Abstract
Verrucae vulgaris in patients with severe combined immunodeficiency (SCID) post-hematopoetic stem cell transplantation (HCST) can be challenging to manage. We describe two brothers with X-linked SCID who both had severe, persistent verrucae that did not respond to traditional topical therapies, including liquid nitrogen, imiquimod, salicylic acid, sinecatechins, 40% urea and 5-fluorourcil. Both brothers had full response to topical 3% cidofovir, which should be considered in recalcitrant warts in post-HSCT SCID patients.
Keywords: severe combined immunodeficiency, warts, hematopoetic stem cell transplantation
Many primary immunodeficiencies present with constellations of infections that include warts(1). After hematopoetic stem cell transplantation (HSCT), patients can have difficult to treat warts, especially in the settings of certain genetic mutations causing SCID, including X-SCID and JAK3 mutations(2). Topical cidofovir has been successfully used with minimal risk in children with refractory warts(3). Of note, there are reports of safe and efficacious use of cidofovir in solid organ transplant patients(4) and cidofovir has been shown in case reports to play a role in treating recalcitrant warts in immunocompromised patients, for example in HIV(5).
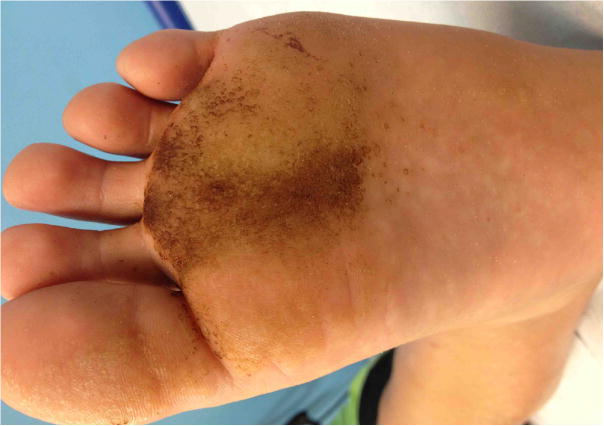
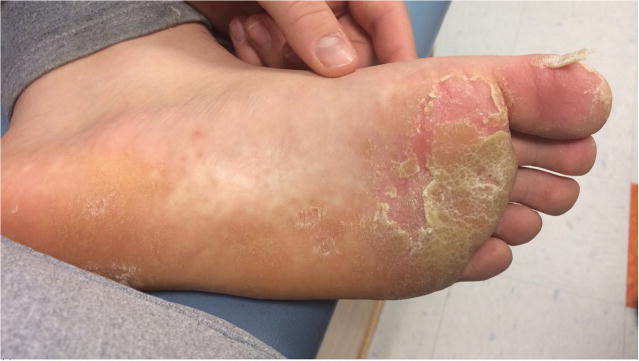
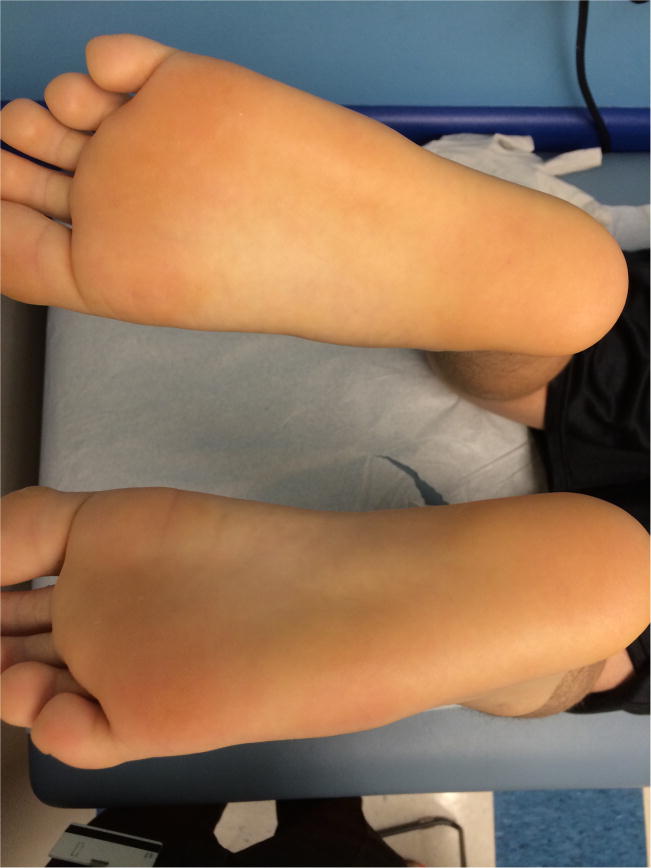
This is a case report of two brothers with X-SCID who had recalcitrant warts that cleared with topical cidofovir. The older sibling received HSCT in infancy for X-linked SCID. He had appropriate T cell engraftment without B cell engraftment and requires ongoing immunoglobulin replacement. He developed bilateral confluent mosaic plantar warts at 11 years of age (figure 1) and presented to Dermatology at 12 years of age after multiple therapies (including imiquimod and salicylic acid) had failed. He was treated with monthly liquid nitrogen in office and daily topical sinecatechins and 40% topical urea at home for 3 months with slight improvement. Topical 5% fluorouracil cream alternating daily with 17% liquid salicylic acid was used for four months but after lack of improvement topical 3% cidofovir in petrolatum with occlusion (using white cloth surgical tape) was started. He continued on topical cidofovir for 5 total months and upon clinic visit at 6 months, he had full clearance and halted cidofovir.
Figure 1.
Response of plantar warts in sibling one to topical cidofovir. a) Prior to therapy b) After initial cidofovir with peeling of keratotic layer c) Full response.
His younger sibling had a newborn haploidentical transplant for X-SCID complicated by inflammatory bowel disease and was retransplanted with full T cell chimerism and appropriate NK chimerism and B cell and myeloid chimerism < 100%. He had onset of bilateral plantar warts at 7 years of age and presented to dermatology after 6 months of symptoms. At that time, he had previously tried topical 17% salicylic acid. A regimen of in-office liquid nitrogen and at home daily 17% salicyclic acid was started. He returned three months later with worsening bilateral plantar warts and new forehead lesions. He had pain with salicylic acid and was started on topical sinecatechins. He returned to clinic in 4 months with stable plantar warts and new lesions on his left index finger and right axilla. He was started on daily 3% topical cidofovir in petrolatum, under occlusion with white cloth surgical tape, and after 5 months he was seen in Immunology clinic with full resolution.
Warts can be challenging to treat in SCID patients after HSCT. Topical cidofovir has been used successfully in treating challenging warts in various types of immunocompromised patients. Topical 3% cidofovir was effective in treatment of recalcitrant warts in X-SCID patients after HSCT.
Acknowledgments
Funding Source: Sarah E. Henrickson was supported by T32HD043021.
Footnotes
Conflict of Interest: None
References
- 1.Leiding JW, Holland SM. Warts and all: human papillomavirus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130:1030–48. doi: 10.1016/j.jaci.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Laffort C, Le Deist F, Favre M, et al. Severe cutaneous papillomavirus disease after HSCT in patients with severe combined immune deficiency caused by common γ cytokine receptor subunit of JAK-3 deficiency. The Lancet. 2005;363:2051–2054. doi: 10.1016/S0140-6736(04)16457-X. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Morano T, del Boz J, Gonzalez-Carrascosa M, et al. Topical cidofovir for viral warts in children. J Eur Acad Dermatol Venereol. 2011;25:1487–1489. doi: 10.1111/j.1468-3083.2010.03961.x. [DOI] [PubMed] [Google Scholar]
- 4.Bonatti H, Aigner F, De Clerq E, et al. Local administration of cidofovir for human papilloma virus associated skin lesions in transplant recipients. Transplant International. 2007;20:238–246. doi: 10.1111/j.1432-2277.2006.00430.x. [DOI] [PubMed] [Google Scholar]
- 5.De Socio GV, Simonetti S, Rosignoli D, et al. Topical Cidofovir for severe warts in a patient affected by AIDS and Hodgkin’s lymphoma. Int J STR AIDS. 2008;19:715–716. doi: 10.1258/ijsa.2008.008065. [DOI] [PubMed] [Google Scholar]