Abstract
The number of clinical trials in regenerative medicine is burgeoning, and stem cell/tissue engineering technologies hold the possibility of becoming the standard of care for a multitude of diseases and injuries. Advances in regenerative biology reveal novel molecular and cellular targets with potential to optimize tissue healing and functional recovery, thereby refining rehabilitation clinical practice. The purpose of this review is to: 1) highlight the potential for synergy between the fields of regenerative medicine and rehabilitation, a convergence of disciplines known as Regenerative Rehabilitation; 2) provide translational examples of Regenerative Rehabilitation within the context of neuromuscular injuries and diseases, and 3) offer recommendations for ways to leverage activity-dependence via combined therapy and technology with the goal of enhancing long-term recovery. The potential clinical benefits of Regenerative Rehabilitation will likely become a critical aspect in the standard of care for many neurological and musculoskeletal disorders.
Keywords: neuroplasticity, regeneration, neuroprosthesis, neural engineering, stem cells, tissue engineering, Regenerative Rehabilitation
Introduction
The combination of rehabilitation together with engineered devices and regenerative therapies holds potential to improve quality of life after neuromuscular injury or disease. The field of regenerative medicine is based on the assumption that the health of our population would benefit from a paradigm shift in the way we approach the treatment of acute and chronic conditions so as to maximize clinical outcomes. As proposed by Daar and Greenwood:
Regenerative medicine is an interdisciplinary field of research and clinical applications focused on the repair, replacement or regeneration of cells, tissues or organs to restore impaired function resulting from any cause, including congenital defects, disease, trauma and ageing. It uses a combination of several converging technological approaches, both existing and newly emerging, that moves it beyond traditional transplantation and replacement therapies. The approaches often stimulate and support the body’s own self-healing capacity1.
Regenerative medicine technologies have been investigated as a means to enhance the functional capacity of a host tissue when endogenous regenerative mechanisms are inadequate or fail altogether. The enthusiasm surrounding regenerative medicine continues to build, and this enthusiasm is being matched with clinical deliverables at an accelerating pace. Over the next decades, stem cell and tissue engineering protocols hold the possibility of becoming the standard of care for a number of diseases and injuries. While early stem cell applications were initially limited to the treatment of potentially fatal conditions, clinical trials are increasingly investigating a diverse array of applications, including musculoskeletal and neurological systems. As an example, the Clinical Trials registry (www.clinicaltrials.gov) lists seven active studies investigating cellular therapies for the treatment of Duchenne Muscular Dystrophy (accessed July 19, 2016). Cell sources for these trials include umbilical cord mesenchymal stem cells and bone marrow derived cells. A similar query using the Boolean search terms, “stroke” and “stem cell”, yields 102 hits (accessed July 19, 2016). With return to normal tissue function as the ultimate goal of these biological therapies, it is clear that regenerative medicine shares an increasingly convergent path with rehabilitation.
Overview of Regenerative Rehabilitation
Physical rehabilitation has foundations in the targeted application of mechanical stimuli to enhance intrinsic tissue healing potential. Mechanobiology is a growing scientific field that seeks to better understand how mechanical forces induce cellular and tissue responses, and how these forces contribute to tissue development, homeostasis and pathophysiology. A central area of study within mechanobiology is mechanotransduction, the process by which mechanical stimuli are sensed, transmitted and translated into biologic responses (reviewed in 2,3). There is robust evidence supporting biologic adaptations in response to both dynamic and static mechanical stimuli. Advances in mechanobiology suggest that changes in cell mechanics, extracellular matrix (ECM) structure and composition, and mechanotransductive sequences may contribute to the pathophysiology of many inheritable and acquired disabling conditions (reviewed in 2,3). Applied mechanical stimuli represent a potent stimulus to harness intrinsic tissue healing capacity. This concept has served as a foundation for the application of rehabilitation protocols for the treatment of diseased or injured tissues.
Similar mechanical and biological stimuli can also be used to activate the nervous system to induce reorganization and potentially repair. Pairing physical movement with activity in the nervous system is the foundation for many therapies aimed at promoting neuroplasticity. These approaches leverage the phenomenon discovery by Donald Hebb in the 1950s, now paraphrased as ‘neurons that fire together wire together’.4 Current approaches to physical therapy promote recovery by leveraging this activity-dependent plasticity via assisted movement and stimulation applied to the muscles, nerves, spinal cord or brain. Going forward, such active and timing-dependent strategies will be needed in combination with stem cell or tissue engineering solutions in order to guide the incorporation of tissue grafts or promote the regeneration and functional organization of endogenous stem cells.
Just as endogenous musculoskeletal and neural tissues benefit from the application of rehabilitation protocols to promote functional tissue recovery after injury and with disease, it is increasingly recognized that the functional efficacy of regenerative medicine technologies may be enhanced when coupled with mechanical and electrical stimuli.5–11 The recognized potential for synergy between the fields of regenerative medicine and rehabilitation science has in recent years launched the birth of a new field, Regenerative Rehabilitation.12–14 The International Consortium for Regenerative Rehabilitation defines Regenerative Rehabilitation as “the integration of principles and approaches from the fields of rehabilitation science and regenerative medicine. Regenerative medicine focuses on the repair or replacement of tissue lost to injury, disease, or age, primarily via the enhancement of endogenous stem cell function or the transplantation of exogenous stem cells. A focus of Rehabilitation science is the use of mechanical and other physical stimuli to promote functional recovery. The integration of these two approaches will optimize independence and participation of individuals with disabilities.” (www.ar3t.pitt.edu).
Successes in Regenerative Rehabilitation and related therapies
Musculoskeletal Regenerative Rehabilitation
Progress in Regenerative Rehabilitation research has arguably been the greatest when considering musculoskeletal applications, such as the treatment of traumatic skeletal muscle injuries. Although skeletal muscle is capable of remarkable regenerative potential, when the injury or disease is extensive and destroys the underlying architecture, regeneration is aborted and is characterized, instead, by scar tissue formation (reviewed in15). The consequence is severely impaired functional capacity of the damaged tissue. In cases such as these, cellular therapies have been investigated as a means to boost tissue regenerative capacity. Unfortunately, the therapeutic benefit of these interventions has often been limited by massive cell death following transplantation and a poor transplantation efficiency,16,17 ultimately resulting in poor functional outcomes. To overcome this barrier, studies have demonstrated that the combination of stem cell transplantation and muscle loading increases the engraftment of donor cells, both in cases of myopathy6,7,18 and injury.5,19
Accordingly, surgical placement of acellular biologic scaffold materials (a tissue engineering approach) composed of mammalian extracellular matrix (ECM) promotes constructive tissue remodeling in cases of volumetric muscle loss (VML 20–22). The mechanisms underlying the reported functional improvements have yet to be elucidated, but it has been hypothesized that donor ECM-mediated response occurs through the recruitment of stem/progenitor cells at the site of implantation.23–26 The application of rehabilitation protocols following ECM implantation has been suggested to be beneficial—even crucial—for providing the needed mechanical signals to encourage site-specific tissue remodeling (reviewed in 27,28). Future randomized studies to determine whether and how optimal rehabilitation protocols may enhance functional outcomes following the application of a tissue engineering device for the treatment of VML are warranted.
Neurological Regenerative Rehabilitation
In the central nervous system (CNS), electrical and chemical signaling are believed to be the strongest drivers of plasticity and remodeling. Following injury to the CNS, fibrosis formation can alter the biophysical tissue properties and may trigger a multitude of downstream cellular responses and strongly influence plasticity and recovery. Indeed, static mechanical and electrical properties of the cellular microenvironment have been shown to exert potent effects on mesenchymal stem cell regenerative potential.29,30 Spinal cord and hippocampal neurons grown on a soft gel substrate were shown to form three times as many branches compared to neurons grown on stiffer gels.31 Together, these studies suggest that complementary methods to optimize the biophysical microenvironment (e.g. through pharmacological or cell-based therapies) may be a critical step in realizing the full potential of rehabilitation protocols after spinal cord injury, stroke, or traumatic brain injury.
More traditional interventions for CNS trauma involve activity dependent therapies. For example, following spinal cord injury or stroke, assisted locomotor training is used with the goal of delivering synchronous input both above and below a lesion.32,33 As reviewed below, such interventions may also employ electrical stimulation of the muscles, peripheral nerves, or spinal cord to activate the affected neuromuscular tissue. In addition to direct efferent activation, such stimulation often also results in activation of sensory afferents, providing coordinated input to the CNS distal to a lesion.32–34
Several methods exist for electrically or magnetically activating the brain and spinal cord after injury. Methods of electrical stimulation include application of current to the dorsal surface of the spinal cord, termed epidural stimulation (Figure 1). Early human studies are possible due to the off-label use of stimulators designed to alleviate chronic pain.35–37 Non-invasive methods of spinal stimulation are also possible using magnetic fields, which have improved spasticity following spinal cord injury for up to 24 hours.38 Magnetic stimulation of the lumber spinal cord can be triggered by upper extremity movement to create an activity-dependent paradigm where stepping movements are synchronized with arm swing in spinally-intact volunteers.39
Figure 1.
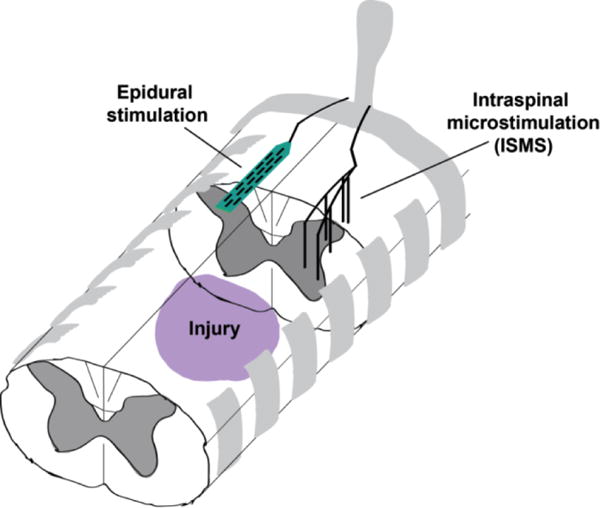
Illustration of spinal stimulation techniques applied distal to an injury. Epidural stimulation is applied to the dorsal surface of the spinal cord, adapting an FDA-approved treatment for chronic pain. Epidural stimulation most likely activates sensory afferents and dorsal roots to recruit spinal networks below the injury. In the presence of constant epidural stimulation, people with otherwise complete paralysis can move their joints individually, and have lasting improvements in autonomic function. Intraspinal stimulation (ISMS) is applied via thin wires implanted within the spinal cord to target the intermediate and ventral lamina where the motor neuron cell bodies are located. ISMS can evoke functional synergies from select stimulating locations and lead to long-term recovery of function when applied therapeutically or in an activity-dependent manner. Reprinted with permission from 71.
Parallel work in animals utilizes hair-like wires within the spinal cord, termed intraspinal microstimulation (ISMS; Figure 1). Intraspinal microstimulation can evoke functional synergies for walking40 and reach/grasp.41,42 Such stimulation can also lead to long-term improvements in forelimb function in animal models of spinal cord injury,11 especially when triggered by residual muscle signals in an activity-dependent paradigm.10 While intraspinal stimulation is more invasive than epidural stimulation, it is currently scheduled for the first human experiments and provides much greater specificity of activation that may benefit the incorporation of regenerative therapies (see below).
In both the brain and spinal cord, pairing of artificial stimulation can benefit individuals recovering from stroke and spinal cord injury.43,44 Application of peripheral nerve stimulation followed by transcranial magnetic stimulation after an appropriate latency can reinforce45,46 or inhibit44 connections either within the intact brain or in subjects recovering from stroke. Similar mechanism have been applied to the cervical spinal cord after injury43 to reinforce weak but spared connections bypassing a lesion. Even stimulation applied directly to the brain surface improves function in animal models of ischemic stroke.47,48 Further, paired stimulation delivered to the brain or brain and spinal cord can lead to long-term changes in synaptic strength in the intact49,50 and injured CNS.51 Building on the success of constraint induced therapy,52,53 if such methods of stimulation can incorporate time- or activity-dependence, they may induce long term plasticity and recovery. Going forward, efforts are required to assure that such stimulation methods are effectively combined with physical therapy, and eventually cellular and regenerative therapies, in order to optimally improve function after injury.
Appropriate neural activity is likely a prerequisite for stem cells to improve function in the damaged central nervous system. Neural activity is critical for avoiding cell death following insult,54,55 improves blood perfusion and the related health of neurons,56 and up-regulates brain-derived neurotrophic factor (BDNF), which is implicated in plasticity and recovery.57,58 In contrast, reduced activity such as that observed in models of spinal muscular atrophy is associated with reduced axon growth.59 Based on this cumulative evidence, one of the most successful stem cell transplant studies coupled brief electrical stimulation of the peripheral nerve with motor neuron cell grafts and demonstrated impressive cell survival and muscle re-innervation.60 This landmark study suggests that the combination of regenerative cell therapies and artificial stimulation may be critical for achieving targeted plasticity and functional recovery following injuries or degeneration of the neuromuscular system.
Obstacles & Barriers to Regenerative Rehabilitation- What is holding us back?
The clinical translation of regenerative medicine approaches for the enhancement of physical functioning presupposes the existence of a critical mass of basic scientists working in close collaboration with rehabilitation clinicians. Unfortunately, while interdisciplinary research is conceptually desirable, there are few opportunities providing rehabilitation scientists with the resources and training necessary to become engaged in the field of regenerative medicine. Of the almost 1300 currently funded studies investigating “stem cell transplantation” or “tissue engineering” listed on NIH reporter, only eight are housed in rehabilitation departments (Accessed July, 2016).
One reason for the disconnect between regenerative biology and rehabilitation studies may be that many rehabilitation programs lack faculty members with the expertise necessary to teach principles and concepts in the domain of cellular and regenerative biology. Physical therapy and occupational therapy departments are often in schools without basic science research programs, thereby limiting opportunities for interaction with basic science colleagues. Similar barriers have impeded those working in the basic sciences from understanding application of their work to clinical practice, as they generally have limited exposure to rehabilitation practice. As a result, regenerative medicine scientists may not consider clinically available approaches, technologies such as robotics and modalities such as neuromuscular electrical stimulation or ultrasound that may be beneficial in targeting the mechano-transductive pathways so fundamental for driving the tissue regenerative cascade. Moreover, given that functional benefit is the ultimate goal of all translational regenerative therapies, basic scientists stand to benefit from the expertise of rehabilitation specialists in functional outcomes assessment.
There is also a large unmet need for better pre-clinical models of rehabilitation. Currently, pre-clinical models of rehabilitation are limited, and the bulk of the studies employ treadmill or wheel running, for example. Yet clinical rehabilitation consists of much more than just the presence or absence of exercise, and investigation into combined rehabilitation modalities such as neuromuscular electrical stimulation, ultrasound, etc., to enhance stem cell transplantation or implantation of a tissue engineering device is needed. Finally, timing, dosing and intensity are all critical variables for both pharmacological and rehabilitation interventions following central nervous system injury, and work is ongoing to determine the optimal paradigm for combining multiple therapies.61,62
Conclusions and charge to the field
As is our tradition, rehabilitation practice must continuously evolve such that it may be responsive to scientific and technological innovations that impact clinical practice. Undoubtedly, progress in the field of rehabilitation will increase proportionately with the pace at which rehabilitation professionals keep up with innovations in medical practice. Just as the prescription of rehabilitation is the standard of care following the onset of most musculoskeletal and neurologic injuries and diseases, it is likely that rehabilitation will necessarily be the standard of care as regenerative medicine technologies increasingly make their way to clinical practice.
To drive knowledge transfer and the technical capabilities of medical rehabilitation researchers to perform cutting edge Regenerative Rehabilitation investigations, we must begin to systematically promote the integration of basic scientists with rehabilitation specialists. We must train rehabilitation clinicians who can help oversee the quality, safety, and validity of these innovative Regenerative Rehabilitation technologies and protocols.
In addition, to be effective in this partnership, there is a need for an improved mechanistic understanding by which mechanical forces and modulation of the tissue microenvironment (e.g. through exercise and modalities) may be used to optimize outcomes following a regenerative medicine intervention. Molecular and cellular mechanisms must be the foundation upon which clinical Regenerative Rehabilitation protocols are derived, and a better understanding of these mechanisms will allow for the more rational design of clinical protocols that elicit targeted and specific cellular and tissue responses. In the absence of these guiding mechanisms, clinical protocols will be left to a trial-and-error approach, an approach that is ineffective both in terms of clinical outcomes as well as economics.
Finally, our ability to utilize engineered devices to interact with the neuromuscular system is beginning to accelerate. Implanted stimulators capable of triggering activity-dependent stimulation are now in early human studies for essential tremor and Parkinson’s disease.63 Experimental devices are already capable of delivering electrical, magnetic,64 optical,65 and pharmacological66 stimulation to targeted locations within the brain and spinal cord, as well as the peripheral nerves and muscles.67 Optogenetics, or light activation of neurons,68,69 is currently under trial to treat blindness.70 This technique may soon be combined with stem cell interventions to enable targeted activation of grafts in situ. Given the current pace of technological advancement, there is tremendous potential to leverage engineered solutions to enhance biological regeneration.
The combination of regenerative therapies such as stem cell or tissue grafts with methods to induce appropriate mechanical or electrical stimuli within the injury or diseased site is likely critical to the success of Regenerative Rehabilitation. If emerging technologies can be effectively coupled with sound physical therapy practice to induce activity-dependent remodeling of injured tissues, Regenerative Rehabilitation therapies may soon dramatically improve plasticity and participation for people with injuries to the neuromuscular system.
Acknowledgments
Chet Moritz is supported by a Paul G. Allen Family Foundation Allen Distinguished Investigator Award, the UW Institute for Neuroengineeirng (UWIN), the Christopher and Dana Reeve Foundation International Consortium on Spinal Cord Repair, GlaxoSmithKlein Bioelectronics Innovation Challenge, and the Center for Sensorimotor Neural Engineering (CSNE), a National Science Foundation Engineering Research Center (EEC-1028725). Fabrisia Ambrosio is supported by the Alliance for Regenerative Rehabilitation Research & Training (AR3T), which is funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), the National Institute of Biomedical Imaging and Bioengineering (NIBIB) and National Institute of Neurological Disorders and Stroke (NINDS) of the National Institutes of Health (P2CHD086843).
Footnotes
Conflict of interest: NA
Contributor Information
Chet T. Moritz, Department of Rehabilitation Medicine, Physiology & Biophysics, Electrical Engineering, UW Institute for Neuroengineering, Center for Sensorimotor Neural Engineering, University of Washington School of Medicine, Seattle, WA.
Fabrisia Ambrosio, Department of Physical Medicine & Rehabilitation, Physical Therapy, Bioengineering, and Microbiology & Molecular Genetics, McGowan Institute for Regenerative Medicine, University of Pittsburgh, Pittsburgh, PA.
References
- 1.Daar AS, Greenwood HL. A proposed definition of regenerative medicine. J Tissue Eng Regen Med. 2007;1:179–84. doi: 10.1002/term.20. [DOI] [PubMed] [Google Scholar]
- 2.Dunn SL, Olmedo ML. Mechanotransduction: Relevance to Physical Therapist Practice-Understanding Our Ability to Affect Genetic Expression Through Mechanical Forces. Physical therapy. 2016;96:712–21. doi: 10.2522/ptj.20150073. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WR, Scott A, Loghmani MT, Ward SR, Warden SJ. Understanding Mechanobiology: Physical Therapists as a Force in Mechanotherapy and Musculoskeletal Regenerative Rehabilitation. Physical therapy. 2016;96:560–9. doi: 10.2522/ptj.20150224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hebb DO. The Organization of Behaviour: a Neuropsychological Theory. New York: Wiley; 1949. [Google Scholar]
- 5.Ambrosio F, Ferrari RJ, Distefano G, et al. The Synergistic Effect of Treadmill Running on Stem Cell Transplantation To Heal Injured Skeletal Muscle. Tissue Eng Part A. 2009 doi: 10.1089/ten.tea.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ambrosio F, Ferrari RJ, Fitzgerald GK, Carvell G, Boninger ML, Huard J. Functional overloading of dystrophic mice enhances muscle-derived stem cell contribution to muscle contractile capacity. Arch Phys Med Rehabil. 2009;90:66–73. doi: 10.1016/j.apmr.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Distefano G, Ferrari RJ, Weiss C, et al. Neuromuscular electrical stimulation as a method to maximize the beneficial effects of muscle stem cells transplanted into dystrophic skeletal muscle. PloS one. 2013;8:e54922. doi: 10.1371/journal.pone.0054922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imura T, Matsumoto M, Fukazawa T, et al. Interactive effects of cell therapy and rehabilitation realize the full potential of neurogenesis in brain injury model. Neuroscience letters. 2013;555:73–8. doi: 10.1016/j.neulet.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Zhang YX, Yuan MZ, Cheng L, et al. Treadmill exercise enhances therapeutic potency of transplanted bone mesenchymal stem cells in cerebral ischemic rats via anti-apoptotic effects. BMC neuroscience. 2015;16:56. doi: 10.1186/s12868-015-0196-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McPherson JG, Miller RR, Perlmutter SI. Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proc Natl Acad Sci U S A. 2015;112:12193–8. doi: 10.1073/pnas.1505383112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasten MR, Sunshine MD, Secrist ES, Horner PJ, Moritz CT. Therapeutic intraspinal microstimulation improves forelimb function after cervical contusion injury. J Neural Eng. 2013;10:044001. doi: 10.1088/1741-2560/10/4/044001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosio F, Russell A. Regenerative rehabilitation: a call to action. J Rehabil Res Dev. 47:xi–xv. doi: 10.1682/jrrd.2010.03.0021. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Terzic C, Childers MK. Regenerative rehabilitation: a new future? American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 93:S73–8. doi: 10.1097/PHM.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosio F, Wolf SL, Delitto A, et al. The emerging relationship between regenerative medicine and physical therapeutics. Physical therapy. 90:1807–14. doi: 10.2522/ptj.20100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huard J, Li Y, Fu FH. Current concepts review - Muscle injuries and repair: Current trends in research. Journal of Bone and Joint Surgery-American Volume. 2002;84A:822–32. [PubMed] [Google Scholar]
- 16.Gussoni E, Pavlath GK, Lanctot AM, et al. Normal dystrophin transcripts detected in Duchenne muscular dystrophy patients after myoblast transplantation. Nature. 1992;356:435–8. doi: 10.1038/356435a0. [DOI] [PubMed] [Google Scholar]
- 17.Huard J, Bouchard JP, Roy R, et al. Human myoblast transplantation: preliminary results of 4 cases. Muscle & Nerve. 1992;15(5):550–60. doi: 10.1002/mus.880150504. [DOI] [PubMed] [Google Scholar]
- 18.Bouchentouf M, Benabdallah BF, Mills P, Tremblay JP. Exercise improves the success of myoblast transplantation in mdx mice. Neuromuscular Disorders. 2006;16(8):518–29. doi: 10.1016/j.nmd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Palermo AT, Labarge MA, Doyonnas R, Pomerantz J, Blau HM. Bone marrow contribution to skeletal muscle: a physiological response to stress. DevelopmentalBiology. 2005;279(2):336–44. doi: 10.1016/j.ydbio.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 20.Gilbert TW, Nieponice A, Spievack AR, Holcomb J, Gilbert S, Badylak SF. Repair of the thoracic wall with an extracellular matrix scaffold in a canine model. J Surg Res. 2008;147:61–7. doi: 10.1016/j.jss.2007.04.035. [DOI] [PubMed] [Google Scholar]
- 21.Turner NJ, Badylak SF. Biologic scaffolds for musculotendinous tissue repair. Eur Cell Mater. 25:130–43. doi: 10.22203/ecm.v025a09. [DOI] [PubMed] [Google Scholar]
- 22.Turner NJ, Badylak JS, Weber DJ, Badylak SF. Biologic scaffold remodeling in a dog model of complex musculoskeletal injury. J Surg Res. 176:490–502. doi: 10.1016/j.jss.2011.11.1029. [DOI] [PubMed] [Google Scholar]
- 23.Badylak SF. The extracellular matrix as a scaffold for tissue reconstruction. Semin Cell Dev Biol. 2002;13:377–83. doi: 10.1016/s1084952102000940. [DOI] [PubMed] [Google Scholar]
- 24.Badylak SF, Gilbert TW. Immune response to biologic scaffold materials. Semin Immunol. 2008;20:109–16. doi: 10.1016/j.smim.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sicari BM, Rubin JP, Dearth CL, et al. An acellular biologic scaffold promotes skeletal muscle formation in mice and humans with volumetric muscle loss. Science translational medicine. 2014;6:234ra58. doi: 10.1126/scitranslmed.3008085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dziki J, Badylak MY, Sicari B, et al. An acellular biologic scaffold treatment for volumetric muscle loss: results of a 13-patient cohort study. Regenerative Medicine. 2016 doi: 10.1038/npjregenmed.2016.8. Article number: 16008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gentile NE, Stearns KM, Brown EH, et al. Targeted rehabilitation after extracellular matrix scaffold transplantation for the treatment of volumetric muscle loss. American journal of physical medicine & rehabilitation / Association of Academic Physiatrists. 93:S79–87. doi: 10.1097/PHM.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 28.Badylak SF, Dziki JL, Sicari BM, Ambrosio F, Boninger ML. Mechanisms by which acellular biologic scaffolds promote functional skeletal muscle restoration. Biomaterials. 2016;103:128–36. doi: 10.1016/j.biomaterials.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 29.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Zhao M, Song B, Pu J, et al. Electrical signals control wound healing through phosphatidylinositol-3-OH kinase-gamma and PTEN. Nature. 2006;442:457–60. doi: 10.1038/nature04925. [DOI] [PubMed] [Google Scholar]
- 31.Chen WH, Cheng SJ, Tzen JT, Cheng CM, Lin YW. Probing relevant molecules in modulating the neurite outgrowth of hippocampal neurons on substrates of different stiffness. PLoS One. 8:e83394. doi: 10.1371/journal.pone.0083394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Behrman AL, Nair PM, Bowden MG, et al. Locomotor training restores walking in a nonambulatory child with chronic, severe, incomplete cervical spinal cord injury. Phys Ther. 2008;88:580–90. doi: 10.2522/ptj.20070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil. 2011;93:1508–17. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 34.Dobkin BH, Duncan PW. Should body weight-supported treadmill training and robotic-assistive steppers for locomotor training trot back to the starting gate? Neurorehabil Neural Repair. 2012;26:308–17. doi: 10.1177/1545968312439687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harkema S, Gerasimenko Y, Hodes J, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377:1938–47. doi: 10.1016/S0140-6736(11)60547-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137:1394–409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herman R, He J, D’Luzansky S, Willis W, Dilli S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord. 2002;40:65–8. doi: 10.1038/sj.sc.3101263. [DOI] [PubMed] [Google Scholar]
- 38.Krause P, Edrich T, Straube A. Lumbar repetitive magnetic stimulation reduces spastic tone increase of the lower limbs. Spinal Cord. 2004;42:67–72. doi: 10.1038/sj.sc.3101564. [DOI] [PubMed] [Google Scholar]
- 39.Sasada S, Kato K, Kadowaki S, et al. Volitional walking via upper limb muscle-controlled stimulation of the lumbar locomotor center in man. J Neurosci. 2014;34:11131–42. doi: 10.1523/JNEUROSCI.4674-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mushahwar VK, Collins DF, Prochazka A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Exp Neurol. 2000;163:422–9. doi: 10.1006/exnr.2000.7381. [DOI] [PubMed] [Google Scholar]
- 41.Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J Neurophysiol. 2007;97:110–20. doi: 10.1152/jn.00414.2006. [DOI] [PubMed] [Google Scholar]
- 42.Sunshine MD, Cho FS, Lockwood DR, Fechko AS, Kasten MR, Moritz CT. Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J Neural Eng. 2013;10:036001. doi: 10.1088/1741-2560/10/3/036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bunday KL, Perez MA. Motor recovery after spinal cord injury enhanced by strengthening corticospinal synaptic transmission. Curr Biol. 2012;22:2355–61. doi: 10.1016/j.cub.2012.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jayaram G, Stinear JW. Contralesional paired associative stimulation increases paretic lower limb motor excitability post-stroke. Exp Brain Res. 2008;185:563–70. doi: 10.1007/s00221-007-1183-x. [DOI] [PubMed] [Google Scholar]
- 45.Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123(Pt 3):572–84. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 47.Teskey GC, Flynn C, Goertzen CD, Monfils MH, Young NA. Cortical stimulation improves skilled forelimb use following a focal ischemic infarct in the rat. Neurol Res. 2003;25:794–800. doi: 10.1179/016164103771953871. [DOI] [PubMed] [Google Scholar]
- 48.Kleim JA, Bruneau R, VandenBerg P, MacDonald E, Mulrooney R, Pocock D. Motor cortex stimulation enhances motor recovery and reduces peri-infarct dysfunction following ischemic insult. Neurol Res. 2003;25:789–93. doi: 10.1179/016164103771953862. [DOI] [PubMed] [Google Scholar]
- 49.Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- 50.Nishimura Y, Perlmutter SI, Eaton RW, Fetz EE. Spike-timing-dependent plasticity in primate corticospinal connections induced during free behavior. Neuron. 2013;80:1301–9. doi: 10.1016/j.neuron.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guggenmos DJ, Azin M, Barbay S, et al. Restoration of function after brain damage using a neural prosthesis. Proc Natl Acad Sci U S A. 2013;110:21177–82. doi: 10.1073/pnas.1316885110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.DeBow SB, Davies ML, Clarke HL, Colbourne F. Constraint-induced movement therapy and rehabilitation exercises lessen motor deficits and volume of brain injury after striatal hemorrhagic stroke in rats. Stroke. 2003;34:1021–6. doi: 10.1161/01.STR.0000063374.89732.9F. [DOI] [PubMed] [Google Scholar]
- 53.Wolf SL, Lecraw DE, Barton LA, Jann BB. Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke and head-injured patients. Exp Neurol. 1989;104:125–32. doi: 10.1016/s0014-4886(89)80005-6. [DOI] [PubMed] [Google Scholar]
- 54.Morimoto T, Jeffrey LG, Ephraim FT. International Review of Neurobiology. Academic Press; 2012. Role of Electrical Activity of Neurons for Neuroprotection; pp. 19–38. [DOI] [PubMed] [Google Scholar]
- 55.Hartshorn DO, Miller JM, Altschuler RA. Protective effect of electrical stimulation in the deafened guinea pig cochlea. Otolaryngol Head Neck Surg. 1991;104:311–9. doi: 10.1177/019459989110400305. [DOI] [PubMed] [Google Scholar]
- 56.Leybaert L. Neurobarrier coupling in the brain: a partner of neurovascular and neurometabolic coupling? J Cereb Blood Flow Metab. 2005;25:2–16. doi: 10.1038/sj.jcbfm.9600001. [DOI] [PubMed] [Google Scholar]
- 57.Al-Majed AA, Brushart TM, Gordon T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur J Neurosci. 2000;12:4381–90. [PubMed] [Google Scholar]
- 58.Al-Majed AA, Tam SL, Gordon T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol Neurobiol. 2004;24:379–402. doi: 10.1023/B:CEMN.0000022770.66463.f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jablonka S, Beck M, Lechner BD, Mayer C, Sendtner M. Defective Ca2+ channel clustering in axon terminals disturbs excitability in motoneurons in spinal muscular atrophy. J Cell Biol. 2007;179:139–49. doi: 10.1083/jcb.200703187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grumbles RM, Liu Y, Thomas CM, Wood PM, Thomas CK. Acute stimulation of transplanted neurons improves motoneuron survival, axon growth, and muscle reinnervation. J Neurotrauma. 2013;30:1062–9. doi: 10.1089/neu.2012.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahl AS, Omlor W, Rubio JC, et al. Neuronal repair. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science. 2014;344:1250–5. doi: 10.1126/science.1253050. [DOI] [PubMed] [Google Scholar]
- 62.Wahl AS, Schwab ME. Finding an optimal rehabilitation paradigm after stroke: enhancing fiber growth and training of the brain at the right moment. Front Hum Neurosci. 2014;8:381. doi: 10.3389/fnhum.2014.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shute JB, Okun MS, Opri E, et al. Thalamocortical network activity enables chronic tic detection in humans with Tourette syndrome. Neuroimage Clin. 2016;12:165–72. doi: 10.1016/j.nicl.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen R, Romero G, Christiansen MG, Mohr A, Anikeeva P. Wireless magnetothermal deep brain stimulation. Science. 2015;347:1477–80. doi: 10.1126/science.1261821. [DOI] [PubMed] [Google Scholar]
- 65.Lu C, Froriep UP, Koppes RA, et al. Polymer Fiber Probes Enable Optical Control of Spinal Cord and Muscle Function In Vivo. Advanced Functional Materials. 2014 n/a-n/a. [Google Scholar]
- 66.Canales A, Jia X, Froriep UP, et al. Multifunctional fibers for simultaneous optical, electrical and chemical interrogation of neural circuits in vivo. Nat Biotechnol. 2015;33:277–84. doi: 10.1038/nbt.3093. [DOI] [PubMed] [Google Scholar]
- 67.Peckham PH, Kilgore KL, Keith MW, Bryden AM, Bhadra N, Montague FW. An advanced neuroprosthesis for restoration of hand and upper arm control using an implantable controller. J Hand Surg [Am] 2002;27:265–76. doi: 10.1053/jhsu.2002.30919. [DOI] [PubMed] [Google Scholar]
- 68.Klapoetke NC, Murata Y, Kim SS, et al. Independent optical excitation of distinct neural populations. Nat Methods. 2014;11:338–46. doi: 10.1038/nmeth.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011;71:9–34. doi: 10.1016/j.neuron.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 70.https://www.clinicaltrials.gov/ct2/show/NCT02556736?term=retrosense&rank=1 2016 (Accessed at
- 71.Mondello SE, Kasten MR, Horner PJ, Moritz CT. Therapeutic intraspinal stimulation to generate activity and promote long-term recovery. Front Neurosci. 2014;8:21. doi: 10.3389/fnins.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]


