholar]
- 93.Balla J, et al. Endothelial cell heme oxygenase and ferritin induction by heme proteins: a possible mechanism for limiting shock damage. Trans. Assoc. Am. Phys. 1992;105:1–7. [PubMed] [Google Scholar]
- 94.Eisenstein RS, et al. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc. Natl. Acad. Sci. U. S. A. 1991;88:688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen YH, et al. Serum bilirubin and ferritin levels link heme oxygenase-1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care. 2008;31:1615–1620. doi: 10.2337/dc07-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vile GF, Tyrrell RM. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J. Biol. Chem. 1993;268:14678–14681. [PubMed] [Google Scholar]
- 97.Ren H, et al. Induction of haem oxygenase-1 causes cortical non-haem iron increase in experimental pneumococcal meningitis: evidence that concomitant ferritin up-regulation prevents iron-induced oxidative damage. J. Neurochem. 2007;100:532–544. doi: 10.1111/j.1471-4159.2006.04230.x. [DOI] [PubMed] [Google Scholar]
- 98.Erdmann K, et al. L-methionine reduces oxidant stress in endothelial cells: role of heme oxygenase-1, ferritin, and nitric oxide. AAPS. J. 2005;7:E195–E200. doi: 10.1208/aapsj070118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gozzelino R, Soares MP. Coupling heme and iron metabolism via ferritin H chain. Antioxid. Redox. Signal. 2014;20:1754–1769. doi: 10.1089/ars.2013.5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Balla G, et al. Ferritin: a cytoprotective antioxidant strategm of endothelium. J. Biol. Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 101.Maccarinelli F, et al. Mice lacking mitochondrial ferritin are more sensitive to doxorubicin-mediated cardiotoxicity. J. Mol. Med. (Berl) 2014;92:859–869. doi: 10.1007/s00109-014-1147-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yunoki K, et al. Association between hemoglobin scavenger receptor and heme oxygenase-1-related anti-inflammatory mediators in human coronary stable and unstable plaques. Hum. Pathol. 2013;44:2256–2265. doi: 10.1016/j.humpath.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 103.Puri N, et al. Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J. Cell Biochem. 2012;113:1926–1935. doi: 10.1002/jcb.24061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chertkov JL, et al. Hemin stimulation of hemopoiesis in murine long-term bone marrow culture. Exp. Hematol. 1991;19:905–909. [PubMed] [Google Scholar]
- 105.Lutton JD, et al. Synergistic effect of heme and IL-1 on hematopoietic stromal regeneration after radiation. Am. J Hematol. 1993;44:172–178. doi: 10.1002/ajh.2830440307. [DOI] [PubMed] [Google Scholar]
- 106.Barbagallo I, et al. Overexpression of heme oxygenase-1 increases human osteoblast stem cell differentiation. J Bone Miner Metab. 2010;28:276–288. doi: 10.1007/s00774-009-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vanella L, et al. HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236–243. doi: 10.1016/j.bone.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burgess AP, et al. Heme oxygenase (HO-1) rescue of adipocyte dysfunction in HO-2 deficient mice via recruitment of epoxyeicosatrienoic acids (EETs) and adiponectin. Cell Physiol Biochem. 2012;29:99–110. doi: 10.1159/000337591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim DH, et al. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev. 2010;19:1863–1873. doi: 10.1089/scd.2010.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vanella L, et al. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 2011;96:54–62. doi: 10.1016/j.prostaglandins.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Galbraith RA, Kappas A. Regulation of food intake and body weight in rats by the synthetic heme analogue cobalt protoporphyrin. Am J Physiol. 1991;261:R1388–R1394. doi: 10.1152/ajpregu.1991.261.6.R1388. [DOI] [PubMed] [Google Scholar]
- 112.Kronke G, et al. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler. Thromb. Vasc. Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- 113.Burgess A, et al. Adipocyte heme oxygenase-1 induction attenuates metabolic syndrome in both male and female obese mice. Hypertension. 2010;56:1124–1130. doi: 10.1161/HYPERTENSIONAHA.110.151423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peterson SJ, et al. L-4F treatment reduces adiposity, increases adiponectin levels, and improves insulin sensitivity in obese mice. J. Lipid Res. 2008;49:1658–1669. doi: 10.1194/jlr.M800046-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nicolai A, et al. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 2009;53:508–515. doi: 10.1161/HYPERTENSIONAHA.108.124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vanella L, et al. Increased heme-oxygenase 1 expression decreases adipocyte differentiation and lipid accumulation in mesenchymal stem cells via upregulation of the canonical Wnt signaling cascade. Stem Cell Res. Ther. 2013;4:28. doi: 10.1186/scrt176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Elmarakby AA, et al. Induction of hemeoxygenase-1 reduces renal oxidative stress and inflammation in diabetic spontaneously hypertensive rats. Int. J. Hypertens. 2012;2012:957235. doi: 10.1155/2012/957235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Elmarakby AA, et al. Induction of hemeoxygenase-1 reduces glomerular injury and apoptosis in diabetic spontaneously hypertensive rats. Am. J. Physiol Renal Physiol. 2012;302:F791–F800. doi: 10.1152/ajprenal.00472.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Iwai T, et al. Cobalt protoporphyrin attenuates rat obstructive nephropathy: role of cellular infiltration. Urology. 2008;72:432–438. doi: 10.1016/j.urology.2007.11.123. [DOI] [PubMed] [Google Scholar]
- 120.Liu X, et al. Sirt1 Mediates the Effect of the Heme Oxygenase Inducer, Cobalt Protoporphyrin, on Ameliorating Liver Metabolic Damage Caused by a High-fat Diet. J. Hepatol. 2015 doi: 10.1016/j.jhep.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 121.Sodhi K, et al. Fructose Mediated Non-Alcoholic Fatty Liver Is Attenuated by HO-1-SIRT1 Module in Murine Hepatocytes and Mice Fed a High Fructose Diet. PLoS. One. 2015;10:e0128648. doi: 10.1371/journal.pone.0128648. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 122.Sodhi K, et al. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prost. Other Lipid Mediat. 2012;98:133–142. doi: 10.1016/j.prostaglandins.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vivot K, et al. Pro-inflammatory and pro-oxidant status of pancreatic islet in vitro is controlled by TLR-4 and HO-1 pathways. PLoS. One. 2014;9:e107656. doi: 10.1371/journal.pone.0107656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55:2319–2326. doi: 10.1007/s00125-012-2598-x. [DOI] [PubMed] [Google Scholar]
- 125.Sharma K, et al. Adiponectin regulates albuminuria and podocyte function in mice. J. Clin. Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Konrad FM, et al. Heme oxygenase-1 attenuates acute pulmonary inflammation by decreasing the release of segmented neutrophils from the bone marrow. Am. J. Physiol Lung Cell Mol. Physiol. 2014;307:L707–L717. doi: 10.1152/ajplung.00145.2014. [DOI] [PubMed] [Google Scholar]
- 127.Turkseven S, et al. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am. J Physiol Heart Circ. Physiol. 2005;289:H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- 128.Bakhautdin B, et al. Protective role of HO-1 and carbon monoxide in ethanol-induced hepatocyte cell death and liver injury in mice. J. Hepatol. 2014;61:1029–1037. doi: 10.1016/j.jhep.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ungvari Z, et al. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol Heart Circ. Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Barbagallo I, et al. Potential therapeutic effects of natural heme oxygenase-1 inducers in cardiovascular diseases. Antioxid. Redox. Signal. 2013;18:507–521. doi: 10.1089/ars.2011.4360. [DOI] [PubMed] [Google Scholar]
- 131.Jamal UM, et al. A functional link between heme oxygenase-1 and tristetraprolin in the anti-inflammatory effects of nicotine. Free Radic. Biol. Med. 2013;65:1331–1339. doi: 10.1016/j.freeradbiomed.2013.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kim SK, et al. Resveratrol induces hepatic mitochondrial biogenesis through the sequential activation of nitric oxide and carbon monoxide production. Antioxid. Redox. Signal. 2014;20:2589–2605. doi: 10.1089/ars.2012.5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen TM, et al. Effects of heme oxygenase-1 upregulation on blood pressure and cardiac function in an animal model of hypertensive myocardial infarction. Int. J. Mol. Sci. 2013;14:2684–2706. doi: 10.3390/ijms14022684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Clerigues V, et al. Haem oxygenase-1 counteracts the effects of interleukin-1beta on inflammatory and senescence markers in cartilage-subchondral bone explants from osteoarthritic patients. Clin. Sci. (Lond) 2012;122:239–250. doi: 10.1042/CS20100519. [DOI] [PubMed] [Google Scholar]
- 135.Yin H, et al. Heme oxygenase-1 upregulation improves lipopolysaccharide-induced acute lung injury involving suppression of macrophage migration inhibitory factor. Mol. Immunol. 2010;47:2443–2449. doi: 10.1016/j.molimm.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 136.Pae HO, et al. Heme oxygenase-1 attenuates contact hypersensitivity induced by 2,4-dinitrofluorobenzene in mice. Immunopharmacol. Immunotoxicol. 2008;30:207–216. doi: 10.1080/08923970801946824. [DOI] [PubMed] [Google Scholar]
- 137.George EM, et al. The heme oxygenases: important regulators of pregnancy and preeclampsia. Am. J. Physiol Regul. Integr. Comp Physiol. 2014;307:R769–R777. doi: 10.1152/ajpregu.00132.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yet SF, et al. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J. Clin. Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cai C, et al. The heme oxygenase 1 inducer (CoPP) protects human cardiac stem cells against apoptosis through activation of the extracellular signal-regulated kinase (ERK)/NRF2 signaling pathway and cytokine release. J. Biol. Chem. 2012;287:33720–33732. doi: 10.1074/jbc.M112.385542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dawn B, Bolli R. HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? Am. J Physiol Heart Circ. Physiol. 2005;289:H522–H524. doi: 10.1152/ajpheart.00274.2005. [DOI] [PubMed] [Google Scholar]
- 141.Stein AB, et al. Carbon monoxide induces a late preconditioning-mimetic cardioprotective and antiapoptotic milieu in the myocardium. J Mol. Cell Cardiol. 2012;52:228–236. doi: 10.1016/j.yjmcc.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheng C, et al. Heme oxygenase 1 determines atherosclerotic lesion progression into a vulnerable plaque. Circulation. 2009;119:3017–3027. doi: 10.1161/CIRCULATIONAHA.108.808618. [DOI] [PubMed] [Google Scholar]
- 143.Li P, et al. Epoxyeicosatrienoic acids enhance embryonic haematopoiesis and adult marrow engraftment. Nature. 2015;523:468–471. doi: 10.1038/nature14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lavrovsky Y, et al. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc. Natl. Acad. Sci. U. S. A. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lavrovsky Y, et al. Downregulation of the human heme oxygenase gene by glucocorticoids and identification of 56b regulatory elements. Biochem. Biophys. Res. Commun. 1996;218:759–765. doi: 10.1006/bbrc.1996.0135. [DOI] [PubMed] [Google Scholar]
- 146.Abraham NG, et al. Modulation of erythropoiesis by novel human bone marrow cytochrome P450-dependent metabolites of arachidonic acid. Blood. 1991;78:1461–1466. [PubMed] [Google Scholar]
- 147.Kang L, et al. Induction and functional significance of the heme oxygenase system in pathological shear stress in vivo. Am. J. Physiol Heart Circ. Physiol. 2015;308:H1402–H1413. doi: 10.1152/ajpheart.00882.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 2006;70:432–443. doi: 10.1038/sj.ki.5001565. [DOI] [PubMed] [Google Scholar]
- 149.Agarwal A, Nick HS. Renal response to tissue injury: lessons from heme oxygenase-1 GeneAblation and expression. J. Am. Soc. Nephrol. 2000;11:965–973. doi: 10.1681/ASN.V115965. [DOI] [PubMed] [Google Scholar]
- 150.Deshane J, et al. Heme oxygenase-1 expression in disease states. Acta Biochim. Pol. 2005;52:273–284. [PubMed] [Google Scholar]
- 151.Kaide J-I, et al. Carbon monoxide of vascular origin attenuates the sensitivity of renal arterial vessels to vasoconstrictors. J. Clin. Invest. 2001;107:1163–1171. doi: 10.1172/JCI11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sabaawy HE, et al. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 153.Li N, et al. Role of renal medullary heme oxygenase in the regulation of pressure natriuresis and arterial blood pressure. Hypertension. 2007;49:148–154. doi: 10.1161/01.HYP.0000250086.06137.fb. [DOI] [PubMed] [Google Scholar]
- 154.Rodriguez F, et al. Effects of exogenous heme on renal function. Role of heme oxygenase and cyclooxygenase. Hypertension. 2003;42:680–684. doi: 10.1161/01.HYP.0000085785.40581.1A. [DOI] [PubMed] [Google Scholar]
- 155.Rodriguez F, et al. Nitric oxide synthesis inhibition promotes renal production of carbon monoxide. Hypertension. 2004;43:347–351. doi: 10.1161/01.HYP.0000111721.97169.97. [DOI] [PubMed] [Google Scholar]
- 156.Johnson RA, et al. Heme oxygenase substrates acutely lower blood pressure in hypertensive rats. Am. J. Physiol. 1996;271:H1132–H1138. doi: 10.1152/ajpheart.1996.271.3.H1132. [DOI] [PubMed] [Google Scholar]
- 157.Li Z, et al. Upregulation of heme oxygenase-1 potentiates EDH-type relaxations in the mesenteric artery of the spontaneously hypertensive rat. Am. J. Physiol Heart Circ. Physiol. 2013;305:H1471–H1483. doi: 10.1152/ajpheart.00962.2012. [DOI] [PubMed] [Google Scholar]
- 158.Kaide J, et al. Vascular CO counterbalances the sensitizing influence of 20-HETE on agonist-induced vasoconstriction. Hypertension. 2004;44:210–216. doi: 10.1161/01.HYP.0000135658.57547.bb. [DOI] [PubMed] [Google Scholar]
- 159.Stec DE, et al. Expression of heme oxygenase-1 in thick ascending loop of henle attenuates angiotensin II-dependent hypertension. J Am. Soc. Nephrol. 2012;23:834–841. doi: 10.1681/ASN.2011050455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J. Biol. Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 161.Capdevila JH, et al. Cytochrome P450 and arachidonic acid bioactivation. Molecular and functional properties of the arachidonate monooxygenase. J. Lipid Res. 2000;41:163–181. [PubMed] [Google Scholar]
- 162.Node K, et al. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science. 1999;285:1276–1279. doi: 10.1126/science.285.5431.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spiecker M, Liao JK. Vascular protective effects of cytochrome p450 epoxygenase-derived eicosanoids. Arch. Biochem. Biophys. 2005;433:413–420. doi: 10.1016/j.abb.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 164.Burgess A, et al. Epoxyeicosatrienoic acids and heme oxygenase-1 interaction attenuates diabetes and metabolic syndrome complications. Prostaglandins Other Lipid Mediat. 2012;97:1–16. doi: 10.1016/j.prostaglandins.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Abraham NG, et al. CYP2J2 targeting to endothelial cells attenuates adiposity and vascular dysfunction in mice fed a high-fat diet by reprogramming adipocyte phenotype. Hypertension. 2014;64:1352–1361. doi: 10.1161/HYPERTENSIONAHA.114.03884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fedorenko A, et al. Mechanism of fatty-acid-dependent UCP1 uncoupling in brown fat mitochondria. Cell. 2012;151:400–413. doi: 10.1016/j.cell.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kusmic C, et al. Improved myocardial perfusion in chronic diabetic mice by the up-regulation of pLKB1 and AMPK signaling. J. Cell Biochem. 2010;109:1033–1044. doi: 10.1002/jcb.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Otterbein LE, et al. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J. Clin. Invest. 1999;103:1047–1054. doi: 10.1172/JCI5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fain JN, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273–2282. doi: 10.1210/en.2003-1336. [DOI] [PubMed] [Google Scholar]
- 170.Tiwari S, Ndisang JF. The heme oxygenase system and type-1 diabetes. Curr. Pharm. Des. 2014;20:1328–1337. doi: 10.2174/13816128113199990552. [DOI] [PubMed] [Google Scholar]
- 171.Mishra M, Ndisang JF. A critical and comprehensive insight on heme oxygenase and related products including carbon monoxide, bilirubin, biliverdin and ferritin in type-1 and type-2 diabetes. Curr. Pharm. Des. 2014;20:1370–1391. doi: 10.2174/13816128113199990559. [DOI] [PubMed] [Google Scholar]
- 172.Cao J, et al. High-fat diet exacerbates renal dysfunction in SHR: reversal by induction of HO-1-adiponectin axis. Obesity. (Silver. Spring) 2012;20:945–953. doi: 10.1038/oby.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Csongradi E, et al. Chronic HO-1 induction with cobalt protoporphyrin (CoPP) treatment increases oxygen consumption, activity, heat production and lowers body weight in obese melanocortin-4 receptor-deficient mice. Int. J. Obes. (Lond) 2012;36:244–253. doi: 10.1038/ijo.2011.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Peterson SJ, et al. The L-4F mimetic peptide prevents insulin resistance through increased levels of HO-1, pAMPK, and pAKT in obese mice. J. Lipid Res. 2009;50:1293–1304. doi: 10.1194/jlr.M800610-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ndisang JF, et al. The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in Zucker diabetic fatty rats. PLoS. One. 2014;9:e87936. doi: 10.1371/journal.pone.0087936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Salley TN, et al. The heme oxygenase system rescues hepatic deterioration in the condition of obesity co-morbid with type-2 diabetes. PLoS. One. 2013;8:e79270. doi: 10.1371/journal.pone.0079270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kim DH, et al. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J Pharmacol. Exp. Ther. 2008;325:833–840. doi: 10.1124/jpet.107.135285. [DOI] [PubMed] [Google Scholar]
- 178.Cao J, et al. Lentiviral-human heme oxygenase targeting endothelium improved vascular function in angiotensin II animal model of hypertension. Hum. Gene Ther. 2011;22:271–282. doi: 10.1089/hum.2010.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Huang JY, et al. Adipose overexpression of heme oxygenase-1 does not protect against high fat diet-induced insulin resistance in mice. PLoS. One. 2013;8:e55369. doi: 10.1371/journal.pone.0055369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Abraham NG, Drummond G. CD163-Mediated hemoglobin-heme uptake activates macrophage HO-1, providing an antiinflammatory function. Circ. Res. 2006;99:911–914. doi: 10.1161/01.RES.0000249616.10603.d6. [DOI] [PubMed] [Google Scholar]
- 181.Philippidis P, et al. Hemoglobin scavenger receptor CD163 mediates interleukin-10 release and heme oxygenase-1 synthesis: antiinflammatory monocyte-macrophage responses in vitro, in resolving skin blisters in vivo, and after cardiopulmonary bypass surgery. Circ. Res. 2004;94:119–126. doi: 10.1161/01.RES.0000109414.78907.F9. [DOI] [PubMed] [Google Scholar]
- 182.Shakeri-Manesch S, et al. Diminished upregulation of visceral adipose heme oxygenase-1 correlates with waist-to-hip ratio and insulin resistance. Int. J. Obes. (Lond) 2009;33:1257–1264. doi: 10.1038/ijo.2009.160. [DOI] [PubMed] [Google Scholar]
- 183.Nielsen VG, et al. Bariatric patients have plasmatic hypercoagulability and systemic upregulation of heme oxygenase activity. Blood Coagul. Fibrinolysis. 2015;26:200–204. doi: 10.1097/MBC.0000000000000194. [DOI] [PubMed] [Google Scholar]
- 184.Rodgers PA, et al. Sources of carbon monoxide (CO) in biological systems and applications of CO detection technologies. Semin. Perinatol. 1994;18:2–10. [PubMed] [Google Scholar]
- 185.Di Noia MA, et al. Heme oxygenase-1 enhances renal mitochondrial transport carriers and cytochrome C oxidase activity in experimental diabetes. J Biol. Chem. 2006;281:15687–15693. doi: 10.1074/jbc.M510595200. [DOI] [PubMed] [Google Scholar]
- 186.Shen X, et al. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am. J. Physiol Endocrinol. Metab. 2004;287:E896–E905. doi: 10.1152/ajpendo.00047.2004. [DOI] [PubMed] [Google Scholar]
- 187.Sparks LM, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 188.Detaille D, et al. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 189.Converso DP, et al. HO-1 is located in liver mitochondria and modulates mitochondrial heme content and metabolism. FASEB J. 2006 doi: 10.1096/fj.05-4204fje. [DOI] [PubMed] [Google Scholar]
- 190.Kim J, et al. In vivo regulation of the heme oxygenase-1 gene in humanized transgenic mice. Kidney Int. 2012;82:278–291. doi: 10.1038/ki.2012.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Varma S, et al. Hyperglycemia Alters PI3k and Akt Signaling and Leads to Endothelial Cell Proliferative Dysfunction. Am. J. Physiol Heart Circ. Physiol. 2005;289:H1744–H1751. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bojunga J, et al. Antioxidative treatment prevents activation of death-receptor- and mitochondrion-dependent apoptosis in the hearts of diabetic rats. Diabetologia. 2004;47:2072–2080. doi: 10.1007/s00125-004-1572-7. [DOI] [PubMed] [Google Scholar]
- 193.Chen W, et al. Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology. 2004;145:627–638. doi: 10.1210/en.2003-1274. [DOI] [PubMed] [Google Scholar]
- 194.Srinivasan S, et al. Diabetic peripheral neuropathy: evidence for apoptosis and associated mitochondrial dysfunction. Diabetes. 2000;49:1932–1938. doi: 10.2337/diabetes.49.11.1932. [DOI] [PubMed] [Google Scholar]
- 195.Huang TJ, et al. Insulin enhances mitochondrial inner membrane potential and increases ATP levels through phosphoinositide 3-kinase in adult sensory neurons. Mol. Cell Neurosci. 2005;28:42–54. doi: 10.1016/j.mcn.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 196.Nascimento AL, et al. Ultraviolet A (320–380 nm) radiation causes an alteration in the binding of a specific protein/protein complex to a short region of the promoter of the human heme oxygenase 1 gene. Nucleic Acids Res. 1993;21:1103–1109. doi: 10.1093/nar/21.5.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Dulak J, et al. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rabinovitch A, et al. Cytotoxic effects of cytokines on rat islets: evidence for involvement of free radicals and lipid peroxidation. Diabetologia. 1992;35:409–413. doi: 10.1007/BF02342435. [DOI] [PubMed] [Google Scholar]
- 199.Choi BM, et al. Critical role of heme oxygenase-1 in Foxp3-mediated immune suppression. Biochem. Biophys. Res. Commun. 2005;327:1066–1071. doi: 10.1016/j.bbrc.2004.12.106. [DOI] [PubMed] [Google Scholar]
- 200.Pae HO, et al. Carbon monoxide produced by heme oxygenase-1 suppresses T cell proliferation via inhibition of IL-2 production. J. Immunol. 2004;172:4744–4751. doi: 10.4049/jimmunol.172.8.4744. [DOI] [PubMed] [Google Scholar]
- 201.Chen S, et al. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc. Natl. Acad. Sci. U. S. A. 2005;102:7251–7256. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chauveau C, et al. Heme oxygenase-1 expression inhibits dendritic cell maturation and proinflammatory function but conserves IL-10 expression. Blood. 2005;106:1694–1702. doi: 10.1182/blood-2005-02-0494. [DOI] [PubMed] [Google Scholar]
- 203.Choudhary AK, et al. Administration of heme arginate ameliorates murine type 2 diabetes independently of heme oxygenase activity. PLoS. One. 2013;8:e78209. doi: 10.1371/journal.pone.0078209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Pileggi A, et al. Heme oxygenase-1 induction in islet cells results in protection from apoptosis and improved in vivo function after transplantation. Diabetes. 2001;50:1983–1991. doi: 10.2337/diabetes.50.9.1983. [DOI] [PubMed] [Google Scholar]
- 205.Harrison EM, et al. Insulin induces heme oxygenase-1 through the phosphatidylinositol 3-kinase/Akt pathway and the Nrf2 transcription factor in renal cells. FEBS J. 2006;273:2345–2356. doi: 10.1111/j.1742-4658.2006.05224.x. [DOI] [PubMed] [Google Scholar]
- 206.Qi Y, et al. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004;10:524–529. doi: 10.1038/nm1029. [DOI] [PubMed] [Google Scholar]
- 207.Halberg N, et al. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009;58:1961–1970. doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kim JY, et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin. Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nawrocki AR, et al. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol. Chem. 2006;281:2654–2660. doi: 10.1074/jbc.M505311200. [DOI] [PubMed] [Google Scholar]
- 210.Yamada N, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am. J. Hum. Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Exner M, et al. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J. Endovasc. Ther. 2001;8:433–440. doi: 10.1177/152660280100800501. [DOI] [PubMed] [Google Scholar]
- 212.Inoguchi T, et al. Relationship between Gilbert syndrome and prevalence of vascular complications in patients with diabetes. JAMA. 2007;298:1398–1400. doi: 10.1001/jama.298.12.1398-b. [DOI] [PubMed] [Google Scholar]
- 213.Chen YH, et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum. Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 214.Kaneda H, et al. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler. Thromb. Vasc. Biol. 2002;22:1680–1685. doi: 10.1161/01.atv.0000033515.96747.6f. [DOI] [PubMed] [Google Scholar]
- 215.Endler G, et al. A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb. Haemost. 2004;91:155–161. doi: 10.1160/TH03-05-0291. [DOI] [PubMed] [Google Scholar]
- 216.Tiroch K, et al. Heme oxygenase-1 gene promoter polymorphism and restenosis following coronary stenting. Eur. Heart J. 2007;28:968–973. doi: 10.1093/eurheartj/ehm036. [DOI] [PubMed] [Google Scholar]
- 217.Courtney AE, et al. Association of functional heme oxygenase-1 gene promoter polymorphism with renal transplantation outcomes. Am. J Transplant. 2007;7:908–913. doi: 10.1111/j.1600-6143.2006.01726.x. [DOI] [PubMed] [Google Scholar]
- 218.Ono K, et al. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173:315–319. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 219.Wijpkema JS, et al. Restenosis after percutaneous coronary intervention is associated with the angiotensin-II type-1 receptor 1166A/C polymorphism but not with polymorphisms of angiotensin-converting enzyme, angiotensin-II receptor, angiotensinogen or heme oxygenase-1. Pharmacogenet. Genomics. 2006;16:331–337. doi: 10.1097/01.fpc.0000205001.07054.fa. [DOI] [PubMed] [Google Scholar]
- 220.Pechlaner R, et al. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with progressive atherosclerosis and incident cardiovascular disease. Arterioscler. Thromb. Vasc. Biol. 2015;35:229–236. doi: 10.1161/ATVBAHA.114.304729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gibbs PE, et al. Human biliverdin reductase-based peptides activate and inhibit glucose uptake through direct interaction with the kinase domain of insulin receptor. FASEB J. 2014;28:2478–2491. doi: 10.1096/fj.13-247015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yang L, et al. Heme oxygenase-1 gene expression modulates angiotensin II-induced increase in blood pressure. Hypertension. 2004;43:1221–1226. doi: 10.1161/01.HYP.0000126287.62060.e6. [DOI] [PubMed] [Google Scholar]
- 223.Melo LG, et al. Gene therapy strategy for long-term myocardial protection using adeno-associated virus-mediated delivery of heme oxygenase gene. Circulation. 2002;105:602–607. doi: 10.1161/hc0502.103363. [DOI] [PubMed] [Google Scholar]
- 224.Tang YL, et al. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J Cardiovasc. Pharmacol. Ther. 2005;10:251–263. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- 225.Adin CA, et al. Protective effects of exogenous bilirubin on ischemia-reperfusion injury in the isolated, perfused rat kidney. Am. J Physiol Renal Physiol. 2005;288:F778–F784. doi: 10.1152/ajprenal.00215.2004. [DOI] [PubMed] [Google Scholar]
- 226.Wu BJ, et al. Niacin inhibits vascular inflammation via the induction of heme oxygenase-1. Circulation. 2012;125:150–158. doi: 10.1161/CIRCULATIONAHA.111.053108. [DOI] [PubMed] [Google Scholar]
- 227.Mrad MF, et al. Statins modulate transcriptional activity of heme-oxygenase-1 promoter in NIH 3T3 Cells. J. Cell Biochem. 2012;113:3466–3475. doi: 10.1002/jcb.24223. [DOI] [PubMed] [Google Scholar]
- 228.Abraham NG. Molecular regulation--biological role of heme in hematopoiesis. Blood Rev. 1991;5:19–28. doi: 10.1016/0268-960x(91)90004-v. [DOI] [PubMed] [Google Scholar]
- 229.Brusko TM, et al. An integral role for heme oxygenase-1 and carbon monoxide in maintaining peripheral tolerance by CD4+CD25+ regulatory T cells. J Immunol. 2005;174:5181–5186. doi: 10.4049/jimmunol.174.9.5181. [DOI] [PubMed] [Google Scholar]
- 230.George JF, et al. Suppression by CD4+CD25+ regulatory T cells is dependent on expression of heme oxygenase-1 in antigen-presenting cells. Am. J Pathol. 2008;173:154–160. doi: 10.2353/ajpath.2008.070963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Minamino T, et al. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc. Natl. Acad. Sci. U. S. A. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Soares MP, et al. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J. Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 233.Sacerdoti D, et al. Rat mesenteric arterial dilator response to 11,12-epoxyeicosatrienoic acid is mediated by activating heme oxygenase. Am. J Physiol Heart Circ. Physiol. 2006;291:H1999–H2002. doi: 10.1152/ajpheart.00082.2006. [DOI] [PubMed] [Google Scholar]
- 234.Abraham NG, et al. Modulation of cGMP by human HO-1 retrovirus gene transfer in pulmonary microvessel endothelial cells. Am. J Physiol Lung Cell Mol. Physiol. 2002;283:L1117–L1124. doi: 10.1152/ajplung.00365.2001. [DOI] [PubMed] [Google Scholar]
- 235.Sambuceti G, et al. Diabetes Impairs the Vascular Recruitment of Normal Stem Cells by Oxidant Damage; Reversed by Increases in pAMPK, Heme Oxygenase-1 and Adiponectin. Stem Cells. 2009;27:399–407. doi: 10.1634/stemcells.2008-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Cao J, et al. Upregulation of Heme Oxygenase-1 Combined with Increased Adiponectin Lowers Blood Pressure in Diabetic Spontaneously Hypertensive Rats through a Reduction in Endothelial Cell Dysfunction, Apoptosis and Oxidative Stress. Int. J. Mol. Sci. 2008;9:2388–2406. doi: 10.3390/ijms9122388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Kruger AL, et al. D-4F induces heme oxygenase-1 and extracellular superoxide dismutase, decreases endothelial cell sloughing, and improves vascular reactivity in rat model of diabetes. Circulation. 2005;111:3126–3134. doi: 10.1161/CIRCULATIONAHA.104.517102. [DOI] [PubMed] [Google Scholar]
- 238.Liu X, et al. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J. 2006;20:207–216. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- 239.Basuroy S, et al. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-alpha in cerebral vascular endothelial cells. Am. J. Physiol Cell Physiol. 2006;291:C897–C908. doi: 10.1152/ajpcell.00032.2006. [DOI] [PubMed] [Google Scholar]
- 240.Agarwal R. Proinflammatory effects of oxidative stress in chronic kidney disease: role of additional angiotensin II blockade. Am. J Physiol Renal Physiol. 2003;284:F863–F869. doi: 10.1152/ajprenal.00385.2002. [DOI] [PubMed] [Google Scholar]
- 241.Mazza F, et al. Heme oxygenase-1 gene expression attenuates angiotensin II-mediated DNA damage in endothelial cells. Exp. Biol. Med. (Maywood. ) 2003;228:576–583. doi: 10.1177/15353702-0322805-31. [DOI] [PubMed] [Google Scholar]
- 242.Abraham NG. Heme oxygenase attenuated angiotensin II-mediated increase in cyclooxygenase activity and decreased isoprostane F2alpha in endothelial cells. Thromb. Res. 2003;110:305–309. doi: 10.1016/s0049-3848(03)00417-1. [DOI] [PubMed] [Google Scholar]
- 243.Quan S, et al. Expression of human heme oxygenase-1 in the thick ascending limb attenuates angiotensin II-mediated increase in oxidative injury. Kidney Int. 2004;65:1628–1639. doi: 10.1111/j.1523-1755.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 244.Durante W, et al. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth cells. Circ. Res. 1997;80:557–564. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 245.Li Volti G, et al. Differential effect of heme oxygenase-1 in endothelial and smooth muscle cell cycle progression. Biochem. Biophys Res Commun. 2002;296:1077–1082. doi: 10.1016/s0006-291x(02)02054-5. [DOI] [PubMed] [Google Scholar]
- 246.Liu XM, et al. Adenovirus-mediated heme oxygenase-1 gene expression stimulates apoptosis in vascular smooth muscle cells. Circulation. 2002;105:79–84. doi: 10.1161/hc0102.101369. [DOI] [PubMed] [Google Scholar]
- 247.Liu XM, et al. Carbon monoxide inhibits apoptosis in vascular smooth muscle cells. Cardiovasc. Res. 2002;55:396–405. doi: 10.1016/s0008-6363(02)00410-8. [DOI] [PubMed] [Google Scholar]
- 248.Morita T, et al. Carbon monoxide controls the proliferation of hypoxic smooth muscle cells. J. Biol. Chem. 1997;272:32804–32809. doi: 10.1074/jbc.272.52.32804. [DOI] [PubMed] [Google Scholar]
- 249.Govindaraju V, et al. Interaction between endothelial heme oxygenase-2 and endothelin-1 in altered aortic reactivity after hypoxia in rats. Am. J. Physiol Heart Circ. Physiol. 2005;288:H962–H970. doi: 10.1152/ajpheart.01218.2003. [DOI] [PubMed] [Google Scholar]
- 250.Stanford SJ, et al. Carbon monoxide inhibits endothelin-1 release by human pulmonary artery smooth muscle cells. Eur. J Pharmacol. 2004;486:349–352. doi: 10.1016/j.ejphar.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 251.Zhang F, et al. CO modulates pulmonary vascular response to acute hypoxia: relation to endothelin. Am. J Physiol Heart Circ. Physiol. 2004;286:H137–H144. doi: 10.1152/ajpheart.00678.2002. [DOI] [PubMed] [Google Scholar]
- 252.Zabalgoitia M, et al. Carbon monoxide donors or heme oxygenase-1 (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death. Free Radic. Biol. Med. 2008;44:284–298. doi: 10.1016/j.freeradbiomed.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]