Abstract
Osteosarcoma (OS) is the most commonly diagnosed tumor of the bones in children and young adults. Even with conventional therapies the 5-year survival rate is ~65% in patients with OS. Considering the side effects and aggressiveness of malignant bone tumors, research is focussing on multi-targeted strategies in treatment. Cucurbitacin B, a triterpenoid compound has been demonstrated to induce apoptosis in various cancer cell types. The Janus kinase 2/signal transducer and activator of transcription 3 (JAK2/STAT3) signalling cascades and mitogen activated protein kinases (MAPK) signalling cascades are critical regulators of tumorigenesis. The present study assessed the influence of cucurbitacin B on the viability and expression of MAPKs and proteins of the JAK2/STAT3 cascades in human OS cells (U-2 OS). Cucurbitacin B (20–100 µM) significantly reduced cell viability (P<0.05) and induced apoptosis, as assessed by MTT and Annexin V/propidium iodide staining, along with inhibiting cell migration. Gelatin zymography revealed supressed activities of matrix metalloproteinase (MMP-)2 and 9. Furthermore, cucurbitacin B effectively upregulated the apoptotic pathway and caused the effective inhibition of MAPK signalling and JAK2/STAT3 cascades. Multifold suppression of vascular endothelial growth factor by cucurbitacin B was also observed, indicating inhibition of angiogenesis. Thus, by downregulating major pathways-MAPK and JAK2/STAT3 and MMPs, cucurbitacin B has potent anti-proliferative and anti-metastatic effects that require further investigation with regards to cancer treatment.
Keywords: apoptosis, cucurbitacin, Janus kinase/signal transducer and activator of transcription pathway, mitogen activated protein kinases, osteosarcoma
Introduction
Osteosarcoma (OS) a primary sarcoma of the bones that primarily affects children and adolescents accounting for ~5% of pediatric tumors (1). OS affects the distal long bones, the femur and tibia (1) and is generally characterized by its local invasion of bone and soft tissues, loss of the affected extremity's functions and distant metastasis (2). Multimodal conventional therapies including radiation, surgical resection and chemotherapy are employed to treat OS (3,4). Despite the combination therapy approach, limited improvement has been observed in the 5-year survival rate (65%) of patients with OS (5). Furthermore, these therapeutic approaches often lead to severe side effects such as cardiotoxicity, hearing loss and nephrotoxicity, and may also cause drug resistance and increase the risk of local relapse (6). Thus, improved targeted approaches are required, with no or less side effects.
The Janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signalling pathway serves a critical role in cell survival and division. Activated JAK2/STAT3 signalling cascade influences the expression of numerous proteins that are associated with various physiological functions, including cell cycle regulation and apoptosis. The JAK/STAT3 pathway regulates the expression level of anti-apoptotic proteins [B-cell lymphoma-extra large (Bcl-xL) and myeloid leukemia cell differentiation protein (Mcl-1)] (7), cell cycle regulatory proteins (cyclin D1, p21, and p27) (8) and mitochondrial apoptosis pathway related proteins [Bcl-2 associated X (Bax), cytochrome c and caspase-3] (9). Furthermore, STAT3 activation promotes angiogenesis by inducing vascular endothelial growth factor (VEGF) (10), and also stimulates invasion and metastasis by increasing the expression of matrix metalloproteinases (MMPs) (11). The pathway is considered as a major molecular target of interest in a number of cancer types, including melanoma (12), renal carcinoma (13) and breast cancer (14).
The mitogen-activated protein kinases (MAPKs) signalling cascades are a large family of serine/threonine kinases that control and regulate various physiological process, including cell survival and apoptosis, and are also involved in tumorigenesis. The functions of MAPK signalling in cancer development are complex, as they regulate wide range of cellular responses (15). Activated MAPK pathway stimulates cell growth or induces apoptosis based on the stimuli (16,17). Numerous studies have reported that the c-Jun N-terminal kinases (JNK), p38 and extracellular signal-regulated kinases (ERK1/2) cascades exert a vital role in regulating cytotoxic drug induced apoptosis in OS (18,19). Thus, targeting the pathway may have clinical value in the treatment of OS.
Accumulating research data indicate that plant-derived compounds are much more effective in inhibiting cancer cell proliferation and inducing apoptosis (20). Phytochemicals are reported to elicit antitumor effects by inducing cellular defense system, antioxidant enzymes system and also inhibition of anti-cell growth signalling and anti-inflammatory pathways culminating in apoptosis and/or cell cycle arrest (21–24).
Cucurbitacins were initially identified in the Cucurbitaceae plant family, which includes cucumber and are also isolated from various plant families (25). Owing to their effective pharmacological properties, plants rich in cucurbitacins have been widely used in traditional Chinese medicine for their analgesic, anti-inflammatory, antimicrobial, antipyretic, antitumor activities and hepatoprotective effects (25–28). Researchers have reported that cucurbitacin I may inhibit cancer cell growth by disrupting the JAK/STAT3 signaling pathway in both in vitro and in vivo tumor models (29,30). Studies have reported that cucurbitacin B inhibits the growth of various human cancer cell lines and tumor xenografts including breast, prostate, lung, uterine cervix, liver, skin and brain cancer (18,31,32). Considering the biological effects cucurbitacin B, the present study aimed investigate the effects of cucurbitacin B on human OS cells and assess whether it modulates the JAK/STAT3 and MAPK signalling pathways.
Materials and methods
Reagents and chemicals
Cucurbitacin B, glutamine, RPMI 1640 medium, fetal calf serum (FCS), penicillin, streptomycin and 0.25% trypsin were purchased from Sigma Aldrich; Merck KGaA (Darmstadt, Germany). Antibodies against ERK1/2 (cat. no. 9102), phospho-ERK1/2 (cat. no. 9101), p38 (rabbit mAb, cat. no. 8690), phospho-p38 (rabbit mAb, cat. no. 4511), JNK (cat. no. 9252), phospho-JNK (mouse mAb, cat. no. 9255), MMP-2 (rabbit mAb, cat. no. 87809), MMP-9 (cat. no. 852) (all from Cell Signaling Technology, Danvers, MA, USA), Bcl-xL (cat. no. sc-8392), Bcl-2-associated death promoter (Bad) (cat. no. sc-8044), Bax (cat. no. sc-4239), Bcl-2 (cat. no. sc-509), VEGF (cat. no. sc-4571), caspase-9 (cat. no. sc-81663), caspase-8 (cat. no. sc-81656), caspase-3 (cat. no. sc-176260), p-JAK2 (cat. no. sc-34479), JAK2 (cat. no. sc-34479), p-STAT3 (cat. no. sc-56747) and STAT3 (cat. no. sc-482) (all from Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were used for expression analysis. All other reagents used in the study were purchased from Sigma Aldrich; Merck KGaA, unless otherwise stated.
Cell lines and culture
Human osteosarcoma cell line, U-2 OS was obtained from the Cell Bank of Shanghai Institute of Biochemistry and Cell Biology (Chinese Academy of Sciences, Shanghai, China). The cells were cultured and maintained in RPMI-1640 medium supplemented with 10% FCS, 2 mM glutamine, streptomycin (100 µg/ml) and penicillin (100 U/ml) at 37°C in a humidified atmosphere with 5% CO2.
Measurement of cell viability
OS cells were seeded into 96-well plates (5×105 cells/well) and incubated at 37°C for 24 h. On reaching 70% confluence, the cells were treated with different concentrations of cucurbitacin B (20, 40, 80 and 100 µM) for 24 h. Cells were then incubated at 37°C with MTT for 4 h. The formazan crystals formed were dissolved with dimethyl sulfoxide and the absorbance was measured at 570 nm using a Thermo Multiskan Spectrum (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Analysis of apoptosis by Annexin V assay
OS cells were incubated at 37°C with various concentrations of cucurbitacin B (20, 40, 80 and 100 µM) for 24 h. Following incubation, cells were treated with 0.25% trypsin, washed twice with ice-cold PBS and harvested for detection of apoptosis using an Annexin V-FITC apoptosis detection kit (BD Biosciences, Franklin Lakes, NJ, USA), according to the manufacturer's instructions. The cells were washed in PBS at 1×106 cells/ml and suspended at 1×105/100 µl binding buffer (BD Biosciences) containing FITC-conjugated anti-Annexin V antibody (provided in the kit) and PI, and analyzed for fluorescence using a flow cytometer (FACSCalibur; BD Biosciences).
Determination of morphological changes by Hoechst staining
Morphological changes of the U-2 OS cells following treatment with cucurbitacin B was determined by Hoechst staining. Briefly, the OS cells were seeded at a density of 5×104 cells per well in a 6-well plate and cultured at 37°C for 12 h and treated with cucurbitacin B (20, 40, 80 and 100 µM) for 48 h. The cells were then fixed with 4% paraformaldehyde in PBS for 10 min, washed with PBS and stained with Hoechst 33258 (5 mg/l) for 15 min at room temperature in the dark. The cells were observed under a a fluorescence microscope (Nikon Eclipse TiS coupled with NIS-Elements imaging software; Nikon Corporation, Tokyo, Japan). The cells undergoing apoptosis were characterized by condensed or fragmented nuclei.
Wound healing assay
The influence of cucurbitacin B on the cell migration of U-2 OS cells was assessed. In brief, 5×105 cells were maintained in 10 cm Petri plates and incubated for 24–72 h until they reached complete confluency and were wounded using a 200 µl pipette tip. All cells in the plates were treated with final concentrations of cucurbitacin B (25, 50 and 100 µM; 3 plates for each concentration) and then were incubated at 37°C in fresh culture medium for 24 h. The migration of the cells in the wounded area was measured as previously described (33). Cell migration was determined as a percentage of the cell-free area compared with the area of the initial wounded area. The fields were photographed using an inverted microscope (Nikon Eclipse TS2; Nikon Corporation).
Gel zymography
Gel zymography was performed to evaluate the activities of MMP-2 and MMP-9. Sample preparation was done as previously described by Mizoguchi et al (34). Samples were run on SDS-PAGE (10%) gels that contained 0.1% gelatin under non-reducing conditions. Triton X-100 (2.5%) was used to wash the gels to remove SDS, then the gels were washed further in incubation buffer (50 mM Tris HCl, 0.15 M NaCl, 10 mM CaCl2) (25±2°C) for 30 min and incubated (24 h, 37°C). Following incubation, the gels were stained with Coomassie Brilliant Blue (1% Coomassie Brilliant Blue G-250, 30% methanol and 10% acetic acid) for 3 h and then were destained (7% acetic acid, 40% methanol) until clear bands representing gelatinolysis were seen against dark background. By using an Atto Densitograph Software Library Lane Analyser (Atto Instruments, Inc., Rockville, MD, USA) the total activity was determined.
Western blot analysis
Following treatment with cucurbitacin B (25, 50 and 100 µM), U-2 OS cells were washed twice with ice-cold PBS and centrifuged at 10,000 × g for 5 min at 4°C. The cells were treated in ice-cold hypotonic lysis buffer (10 mM HEPES pH 7.9, 1.5 mM MgCl2, 0.2 mM KCl, 0.5 mM dithiothreitol, 0.2 mM phenylmethyl-sulphonylfluoride), vortexed for 2 min, centrifuged at 12,000 × g for 10 min at 4°C and the supernatants were collected and assayed for protein content using the Bradford method (35). Equal amounts of protein samples (60 µg) were subjected to electrophoresis in 12% SDS-PAGE and the bands were then transferred to nitrocellulose membranes (GE Healthcare Lifesciences, Chalfont, UK). The membranes were washed and blocked using blocking buffer (5% non-fat dry milk/0.1% Tween-20 in TBS) for 1 h at room temperature. They were then incubated overnight at 4°C with respective primary antibodies followed by incubation at room temperature for 40 min with horseradish peroxidase-conjugated-secondary antibody (mouse mAb; Cell Signaling Technology, cat. no. 7076; dilution 1:1,000). The immunoreactive bands were detected by enhanced chemiluminescence procedure (GE Healthcare Lifesciences). Protein expression levels were normalized with the expression levels of β-actin using anti-β-actin antibody (AC-15) from Santa Cruz Biotechnology, Inc. (cat. no. sc-69879; dilution 1:1,000). The positive signals from the immunoblots (n=3) were quantified using Image Lab Software (v5.1) (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
The data are presented as mean ± standard deviation, from three or six individual experiments. The values were analyzed by one-way analysis of variance followed by Duncan's Multiple Range Test. All statistical analyses were performed using the SPSS software (version 22.0; IBM SPSS, Armonk, NY, USA). P<0.05 was considered to indicate a statistically significant difference.
Results
Cucurbitacin B inhibits U-2 OS cell proliferation
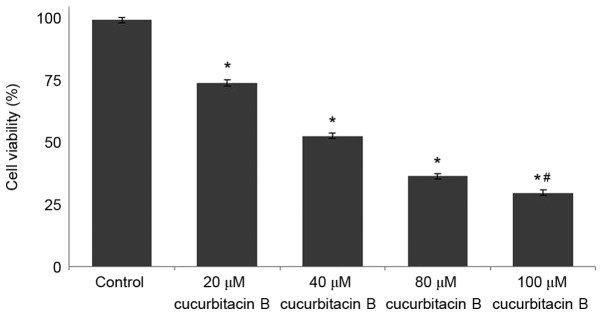
The viability of osteosarcoma cells following treatment with cucurbitacin B was determined by MTT assay, and the findings are presented in Fig. 1. Osteosarcoma cells treated with different concentrations of cucurbitacin B (20, 40, 80 and 100 µM) exhibited reduced cell proliferation in a concentration-dependent manner. The viability percentage of U-2 OS cells treated with cucurbitacin B at 20 µM was 83%, whereas following the 100 µM dose, the cell viability significantly decreased to 21% (P<0.05; Fig. 1).
Figure 1.
Cucurbitacin B reduces the cell viability of U-2 OS cells. *P<0.05 vs. control; #P<0.05 vs. 20 µM. Data are presented as mean ± standard deviation, n=3. OS, osteosarcoma.
Influence of cucurbitacin B on cell viability counts and morphological changes in U-2 OS cells
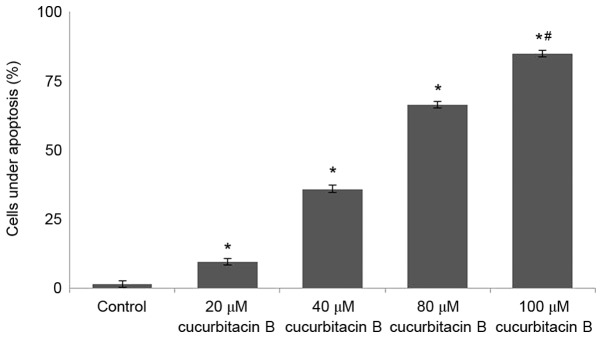
The results of MTT assay revealed that cucurbitacin B may effectively reduce the viability of U-2 OS cells. The present study further analyzed whether cucurbitacin B reduced viability by inducing apoptosis. Annexin V/PI staining assay was performed to determine apoptotic cell counts. Exposure to cucurbitacin B was identified to significantly increase the apoptotic cell counts (P<0.05; Fig. 2), in a dose-dependent manner with 100 µM concentration exhibiting maximum effects.
Figure 2.
Cucurbitacin B induces apoptosis of human osteosarcoma U-2 OS cells. Cucurbitacin B was observed to significantly increase the apoptotic cell count and reduce cell viability in U-2 OS cells. *P<0.05 vs. control; #P<0.05 vs. 20 µM. Data are presented as mean ± standard deviation, n=3. OS, osteosarcoma.
Furthermore, Hoechst 33258 staining was performed to assess morphological changes in the U-2 OS cells following cucurbitacin B treatment. Following treatment for 48 h, the chromatin of U-2 OS cells was condensed and appeared to be brighter and deeper. Notably, treatment with cucurbitacin at higher concentrations of 80 and 100 µM demonstrated deeper staining of nuclear chromatin than the lower doses (20 and 40 µM). Highly condensed and stained chromatin indicates characteristics of apoptotic cells, thus suggesting that cucurbitacin B effectively induced apoptosis in U-2 OS cells. The percentage of apoptotic cells increased significantly following cucurbitacin B treatment (20, 40, 80 or 100 µM) compared with the control (P<0.05; Fig. 3).
Figure 3.
Influence of Cucurbitacin B on the viability of U-2 OS cells under Hoechst staining. Hoechst staining using a fluorescence microscope (Nikon Eclipse TiS coupled with NIS-Elements imaging software) revealed a significantly increased number of cells under apoptosis following cucurbitacin B treatment. *P<0.05 vs. control; #P<0.05 vs. 20 µM. Data are presented as mean ± standard deviation, n=3. OS, osteosarcoma.
Cucurbitacin B inhibits migration of U-2 OS cells
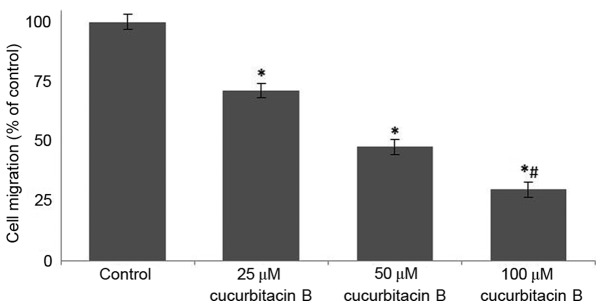
Following incubation with cucurbitacin B (25, 50 and 100 µM) for 24 h, the area of the wound was measured. In the cells that were not treated with cucurbitacin B, the distance between the scratches was significantly reduced when compared with U-2 OS cells that were exposed to cucurbitacin, in a dose-dependent manner (P<0.05). In the OS cells that were incubated with cucurbitacin B the cell free area was evidently increased, the distance increased with concentration of cucurbitacin B (Fig. 4). The growth and movement of the cells in the cell free scratch area observed in cells that were not treated with cucurbitacin suggests cell division and migration were occuring, which were supressed following cucurbitacin B treatment.
Figure 4.
Influence of cucurbitacin B on cell migration determined by a wound healing assay. Cucurbitacin B exposure significantly reduced cell migration of the U-2 OS cells over the wound area in a dose-dependent manner. *P<0.05 vs. control; #P<0.05 vs. 25 µM. Data are presented as mean ± standard deviation, n=6. OS, osteosarcoma.
Cucurbitacin B downregulates MMPs
It has been established that MMPs principally contribute to the invasion and metastasis of tumor cells (36–38). In the present study, to further assess whether cucurbitacin inhibits MMPs, the expression of MMP-2 and −9 were determined by gel zymography and western blot analysis. The result of the current study indicated that cucurbitacin B exposure at all assessed concentrations caused marked inhibitions in the expressions of MMPs (Fig. 5A). The zymography analysis revealed significantly supressed activities of MMP-2 and −9 in a dose-dependent manner (Fig. 5B). Furthermore, western blot analysis revealed a markedly decreased expression level of MMP2 and MMP9 in cucurbitacin B-treated cells. However, relatively little inhibition was observed following treatment with 25 µM cucurbitacin when compared with higher doses, 50 and 100 µM. Furthermore, marked suppression of VEGF levels was also observed following cucurbitacin B exposure in a dose-dependent manner (Fig. 5C). These observations suggest that cucurbitacin B was able to markedly supress cell migration and inhibit angiogenesis, potentially by downregulating the expression of MMP2, MMP9 and VEGF.
Figure 5.
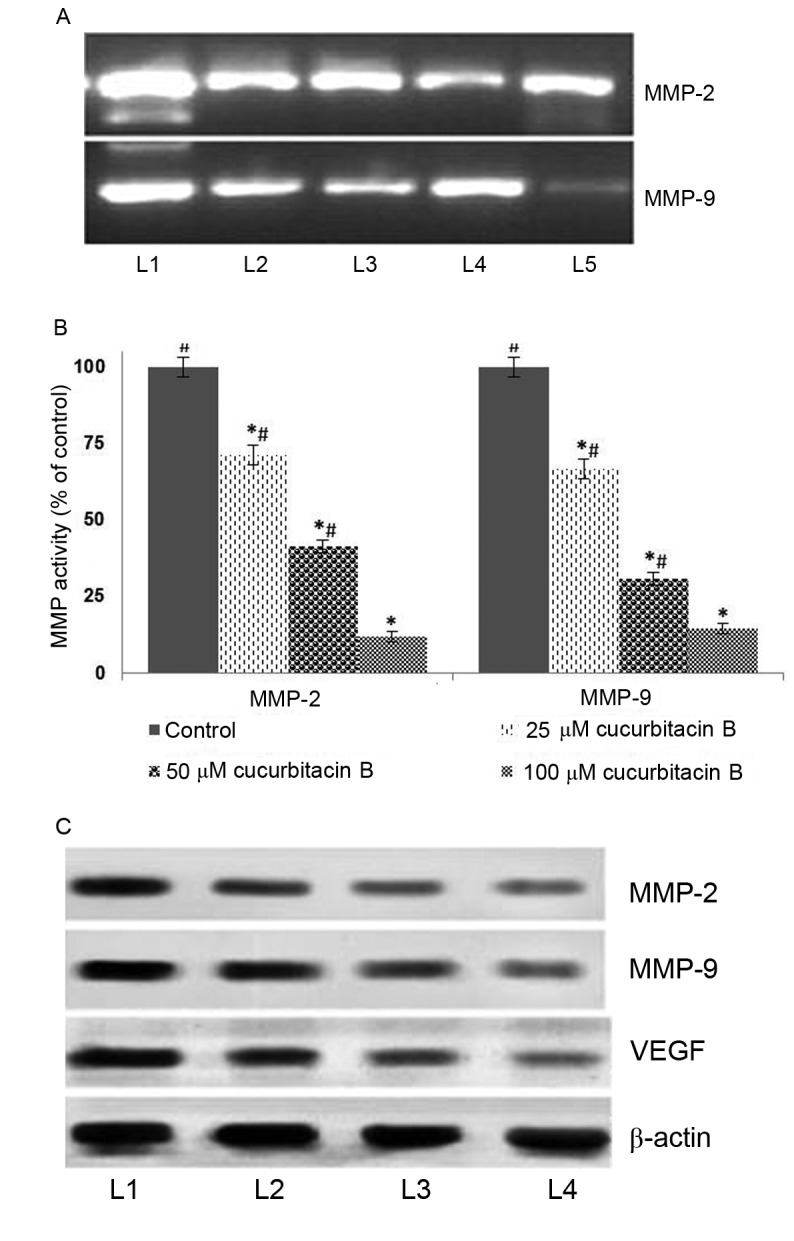
Cucurbitacin B reduces activities of MMP-2, MMP-9 and VEGF in U-2 OS cells. This was determined by (A) gelatin zymography following exposure to cucurbitacin B, indicating marked downregulation in the expressions of MMP-2 and −9, (B) quantified results from the gelatin zymography indicating a significant downregulation of MMP activity. (C) Western blot analysis indicates a marked reduction in the expression of MMP-2, MMP-9 and VEGF. *P<0.05 vs. control; #P<0.05 vs. 25 µM. Data are presented as mean ± standard deviation, n=3. MMP, matrix metalloproteinases; VEGF, vascular endothelial growth factor; OS, osteosarcoma; L1, control; L2, 20 µM cucurbitacin B; L3, 40 µM cucurbitacin B; L4, 80 µM cucurbitacin B; L5, 100 µM cucurbitacin B.
Influence of cucurbitacin B on the expressions of apoptotic pathway proteins
Apoptosis is a coordinated network of genes that lead to cell death and is the target pathway in many anticancer therapies. Anticancer drugs are known to induce apoptosis by targeting cells that harbour genetic damage or that divide inappropriately (39). The present study assessed the influence of cucurbitacin B on the expression of apoptotic pathway proteins in U-2 OS cells to investigate the molecular events associated with cucurbitacin-induced cell death. A significant increase in the expression of caspase-3, −8 and −9 in the U-2 OS cells exposed to cucurbitacin B was observed following western blot analysis (P<0.05; Fig. 6). The activation of caspase-9 and −8 have previously been documented, suggesting the involvement of intrinsic and extrinsic apoptotic pathways that subsequently lead to the activation of caspase-3 (40). Therefore, in the present study the enhanced expression of caspases by cucurbitacin B indicates an increased level of apoptosis in U-2 OS cells.
Figure 6.
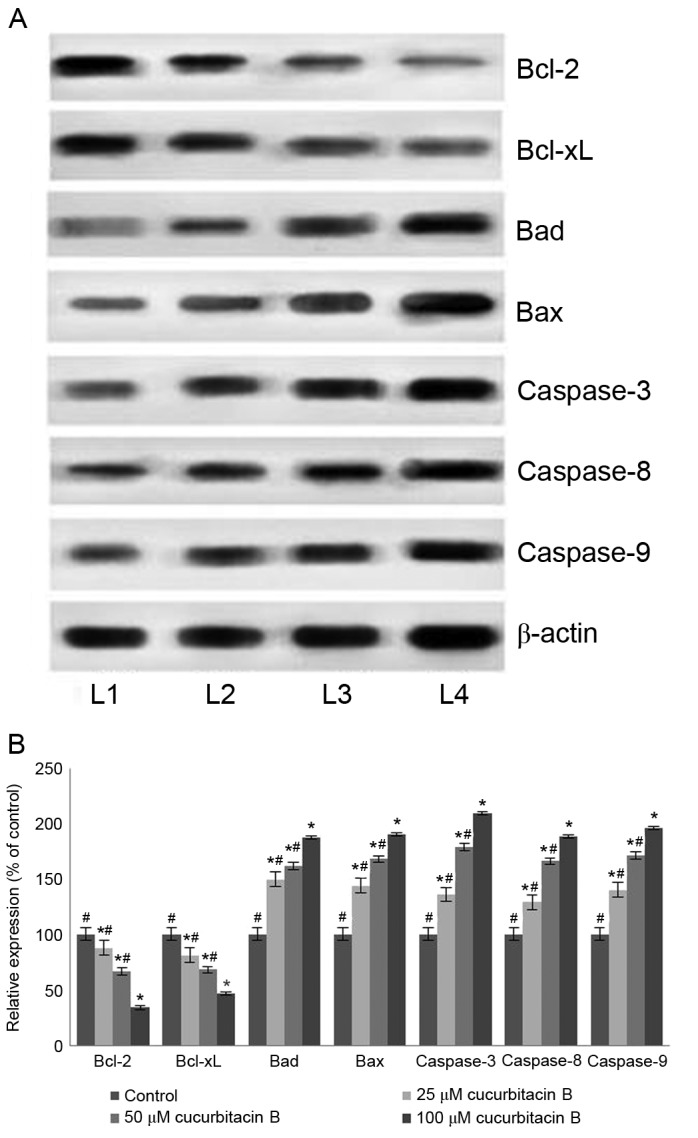
Cucurbitacin B modulates the expression of apoptosis pathway proteins. Cucurbitacin B caused effective upregulation of caspase-3, −8 and −9 in addition to an increase in the expression of Bax and Bad, while suppressing the expression of Bcl-2 and Bcl-xL, demonstrated in (A) western blot analysis and (B) quantification of the results. *P<0.05 vs. control; #P<0.05 vs. 100 µM. Data are presented as mean ± standard deviation, n=3. Bcl-2, B-cell lymphoma 2; Bcl-xL, Bcl-2 extra large; Bad, Bcl-2-associated death promoter; Bax, Bcl-2 associated X; L1, control; L2, 25 µM cucurbitacin B; L3, 50 µM cucurbitacin B; L4, 100 µM cucurbitacin B.
Furthermore, the expression of Bcl-2 family members was also assessed under the influence of cucurbitacin B. The Bcl-2 family are key proteins in the regulation of intrinsic mitochondrial pathways of apoptosis, specifically controlling the release of cytochrome c, which further regulates the caspases cascade (41,42). The Bcl-2 family comprises anti-apoptotic (Bcl-2 and Bcl-xL) and pro-apoptotic proteins [Bax, Bcl-2 antagonist killer (Bak) and Bad] (43). Cucurbitacin B exposure resulted in a multi-fold increase in the Bad and Bax protein expression (P<0.05; Fig. 6B) with significant downregulation of Bcl-2 and Bcl-xL expression (P<0.05; Fig. 6B). The upregulated expression of Bax and Bad indicates the activation of the apoptotic pathway. Therefore, with enhanced levels of pro-apoptotic proteins, cucurbitacin B aids in elevated levels of caspases, which eventually induces apoptosis.
Cucurbitacin modulates the MAPK signalling cascades
It has been well documented that activation of MAPK pathways serves a critical role in inhibiting apoptosis. In the present study, in order to elucidate the mechanisms underlying cucurbitacin B-induced apoptosis, the expression levels of MAPK cascade proteins, JNK, p-JNK, p38, p-p38, ERK1/2, and p-ERK1/2 were analyzed by western blot analysis. The results demonstrated an enhanced expression of the proteins in the U-2 OS cells that were not exposed to cucurbitacin (Fig. 7). However, U-2 OS cells treated with cucurbitacin B exhibited marked downregulation in the phosphorylated levels of ERK1/2, p38 and JNK. Whereas substantial decreases were observed in the expression levels of p38 and ERK1/2, JNK and p-JNK levels were decreased to a lesser extent. However, marked inhibitions were observed following cucurbitacin B treatment at all the three assessed concentrations, with 100 µM exerting maximum effects. The results indicated that the downregulation of the MAPK signalling pathway may have aided in induction of apoptosis of the U-2 OS cells.
Figure 7.
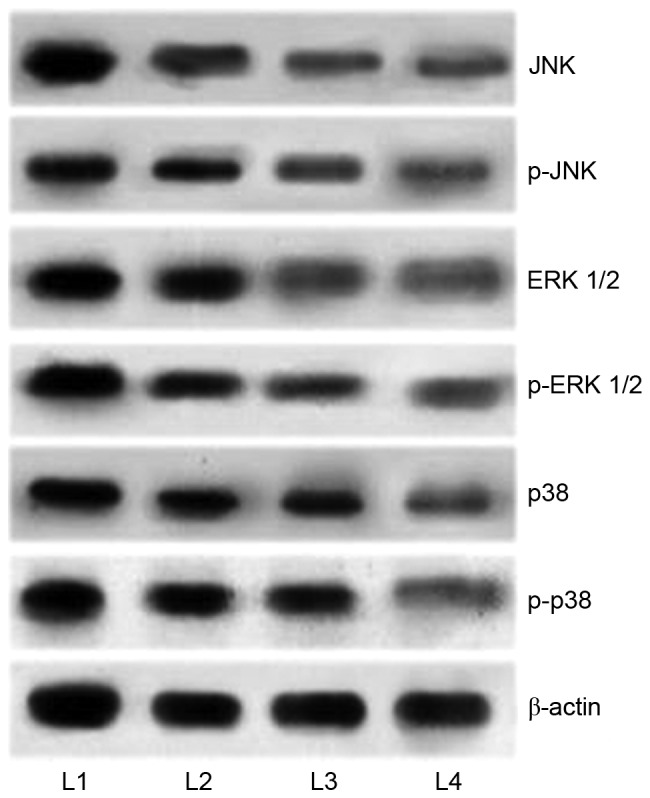
Cucurbitacin B downregulates the expression of MAPK signalling cascades. Cucurbitacin B exposure markedly inhibited the activation of JNK, ERK1/2 and p38, thus inhibiting the signaling pathway, demonstrated by western blot analysis. MAPK, mitogen activated protein kinases; JNK, c-Jun N-terminal kinases; ERK, extracellular signal-regulated kinases; p-JNK, phosphorylated-JNK; p-ERK, phosphorylated ERK; L1, control; L2, 25 µM cucurbitacin B; L3, 50 µM cucurbitacin B; L4, 100 µM cucurbitacin B.
Effects of cucurbitacin B on the JAK2/STAT3 signalling pathway
The JAK/STAT3 pathway serves a pivotal role in the transduction of a multitude of signals that are critically involved in the development and homeostasis in mammals (44). STAT3 has been reported to be constitutively activated in different types of cancer in humans (45). It has been demonstrated that the inhibition of STAT3 leads to apoptosis and inhibition of cancer cell proliferation (46). In the present study, western blot analysis was performed to assess the expression level of phosphorylated forms of JAK2 and STAT3 following exposure to cucurbitacin B. The results of the current study indicate that cucurbitacin B treatment significantly reduced the levels of activated JAK2 and STAT3 (P<0.05; Fig. 8). Cucurbitacin B at 100 µM concentration caused a multi-fold decrease in the expression of STAT3 and JAK2. Furthermore, the inhibition of STAT3 and JAK2 expression was dose-dependent. Therefore, these observations indicate that cucurbitacin significantly inhibited the STAT3/JAK2 pathway and this may have a part in the increase rate of apoptosis observed.
Figure 8.
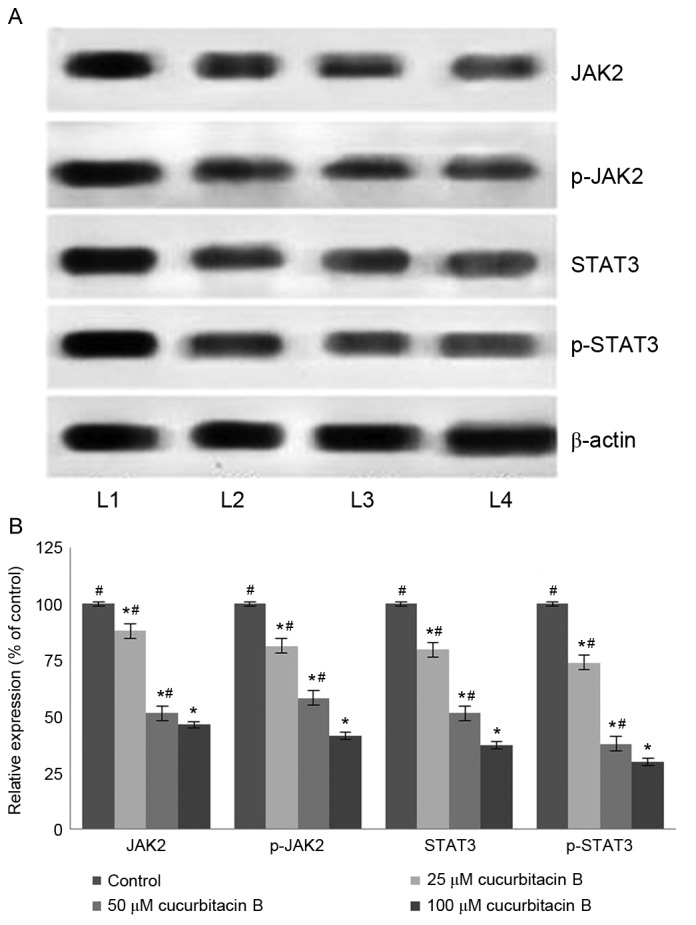
Cucurbitacin B downregulates the expression of JAK2/STAT3 signalling cascades. (A) Western blot analysis indicates that cucurbitacin B induced marked downregulation of JAK2 and STAT3 expression, thereby inhibiting the signaling cascade and (B) quantified results indicate a significant reduction in expression of JAK2/STAT3 signaling cascades. *P<0.05 vs. control; #P<0.05 vs. 100 µM. Data are presented as mean ± standard deviation, n=3. JAK2, Janus kinase 2; STAT3, signal transducer and activator of transcription 3; L1, control; L2, 25 µM cucurbitacin B; L3, 50 µM cucurbitacin B; L4, 100 µM cucurbitacin B.
Discussion
Cucurbitacins are widely used in traditional Chinese medicines and are the bitter principles of Cucurbitaceae (25,47). Cucurbitacin B, which is one of the most abundant cucurbitacins (27) exhibits anti-inflammatory activity and is used traditionally to treat hepatitis (28,48). Previous studies have demonstrated the antitumor activities of cucurbitacin B in various human cancer cell lines and tumor xenografts (49,50). However, the mechanisms underlying the anticancer activities are yet to be elucidated. Studies are currently focussing on the identification and development of small molecules that inhibit major cell signalling pathways, as an important strategy in anticancer drug research (51–53). The present study investigated the effects of cucurbitacin B on cell proliferation and the MAPK and JAK2/STAT3 pathways in U-2 OS cells.
Inhibition of cell proliferation is an important indicator of anticancer activity. Treatment with cucurbitacin B (20–100 µM) exhibited evident growth inhibition. Furthermore, cucurbitacin B markedly induced the apoptosis of U-2 OS, as observed by Hoechst staining and flow cytometry analysis followed by Annexin V/PI staining. The events underlying the induction of apoptosis were also analysed. Apoptosis occurs via the intrinsic mitochondrial pathway and the extrinsic membrane death receptor pathways. Cucurbitacin B exposure caused marked activation and enhanced expression of caspase-9, −8 and −3. These observations suggest that cucurbitacin B induces apoptosis via the intrinsic pathway, which is in accordance with a previous study using chemotherapeutic drugs as gallic acid (54). Furthermore, Liu et al (55) reported that the majority of chemotherapeutic drugs were able to induce apoptosis through the intrinsic mitochondrial pathway.
The mitochondria-dependent apoptotic pathway is governed by Bcl-2-family proteins that includes pro-apoptotic (BH3-interacting domain death agonist, Bax, and Bak) and anti-apoptotic (Bcl-2 and Bcl-xL) proteins (56,57). Bax promotes apoptotic factors and induces apoptosis, whereas Bcl-2 inhibits the release of pro-apoptotic proteins (58). Cucurbitacin B notably enhanced the expression of Bad and Bax and supressed the levels of Bcl-xL and Bcl-2 indicating activation of the intrinsic apoptotic cascade.
Cancer metastasis presents a huge challenge in cancer therapy and thus, blocking cancer cell metastasis is an important approach. MMPs serve crucial roles in metastasis, angiogenesis and also cause the release of growth factors from the extra cellular matrix (59). MMP-2 and −9 are well documented to be associated with the invasive metastatic potential of tumor cells (60). In U-2 OS cells a markedly enhanced expression of MMP-2 and −9 was observed. Cucurbitacin B supressed the levels of MMP-2 and −9 at all the tested doses and inhibited the activities of the enzymes, thus exhibiting anti-metastatic activity. The observations of the cell migration assay also reveal the potent effects of cucurbitacin B on the inhibition of cell migration and therefore, metastasis.
The JAK/STAT3 pathway is one of the major pathways that regulate and control various vital physiological processes. JAK activation exerts critical roles in cell proliferation, differentiation, migration and apoptosis (44,61). Activated JAKs phosphorylate cellular substrates, including the STAT family, which are vitally associated with oncogenic signalling pathways (62). Constitutive activation of STAT3 is typically observed in cancer cells and has been demonstrated to serve a critical role in tumor cell growth and survival in human solid tumors (62,63). STAT3 also upregulates anti-apoptotic proteins as Mcl-1 and Bcl-xL (63,64). Therefore, blocking the pathway may aid in the inhbition of cancer cell growth and promotion of apoptosis. Treatment with cucurbitacin B was observed to markedly downregulate the level of phosphorylated STAT3 and JAK2 in a dose-dependent manner. The reduced level of Bcl-2 and Bcl-xL were also in line with STAT3 levels. Treatment with cucurbitacin B exhibited a marked reduction in the level of phosphorylated STAT3 and its downstream targets, such as cyclin B1 and Bcl-2 in the human laryngeal cell line Hep-2 (65). Furthermore, downregulated expression of VEGF was observed, which may be due to potent inhibition of STAT3. In the present study cucurbitacin B treatment downregulated JAK2/STAT3 signalling and also significantly upregulated mitochondrial apoptotic pathway-related proteins (Bax, Bad and cleaved caspases).
It has also been demonstrated that members of the MAPK family are important regulators of stress responses, in addition to being associated with cell survival and modulating the induction of apoptosis (66,67). Cucurbitacin B was identified to effectively inhibit the activation of JNK, ERK1/2 and p38 in U-2 OS cells. Studies have reported that ERK1/2, the key molecule of the MAPK signalling pathway (68), is associated with promotion of tumor invasion and metastasis (69). Therefore, by inhibiting the activation of ERK1/2, cucurbitacin B effectively contributes to the inhibition of metastasis in line with MMP suppression.
In conclusion, cucurbitacin B significantly downregulates MAPK signalling and JAK2/STAT3 cascades and induces apoptosis in U-2 OS cells. The downregulation of MMPs and VEGF aid in the inhibition of invasion, metastasis and angiogenesis. Therefore, these findings indicate that cucurbitacin B is a potential potent candidate for OS therapy in the future. Further experiments under in vivo conditions will be required to confirm the effects of cucurbitacin B. Furthermore, the mechanisms associated with the protective effects of cucurbitacin B should be explored in more detail.
References
- 1.Thayanithy V, Park C, Sarver AL, Kartha RV, Korpela DM, Graef AJ, Steer CJ, Modiano JF, Subramanian S. Combinatorial treatment of DNA and chromatin-modifying drugs cause cell death in human and canine osteosarcoma cell lines. PLoS One. 2012;7:e43720. doi: 10.1371/journal.pone.0043720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guijarro MV, Ghivizzani SC, Gibbs CP. Animal models in osteosarcoma. Front Oncol. 2014;4:189. doi: 10.3389/fonc.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Federman N, Bernthal N, Eilber FC, Tap WD. The multidisciplinary management of osteosarcoma. Curr Treat Options Oncol. 2009;10:82–93. doi: 10.1007/s11864-009-0087-3. [DOI] [PubMed] [Google Scholar]
- 4.Jaffe N. Osteosarcoma: Review of the past, impact on the future. The American experience. Cancer Treat Res. 2009;152:239–262. doi: 10.1007/978-1-4419-0284-9_12. [DOI] [PubMed] [Google Scholar]
- 5.Han XR, Sun Y, Bai XZ. The anti-tumor role and mechanism of integrated and truncated PDCD5 proteins in osteosarcoma cells. Cell Signal. 2012;24:1713–1721. doi: 10.1016/j.cellsig.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment-where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–532. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran P, Li F, Manu KA, Shanmugam MK, Loo SY, Kumar AP, Sethi G. γ-tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: Potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br J Pharmacol. 2011;163:283–298. doi: 10.1111/j.1476-5381.2010.01187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang JS, Chuang LY, Guh JY, Huang YJ, Hsu MS. Antioxidants attenuate high glucose-induced hypertrophic growth in renal tubular epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1072–F1082. doi: 10.1152/ajprenal.00020.2007. [DOI] [PubMed] [Google Scholar]
- 9.Du W, Hong J, Wang YC, Zhang YJ, Wang P, Su WY, Lin YW, Lu R, Zou WP, Xiong H, et al. Inhibition of JAK2/STAT3 signalling induces colorectal cancer cell apoptosis via mitochondrial pathway. J Cell Mol Med. 2012;16:1878–1888. doi: 10.1111/j.1582-4934.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J, Zhang S, Wang T, Sinibaldi D, Coppola D, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 11.Xie TX, Huang FJ, Aldape KD, Kang SH, Liu M, Gershenwald JE, Xie K, Sawaya R, Huang S. Activation of stat3 in human melanoma promotes brain metastasis. Cancer Res. 2006;66:3188–3196. doi: 10.1158/0008-5472.CAN-05-2674. [DOI] [PubMed] [Google Scholar]
- 12.Nam S, Xie J, Perkins A, Ma Y, Yang F, Wu J, Wang Y, Xu RZ, Huang W, Horne DA, et al. Novel synthetic derivatives of the natural product berbamine inhibit Jak2/Stat3 signaling and induce apoptosis of human melanoma cells. Mol Oncol. 2012;6:484–493. doi: 10.1016/j.molonc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Um HJ, Min KJ, Kim DE, Kwon TK. Withaferin A inhibits JAK/STAT3 signaling and induces apoptosis of human renal carcinoma Caki cells. Biochem Biophys Res Commun. 2012;427:24–29. doi: 10.1016/j.bbrc.2012.08.133. [DOI] [PubMed] [Google Scholar]
- 14.Park JH, Darvin P, Lim EJ, Joung YH, Hong DY, Park EU, Park SH, Choi SK, Moon ES, Cho BW, et al. Hwanggeumchal sorghum induces cell cycle arrest, and suppresses tumor growth and metastasis through Jak2/STAT pathways in breast cancer xenografts. PLoS One. 2012;7:e40531. doi: 10.1371/journal.pone.0040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 16.Park WH. MAPK inhibitors differentially affect gallic acid-induced human pulmonary fibroblast cell growth inhibition. Mol Med Rep. 2011;4:193–204. doi: 10.3892/mmr.2010.361. [DOI] [PubMed] [Google Scholar]
- 17.You BR, Park WH. The effects of mitogen-activated protein kinase inhibitors or small interfering RNAs on gallic acid induced HeLa cell death in relation to reactive oxygen species and glutathione. J Agric Food Chem. 2011;59:763–771. doi: 10.1021/jf103379d. [DOI] [PubMed] [Google Scholar]
- 18.Chen YC, Chang CN, Hsu HC, Chiou SJ, Lee LT, Hseu TH. Sennoside B inhibits PDGF receptor signaling and cell proliferation induced by PDGF-BB in human osteosarcoma cells. Life Sci. 2009;84:915–922. doi: 10.1016/j.lfs.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Noh K, Kim KO, Patel NR, Staples JR, Minematsu H, Nair K, Lee FY. Targeting inflammatory kinase as an adjuvant treatment for osteosarcomas. J Bone Joint Surg Am. 2011;93:723–732. doi: 10.2106/JBJS.J.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordaliza M. Natural products as leads to anticancer drugs. Clin Transl Oncol. 2007;9:767–776. doi: 10.1007/s12094-007-0138-9. [DOI] [PubMed] [Google Scholar]
- 21.Pezzuto JM. Plant-derived anticancer agents. Biochem Pharmacol. 1997;53:121–133. doi: 10.1016/S0006-2952(96)00654-5. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad A, Sakr WA, Rahman KM. Novel targets for detection of cancer and their modulation by chemopreventive natural compounds. Front Biosci (Elite Ed) 2012;4:410–425. doi: 10.2741/e388. [DOI] [PubMed] [Google Scholar]
- 23.Hijová E, Szabadosova V, Štofilová J, Hrčková G. Chemopreventive and metabolic effects of inulin in colon cancer development. J Vet Sci. 2013;14:387–393. doi: 10.4142/jvs.2013.14.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park EJ, Pezzuto JM. Antioxidant marine products in cancer chemoprevention. Antioxid Redox Signal. 2013;19:115–138. doi: 10.1089/ars.2013.5235. [DOI] [PubMed] [Google Scholar]
- 25.Chen JC, Chiu MH, Nie RL, Cordell GA, Qiu SX. Cucurbitacins and cucurbitane glycosides: Structures and biological activities. Nat Prod Rep. 2005;22:386–399. doi: 10.1039/b418841c. [DOI] [PubMed] [Google Scholar]
- 26.Geissman TA. New substances of plant origin. Annu Rev Pharmacolog. 1964;4:305–316. doi: 10.1146/annurev.pa.04.040164.001513. [DOI] [Google Scholar]
- 27.Farias MR, Schenkel EP, Mayer R, Rücker G. Cucurbitacins as constituents of Wilbrandia ebracteata. Planta Med. 1993;59:272–275. doi: 10.1055/s-2006-959668. [DOI] [PubMed] [Google Scholar]
- 28.Peters RR, Farias MR, Ribeiro-do-Valle RM. Anti-inflammatory and analgesic effects of cucurbitacins from Wilbrandia ebracteata. Planta Med. 1997;63:525–528. doi: 10.1055/s-2006-957755. [DOI] [PubMed] [Google Scholar]
- 29.Blaskovich MA, Sun J, Cantor A, Turkson J, Jove R, Sebti SM. Discovery of JSI-124 (cucurbitacin I), a selective Janus kinase/signal transducer and activator of transcription 3 signaling pathway inhibitor with potent antitumor activity against human and murine cancer cells in mice. Cancer Res. 2003;63:1270–1279. [PubMed] [Google Scholar]
- 30.Shi X, Franko B, Frantz C, Amin HM, Lai R. JSI-124 (cucurbitacin I) inhibits Janus kinase-3/signal transducer and activator of transcription-3 signalling, downregulates nucleophosmin-anaplastic lymphoma kinase (ALK), and induces apoptosis in ALK-positive anaplastic large cell lymphoma cells. Br J Haematol. 2006;135:26–32. doi: 10.1111/j.1365-2141.2006.06259.x. [DOI] [PubMed] [Google Scholar]
- 31.Tannin-Spitz T, Grossman S, Dovrat S, Gottlieb HE, Bergman M. Growth inhibitory activity of cucurbitacin glucosides isolated from Citrullus colocynthis on human breast cancer cells. Biochem Pharmacol. 2007;73:56–67. doi: 10.1016/j.bcp.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Yin D, Wakimoto N, Xing H, Lu D, Huynh T, Wang X, Black KL, Koeffler HP. Cucurbitacin B markedly inhibits growth and rapidly affects the cytoskeleton in glioblastoma multiforme. Int J Cancer. 2008;123:1364–1375. doi: 10.1002/ijc.23648. [DOI] [PubMed] [Google Scholar]
- 33.Ho YT, Yang JS, Li TC, Lin JJ, Lin JG, Lai KC, Ma CY, Wood WG, Chung JG. Berberine suppresses in vitro migration and invasion of human SCC-4 tongue squamous cancer cells through the inhibitions of FAK, IKK, NF-kappaB, u-PA and MMP-2 and -9. Cancer Lett. 2009;279:155–162. doi: 10.1016/j.canlet.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 34.Mizoguchi H, Nakade J, Tachibana M, Ibi D, Someya E, Koike H, Kamei H, Nabeshima T, Itohara S, Takuma K, et al. Matrix metalloproteinase-9 contributes to kindled seizure development in pentylenetetrazole-treated mice by converting pro-BDNF to mature BDNF in the hippocampus. J Neurosci. 2011;31:12963–12971. doi: 10.1523/JNEUROSCI.3118-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 36.Güllü IH, Kurdoğlu M, Akalin I. The relation of gelatinase (MMP-2 and -9) expression with distant site metastasis and tumour aggressiveness in colorectal cancer. Br J Cancer. 2000;82:249. doi: 10.1054/bjoc.1999.0909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kilian M, Gregor JI, Heukamp I, Hanel M, Ahlgrimm M, Schimke I, Kristiansen G, Ommer A, Walz MK, Jacobi CA, Wenger FA. Matrix metalloproteinase inhibitor RO 28–2653 decreases liver metastasis by reduction of MMP-2 and MMP-9 concentration in BOP-induced ductal pancreatic cancer in Syrian Hamsters: Inhibition of matrix metalloproteinases in pancreatic cancer. Prostaglandins Leukot Essent Fatty Acids. 2006;75:429–434. doi: 10.1016/j.plefa.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Mizutani K, Kofuji K, Shirouzu K. The significance of MMP-1 and MMP-2 in peritoneal disseminated metastasis of gastric cancer. Surg Today. 2000;30:614–621. doi: 10.1007/s005950070101. [DOI] [PubMed] [Google Scholar]
- 39.Qazi A, Pal J, Maitah M, Fulciniti M, Pelluru D, Nanjappa P, Lee S, Batchu RB, Prasad M, Bryant CS, et al. Anticancer activity of a broccoli derivative, sulforaphane, in barrett adenocarcinoma: Potential use in chemoprevention and as adjuvant in chemotherapy. Transl Oncol. 2010;3:389–399. doi: 10.1593/tlo.10235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hartojo W, Silvers AL, Thomas DG, Seder CW, Lin L, Rao H, Wang Z, Greenson JK, Giordano TJ, Orringer MB, et al. Curcumin promotes apoptosis, increases chemosensitivity, and inhibits nuclear factor kappaB in esophageal adenocarcinoma. Transl Oncol. 2010;3:99–108. doi: 10.1593/tlo.09235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monian P, Jiang X. Clearing the final hurdles to mitochondrial apoptosis: Regulation post cytochrome C release. Exp Oncol. 2012;34:185–191. [PubMed] [Google Scholar]
- 43.Soriano ME, Scorrano L. The interplay between BCL-2 family proteins and mitochondrial morphology in the regulation of apoptosis. Adv Exp Med Biol. 2010;687:97–114. doi: 10.1007/978-1-4419-6706-0_6. [DOI] [PubMed] [Google Scholar]
- 44.Kiu H, Nicholson SE. Biology and significance of the JAK/STAT signalling pathways. Growth Factors. 2012;30:88–106. doi: 10.3109/08977194.2012.660936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chau MN, Banerjee PP. Development of a STAT3 reporter prostate cancer cell line for high throughput screening of STAT3 activators and inhibitors. Biochem Biophys Res Commun. 2008;377:627–631. doi: 10.1016/j.bbrc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yue P, Turkson J. Targeting STAT3 in cancer: How successful are we? Expert Opin Investig Drugs. 2009;18:45–56. doi: 10.1517/13543780802565791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chambliss OL, Jone CM. Cucurbitacins: Specific insect attractants in Cucurbitaceae. Science. 1966;153:1392–1393. doi: 10.1126/science.153.3742.1392. [DOI] [PubMed] [Google Scholar]
- 48.Yesilada E, Tanaka S, Sezik E, Tabata M. Isolation of an anti-inflammatory principle from the fruit juice of Ecballium elaterium. J Nat Prod. 1988;51:504–508. doi: 10.1021/np50057a008. [DOI] [PubMed] [Google Scholar]
- 49.Zhang M, Zhang H, Sun C, Shan X, Yang X, Li-Ling J, Deng Y. Target constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B. Cancer Chemother Pharmacol. 2009;63:635–642. doi: 10.1007/s00280-008-0780-0. [DOI] [PubMed] [Google Scholar]
- 50.Wakimoto N, Yin D, O'Kelly J, Haritunians T, Karlan B, Said J, Xing H, Koeffler HP. Cucurbitacin B has a potent antiproliferative effect on breast cancer cells in vitro and in vivo. Cancer Sci. 2008;99:1793–1797. doi: 10.1111/j.1349-7006.2008.00899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li Y, Li W, Chen X, Jiang H, Sun J, Chen H, Lv S. Integrated analysis identifies interaction patterns between small molecules and pathways. Biomed Res Int. 2014;2014:931825. doi: 10.1155/2014/931825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wen D, Danquah M, Chaudhary AK, Mahato RI. Small molecules targeting MicroRNA for cancer therapy: Promises and obstacles. J Control Release. 2015;219:237–247. doi: 10.1016/j.jconrel.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wondrak GT, Villeneuve NF, Lamore SD, Bause AS, Jiang T, Zhang DD. The cinnamon-derived dietary factor cinnamic aldehyde activates the Nrf2-dependent antioxidant response in human epithelial colon cells. Molecules. 2010;15:3338–3355. doi: 10.3390/molecules15053338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liang CZ, Zhang X, Li H, Tao YQ, Tao LJ, Yang ZR, Zhou XP, Shi ZL, Tao HM. Gallic acid induces the apoptosis of human osteosarcoma cells in vitro and in vivo via the regulation of mitogen-activated protein kinase pathways. Cancer Biother Radiopharm. 2012;27:701–710. doi: 10.1089/cbr.2012.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z, Li D, Zhao W, Zheng X, Wang J, Wang E. A potent lead induces apoptosis in pancreatic cancer cells. PLoS One. 2012;7:e37841. doi: 10.1371/journal.pone.0037841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gross A, McDonnell JM, Korsmeyer SJ. Bcl-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 57.Reed JC. Apoptosis-regulating proteins as targets for drug discovery. Trends Mol Med. 2001;7:314–319. doi: 10.1016/S1471-4914(01)02026-3. [DOI] [PubMed] [Google Scholar]
- 58.Yang SH, Chien CM, Lu MC, Lin YH, Hu XW, Lin SR. Upregulation of Bax and endonuclease G, and down-modulation of Bcl-XL involved in cardiotoxin III-induced apoptosis in K562 cells. Exp Mol Med. 2006;38:435–444. doi: 10.1038/emm.2006.51. [DOI] [PubMed] [Google Scholar]
- 59.Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3:895–904. doi: 10.1016/S1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Shi J, Feng J, Klocker H, Lee C, Zhang J. Type IV collagenase (matrix metalloproteinase-2 and -9) in prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:327–332. doi: 10.1038/sj.pcan.4500750. [DOI] [PubMed] [Google Scholar]
- 61.O'Shea JJ, Gadina M, Schreiber RD. Cytokine signaling in 2002: New surprises in the Jak/Stat pathway. Cell. 2002;109(Suppl):121–131. doi: 10.1016/S0092-8674(02)00701-8. [DOI] [PubMed] [Google Scholar]
- 62.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu H, Jove R. The STATs of cancer-new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 64.Epling-Burnette PK, Liu JH, Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang JM, Yang-Yen HF, Karras J, et al. Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J Clin Invest. 2001;107:351–362. doi: 10.1172/JCI9940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu T, Zhang M, Zhang H, Sun C, Deng Y. Inhibitory effects of cucurbitacin B on laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2008;265:1225–1232. doi: 10.1007/s00405-008-0625-9. [DOI] [PubMed] [Google Scholar]
- 66.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 67.Kim BM, Chung HW. Desferrioxamine (DFX) induces apoptosis through the p38-caspase8-Bid-Bax pathway in PHA stimulated human lymphocytes. Toxicol Appl Pharmacol. 2008;228:24–31. doi: 10.1016/j.taap.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 68.Mendes O, Kim HT, Lungu G, Stoica G. MMP2 role in breast cancer brain metastasis development and its regulation by TIMP2 and ERK1/2. Clin Exp Metastasis. 2007;24:341–351. doi: 10.1007/s10585-007-9071-0. [DOI] [PubMed] [Google Scholar]
- 69.Peng L, Xing X, Li W, Qu L, Meng L, Lian S, Jiang B, Wu J, Shou C. PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin beta1-ERK1/2 and-MMP2 signaling. Mol Cancer. 2009;8:110. doi: 10.1186/1476-4598-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]