Abstract
Rhubarb has been used as an evacuant for thousands of years. However, recent research has indicated that rhubarb inhibits inflammation and protects organ function. In the current study, the use of rhubarb was investigated in patients with intra-abdominal hypertension (IAH). Specifically, its dual role in attenuating lung and bowel injury by catharsis and inhibiting inflammation was evaluated. Patients in the glycerin group (n=56) received 110 ml of glycerin enema by coloclysis once daily for 7 to 9 days. Patients in the rhubarb group (n=56) were treated with a mixture of 0.3 g/kg body weight rhubarb powder in 100 ml warm water. The Acute Physiology and Chronic Health Evaluation II (APACHE II), Sepsis-Related Organ Failure Assessment (SOFA), intra-abdominal pressure, procalcitonin (PCT), C-reactive protein (CRP), tumor necrosis factor-α (TNF-α) and interleukin (IL)-6 levels were recorded. The duration of mechanical ventilation (MV), respiratory parameters, first day of enteral nutrition (EN), intensive care unit (ICU) hospital stay and 30-day mortality were also recorded. The APACHE II scores were significantly lower in the rhubarb group compared with the glycerin group from day 3 to 9 (P<0.05 at day 3 and 4; P<0.01 at day 5, 7 and 9). The SOFA scores were significantly lower in the rhubarb group compared with the glycerin group from day 5 to 9 (P<0.05). PCT levels were significantly lower from day 4 to 9 (P<0.05) and the CRP level was significantly lower from day 3 to 9 (P<0.05) in the rhubarb group compared with the glycerin group. The TNF-α and IL-6 were significantly lower in the rhubarb group compared with the glycerin group from day 3 to 9 (P<0.05 at day 3 and 4, P<0.01 at day 5, 7 and 9). The positive end-expiratory pressure and peak inspiratory pressure were significantly lower in the rhubarb group compared with the glycerin group at day 3, 5 and 7 (P<0.05 at day 3 and 5, P<0.01 at day 7), while the oxygenation index (P<0.05) and alveolar-arterial partial pressure of oxygen (P<0.05 at day 3 and 5, P<0.01 at day 7) were significantly improved. Significantly shorter durations of MV and ICU hospital stay, and earlier EN, were observed in the rhubarb group compared with the glycerin group (all P<0.05). Rhubarb treatment was indicated to be beneficial in IAH, by inhibiting inflammation and restoring intestinal function.
Keywords: intra-abdominal hypertension, rhubarb, enema, respiratory failure, enteral nutrition
Introduction
Critically ill patients are prone to developing intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS). IAH has been reported in 35% of intensive care unit (ICU) patients, 5% of which also manifest ACS (1), which is an important factor leading to an increased mortality rate in ICU patients. In critically ill patients, contributing factors often overlap, leading to elevated intra-abdominal pressure (IAP), and resulting in multiple organ failure (2–4).
The severity and duration of IAH is associated with the incidence and mortality of multiple organ dysfunction syndrome (MODS) (5). Previous results have indicated that global hemodynamics, oxygenation and organ function are notably affected by sustained IAH of 12 h combined with severe acute pancreatitis (SAP) (6). Animal models of SAP treated with 25 mmHg IAH/ACS undergoing delayed decompression had a higher grade of lung and intestinal injury (7). Increased oxygenation index and urinary output were the most pronounced effects of decompressive laparotomy, with a mortality rate of 49.2% (8). Surgical abdominal incision decompression resulted in a rapid decline in intra-abdominal pressure by 50%, without improving the Sepsis-Related Organ Failure Assessment (SOFA) score (9).
Patients with bowel dysfunction, which leads to IAH/ACS, are typically treated using conservative therapy to reduce intra-abdominal pressure. Currently, glycerin enema is commonly used for catharsis. The content of 1,2,3-propanetriol is 42.7 g per 100 g of glycerin enema, which is not absorbed after entering the rectum and acts as a laxative.
IAH/ACS is characterized by declining gastrointestinal peristalsis as well as intestinal mucosal edema and damage to the intestinal mucosal barrier, resulting in acute lung injury/acute respiratory distress syndrome (ALI/ARDS), acute renal failure and serious systemic inflammatory response syndrome (SIRS) (10,11). Therefore, catharsis alone may not be adequate therapy for IAH/ACS. Rhubarb is associated with multiple therapeutic effects and is often used to relieve constipation (12). A recent study also indicated that rhubarb also promotes intestinal epithelial proliferation and improves intestinal function in sepsis (13). It has been reported to inhibit the expression of inflammatory markers and exert a protective effect against organ damage in sepsis (14).
Based on these previous results, the current study aimed to investigate the pharmacological effects of raw rhubarb in reducing IAP and improving the function of the intestinal tract and other organs. To this end, a randomized and controlled clinical trial was conducted to compare rhubarb and glycerin enemas in IAH/ACS patients. The protective role of rhubarb in IAH/ACS were evaluated in order to assess its potential as a therapeutic alternative.
Patients and methods
Patients and groups
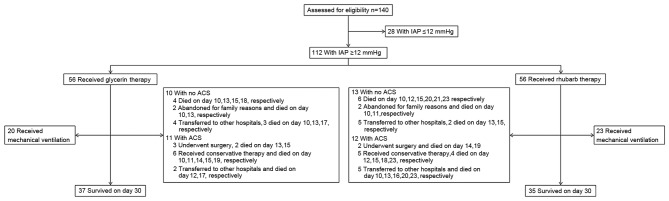
The current study was conducted in the Departments of General Surgery, ICU and Emergency of the Affiliated Hospital of Jiangsu University. Critically ill patients with IAH and acute gastrointestinal injury (AGI) stage I–III, aged 18–80 years, were consecutively recruited into this study. AGI was defined by the criteria of the European Society of Intensive Care Medicine Working Group (15). IAH was defined as a consistently increased IAP value of ≥12 mmHg. ACS was defined as IAP >20 mmHg. Single organ dysfunction or MODS/ACS was diagnosed using the criteria of the World Society of ACS (16). Patients with mechanical intestinal obstruction or AGI at stage IV were excluded. Patients with a high probability of receiving abdominal surgery were also excluded. From October 2011 to July 2015, 140 patients were assessed for eligibility, and 112 patients were selected for inclusion in the trial. According to the order of admission, patients were randomly assigned to the glycerin enema group or the rhubarb group (Fig. 1). Patients' clinical demographics at admission are summarized in Table I.
Figure 1.
Flow diagram describing enrollment, grouping and follow-up results of the clinical trial. IAP, intra-abdominal pressure.
Table I.
Patient clinical demographics.
| Parameter | Glycerin group | Rhubarb group | P-value |
|---|---|---|---|
| Number of cases (n) | 56 | 56 | |
| Sex (male/female) | 46/10 | 43/13 | 0.483 |
| Age (years) | 43±16 | 45±15 | 0.673 |
| Class of intra-abdominal hypertension at admission, n (%) | |||
| I | 28 (50%) | 27 (48.2%) | 0.850 |
| II | 18 (32.1%) | 21 (37.5%) | 0.552 |
| III | 10 (17.9%) | 8 (14.3%) | 0.607 |
| Abdominal compartment syndrome at day 30, n (%) | 10 (17.9%) | 13 (23.2%) | 0.483 |
| Diagnosis, n (%) | |||
| Severe acute pancreatitis | 36 (64.3%) | 30 (53.6%) | 0.249 |
| Septic shock | 10 (17.9%) | 13 (23.2%) | 0.483 |
| Post-cardiopulmonary resuscitation | 5 (8.9%) | 10 (17.9%) | 0.165 |
| Bowel obstruction | 5 (8.9%) | 3 (5.4%) | 0.463 |
| Co-morbidities, n (%) | |||
| Hypertension | 11 (19.6%) | 6 (10.7%) | 0.188 |
| Diabetes | 7 (12.5%) | 9 (16.1%) | 0.589 |
| Hyperthyroidism | 2 (3.6%) | 0 (0.0%) | 0.154 |
| Schistosomal cirrhosis | 1 (1.8%) | 2 (3.6%) | 0.558 |
| Chronic obstructive pulmonary disease | 3 (5.4%) | 5 (8.9%) | 0.463 |
| No co-morbidities | 32 (57.1%) | 34 (60.7%) | 0.701 |
| Liver function | |||
| Alanine aminotransferase (U/l) | 89.60±25.81 | 95.18±27.23 | 0.268 |
| Aspartate aminotransferase (U/l) | 88.75±20.56 | 96.20±26.64 | 0.101 |
| Kidney function | |||
| Blood urea nitrogen (mmol/l) | 19.52±7.70 | 18.85±9.84 | 0.691 |
| Creatinine (µmol/l) | 151.39±45.93 | 139.96±27.44 | 0.113 |
| White blood cells (×109/l) | 17.06±8.51 | 16.35±7.37 | 0.636 |
| Body mass index (kg/m2) | 23.73±4.38 | 25.09±3.77 | 0.080 |
Treatment
All patients with IAH/ACS underwent necessary treatment (17). Patients with respiratory failure received mechanical ventilation (MV) until recovery. The initial parameters of MV included a tidal volume of 6–8 ml/kg, platform pressure of 30–35 cm H2O and a positive end-expiratory pressure (PEEP) of 8–16 cm H2O. The parameters were adjusted according to arterial blood gas (ABG) analysis.
Patients in the glycerin group received 110 ml glycerin enema (Winguide Huangpu Pharmaceutical, Shanghai, China) via coloclysis once daily for 7–9 days. Patients were maintained in the supine position to retain glycerin in the intestine for more than 10 min.
Patients in the rhubarb group received 0.3 g/kg body weight rhubarb powder (Gansu Yalan Pharmaceutical Co., Ltd., Gansu, China) soaked in 100 ml warm water (80–100°C, for 30 min and cooled down to 37°C) via coloclysis once daily for 7–9 days. Enteral nutrition (EN) was administrated to patients via the nasogastric tube or oral route. EN suspension was initiated with Peptison (1 kcal/ml; Nutricia Pharmaceutical Wuxi Co., Ltd) and switched to Nutrition Fibre (1.5 kcal/ml) with feeding speed from 30 ml/h to 100 ml/h by using a nutrition pump.
Testing
Acute Physiology and Chronic Health Evaluation II (APACHE II) (18) and SOFA scores (19) were conducted twice on days 1–5, 7 and 9 post-admission, and the scores indicating greatest severity on each day were recorded.
Peripheral venous blood samples were collected on days 1–5, 7 and 9 post-admission to measure the concentrations of serum interleukin (IL)-6, tumor necrosis factor-α (TNF-α), procalcitonin (PCT) and C-reactive protein (CRP) using ELISA kits (ab178013, ab181421, ab100630 and ab99995; Abcam, Cambridge, MA, USA).
Arterial blood samples were tested at least twice per day in patients that received MV and the most severe results of ABG were used to calculate the oxygenation index (PaO2/FiO2). PEEP, alveolar-arterial partial pressure of oxygen (P(A-a)O2) and peak inspiratory pressure (PIP), duration of MV, duration of ICU hospital stay, first day of EN and 30-day mortality were recorded.
The bladder inner pressure measured in a recumbent position was used to assess the level of IAP. A Foley catheter (18 Fr) was inserted into the bladder, to drain urine, followed by injection of 25 ml saline. The catheter was connected to a pressure transducer. The operator selected the zero level of axillary midline for measurement at the end of the breath. The measurement was performed three times in total and the mean of the data was recorded (16,17).
Statistical analysis
The primary endpoints of this study were the duration of MV, ICU hospital stay, first day of EN and 30-day mortality. The secondary endpoints were the level of IAP, systemic inflammation and disease severity. Data were expressed as the mean ± standard deviation. Differences between groups were analyzed using the chi-squared test, a rank sum test and repeated analysis of variance, on SPSS 20.0 software (IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate statistical difference.
Results
Effect of rhubarb and glycerin therapies on IAP
After intervention with rhubarb or glycerin enema, the IAP of patients in both groups began to decrease from day 3 (Fig. 2). No significant differences were observed between the two groups at any time point.
Figure 2.
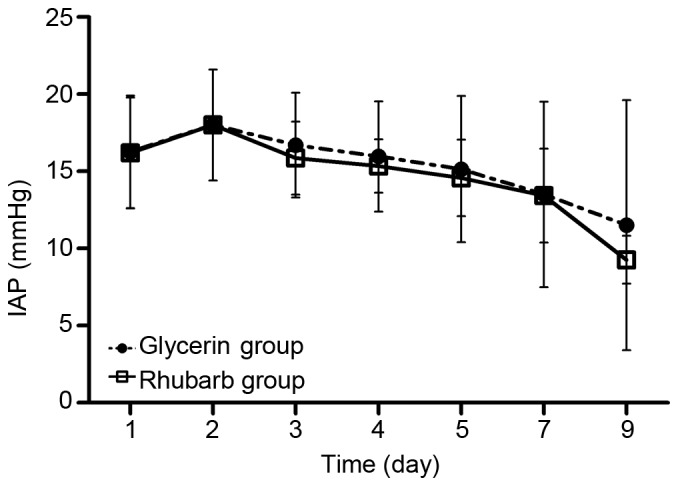
Effect of rhubarb or glycerin enema on IAP. No significant differences between the groups were found at any time point. IAP, intra-abdominal pressure.
Effect of rhubarb and glycerin therapies on severity scores
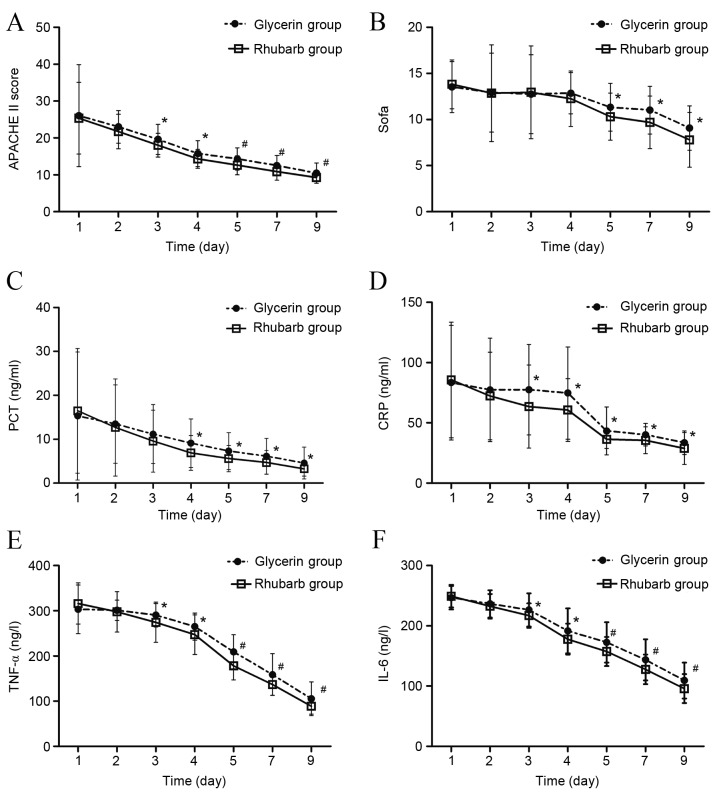
The APACHE II scores in both groups showed a declining trend (Fig. 3A). The APACHE II scores in the rhubarb group were significantly lower compared with the glycerin group from day 3 to day 9 (day 3–4, P<0.05; day 5–9, P<0.01). The SOFA scores showed no significant difference between the two groups from day 1 to day 4 however, from day 5 to 9, the SOFA scores of the rhubarb group were significantly lower compared with the glycerin group (P<0.05; Fig. 3B).
Figure 3.
Effect of rhubarb or glycerin enema on severity scores and inflammatory marker levels. (A) APACHE II score, (B) SOFA score, (C) PCT level, (D) CRP level, (E) TNF-α level and (F) IL-6 level after rhubarb or glycerin enema. *P<0.05 and #P<0.01 vs. glycerin group. APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sepsis-Related Organ Failure Assessment; PCT, procalcitonin; CRP, C-reactive protein, TNF-α, tumor necrosis factor-α; IL-6, interleukin-6.
Effect of rhubarb and glycerin therapies on the levels of inflammatory markers
PCT, CRP, TNF-α and IL-6 levels in both groups showed a declining trend (Fig. 3C-F). From day 4 to 9, the PCT levels of the rhubarb group were significantly lower compared with the glycerin group (P<0.05; Fig. 3C). From day 3 to 9, the CRP levels of the rhubarb group were significantly lower compared with the glycerin group (P<0.05; Fig. 3D). Similarly, from day 3 to 9, the TNF-α and IL-6 levels of the rhubarb group were significantly lower compared with the glycerin group (day 3–4, P<0.05; day 5–9, P<0.01; Fig. 3E and F).
Effect of rhubarb and glycerin therapies on the duration of MV, first day of EN and ICU hospital stay
A total of 20 patients in the glycerin group and 23 patients in the rhubarb group received MV (20/56 vs. 23/56, χ2=0.340, P=0.698). The duration of MV and the ICU hospital stay in the rhubarb group were significantly lower compared with the glycerin group (both P<0.05; Table II). The first day of EN administration was significantly earlier in the rhubarb group compared with the glycerin group (P<0.05).
Table II.
Timing of patient treatment.
| Parameter | Glycerin group | Rhubarb group | P-value |
|---|---|---|---|
| Duration of mechanical ventilation (days) | 8.20±3.93 | 4.13±2.36a | 0.034 |
| First day of enteral nutrition (day) | 7.64±2.94 | 5.91±1.99a | 0.017 |
| Intensive care unit hospital stay (days) | 16.02±4.93 | 14.29±3.53a | 0.039 |
P<0.05 vs. glycerin group.
Effect of rhubarb and glycerin therapies on respiratory parameters in patients with MV
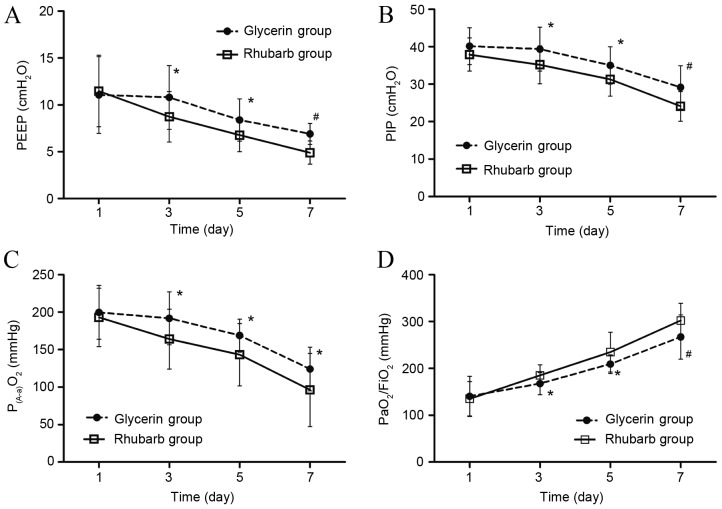
From day 3 to day 7, the level of PEEP and PIP in the two groups declined. The levels of PEEP and PIP in the rhubarb group were significantly lower compared with the glycerin group (P<0.05 on day 3 and 5; P<0.01 on day 7; Fig. 4A and B). The level of P(A-a)O2 declined from day 1 to 7, and was significantly lower in the rhubarb group compared with the glycerin group from day 3 to 7 (P<0.05; Fig. 4C). The oxygenation index increased from day 1 to 7, and was significantly higher in the rhubarb group compared with the glycerin group from day 3 to 7 (P<0.05 on day 3 and 5; P<0.01 on day 7; Fig. 4D).
Figure 4.
Effect of rhubarb or glycerin enema on respiratory parameters. (A) PEEP, (B) PIP, (C) P(A-a) O2 and (D) oxygenation index (PaO2/FiO2) after rhubarb or glycerin enema. *P<0.05 and #P<0.01 vs. glycerin group. PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure; P(A-a) O2, alveolar-arterial partial pressure of oxygen; PaO2, partial pressure of oxygen; FiO2, fraction of inspired oxygen.
30-day mortality
No significant difference in 30-day mortality was indicated between the groups (33.9 vs. 37.5%, χ2=0.156, P=0.693; Fig. 5).
Figure 5.
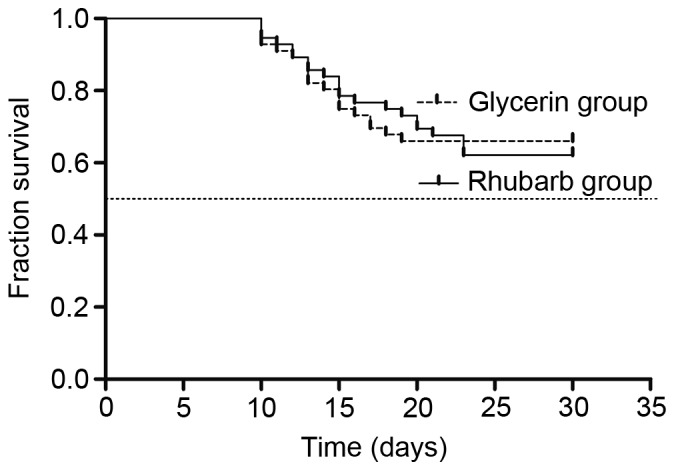
Effect of rhubarb and glycerin enema on 30-day mortality rate. The 30-day mortality rate was 33.9% in the glycerin group and 37.5% in the rhubarb group, with no significant difference in survival between the groups. χ2=0.156, P=0.693.
Adverse events
In the rhubarb group, there were 23 patients with abdominal pain (41.1%), 10 patients with nausea (17.9%) and five patients with vomiting (8.9%). One patient suffered bleeding from hemorrhoids, which stopped after cessation of enema. In the glycerin group, there were 30 patients with abdominal pain (53.6%), 13 patients with nausea (23.2%) and 3 patients with vomiting (5.4%). No specific treatment was administered to eliminate these adverse symptoms. No significant difference in adverse event rate was observed between the two groups (χ2=0.104, P=0.588).
Discussion
IAH developed with MODS may lead to ACS in critically ill patients (20). In addition to the abdominal cavity, multiple organs are affected directly and indirectly, resulting in decreased cardiac output, pulmonary edema, atelectasis, increased intracranial pressure, deterioration of consciousness, reduced renal blood flow and even kidney failure (17,21).
APACHE II, CRP and IAP levels are good predictors of disease severity in critical acute pancreatitis (22). Previous results indicated that APACHE II and SOFA scores were positively associated with the severity of IAP (20). The current study demonstrated that rhubarb inhibited inflammatory factors, improved APACHE II and SOFA scores, increased the intestinal tolerance to EN (allowing earlier administration) and decreased the duration of hospital stay in ICU. Rhubarb and its active ingredients not only reduce IAP, but also inhibit inflammatory factors and protect organ function, particularly gastrointestinal function. (12,23).
ALI is considered to be a risk factor for IAH (9). IAH has also been reported to dramatically increase the incidence of ALI/ARDS (24). In previous studies, rhubarb and dexamethasone significantly reduced the edema of lung tissue, decreased the red blood cell exudation, neutrophil infiltration and plasma protein exudation in the alveoli, and inhibited nitric oxide (NO) and the activities of inducible NO synthase in acute lung injury (21,25). Rhubarb has also been reported to protect lung fibroblast cells against oxidative damage by enhancing cellular antioxidant activity and modulating cellular signal transduction pathways (26) These studies could potentially explain the improvement of oxygenation index and P(A-a)O2 in the rhubarb group of the current study.
In conclusion, rhubarb enema had a protective effect against IAH and may be more effective compared with glycerin enema. However, the current study was limited by a relatively small sample size and a trial design that was not fully rigorous due to restrictions involving clinical research and ethics. The dose of rhubarb could not be further increased due to ethical restrictions. These factors may have led to bias in this research results. To address these issues, the current authors intend to develop an optimized design and conduct a multicenter observational study. Further clinical and basic studies are required to confirm these findings and elucidate the mechanism of action of rhubarb.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (grants nos. 81370172 and 81570078), the Jiangsu Province TCM Project (grant no. YB2015184) and Jiangsu Provincial Key Research and Development Program (grant no. BE2016721).
References
- 1.Sugrue M. Abdominal compartment syndrome. Curr Opin Crit Care. 2005;11:333–338. doi: 10.1097/01.ccx.0000170505.53657.48. [DOI] [PubMed] [Google Scholar]
- 2.Schachtrupp A, Lawong G, Afify M, Graf J, Toens C, Schumpelick V. Fluid resuscitation preserves cardiac output but cannot prevent organ damage in a porcine model during 24 h of intraabdominal hypertension. Shock. 2005;24:153–158. doi: 10.1097/01.shk.0000172094.73918.c2. [DOI] [PubMed] [Google Scholar]
- 3.Balogh ZJ, Lumsdaine W, Moore EE, Moore FA. Postinjury abdominal compartment syndrome: From recognition to prevention. Lancet. 2014;384:1466–1475. doi: 10.1016/S0140-6736(14)61689-5. [DOI] [PubMed] [Google Scholar]
- 4.Ganeshanantham G, Walsh SR, Varty K. Abdominal compartment syndrome in vascular surgery-A review. Int J Surg. 2010;8:181–185. doi: 10.1016/j.ijsu.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Malbrain ML, Chiumello D, Pelosi P, Wilmer A, Brienza N, Malcangi V, Bihari D, Innes R, Cohen J, Singer P, et al. Prevalence of intra-abdominal hypertension in critically ill patients: A multicentre epidemiological study. Intensive Care Med. 2004;30:822–829. doi: 10.1007/s00134-004-2169-9. [DOI] [PubMed] [Google Scholar]
- 6.Ke L, Tong ZH, Ni HB, Ding WW, Sun JK, Li WQ, Li N, Li JS. The effect of intra-abdominal hypertension incorporating severe acute pancreatitis in a porcine model. PLoS One. 2012;7:e33125. doi: 10.1371/journal.pone.0033125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ke L, Ni HB, Tong ZH, Li WQ, Li N, Li JS. The importance of timing of decompression in severe acute pancreatitis combined with abdominal compartment syndrome. J Trauma Acute Care Surg. 2013;74:1060–1066. doi: 10.1097/TA.0b013e318283d927. [DOI] [PubMed] [Google Scholar]
- 8.de Waele JJ, Hoste EA, Malbrain ML. Decompressive laparotomy for abdominal compartment syndrome-a critical analysis. Crit Care. 2006;10:R51. doi: 10.1186/cc4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malbrain ML, Chiumello D, Pelosi P, Bihari D, Innes R, Ranieri VM, Del Turco M, Wilmer A, Brienza N, Malcangi V, et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: A multiple-center epidemiological study. Crit Care Med. 2005;33:315–322. doi: 10.1097/01.CCM.0000153408.09806.1B. [DOI] [PubMed] [Google Scholar]
- 10.Kirkpatrick AW, Roberts DJ, De Waele J, Jaeschke R, Malbrain ML, De Keulenaer B, Duchesne J, Bjorck M, Leppaniemi A, Ejike JC, et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013;39:1190–1206. doi: 10.1007/s00134-013-2906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohmand H, Goldfarb S. Renal dysfunction associated with intra-abdominal hypertension and the abdominal compartment syndrome. J Am Soc Nephrol. 2011;22:615–621. doi: 10.1681/ASN.2010121222. [DOI] [PubMed] [Google Scholar]
- 12.Wan B, Fu H, Yin J, Xu F. Efficacy of rhubarb combined with early enteral nutrition for the treatment of severe acute pancreatitis: A randomized controlled trial. Scand J Gastroenterol. 2014;49:1375–1384. doi: 10.3109/00365521.2014.958523. [DOI] [PubMed] [Google Scholar]
- 13.Cui YL, Wang L, Tian ZT, Lin ZF, Chen DC. Effect of rhubarb pre-treatment on intestinal microcirculation in septic rats. Am J Chin Med. 2014;42:1215–1227. doi: 10.1142/S0192415X14500761. [DOI] [PubMed] [Google Scholar]
- 14.Chen DC, Wang L. Mechanisms of therapeutic effects of rhubarb on gut origin sepsis. Chin J Traumatol. 2009;12:365–369. [PubMed] [Google Scholar]
- 15.Blaser A Reintam, Malbrain ML, Starkopf J, Fruhwald S, Jakob SM, De Waele J, Braun JP, Poeze M, Spies C. Gastrointestinal function in intensive care patients: Terminology, definitions and management. Recommendations of the ESICM Working Group on Abdominal Problems. Intensive Care Med. 2012;38:384–394. doi: 10.1007/s00134-011-2459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malbrain ML, Cheatham ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006;32:1722–1732. doi: 10.1007/s00134-006-0349-5. [DOI] [PubMed] [Google Scholar]
- 17.Cheatham ML, Malbrain ML, Kirkpatrick A, Sugrue M, Parr M, De Waele J, Balogh Z, Leppäniemi A, Olvera C, Ivatury R, et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. II. Recommendations. Intensive Care Med. 2007;33:951–962. doi: 10.1007/s00134-007-0592-4. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Vincent JL, De Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on ‘sepsis-related problems’ of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26:1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 20.de Waele JJ, De Laet I, Kirkpatrick AW, Hoste E. Intra-abdominal Hypertension and Abdominal Compartment Syndrome. Am J Kidney Dis. 2011;57:159–169. doi: 10.1053/j.ajkd.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 21.Malbrain ML, Cheatham ML. Definitions and pathophysiological implications of intra-abdominal hypertension and abdominal compartment syndrome. Am Surg. 2011;77(Suppl 1):S6–S11. [PubMed] [Google Scholar]
- 22.Ke L, Tong ZH, Li WQ, Wu C, Li N, Windsor JA, Li JS, Petrov MS. Predictors of critical acute pancreatitis: A prospective cohort study. Medicine (Baltimore) 2014;93:e108. doi: 10.1097/MD.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li H, Yang T, Zhou H, Du J, Zhu B, Sun Z. Emodin Combined with Nanosilver Inhibited Sepsis by Anti-inflammatory Protection. Front Pharmacol. 2017;7:536. doi: 10.3389/fphar.2016.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Gui P, He X, Yang H. Actions of NO and INOS on endotoxin induced rat acute lung injury and effect of rhubarb on them. J Tradit Chin Med. 2000;20:216–222. [PubMed] [Google Scholar]
- 25.Li C, Zhou J, Gui P, He X. Protective effect of rhubarb on endotoxin-induced acute lung injury. J Tradit Chin Med. 2001;21:54–58. [PubMed] [Google Scholar]
- 26.Zhang R, Kang KA, Piao MJ, Lee KH, Jang HS, Park MJ, Kim BJ, Kim JS, Kim YS, Ryu SY, et al. Rhapontigenin from Rheum undulatum protects against oxidative-stress-induced cell damage through antioxidant activity. J Toxicol Environ Health A. 2007;70:1155–1166. doi: 10.1080/15287390701252766. [DOI] [PubMed] [Google Scholar]