Abstract
The toxic dinoflagellate Alexandrium minutum Halim is one of three species that comprise the “minutum” species complex. This complex is notable due to its role in the etiology of paralytic shellfish poisoning (PSP). Recent increases in PSP incidence and the geographic expansion of toxin-producing Alexandrium dinoflagellates have prompted the intensive examination of genetic relationships among globally distributed strains to address questions regarding their present distribution and reasons for their apparent increase. The biogeography of A.minutum was studied using large subunit ribosomal DNA gene (LSU rRNA) and internal transcribed spacer (ITS) sequences and genotypic data from 12 microsatellite loci. rRNA gene and ITS sequencing data distinguished between two clades, herein termed the “Global” and the “Pacific”; however, little to no resolution was seen within each clade. Genotypic data from 12 microsatellite loci provided additional information regarding genetic relationships within the Global clade, but it was not possible to amplify DNA from the Pacific clade using these markers. With the exception of isolates from Italy and Spain, strains generally clustered according to origin, revealing geographic structuring within the Global clade. Additionally, no evidence supported the separation of A. lusitanicum and A. minutum as different species. With the use of microsatellites, it is now possible to initiate studies on the origin, history, and genetic heterogeneity of A. minutum that were not previously possible using only rRNA gene sequence data. This study demonstrates the power of combining a marker with intermediate resolution (rRNA sequences) with finer-scale markers (microsatellites) to examine intraspecies variability among globally distributed isolates and represents the first effort to employ this technique in A. minutum.
Keywords: A. lusitanicum, A. minutum, biogeography, genotype, harmful algal blooms, LSU rRNA, microsatellites, phylogeny
Introduction
Harmful algal blooms (HABs) are a serious and growing problem in the U.S. and the world. Impacts include the illness and death of human consumers of contaminated seafood, mass mortalities of fish and marine animals, deterioration of coastal aesthetics and water quality, and broadly based ecosystem impacts. Among the multiple poisoning syndromes linked to HABs, PSP is arguably the most widespread and significant on a global basis. Dinoflagellates within the genus Alexandrium, and in particular within the “tamarensis” (A. tamarense, A. fundyense and A. catenella) and “minutum” (A. minutum, A. lusitanicum, and… angustitabulatum) species complexes, are responsible for many of these PSP outbreaks worldwide (Cembella 1998).
In recent decades, the frequency of toxic Alexandrium blooms has increased, as have HABs in general (Anderson 1989, Hallegraeff 1993). Concomitant with this apparent increase, species within both the tamarensis and minutum groups appear to have expanded their geographic distribution (Lilly et al. 2005, 2007). Prior to 1970, dinoflagellates in the tamarense complex were only known from temperate waters of Europe, North America, and Japan (Dale and Yentsch 1978); however, their range has since expanded to include South America, South Africa, Australia, the Pacific Islands, India, Asia, and the Mediterranean (Hallegraeff 2003). Similarly, …minutum species were previously known only from the waters of the Mediterranean Sea and South Australia (Hallegraeff et al. 1988). Since the mid-1980s, however, A. minutum has been linked to PSP in Ireland (Gross 1989), and toxic populations have been identified in northern France (Belin, 1993), northwestern Spain (Franco et al. 1994), the North Sea (Nehring 1998, Elbrachter 1999, Persson et al. 2000, Hansen et al. 2003), India (Godhe et al. 2000, Godhe et al. 2001), Malaysia (Usup et al. 2002), and Vietnam (Yoshida et al. 2000). In Europe, monitoring records document the expansion of A. minutum from France to Ireland, England, and Denmark (Nehring 1998, Hansen et al. 2003,). Similar expansions are reported in Taiwan (Hwang et al. 1999) and New Zealand (Chang et al. 1997, Chang et al. 1999).
Anderson (1989) presented several explanations for the expansion of HABs that relate to increased scientific study and/or increased exposure due to human activities, including: (1) improved scientific awareness and analytical capabilities; (2) increased use of coastal waters for aquaculture; and (3) improved detection, preservation and quantification methods. In addition to these factors, long-term increases in nutrient loading of coastal waters and unusual climatological conditions are believed to favor certain species and stimulate blooms (Anderson 1989, Hallegraeff 1993). These explanations cannot, however, account for all increases in PSP, and both natural dispersal (Vila et al. 2001, Persich et al. 2003) and human-assisted transport (e.g., Lilly et al. 2002) have been documented. Although it is difficult to characterize the incidence of these latter mechanisms, molecular methods provide an effective means for studying dispersal in Alexandrium and its relative contribution to rising PSP incidence.
To understand better why HABs caused by A. minutum are increasing in biogeographic range and frequency, we first need to characterize the genetic relatedness among geographically diverse isolates. This process will allow us to begin to address questions regarding the present distribution of these organisms and whether or not this apparent increase is attributable to natural dispersal, human-assisted transport, eutrophication, or simply the discovery of previously unknown populations. However, due to the lack of fine-scale genetic markers, studies have been limited in scope to broad phylogenetic analyses.
Past studies on Alexandrium have primarily examined rRNA gene sequences to address questions regarding taxonomy and phylogeography. John et al. (2003) and Lilly et al. (2007) examined the phylogeny and historical biogeography of the A, tamarense species complex using large and small subunit rRNA gene sequences. These studies provided an evolutionary framework within which the genetic relationships among Alexandrium morphospecies comprising the complex were examined. John et al. (2003) proposed a paleobiogeographic scenario that described how vicariant events (e.g., geological events, changing ocean currents, and paleoclimatic changes) resulted in the present distribution of the tamarense complex.
Lilly et al. (2005) characterized the phylogenetic relationships of globally distributed strains of A. minutum using hypervariable sequences of the D1–D2 domain of the LSU rRNA. Their analyses recovered two monophyletic groups, termed “Global” and “Pacific” clades; however, the sequence data were insufficient to resolve finer-scale differences among isolates within each clade. Given these limitations, many biogeographic and genetic questions could not be addressed. The objectives of this study were therefore to (1) identify high-resolution genetic markers to distinguish among strains of A. minutum on a global scale; (2) use these markers to identify genetic similarities and differences among strains of A. minutum; (3) explore the relationship between A. lusitanicum and A. minutum; and (4) provide a greater understanding of the relationship between A. minutum strains in the Global and Pacific clades.
Methods
Isolates
Clonal cultures of A. minutum and A. lusitanicum used in this study are listed in Table S1 in the supplementary material. Four additional strains (two A. sp, one A. tamutum and one A. ostenfeldii) were chosen for use in the phylogenetic analysis. All cultures were maintained at 15 or 20°C at 250 μmol•photons•m−2•sec−1 on a 14h:10h light:dark cycle in modified f/2 medium without silica (Guillard 1975, Anderson et al. 1994,).
DNA extraction
When cultures reached mid-exponential phase, ~20 mL was harvested by centrifugation (3000. for 5 min; Eppendorf 5702 Centrifuge: Eppendorf, Westbury, NY, USA). DNA was extracted from cell pellets following the manufacturer’s instructions for the Qiagen DNeasy Tissue Kit (Valencia, CA, USA) with an elution volume of 200 μL. Whole genomic DNA was stored at −20°C until used for PCR amplification.
PCR amplification of D1–D6 and D8–D10 LSU rRNA and ITS regions
The highly variable D1–D6 (~1450 bp) and D8–D10 (~850 bp) domains of the large subunit ribosomal RNA gene (LSU rRNA) and the internal transcribed spacer (ITS) ITS1, 5.8S and ITS2 (herein collectively called ITS, ~550 bp) were amplified from whole genomic DNA. Amplification was performed using the PCR and the previously reported primers D1R (Scholin and Anderson 1994) and 28-1483R (Daugbjerg et al. 2000), FD8 and RB, and ITS1 and ITS4 (Chinain et al. 1998, D’Onofrio et al. 1999), respectively. PCR reactions contained 1X buffer, 1.0 μM of each dNTP, 2.5 μM forward and reverse primer, 1 μL DNA template, and nuclease-free water to a total volume of 50 μL. Cycling conditions were as follows: hotstart at 96 °C for 5 min then 1.0 units of Taq DNA polymerase (New England Biolabs, Ipswich, MA, USA) was added; 40 cycles of 95°C for 45 s, 55°C for 45 s, 72°C for 1 min, with a final extension of 72°C for 10 min. To determine if amplification was successful, PCR products were separated on a 1% TAE agarose gel adjacent to a 100 bp DNA ladder. Positive PCR products were purified using the Qiagen MinElute PCR purification kit following the manufacturer’s instructions. Purified products were stored at −20°C until needed for sequencing. An estimate of the concentration was determined relative to the 100 bp DNA ladder.
DNA sequencing
DNA sequencing was performed using ABI BigDye version 3.0 (Applied Biosytems Inc., Foster City, CA, USA). The reactions consisted of 0.5 μL BigDye, 0.6 μL of 10 μM forward or reverse PCR primer, 0.1 μL DMSO and 1–3 μL purified PCR product to a volume of 6 μL with nuclease-free water. Thermocycling conditions were 60 cycles of 96°C for 15 s, 50°C for 5 s, and 60°C for 4 min. Reactions were precipitated using isopropanol, air dried, and resuspended in Hi-Di Formamide before being analyzed on an ABI 3730xl. Products were sequenced in both the forward and reverse direction.
DNA sequence analysis
Sequences were edited by eye for base-calling errors and consensus sequences were assembled using Sequencher 4.2.2 (Gene Codes, Ann Arbor, MI, USA), a DNA sequence editing program. The accession numbers of the consensus sequences deposited in GenBank are listed in Table S2 in the supplementary material. Once consensus sequences were complete for each region (D1–D6 and D8–D10 LSU rRNA, and ITS) they were concatenated by hand. Full consensus sequences were imported into the multiple alignment software program, MacClade 3.06 (Maddison and Maddison 2001), where alignments were checked by eye.
Modeltest V. 3.7 (Posada and Crandall 1998) was used to select the appropriate model of nucleotide substitution for phylogenetic analyses. Phylogenetic trees were constructed with PAUP* version 4.0b 10 (Swofford, 2000) using maximum likelihood (ML) analyses with A. species (D163, D164), A. tamutum (AL2T), and A. ostenfeldii (LK-E6) as outgroups. LSU rRNA/5.8S concatenated sequences, ITS sequences, and the full concatenated alignment were examined separately. Heuristic searches using ML employed the following model parameters: (1) for the LSU rRNA/5.8S data the Tamura-Nei model (TrN + I) (Tamura and Nei 1993) was used with base frequencies A=0.2813, C=0.2617, G=0.1755, T=0.2815, variable substitution rates (AC=1, AG=6.2420, AT=1, CG=1, CT =2.5611, and GT = 1), and invariable (I) sites=0.7985; (2) for the ITS data the general-time-reversible model (GTR + Γ) (Lanave et al. 1984, Tavare 1986, Rodriguez et al. 1990) was used with base frequencies A=0.3515, C=0.2450, G=0.2107, T=0.1928, variable substitution rates (AC=0.5769, AG=1.4945, AT=0.6848, CG=0.1209, CT =0.9571, and GT = 1), and gamma (Γ) distribution=0.9196; and (3) for the LSU rRNA/ITS alignment the GTR+Γ+I model was used with base frequencies A=0.2901, C=0.2594, G=0.1802, T=0.2703, variable substitution rates (AC = 0.8815, AG = 3.5537, AT = 0.7381, CG = 0.2915, CT = 1.298, GT = 1), Γ=0.8013, and I=0.6069. Bootstrap support values were determined for the concatenated alignment using 100 replicates.
PCR amplification of microsatellite regions
Twelve microsatellite loci were used to examine the genetic heterogeneity of A. minutum and A. lusitanicum using previously designed primers (Nagai et al. 2006a). Characterizations of these loci are shown in Table 1. PCR reactions contained 5 ng of template DNA, 0.2 mM of each dNTP, 0.5 μM of each designed primer pair, with one primer labelled with 6FAM, NED, PET, or VIC, 1× PCR buffer (10mM Tris-HCl, pH 8.3, 500mM KCl, 15 mM MgCl2, 0.01% w/v gelatin), and 0.25 U of ABI Ampli Taq Gold to a total volume of 10 μL. The PCR cycling conditions were as follows: 10 min at 94°C, 38 cycles of 30 s at 94°C, 30 s at 60°C for the first 10 cycles then at the primer-specific annealing temperature (see Table 1) for the last 28 cycles, and 1 min at 72°C, and a final elongation for 5 min at 72°C.
Table 1.
Species, strain identification, toxicity, collection site, and GenBank accession number for cultures and sequences used in this study. Y = yes; N = no; ND = no data.
| Genus species | Strain ID | Collection Site | Toxic | GenBank Accession No. |
|---|---|---|---|---|
| A. lusitanicum | ||||
| 18-1NT | Obidos Lagoon, Portugal | N | EU707455, EU707488–489 | |
| 18-1T | Obidos Lagoon, Portugal | Y | EU707456, EU707490–491 | |
| AL2V | Ria de Vigo, Spain | Y | EU707460, EU707498–499 | |
| GT PORT | Obidos Lagoon, Portugal | Y | EU707482, EU707538–539 | |
| A. minutum | ||||
| AL1T | Punta Sottile, Gulf of Trieste, Northern Adriatic | N | EU707457, EU707492–493 | |
| AL1V | Ria de Vigo, Spain | Y | EU707458, EU707494–495 | |
| AL3V | Ria de Vigo, Spain | Y | EU707461, EU707500–501 | |
| AL4V | Ria de Vigo, Spain | Y | EU707462, EU707502–503 | |
| AL5T | Punta Sottile, Gulf of Trieste, Northern Adriatic Sorgenti di Aurisina, Gulf of Trieste, Northern | N | EU707463, EU707504–505 | |
| AL8T | Adriatic Sorgenti di Aurisina, Gulf of Trieste, Northern |
Y | EU707464, EU707506–507 | |
| AL9T | Adriatic | Y | EU707465, EU707508–509 | |
| AM1 | Morlaix Bay, France | Y | EU707466, EU707510–511 | |
| AM2 | Morlaix Bay, France | Y | ND | |
| AM3 | Morlaix Bay, France | Y | ND | |
| AMAD01 | Port River, Australia | Y | EU707467, EU707512–513 | |
| AMAD06 | Port River, Australia | Y | ND | |
| AMBOPO06 | Pio’s Ocean Beach, western Bay of Plenty, New Zealand | Y | EU707468, EU707514–515 | |
| AMBOPO14 | Tauranga Harbor, western Bay of Plenty, New Zealand | Y | EU707469, EU707516–517 | |
| AMD21 | Port River, Australia | Y | EU707470, EU707518–519 | |
| AMFL | Fleet Lagoon, England | Y | EU707471, EU707520–521 | |
| AMI A1 | Syracuse, Ionian Sea, Sicily, Italy | Y | EU707472, EU707550–551 | |
| AMI A4 | Syracuse, Ionian Sea, Sicily, Italy | Y | ND | |
| AMI A5 | Syracuse, Ionian Sea, Sicily, Italy | Y | ND | |
| AMIR-1 | Cork Harbor, Ireland | Y | EU707473, EU707522–523 | |
| AMIR-3 | Cork Harbor, Ireland | Y | EU707474, EU707552–553 | |
| AMITA | Adriatic Sea | Y | EU707475, EU707524–525 | |
| AMNZ01 | Croisilles Harbor, New Zealand | Y | EU707476, EU707526–527 | |
| AMNZ02 | Anakoha Bay, New Zealand | Y | EU707477, EU707528–529 | |
| AMNZ03 | Anakoha Bay, New Zealand | Y | EU707478, EU707530–531 | |
| AMP4 | Palma de Mallorca, Spain | Y | EU707479, EU707532–533 | |
| LAC27 | Gulf of Trieste, Northern Adriatic | Y | ND | |
| SAAM01 | Cape Town, South Africa | Y | ND | |
| SAAM02 | Cape Town, South Africa Y | EU707484, EU707542–543 | ||
| SAAM06 | Cape Town, South Africa | Y | ND | |
| SAAM07 | Cape Town, South Africa | Y | ND | |
| SAAM08 | Cape Town, South Africa | Y | EU707485, EU707544–545 | |
| SAAM11 | Cape Town, South Africa | Y | ND | |
| SAAM12 | Cape Town, South Africa | Y | EU707486, EU707546–547 | |
| SAAM15 | Cape Town, South Africa | Y | ND | |
| SZN 030 | Gulf of Naples, Italy | Y | EU707487, EU707548–549 | |
| A. species | ||||
| D163 C5 | Iwate, Japan | N | EU707480, EU707534–535 | |
| D164 C6 | Iwate, Japan | N | EU707481, EU707536–537 | |
| A. tamutum | ||||
| AL2T | Punta Sottile, Gulf of Trieste, Northern Adriatic | N | EU707459, EU707496–497 | |
| A. ostenfeldii | ||||
| LK-E6 | Gulf of Maine, USA | EU707483, EU707540–541 |
Fragment analysis
PCR products were diluted 3–5 X with nuclease-free water and 1 μL of diluted product was mixed with 0.25 μL 500 LIZ Size Standard and 8.75 μL Hi-Di Formamide, and then analyzed using an ABI 3730xl DNA Analyzer. Allele sizes were determined using the program FPMiner, BioinforSoft, LLC, 2005.
Cluster analysis
Cluster analysis was performed on microsatellite data by first converting the allele size data into binary format, scoring each strain for the presence (1) or absence (0) of each allele. The binary data was imported into Phyltools (www.dpw.wau.nl/pv/PUB/pt/) and a distance matrix was created using the Nei algorithm (Nei and Li, 1979). This matrix was imported into Phylip 3.6 (Felsenstein 1986) where it was analyzed using Neighbor. The non-toxic AL1T strain was used as an outgroup because it formed a sister to the Global Clade on the LSU rRNA and ITS phylogenetic tree. Draw Gram was selected to create a tree from the Neighbor file. Bootstrap analysis was also performed by creating 100 distance matrices in Phyltools, with analysis performed in Phylip using Neighbor and Consense.
Results
Phylogenetic analysis of LSU rRNA and ITS sequences
The concatenated LSU rRNA and ITS sequence alignment included 33 taxa covering 2833 bases total, of which 2453 bases were conserved, 106 bases were variable but parsimony-uninformative, and 274 bases were parsimony-informative. The phylogenetic tree produced using maximum likelihood is shown in Figure 1, with a map of origin locations shown in Figure 2. Trees produced using maximum likelihood analyses of the LSU rRNA/5.8S and ITS regions exhibited identical topology (data not shown). Our analyses identified two major clades, both supported by high bootstrap values (100%). The larger clade included all of the European, South African, and Australian A. minutum and A. lusitanicum strains. Genetic distances for the entire clade, excluding AL1T, were less than 1%, and the few sequence differences were distributed throughout the gene regions examined. The genetic distance between the non-toxic strain AL1T and the rest of this large clade was 2%, and those sequence differences clustered in the ITS1 and ITS2 regions. The smaller clade contained only A. minutum from New Zealand. The sequences for all five strains in this clade were at least 99.9% identical (2830 of 2833 bp were constant). The small clade differed from the larger clade by approximately 9–11%.
Figure 1.
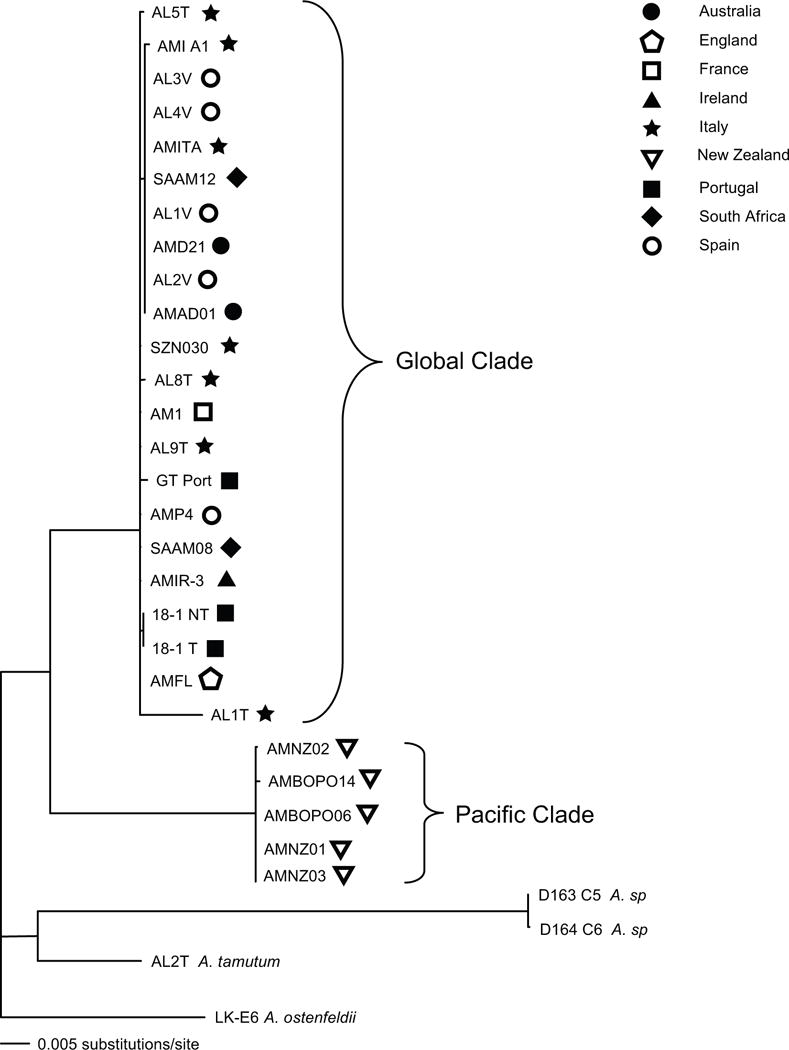
Maximum likelihood tree using the GTR + Γ + I model on the combined LSU and ITS sequence alignment (2833 bp). Bootstrap values were determined using 100 replicates and values greater that 50% are shown above the branches. The outgroups used for this tree are D163 C5, D164 C6, AL2T, and LK-E6. Symbols indicate general collection location of strains, consistent with the symbols in Figure 2. Toxin producing characteristics are shown at the end of each branch (N = toxic, NT = non-toxic, ND = No data).
Figure 2.
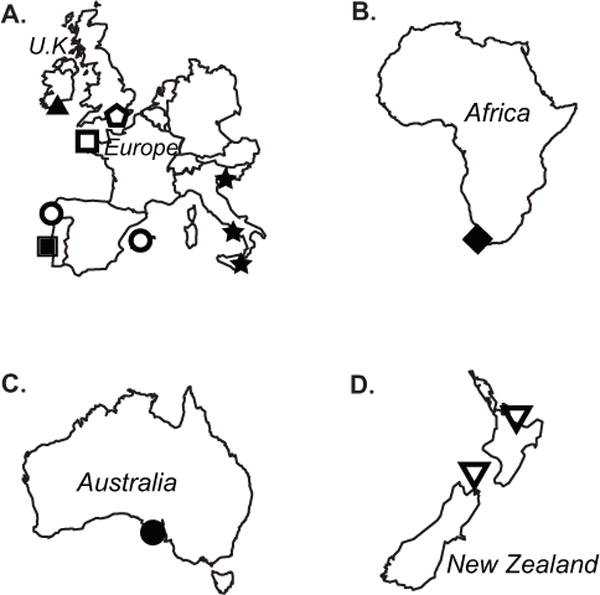
Regional maps showing the general collection location of strains used in this study: (a) England, Ireland, Italy, France, Portugal, and Spain; (b) South Africa; (c) Australia; and (d) New Zealand. Symbols are consistent with those of Figures 1 and 3. Note that sizes of the countries and their geographic proximity are not to scale.
Cluster analysis of genotypic data (microsatellites)
A dendrogram based upon cluster analysis of microsatellite genotypes is shown in Figure 3, with symbols to represent the strain origins. A few notable features were evident in the dendrogram. First, strains from South Africa, Portugal, Ireland, Australia, and France grouped according to collection location. While there is genetic similarity among isolates from each location, the individual genotypes from each strain were still unique, except for three pairs of strains that share the same genotype (SAAM01/SAAM02, SAAM06/SAAM07, and ALUS 18-1/ALUS USA).
Figure 3.
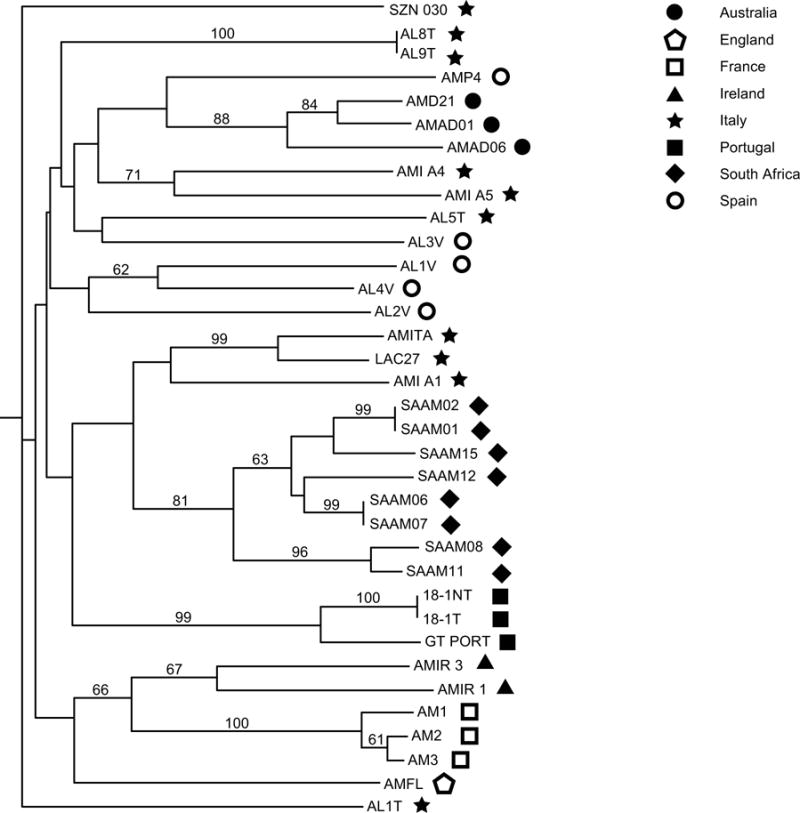
Dendrogram generated from data collected from 12 microsatellite loci using the Nei algorithm (Nei 1979). Bootstrap values above 50% are shown. Strain AL1T was used as an outgroup. Symbols represent the general collection location and are consistent with symbols in Figures 1 and 2. Toxin producing characteristics are shown at the end of each branch (N = toxic, NT = non-toxic, ND = No data).
Second, isolates from Italy and Spain were scattered throughout the dendrogram, with multiple groupings found from these regions. It is worth noting, however, that strains were collected from more than one site around these two countries. The Italian strains were collected from the Northern Adriatic (northeastern Italy), the Ionian Sea near Sicily (southern Italy), and the Gulf of Naples (western Italy). Three of the Italian strains grouped together – the two that were collected from the Northern Adriatic (AMITA and LAC27) and the other from the Ionian Sea (AMI A1). Two strains collected from Sorgenti di Aurisina in the Northern Adriatic had identical genotypes and form a small group, and another small cluster included two strains collected from the Ionian that did not have identical genotypes. Two of the remaining three Italian strains (SZN 030 from the Gulf of Naples and AL1T from the Northern Adriatic), fell outside of the main branching point of the dendrogram, as AL1T was used as the outgroup and SZN 030 is apparently most similar to this outgroup. The Spanish strains were collected from Ria de Vigo in western Spain and Palma de Mallorca in the Mediterranean. Three of the strains from Ria de Vigo grouped together (AL1V, AL4V, and AL2V), while the remaining strain from this location grouped with an Italian strain as mentioned above. The only strain isolated from Palma de Mallorca formed a sister to the group of Australian strains.
Lastly, New Zealand A. minutum isolates were not included in the dendrogram because they did not amplify using these specific microsatellite primers. Similarly, the primers did not generate products for the A. sp., A. ostenfeldii, and A. tamutum isolates that were used as outgroups to the LSU rRNA and ITS tree.
The South African strains were also noteworthy because the following pairs of strains were isolated from the germinated progeny of individual cysts: SAAM01/SAAM02; SAAM06/SAAM07; SAAM08/SAAM11; SAAM12/SAAM15. For pairs SAAM01/SAAM02 and SAAM06/SAAM07, each strain had the same genotype as its partner, whereas SAAM08/SAAM11 and SAAM12/SAAM15 shared many common alleles with their partners but were not genotypically identical (Fig 3). Another interesting feature of the South African strains was that they contained alleles that are unique to that region, at 5 of the 12 loci, showing a clear genetic distinction between strains from South Africa and those from Europe and South Australia.
Discussion
A. minutum is globally distributed, yet strains of this dinoflagellate group into only two clades, termed the Global and the Pacific (Lilly et al. 2005). These clades were recovered in the analysis reported here, which examined a larger portion of the rRNA gene and its ITS region. To provide additional resolution, we used 12 microsatellite markers to assess the genetic diversity of this species at a finer- scale. Isolates from Australia, South Africa, France, and Portugal formed separate geographic clades in our microsatellite analyses, thus demonstrating the power of these finer-scale markers to examine intra-species variability in A. minutum. Similar to DNA sequence data, microsatellite analyses were also unable to distinguish between A. lusitanicum and A. minutum. These markers make it possible to initiate studies on the origin, history, and genetic heterogeneity of A. minutum that previously were not possible using only ribosomal RNA gene sequence data. These findings are discussed in more detail below.
Intra-clade Variability
Previous work by Lilly et al. (2005) used sequences from the D1–D2 hypervariable domains (700 bp) of LSU rRNA to study the global phylogeny of A. minutum. Two morphologically homogenous clades were identified, termed the Global and Pacific clades. Hoping to assess phylogenetic relationships within these clades, we examined a larger region of DNA (Fig 1), spanning 2833 bases from the hypervariable domains D1–D6 and D8–D10 of the LSU rRNA gene combined with the ITS1, 5.8S, and ITS2 regions. Our analyses recovered the Global and Pacific clades identified by Lilly et al. (2005), but provided no additional phylogenetic resolution.
These results prompted an analysis using microsatellite markers, which evolve much more rapidly. Microsatellite analyses of marine phytoplankton have expanded greatly in recent years as these markers have been successfully used to examine genetic relationships in Haptophyceae (Iglesias-Rodriguez et al., 2002), Bacillariophyceae Rynearson & Armbrust, 2004), Raphidophyceae (Nagai et al., 2006c), and Dinophyceae (Santos et al., 2003), including Alexandrium spp. (Nagai et al., 2004, Nagai et al. 2006b, Nagai et al. 2007, Nishitani et al. 2007). Here we used microsatellite markers to examine the relationships between globally distributed A. minutum strains in more detail than was possible using rRNA sequences. A dendrogram generated using microsatellite data from 12 loci showed that isolates from geographically diverse areas are also genetically distinct from one another (Fig 3). Strains from Australia, South Africa, France, and Portugal formed separate geographic clades; furthermore, the South African strains contained alleles that were unique to that region. In contrast, isolates from Italy and Spain were genetically diverse.
We could not investigate relationships within the Pacific clade of A. minutum, or between the two closely related Alexandrium sp. strains (D163 C5 and D164 C6) because we were unable to amplify the microsatellite markers from these strains, despite multiple attempts. There may be technical issues, such as DNA quality or the choice of PCR conditions, that prevented amplification of the microsatellite markers from the Pacific clade and A. sp. It is also possible that the genetic differences seen at the rRNA level reflect the overall genetic divergence amongst the clades, such that different markers would be needed for strains outside of the Global clade.
The primary strength of the microsatellites markers, as used in this study, is the ability to discriminate amongst members of the Global clade, providing a novel approach for examining genetic diversity and biogeography of these isolates. Furthermore, these loci may foster new hypotheses regarding natural dispersal and human-assisted transport in this species, as has been demonstrated with other Alexandrium dinoflagellates (e.g., evaluating the potential human-assisted introduction of A. catenella in France (Lilly et al. 2002) and Australia (de Salas 2000)).
Toxicity Patterns
In contrast with the tamarensis complex, major phylogenetic lineages within the minutum complex contain both toxic and non-toxic strains (Lilly et al. 2005 and this study). Within the Global clade, DNA sequences were nearly identical except for the non-toxic AL1T collected from the Northern Adriatic, which forms a sister to the larger clade. However, the other non-toxic strains used in this study, including AL5T (also from the Northern Adriatic), and ALUS 18-1 NT (from Portugal) are within the Global clade and do not group separately based on toxicity. This suggests that the divergence of AL1T is probably not a reflection of toxicity.
We wondered if a different, much more rapidly evolving, genetic marker like microsatellite markers would be able to resolve patterns in the toxigenicity of the strains in the Global clade, as was seen with geographic origin. However, there was no grouping by toxicity using the 12 microsatellite loci examined in this study and no alleles were found to be specific to either toxic or non-toxic strains. Thus, as with the rRNA sequences in this study and that of Lilly et al. (2005), the microsatellite data do not delineate toxic from non-toxic strains. However, as mentioned earlier, with the small number of non-toxic strains available to us, this inference lacks rigor, and should be evaluated with additional non-toxic cultures.
A. lusitanicum
Strains of both A. minutum and A. lusitanicum are present within the Global clade with virtually no resolution between these strains based on our rRNA and ITS sequence data (Fig 1). This concurs with the growing body of evidence (Franco et al. 1995, Hansen et al. 2003, Lilly et al. 2005, Mendoza et al. 1995, Zardoya et al. 1995) indicating that A. lusitanicum is not distinct from A. minutum. Further support for this claim is derived from the 12 microsatellite loci examined in this study. The four A. lusitanicum strains fall into two different clusters according to their isolation location rather than morphospecies designation. These two clusters are within the larger group of A. minutum strains, such that A. lusitanicum is indistinguishable from the European, South Australian, and South African A. minutum strains on the basis of microsatellite genotypes.
Two A. lusitanicum strains used in this study are a somewhat unusual case, as they represent separate daughter lines of a single parental culture. These two samples were derived from the toxic parent culture 18-1, isolated from Obidos Lagoon, Portugal in 1962. A subculture of this parent was sent to a different laboratory in 1992 where it remained toxic (18-1T). At sometime between 1995 (Franca et al. 1995, Mascarenhas et al. 1995) and 2000 (Pereira et al. 2000) the 18-1 culture became non-toxic and has since been renamed by Martins et al. (2004) as 18-1NT. Examination of these strains show that they are indistinguishable using LSU rRNA sequence comparisons and morphological analysis of thecal plate patterns (Martins et al. 2004). Our data found no LSU rRNA or ITS nucleotide differences, which concurs with Martins et al. (2004). Additionally, microsatellite data show that these two strains are genotypically identical in the 12 loci examined (Fig 3). During the 14 years in which they were cultured separately, the microsatellite loci used in this study apparently did not accumulate significant changes. While mutation rates for microsatellites in A. minutum (or any other dinoflagellate) have not been determined, these results seem reasonable, as reported mutation rates in a variety of organisms range from 10−2 to 5 × 10−6 mutations/generation and have been shown to vary widely among loci and species (Dallas 1992, Dietrich et al., 1992, Schug et al. 1998, Kovalchuck et al. 2000, Vazquez et al. 2000, Udupa and Baum, 2001).
Conclusions
DNA sequence data confirm the results of Lilly et al. (2005) and lend support to their proposal that members of the Pacific clade comprise a distinct Alexandrium species. In addition, our sequencing and microsatellite data provide no distinction between A. lusitanicum and A. minutum, supporting the reclassification of A. lusitanicum as A. minutum, as was also noted by Lilly et al. (2005). Most significantly, microsatellite data revealed geographic structuring of isolates within the Global clade, permitting the examination of genetic relationships in this group, which previously was not possible using rDNA sequence data. This expanded capability enables novel investigations of natural versus human-assisted species dispersal of A. minutum to identify the sources and determine the causes of its recent geographic expansion.
Supplementary Material
Table 2.
Primer pairs for amplification of twelve polymorphic microsatellite regions in the toxic dinoflagellate Alexandrium minutum and some characteristics of the loci. Ta indicates annealing temperature; gene diversity was calculated after Nei (1987). Thirty-five clonal strains were screened at each locus.
| Locus | Repeat motif | Primer sequence | Ta (°C) | No. of non-amplifying samples | No. of alleles | Size range (bp) | Gene diversity | Genbank accession number |
|---|---|---|---|---|---|---|---|---|
| Aminu08 | (CT)7GTC3(CT)3 | F: AGCCTCCTTGTCTCACTTCGTTTC R: PET-GTTATGCTATGCCATGCCTTGCC |
52 | 16 | 6 | 187–223 | 0.6094 | AB242303 |
| Aminu10 | (GT)5G11CA5 | F: 6FAM-GCTTGAGATGGAGTGGATAACGG R: GATACAATTTCGGGGGTAGAAGACTGG |
52 | 10 | 6 | 148–162 | 0.7264 | AB242304 |
| Aminu11 | (CT)13 | F: AGGAGAAATCACAAGCGGTGG R: VIC-GCAAACAAACAGGACTCTGAGAGC |
52 | 0 | 13 | 224–256 | 0.8180 | AB242305 |
| Aminu15 | (CT)14 | F:6FAM-CTTTACATACGCCTGTCTAGATCCCTT R: CCACASACAGTCTGACAGGAAGG |
52 | 7 | 7 | 209–235 | 0.7551 | AB242306 |
| Aminu20 | (CT)5C3(CT)13 | F: VIC-ACCTTGACAATGCTCCTGTTGGG R: CSYTGCTCTTGACATCACCATCTTG |
55 | 16 | 7 | 245–285 | 0.7922 | AB242307 |
| Aminu22 | (CT)19 | F: ATTTGGTCAACTGTCTCTCACCCTCAC R: 6FAM-GTAGCCATCACTATCCTCATTCGC |
55 | 0 | 9 | 182–204 | 0.8245 | AB242308 |
| Aminu29 | (CT)4C3(CT)13 | F: NED-GCAAACTGGATTCTGGCGAAAGG R: CTGAACAACTGTATTCGCCATCGC |
52 | 1 | 8 | 232–250 | 0.7059 | AB242309 |
| Aminu39 | (CT)10T6GAG7 | F: TCCTTTTTCTTTGAGGCGCTCG R: 6FAM-CAAGGTGTGATGGCCATCATG |
53 | 0 | 7 | 142–156 | 0.7722 | AB242310 |
| Aminu41 | (CT)13 | F: CTCCTGAGAAATGTGATTAGTGTTCG R: VIC-CAAGGCACGTGTGTTTGAAGTC |
55 | 3 | 14 | 165–247 | 0.8809 | AB242311 |
| Aminu43 | (CTA)2T(CT)14GAG5 | F: CACAAGGTTGCATCAGTAGG R: VIC-GAAAGAATTGCTTCCTCGACTG |
52 | 5 | 9 | 182–224 | 0.8267 | AB242312 |
| Aminu44 | (CT)17(CA)3 | F: CCTTGAACGTAGTAAGTAGCAACC R: 6FAM-GTCTACCCTTTTCTTTCTCAGAGCC |
52 | 2 | 12 | 257–285 | 0.8338 | AB242313 |
| Aminu48 | (GT)2CT(GT)4N4(GT)6(GC)5 | F: 6FAM-GCAGCTGGCAAAGTGATCCGTT R: CAAGGGTCTGGTTGATTCGG |
55 | 5 | 9 | 234–252 | 0.8133 | AB242314 |
Acknowledgments
This work would not have been possible without the generous contributions of cultures by researchers from many countries. We would also like to acknowledge Judy Kleindinst for her assistance with the figures, Katie Libera for her help with the manuscript, and David Kulis for maintaining all cultures used in this study. Funding was provided by the NSF Grants OCE-0430724 and OCE-0402707 and NIEHS grant 1 P50 ES012742.
Abbreviations
- HABs
harmful algal blooms
- PSP
paralytic shellfish poisoning
- LSU
large subunit
- ITS
internal transcribed spacer region
- bp
base pair(s)
Footnotes
Received ________; Accepted ________
Contributor Information
Linda A. R. McCauley, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts 02543, USA
Deana L. Erdner, University of Texas at Austin, Marine Science Institute, Port Aransas, Texas 78373, USA
Satoshi Nagai, National Research Institute of Fisheries and Environment of Inland Sea, 2-17-5 Maruishi, Hatsukaichi, Hiroshima 739-0452, Japan.
Mindy L. Richlen, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts 02543, USA
Donald M. Anderson, Woods Hole Oceanographic Institution, Woods Hole, Massachusetts 02543, USA.
References
- Anderson DM. Toxic algal blooms and red tides: a global perspective. In: Okaichi, Anderson DM, Nemoto, editors. Red tides: biology, environmental science, and toxicology. Elsevier Science Publishing Co., Inc; 1989. [Google Scholar]
- Anderson DM, Kulis DM, Doucette GJ, Gallagher JC, Balech E. Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeastern United States and Canada. Mar Biol. 1994;120:467–78. [Google Scholar]
- Belin C. Distribution of Dinophysis spp. and Alexandrium minutum along French coasts 69 since 1984 and their DSP and PSP toxicity levels. In: Smayda TJ, Shimizu Y, editors. Toxic Phytoplankton Blooms in the Sea. Elsevier; Amsterdam: 1993. pp. 469–74. [Google Scholar]
- Cembella AD. Ecophysiology and metabolism of paralytic shellfish toxins in marine microalgae. In: Anderson DM, Cembella AD, Hallegraeff GM, editors. Physiological Ecology of Harmful Algal Blooms. Springer-Verlag; Heidelberg: 1998. pp. 381–403. [Google Scholar]
- Chang FH, Anderson DM, Kulis DM, Till DG. Toxin Production of Alexandrium minutum (Dinophyceae) from the Bay of Plenty, New Zealand. Toxicon. 1997;35:393–409. doi: 10.1016/s0041-0101(96)00168-7. [DOI] [PubMed] [Google Scholar]
- Chang FH, Garthwaite I, Anderson DM, Towers M, Stewart R, MacKenzie L. Immunofluorescent detection of a PSP-producing dinoflagellate, Alexandrium minutum, from Bay of Plenty, New Zealand N. Z. J. J Mar Fresh Res. 1999;33:533–43. [Google Scholar]
- Chinain M, Germain M, Sako Y, Pauillac S, Legrand AM. Genetic diversity in French Polynesian strains of the ciguatera-causing dinoflagellate Gambierdiscus toxicus: RFLP and sequence analysis on the SSU and LSU rRNA genes. In: Reguera B, Blanco J, Fernández ML, Wyatt Tc, editors. Harmful Algae. Xunta de Galicia and Intergovernmental Oceanographic Commission of UNESCO; Paris: 1998. pp. 287–90. [Google Scholar]
- D’Onofrio G, Marino D, Blanco L, Busico E, Montresor M. Toward an assessment of the taxonomy of dinoflagellates that produce calcerous cysts (Calciodinelloideae, Dinophyceae): a morphological and molecular approach. J Phycol. 1999;35:1063–78. [Google Scholar]
- Dale B, Yentsch CM. Red tide and paralytic shellfish poisoning. Oceanus. 1978;21(3):41–49. [Google Scholar]
- Dallas JF. Estimation of the mutation rates in recombinant inbred strains of mouse. Mamm Genome. 1992;3:452–56. doi: 10.1007/BF00356155. [DOI] [PubMed] [Google Scholar]
- Daugbjerg N, Hansen G, Larsen J, Moestrup O. Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia. 2000;39:302–17. [Google Scholar]
- de Salas M, van Emmerik MJ, Hallegraeff GM, Negri AP, Vaillancourt RE, Bolch CJS. Toxic Australian Alexandrium dinoflagellates: introduced or indigenous? In: Hallegraeff GM, Blackburn SI, Bolch CJS, Lewis RJ, editors. Harmful Algal Blooms 2000. 2001. pp. 214–17. [Google Scholar]
- Dietrich W, Katz H, Lincoln S, Shin H, Friedman J, Dracopoli N, Lander E. A genetic map of the mouse suitable for typing intraspecific crosses. Genetics. 1992;131:423–27. doi: 10.1093/genetics/131.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbrachter M. Exotic flagellates of coastal North Sea waters. Helgol Wiss Meeresunters. 1999;52:235–42. [Google Scholar]
- Felsenstein J. Phylip (Phylogeny Inference Package) University of Washington; Seattle: 1986. [Google Scholar]
- Franca S, Viegas S, Mascarenhas V, Pinto L, Doucette GJ, Lassus P, Arzul G, Erard-Le Denn E. Prokaryotes in association with a toxic Alexandrium lusitanicum in culture. In: Gentien P, Marcaillou-Le Baut C, editors. Harmful Marine Algal Blooms/Proliferation d’Algues Marines Nuisibles. Proceedings of the Sixth International Conference on Toxic Marine Phytoplankton, Nantes (France), Oct 1993. Lavoisier; Paris (France): 1995. pp. 45–51. [Google Scholar]
- Franco JM, Fernandez P, Reguera B. Toxin profiles of natural populations and cultures of Alexandrium minutum Halim from Galician (Spain) coastal waters. J Appl Phycol. 1994;6:275–79. [Google Scholar]
- Franco JM, Fraga S, Zapata M, Bravo I, Fernandez P, Ramilo I. Comparison between different strains of genus Alexandrium of the minutum group. In: Lassus P, Arzul G, Erard E, Gentien P, Marcaillou C, editors. Harmful Marine Algal Blooms. Lavoisier; Paris: 1995. pp. 53–8. [Google Scholar]
- Godhe A, Karunasagar I, Karunasagar I, Karlson B. Dinoflagellate cysts in recent marine sediments from SW India. Bot Mar. 2000;43:39–48. [Google Scholar]
- Godhe A, Otta SK, Rehnstam-Holm AS, Karunasagar I, Karunasagar I. Polymerase chain reaction in detection of Gymnodinium mikimotoi and Alexandrium minutum in field samples from southwest India. Mar Biotech. 2001;3:152–62. doi: 10.1007/s101260000052. [DOI] [PubMed] [Google Scholar]
- Gross J. Re-occurrence of red tide in Cork Harbor, Ireland. Red Tide Newsletter. 1989;2:4–5. [Google Scholar]
- Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: Smith WL, Chanley MH, editors. Culture of marine invertebrate animals. Plenum Publishing Corporation; New York: 1975. pp. 29–60. [Google Scholar]
- Hallegraeff G, Steffensen DA, Wetherbee R. Three estuarine Australian dinoflagellates that can produce paralytic shellfish toxins. J Plankt Res. 1988;10:533–41. [Google Scholar]
- Hallegraeff GM. A review of harmful algal blooms and their apparent global increase. Phycologia. 1993;32:79–99. [Google Scholar]
- Hallegraeff GM. Harmful algal blooms: a global overview. In: Hallegraeff GM, et al., editors. Manual on harmful marine microalgae. Monographs on oceanographic methodology. Vol. 11. 2003. pp. 25–49. 2003. [Google Scholar]
- Hansen G, Daugbjerg N, Franco JM. Morphology, toxin composition and LSU rDNA phylogeny of Alexandrium minutum (Dinophyceae) from Denmark, with some morphological observations on other European strains. Harmful Algae. 2003;2:317–35. [Google Scholar]
- Hwang DF, Tsai YH, Liao HJ, Matsuoka K, Noguchi T, Jeng SS. Toxins of the dinoflagellate Alexandrium minutum Halim from the coastal waters and aquaculture ponds in southern Taiwan. Fish Sci. 1999;65:171–72. [Google Scholar]
- Iglesias-Rodriguez MD, Sáez AG, Groben R, Edwards KJ, Batley J, Medlin LM, Hayes PK. Polymorphic microsatellite loci in global populations of the marine coccolithophorid Emiliania huxleyi. Mol Ecol Notes. 2002;2:495–97. [Google Scholar]
- John U, Fensome RA, Medlin LK. The application of a molecular clock based on molecular sequences and the fossil record to explain biogeographic distributions within the Alexandrium tamarense “species complex” (Dinophyceae) Mol Biol Evol. 2003;20(7):1015–27. doi: 10.1093/molbev/msg105. [DOI] [PubMed] [Google Scholar]
- Kovalchuck O, Dubrova YE, Arkhipov A, Hohn B, Kovalckuck I. Wheat mutation rate after Chernobyl. Nature. 2000;407:583–84. doi: 10.1038/35036692. [DOI] [PubMed] [Google Scholar]
- Lanave C, Preparata G, Saccone C, Serio G. A new method for calculating evolutionary substitution rates. J Mol Evol. 1984;20:86–93. doi: 10.1007/BF02101990. [DOI] [PubMed] [Google Scholar]
- Lilly EL, Halanych KM, Anderson DM. Phylogeny, biogeography, and species boundaries within the Alexandrium minutum group. Harmful Algae. 2005;4:1004–20. [Google Scholar]
- Lilly EL, Halaynch KM, Anderson DM. Species boundaries and global biogeography of the Alexandrium tamarense complex (Dinophyceae) J Phycol. 2007;43:1329–1338. [Google Scholar]
- Lilly EL, Kulis DM, Gentien P, Anderson DM. Paralytic shellfish poisoning toxins in France linked to a human-introduced strain of Alexandrium catenella from the western Pacific: evidence from DNA and toxin analysis. J Plankt Res. 2002;24:443–52. [Google Scholar]
- Maddison DR, Maddison W. MacClade 4: Analysis of phylogeny and character evolution. evolution. 4.03 ed. Sinauer Associates; Sunderland, MA: 2001. [Google Scholar]
- Martins CA, Kulis D, Franca S, Anderson DM. The loss of PSP toxin production in a formerly toxic Alexandrium lusitanicum clone. Toxicon. 2004;43:195–205. doi: 10.1016/j.toxicon.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Mascarenhas V, Alvito P, Franca S, Sousa I, Rodriguez-Vasquez JA, Martinez AG. The dinoflagellate Alexandrium lusitanicum isolated from the coast of Portugal: observations on toxicity and ultrastructure during growth phases. In: Lassus P, Arzul G, Erard-Le Denn E, Gentien P, Marcaillou-Le Baut C, editors. Harmful Marine Algal Blooms/Proliferation d’Algues Marines Nuisibles. Proceedings of the Sixth International Conference on Toxic Marine Phytoplankton, Nantes (France), Oct 1993. Lavoisier; Paris (France): 1995. pp. 71–6. [Google Scholar]
- Mendoza H, López-Rodas V, González-Gil S, Aguilera A, Costas E. The use of polyclonal antisera and blocking of antibodies in the identification of marine dinoflagellates: species-specific and clone-specific antisera against Gymnodinium and Alexandrium. J Exp Mar Biol Ecol. 1995;186:103–15. [Google Scholar]
- Nagai S, Lian LC, Hamaguchi M, Matsuyama Y, Itakura S, Hogetsu T. Development of microsatellite markers in the toxic dinoflagellate Alexandrium tamarense (Dinophyceae) Mol Ecol Notes. 2004;4:83–85. [Google Scholar]
- Nagai S, McCauley L, Yasuda N, Erdner DL, Kulis DM, Matsuyama Y, Itakura S, Anderson DM. Development of microsatellite markers in the toxic dinoflagellate Alexandrium minutum (Dinophyceae) Mol Ecol Notes. 2006a;6:756–8. [Google Scholar]
- Nagai S, Sekino M, Matsuyama Y, Itakura S. Development of microsatellite markers in the toxic dinoflagellate Alexandrium catenella (Dinophyceae) Mol Ecol Notes. 2006b;6:120–22. [Google Scholar]
- Nagai S, Yamaguchi S, Lian LC, Matsuyama Y, Itakura S. Development of microsatellite markers in the noxious red tide-causing alga Heterosigma akashiwo (Raphidophyceae) Mol Ecol Notes. 2006c;6:477–79. [Google Scholar]
- Nagai S, Lian LC, Yamaguchi S, Hamaguchi M, Matsuyama Y, Itakura S, Shimada H, Kaga S, Yamauchi H, Sonda Y, Nishikawa T, Kim CH, Hogetsu T. Microsatellite markers reveal population genetic structure of the toxic dinoflagellate Alexandrium tamarense (Dinophyceae) in Japanese coastal waters. J Phycol. 2007;43:43–54. [Google Scholar]
- Nehring S. Non-indigenous phytoplankton species in the North Sea: supposed region of origin and possible transport vector. Arch Fish Mar Res. 1998;46:181–94. [Google Scholar]
- Nei MH, Li W-H. Mathematical model for studying genetic variation in terms restriction endonucleases. PNAS. 1979;76:5269–73. doi: 10.1073/pnas.76.10.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani G, Nagai S, Masseret E, Lian CL, Yamaguchi S, Yasuda N, Itakura S, Grzebyk D, Berrebi P, Sekino M. Development of compound microsatellite markers in the toxic dinoflagellate Alexandrium catenella (Dinophyceae) Plankton & Benthos Res. 2007;2:128–133. [Google Scholar]
- Pereira P, Andrinolo D, Sam-Bento F, Alvito P, Martins C, Franca S. VI Reunion Iberica sobre Fitoplancton Toxico y Biotoxinas. CAPDG, Junta de Andalucia; Sevilha: 2000. Novos resultados sobre estudos de toxicidade PSP em dinoflagelados e bacterias associadas; pp. 139–48. [Google Scholar]
- Persich GR, Kulis DM, Lilly EL, Anderson DM, Garcia VMT. Probable origin and toxin profile of Alexandrium tamarense (Lebour) Balech from southern Brazil. Harmful Algae. 2003;5:36–44. [Google Scholar]
- Persson A, Godhe A, Karlson B. Dinoflagellate cysts in recent sediments from the west coast of Sweden. Bot Mar. 2000;43:69–79. [Google Scholar]
- Posada D, Crandall KA. MODEL TEST: testing the model of DNA substitution. Bioinformatics. 1998;14:817–18. doi: 10.1093/bioinformatics/14.9.817. [DOI] [PubMed] [Google Scholar]
- Rodriguez F, Oliver JF, Marin A, Medina JR. The general stochastic model of nucleotide substitutions. J Theor Biol. 1990;142:485–501. doi: 10.1016/s0022-5193(05)80104-3. [DOI] [PubMed] [Google Scholar]
- Rynearson TA, Armbrust EV. Genetic differentiation among populations of the planktonic marine diatom Ditylum brightwellii (Bacillariophyceae) J Phycol. 2004;40:34–43. [Google Scholar]
- Santos SR, Rodríguez CG, Lasker HR, Coffroth MA. Symbiodinium sp. association in the gorgonian Pseudopterogorgia elisabethae in the Bahamas: high levels of genetic variability and population structure in symbiotic dinoflagellates. Mar Biol. 2003;143:111–20. [Google Scholar]
- Scholin CA, Anderson DM. Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). I RFLP analysis of SSU rRNA genes. J Phycol. 1994;30:744–54. [Google Scholar]
- Schug MD, Hutter CM, Wetterstrand KA, Gaudette MS, Mackay TFC, Aquadro CF. The mutation rates of di-, tri-, tetranucleotide repeats in Drosophila melanogaster. Mol Biol Evol. 1998;15:1751–60. doi: 10.1093/oxfordjournals.molbev.a025901. [DOI] [PubMed] [Google Scholar]
- Swofford DL. PAUP: Phylogenetic analysis using parsimony and other methods (software) 4.0 Beta 10 ed. Sinauer Associates; Sunderland MA: 2000. [Google Scholar]
- Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tavare S. Some probabilistic and statistical problems in the analysis of DNA sequences. Lect Notes Math Life Sci. 1986;17:57–86. [Google Scholar]
- Udupa SM, Baum M. High mutation rate and mutational bias at (TAA)n microsatellite loci in chickpea (Cicer arietinum L.) Mol Genet Genomics. 2001;265:1097–103. doi: 10.1007/s004380100508. [DOI] [PubMed] [Google Scholar]
- Usup G, Pin LC, Ahmad A, Teen LP. Alexandrium (Dinophyceae) species in Malaysian waters. Harmful Algae. 2002;1:265–75. [Google Scholar]
- Vazquez F, Perez T, Albornoz J, Dominquez A. Estimation of the mutation rates in Drosophila melanogaster. Genet Res. 2000;76:323–26. doi: 10.1017/s0016672300004791. [DOI] [PubMed] [Google Scholar]
- Vila M, Garces E, Maso M, Camp J. Is the distribution of the toxic dinoflagellate Alexandrium catenella expanding along the NW Mediterranean coast? Mar Ecol Prog Ser. 2001;222:73–83. [Google Scholar]
- Yoshida M, Ogata T, Thuoc CV, Matsuoka K, Fukuyo Y, Hoi NC, Kodama M. The first finding of toxic dinoflagellate Alexandrium minutum in Vietnam. Fish Sci. 2000;66:177–79. [Google Scholar]
- Zardoya R, Costas E, López-Rodas V, Garrido-Pertierra A, Bautista JM. Revised dinoflagellate phylogeny inferred from molecular analysis of large-subunit ribosomal RNA gene sequences. J Mol Evol. 1995;41:637–45. doi: 10.1007/BF00175822. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


