Abstract
Purpose
This study was designed to test the short-term toxicity of DHA-dFdC in a mouse model and its efficacy in a mouse model of leukemia at or below its repeat-dose maximum tolerated dose (RD-MTD).
Method
A repeat-dose dose-ranging toxicity study was designed to determine the tolerability of DHA-dFdC when administered to DBA/2 mice by intravenous (i.v.) injection on a repeat-dose schedule (i.e. injections on days 0, 3, 7, 10, and 13). In order to determine the effect of a lethal dose of DHA-dFdC, mice were injected i.v. with three doses of DHA-dFdC at 100 mg/kg on days 0, 3, and 5 (i.e. a lethal-RD). The body weight of mice was recorded two or three times a week. At the end of the study, major organs (i.e. heart, liver, spleen, kidneys, lung, and pancreas) of mice that received the lethal-RD or RD-MTD were weighed, and blood samples were collected for analyses. Finally, DHA-dFdC was i.v. injected into DBA/2 mice with syngeneic L1210 mouse leukemia cells to evaluate its efficacy at or below RD-MTD.
Results
The RD-MTD of DHA-dFdC is 50 mg/kg. At 100 mg/kg, a lethal-RD, DHA-dFdC decreases the weights of mouse spleen and liver and significantly affected certain blood parameters (i.e. white blood cells, lymphocytes, eosinophils, and neutrophil segmented). At or below its RD-MTD, DHA-dFdC significantly prolonged the survival of L1210 leukemia-bearing mice.
Conclusion
DHA-dFdC has dose-dependent toxicity, affecting mainly spleen at a lethal-RD. At or below its RD-MTD, DHA-dFdC is effective against leukemia in a mouse model.
Keywords: DHA, gemcitabine, repeat dose-maximum tolerated dose, lethal-repeated dose, leukemia, efficacy
Introduction
Gemcitabine (2′, 2′-difluorodeoxycytidine, dFdC) is a nucleoside analogue approved for the treatment of solid tumors such as pancreatic, non-small lung, breast, and ovarian cancers (1–5). However, many factors limit its use in cancer treatment, such as its short half-life and tumor cell development of resistance (6, 7). Data from clinical studies showed a mild to moderate toxicity of gemcitabine (1, 8). The most common side effects are myelosuppression, high levels of hepatoxicity, renal toxicity, thrombocytopaenia, and anemia (1, 8, 9).
Docosahexaenoic acid (DHA) is a natural omega-3 polyunsaturated fatty acid (PUFAs) with six cis double bonds (10). Since DHA cannot be synthesized by mammals, it is obtained from dietary sources such as fatty cold-water fish and fish oils (10). Data from many studies suggested that DHA has anticancer activity. For example, DHA has an inhibitory effect on breast cancer cells lines (11), induces apoptosis in breast and colon cancer cell lines (12), and inhibits angiogenesis through the inhibition of important angiogenic mediators (e.g. vascular endothelial growth factor, cyclo-oxygenase 2, nitric oxide, matrix metalloproteinases, etc.) (13). In addition, there are reports that DHA is able to inhibit tumor cell invasion and metastasis (14, 15), and data from a recent study suggested that the antitumor activity of DHA may be related to its ability to increase drug transportation across tumor cell membrane (16). Toxicity study in animals and human demonstrated that the consumption of DHA is safe. For example, data from a 90-day subchronic toxicity study showed that DHA is safe in rats at 0.5 and 1.25 g/kg body weight/day (17). Clinical studies of DHA supplementation in adults did not show adverse effects in lipid levels (18, 19), platelet function (20, 21), or immune function (22, 23).
In spite of the promising results of the antitumor activity of omega-3 PUFAs in cell culture and in animal models, data from clinical studies do not support an anti-neoplastic activity by omega-3 PUFAs. Most clinical trials supported the role of PUFAs in cancer prevention, as adjuvants in improving chemotherapy, and preventing cachexia (24–30). For example, a VITAL cohort study reported that the incidence of pancreatic cancer was inversely related to the uptake of DHA (24). Another study showed that the supplementation of omega-3 PUFAs such as eicosapentaenoic acid and DHA through parental nutrition improves hepatic and pancreatic function in patients with major abdominal surgery for pancreatic or gastrointestinal cancer (31). This improvement has been associated with the role of omega-3 PUFAs in inflammation. For example, the use of gemcitabine plus i.v. omega-3 fatty acid rich lipid emulsion (e.g. Lipidem®) in pancreatic cancer patients improves their outcomes through reduction in circulating pro-angiogenic platelet-derived growth factor and fibroblast growth factor and pro-inflammatory factors (e.g. interleukin-6 and -8) (26, 27).
Previously, in an effort to exploit the antitumor activity of both gemcitabine and DHA, we synthesized a new compound, DHA-dFdC, by covalently conjugating DHA to dFdC at the 4-N position of dFdC (32). DHA-dFdC is a pale yellow waxy solid with a melting point of 96º C. It is poorly soluble in water (i.e. 25.2 ± 11.2 μg/ml) and has a logP value of 2.2 ± 0.3 (32). DHA-dFdC has potent cytotoxicity against a broad-spectrum of human and mouse tumor cell lines and showed significant antitumor activity in several mouse models of pancreatic cancer, including Kras-Ink4a genetically-modified mice and nude mice with orthotopically implanted human Panc-1 tumor cells (32). Importantly, in both tumor cells in culture and in animals, DHA-dFdC is significantly more effective than the molar equivalent DHA and dFdC physically mixed (32), suggesting a unique mechanism of antitumor activity by the DHA-dFdC conjugate. In the present study, we tested the short-term toxicity of DHA-dFdC in healthy DBA/2 mice by identifying its RD-MTD, a lethal-RD, and the major organ(s) and blood and serum parameters affected by DHA-dFdC at the lethal-RD. Finally, the antitumor activity of DHA-dFdC at or below its RD-MTD was also evaluated in DBA/2 mice with syngeneic leukemia to confirm that DHA-dFdC is active at or below its RD-MTD.
Materials and Methods
Synthesis and formulation of DHA-dFdC
DHA-dFdC was synthesized following our previously reported conjugation scheme (32). The purity of the resultant DHA-dFdC was confirmed by NMR and Mass Spectrum analyses. DHA-dFdC was dissolved in a vehicle solution that consists of Tween 80 (10%, w/v), ethanol (5.2 %, v/v), and mannitol (5%, w/v) in water.
Cell lines
L1210 cells (mouse leukemia cell line) were from the American Type Culture Collection (Manassas, VA). Cells were grown in DMEM supplemented with 10% horse serum, 100 U/ml of penicillin, and 100 mg/ml of streptomycin. All cell culture medium and reagents were from Invitrogen (Carlsbad, CA).
Short-term repeated dose toxicity studies
The animal protocol was approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin. Toxicity studies were performed using healthy DBA/2 mice (5–6 weeks) from Charles River Laboratories (Wilmington, MA). For all the toxicity studies, a mouse weight loss of ≥ 20% of its initial body weight on day 0 was considered as the end point (33, 34).
Initially, a single dose acute toxicity study was completed. Mice (n = 6, half male, half female) were intravenously (i.v.) injected once with DHA-dFdC at 85 mg/kg or 100 mg/kg. Untreated mice were used as a negative control. Mice were regularly monitored for overall health, and body weight was measured on days 3 and 6. On days 6, mice in the 100 mg/kg dose group and the untreated group were euthanized; major organs (i.e. heart, kidneys, liver, spleen, pancreas, and lung) were collected and weighed. Experimental data for organ weights were expressed as the relative organ weight calculated as: (organ weight (g)/body weight (g)) × 100%. The percent of body weight change was similar in both treated groups, and there is not any significant difference in the relative organ weights in mice that received 100 mg/kg of DHA-dFdC or were left untreated (data not shown). An i.v. bolus dose of more than 100 mg/kg of DHA-dFdC was not attempted due to the limited solubility of DHA-dFdC in the vehicle solution, prompting us to focus on testing the toxicity of the DHA-dFdC on a repeat-dose schedule only.
A five-dose schedule (e.g. injection on days 0, 3, 7, 10, and 13) was adopted for short-term repeated dose toxicity studies. Mice (n = 8, half male, half female) were i.v. injected with DHA-dFdC at different doses: 70 mg/kg, 60 mg/kg, 53 mg/kg, 50 mg/kg, 40 mg/kg, or 0 mg/kg (i.e. vehicle control). An additional group of mice was left untreated as a negative control. Mice were regularly monitored for overall health, body weight change, and survival until 2 weeks after the last injection. The body weight of mice was recorded on days 0, 3, 7, 10, 13, 17, 21, 24, 28, and 31.
To identify a lethal-RD of DHA-dFdC, mice (n = 8, half male, half female) were i.v. injected with 100 mg/kg of DHA-dFdC three times on days 0, 3, and 5. Again, this dose was selected due to the limited solubility of DHA-dFdC in the vehicle solution. Mice in the control groups were left untreated or i.v. injected with vehicle solution (i.e. 0 mg/kg of DHA-dFdC). Mice were monitored every day, weighed on days 0, 2, 4, and 6, and euthanized on day 6.
Effect of DHA-dFdC at RD-MTD or a lethal-RD on major mouse organ weights and blood and serum parameters
Two experiments were carried out. In the first experiment, DBA/2 mice (n = 8, half male, half female) were i.v. injected with DHA-dFdC at 50 mg/kg on days 0, 3, 7, 10, and 13. As a control, another group of mice were left untreated. Five days after the last injection, mice were euthanized. Major organs were harvested, weighed, and reported as the weights relative to the body weight of individual mouse. Blood samples were collected and shipped to IDEXX Laboratories (Sacramento, CA) for blood and serum parameter analyses.
In another experiment, mice (n = 8, half male, half female) were i.v. injected with DHA-dFdC at 100 mg/kg on days 0, 3, and 5. As controls, mice were either left untreated or i.v. injected with the vehicle solution alone. On day 6 mice were euthanized. Major organs were harvested and weighed. Blood samples were collected and shipped to IDEXX Laboratories for blood and serum parameter analyses.
Evaluation of the antitumor activity of DHA-dFdC in a mouse model of leukemia
Female DBA/2 mice (5–6 weeks) were intraperitoneally (i.p.) injected with the syngeneic L1210 leukemia cells (1 × 105) on day 0 to establish a mouse leukemia model (35, 36). Mice were then randomized into groups (n = 7) of untreated, vehicle alone, 1 × RD-MTD (i.e. 50 mg/kg), ¾ × RD-MTD (i.e. 37.5 mg/kg), and ½ × RD-MTD (i.e. 25 mg/kg), i.v. injected on days 1, 4, 8, 11, and 14, and monitored daily for overall health, weight change and survival (37). A mouse weight gain ≥ 60% of its initial body weight on day 0 was considered the end point (33, 34). Experimental data were reported as the mean survival time and T/U, which is defined as the ratio of the mean survival time of the treated group (T) divided by the mean survival time of the untreated group (U).
Statistical analysis
All statistical analyses involving comparing two groups were completed using Student’s t-test. For comparison of more than two groups, one-way ANOVA followed by a Bonferroni post hoc test was used to determine statistical significance between groups. Mouse survival data were analyzed using the Log-rank (Mantel-Cox) test to determine the level of significance. All of the analyses were performed with GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Data shown are mean ± S.D., and a p value of ≤ 0.5 is considered significant.
Results
Identification of the RD-MTD of DHA-dFdC in healthy mice
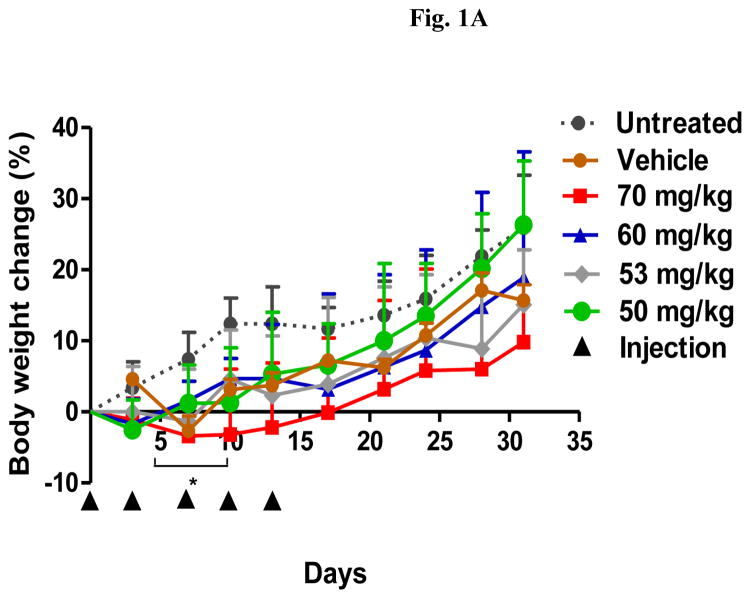
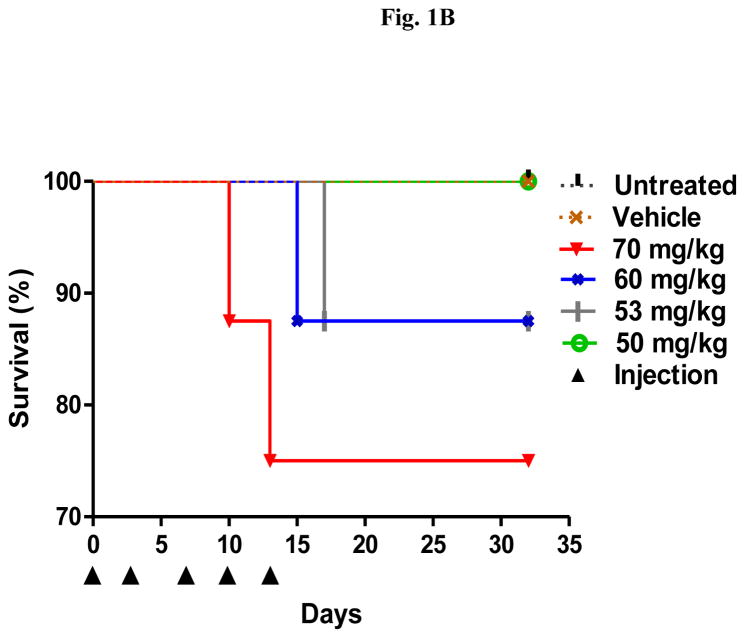
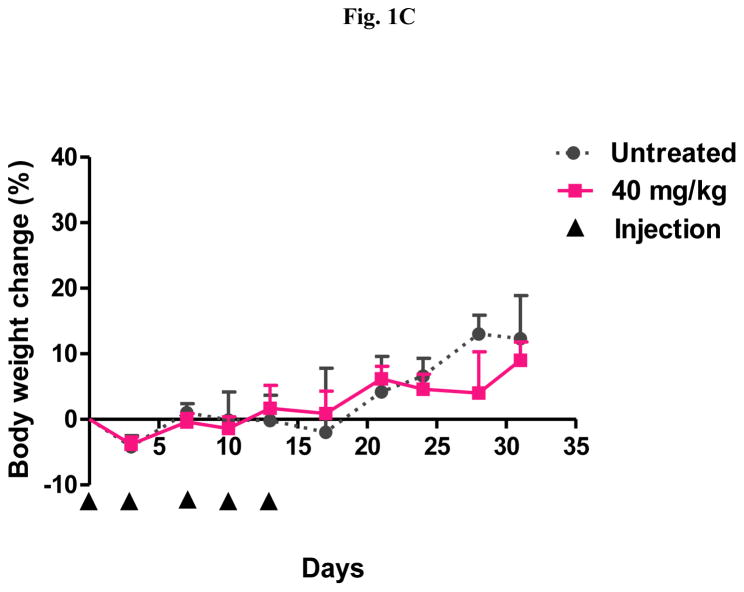
A repeat-dose range finding study was performed to identify the RD-MTD of DHA-dFdC. Mice were i.v. injected with DHA-dFdC at various doses on days 0, 3, 7, 10, and 13. As shown in Fig. 1A, the highest dose tested (i.e. 70 mg/kg) caused significant body weight loss in mice, as compared with untreated groups (e.g. p < 0.05 vs. Untreated, from day 5 to day 10). In addition, in the 70 mg/kg, 60 mg/kg, and 53 mg/kg groups, some mice reached the end point (i.e. body weight loss of ≥ 20%) (Fig. 1B). On the other hand, DHA-dFdC at 50 mg/kg did not cause any mortality or body weight loss of ≥ 20% (Fig. 1A and 1B), and the mean mouse body weight changes did not show any difference when compared with the untreated group (Fig. 1A). Moreover, at 40 mg/kg, DHA-dFdC did not cause mortality, body weight loss of ≥ 20%, or any sign of toxicity in a separate experiment (Fig. 1C). Therefore, 50 mg/kg is considered the RD-MTD of DHA-dFdC in DBA/2 mice when administered intravenously.
Fig. 1.
Identification of the RD-MTD of DHA-dFdC following five i.v. injections (on days 0, 3, 7, 10, and 13 (▲)) in healthy DBA/2 mice (n = 8). (A) Percent of body weight change (* p < 0.05, 70 mg/kg vs. Untreated, one-way ANOVA followed by a Bonferroni post hoc test). (B) Survival curves. Log-rank (Mantel-Cox) test did not reveal any significant difference among groups. (C) Percent of body weight change at 40 mg/kg of DHA-dFdC. Student’s t-test did not reveal any difference. In A and C, data shown are mean ± S.D.
Effect of DHA-dFdC at its RD-MTD on organ weights and blood and serum parameters in healthy mice
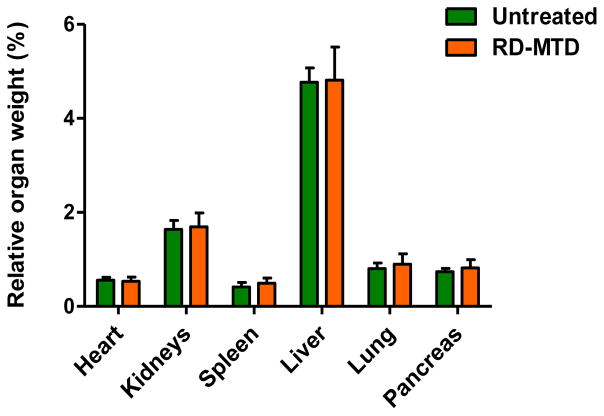
DHA-dFdC was given (i.v.) to healthy mice at its RD-MTD and the weights of major organs were measured at the end of the study. As shown in Fig. 2, at RD-MTD, DHA-dFdC did not significantly affect the weight of any of the major organs tested. In addition, at RD-MTD, DHA-dFdC did not cause any significant change in the blood or serum parameters tested, as compared to mice in the untreated group (Table I).
Fig. 2.
Effect of DHA-dFdC at its RD-MTD on major mouse organ weights. Shown are relative organ weights (vs. body weights) 6 days after the completion of a schedule of five i.v. doses of DHA-dFdC at its RD-MTD. Data shown are mean ± S.D. (n = 8). Student’s t-test did not reveal any significant difference between the Untreated and RD-MTD groups.
Table I.
Blood and serum parameters in healthy mice 5 days after the last dose of DHA-dFdC at its RD-MTD. Data are mean ± S.D. (n = 6–8).
| Parameter | Unit | RD-MTD (50 mg/kg) | |
|---|---|---|---|
| Untreated | DHA-dFdC | ||
| Blood | |||
| White blood cells (WBC) | k/μl | 8.0 ± 3.5 | 5.4 ± 2.3 |
| Red blood cell count (RBC) | M/μl | 10.1 ± 1.1 | 9.4 ± 1.0 |
| Hematocrit (HCT) | % | 40.0 ± 3.5 | 39.2 ± 4.2 |
| Mean globular volume (MCV) | fL | 39.8 ± 1.9 | 41.8 ± 1.7 |
| Mean corpuscular hemoglobin (MCH) | pg | 14.0 ± 0.3 | 14.5 ± 1.0 |
| Mean corpuscular hemoglobin concentration (MCHC) | g/dL | 35.3 ± 2.1 | 34.8 ± 2.7 |
| Lymphocytes | % | 79.9 ± 9.6 | 84.4 ± 7.8 |
| Monocytes | % | 5.5 ± 2.6 | 7.0 ± 3.9 |
| Basophil | % | 0.6 ± 1.0 | 0.2 ± 0.4 |
| Eosinophil | % | 2.9 ± 2.4 | 1.7 ± 1.6 |
| Neutrophil Segmented | % | 11.1 ± 7.6 | 6.8 ± 6.9 |
| Platelet estimate | Adequate | Adequate | |
| Serum | |||
| Cholesterol | mg/dL | 116.7 ± 17.0 | 127.6 ± 23.3 |
| Glucose | mg/dL | 262.5 ± 70.9 | 270.8 ± 39.7 |
| Calcium | mg/dL | 9.4 ± 2.2 | 7.0 ± 5.6 |
| Phosphorus | mg/dL | 14.6 ± 3.3 | 16.4 ± 1.5 |
| Blood urea nitrogen (BUN) | mg/dL | 23.5 ± 4.0 | 23.4 ± 1.7 |
| Globulin | g/dL | 2.8 ± 0.5 | 2.7 ± 0.2 |
| Total bilirubin | mg/dL | 0.3 ± 0.1 | 0.2 ± 0.04 |
| Total proteins | g/dL | 6.0 ± 0.6 | 6.0 ± 0.2 |
| Aspartate transaminase (AST) | U/L | 689.5 ± 599.5 | 1266.0 ± 808.9 |
| Alanine transaminase (ALT) | U/L | 116.7 ± 115.2 | 154.2 ± 115.2 |
| Alkaline phosphatase (ALP) | U/L | 158 ± 39.4 | 145.0 ± 66.3 |
| Albumin | g/dL | 3.2 ±0.3 | 3.3 ± 0.2 |
Student’s t-test did not reveal any significant difference between Untreated and DHA-dFdC groups.
Effect of a lethal-RD of DHA-dFdC on healthy mice
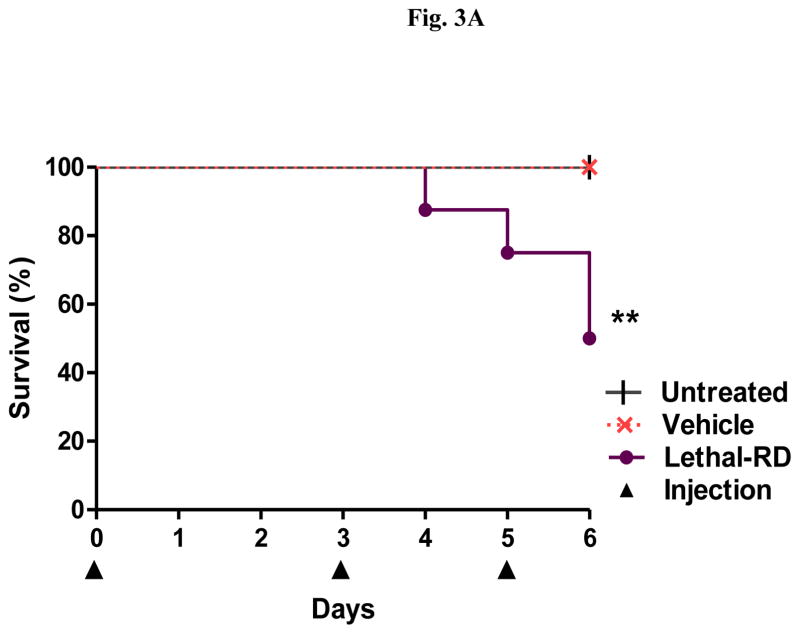
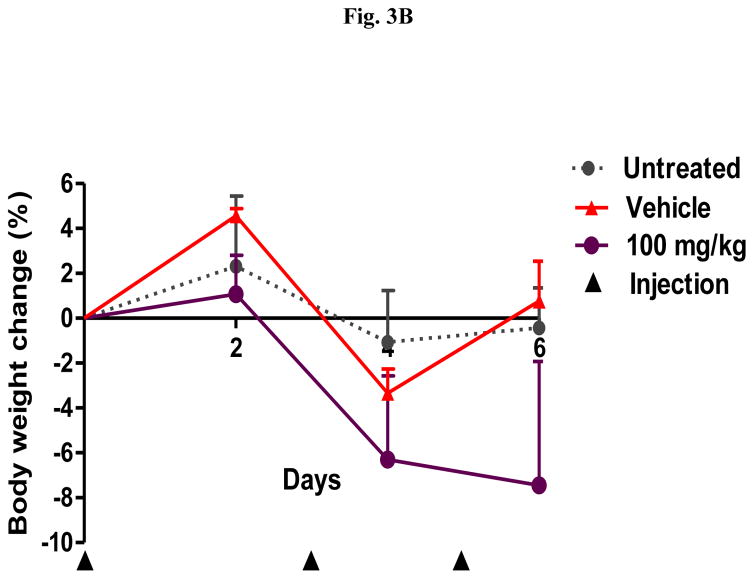
In order to identify a dose of DHA-dFdC that is lethal to DBA/2 mice, healthy mice were i.v. injected with three doses of DHA-dFdC at 100 mg/kg (i.e. on days 0, 3, and 5). Following the second dose, physical signs of toxicity observed include marked piloerection, transient paralysis, and little peer interactions. One day after the third dose, 50% of mice reached the end point (i.e. mortality or body weight loss of ≥ 20%) (Figs. 3A and B). Therefore, 100 mg/kg in a spaced schedule is considered a lethal-RD of DHA-dFdC in DBA/2 mice.
Fig. 3.
Effect of a lethal-RD of DHA-dFdC following three i.v. injections (on days 0, 3, and 5 (▲)) on healthy DBA/2 mice (n = 8, half male, half female). (A) Survival curves (** p < 0.01, Lethal-RD vs. Untreated, Log-rank (Mantel-Cox) test). (B) Percent of body weight change. Data are mean ± S.D.
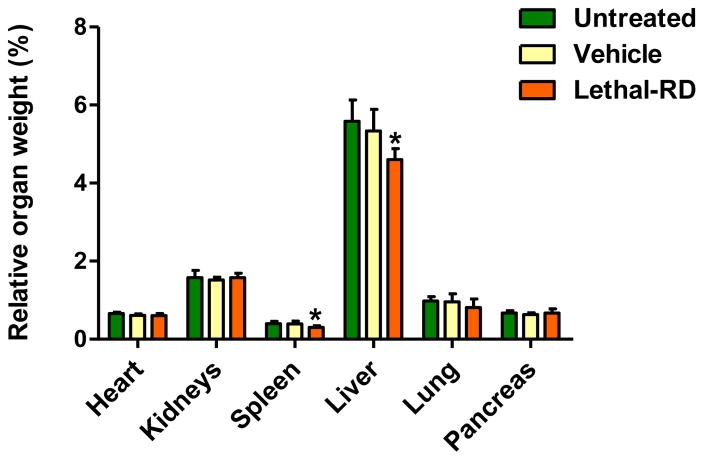
To identify the effect of a lethal-RD of DHA-dFdC on the main organs of mice, DHA-dFdC was i.v. injected to healthy mice at 100 mg/kg on days 0, 3, and 5, and the weights of major organs were measured at the end of the study (i.e. day 6). As shown in Fig. 4, the relative weights of the spleen and liver in mice that received the DHA-dFdC at the lethal-RD were significantly lower than that of the untreated mice, whereas the vehicle in which the DHA-dFdC was dissolved in did not significantly affect the weights of any of the organs. At the lethal-RD, DHA-dFdC significantly affected some blood parameters, including the white blood cells (WBC), red blood cell count (RBC), hemoglobin level (HGB), hematocrit value (HCT), lymphocytes, eosinophil, and neutrophil segmented (i.e. neutrophil segs), as compared to mice in the untreated group (Table II). In addition, the levels of some serum parameters such as phosphorus, blood urea nitrogen (BUN), and creatine phosphokinase (CPK) decreased in the lethal-RD group, as compared with the untreated group (Table II). Albumin also decreased in the lethal-RD group as compared with the untreated group, but was not different from that in the vehicle group (Table II).
Fig. 4.
Effect of DHA-dFdC at a lethal-RD on major organ weights of healthy mice. Shown are relative organ weights one day after the completion of three i.v. doses of DHA-dFdC at 100 mg/kg. Data shown are mean ± S.D. (n = 6–8) (* p < 0.05, Lethal-RD vs. Untreated, one-way ANOVA followed by Bonferroni post hoc test).
Table II.
Blood and serum parameters in healthy mice one day after three i.v. doses of DHA-dFdC at a lethal-RD (100 mg/kg). Data are mean ± S.D. (n = 8).
| Parameter | Unit | Treatment | ||
|---|---|---|---|---|
| Untreated | Vehicle | DHA-dFdC | ||
| Blood | ||||
| White blood cells (WBC) | k/μl | 5.2 ± 1.7 | 5.3 ± 2.1 | 3.2 ±1.4a,b |
| Red blood cell count (RBC) | M/μl | 9.7 ± 0.5 | 9.2 ± 0.7 | 7.9 ± 2.0a |
| Hemoglobin (HGB) | (g/dL) | 13.8 ± 0.9 | 13.8 ± 1.1 | 11.7 ± 2.5a,b |
| Hematocrit (HCT) | % | 39.8 ± 3.2 | 38.2 ± 2.1 | 32.0 ± 7.5a,b |
| Mean globular volume (MCV) | fL | 41.0 ± 3.8 | 41.4 ± 1.8 | 41.0 ± 2.0 |
| Mean hemoglobin quantity (MCH) | pg | 14.3 ± 1.1 | 14.9 ± 0.5 | 15.1 ± 1.2 |
| Mean cellular hemoglobin concentration (MCHC) | g/dL | 35.0 ± 4.1 | 36.2 ± 2.3 | 36.9 ± 2.5 |
| Lymphocytes | % | 69.9 ± 9.7 | 75.1 ± 14.4 | 84.7 ± 6.2a |
| Monocytes | % | 9.2 ± 9.0 | 6.7 ± 2.0 | 8.5 ± 2.9 |
| Basophil | % | 1.0 ± 1.5 | 2.1 ± 2.6 | 1.4 ± 2.9 |
| Eosinophil | % | 3.8 ± 3.0 | 2.8 ± 1.6 | 0.8 ± 1.2a,b |
| Neutrophil Segmented | % | 16.1 ± 5.6 | 13.4 ± 12.3 | 4.6 ± 3.6a |
| Platelet estimate | Adequate | Adequate | Adequate∞ | |
| Serum | ||||
| Cholesterol | mg/dL | 103.7 ± 22.3 | 99.7 ± 9.2 | 94.6 ± 17.5 |
| Glucose | mg/dL | 230.4 ± 44.0 | 199.7 ± 37.5 | 210.1 ± 45.4 |
| Calcium | mg/dL | 9.9 ± 1.7 | 8.8 ± 3.4 | 9.9 ± 0.8 |
| Phosphorus | mg/dL | 14.3 ± 1.7 | 13.8 ± 1.5 | 11.9 ± 1.7a |
| Bicarbonate | (mmol/L) | 28.9 ± 2.7 | 33.3 ± 2.8 | 31.6 ± 4.8 |
| Blood urea nitrogen (BUN) | mg/dL | 22.1 ± 3.0 | 20.5 ± 2.2 | 17.9 ± 1.96a,b |
| Globulin | g/dL | 2.7 ± 0.1 | 2.7 ± 0.2 | 2.7 ± 0.1 |
| Total bilirubin | mg/dL | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.3 ± 0.1 |
| Direct bilirubin | mg/dL | 0.1 ± 0.04 | 0.1 ± 0.05 | 0.1 ± 0.05 |
| Indirect bilirubin | mg/dL | 0.2 ± 0.08 | 0.3 ± 0.1 | 0.2 ± 0.08 |
| Total proteins | g/dL | 5.5 ± 0.2 | 5.3 ± 0.4 | 5.1 ± 0.2 |
| Aspartate transaminase (AST) | U/L | 200.6 ± 91.6 | 279.9 ± 165.3 | 193.9 ± 69.3 |
| Alanine transaminase (ALT) | U/L | 21.3 ± 3.0 | 25.9 ± 8.0 | 18.8 ± 5.1 |
| Alkaline phosphatase (ALP) | U/L | 200.3 ± 29.1 | 161.9 ± 70.2 | 182.6 ± 21.6 |
| Creatine phosphokinase (CPK) | U/L | 1033.7 ± 609.6 | 1923.3 ± 1248.2 | 822.4 ± 451.6b |
| Albumin | g/dL | 2.8 ± 0.1 | 2.5 ± 0.3a | 2.4 ± 0.2a |
Statistical analysis was performed using one-way ANOVA followed by a Bonferroni post hoc test.
p < 0.05 vs. Untreated;
p < 0.05 vs. Vehicle;
7 adequate, 1 decreased.
Efficacy of DHA-dFdC at or below its RD-MTD in vivo
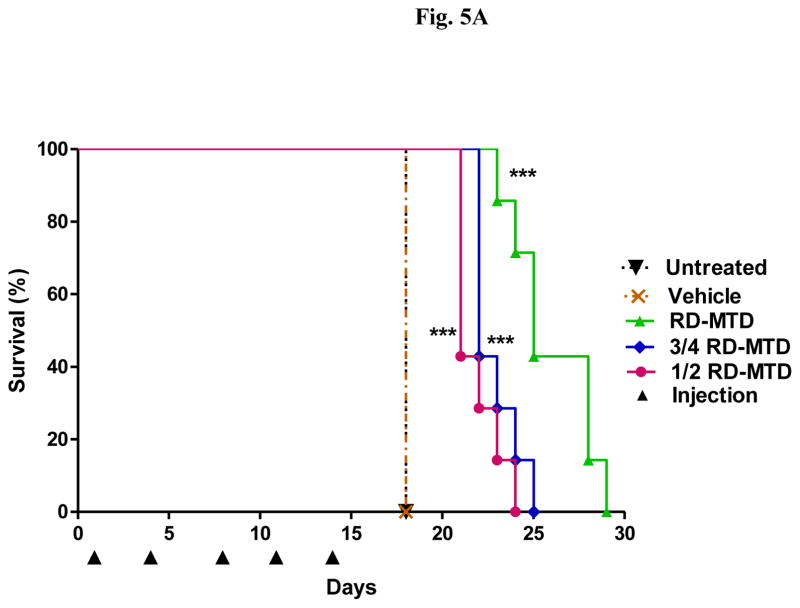
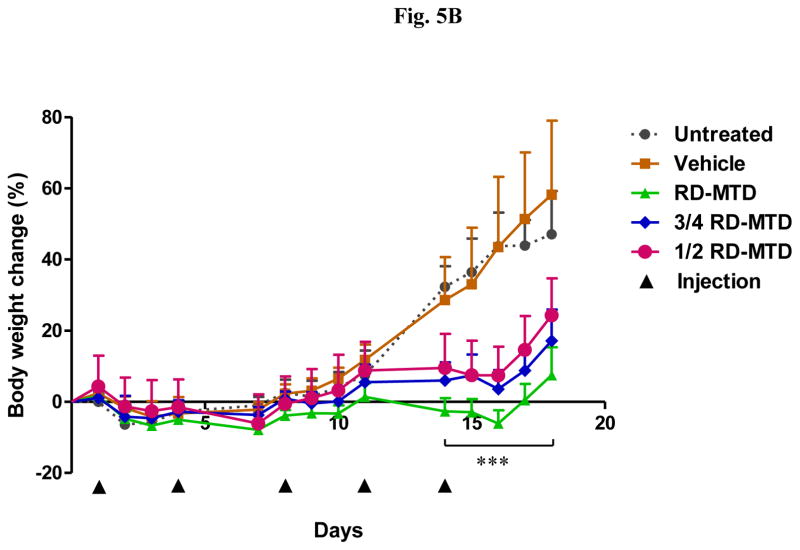
The RD-MTD identified above was in DBA/2 mice, and therefore DBA/2 mice were i.p. injected (on day 0) with the syngeneic L1210 leukemia cells to establish a mouse model of leukemia to evaluate the antitumor activity of DHA-dFdC at or below its RD-MTD. Mice were inoculated on day 0, and then received (i.v.) five doses of DHA-dFdC on days 1, 4, 8, 11, and 14. Mice in the control groups (untreated mice and vehicle alone) were sacrificed on day 18, because they reached our end point (i.e. body weight increase of ≥ 60%) (Figs. 5A and B). Those mice presented larger distended abdomens, urine staining, and thin body conditions. In contrast, mice treated with DHA-dFdC at or below its RD-MTD did not gain more than 10% of body weight (Fig. 5B), and they appeared normal on day 18. Mice that were treated with DHA-dFdC at or below its RD-MTD survived longer than mice in the control groups (Fig. 5A and Table III). DHA-dFdC at its RD-MTD (50 mg/kg) showed a higher T/U ratio than at 3/4 × RD-MTD (37.5 mg/kg) or at 1/2 × RD-MTD (25 mg/kg) (Table III). Furthermore, DHA-dFdC at its RD-MTD increased mouse life span by 44.4%, 27.0% at 3/4 × RD-MTD, and 21.4% at 1/2 × RD-MTD (Table III).
Figure 5.
Efficacy of DHA-dFdC against L1210 leukemia cells in a mouse model. Tumor cells were injected (i.p.) on day 0. On days 1, 4, 8, 11, and 14 (▲), mice (n = 7) were i.v. injected with DHA-dFdC at 1 × RD-MTD, ¾ × RD-MTD, or ½ × RD-MTD. Mice in control groups were i.v. injected with vehicle alone or left untreated. (A) Survival curves (*** p < 0.0001, all DHA-dFdC groups vs. Untreated, Log-rank (Mantel-Cox) test). (B) Percent of body weight change. Data shown are mean ± S.D. (*** p < 0.0001, all DHA-dFdC groups vs. Untreated, one-way ANOVA followed by Bonferroni post hoc test).
Table III.
Antitumor activity of DHA-dFdC at 1 × RD-MTD, ¾ × RD-MTD, and ½ × RD-MTD in mice with syngeneic L1210 leukemia cells. Data are mean ± S.D. (n = 7).
| Groups | Dose (mg/kg) | Days of treatment | Survival (day) | T/U* | % ILS+ |
|---|---|---|---|---|---|
| Untreated | - | - | 18 | 1.0 | 0 |
| Vehicle | 0 | 1, 4, 8, 11, 14 | 18 | 1.0 | 0 |
| 1 × RD-MTD | 50 | 1, 4, 8, 11, 14 | 26.0 ± 2.3a | 1.4 ± 0.13a | 44.4 |
| 3/4 × RD-MTD | 37.5 | 1, 4, 8, 11, 14 | 22.9 ± 1.2a,b | 1.3 ± 0.07a,b | 27.0 |
| 1/2 × RD-MTD | 25 | 1, 4, 8, 11, 14 | 21.9 ± 1.2a,b | 1.2 ± 0.07a,b | 21.4 |
The ratio of the mean survival time of treated group to the median survival time of the untreated group.
Increase in life span compared to untreated group. Statistical analysis was performed using one-way ANOVA followed by a Bonferroni post hoc test.
p < 0.0001 vs. Control groups (i.e. Untreated and Vehicle);
p< 0.0001 vs. 50 mg/kg.
Discussion
The repeat-dose range finding study showed that DHA-dFdC has a dose-dependent toxicity in terms of body weight and survival (i.e. weight loss of > 20%). Its RD-MTD is 50 mg/kg (Figs. 1–2 and Table I). At or below its RD-MTD, DHA-dFdC is effective in prolonging mouse survival in a mouse leukemia model (Fig. 5). However, higher doses of DHA-dFdC following a repeat-dose schedule affected the survival and body weight of the mice significantly (Figs. 1A and 1B).
A single i.v. dose of DHA-dFdC at 100 mg/kg was not lethal to mice (data not shown). Due to the limited solubility of DHA-dFdC in the vehicle solution, we tested whether multiple doses of DHA-dFdC at 100 mg/kg in a spaced schedule is lethal to mice. Mice were i.v. injected with DHA-dFdC at 100 mg/kg three times on days 0, 3, and 5. At this dosing regimen, around 50% of mice reached the end point (i.e. ≥ 20% of body weight loss) as shown Figs. 3A and 3B. In addition, the spleen and liver weights of those mice decreased significantly in comparison to mice in the control groups (Fig. 4A). Furthermore, at this lethal-RD, DHA-dFdC showed hematological toxicity, because certain blood parameters (e.g. WBC, RBC, HGB, HCT, lymphocytes, eosinophil, and neutrophil segmented) were significantly different from that of mice in the untreated group (Table II). Gemcitabine has dose-limiting toxicities in clinics. For example, data from clinical studies showed that gemcitabine causes dose-limiting myelosuppression that is characterized mainly by thrombocytopenia (1, 8, 17). In our study, at a lethal-RD dose, DHA-dFdC significantly decreased the levels of WBC as well as RBC, when compared to the control groups (40% and 18%, respectively) (Table II). On the other hand, our data showed that platelet estimate was adequate, indicating that DHA-dFdC may not cause significant thrombocytopenia. At the lethal-RD used, DHA-dFdC also induced a significant decrease in the levels of HGB and HCT (15% and 20%, respectively), indicating DHA-dFdC induces anemia in DBA/2 mice (38, 39). The neutrophil levels in mice that received the lethal-RD of DHA-dFdC decreased significantly as compared to the untreated group (71%), indicating an acute neutropenia. It was reported that the number of WBC decreased in healthy men when they received a diet supplemented with DHA (22). The main cause of reduction of WBC number was attributed to a reduction in the number of circulating neutrophils (22). In our study, we found that at lethal-RD, DHA-dFdC caused a decrease in the number of WBC as well as neutrophils segmented (Table II). Both the DHA and gemcitabine moieties in the DHA-dFdC may have contributed to the observed hematological toxicity. Some serum parameters such as phosphorus, BUN, CPK and albumin in mice that received DHA-dFdC at the lethal-RD were also decreased compared to the untreated group (Table II), but these changes were not more than 20%. According a previous study (40), small changes in some parameters in toxicity studies are due to animals having a poor tolerance to the test article. Indeed, one of the most common effects of toxicity studies in rodents is decreasing serum albumin concentration because small species have a rapid turnover of albumin (41). Finally, DHA-dFdC at the lethal-RD did not significantly affect serum levels of aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP), when compared to the untreated control, in spite of the finding that it caused a decrease in the relative weight of mouse liver (Fig. 4). Data from a previous phase II clinical study showed that the liver toxicity of gemcitabine was mild and transient (1).
DHA is considered a generally recognized as safe material for inclusion in diet by the U.S. Food and Drug Administration (42). However, some side effects have been reported such as gastrointestinal disturbances, nausea, increased bleeding time, effects in the glycemic control in non-insulin-dependent diabetics, and increased levels of low-density lipoprotein cholesterol (42). Parenteral omega-3 fatty acid preparations have been used for years as part of total parenteral nutrition and are generally well tolerated (27, 43). In addition, an in vitro study reported that pancreatic stellate cells (PSCs) are highly sensitive to gemcitabine plus Lipidem® (i.e. emulsion for infusion that contains omega-3-acid triglycerides) (44). As PSCs are responsible for therapeutic resistance of pancreatic cancer due to their contribution to secretion of extracellular matrix, the anti-proliferative and anti-invasive efficacy of gemcitabine plus Lipidem® treatment reported in PSC culture might be a good alternative to targeting pancreatic cancer.
Conclusion
The RD-MTD of DHA-dFdC in a spaced schedule is 50 mg/kg when intravenously injected to DBA/2 mice. DHA-dFdC mainly affects mouse spleen at a lethal repeat-dose. At or below its RD-MTD, DHA-dFdC shows anticancer activity in a mouse model of leukemia, improving the survival of leukemia-bearing mice.
Acknowledgments
This work was supported in part by grants from the U.S. National Institutes of Health (CA135274 and CA179362 to Z.C.). Solange Valdes is a winner of the Becas-Chile Scholarship from the Government of Chile.
Abbreviations
- dFdC
2′, 2′-difluorodeoxycytidine HCl
- DHA
docosahexaenoic acid
- PUFA
polyunsaturated fatty acid
- DHA-dFdC
4-(N)-docosahexaenoyl 2′, 2′-difluorodeoxycytidine
- RD-MTD
Repeat-dose maximum tolerated dose
- Lethal-RD
lethal repeat-dose
- i.v
intravenous
- i.p
intraperitoneally
- ILS
increase in life span
- T/U
the ratio of the mean survival time of treated group to the median survival time of the untreated group
- WBC
white blood cells
- RBC
red blood cell count
- HGB
hemoglobin level
- HCT
hematocrit value
- BUN
blood urea nitrogen
- CPK
creatine phosphokinase
- AST
aspartate transaminase
- ALT
alanine transaminase
- ALP
alkaline phosphatase
- PSCs
pancreatic stellate cells
References
- 1.Carmichael J, Fink U, Russell RC, Spittle MF, Harris AL, Spiessi G, Blatter J. Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer. 1996;73(1):101–105. doi: 10.1038/bjc.1996.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, Nelson R, Dorr FA, Stephens CD, Von Hoff DD. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 3.Hoang T, Kim K, Jaslowski A, Koch P, Beatty P, McGovern J, Quisumbing M, Shapiro G, Witte R, Schiller JH. Phase II study of second-line gemcitabine in sensitive or refractory small cell lung cancer. Lung Cancer. 2003;42(1):97–102. doi: 10.1016/s0169-5002(03)00273-3. [DOI] [PubMed] [Google Scholar]
- 4.Pfisterer J, Plante M, Vergote I, du Bois A, Hirte H, Lacave AJ, Wagner U, Stahle A, Stuart G, Kimmig R, Olbricht S, Le T, Emerich J, Kuhn W, Bentley J, Jackisch C, Luck HJ, Rochon J, Zimmermann AH, Eisenhauer E. Gemcitabine plus carboplatin compared with carboplatin in patients with platinum-sensitive recurrent ovarian cancer: an intergroup trial of the AGO-OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24(29):4699–4707. doi: 10.1200/JCO.2006.06.0913. [DOI] [PubMed] [Google Scholar]
- 5.Yardley DA. Gemcitabine plus paclitaxel in breast cancer. Semin Oncol. 2005;32(4 Suppl 6):S14–21. doi: 10.1053/j.seminoncol.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 6.Wong A, Soo RA, Yong WP, Innocenti F. Clinical pharmacology and pharmacogenetics of gemcitabine. Drug Metab Rev. 2009;41(2):77–88. doi: 10.1080/03602530902741828. [DOI] [PubMed] [Google Scholar]
- 7.de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014;741:8–16. doi: 10.1016/j.ejphar.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 8.Casper ES, Green MR, Kelsen DP, Heelan RT, Brown TD, Flombaum CD, Trochanowski B, Tarassoff PG. Phase II trial of gemcitabine (2,2′-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs. 1994;12(1):29–34. doi: 10.1007/BF00873232. [DOI] [PubMed] [Google Scholar]
- 9.Storniolo AM, Allerheiligen SR, Pearce HL. Preclinical, pharmacologic, and phase I studies of gemcitabine. Semin Oncol. 1997;24(2 Suppl 7):S7-2–S7-7. [PubMed] [Google Scholar]
- 10.Merendino N, Costantini L, Manzi L, Molinari R, D’Eliseo D, Velotti F. Dietary omega -3 polyunsaturated fatty acid DHA: a potential adjuvant in the treatment of cancer. Biomed Res Int. 2013;2013:310186. doi: 10.1155/2013/310186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chamras H, Ardashian A, Heber D, Glaspy JA. Fatty acid modulation of MCF-7 human breast cancer cell proliferation, apoptosis and differentiation. J Nutr Biochem. 2002;13(12):711–716. doi: 10.1016/s0955-2863(02)00230-9. [DOI] [PubMed] [Google Scholar]
- 12.Serini S, Piccioni E, Merendino N, Calviello G. Dietary polyunsaturated fatty acids as inducers of apoptosis: implications for cancer. Apoptosis. 2009;14(2):135–152. doi: 10.1007/s10495-008-0298-2. [DOI] [PubMed] [Google Scholar]
- 13.Spencer L, Mann C, Metcalfe M, Webb M, Pollard C, Spencer D, Berry D, Steward W, Dennison A. The effect of omega-3 FAs on tumour angiogenesis and their therapeutic potential. Eur J Cancer. 2009;45(12):2077–2086. doi: 10.1016/j.ejca.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 14.Horia E, Watkins BA. Complementary actions of docosahexaenoic acid and genistein on COX-2, PGE2 and invasiveness in MDA-MB-231 breast cancer cells. Carcinogenesis. 2007;28(4):809–815. doi: 10.1093/carcin/bgl183. [DOI] [PubMed] [Google Scholar]
- 15.D’Eliseo D, Manzi L, Merendino N, Velotti F. Docosahexaenoic acid inhibits invasion of human RT112 urinary bladder and PT45 pancreatic carcinoma cells via down-modulation of granzyme B expression. J Nutr Biochem. 2012;23(5):452–457. doi: 10.1016/j.jnutbio.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 16.Li S, Qin J, Tian C, Cao J, Fida G, Wang Z, Chen H, Qian Z, Chen WR, Gu Y. The targeting mechanism of DHA ligand and its conjugate with Gemcitabine for the enhanced tumor therapy. Oncotarget. 2014;5(11):3622–3635. doi: 10.18632/oncotarget.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abbruzzese JL, Grunewald R, Weeks EA, Gravel D, Adams T, Nowak B, Mineishi S, Tarassoff P, Satterlee W, Raber MN, et al. A phase I clinical, plasma, and cellular pharmacology study of gemcitabine. J Clin Oncol. 1991;9(3):491–498. doi: 10.1200/JCO.1991.9.3.491. [DOI] [PubMed] [Google Scholar]
- 18.Nelson GJ, Schmidt PC, Bartolini GL, Kelley DS, Kyle D. The effect of dietary docosahexaenoic acid on plasma lipoproteins and tissue fatty acid composition in humans. Lipids. 1997;32(11):1137–1146. doi: 10.1007/s11745-997-0146-5. [DOI] [PubMed] [Google Scholar]
- 19.Ryan AS, Keske MA, Hoffman JP, Nelson EB. Clinical overview of algal-docosahexaenoic acid: effects on triglyceride levels and other cardiovascular risk factors. Am J Ther. 2009;16(2):183–192. doi: 10.1097/MJT.0b013e31817fe2be. [DOI] [PubMed] [Google Scholar]
- 20.Nelson GJ, Schmidt PS, Bartolini GL, Kelley DS, Kyle D. The effect of dietary docosahexaenoic acid on platelet function, platelet fatty acid composition, and blood coagulation in humans. Lipids. 1997;32(11):1129–1136. doi: 10.1007/s11745-997-0145-6. [DOI] [PubMed] [Google Scholar]
- 21.Park Y, Harris W. EPA, but not DHA, decreases mean platelet volume in normal subjects. Lipids. 2002;37(10):941–946. doi: 10.1007/s11745-006-0984-1. [DOI] [PubMed] [Google Scholar]
- 22.Kelley DS, Taylor PC, Nelson GJ, Mackey BE. Dietary docosahexaenoic acid and immunocompetence in young healthy men. Lipids. 1998;33(6):559–566. doi: 10.1007/s11745-998-0240-8. [DOI] [PubMed] [Google Scholar]
- 23.Thies F, Nebe-von-Caron G, Powell JR, Yaqoob P, Newsholme EA, Calder PC. Dietary supplementation with gamma-linolenic acid or fish oil decreases T lymphocyte proliferation in healthy older humans. J Nutr. 2001;131(7):1918–1927. doi: 10.1093/jn/131.7.1918. [DOI] [PubMed] [Google Scholar]
- 24.He K, Xun P, Brasky TM, Gammon MD, Stevens J, White E. Types of fish consumed and fish preparation methods in relation to pancreatic cancer incidence: the VITAL Cohort Study. Am J Epidemiol. 2013;177(2):152–160. doi: 10.1093/aje/kws232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. Bmj. 2013;346:f3706. doi: 10.1136/bmj.f3706. [DOI] [PubMed] [Google Scholar]
- 26.Arshad A, Chung WY, Steward W, Metcalfe MS, Dennison AR. Reduction in circulating pro-angiogenic and pro-inflammatory factors is related to improved outcomes in patients with advanced pancreatic cancer treated with gemcitabine and intravenous omega-3 fish oil. HPB (Oxford) 2013;15(6):428–432. doi: 10.1111/hpb.12002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arshad A, Isherwood J, Mann C, Cooke J, Pollard C, Runau F, Morgan B, Steward W, Metcalfe M, Dennison A. Intravenous omega-3 Fatty Acids Plus Gemcitabine: Potential to Improve Response and Quality of Life in Advanced Pancreatic Cancer. JPEN J Parenter Enteral Nutr. 2015 doi: 10.1177/0148607115595221. [DOI] [PubMed] [Google Scholar]
- 28.de Camargo CQ, Mocellin MC, de Pastore Silva JA, Fabre ME, Nunes EA, Trindade EB. Fish oil supplementation during chemotherapy increases posterior time to tumor progression in colorectal cancer. Nutr Cancer. 2016;68(1):70–76. doi: 10.1080/01635581.2016.1115097. [DOI] [PubMed] [Google Scholar]
- 29.Murphy RA, Mourtzakis M, Chu QS, Baracos VE, Reiman T, Mazurak VC. Supplementation with fish oil increases first-line chemotherapy efficacy in patients with advanced nonsmall cell lung cancer. Cancer. 2011;117(16):3774–3780. doi: 10.1002/cncr.25933. [DOI] [PubMed] [Google Scholar]
- 30.Colomer R, Moreno-Nogueira JM, Garcia-Luna PP, Garcia-Peris P, Garcia-de-Lorenzo A, Zarazaga A, Quecedo L, del Llano J, Usan L, Casimiro C. N-3 fatty acids, cancer and cachexia: a systematic review of the literature. Br J Nutr. 2007;97(5):823–831. doi: 10.1017/S000711450765795X. [DOI] [PubMed] [Google Scholar]
- 31.Heller AR, Rossel T, Gottschlich B, Tiebel O, Menschikowski M, Litz RJ, Zimmermann T, Koch T. Omega-3 fatty acids improve liver and pancreas function in postoperative cancer patients. Int J Cancer. 2004;111(4):611–616. doi: 10.1002/ijc.20291. [DOI] [PubMed] [Google Scholar]
- 32.Naguib YW, Lansakara PD, Lashinger LM, Rodriguez BL, Valdes S, Niu M, Aldayel AM, Peng L, Hursting SD, Cui Z. Synthesis, Characterization, and In Vitro and In Vivo Evaluations of 4-(N)-Docosahexaenoyl 2′, 2′-Difluorodeoxycytidine with Potent and Broad-Spectrum Antitumor Activity. Neoplasia. 2016;18(1):33–48. doi: 10.1016/j.neo.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery CA. Oncological and toxicological research: Alleviation and control of pain and distress in laboratory animals. Cancer Bulletin. 1990;42:230–237. [Google Scholar]
- 34.NRC. The Guide for the Care and Use of Laboratory Animals. Washington, D.C: National Academies Press; 2011. [PubMed] [Google Scholar]
- 35.Gianasi E, Wasil M, Evagorou EG, Keddle A, Wilson G, Duncan R. HPMA copolymer platinates as novel antitumour agents: in vitro properties, pharmacokinetics and antitumour activity in vivo. Eur J Cancer. 1999;35(6):994–1002. doi: 10.1016/s0959-8049(99)00030-1. [DOI] [PubMed] [Google Scholar]
- 36.Andersson L, Davies J, Duncan R, Ferruti P, Ford J, Kneller S, Mendichi R, Pasut G, Schiavon O, Summerford C, Tirk A, Veronese FM, Vincenzi V, Wu G. Poly(ethylene glycol)-poly(ester-carbonate) block copolymers carrying PEG-peptidyl-doxorubicin pendant side chains: synthesis and evaluation as anticancer conjugates. Biomacromolecules. 2005;6(2):914–926. doi: 10.1021/bm049381p. [DOI] [PubMed] [Google Scholar]
- 37.Reddy LH, Marque PE, Dubernet C, Mouelhi SL, Desmaele D, Couvreur P. Preclinical toxicology (subacute and acute) and efficacy of a new squalenoyl gemcitabine anticancer nanomedicine. J Pharmacol Exp Ther. 2008;325(2):484–490. doi: 10.1124/jpet.107.133751. [DOI] [PubMed] [Google Scholar]
- 38.Raabe BM, Artwohl JE, Purcell JE, Lovaglio J, Fortman JD. Effects of weekly blood collection in C57BL/6 mice. J Am Assoc Lab Anim Sci. 2011;50(5):680–685. [PMC free article] [PubMed] [Google Scholar]
- 39.Oliver SAG, Smith JE, Kaneko JJ. Erythrocyte Structure and Function. In: Douglas J, Weiss KJW, editors. Schalm’s Veterinary Hematology. Ames: John Wiley & Sons; 2010. pp. 123–130. [Google Scholar]
- 40.Hall R, Everds N. Principles and Methods of Toxicology. 5. CRC Press; 2007. Principles of Clinical Pathology for Toxicology Studies; pp. 1317–1358. [Google Scholar]
- 41.MacNeill A. Clinical Biochemistry of Domestic Animals, 6th Edition by Editors: J. Jerry Kaneko, John W. Harvey, and Michael L. Bruss. Veterinary Clinical Pathology. 2009;38(4):545. [Google Scholar]
- 42.Department of Health and Human Services UFaDA. Substances affirmed as generally recognized as safe: menhaden oil. Washington: Food and Drug Administration, HHS; 1997. pp. 30751–30757. [Google Scholar]
- 43.Klek S. Omega-3 Fatty Acids in Modern Parenteral Nutrition: A Review of the Current Evidence. J Clin Med. 2016;5(3) doi: 10.3390/jcm5030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haqq J, Howells LM, Garcea G, Dennison AR. Targeting pancreatic cancer using a combination of gemcitabine with the omega-3 polyunsaturated fatty acid emulsion, Lipidem. Mol Nutr Food Res. 2016;60(6):1437–1447. doi: 10.1002/mnfr.201500755. [DOI] [PubMed] [Google Scholar]