Abstract
There are a wide variety of synthetic and naturally occurring nanomaterials under development for nanoscale cargo-delivery applications. Viruses play a special role in these developments, because they can be regarded as naturally occurring nanomaterials evolved to package and deliver cargos. While any nanomaterial has its advantage and disadvantages, viral nanoparticles (VNPs), in particular the ones derived from plant viruses and bacteriophages, are attractive options for cargo-delivery as they are biocompatible, biodegradable, and non-infectious to mammals. Their protein-based structures are often understood at atomic resolution and are amenable to modification with atomic-level precision through chemical and genetic engineering. Here we present a focused review of the emerging technology development of plant viruses and bacteriophages targeting human health and agricultural applications. Key target areas of development are their use in chemotherapy-, photodynamic therapy-, pesticide-delivery, gene therapy, vaccine carriers, and immunotherapy.
Introduction
Advances in nanotechnology have allowed development of nanoscale materials that can be tailored for delivery of medical cargo to molecular signatures on specific cells or tissues. Nanoparticles also improve drug stability and pharmacokinetics, and the formulation of therapeutics into nanoparticles often leads to reduced toxicity due to favorable biodistribution. While the development pipeline is moving rapidly with many novel chemistries and (bio)materials emerging, only a few nanoparticles have been approved for clinical use. Translation of novel drug carriers from bench-to-bedside is a long and costly process; challenges in toxicology and large-scale manufacture remain hurdles to success. Addressing the latter point, biologics offer a promising alternative to synthetic materials, because the genetically controlled design and manufacture provides a high degree of quality control and assurance and scale-up can be achieved through fermentation or production in plants [1]. Here, we highlight the development of plant viruses and bacteriophages (i.e., viruses that infect bacteria) as an emerging class of biologics for use in drug delivery.
Viruses can be regarded as natural carrier systems; they have evolved to package, protect, and deliver nucleic acid cargo to host cells. A number of mammalian viral vectors are under development for gene therapy [2,3]. Glybera, a viral vector derived from adeno-associated virus (AAV) was the first gene therapy approved for clinical use in Europe in 2012 for the treatment of lipoprotein lipase deficiency [4]. Plant viruses and bacteriophages may offer safety advantages as these systems are non-integrating and non-replicating in mammalian systems. Furthermore, because viruses are protein-based, they are biodegradable and thus have a lower risk of tissue persistence compared to some synthetic materials [5,6]. Plant viruses and bacteriophages can be produced and engineered as viral nanoparticles (VNPs) and non-replicative, virus-like particles (VLPs). VLPs are a subclass of VNPs and in the remainder of this article we will refer to viral or virus-like systems as VNPs.
Because viruses comes in many shapes, sizes, and surface properties, the nanomedical engineer can pick and choose the most appropriate platform for a given application (Figure 1) [1,7,8]. For example, higher aspect ratio materials have propensity to marginate and target the diseased vessel wall [9], while flexuous rods allow penetration deep into tumor tissue [10]; and low aspect ratio materials or icosahedrons interact effectively with the immune system [11]. Many platforms are known to atomic resolution; the well-characterized surface groups on the exterior and interior capsid surfaces allow genetic and chemical tailoring in a predictable way [12]. Additionally, the multidendate nature of the capsids offers an array of surface resides making it possible to introduce multiple ligands and cargos, including therapeutic compounds, contrast agents, and targeting ligands into a single formulation [13,14]. Functionalization can be achieved through genetic programming, for example amino acids can be introduced to function as conjugation handles (e.g. [15]) or peptides with specificity for cell surface receptors can be added to give the particle directions to target cancer cells (e.g. [16]). Some viruses are also amenable to physical modifications; tobacco mosaic virus (TMV), for example, can be transformed from its native rod-shape to a spherical form through heating [17].
Figure 1.
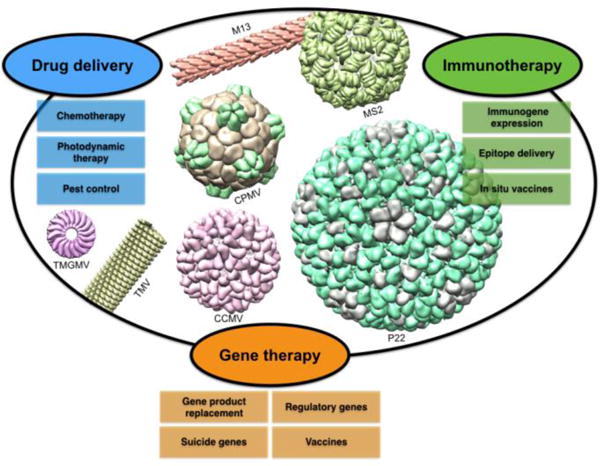
Plant viruses and bacteriophages come in a variety of shapes, sizes, and surface properties and have been explored for a number of biomedical and biotechnology applications. Here we show a number of plant viruses and bacteriophages with elongated and spherical structures and a range of sizes.
For implementation in nanomedicine, the therapeutic cargo is loaded either into the interior cavity of the capsid or conjugated the capsid’s interior or exterior surfaces. Targeted delivery of the cargo is then achieved through a combination of carrier shape and surface chemistry. Carrier size and shape dictate passive targeting to tissues making use of physiopathological features, e.g. enhanced permeability and retention effect observed in some tumors [18]. Moreover, active targeting is achieved making use of naturally occurring VNP–cell/tissue interactions (i.e. tropism of cowpea mosaic virus (CPMV) to target and bind to cell-surface vimentin [19]), or active targeting strategies achieved by incorporation of antibodies [20], aptamers [21], or peptides [22] into the capsid scaffold. With increasing understanding of the underlying biology of diseases, there is an ever-expanding capability to target diseased tissue allowing the development of personalized medicine.
Drug delivery
Chemotherapy
VNPs have been explored as nanocarriers for a number of clinically approved and experimental chemotherapeutics. Conjugation or complexation of the therapeutic compound to VNPs can have several advantages including improvement of pharmacokinetics and advantageous biodistribution enabling enhanced drug delivery to disease sites while overcoming off-target effects. Passive and active targeting strategies as mentioned above allow tailored tissue specificity. Several VNP platforms have been developed as a carrier for the anthracycline doxorubicin. In the clinic doxorubicin is used to treat a variety of cancer types including breast, ovarian, prostate, and thyroid, among others. High off-target toxicity of the drug however, limits the administered dose reducing treatment efficacy [23]. Liposomal formulation of doxorubicin is one of the few examples of nanoparticle-based drugs used in the clinic today [24]. Examples of VNP-doxorubicin complexes include high aspect ratio TMV-based nanotubes as well as TMV-derived disk-shaped nanoparticles, both of which have been conjugated with doxorubicin through covalent attachment [25–27]. Spherical TMV nanoparticles have also been infused with doxorubicin through mixing of doxorubicin and TMV during the heat-mediated rod-to-sphere shape transition. Either method yielded doxorubicin-loaded TMV formulations that exhibited potent cytotoxicity against cancer cells when tested in vitro [25]. Another example is cucumber mosaic virus (CMV); here doxorubicin was infused and trapped inside the capsid through non-covalent loading into the encapsidated RNA genome, leading to increased drug concentration in ovarian cancer tissue and decreased cardiotoxicity in a mouse model [28]. Similarly, the reversible pore-opening process of the red clover necrotic mosaic virus (RCNMV) capsid allowed loading and release of doxorubicin (as well as other drugs) in a pH- and divalent cation-dependent manner [29]. More recently, we also demonstrated the non-covalent complexation of doxorubicin to high aspect ratio, flexuous potato virus X (PVX); the formulation exhibited enhanced efficacy vs. free drug in a mouse model of triple negative breast cancer [30].
While doxorubicin is a good entry point to the field of drug delivery, allowing comparison of the different platform technologies, newer approaches and alternate nanoparticle-drug formulations delivering more potent drugs are urgently needed to overcome drug resistance. Toward this goal, we recently demonstrated TMV-assisted delivery of the drug candidate phenanthriplatin (Figure 2). Phenanthriplatin is a cisplatin derivative up to 40× more potent than contemporary platinum therapeutics, including among platinum resistant cell lines [31]. To enable its delivery to tumors, we established a non-covalent drug loading protocol yielding therapeutic TMV carrying ~2,000 phenanthriplatin moieties in its central channel. Loading was achieved through electrostatic-driven assembly (the dication of phenanthriplatin interacts with the negatively-charged interior of TMV). Phenanthriplatin-loaded TMV outperformed both free phenanthriplatin and the current standard of care, cisplatin, when used to treat a mouse model of triple negative breast cancer [32].
Figure 2.
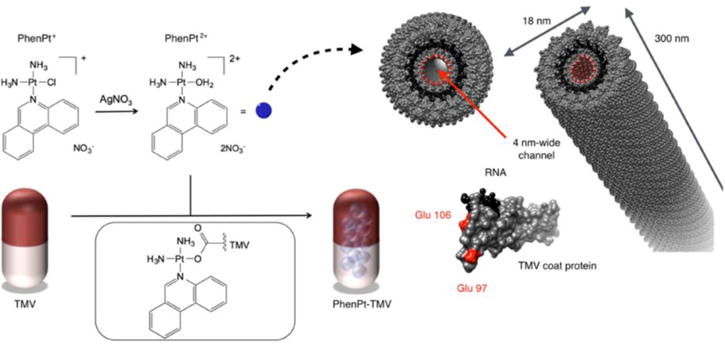
Schematic for loading of phenanthriplatin into TMV. Reprinted with permission from Czapar et al.: Tobacco Mosaic Virus Delivery of Phenanthriplatin for Cancer therapy in ACS Nano [32]. Copyright (2016) American Chemical Society.
Photodynamic therapy
Another avenue is the use of VNPs for the delivery of photodynamic therapies (PDT), an emerging therapeutic regimen used to treat infectious disease and cancer. PDT relies on photosensitizers, often derived from porphyrins, which produce reactive oxygen species following activation by specific wavelengths of light. Targeted delivery of PDT allows for increased clinical applications through reduction of host-cell/non-target tissue damage. Several VNP platforms are undergoing development of PDT. For example, Staphylococcus aureus, an infectious agent that can cause persistent biofilms, was treated using cowpea chlorotic mosaic virus (CCMV) dual-functionalized with a photosensitizer and a targeting ligand [33]. PDT has also been loaded into the tumor cell-targeted bacteriophages Qβ and MS2. In brief, aptamer-targeted MS2-delivered PDT allowed for preferential killing of the Jurkat leukemia cell line [21]. Similarly, glycan-conjugated Qβ delivered PDT enabled highly specific CD22+ B cell lineage targeting and killing. Potential applications are lymphoma chemotherapy and certain immune disorders [34].
Two recent examples used TMV and CPMV to target PDT agents to melanoma. TMV was loaded with cationic photosensitizers using the electrostatically-driven loading protocol described above (see Figure 2) [32]; the resultant PDT-carrier system showed increased cell uptake and cell killing efficiency when tested with melanoma cells in vitro [35]. CPMV modified with a corona of negatively charged dendrons was used to complex and load positively charged PDT agents. This VNP-synthetic hybrid material was effective in killing both a melanoma cell line as well as macrophages – the combined efficacy on cancer cells and tumor-associated macrophages could be a powerful strategy for cancer therapy [36].
Pest control
VNPs can also be used to improve pesticide delivery for agriculture. Currently, tobacco mottle green mosaic virus (TMGMV) is approved by the EPA as a biopesticide for control of the weed tropical soda apple, demonstrating the potential utility of plant virus-based pest control [37]. In another example, a plant VNP was used to target pesticides to parasitic nematodes. Nematodes cause an estimated $157 billion in crop damage annually and, while abamectin is effective in killing these pests, it has poor mobility in the soil, leading to a limited zone of protection. To overcome this challenge, abamectin was loaded into RCNMV through a pH- and salt-dependent gating mechanism (Figure 3); the RCNMV carrier was shown to be more effective than free abamectin in nematode killing protecting plant roots from disease [29,38].
Figure 3.
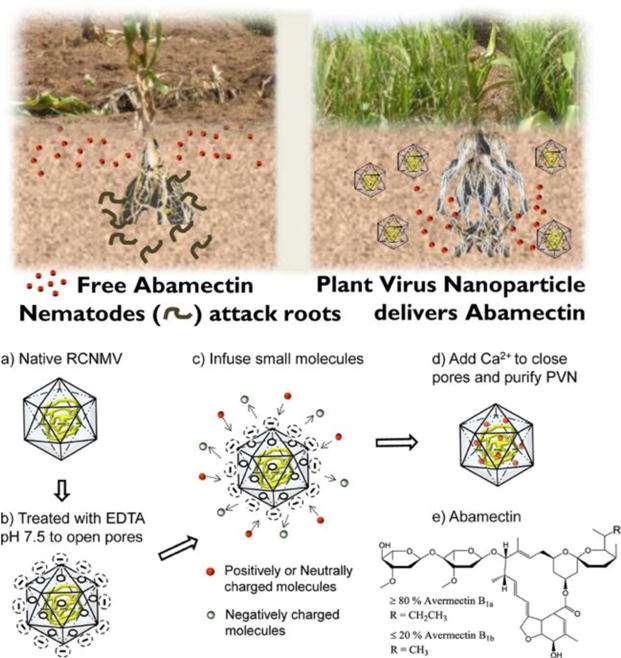
Loading of RCNMV with abamectin for nematode control. Reprinted with permission from Cao J, et al.: Development of Abamectin Loaded Plant Virus Nanoparticles for Efficacious Plant Parasitic Nematode Control in ACS Appl. Mater. Interfaces [38]. Copyright (2015) American Chemical Society.
Gene therapy
While mammalian viral vectors are already established for gene delivery newer approaches include the development of plant viruses and bacteriophages targeting diverse applications. A prominent example is the development of CCMV to deliver mRNA cargos. CCMV, like other VNP platform technologies, has the capability to package non-native RNA of a range of lengths and sequences. In a proof-of-concept study, VLPs consisting of a CCMV capsid containing heterologous RNA were delivered to cells using lipofectamine; upon cell entry packaged nucleic acids were released in the cytoplasm of mammalian cells leading to expression of the reporter enhanced yellow fluorescent protein. Loading of these RNAs into the VNP carrier protects the genetic material from degradation during storage and delivery [39]. Bacteriophage M13 has also been used as a nanocarrier for genetic material; through genetic engineering the bacteriophage was modified to carry a transgene cassette for herpes simplex virus thymidine kinase, a conditionally lethal enzyme that causes cell death when expressed in the presence of the pro-drug ganciclovir. This M13 gene delivery vector was also modified to express a RGD4C peptide on its surface to enable cancer cell targeting. By combining this targeted bacteriophage with a cationic polymer, cell delivery and efficacy was demonstrated in vitro [16]. Another area of development is the delivery of mRNAs for vaccination, a topic discussed in the next section (Immunotherapies and vaccines).
Bacteriophages have also been developed for gene-silencing applications. For example MS2 has been developed as a carrier for micro-RNAs (miRNAs). Like the plant virus CCMV, the bacteriophage has not developed a natural mechanism for trafficking in mammalian cells; however targeting of the nucleic acid cargo to the cytoplasm was achieved through conjugation of the cell penetrating peptide Tat (trans-activator of transcription) from human immunodeficiency virus 1. In vivo administration of this nanocarrier in a murine model of systemic lupus erthythematous achieved a significant increase in the expression of miR146a, a miRNA involved in regulation of the innate immune response and found to be a regulator of autoimmune disorders, myeloproliferation, and cancer. Delivery of the MS2 encapsulated regulatory miR146a was found to have a therapeutic effect as it reduced the total antibody production [40].
Immunotherapies and vaccines
Vaccination by means of gene expression
Plant virus- and bacteriophage- derived VNPs are well suited to use as vaccines and immunotherapy because they are capable of stimulating the immune system without the risk of integration or replication – the latter remain safety concerns for attenuated or inactivated vaccines. The size and multivalent nature of VNPs allows for increased immune stimulation, including enhanced B cell activation and uptake by antigen presenting cells. Additionally, even viruses which do not infect mammalian cells stimulate pathogen associated molecular patterns receptors thus priming the innate immune system, resulting in chemo/cytokine profiles that also stimulate T cell activation [8]. Building on their ability to interact efficiently with immune cells and their natural capacity to encapsulate nucleic acid cargo, VNPs are undergoing development for delivery and expression of immunogenic transgenes in host cells as a vaccination strategy. In particular, RNA viral vectors have the advantage of inducing high levels of epitope expression in target cells without integration into host DNA. By inducing expression of an antigen, a high level of long-lasting cellular immunity can be achieved. Nevertheless, based on the inherent instability of RNA a delivery system is required. Based on their natural interaction and priming of immune cells, plant-based VNPs provide an intriguing platform for vaccine applications. In one example, a RNA vaccine system was created using a combination of modified RNA-1 from insect virus Flockhouse virus and coat proteins from plant virus TMV. In brief, a TMV origin of assembly was inserted into the target RNA sequence containing open reading frames of the gene of interest. The presence of the origin of assembly triggered assembly of TMV coat proteins allowing for production of hybrid VNPs in planta. In a proof-of-concept study using the model immunogen enhanced green fluorescent protein, this VNP-delivered vaccine strategy yielded an effective humoral response against the immunogen when tested in mice [41].
Vaccination by means of epitope delivery
In addition to their capability to deliver the coding sequences of immunogens, peptide antigens can also be directly displayed on the surface of VNPs either through genetic fusion or chemical bioconjugation to the coat proteins. The multivalent display of epitope in combination with the immunogenic nature of the carrier helps to boost epitope-specific B and T cell responses. Developments of VNPs epitope display platform are wide-ranging and applications include vaccination against infectious disease, cancer, chronic diseases and addiction. For a comprehensive review on this topic, we would like to refer the reader to the following review [42]. Here we will highlight two recent examples.
First, in the setting of infectious disease, the bacteriophage P22 has been explored as a platform for a universal influenza vaccine. Influenza nucleoprotein (NP), a protein that is conserved among different strains, was incorporated into the VNP through genetic engineering. Immunization of mice with purified resultant VNPs elicited a humoral and cellular response with anti-NP antibodies and CD8+ T cells specific to NP produced, resulting in protection of immunized mice against both H1N1 and H3N2 influenza strain intranasal challenges [43].
Second, efforts have also focused on the development of VNPs displaying tumor-associated antigens to elicit therapeutic and prophylactic immunity. One example is the display of an epitope for the idiotypic (Id) immunoglobulin from B-cell lymphoma using the PVX platform technology. Conjugation of the Id epitope to PVX through biotin-streptavidin interactions allowed for a multivalent presentation of the epitope. The multivalent display alongside with immune-stimulation through toll-like receptor-7 activation based on the encapsidated ssRNA genome of PVX led to enhanced immune stimulation, increased production of anti-Id antibodies, and improved resistance to Id-expressing BCL1 lymphoma challenge when compared to Id-streptavidin alone [44].
In situ vaccines
In situ vaccination is a technique where an immune-stimulatory agent is introduced directly into an identified tumor or metastatic site to overcome the immunesuppressive tumor microenvironment and mount an anti-tumor response [45]. With this approach the tumor itself is used as the antigen source and what is introduced is an adjuvant. In situ vaccination with mycobacteria has been used over 40 years for bladder cancer [46]. Recently FDA-approved, is TVEC [Amgen], an oncolytic viral therapy based on an attenuated herpes simplex virus engineered to express granulocyte-macrophage colony-stimulating factor (GM-CSF) [47,48].
We and others have recently demonstrated that plant VNPs also induce an anti-tumor immune response when applied as in situ vaccine. First, CPMV has been found to be effective in stimulating an anti-tumor immune response when administered into the tumor microenvironment – efficacy was demonstrated in mouse models of metastatic melanoma, breast cancer, ovarian cancer, and colon cancer. The in situ vaccination using CPMV stimulates the innate immune system, in particular CPMV triggers activation and infiltration of neutrophils, resulting in a chemo/cytokine profile that leads to activation of adaptive immunity. Data indicate that CPMV in situ vaccination of dermal melanoma in mice elicits a durable anti-tumor response: treated animals were protected from tumor re-challenge (Figure 4) [11]. Similarly to our finding, others have shown that filamentous papaya mosaic virus (PapMV) also exhibited efficacy in treating a mouse model of melanoma when administered intratumorally. The efficacy of PapMV was further improved by synergistic co-administration with dendritic cell vaccination and programmed death ligand 1, a checkpoint inhibitor [49].
Figure 4.
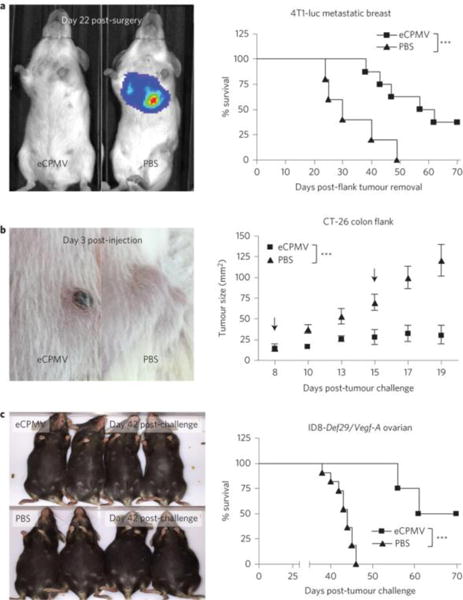
eCPMV administered at the site of the tumor was shown to be effective in treating mouse models of (a) metastatic breast, (b) colon, and (c) ovarian cancer. Reprinted with permission from Macmillan Publishers Ltd: Nature Nanotechnology [11], copyright 2016.
Conclusion
It is clear that there are many exciting opportunities for potential applications of VNPs in medicine and biotechnology. The field has grown out of its infancy: through collaboration between structural biologists, chemists, physicists, and bioengineers a toolbox of engineering principles has been developed and we are beginning to understand the underlying design concepts to tailor VNPs as cargo-delivery vehicles. Multiple platform technologies are being developed with translational and/or commercial applications on the horizon. Yet there is much work to do to realize the bench-to-bedside/field application. First, many works are still being carried out in tissue culture or small animal models, which may not mimic human disease in a realistic fashion and therefore do not always provide a realistic and predictive testbed, in particular for toxicology assessment. As for any biologic (as well as synthetic nanomaterials) immunogenicity needs to be carefully evaluated – while a desired property for immunotherapies, immune surveillance must be overcome for therapeutic delivery in which immune-targeting is not desired (e.g. chemotherapy delivery). To some extent, pharmacokinetics and immunogenicity of VNPs can be tailored through conjugation of a polymer stealth coating such as polyethylene glycol or polyoxazoline [18,50] or a camouflage coating such as albumin [51]. Yet newer and more modern approaches are required to make an impact in the clinic. Another consideration is the manufacture of ever more complex formulations harboring cargos, targeting ligands, and stealth/camouflage coatings. Any functionalization applied post-harvest will lower the yield, increase manufacturing costs, and add the risk of batch-to-batch variability [52]. As the field matures and translation/commercialization become targets in reach, careful design considerations must be taken to yield products that are effective and safe and that have a realistic and cost-effective path of nanomanufacture.
Highlights.
Plant viruses and bacteriophages can be regarded as natural nanoparticles.
Viral nanoparticles are utilized in nanotechnology targeting diverse applications.
Viral capsids offer a scaffold to be modified using a multitude of approaches.
We highlight developments of viral nanoparticles for applications in human health and agriculture.
Acknowledgments
This work was supported in part by a grant from the National Science Foundation (CHE 1306447 to N.F.S.) and by a Research Scholar Award from the American Cancer Society (128319-RSG-15-144-01-CDD to N.F.S.). A.E.C. was supported in part by NIH grants T32 GM007250 and TL1 TR000441.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yildiz I, Shukla S, Steinmetz NF. Applications of viral nanoparticles in medicine. Current Opinion in Biotechnology. 2011;22:901–908. doi: 10.1016/j.copbio.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Guenther CM, Kuypers BE, Lam MT, Robinson TM, Zhao J, Suh J. Synthetic Virology: Engineering Viruses for Gene Delivery. WIREs Nanomed Nanobiotechnol. 2015;6:548–558. doi: 10.1002/wnan.1287. **Excellent review of methods of engineering of viruses for gene delivery, including genetic engineering and chemical conjugation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez EJ, Gerhardt K, Judd J, Tabor JJ, Suh J. Light-Activated Nuclear Translocation of Adeno-Associated Virus Nanoparticles Using Phytochrome B for Enhanced, Tunable, and Spatially Programmable Gene Delivery. ACS Nano. 2016;10:225–237. doi: 10.1021/acsnano.5b05558. [DOI] [PubMed] [Google Scholar]
- 4.Ylä-Herttuala S. Endgame: Glybera Finally Recommended for Approval as the First Gene Therapy Drug in the European Union. Mol Ther. 2012;20:1831–1832. doi: 10.1038/mt.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser CR, Flenniken ML, Gillitzer E, Harmsen AL, Harmsen AG, Jutila MA, Douglas T, Young MJ. Biodistribution studies of protein cage nanoparticles demonstrate broad tissue distribution and rapid clearance in vivo. IJN. 2007;2:715–733. [PMC free article] [PubMed] [Google Scholar]
- 6.Bruckman MA, Randolph LN, VanMeter A, Hern S, Shoffstall AJ, Taurog RE, Steinmetz NF. Biodistribution, pharmacokinetics, and blood compatibility of native and PEGylated tobacco mosaic virus nano-rods and -spheres in mice. Virology. 2014;449:163–173. doi: 10.1016/j.virol.2013.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla S, Eber FJ, Nagarajan AS, DiFranco NA, Schmidt N, Wen AM, Eiben S, Twyman RM, Wege C, Steinmetz NF. The Impact of Aspect Ratio on the Biodistribution and Tumor Homing of Rigid Soft-Matter Nanorods. Adv Healthcare Mater. 2015;4:874–882. doi: 10.1002/adhm.201400641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lebel M-È, Chartrand K, Leclerc D, Lamarre A. Plant Viruses as Nanoparticle-Based Vaccines and Adjuvants. Vaccines. 2015;3:620–637. doi: 10.3390/vaccines3030620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen AM, Wang Y, Jiang K, Hsu GC, Gao H, Lee KL, Yang AC, Yu X, Simon DI, Steinmetz NF. Shaping bio-inspired nanotechnologies to target thrombosis for dual optical-magnetic resonance imaging. Journal of Materials Chemistry B: Materials for biology and medicine. 2015;3:6037–6045. doi: 10.1039/C5TB00879D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shukla S, Ablack AL, Wen AM, Lee KL, Lewis JD, Steinmetz NF. Increased Tumor Homing and Tissue Penetration of the Filamentous Plant Viral Nanoparticle Potato virus X. Mol Pharm. 2012;10:33–42. doi: 10.1021/mp300240m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11**.Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, Fiering S. In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat Nano. 2015;11:295–303. doi: 10.1038/nnano.2015.292. **This paper describes the use of eCPMV to stimulate systemic anti-cancer immunity. Through activation of the immune system, survival was dramatically extended in a number of mouse cancer models. Further, the majority of mice re-challenged with tumors on the opposite flank completely rejected the re-challenged tumor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruckman MA, Steinmetz NF. Chemical Modification of the Inner and Outer Surfaces of Tobacco Mosaic Virus (TMV) In: McNeil SE, editor. Characterization of Nanoparticles Intended for Drug Delivery. Humana Press; 2013. pp. 173–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cho K, Wang X, Nie S, Chen Z, Shin DM. Therapeutic Nanoparticles for Drug Delivery in Cancer. Clinical Cancer Research. 2008;14:1310–1316. doi: 10.1158/1078-0432.CCR-07-1441. [DOI] [PubMed] [Google Scholar]
- 14.Bruckman MA, Jiang K, Simpson EJ, Randolph LN, Luyt LG, Yu X, Steinmetz NF. Dual-Modal Magnetic Resonance and Fluorescence Imaging of Atherosclerotic Plaques in Vivo Using VCAM-1 Targeted Tobacco Mosaic Virus. Nano Lett. 2014;14:1551–1558. doi: 10.1021/nl404816m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith ML, Lindbo JA, Dillard-Telm S, Brosio PM, Lasnik AB, McCormick AA, Nguyen LV, Palmer KE. Modified Tobacco mosaic virus particles as scaffolds for display of protein antigens for vaccine applications. Virology. 2006;348:475–488. doi: 10.1016/j.virol.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 16**.Yata T, Lee K-Y, Dharakul T, Songsivilai S, Bismarck A, Mintz PJ, Hajitou A. Hybrid Nanomaterial Complexes for Advanced Phage-guided Gene Delivery. Mol Ther Nucleic Acids. 2014;3:e185–13. doi: 10.1038/mtna.2014.37. **This paper describes use of M13 bacteriophage that has been genetically altered to express a targeting ligand for gene delivery applications. The incorporation of a genetically engineered targeting ligand and a cationic polymer for increased uptake demonstrates the potential for synthetic and biomaterial hybrids for gene delivery. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bruckman MA, VanMeter A, Steinmetz NF. Nanomanufacturing of Tobacco Mosaic Virus-Based Spherical Biomaterials Using a Continuous Flow Method. ACS Biomater Sci Eng. 2015;1:13–18. doi: 10.1021/ab500059s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee KL, Shukla S, Wu M, Ayat NR, Sanadi El CE, Wen AM, Edelbrock JF, Pokorski JK, Commandeur U, Dubyak GR, Steinmetz NF. Stealth filaments: Polymer chain length and conformation affect the in vivo fate of PEGylated potato virus X. Acta Biomaterialia. 2015;19:166–179. doi: 10.1016/j.actbio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinmetz NF, Cho C-F, Ablack A, Lewis JD, Manchester M. Cowpea mosaic virus nanoparticles target surface vimentin on cancer cells. Nanomedicine. 2011;6:351–364. doi: 10.2217/nnm.10.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ElSohly AM, Netirojjanakul C, Aanei IL, Jager A, Bendall SC, Farkas ME, Nolan GP, Francis MB. Synthetically Modified Viral Capsids as Versatile Carriers for Use in Antibody-Based Cell Targeting. Bioconjugate Chem. 2015;26:1590–1596. doi: 10.1021/acs.bioconjchem.5b00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stephanopoulos N, Tong GJ, Hsiao SC, Francis MB. Dual-Surface Modified Virus Capsids for Targeted Delivery of Photodynamic Agents to Cancer Cells. ACS Nano. 2010;4:6014–6020. doi: 10.1021/nn1014769. [DOI] [PubMed] [Google Scholar]
- 22.Pan Y, Zhang Y, Jia T, Zhang K, Li J, Wang L. Development of a microRNA delivery system based on bacteriophage MS2 virus-like particles. FEBS Journal. 2012;279:1198–1208. doi: 10.1111/j.1742-4658.2012.08512.x. [DOI] [PubMed] [Google Scholar]
- 23.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. Journal of Pharmacy and Pharmacology. 2012;65:157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 24.Marchal S, Hor El A, Millard M, Gillon V, Bezdetnaya L. Anticancer Drug Delivery: An Update on Clinically Applied Nanotherapeutics. Drugs. 2015;75:1601–1611. doi: 10.1007/s40265-015-0453-3. [DOI] [PubMed] [Google Scholar]
- 25.Bruckman MA, Czapar AE, VanMeter A, Randolph LN, Steinmetz NF. Tobacco mosaic virus-based protein nanoparticles and nanorods for chemotherapy delivery targeting breast cancer. Journal of Controlled Release. 2016;231:103–113. doi: 10.1016/j.jconrel.2016.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finbloom JA, Han K, Aanei IL, Hartman EC, Finley DT, Dedeo MT, Fishman M, Downing KH, Francis MB. Stable Disk Assemblies of a Tobacco Mosaic Virus Mutant as Nanoscale Scaffolds for Applications in Drug Delivery. Bioconjugate Chem. 2016;27:2480–2485. doi: 10.1021/acs.bioconjchem.6b00424. [DOI] [PubMed] [Google Scholar]
- 27.Tian Y, Gao S, Wu M, Liu X, Qiao J, Zhou Q, Jiang S, Niu Z. Tobacco Mosaic Virus-Based 1D Nanorod-Drug Carrier via the Integrin-Mediated Endocytosis Pathway. ACS Appl Mater Interfaces. 2016;8:10800–10807. doi: 10.1021/acsami.6b02801. [DOI] [PubMed] [Google Scholar]
- 28.Zeng Q, Wen H, Wen Q, Chen X, Wang Y, Xuan W, Liang J, Wan S. Cucumber mosaic virus as drug delivery vehicle for doxorubicin. Biomaterials. 2013;34:4632–4642. doi: 10.1016/j.biomaterials.2013.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Cao J, Guenther RH, Sit TL, Opperman CH, Lommel SA, Willoughby JA. Loading and Release Mechanism of Red Clover Necrotic Mosaic Virus Derived Plant Viral Nanoparticles for Drug Delivery of Doxorubicin. Small. 2014;10:5126–5136. doi: 10.1002/smll.201400558. [DOI] [PubMed] [Google Scholar]
- 30.Le DHT, Lee KL, Shukla S, Commandeur U, Steinmetz NF. Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale. 2017;9:2348–2357. doi: 10.1039/c6nr09099k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park GY, Wilson JJ, Song Y, Lippard SJ. Phenanthriplatin, a monofunctional DNA-binding platinum anticancer drug candidate with unusual potency and cellular activity profile. Proc Natl Acad Sci USA. 2012;109:11987–11992. doi: 10.1073/pnas.1207670109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32**.Czapar AE, Zheng Y-R, Riddell IA, Shukla S, Awuah SG, Lippard SJ, Steinmetz NF. Tobacco Mosaic Virus Delivery of Phenanthriplatin for Cancer therapy. ACS Nano. 2016;10:4119–4126. doi: 10.1021/acsnano.5b07360. **This paper describes the use of TMV increase delivery of a novel cisplatin derivative, phenanthriplatin, to a mouse model of triple negative breast cancer. This is the first paper to describe in vivo delivery of a platinum compound with a plant viral nanoparticle. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suci PA, Varpness Z, Gillitzer E, Douglas T, Young M. Targeting and Photodynamic Killing of a Microbial Pathogen Using Protein Cage Architectures Functionalized with a Photosensitizer. Langmuir. 2007;23:12280–12286. doi: 10.1021/la7021424. [DOI] [PubMed] [Google Scholar]
- 34.Rhee J-K, Baksh M, Nycholat C, Paulson JC, Kitagishi H, Finn MG. Glycan-Targeted Virus-like Nanoparticles for Photodynamic Therapy. Biomacromolecules. 2012;13:2333–2338. doi: 10.1021/bm300578p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee KL, Carpenter BL, Wen AM, Ghiladi RA, Steinmetz NF. High Aspect Ratio Nanotubes Formed by Tobacco Mosaic Virus for Delivery of Photodynamic Agents Targeting Melanoma. ACS Biomater Sci Eng. 2016;2:838–844. doi: 10.1021/acsbiomaterials.6b00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wen AM, Lee KL, Cao P, Pangilinan K, Carpenter BL, Lam P, Veliz FA, Ghiladi RA, Advincula RC, Steinmetz NF. Utilizing Viral Nanoparticle/Dendron Hybrid Conjugates in Photodynamic Therapy for Dual Delivery to Macrophages and Cancer Cells. Bioconjugate Chem. 2016;27:1227–1235. doi: 10.1021/acs.bioconjchem.6b00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charudattan R, Hiebert E. A Plant Virus as a Bioherbicide for Tropical Soda Apple, Solanum Viarum. Outlook Pest Man. 2007;18:167–171. [Google Scholar]
- 38.Cao J, Guenther RH, Sit TL, Lommel SA, Opperman CH, Willoughby JA. Development of Abamectin Loaded Plant Virus Nanoparticles for Efficacious Plant Parasitic Nematode Control. ACS Appl Mater Interfaces. 2015;7:9546–9553. doi: 10.1021/acsami.5b00940. [DOI] [PubMed] [Google Scholar]
- 39.Azizgolshani O, Garmann RF, Cadena-Nava R, Knobler CM, Gelbart WM. Reconstituted plant viral capsids can release genes to mammalian cells. Virology. 2013;441:12–17. doi: 10.1016/j.virol.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Pan Y, Jia, Zhang, Zhang, Zhang, Li, Wang L. MS2 VLP-based delivery of microRNA-146a inhibits autoantibody production in lupus-prone mice. IJN. 2012;7:5957–5967. doi: 10.2147/IJN.S37990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41**.Zhou Y, Maharaj PD, Mallajosyula JK, McCormick AA, Kearney CM. In planta Production of Flock House Virus Transencapsidated RNA and Its Potential Use as a Vaccine. Mol Biotechnol. 2014;57:325–336. doi: 10.1007/s12033-014-9826-1. **This paper describes a highly interesting system of packaging FHV RNA into VLPs derived from TMV coat proteins in plants. [DOI] [PubMed] [Google Scholar]
- 42.Lee KL, Twyman RM, Fiering S, Steinmetz NF. Virus-based nanoparticles as platform technologies for modern vaccines. WIREs Nanomed Nanobiotechnol. 2016;8:554–578. doi: 10.1002/wnan.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson DP, Rynda-Apple A, Harmsen AL, Harmsen AG, Douglas T. Biomimetic Antigenic Nanoparticles Elicit Controlled Protective Immune Response to Influenza. ACS Nano. 2013;7:3036–3044. doi: 10.1021/nn4006544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, Lomonossoff GP, Ottensmeier C, Diebold SS, Stevenson FK, Savelyeva N. Plant Virus Particles Carrying Tumour Antigen Activate TLR7 and Induce High Levels of Protective Antibody. PLoS ONE. 2015;10:e0118096–16. doi: 10.1371/journal.pone.0118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hammerich L, Binder A, Brody JD. In situ vaccination: Cancer immunotherapy both personalized and off-the-shelf. Molecular Oncology. 2015;9:1966–1981. doi: 10.1016/j.molonc.2015.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamat AM, Flaig TW, Grossman HB, Konety B, Lamm D, O’Donnell MA, Uchio E, Efstathiou JA, Taylor JA. Expert consensus document: Consensus statement on best practice management regarding the use of intravesical immunotherapy with BCG for bladder cancer. Nature Publishing Group. 2015;12:225–235. doi: 10.1038/nrurol.2015.58. [DOI] [PubMed] [Google Scholar]
- 47.Harrington KJ, Puzanov I, Hecht JR, Hodi FS, Szabo Z, Murugappan S, Kaufman HL, Harrington KJ, Puzanov I, Hecht JR, et al. Clinical development of talimogene laherparepvec (T-VEC): a modified herpes simplex virus type-1–derived oncolytic immunotherapy. Expert Review of Anticancer Therapy. 2015;15:1389–1403. doi: 10.1586/14737140.2015.1115725. [DOI] [PubMed] [Google Scholar]
- 48.Andtbacka RHI, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, Delman KA, Spitler LE, Puzanov I, Agarwala SS, et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. Journal of Clinical Oncology. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 49.Lebel M-È, Chartrand K, Tarrab E, Savard P, Leclerc D, Lamarre A. Potentiating Cancer Immunotherapy Using Papaya Mosaic Virus-Derived Nanoparticles. Nano Lett. 2016;16:1826–1832. doi: 10.1021/acs.nanolett.5b04877. [DOI] [PubMed] [Google Scholar]
- 50.Bludau H, Czapar AE, Pitek AS, Shukla S, Jordan R, Steinmetz NF. POxylation as an alternative stealth coating for biomedical applications. European Polymer Journal. 2016 doi: 10.1016/j.eurpolymj.2016.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pitek AS, Jameson SA, Veliz FA, Shukla S, Steinmetz NF. Serum albumin “camouflage” of plant virus based nanoparticles prevents their antibody recognition and enhances pharmacokinetics. Biomaterials. 2016;89:89–97. doi: 10.1016/j.biomaterials.2016.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McNeil SE. Challenges for Nanoparticle Characterization. In: McNeil SE, editor. Characterization of Nanoparticles Intended for Drug Delivery. Humana Press; 2010. pp. 9–15. [Google Scholar]


