Abstract
Stem-boring insects and methyl jasmonate (MeJA) are thought to induce similar complex chemical and anatomical defenses in conifers. To compare insect- and MeJA-induced terpenoid responses, we analyzed traumatic oleoresin mixtures, emissions of terpenoid volatiles, and expression of terpenoid synthase (TPS) genes in Sitka spruce (Picea sitchensis) following attack by white pine weevils (Pissodes strobi) or application of MeJA. Both insects and MeJA caused traumatic resin accumulation in stems, with more accumulation induced by the weevils. Weevil-induced terpenoid emission profiles were also more complex than emissions induced by MeJA. Weevil feeding caused a rapid release of a blend of monoterpene olefins, presumably by passive evaporation of resin compounds from stem feeding sites. These compounds were not found in MeJA-induced emissions. Both weevils and MeJA caused delayed, diurnal emissions of (−)-linalool, indicating induced de novo biosynthesis of this compound. TPS transcripts strongly increased in stems upon insect attack or MeJA treatment. Time courses and intensity of induced TPS transcripts were different for monoterpene synthases, sesquiterpene synthases, and diterpene synthases. Increased levels of weevil- and MeJA-induced TPS transcripts accompanied major changes in terpenoid accumulation in stems. Induced TPS expression profiles in needles were less complex than those in stems and matched induced de novo emissions of (−)-linalool. Overall, weevils and MeJA induced similar, but not identical, terpenoid defense responses in Sitka spruce. Findings of insect- and MeJA-induced accumulation of allene oxide synthase-like and allene oxide cyclase-like transcripts are discussed in the context of traumatic resinosis and induced volatile emissions in this gymnosperm system.
Chemical and physical defense of conifers against potential herbivores and pathogens depends on a large array of structurally diverse monoterpenoids (C10), sesquiterpenoids (C15), and diterpenoids (C20; Bohlmann and Croteau, 1999; Phillips and Croteau, 1999; Trapp and Croteau, 2001; Martin et al., 2002, 2003). These terpenoids are major components of conifer oleoresins, which can function as repellents or toxins against herbivores or pathogens (Langenheim, 2003). Some conifer terpenoids can act as insect juvenile hormone analogs. Terpenoid volatiles emitted from conifers into the atmosphere can serve as signals in host recognition for insect herbivores such as bark beetles (Seybold et al., 2000). Upon herbivory by foliage-feeding or stem-boring insects, induced volatile emissions can attract natural enemies of the herbivore (Hilker et al., 2002; Mumm et al., 2003). Qualitative and quantitative composition of monoterpenoids, sesquiterpenoids, and diterpenoids have been characterized in Norway spruce (Picea abies) in constitutive and induced stems (Persson et al., 1996; Martin et al., 2002) and needles (Schönwitz et al., 1990; Martin et al., 2003) and in constitutive and induced volatile emissions (Martin et al., 2003).
In recent studies of the complex anatomical and chemical terpenoid defenses in conifers, Norway spruce trees were treated with methyl jasmonate (MeJA) to simulate insect or pathogen attack. This approach allowed detailed histological (Franceschi et al., 2002; Martin et al., 2002), biochemical (Martin et al., 2002, 2003), and preliminary molecular characterization (Fäldt et al., 2003) of the induced terpenoid defense system. Treatment with MeJA induced formation of traumatic resin ducts (TDs) in developing stem xylem (Franceschi et al., 2002; Martin et al., 2002) and increased accumulation of oleoresin terpenoids in inner and outer stem tissues (Martin et al., 2002). TDs were initiated within a few days of MeJA treatment in close proximity to the stem cambium and appeared within 3 weeks after MeJA treatment as fully developed resin ducts in the outer xylem tissues (Franceschi et al., 2002; Martin et al., 2002). Monoterpenoids and sesquiterpenoids were identified as major MeJA-induced volatile emissions, dominated by the oxygenated monoterpene (−)-linalool and the sesquiterpenes (E)-α-bisabolene and (E)-β-farnesene (Martin et al., 2003). Associated with MeJA-induced formation of TDs and terpenoid volatile emissions were increased terpenoid synthase (TPS) enzyme activities in stems and needles (Martin et al., 2002, 2003). Initial characterization at the molecular level revealed accumulation of monoterpene synthase (mono-TPS) and diterpene synthase (di-TPS) transcripts in Norway spruce stems upon treatment with MeJA (Fäldt et al., 2003).
In most biochemical or molecular studies of induced terpenoid defenses in conifers to date, insect attack was simulated by mechanical wounding (Gijzen et al., 1992; Bohlmann et al., 1997; Steele et al., 1998b) or by MeJA treatment (Richard et al., 2000; Martin et al., 2002, 2003; Fäldt et al., 2003). While MeJA treatment has proven useful as a noninvasive method for induction of conifer defense responses, terpenoid defenses induced by real insect attack have not been characterized adequately at the biochemical or molecular levels, despite the numerous interactions occurring between conifers and a large array of insect pests, including bark beetles, stem and root weevils, budworms, or sawflies. Notable exceptions are works by Litvak and Monson (1998), which showed increased mono-TPS activity in foliage of Ponderosa pine (Pinus ponderosa) induced by tiger moth larvae (Halisdota ingens), and a recent study in Sitka spruce (Picea sitchensis), which showed increased levels of transcripts hybridizing with (−)-pinene synthase cDNA in response to stem feeding by white pine weevil (Pissodes strobi; Byun McKay et al., 2003).
The white pine weevil is the most destructive insect pest of Sitka spruce in much of the tree's natural range of distribution in western North America (Alfaro et al., 2002). Severe deformation and mortality of Sitka spruce is caused by feeding and ovipositing adult weevils and by larvae that feed under the bark on phloem, vascular cambium, and developing xylem of the apical shoot leaders of young trees (Alfaro et al., 2002) and thus girdle growing apical leaders. The white pine weevil is also a major pest of planted Norway spruce forests and attacks other species of spruce and pine in North America (Alfaro, 1995; Alfaro et al., 2002). If introduced by transport of plants, soils, or forest products, the weevil could spread rapidly as an exotic pest of conifer forests in other parts of the world. While constitutive and induced terpenoid defenses are known to contribute to the resistance of Sitka spruce against white pine weevils (Alfaro et al., 2002), very little is known about molecular mechanisms of terpenoid defenses that are activated in response to the white pine weevil (Byun McKay et al., 2003). It is also not clear how well treatment of trees with MeJA mimics the effect of real insects in inducing terpenoid defense responses in any conifer species.
Here we compare effects of attack by adult white pine weevils with effects of MeJA treatment on terpenoid resin accumulation, terpenoid volatile emissions, and TPS transcript accumulation in stems and foliage of young Sitka spruce trees. A possible role for octadecanoid signaling in weevil-induced conifer defense is discussed based on the similar terpenoid responses induced by insects and by MeJA, and based on findings of insect- and MeJA-induced transcript accumulation of allene oxide synthase (AOS) and allene oxide cyclase (AOC) in Sitka spruce.
RESULTS
Weevil- and MeJA-Induced Terpenoid Accumulation in Stems of Sitka Spruce
Weevil attack and mechanical wounding induce the formation of one or more rings of resin-filled, axial TDs in stems of Sitka spruce (Alfaro et al., 2002; Byun McKay et al., 2003). Similarly, formation of TDs is induced in Norway spruce upon treatment with MeJA (Franceschi et al., 2002; Martin et al., 2002). Early stages of TDs appear within a few days after MeJA treatment due to developmental changes in xylem mother cells on the interior side of the stem cambium (Franceschi et al., 2002; Martin et al., 2002). After approximately 3 weeks, following initiation of TDs, and after switching back of the cambium zone to normal tracheid formation, one or more rings of fully developed, resin-filled TDs become embedded in the outer xylem tissue (Franceschi et al., 2002; Martin et al., 2002). When outer stem tissues (bark, phloem, cambium) are manually separated from sapling stems within a few days after MeJA treatment, TD-forming cells remain attached with the outer stem tissues. After about 3 weeks, upon induction, newly formed TDs remain attached with the inner stem tissues (primary and secondary xylem).
We analyzed constitutive and induced accumulation of mono-, sesqui-, and diterpenoids in outer and inner stem tissues of Sitka spruce saplings in response to feeding by adult white pine weevils and in response to MeJA 3 weeks after onset of treatment (Fig. 1). Constitutive and induced resin terpenoids were measured in outer and inner stem tissues by qualitative and quantitative gas chromatographic (GC) analyses, with mass-spectrometry, and with flame ionization detection (Supplemental Tables I and II; Fig. 1). Previous time-course studies showed that MeJA-induced traumatic resin accumulation reaches a maximum about 20 d after treatment in Norway spruce (Martin et al., 2002). Sitka spruce saplings used in this study sustained weevil feeding for at least 20 d without signs of tree mortality.
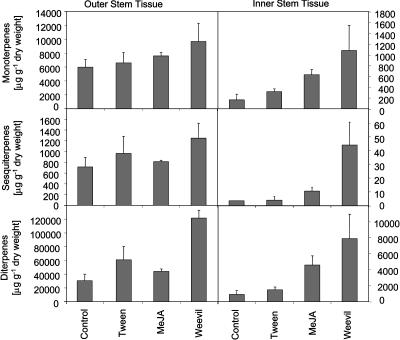
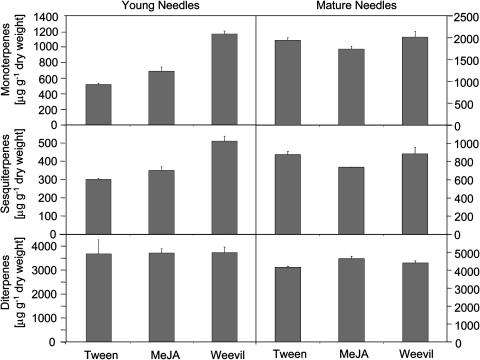
Figure 1.
Accumulation of monoterpenoids, sesquiterpenoids, and diterpenoids in outer and inner stem tissues of Sitka spruce after feeding by white pine weevil or MeJA treatment. Terpenoids were analyzed from samples harvested 20 d after treatment of sapling trees with 0.1% Tween 20 (Tween), treatment with 0.01% MeJA in 0.1% Tween (MeJA), or after feeding by white pine weevils (Weevil). Untreated trees served as controls (Control). Each bar represents the mean total + se of three samples.
Accumulation of all three classes of resin terpenoids, mono-, sesqui-, and diterpenoids, was strongly increased in inner stem tissues upon weevil feeding or MeJA treatment (Fig. 1). MeJA caused a 3.8-fold increase of total monoterpenoids, a 2.8-fold increase of total sesquiterpenoids, and a 5.1-fold increase of total diterpenoids compared with untreated controls after 20 d (Fig. 1). Weevil feeding had an even stronger effect on resin accumulation in inner stem tissues, causing a 6.5-fold increase of total monoterpenoids, an 11.9-fold increase of total sesquiterpenoids, and an 8.8-fold increase of total diterpenoids compared with untreated controls (Fig. 1). Treatment with Tween 20 resulted only in a weak increase of resin accumulation. Analysis of inner stem tissues 20 d after MeJA treatment or 20 d after onset of weevil feeding revealed a more complex composition of mono-, sesqui-, and diterpenoids compared with controls (Supplemental Table I). Several compounds were only detected, in some cases in substantial amounts, in the MeJA- and weevil-induced tissues.
Levels of total constitutive mono-, sesqui-, and diterpenoids in outer stem tissues were at least one order of magnitude higher than those in inner stem tissues (Fig. 1). High levels of constitutive resin terpenoids are associated with large constitutive, axial resin ducts in outer stem tissues (Alfaro et al., 2002). A 4-fold increase of resin terpenoids in the outer stem tissues was found for diterpenoids induced by insect attack. Although relative increases of each class of resin terpenoids in outer stem tissues were less pronounced than in inner stem tissues, the absolute increase of all three combined classes of resin terpenoids induced by weevils in outer stem tissues (an increase of approximately 95 mg g−1 dry weight) was more than 10 times higher than the weevil-induced increase of all three classes of terpenoids in inner stem tissues (approximately 8 mg g−1 dry weight; Fig. 1).
Weevil- and MeJA-Induced TPS Transcript Accumulation in Stems of Sitka Spruce
To test for effects of weevil-feeding and MeJA treatment on TPS gene expression by northern-blot analyses (Fig. 2), we used a set of eleven Norway spruce cDNA probes (Supplemental Table III; Fäldt et al., 2003; Martin et al., 2004) encoding six different mono-TPS, three different sesquiterpene synthases (sesqui-TPS), and two different di-TPS. A comparable large set of Sitka spruce TPS probes was not available. High similarity of Sitka spruce and Norway spruce TPS sequences support the suitability of Norway spruce TPS probes for northern analyses in these closely related species. For example, the cDNAs encoding (−)-pinene synthases from Sitka spruce (Byun MacKay et al., 2003) and from Norway spruce (Martin et al., 2004) are 98.3% identical at the nucleic acid level and cluster as closest neighbors in a phylogenetic tree of the conifer TPSd gene family (Martin et al., 2004). Similarly, (−)-limonene synthase cDNAs from Sitka spruce and from Norway spruce are 98.5% identical (Martin et al., 2004). Dot-blot assays showed little or no cross-hybridization between TPS probes of the three different classes, mono-TPS, sesqui-TPS, and di-TPS, but some cross-hybridization was found for probes within each of the three classes (data not shown).
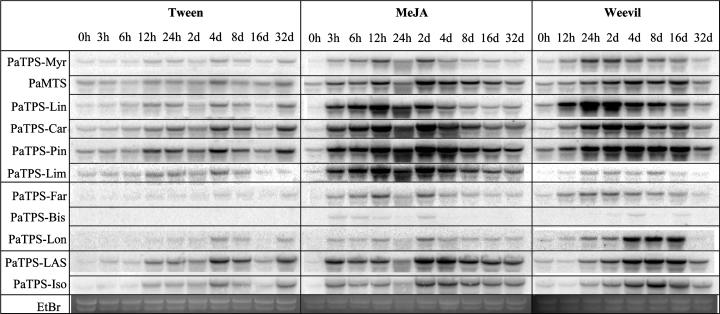
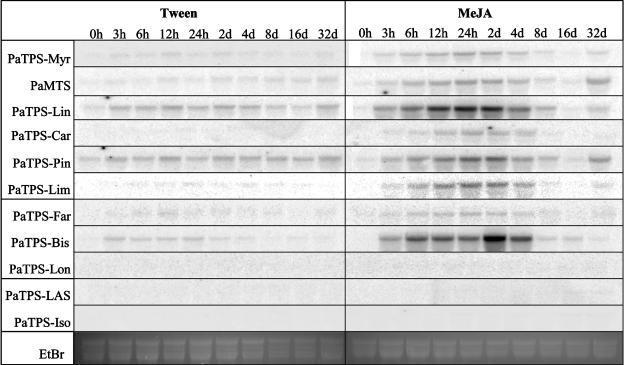
Figure 2.
Transcript accumulation of mono-TPS, sesqui-TPS, and di-TPS in outer stem tissues of Sitka spruce after feeding by white pine weevils or MeJA treatment. RNA for northern hybridizations was isolated from outer stem samples harvested over a time course of 32 d after treatment of sapling trees with 0.1% Tween 20 (Tween), treatment with 0.01% MeJA in 0.1% Tween (MeJA), or after feeding by white pine weevils (Weevil). Mono-TPS probes (Supplemental Table III) are PaTPS-Myr, PaMTS, PaTPS-Lin, PaTPS-Car, PaTPS-Pin, and PaTPS-Lim. Sesqui-TPS probes are PaTPS-Far, PaTPS-Bis, and PaTPS-Lon. Di-TPS probes are PaTPS-LAS and PaTPS-Iso. Ethidium bromide-stained major ribosomal RNA bands serve for evaluation of equal gel loading.
We measured TPS transcripts in outer stem tissues (Fig. 2) to capture transcripts associated with early events of weevil- and MeJA-induced TD formation and to capture transcripts associated with the strong, absolute increase of resin terpenoids in outer stem tissues. Constitutively, all TPS probes revealed low transcript levels. A weak increase of transcript accumulation was observed in the Tween 20-treated trees. Strongly increased accumulation of TPS transcripts was found in weevil-attacked and in MeJA-induced trees with mono-TPS probes (Fig. 2). Mono-TPS probes showed rapidly increased transcript levels within 3 h in MeJA-treated trees and within 12 to 24 h in weevil-induced trees, in each case at the first time point of tissue collection. In MeJA-induced trees, mono-TPS transcript levels remained high until at least 2 to 4 d after treatment, while in weevil-infested trees high levels of mono-TPS were still found at 8 to 16 d probably due to continuous exposure of trees to insects compared to a single MeJA treatment.
Sesqui-TPS probes showed weaker increases of transcript accumulation in outer stem tissues of MeJA-treated and weevil-induced trees compared to most mono-TPS signals (Fig. 2). PaTPS-Lon, which encodes a typical conifer multiproduct sesqui-TPS of the TPS-d2 cluster (Steele et al., 1998a; Martin et al., 2004), revealed a stronger increase of transcripts in weevil-attacked stems than in MeJA-treated trees. For PaTPS-Bis, which encodes E-α-bisabolene synthase (Martin et al., 2004), only very low transcript levels were detected in MeJA- or weevil-induced outer stem tissues.
Transcripts hybridizing with two very similar di-TPS probes, PaTPS-LAS and PaTPS-Iso (Martin et al., 2004), showed strong increases in weevil- and MeJA-treated stem tissues (Fig. 2). Highest transcript levels were achieved only 2 to 4 d after MeJA treatment and 8 d after initiation of insect feeding, suggesting the kinetics of accumulation of induced di-TPS transcripts were slower than those for mono-TPS transcripts.
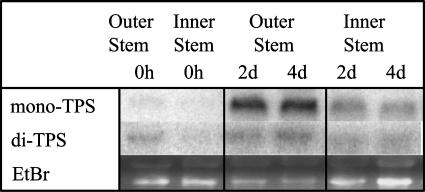
Because we found high transcript levels of mono-TPS and di-TPS at 2 and 4 d after MeJA treatment (Fig. 2), we also measured mono-TPS and di-TPS transcripts for these time points in inner stem tissues of MeJA-treated trees where we found weaker transcript accumulation (Fig. 3). Induced TPS transcripts in outer stem tissues (Fig. 2), which match well with the levels of induced terpenoid accumulations, could be associated with activation of existing resin ducts, de novo formation of TDs, and possibly TPS expression in cells that are not part of resin ducts. In future work, we will test if constitutive and insect- or MeJA-induced TPS transcripts are restricted to the epithelial cells of constitutive and traumatic resin ducts.
Figure 3.
Accumulation of TPS transcripts in inner and outer stem tissues after MeJA treatment. RNA for northern hybridizations was isolated from inner and outer stem samples harvested over a time course of 4 d after treatment with 0.01% MeJA in 0.1% Tween. Ethidium bromide-stained major ribosomal RNA bands serve for evaluation of equal gel loading.
Spatial Patterns of Terpenoid and TPS Transcript Accumulation in Stems upon Weevil Attack
In natural infestations, weevils attack the uppermost parts of apical shoot leaders for feeding and egg deposition (Alfaro, 1995). In experiments to evaluate spatial patterns of local and distal weevil-induced accumulation of resin terpenoids and TPS transcripts, insects were caged on the upper half of sapling trees to induce their natural feeding behavior. Twenty days after placing the weevils, we analyzed terpenoid accumulation in inner and outer stem tissues of local, infested and distal, noninfested stem sections. Compared with strong accumulation of total resin terpenoids in local, infested stem tissues (Fig. 1), we did not find a similar strong increase of terpenoid accumulation in inner or outer tissues of distal, noninfested stem sections 10 to 20 cm below the sites of insect feeding (Fig. 4A). These results suggested that weevil feeding on the apical shoot leader causes a local, but not a systemic, response at the level of terpenoid accumulation.
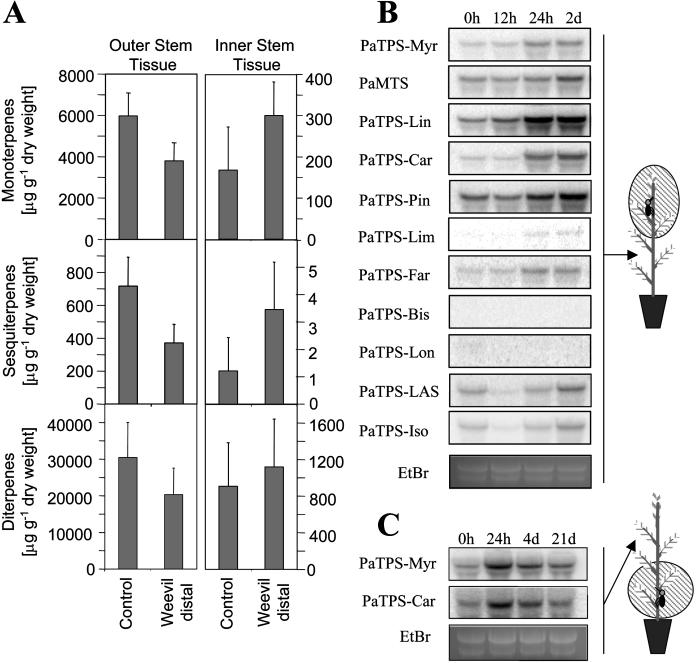
Figure 4.
Terpenoid accumulation and TPS transcripts in distal, noninfested stem sections upon local feeding by weevils. A, Terpenoids were analyzed in inner and outer tissues of stem sections below the site of weevil feeding at day 20. Data represent mean + se from three samples. B, Northern hybridizations of total RNA from outer stem tissue below the site of weevil exposure harvested over a time course of 2 d after initiation of weevil feeding. C, Northern hybridizations of total RNA from outer stem tissue above the site of weevil exposure. Ethidium bromide-stained major ribosomal RNA bands serve for evaluation of equal gel loading.
At the level of TPS transcripts, local, infested stem sections (Fig. 2) and distal, noninfested stem sections (Fig. 4B) showed similar increases of mono-TPS and di-TPS transcript accumulation during the first 2 d of weevil attack. In contrast with infested stem sections (Fig. 2), the distal noninfested parts of stems did not reveal induced transcript accumulation with the PaTPS-Lon sesqui-TPS probe (Fig. 4B). When weevils were caged on lower stem sections, they also caused increased mono-TPS transcript accumulation in the upper, noninfested parts of the stems as shown with two probes (Fig. 4C). These experiments demonstrated that weevil-induced TPS transcript accumulation is not restricted to the sites of local insect attack but is detectable below and above feeding sites.
Terpenoid Accumulation in Needles of Sitka Spruce
To test if weevils and MeJA treatment affect terpenoid accumulation in foliage, we analyzed terpenoids in young needles of current-year developing shoots (Supplemental Table IV; Fig. 5) and in 1-year-old mature needles (Supplemental Table V; Fig. 5). While constitutive amounts of mono-, sesqui-, and diterpenoids were higher in mature needles compared to young needles (Fig. 5), there was no substantial increase of terpenoid accumulation in mature needles 20 d after MeJA treatment or weevil attack. By contrast, in young needles weevils caused increased accumulation of total monoterpenoids and of total sesquiterpenoids 20 d after onset of weevil feeding (Fig. 5).
Figure 5.
Accumulation of monoterpenoids, sesquiterpenoids, and diterpenoids in young and mature needles after feeding by weevils on stems or after treatment of trees with MeJA. Terpenoids were analyzed from samples harvested 20 d after treatment of sapling trees with 0.1% Tween 20 (Tween), treatment with 0.01% MeJA in 0.1% Tween (MeJA), or after exposure of trees to weevils (Weevil). Each bar represents the mean total + se from three samples.
Emission of Terpenoid Volatiles Induced by MeJA
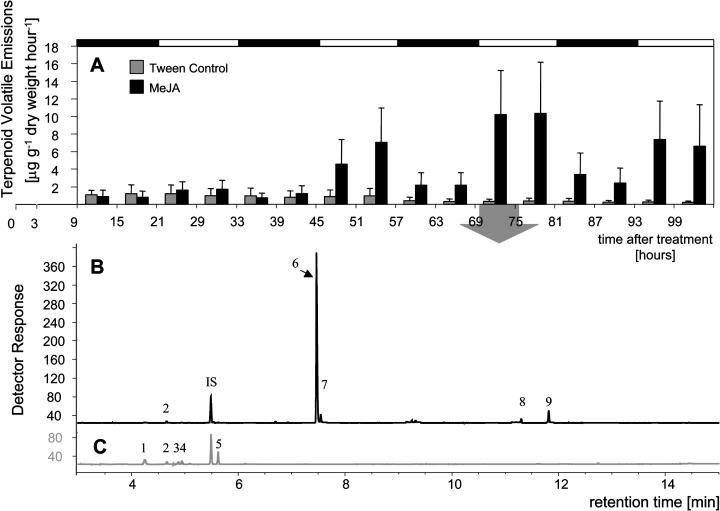
Following the finding of MeJA-induced volatile emissions of (−)-linalool, E-β-farnesene, and E-α-bisabolene, along with minor amounts of other mono- and sesquiterpenes, in Norway spruce (Martin et al., 2003), we compared time courses and composition of volatile emissions induced by MeJA treatment (Fig. 6) or by weevil feeding (Fig. 7) in Sitka spruce. We monitored emissions of mono- and sesquiterpenoids over a period of 4 d after MeJA treatment using a headspace volatile collection system as described previously (Arimura et al., 2004). In three independent experiments, treatment with MeJA induced increased diurnal terpenoid emissions that were clearly detectable during the second light period of the collection time course, 45 to 57 h after treatment (Fig. 6A). Volatile emissions increased further during the light period of the 3rd day of volatile collection, 69 to 81 h after treatment, reaching emission rates of 10.3 ± 3.4 μg g−1 dry weight h−1 (mean emission ± se from 3 replicates), and remained high for the light period of day 4 of volatile collection, 93 to 105 h after treatment. Volatile emissions from control trees did not change. Qualitative analysis of volatiles collected over a 6-h period at the time of maximum emission, 69 to 75 h after treatment, revealed a terpenoid emission profile dominated by the monoterpene alcohol (−)-linalool (72.7%–86.0% of total volatiles detected) along with minor emissions of (+)-linalool, and the sesquiterpenes (E,E)-α-farnesene and (Z)-α-bisabolene (Fig. 6B). None of these compounds was found in the volatile emissions of control trees (Fig. 6C).
Figure 6.
Terpenoid volatile emission from Sitka spruce saplings upon treatment with MeJA. A, Time course of volatile emissions of trees treated with 0.1% Tween 20 (Tween Control) and 0.01% MeJA in 0.1% Tween 20 (MeJA). Volatile collections started 9 h after treatment of trees to allow for evaporation of excessive amounts of MeJA. Trees were placed into collection chambers 6 h before onset of volatile collections. Data represent mean + se from three independent volatile collection experiments. Black and white bars on top of the graph represent day and night periods. B, Representative chromatogram of terpenoids collected from MeJA-treated trees and from (C) Tween 20-treated control trees on the 4th day after treatment between 6 am and 12 pm, 69 to 75 h after treatment. Peaks were identified as: 1, (−)-α-pinene; 2, myrcene; 3, (+)-β-pinene; 4, (−)-β-pinene; 5, sabinene; 6, (−)-linalool; 7, (+)-linalool; 8, (E,E)-α-farnesene; 9, (Z)-α-bisabolene; and IS, internal standard.
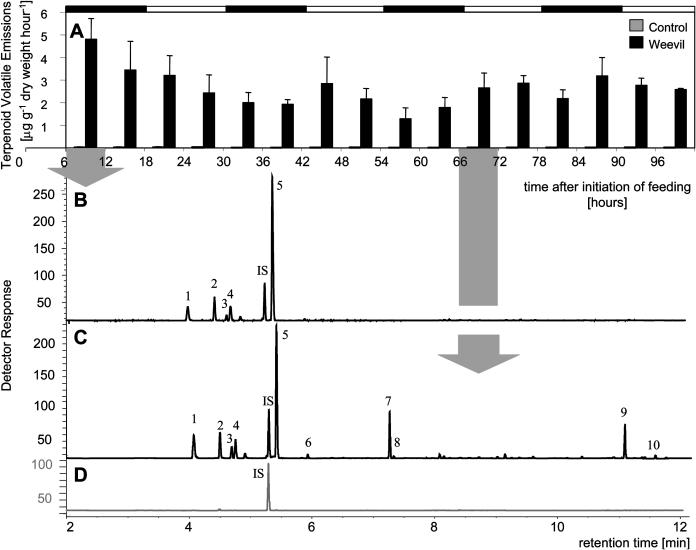
Figure 7.
Terpenoid volatile emission from Sitka spruce saplings upon feeding by white pine weevils. A, Time course of volatile emissions of trees with five weevils feeding per tree (Weevil), and time course of volatile emissions from control trees with weevils separated from trees by mesh bag (Control). Volatile collections started 6 h after onset of weevil feeding at the time when trees were placed into the collection system. Black and white bars on top of the graph represent day and night periods. Data represent mean + se from three independent volatile collection experiments. B, Representative chromatogram of terpenoids collected from weevil infested trees on the 1st day, 6 to 12 h after begin of weevil feeding. C, Representative chromatogram of terpenoids collected from a weevil-treated tree on the 4th day between 6 am and 12 pm, 66 to 72 h after onset of treatment. D, Representative chromatogram of terpenoids collected from a control tree independent of time point during the course of the volatile collection experiment. Peaks were identified as: 1, (−)-α-pinene; 2, myrcene; 3, (+)-β-pinene; 4, (−)-β-pinene; 5, sabinene; 6, terpinolene; 7, (−)-linalool; 8, (+)-linalool; 9, (E,E)-α-farnesene; 10, (Z)-α-bisabolene; and IS, internal standard.
Emission of Terpenoid Volatiles upon Weevil Feeding
MeJA has been proven a useful elicitor for studies of induced volatile emissions and their molecular and biochemical regulation (e.g. Kessler and Baldwin, 2001; Martin et al., 2003; Arimura et al., 2004). However, it is not known if stem-boring insects and MeJA treatment induce similar volatile emission profiles. This question is of particular relevance for studies in conifers because conifers sequester large amounts of volatile terpenoids in specialized anatomical structures. These terpenoids evaporate passively at sites of mechanical damage caused by stem-boring insects. To our knowledge, additional, insect-induced de novo formation of altered volatile profiles has not been shown in conifers. Knowledge of insect-induced volatile emissions and their mechanisms of release will be important for future ecological and insect-behavioral studies in the Sitka spruce/white pine weevil system, as well as for future studies in other conifer-insect interactions. We therefore analyzed volatile emissions of sapling trees under attack by adult weevils (Fig. 7).
Trees infested with weevils showed strongly increased emissions of terpenoid volatiles compared to headspace samples of noninfested control trees (Fig. 7A). We found several substantial differences between insect- and MeJA-induced volatile emissions. Weevils caused a very rapid emission of volatiles, with highest emission rates detected during the first collection period 6 to 12 h after onset of feeding (Fig. 7A), while MeJA-induced emissions were released with a delay of 2 d (Fig. 6A). In contrast with MeJA-induced emissions, total weevil-induced terpenoid emissions did not follow obvious diurnal patterns (Fig. 7A). Weevil-attacked trees emitted terpenoid mixtures during the first sampling period, 6 to 12 h after initiation of insect feeding, that contained as major components the monoterpene olefins (−)-α-pinene (4.9%–9.1%), myrcene (10.6%–12.8%), (+)-β-pinene (2.5%–4.4%), (−)-β-pinene (3.8%–7.2%), and sabinene (62.7%–68.4%) with a total rate of emission of 4.8 ± 0.9 μg g−1 dry weight h−1 (mean emission ± se from 3 replicates; Fig. 7, A and B). The monoterpenes detected in early emissions of weevil-infested trees were also abundant in oleoresins of Sitka spruce stems (Supplemental Tables I and II) and foliage (Supplemental Tables IV and V).
During a later collection period, 66 to 72 h after weevils were placed on trees (Fig. 7C), we found a qualitative change of volatile emissions compared with the early emissions of the 6 to 12-h collection period (Fig. 7B) and compared with controls (Fig. 7D). (−)-Linalool and (E,E)-α-farnesene were strongly increased in the volatile terpenoid profile of weevil-infested trees at 66 to 72 h after onset of feeding (Fig. 7C). The presence of (−)-linalool in weevil-induced trees resembled the MeJA-induced (−)-linalool emissions at 60 to 66 h past treatment (Fig. 6B). Weevil-induced terpenoid emissions at 66 to 72 h were composed of (−)-α-pinene (10.8%–22.3%), myrcene (6.3%–8.6%), (+)-β-pinene (2.5%–7.9%), (−)-β-pinene (6.6%–10.9%), sabinene (41.9%–50.5%), terpinolene (0.8%–2.5%), (−)-linalool (1.7%–12.0%), (E,E)-α-farnesene (0.5%–9.2%), and (Z)-α-bisabolene (0.8%–1.0%), with a total rate of emission of 2.7 ± 0.7 μg g−1 dry weight h−1 (mean emission ± se from 3 replicates; Fig. 7, A and C). Since linalool is not present in the early weevil-induced emissions and is not a major oleoresin terpenoid (Supplemental Tables I, II, IV, and V) but appears strongly in delayed weevil- and MeJA-induced emissions, it is likely that release of this compound is not due to passive volatilization of previously accumulated oleoresin terpenes but involves additional active mechanisms of formation or release.
Weevil-Induced Diurnal Emissions of (−)-Linalool
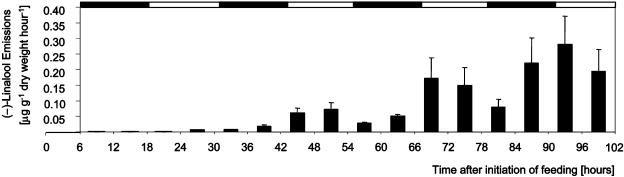
Linalool was not detected in the early emissions of weevil-infested trees (Figs. 7B and 8), consistent with the finding that linalool was not a major compound of the oleoresin sequestration. The result of a delayed emission of (−)-linalool in weevil-infested trees (Fig. 7B) prompted us to analyze linalool emissions further for diurnal patterns. Although total terpene emissions from weevil-infested trees did not show diurnal emission profiles (Fig. 7A), we found increased emissions of (−)-linalool during the light phases 42 to 54 h and 66 to 78 h after onset of insect feeding, with reduced emissions during the first half of the following dark phases (Fig. 8). This result supports the hypothesis that emission of (−)-linalool is controlled by active mechanisms that are different from passive volatilization of oleoresin monoterpenes at sites of insect-caused tissue damage.
Figure 8.
Time course of (−)-linalool emission from Sitka spruce saplings upon feeding by white pine weevils. Time course of (−)-linalool emissions of trees with five weevils feeding per tree. Volatile collections started 6 h after onset of weevil feeding at the time when trees were placed into the collection system. Black and white bars on top represent day and night periods. Data represent mean + se from three independent volatile collection experiments. No emission of linalool was detected with control trees.
MeJA-Induced TPS Transcript Accumulation in Needles of Sitka Spruce
In addition to analyses of TPS transcripts in stems (Fig. 2), we further measured TPS transcript accumulation in 1-year-old needles of Sitka spruce to test if increased volatile emissions correlate with increased transcript levels in foliage (Fig. 9). In contrast with MeJA-induced TPS transcript accumulation in stems (Fig. 2), MeJA treatment did not cause an increase of transcripts hybridizing with di-TPS probes, PaTPS-LAS and PaTPS-Iso, in needles (Fig. 9). This result agreed with the lack of induction of diterpene accumulation in needles (Fig. 5) and the absence of diterpenes in induced volatile emissions (Fig. 6B). Induction of most mono-TPS transcripts was also less pronounced in MeJA-treated needles (Fig. 9) compared with stems (Fig. 2). However, transcripts hybridizing to the PaTPS-Lin (−)-linalool synthase probe were substantially increased in needles upon treatment with MeJA (Fig. 9), matching the major induced emission of (−)-linalool in MeJA-treated trees (Fig. 6B). Transcripts hybridizing with PaTPS-Bis, encoding (E)-α-bisabolene synthase, were also strongly induced in MeJA-treated needles (Fig. 9), possibly associated with the minor, induced sesquiterpene emission in the trees of this study.
Figure 9.
Transcript accumulation of mono-TPS, sesqui-TPS, and di-TPS in needles of Sitka spruce after treatment with MeJA. RNA for northern hybridizations was isolated from needles over a time course of 32 d after treatment of sapling trees with 0.1% Tween 20 (Tween) or treatment with 0.01% MeJA in 0.1% Tween (MeJA). The mono-TPS probes (Supplemental Table III) are PaTPS-Myr, PaMTS, PaTPS-Lin, PaTPS-Car, PaTPS-Pin, and PaTPS-Lim. Sesqui-TPS probes are PaTPS-Far, PaTPS-Bis, and PaTPS-Lon. Di-TPS probes are PaTPS-LAS and PaTPS-Iso. Ethidium bromide-stained major ribosomal RNA bands serve for evaluation of equal gel loading.
MeJA-induced accumulation of transcripts hybridizing with (−)-linalool synthase cDNA in needles provides strong evidence that needles are a source for induced linalool emissions and that induced release of linalool depends on de novo formation rather than release from previously accumulated compounds. This result fits well with earlier findings of MeJA-induced emission of linalool associated with strongly increased linalool synthase enzyme activities in Norway spruce needles (Martin et al., 2003). De novo formation of induced linalool is further corroborated by data from resin analyses that did not detect linalool in mature and young needles (Supplemental Tables IV and V). Apparently, linalool does not accumulate in large amounts but is rapidly released upon induced de novo formation. The exact tissues or cells involved in linalool formation and the mechanisms of emission in spruce needles remain to be elucidated. It is possible that cell types other than the epithelial cells of resin ducts are contributing to the formation of induced emission of (−)-linalool.
Weevils and MeJA Induced Putative Allene Oxide Synthase and Allene Oxide Cyclase Transcripts in Stems and Needles
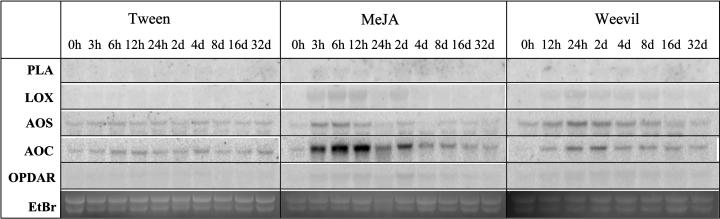
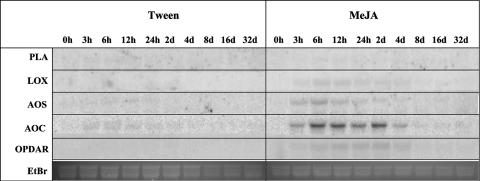
The similar profiles of TPS gene expression, terpenoid accumulation, and volatile emissions induced by weevils and MeJA in Sitka spruce suggest that octadecanoids and the octadecanoid pathway could be active in insect-induced defense signaling in this system. Mining for octadecanoid pathway genes in a spruce expressed sequence tag (EST) database that is derived to some large extent from cDNA libraries of insect-, wound-, or elicitor-induced tissues of Sitka spruce and interior spruce (Picea glauca x engelmannii; http://www.treenomix.com) revealed cDNAs with similarity to known genes for several steps in octadecanoid biosynthesis (Supplemental Table VI). Northern-blot analyses showed that weevils and MeJA caused increased accumulation of AOS-like and AOC-like transcripts in outer stems of Sitka spruce (Fig. 10). Similarly, treatment with MeJA also caused increased levels of these transcripts in mature needles (Fig. 11). Induction of transcripts hybridizing with spruce AOC was stronger in response to MeJA than in response to weevils (Fig. 10). These data could suggest a positive feedback control of octadecanoid signal activation in Sitka spruce similar to octadecanoid signaling in herbaceous angiosperms (Kessler and Baldwin, 2002). Another possibility is that weevils suppress a strongly induced accumulation of AOC-like transcripts.
Figure 10.
Transcript accumulation of genes of octadecanoid biosynthesis in stems of Sitka spruce after feeding by white pine weevil or treatment with MeJA. RNA for northern hybridizations was isolated from outer stem samples harvested over a time course of 32 d after treatment of sapling trees with 0.1% Tween 20 (Tween), treatment with 0.01% MeJA in 0.1% Tween (MeJA), or after feeding by white pine weevils (Weevil). EST cDNA clones used as probes are described in Supplemental Table VI. PLA, phospholipaseA; LOX, lipoxygenase; AOS, allene oxide synthase; AOC, allene oxide cyclase; OPDAR, 12-oxo-phytodienoic acid reductase. Ethidium bromide-stained major ribosomal RNA bands serve for evaluation of equal gel loading.
Figure 11.
Transcript accumulation of genes of octadecanoid biosynthesis in needles of Sitka spruce after treatment with MeJA. RNA for northern hybridizations was isolated from needles over a time course of 32 d after treatment of sapling trees with 0.1% Tween 20 (Tween) or treatment with 0.01% MeJA in 0.1% Tween (MeJA). EST cDNA clones used as probes are described in Supplemental Table VI. PLA, phospholipaseA; LOX, lipoxygenase; AOS, allene oxide synthase; AOC, allene oxide cyclase; OPDAR, 12-oxo-phytodienoic acid reductase. Ethidium bromide-stained major ribosomal RNA bands serve for evaluation of equal gel loading.
DISCUSSION
MeJA Induces Similar Terpenoid Responses in Two Species of Spruce
Feeding by white pine weevil and MeJA treatment caused massive up-regulation of oleoresin defenses and terpenoid volatile emissions in Sitka spruce. Induced traumatic resin accumulation in stems was associated with previously documented formation of TDs (Alfaro et al., 2002; Byun McKay et al., 2003) and possibly with activation of existing resin ducts in outer stem tissues. This oleoresin response was further associated with increased levels of TPS transcripts hybridizing with a series of mono-TPS, sesqui-TPS, and di-TPS probes. Induction of terpenoid accumulation in stems of Sitka spruce by MeJA and weevils was very similar to MeJA-induced oleoresin accumulation in Norway spruce stems (Martin et al., 2002). In both species, inner stem tissues showed strong, relative increases of resin terpenoids. Both species responded with an even stronger absolute increase of constitutively high levels of resin terpenoids in outer stem tissues. The possibility that a MeJA-induced increase of terpenoids in stems is due to de novo terpenoid formation, as opposed to translocation of existing resin alone, was supported with measurements of increased mono-TPS and di-TPS enzyme activities in Norway spruce (Martin et al., 2002) and was further supported by findings of strongly increased TPS transcripts described here for Sitka spruce and by previous, preliminary findings of MeJA-induced TPS transcripts in Norway spruce (Fäldt et al., 2003).
MeJA-induced terpenoid emissions were also very similar in Sitka spruce and in Norway spruce (Martin et al., 2003), with a prominence of the monoterpenol (−)-linalool among the induced volatiles. It has previously been suggested that MeJA-induced emissions of linalool are due to de novo formation in needles of Norway spruce because (−)-linalool synthase enzyme activity was strongly increased in needles (Martin et al., 2003). A (−)-linalool synthase cDNA, PaTPS-Lin, has recently been cloned and characterized in Norway spruce (Martin et al., 2004). In foliage of Sitka spruce, MeJA causes up-regulation of TPS transcripts that hybridize with the PaTPS-Lin cDNA. Results from analyses of volatile emissions, enzyme activities, and transcript levels all agree with MeJA-induced de novo biosynthesis of linalool emissions in needles, without excluding the possibility of additional formation and emission of linalool in stems.
In conclusion, MeJA induces very similar, massive terpenoid defense responses in two species of spruce. The combined data for Sitka spruce and Norway spruce firmly establish that MeJA-induced terpenoid accumulations and emissions involve substantial increases of TPS transcripts and increased TPS activities. The use of MeJA as a noninvasive elicitor has been instrumental in demonstrating de novo formation of linalool, as opposed to release from existing terpenoid storage sites, in two species of spruce.
Weevils and MeJA Induce Similar Traumatic Resin Defenses in Sitka Spruce Stems
A few recent papers described MeJA-induced oleoresin defenses in Norway spruce. These studies suggest that MeJA mimics the effect of insect infestation in conifers at the levels of induced TD formation (Franceschi et al., 2002; Martin et al., 2002), terpenoid accumulation (Martin et al., 2002), TPS gene expression (Fäldt et al., 2003), and TPS enzyme activities (Martin et al., 2002). Effects of attack by a stem-boring insect, the white pine weevil, have been studied at the level of TD formation in Sitka spruce (Alfaro et al., 2002; Byun McKay et al., 2003) and at the levels of terpenoid accumulation and TD formation in white spruce (P. glauca; Alfaro, 1995; Tomlin et al., 1998, 2000). Initial studies in Sitka spruce also showed some induced TPS transcript accumulation in response to weevil feeding and mechanical wounding (Byun McKay et al., 2003). However, to our knowledge, a comprehensive analysis of conifer terpenoid defenses induced by real insect attack, including characterization of traumatic resinosis and volatile emissions at the molecular and biochemical levels, has not previously been reported. By comparison of weevil-infested and MeJA-treated Sitka spruce sapling stems, we demonstrated a very similar traumatic oleoresin response at the level of mono-, sesqui-, and diterpenoid accumulation, and at the level of mono-TPS, sesqui-TPS, and di-TPS transcript profiles upon real and simulated insect attack. A stronger accumulation of traumatic resin terpenoids in stems in response to weevils, as compared with that induced by MeJA, could be due to continuous exposure to insects for up to 20 d compared to a one-time treatment with MeJA. In addition, the specific mode of insect feeding, insect-derived elicitors, or fungal symbionts associated with weevils may have lead to a stronger insect-induced response.
The overall kinetics of weevil- and MeJA-induced mono-TPS, sesqui-TPS, and di-TPS transcript accumulation in stems of Sitka spruce resembled those found for wound-induced TPS in stems of grand fir (Steele et al., 1998b), with a slightly slower induction of di-TPS compared to mono-TPS and a weaker induction of sesqui-TPS. These data suggest that wounding or MeJA treatment can be useful to some degree to simulate stem-feeding insects in conifers. One noticeable difference between weevil- and MeJA-induced responses was with the induction of transcripts hybridizing to PaTPS-Lon, which represents a class of conifer multiproduct sesqui-TPS of the TPS-d2 group (Martin et al., 2004). MeJA treatment in Sitka spruce as well as mechanical wounding of grand fir stems (Steele et al., 1998b) induced only very weak or no accumulation of transcripts of the TPS-d2 group. By contrast, weevils induced substantial accumulation of this type of transcript. At the level of terpenoid accumulation, weevils also induced a much stronger increase of sesquiterpenoids in Sitka spruce stem tissues than did MeJA in either Sitka spruce or in Norway spruce (Martin et al., 2002).
Patterns of Volatile Emissions Induced by Weevils and MeJA Suggest Both Active and Passive Mechanisms of Terpenoid Volatile Release in Conifers
Insect-induced terpenoid emissions, their significance in indirect plant defense, and their possible functions in plant-plant-herbivore signaling, have been studied in a number of angiosperm species (for review, see Paré and Tumlinson, 1999; Baldwin et al., 2002; Kessler and Baldwin, 2002; Pichersky and Gershenzon, 2002). More recently, insect-induced terpenoid emissions induced by egg deposition of sawflies and attraction of egg parasitoids have been shown in the conifer Pinus sylvestris (Hilker et al., 2002; Mumm et al., 2003). In a few angiosperm systems that were analyzed for molecular or biochemical mechanisms of herbivore-induced volatile emissions, increased TPS transcript accumulation or increased TPS enzyme activities were associated with de novo terpenoid emissions (e.g. Bouwmeester et al., 1999; Shen et al., 2000; Schnee et al., 2002; Arimura et al., 2004). Similarly, MeJA treatment, as a noninvasive mimic of insect attack, induced de novo terpenoid volatile emissions in Norway spruce (Martin et al., 2003) and in Sitka spruce (this study). Specifically, MeJA application caused the release of (−)-linalool and a variable bouquet of sesquiterpenoids that involved induced mono-TPS and sesqui-TPS enzyme activities (Martin et al., 2003) and induced TPS transcript accumulations.
In contrast with most angiosperms that have been studied for molecular or biochemical mechanisms of herbivore-induced volatile release, conifers constitutively sequester massive amounts of volatile terpenoids in resin ducts or blisters in stems and foliage. Based on results presented in this study, we suggest two different mechanisms of induced volatile release to function in conifers. One mechanism relies on passive evaporation of previously formed oleoresin compounds at sites of stem damage, while a second mechanism is regulated by molecular, biochemical, and physiological control. In Norway spruce (Martin et al., 2003) and in Sitka spruce, induced emissions of (−)-linalool involve de novo terpenoid formation accompanied by increased (−)-linalool synthase transcript accumulation and enzyme activity. Induced de novo formation was reflected in delayed and diurnal emissions of (−)-linalool in Sitka spruce compared with emission of several other monoterpenes. Diurnal emission profiles of linalool in Sitka spruce and Norway spruce suggested physiological control of the release. In addition to delayed emissions of linalool, weevil feeding triggered a faster release of monoterpene olefins that was most likely due to rapid volatilization from damaged resin storage sites of infested trees. Passive volatile emission, as a consequence of tissue damage, has also been suggested to occur together with events of de novo formation of volatile emissions in other systems (Baldwin et al., 2002). Possible differences or synergies in ecological functions of actively and passively released volatiles remain to be tested in conifer-insect interactions. In future work, we will also test if the release of (−)-linalool in Sitka spruce is systemically induced, similar to de novo induced terpenoid emissions in other systems (Röse et al., 1996; Arimura et al., 2004).
Localized Feeding by Weevils Causes Increased TPS Transcript Accumulation in Acropetal and Basipetal Directions
We demonstrated that localized feeding by weevils, which attack the tip of apical spruce leaders (Alfaro, 1995), causes local and distal increase of TPS transcripts. However, despite some basipetal induction of TPS transcript accumulation, terpenoids were not reproducibly increased in distal stem sections. Apparently other mechanisms, in addition to TPS transcript accumulation, also control weevil-induced terpenoid accumulation in the traumatic oleoresin response. In contrast with the strictly acropetal direction of herbivore-induced TPS transcript accumulation recently described in forest tent caterpillar-poplar interactions (Arimura et al., 2004), weevils caused both acropetal and basipetal induction of TPS transcripts in stems of Sitka spruce, suggesting that the nature or transport of herbivore-induced signals differ in these two systems.
Possible Endogenous Signals in Insect-Induced Conifer Terpenoid Defense
Although MeJA has been used successfully in this and in previous work to induce and characterize defense responses in conifers, endogenous roles of octadecanoids in conifer defense have, to our knowledge, not been demonstrated. A number of candidate genes and ESTs for the octadecanoid pathway in spruce are now available for further evaluation and possible manipulation of defense signaling in this gymnosperm system. Other defense signals are likely also involved in the orchestration of a complex traumatic oleoresin response and induced volatile emissions in conifers. Recently, Hudgins and Franceschi (2004) have provided convincing evidence for a role of ethylene in MeJA- and wound-induced TD formation in Douglas fir (Pseudotsuga menziesii) and in giant redwood (Sequoiadendrum giganteum). Since TD formation results from a transient change of xylem differentiation, other phytohormones that regulate xylem development could also have a function in this process. Future knowledge of insect-induced traumatic resin defense in species of spruce and its regulation at the expense of regular xylem development will be advanced by tissue-specific transcript, protein, and signal metabolite profiling, and by manipulation of the candidate signaling pathways.
MATERIALS AND METHODS
Plant Material and Insects
Sitka spruce (Picea sitchensis [Bong.] Carriere; clone FB3-425) seedlings were propagated by somatic embryogenesis and generously provided by Dr. David Ellis (CellFor, Vancouver). Unless noted otherwise, trees were grown outside at the University of British Columbia horticulture greenhouse for 1 or 2 years under natural light and environmental conditions to a height of 40 to 50 cm. Trees were grown in 3:1 (v/v) peat:vermiculite, 2.4 g L−1 Dolomite, 0.5 g L−1 Nutritrace micronutrients, and 3.6 g L−1 Osmocoat in 656-mL conetainers (Stuewe & Sons, Corvallis, OR), and were watered daily. Four weeks prior to the experiments, which commenced in May 2003, trees were moved inside a greenhouse with constant 16/8-h photoperiod provided by high-pressure sodium lamps. Average greenhouse temperatures were 24°C/20°C (day/night). Average humidity was 57%. Adult white pine weevils (Pissodes strobi Peck) were generously provided by Dr. Rene I. Alfaro (Pacific Forestry Centre, Canadian Forest Service, Victoria, Canada). Weevils were reared from larvae of infested Sitka spruce shoots collected at natural infestation sites in British Columbia in 2002 and maintained on fresh Sitka spruce shoots as food source.
Treatment of Trees with MeJA or Weevils and Tissue Harvest
MeJA treatment was as described previously (Martin et al., 2002, 2003; Fäldt et al., 2003). In brief, each tree was sprayed with 150 mL of 0.01% (v/v) MeJA (Sigma-Aldrich, St. Louis; 95% pure, w/w) dissolved in 0.1% Tween 20. To control for solvent effects, a set of trees were sprayed with 150 mL of 0.1% Tween 20. For insect treatment, weevils were kept without food on moist filter paper for 48 h prior to placing them in groups of five insects per tree under mesh bags on individual trees. Unless otherwise noted, weevils were caged for 20 d on the upper half of sapling trees. For terpenoid analysis, weevils were removed from trees after 4 d. An additional set of trees was left untreated. MeJA-treated trees, insect-treated trees, and control trees were kept in separate treatment groups in a well-ventilated greenhouse. Stems and needles were harvested 20 d after treatment for terpenoid analysis and at 0 h, 3 h, 6 h, 12 h, 24 h, 2 d, 4 d, 8 d, 16 d, and 32 d for RNA isolation. To harvest stem tissues, saplings were cut at the base, lateral branches removed, and the stem divided into 10-cm-long sections. The bark of each section was cut longitudinally with a razor blade, and the outer tissue was peeled off the woody inner stem tissues. For isolation of RNA from the inner stem tissue (Fig. 3), developing xylem was scraped off with a razor blade. Outer stem tissues, inner stem tissues, previous-year mature needles, and young needles from the current year shoot were separately flash frozen in liquid nitrogen and stored at −80°C for RNA isolation and terpenoid extractions.
Extraction and Analysis of Terpenoids
Terpenoid extractions were as described previously (Martin et al., 2002). Samples for resin analyses were harvested and pooled from three trees for each time point and treatment. Three separate extracts were prepared for each treatment and tissue. Extractions were carried out in 2-mL glass vials with Teflon/red rubber-coated screw caps (Agilent Technologies, Palo Alto, CA). Samples of inner or outer stem tissues of approximately 1 cm in length, and needles of 0.1 g dry weight, were submerged in 1.5 mL of tert-butyl methyl ether (Aldrich, Milwaukee, WI) spiked with 100 μg mL−1 isobutyl benzene (for monoterpenes and sesquiterpenes) and 200 μg mL−1 dichlorodehydroabietic acid (Helix Biotech, Richmond, Canada; for diterpenes) as internal standards. Methylation of terpenoid acids was as previously described (Martin et al., 2002). Dry weights were determined after drying tissues at 70°C for 48 h.
Analysis of terpenoids was performed on an Agilent 6890A Series GC system (Agilent Technologies) equipped with an Agilent 7683 Series autosampler and flame ionization detector (FID). The injection volume for all samples was 1 μL, the injector temperature was 220°C, and the FID was operated at 300°C. Hydrogen flow was 40 mL min−1, air flow 450 mL min−1, and constant makeup flow 7 mL min−1. Mono- and sesquiterpenes were separated on a DB-WAX column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; J&W Scientific, Folsom, CA). The flow of hydrogen carrier gas was 2 mL min−1. The split ratio was 10:1. The GC was programmed with an initial oven temperature of 40°C (3-min hold), a temperature increase at 3°C min−1 to 110°C, followed by 10°C min−1 to 180°C and 15°C min−1 to 250°C (10-min hold). Diterpenoids were separated on an AT-1000 column (30 m × 0.25 mm i.d. × 0.25 μm film thickness; Alltech, Deerfield, IL). Hydrogen carrier gas was kept constant at a flow rate of 1.7 mL min−1. The split ratio was 50:1. The oven was programmed from an initial temperature of 180°C (1-min hold) to 220°C at a rate of 1°C min−1, followed by 20°C min−1 until 240°C (15-min hold). GC-FID generated peaks were integrated using Agilent ChemStation software, Rev.A.09.01 (Agilent Technologies).
For additional identification of terpenoids, samples were analyzed by GC-mass spectrometry on an Agilent 6890A Series GC system equipped with Agilent 7683 Series autosampler and 5973N mass selective detector (quadrupole analyzer, electron ionization, 70 eV), running Enhanced ChemStation version D.00.00.38 software (Agilent Technologies) using the same GC columns as for GC-FID. The injection volume for all samples was 1 μL, and the injector temperature was 220°C. The temperature program for separation of mono- and sesquiterpenes started at 40°C (hold 3 min), followed by 3°C min−1 to 110°C and 10°C min−1 to 180°C, and finally 15°C min−1 until 250°C (10-min hold). The solvent delay was 12 min. Diterpenes were separated using an initial temperature of 180°C (1-min hold), followed by 10°C min−1 to 220°C and 20°C min−1 until 240°C (20-min hold). The solvent delay was 10 min. Mono- and sesquiterpenes were analyzed splitless, and diterpenes were analyzed with a split ratio of 5:1. The carrier gas was helium with constant flow at 1.0 mL min−1 for diterpenes and 1.3 mL min−1 for mono- and sesquiterpenes. The mass range for analysis of mono- and sesquiterpenes was 40 to 350 m/z, and 40 to 400 m/z for diterpenes.
Identification of terpenes was based on comparison of retention times and mass spectra with those of authentic standards and/or based on matching mass spectra in the Wiley275 Mass Spectral Library (Rev.D.01.00, June 2000; purchased from Agilent Technologies) and the HP1607 Library (purchased from Agilent Technologies). Terpene concentrations were calculated by integration of GC-FID peak area and normalization against peak areas of internal standards. Mean terpenoid concentrations in μg × g−1 dry weight were determined, and ses were calculated.
Collection and Analysis of Terpenoid Volatiles
Dynamic headspace sampling was carried out with an automated volatile collection system (Analytical Research Systems, Gainesville, FL) as described previously (Arimura et al., 2004). The collection system was installed in a Conviron model PGW 36 (Conviron, Winnipeg, Canada) growth chamber with a 12/12-h photoperiod at 500 μmol m−2 s−1 photon flux and 25°C constant temperature. Trees used for volatile collection were maintained outside prior to experiments. MeJA treatment (see above) was done in a fume hood at 9 am, and trees were kept there for 3 h to avoid contamination of the volatile collection system with excessive MeJA evaporations. At 12 pm, trees treated with MeJA or infested with weevils (5 weevils per tree) were moved into the volatile collection chambers and trees were given another 6 h to adjust prior to volatile collections starting at 6 pm. For each experiment, a pair of treated and untreated control trees was used and was placed in the separate glass cylinders of the collection system for parallel sampling. Control trees in MeJA experiments were treated with 0.1% Tween 20. For weevil experiments, noninfested control trees were placed into the collection chamber with five weevils separated from the tree with a mesh bag also inside the chamber. Sampling of volatiles was controlled with an automated exit port system (Analytical Research Systems) that drew efflux air at a rate of 1 L min−1 through glass tubes each containing 30 mg of Super Q (80/100 mesh; Alltech). Purified air influx was 1 L min−1. Headspace volatiles were collected over 6-h intervals for a total of 4 d per experiment. Experiments were replicated three times with new trees for each replicate. After volatile collection, needles and stems of MeJA-treated or weevil-infested trees were harvested and their dry weights were recorded.
Volatiles collected on SuperQ columns were eluted with 0.5 mL of methylene chloride spiked with 12.5 μg mL−1 isobutyl benzene as internal standard and stored in glass vials with Teflon-lined caps at −20°C until GC analysis. Samples were analyzed by GC-FID (Agilent 6890 Series GC system) with an Agilent 7683 Series injector fitted with a CyclosilB capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness; J&W Scientific). Injection volumes were 1 μL, splitless, with an injector temperature of 250°C. The instrument was programmed from an initial temperature of 60°C and increased at 10°C min−1 to 240°C (10-min hold). Hydrogen was used as the carrier gas at a constant flow of 2 mL min−1. GC-FID-generated peaks were integrated using Agilent ChemStation software (Agilent Technologies). Identification of terpenes was based on comparison of retention times with authentic standards. Amounts of individual terpenoids were determined using the following equation:
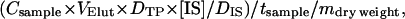 |
with Csample, sampling constant (ratio of air input to air output; in our experiment = 5); VElut, solvent volume used to elute SuperQ column; DTP, area for each terpenoid peak; [IS], amount of IS in sample in micrograms; DIS, area for IS peak; tsample, sampling duration (6 h); and mdry weight, dry weight of tissue in grams. For each sample period, means were determined and se were calculated.
Northern Analyses
Isolation of total RNA from needles and stem tissues of 1-year-old saplings followed the protocol of Kolosova et al. (2004). Aliquots of 10 μg of total RNA were separated in 1% (w/v) formaldehyde agarose gels. Even loading of RNA samples was established by inspecting the ethidium bromide-stained gels for the major ribosomal RNAs using ChemiImager 5500 with AlphaEaseFC software (Alpha Innotech, San Leandro, CA). RNA was transferred by capillary action to positively charged nylon membranes (Hybond-N+; Amersham Pharmacia Biotech, Buckinghamshire, UK) and fixed by UV cross-linking (TL-2000 Ultraviolet Translinker; UVP Ultra-Violet Products, Upland, CA). TPS cDNA probes were amplified by PCR from plasmid clones using primers described in Supplemental Table III. The octadecanoid pathway EST cDNA clones used for probe generation by PCR with T3 and T7 vector primers are described in Supplemental Table VI. PCR products were purified using gel electrophoresis and the QIAquick gel extraction kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. Labeling of probes was performed using Strip-EZ DNA kit (Ambion, Austin, TX) with (α-32P) dATP (3,000 Ci mmol−1, 10 mCi mL−1; Perkin Elmer Applied Biosystems, Streetsville, Canada) according to the manufacturers' protocol. Unincorporated (α-32P) dATP was removed using gel filtration columns (Microspin S-300 HR columns; Amersham Pharmacia Biotech). Membranes were prehybridized for 1 h at 65°C in hybridization buffer (0.05 m Na4P2O7, 0.115 m NaH2PO4, 7% SDS, 1 mm EDTA, 100 μg mL−1 denatured salmon sperm DNA) followed by hybridizations with heat-denatured cDNA probes for 16 h at 65°C. Membranes were rinsed once for 2 min and three times for 45 min in wash buffer (0.05 m Na4P2O7, 0.115 m NaH2PO4, 1% SDS, 1 mm EDTA) at 65°C. Hybridization signals were detected using a Storm 860 phosphor imager (Amersham Pharmacia Biotech). Membranes were stored at −20°C and used for multiple hybridizations after removal of probes according to the manufacturer's protocol (Strip-EZ DNA kit; Ambion).
Distribution of Materials
Upon request, all novel materials described in this publication will be made available in a timely manner under standard material transfer agreements for noncommercial research purposes.
EST data from this article have been deposited with the GenBank data library under accession numbers CO208543, CO208906, CO218750, CO231336, CV720218, and CV720219.
Supplementary Material
Acknowledgments
We thank Dr. David Ellis for the generous gift of Sitka spruce seedlings, Dr. René I. Alfaro for the generous gift of insects, Ms. Natalia Kolosova and Dr. Jun Zhuang for technical assistance, Dr. Diane Martin for technical advice, David Kaplan for excellent greenhouse support, and Dr. Dezene Huber for critical reading of the manuscript.
This work was supported by the Natural Sciences and Engineering Research Council of Canada and the Human Frontier Science Program (grants to J.B.), and infrastructure funds from the Canadian Foundation for Innovation and the British Columbia Knowledge and Development Funds. Expressed sequence tag cDNA clones were from the Treenomix database developed with support from Genome Canada, Genome British Columbia, and the Province of British Columbia.
The online version of this article contains Web-only data.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.104.050187.
References
- Alfaro RI (1995) An induced defense reaction in white spruce to attack by the white-pine weevil, Pissodes strobi. Can J For Res 25: 1725–1730 [Google Scholar]
- Alfaro RI, Borden JH, King JN, Tomlin ES, McIntosh RL, Bohlmann J (2002) Mechanisms of resistance in conifers against shoot infesting insects. In MR Wagner, KM Clancy, F Lieutier, TD Paine, eds, Mechanisms and Deployment of Resistance in Trees to Insects. Kluwer Academic Press, Dordrecht, The Netherlands, pp 101–126
- Arimura G, Huber DPW, Bohlmann J (2004) Forest tent caterpillars (Malacosoma disstria) induce local and systemic diurnal emissions of terpenoid volatiles in hybrid poplar (Populus trichocarpa x deltoides): cDNA cloning, functional characterization, and patterns of gene expression of (−)-germacrene D synthase, PtdTPS1. Plant J 37: 603–616 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Kessler A, Halitschke R (2002) Volatile signaling in plant-plant-herbivore interactions: What is real? Curr Opin Plant Biol 5: 351–354 [DOI] [PubMed] [Google Scholar]
- Bohlmann J, Croteau R (1999) Diversity and variability of terpenoid defenses in conifers: molecular genetics, biochemistry and evolution of the terpene synthase gene family in grand fir (Abies grandis). In DJ Chadwick, JA Goode, eds, Insect Plant Interactions and Induced Plant Defense. John Wiley and Sons, West Sussex, UK, pp 132–146 [DOI] [PubMed]
- Bohlmann J, Steele CL, Croteau R (1997) Monoterpene synthases from grand fir (Abies grandis). cDNA isolation, characterization, and functional expression of myrcene synthase, (−)-(4S)-limonene synthase, and (−)-(1S,5S)-pinene synthase. J Biol Chem 272: 21784–21792 [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Verstappen FWA, Posthumus MA, Dicke M (1999) Spider mite-induced (3S)-(E)-nerolidol synthase activity in cucumber and lima bean. Plant Physiol 121: 173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byun McKay A, Hunter W, Goddard K, Wang S, Martin D, Bohlmann J, Plant A (2003) Insect attack and wounding induce traumatic resin duct development and gene expression of (−)-pinene synthase in Sitka spruce. Plant Physiol 133: 368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fäldt J, Martin D, Miller B, Rawat S, Bohlmann J (2003) Traumatic resin defense in Norway spruce (Picea abies): methyl jasmonate-induced terpene synthase gene expression, and cDNA cloning and functional characterization of (+)-3-carene synthase. Plant Mol Biol 51: 119–133 [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Krekling T, Christiansen E (2002) Application of methyl jasmonate on Picea abies (Pinaceae) stems induces defense-related responses in phloem and xylem. Am J Bot 89: 578–586 [DOI] [PubMed] [Google Scholar]
- Gijzen M, Lewinsohn E, Croteau R (1992) Antigenic cross-reactivity among monoterpene cyclases from grand fir and induction of these enzymes upon stem wounding. Arch Biochem Biophys 294: 670–674 [DOI] [PubMed] [Google Scholar]
- Hilker M, Kobs C, Varma M, Schrank K (2002) Insect egg deposition induces Pinus sylvestris to attract egg parasitoids. J Exp Biol 205: 455–461 [DOI] [PubMed] [Google Scholar]
- Hudgins JW, Franceschi VR (2004) Methyl jasmonate-induced ethylene production is responsible for conifer phloem defense responses and reprogramming of stem cambial zone for traumatic resin duct formation. Plant Physiol 135: 2134–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2001) Defensive function of herbivore-induced plant volatile emissions in nature. Science 291: 2141–2144 [DOI] [PubMed] [Google Scholar]
- Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299–328 [DOI] [PubMed] [Google Scholar]
- Kolosova N, Miller B, Ralph S, Ellis BE, Douglas C, Ritland K, Bohlmann J (2004) Isolation of high-quality RNA from gymnosperm and angiosperm trees. Biotechniques 36: 821–824 [DOI] [PubMed] [Google Scholar]
- Langenheim JH (2003) Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany. Timber Press, Portland, OR
- Litvak ME, Monson RK (1998) Patterns of induced and constitutive monoterpene production in conifer needles in relation to insect herbivory. Oecologia 114: 531–540 [DOI] [PubMed] [Google Scholar]
- Martin DM, Fäldt J, Bohlmann J (2004) Functional characterization of nine Norway spruce TPS genes and evolution of gymnosperm terpene synthases of the TPS-d subfamily. Plant Physiol 135: 1908–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Gershenzon J, Bohlmann J (2003) Induction of volatile terpene biosynthesis and diurnal emission by methyl jasmonate in foliage of Norway spruce (Picea abies). Plant Physiol 132: 1586–1599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DM, Tholl D, Gershenzon J, Bohlmann J (2002) Methyl jasmonate induces traumatic resin ducts, terpenoid resin biosynthesis, and terpenoid accumulation in developing xylem of Norway spruce stems. Plant Physiol 129: 1003–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm R, Schrank K, Wegener R, Schulz S, Hilker M (2003) Chemical analysis of volatiles emitted by Pinus sylvestris after induction by insect oviposition. J Chem Ecol 29: 1235–1252 [DOI] [PubMed] [Google Scholar]
- Paré PW, Tumlinson JH (1999) Plant volatiles as a defense against insect herbivores. Plant Physiol 121: 325–332 [PMC free article] [PubMed] [Google Scholar]
- Persson M, Sjodin K, Borg Karlson AK, Norin T, Ekberg I (1996) Relative amounts and enantiomeric compositions of monoterpene hydrocarbons in xylem and needles of Picea abies. Phytochemistry 42: 1289–1297 [Google Scholar]
- Phillips MA, Croteau RB (1999) Resin-based defenses in conifers. Trends Plant Sci 4: 184–190 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5: 237–243 [DOI] [PubMed] [Google Scholar]
- Richard S, Lapointe G, Rutledge RG, Seguin A (2000) Induction of chalcone synthase expression in white spruce by wounding and jasmonate. Plant Cell Physiol 41: 982–987 [DOI] [PubMed] [Google Scholar]
- Röse USR, Manukian A, Heath RR, Tumlinson JT (1996) Volatile semiochemicals released from undamaged cotton leaves. Plant Physiol 111: 487–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnee C, Köllner TG, Gershenzon J, Degenhardt J (2002) The maize gene terpene synthase 1 encodes a sesquiterpene synthase catalyzing the formation of (E)-β-farnesene, (E)-nerolidol, and (E,E)-farnesol after herbivore damage. Plant Physiol 130: 2049–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönwitz R, Kloos M, Merk L, Ziegler H (1990) Patterns of monoterpenes stored in the needles of Picea abies (L.) Karst. from several locations in mountainous regions of southern Germany. Trees-Struct Funct 4: 27–33 [Google Scholar]
- Seybold SJ, Bohlmann J, Raffa KF (2000) Biosynthesis of coniferophagous bark beetle pheromones and conifer isoprenoids: evolutionary perspective and synthesis. Can Entomol 132: 697–753 [Google Scholar]
- Shen B, Zheng Z, Dooner HK (2000) A maize sesquiterpene cyclase gene induced by insect herbivory and volicitin: characterization of wild-type and mutant alleles. Proc Natl Acad Sci USA 97: 14807–14812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele CL, Crock J, Bohlmann J, Croteau R (1998. a) Sesquiterpene synthases from grand fir (Abies grandis): comparison of constitutive and wound-induced activities, and cDNA isolation, characterization, and bacterial expression of δ-selinene synthase and γ-humulene synthase. J Biol Chem 273: 2078–2089 [DOI] [PubMed] [Google Scholar]
- Steele CL, Katoh S, Bohlmann J, Croteau R (1998. b) Regulation of oleoresinosis in grand fir (Abies grandis): differential transcriptional control of monoterpene, sesquiterpene, and diterpene synthase genes in response to wounding. Plant Physiol 116: 1497–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlin ES, Alfaro RI, Borden JH, He FL (1998) Histological response of resistant and susceptible white spruce to simulated white pine weevil damage. Tree Physiol 18: 21–28 [DOI] [PubMed] [Google Scholar]
- Tomlin ES, Antonejevic E, Alfaro RI, Borden JH (2000) Changes in volatile terpene and diterpene resin acid composition of resistant and susceptible white spruce leaders exposed to simulated white pine weevil damage. Tree Physiol 20: 1087–1095 [DOI] [PubMed] [Google Scholar]
- Trapp SC, Croteau R (2001) Defensive resin biosynthesis in conifers. Annu Rev Plant Physiol Plant Mol Biol 52: 689–724 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.