Abstract
The collectins family encompasses several collagenous Ca2+-dependent defense lectins that are described as pathogen recognition molecules. They play an important role in both adaptive and innate immunity. Surfactant protein A and D are two of these proteins which were initially discovered in association with surfactant in the pulmonary system. The structure, immune and inflammatory functions, and genetic variations have been well described in relation to their roles, function and pathophysiology in the pulmonary system. Subsequently, these proteins have been discovered in a wide range of other organs and organ systems. The role of these proteins outside the pulmonary system is currently an active area of research. This review intends to provide a current overview of the genetics, structure and extra-pulmonary functions of the surfactant collectin proteins.
Keywords: Surfactant Protein A, Surfactant Protein D, Collectins, Extra-pulmonary, Complement pathway
2. Introduction
The immunomodulatory system is comprised of many complex cytokines, scavengers, signaling molecules and intermediary complex families of molecules. One such family of innate immunity proteins is the carbohydrate binding lectins also known as c-type lectins (Hoppe and Reid, 1994). These proteins recognize a host of sugar moieties, which constitute the cell walls of various pathogens, thus also giving them the name of Pathogen Recognition Receptor Proteins (Mayer et al., 2017) or PRRP's. Collectins are a sub-type of c-type lectins which are characterized by a collagen-like domain with a short Cysteine-rich N-terminus (Drickamer et al., 1986). The structures of the majority of these proteins are similar, with a varying number of monomers binding to form multimeric structures. Each monomer is made up of four regions; a cysteine-rich domain at the N-terminus, a collagen-like domain, a coiled neck domain and a C-type lectin domain that is also called a carbohydrate recognition domain (CRD). Recognition of pathogens is mediated by the CRD in the presence of calcium (Petersen et al., 2001; Weis et al., 1992). To date, a host of collectins have been described including MBL, SP-A, SP-D, collectin liver 1 (CL-L1), collectin kidney 1 (CL-K1) and conglutinins (Mayer et al., 2017). These collectins recognize and neutralize pathogens by several different mechanisms including aggregation (Tenner et al., 1989), apoptosis (Liu et al., 2015), opsonization (Ferguson et al., 1999; Ghildyal et al., 1999; Hartshorn et al., 1996; Hickling et al., 1999; McIntosh et al., 1996; McNeely and Coonrod, 1994), activation of phagocytosis (Beharka et al., 2002; Ferguson et al., 1999; Nepomuceno et al., 1997; Tenner et al., 1989), inhibition of microbial growth, or modulation of the inflammatory response (Herbein and Wright, 2001; Liu et al., 2015; Saka et al., 2016; Salminen et al., 2008; Sato et al., 2003). Collectins may compete with factors such as LPS for binding to cell surface receptors (Chuang et al., 2011; Ohya et al., 2006; Saka et al., 2016; Vayrynen et al., 2002; Yamada et al., 2004). Some collectin-receptor interactions result in increased transcription of cytokines (Gardai et al., 2003) while others modulate the adaptive immune system by activation of T-lymphocytes (Gowdy et al., 2012; Pawaria and Binder, 2011), the modulation of antigen presentation by dendritic cells (Awasthi et al.; Steinberger et al., 2002; Yamada et al., 2004), or by mediation of IgE (Madan et al.), or histamine (Herias et al., 2007; Nayak et al., 2012) dependent allergic responses.
Prior to the recognition of surfactant proteins as an independent entity, pulmonary mechanisms were defined in the context of the integrity of the alveolar surface and the substances which contributed to its function. It was in 1929 that Von Neergard, while describing the mechanics of the pulmonary system, suggested that a liquid film present on the alveolar surface might be important for the lowering of surface tension thereby modulating pulmonary mechanics (Von Neergaard, 1929). It was not until 1954, that Macklin elegantly described this “mucoid substance” (Macklin, 1954). He explained that “the mucoid film has been credited with performing vital functions such as assisting in the removal of fine living and dead particulate matter, the maintenance of a constant favorable surface tension, the facilitation of gaseous exchange, the protection of the underlying tissue from desiccation and the suppression of invading microorganisms”. This film, now known as surfactant, is produced by alveolar type II cells in the walls of the terminal respiratory alveolar structure in mature lungs. It is responsible for decreasing surface tension of the alveolar lining allowing the alveolus to remain distended to allow gas exchange at the alveolar epithelial surface. Surfactant is comprised of 80% lipids and 20% proteins, of which there are four surfactant proteins, namely, SP-A, SP-B, SP-C and SP-D (Hawgood and Clements, 1990). Alveolar type II cells, in the alveoli, produce and secrete all four of these surfactant proteins within lamellar bodies (Clements, 1957).
Subsequently, SP-A and SP-D were also found in larger airways, secreted by sub-mucosal and Clara cells (Horowitz et al., 1991). Here, adsorption of the proteins is not necessary for airway function (Wong et al., 1996). SP-B and SP-C are involved in helping the spreading of surfactant across the alveolar lining and are also involved in surfactant metabolism and recycling. SP-A and SP-D, on the other hand are collectins with important roles in the maintenance of the pulmonary immune system and help in the first line of defense against various pathogens in the respiratory tract. This was first suggested by earlier data from Van Iwardeen and colleagues who showed that SP-A was important for rat macrophage phagocytic activity (van Iwaarden et al., 1990). Since then, alterations in SP-A and SP-D structure and function have been implicated in a wide variety of pulmonary diseases. These include pneumonia (Awasthi et al., 2001; Baughman et al., 1993), acute respiratory distress syndrome (ARDS) (Lin et al., 2000), cystic fibrosis (Korfhagen, 2001), and pulmonary interstitial fibrosis (Kuroki et al., 1993; McCormack et al., 1991). In the premature neonate, these proteins are important after birth where there may be protein variants raising the susceptibility of pulmonary dysfunction resulting in acute respiratory distress syndrome (RDS) (Gerdes et al., 1990; Marttila et al., 2003a; Smith et al., 2000). In addition, they are important in the modulation of chronic lung disease (CLD) and/or bronchopulmonary dysplasia (BPD) (Hallman and Haataja, 2006; Pavlovic et al., 2006). Interestingly, over the last three decades, these proteins have become increasingly apparent in other organ systems besides the respiratory system. The focus of this review is to provide a comprehensive description of the structure, receptors, immunomodulatory roles and genetics of surfactant proteins A and D followed by a review of the known extra-pulmonary localization and functions of these proteins.
3. Structure Of SP-A and SP-D
The collectin family of proteins shares homology in their basic monomeric structure, which are comprised of collagen-like regions attached to non-collagenous domains (Hoppe and Reid, 1994). While other immune modulators may also recognize and neutralize pathogensthe term “collectin” refers exclusively to proteins of the extracellular matrix. For example, serum complement protein C1 q, important in the function of binding to immune complexes and activation of complement and the interaction with cell surface receptors, is an example of a protein with a collagen-like region that is not present in the extracellular matrix (Brodsky-Doyle et al., 1976).
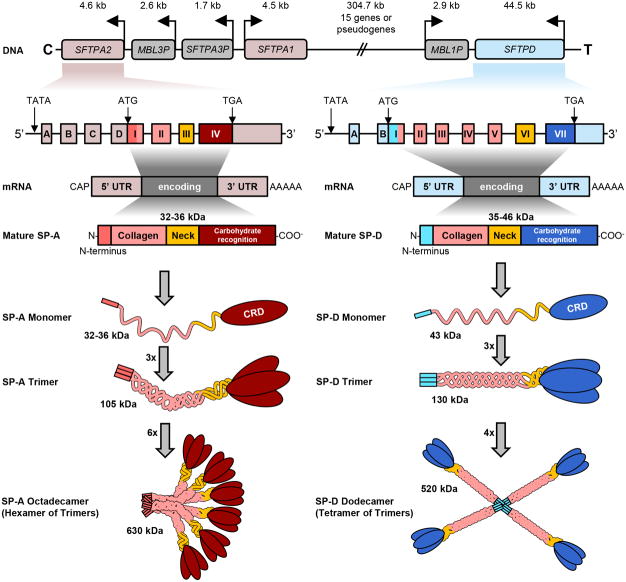
Each polypeptide chain has a C-terminal C-type lectin (carbohydrate binding) domain, a collagen body and an N-terminal domain, as depicted in Figure 1. These are reviewed in more detail below:
Figure 1.
Schematic of gene map, transcription and translational products and final protein structure of SP-A and SP-D. The genes for both SP-A and D are on chromosome 10. The mRNA of both are flanked by 5′ untranslated regions (UTR) and 3′ UTR containing a poly A tail. The UTR's are depicted in light pink for Sftpa and blue for Sftpd. There are four exons in the 5′UTR of SP-A (A-D), whereas the 5′UTR of SP-D has only two (A, B). The four protein coding domains are named I – IV. The neck (III) and collagen (II) domains show high sequence similarities and are shown in identical colors in the translated proteins. The carbohydrate recognitions domain (CRD, IV) and the cysteine-rich N-terminuses (I) are SP-A and SP-D-specific and exhibit low overall similarities. The final arrangement of protein chains for both are depicted with the classical bouquet pattern for SP-A and a cross of arms for SP-D.
3.1 N-terminal domain
This is a short (seven amino acid) non-collagenous, cysteine rich region containing a signal peptide sequence. This domain is critical for oligomerization occurring by disulfide bridging (Haagsman et al., 1989). The inter-chain linkages are formed by cysteine residues (Hoppe and Reid, 1994).
3.2 Collagen Body and neck domain
The C-type lectin domain associates with the collagen body through a strong hydrophobic interaction via the α-helical bundle forming the neck region (Haagsman et al., 1989). The collagen-like region of Gly-X-Y repeats bind in a triple-helix, similar to a zipper-like structure.
3.3 Carbohydrate binding domain
The lectin domain or CRD, is what defines the function of all collectin proteins. Lectins are structures with the ability to bind to carbohydrates and lipids. They are further characterized as C-type and S-type. C-type lectins are extracellular and Ca2+-dependent, however S-type are intra- or extracellular and do not require divalent cations. The CRD is important in mediating immune response, since it recognizes carbohydrate epitope moieties of different size and shape from multiple microorganisms when polymerized in their tridimensional structure (Petersen et al., 2001). The oligomeric assembly facilitated by the N-terminal cross-linking of trimeric arms favors the high affinity interactions of CRD. This can cause agglutination of the pathogens enhancing killing and clearance of bacteria by phagocytic cells, which carry receptors for SP-A or SP-D (Reid, 1998).
For SP-A, the peptide subunits are composed of three, 35-kDa-polypeptide, monomer chains that are held together by disulfide bonds at the N-terminus. Together, the three monomer subunits form a trimer. The trimer has a molecular weight of 105 kDa which, when finally assembled (six trimers) into the mature SP-A protein, yields an octadecameric molecule of 630 kDa (Haagsman et al., 1989). As depicted in Figure 1, mature SP-A has 248 amino acids, of which seven are in the N-terminus, 73 in the collagen region, 34 in the neck region and 123 in the CRD (Floros et al., 2009). SP-D protein is oligomerized into a 520-kDa molecular weight protein, with each trimer having a molecular weight of 130 kDa (Holmskov et al., 1997). The gene transcription and translation to final protein is illustrated in Figure 1. Crouch and colleagues performed electron microscopic and correlative biochemical techniques in order to examine the quaternary structure of rat SP-D (Crouch et al., 1993). They demonstrated that SP-D is assembled as homopolymers of four identical trimeric subunits, and that interchain disulfide bonds stabilize interactions at the amino-terminal domains of the trimers. They also showed that SP-D molecules can associate to form complex multimolecular structures. These observations are imperative as they may explain the difficulty in denaturing the strong inter-sulfide bonds in order to detect the primary protein structure on native gel blots. Antibody bands at varying molecular weights are reported in the literature, spanning molecular weights of 35-46 kDa. Similar variations are found in SP-A immunoblotting in the literature.
By simple analysis of the basic structure of these proteins, the simplest polypeptide chain if reduced to it most native form would yield a band of 35 kDa for monomeric SP-A (Floros et al., 1986). A band of 66 kDa represents a partially reduced dimer (Awasthi et al., 2011), 105 kDa represents a trimer, while a 630-kDa band represents mature SP-A protein with six oligomers bound together (octadecamer) (Kishore et al., 2005; Kishore et al., 2006). Similarly, for SP-D one may anticipate 43 kDa (Kankavi et al., 2008), 130 kDa or 520 kDa molecular weight bands depending on the degree of denaturation achieved.
The process of oligomerization is what differentiates the final tridimensional appearance of SP-A and SP-D. SP-A is assembled by six trimers in a “bouquet” formation, in which the N-terminus bind to each other in parallel at the foot of the molecule. On the other hand, SP-D is formed by four trimers in a radial alignment to impart a cruciform organization (Voss et al., 1988). In animal models, it was demonstrated that the deletion of the collagen-like domain from SP-A impacted the association of the oligomers, thereby impairing liposomal aggregation, one of the primary functions of the SP-A (McCormack et al., 1997). However, modification of the N-terminus by selective disruption of Cys6 in SP-A (McCormack et al., 1999) and substitution of C15S and C20S in SP-D blocks the regular function of the protein (Zhang et al., 2001). In conjunction, these data demonstrate the critical role of the oligomeric and tridimensional structure of SPA and SP-D for proper function.
4. Genetics of SP-A and SP-D
The genes responsible for encoding the collectin proteins SP-A, SP-D, and MBL in humans are located on chromosome 10 (Kolble and Reid, 1993). More specifically, these genes are on the long arm of the chromosome, 10q as shown in Figure 1. In humans, two different but significantly homologous genes, SP-A1 and SP-A2, encode the SP-A protein. At the nucleotide level, they have a similarity of 94% similarity and on the amino acid level, 96%. Interestingly, in the same chromosome there is a pseudogene SP-A, it has significant similarity in intron VI. However, the upstream sequence is not detectable in the western-blot, likely due to a premature stop-codon (Korfhagen et al., 1991).
4.1 Significance of SP-A1 and SP-A2 and Allelic Variants
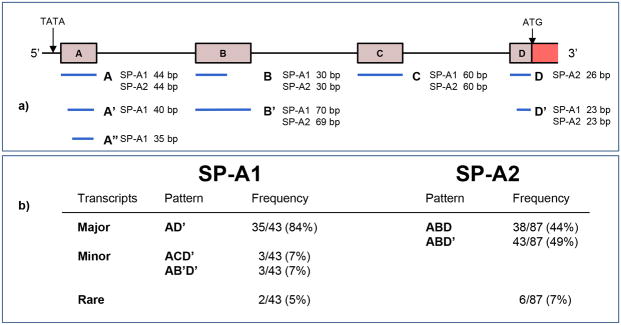
It is believed that the final protein product of SP-A is a result of the expression of two distinct but similar genes, SP-A1 and SP-A2. The complex requires two SP-A1 gene products and one SP-A2 product per trimer (Voss et al., 1991). Both genes have a 5′ untranslated region (UTR) containing exons A-D. The splice variants arising from changes in the 5′UTR are well described. The most common variant in the population is AD' which occurs in 81% of the population (Karinch and Floros, 1995). Other variants are depicted in Figure 2. The highly conserved TATAAA domain is similar in both genes. The first coding exon I contains part of Exon D of the 5′ UTR followed by exons II, II and IV. The 3′ UTR begins at the stop codon TGA within Exon IV. This is followed by a polyadenylation signal sequence. The highest divergence of the two genes are in the upstream region, intron I and exon III, (Katyal et al., 1992).
Figure 2.
Representation of the most common 5′UTR splice variants of SP-A (adapted from Karinch and Floros, 1995). Alternative splicing results in various combinations of exons in SP-A1 and SP-A2 transcripts resulting in varying sizes of the untranslated exons A, B, C and D. For SP-A1 and SP-A2, different combinations of exon variants represent the most common patterns found in human lung tissue.
The allelic variants arising from nucleotide variants are also well known for both genes (Karinch and Floros, 1995). The known SP-A1 allelic variants are 6A, 6A0, 6A1, 6A2, 6A3, 6A4. For SP-A2, the allelic variants are 1A, 1A0, 1A1, 1A2. The clinical significance of these observations is paramount as the frequency of allelic variation can be analyzed via haplotype analysis. Certain variants or haplotypes may either result in an increased risk or confer protection for a particular disease state. To date, all of these studies have been carried out in the pulmonary system. The greatest example of this is observed in neonates born prematurely who develop respiratory distress syndrome (RDS). The major combined haplotypes of SP-A1 and SP-A2 or the 6A2/1A0 haplotype has been shown to increase the risk of RDS (Haataja et al., 2000; Marttila et al., 2003a). On the other hand, a Finnish study of near term twin infants revealed that the same 6A2/1A0 haplotype protected from RDS (Marttila et al., 2003b). As some 50% of twins are born preterm, the 6A2/1A0 haplotype can be regarded as a neonatal survival factor for twins.
4.2 Genetics of SP-D
The SP-D gene is also located on the long arm of chromosome 10. The human SP-D DNA structure was first characterized after structural analysis of SP-D obtained from the amniotic fluid followed by identification of the complimentary DNA (cDNA) (Lu et al., 1992). Later, localization of SP-D gene was identified at 10q22.2-23.1, which has close proximity to SP-A (10q21-24) and MBP (10q11.2-q21) (Crouch et al., 1993). The proximity of the SP-A and SP-D genes has been demonstrated by gene sequencing in a variety of mammals.
4.3 SP-A/SP-D Haplotypes
Looking at bronchopulmonary dysplasia (BPD) in preterm infants, SP-A2 and SP-D genes and disease groups were analyzed by transmission disequilibrium testing (TDT) in babies at 28 days of life (BPD28D) (Pavlovic et al., 2006). The DA160 (G)SP-A2 (1A2) and the DA11 (C)DA160 (G)SP-A2 (1A2) haplotypes of SP-D/SP-A2 were not transmitted to the affected child. Therefore, the authors concluded that these haplotypes may act as protective factors in the BPD.
The risk of developing viral infections may also be modulated by variants in SP-A/D. Lofgren and Lahti et al (Lahti et al., 2002; Lofgren et al., 2002) performed elegant studies in which they studied the risk of RSV in infants in wintertime when disease rates are high. Controlling for confounders such as gestational age at birth, crowding and smoking in the family, they found that the Met11Thr genotype of SP-D was associated with protection from severe RSV, whereas the 11Met allele was associated with RSV bronchiolitis. The SP-A2 223Lys allele was overrepresented in infants with severe RSV infection, whereas 91Pro was underrepresented in severe RSV. Similar haplotype analyses for other extra-pulmonary disease states are currently ongoing. The constraining factor will be that the sequences of these collectin proteins may vary from organ system to another. Therefore, it is imperative that sequence analysis be performed for all collectins identified outside the pulmonary system.
5. Homology of Collectin Biology Across Species
From an evolutionary standpoint, several of the collectin genes have duplicated and clustered, while others have involuted (Seyfarth et al., 2005). Figure 3 depicts the genetic loci for the prominent collectin genes across the species in which they have been most studied. In humans, the genes for SFTPA1, SFTPA2, SFTPD, MBL1 and MBL2 are all in close proximity to one another on chromosome 10 (Kolble and Reid, 1993). In contrast, the murine SFTPA, the SFTPD and Mbl1 genes are found on chromosome 14 while Mbl2 is on chromosome 19 (White et al., 1994). Akiyama et al. characterized the murine genes showing that all three are located within a 55-kb region on chromosome 14 (Motwani et al., 1995). However, murine SP-A and Mbl1 are in closer proximity to each other as opposed to in humans, where SFTPA2, MBL3 and SFTPA1 are in order upstream of SFTPD, which is then followed by MBL1.
Figure 3.
Comparative gene environment of SP-A and SP-D in Homo sapiens (humans), Papio anubis (baboons) and Mus musculus (mice). In all three cases, a gene encoding Mannose-binding lectin (grey) is positioned between Sftpa1 (red) and Sftpd (blue). The genes are depicted as arrows representing the 5′-3′ direction of the coding strand. Lengths of arrows and gaps are to scale. On the human chromosome 10, Sftpa1 and Sftpd are separated by a 304 kbp gap containing 15 genes or pseudogenes (double diagonal lines). Two SP-A encoding genes were reported for P. Anubis (Gao et al., 1996; Li et al., 1998). However, only the one published in the NCBI database (Sftpa1) is shown in this figure.
SP-A protein is encoded by a single gene copy in rabbits (Boggaram and Mendelson, 1988), rats (Fisher et al., 1988), mice (Korfhagen et al., 1992) and dogs (Benson et al., 1985). It has been reported that baboons, similar to humans, carry two slightly different genes for SP-A on chromosome 9 (Gao et al., 1996; Li et al., 1998). The genes of baboons and humans share a high similarity on the amino acid level (92%) when compared to the rabbit (74%). However, the NCBI database contains only one gene sequence for Papio anubis for SFTPA1 [GeneID: 101023773 as of March 7, 2017].
The genomic sequence of murine SP-A also has significant homology to its human counterpart. The exon sequences have approximately 75% concordance between the two species. The intron regions tend to be shorter in the murine sequence, with similarity as high as 70% in some of the same introns. Interestingly, other intron sequencesshow no homology. Regarding the murine SP-A protein, the homology with human SP-A is noticeable in the organization of structural domains and sites of post-translational modification including the crucial 23 Gly-X-Y repeats and 4 cysteine residues of the carboxyl-terminal lectin domain. Exon 3, 4 and 5 encode the collagen-like domain and the lectin domains are encoded entirely in exon 6 in both species (Korfhagen et al., 1992).
Murine and human SFTPD genes also share significant homology, both having the translation initiation codon in exon 2 (Akiyama et al., 1999). The mature SP-D protein of the mouse has 76% concordance to that of humans (Lu et al., 1992). The alignment of the amino acid sequence of mouse, rat, human and bovine SP-D demonstrates that the seven repeats of Gly-X-Y from the collagen domain is the most conserved region across the species (Motwani et al., 1995).
6. Two Newly Identified Surfactant Proteins SP-G and SP-H
Recently, two novel surfactant proteins have been identified in lung tissue. This discovery highlights that our previous understanding of the anatomical constituents of the pulmonary system is incomplete and that the mechanisms of its constituents have not yet been fully elucidated. Surfactant protein G (SP-G) or surfactant associated protein 2 (SFTA2) is encoded in chromosome 6, with a final product of 78 amino acids and a molecular weight of ∼8 kDa (Rausch et al., 2012). Surfactant protein H (SP-H) or surfactant associated protein 3 (SFTA3) is encoded on chromosome 14. The primary translation product of SP-H consists of 94 amino acids with a molecular weight of ∼10 kDa (Schicht et al., 2014).
Neither of the new proteins has significant structural similarity to the previously described four surfactant proteins, however, they both exhibit physicochemical similarities to SP-B and SP-C, including the surface-regulatory properties necessary for adsorption of the lung interface. SP-H may also have immunomodulatory effects, since it is downregulated by cytokines such as IL-1β and IL-23 and is upregulated by LPS via TLR-2 and 4. Similar to SP-A and SP-D, both SP-G and SP-H have been identified in other tissues besides the lung. Rausch et al. found mRNA for SP-G to be present in kidneys, heart, testis, umbilical cord and trophoblast, whereas Schicht et al. amplified SP-H mRNA in eyelid, heart, kidney, testis, multiple areas of the gastrointestinal tract and umbilical cord (Rausch et al., 2012; Schicht et al., 2014).
The discovery of these new surfactant proteins will also have implications for cross reactivity with receptors and downstream effects. The potential for up-regulation of any lectin in order to compensate for inadequate or abnormal functioning of another surfactant protein is an intriguing concept which merits further rigorous study. This is crucial especially as novel therapeutic pathways may be identified for one or more of these targets.
7. Immunological Roles of SP-A and SP-D
SP-A and SP-D have both been shown to play an important role in innate and adaptive immunity. Classically, they have been known to contribute to the primary defense mechanisms of both human and animal pulmonary systems, which are constantly exposed to foreign organisms and noxious stimuli. Over the last three decades, more information has come to light regarding the non-pulmonary organ systems in which these proteins are also found. The mechanisms by which they function outside the lung is an area of active study, both from a pathological and translational clinical standpoint. From the newborn period to adulthood, they are essential in the protection against pathogenic infecting organisms, the regulation of allergic responses, as well as modulation and resolution of inflammation throughout the human body. The ability to modulate these proteins has the potential to provide a new range of therapeutic options in this era of high antimicrobial resistance.
It was mentioned above that the CRD's of both SP-A and D bind to carbohydrate moieties. While the CRD's of all collectins have a high affinity for mannose, SP-A has preference for N-acetylmannosamine and L-fucose, while SP-D binds to inositol, maltose and glucose (Haagsman et al., 1987; Persson et al., 1990). In most vertebrate animals, clusters of oligosaccharides are usually made up of galactose and sialic acid, which are poorly recognized by collectins. This may be significant in distinguishing self from non-self (Wright, 2004).
As we strive to understand the multitude of physiological and pathological roles these collectins may play, it is helpful to breakdown each of the pathways in which they are involved and discuss each one individually. This is by no means intended to be a comprehensive discussion of each functional pathway, but rather an overview of the multitude of complex signaling pathways, which may be affected by the collectin proteins.
7.1 Activation of Complement Pathway
Besides SP-A and SP-D, another member of the collectin family is MBL, which has a high degree of similarity to the complement protein, C1q. Even though, they can be classified structurally as collectins, SP-A and SP-D are distinct from C1q due to differences in their non-collagenous regions (Voss et al., 1988). However, there is still a great similarity in the collagen-like domain and immunological functions of these proteins. For example, SP-A and SP-D mimic ligands for the “Complement Cascade Pathway” which ultimately leads to recruitment of inflammatory cells, activation of phagocytes and opsonization or the direct killing of pathogens (Tenner et al., 1989).
7.2 Opsonization
Opsonization is the process by which foreign invading organisms are targeted for recognition by immune cells such as phagocytes or macrophages. One example of opsonization of pathogens mediated by SP's is via LPS. This is a Gram-negative outer membrane toxin known to elicit a major inflammatory reaction once bound to either SP-A or SP-D (McIntosh et al., 1996). Similarly, the P2 outer membrane protein (OMP P2) of Haemophilus influenzae is known to bind to SP-A (McNeely and Coonrod, 1994), while lipoarabino-mannan (LAM) of Mycobacterium tuberculosis binds to SP-D (Ferguson et al., 1999).
Regarding opsonization of viruses, SP-A binds with respiratory syncytial virus (RSV) through the glycoprotein (GP) in a calcium-dependent manner (Ghildyal et al., 1999). SP-D on the other hand, interacts via the respiratory G-protein (Hickling et al., 1999). This pattern of binding to different structures in the same organisms also applies to other viruses. SP-D binds to influenza virus most likely via the neuraminidase envelope glycoprotein (Hartshorn et al., 1996), whereas the SP-A binds through the N-linked oligosaccharide (Hartshorn et al., 1997).
7.3 Aggregation
SP-A and D can target pathogens by simple aggregation, without a direct interaction with the cell. Similar to complement C1q, SPA can function as an “activation-ligand” which facilitates particle uptake once it is coated by Immunoglobulin G (Tenner et al., 1989).
7.4 Ligand Receptor Association
SP-A and SP-D have both been shown to up regulate the expression of cell-surface receptors, which are responsible for pathogen recognition and phagocytosis. Mannose receptors in the alveolar macrophage are important for phagocytosis of extracellular pathogens in the airway. Beharka et al showed an increased expression of mannose receptors in wild type mice when compared to SP-A knockout mice (Beharka et al., 2002). Similarly, Kudo and colleagues also demonstrated the up-regulation of the same receptor in alveolar macrophages treated with SP-A and SP-D (Kudo et al., 2004). However, the same mannose receptors were shown to facilitate contamination by facultative intracellular pathogens as Mycobacterium tuberculosis and Mycobacterium avium in both studies. In addition, both SP-A and D are known to bind to the family of Toll Like Receptors (TLR's) that are trans-membrane receptors on immune cells such as macrophages and dendritic cells. They are known to recognize microbial molecules but SP-A and D have been shown to have affinity for TLR-2 and 4. This is discussed in more detail below.
7.5 Direct Killing
One very interesting function of surfactant proteins is the direct killing of pathogens. Complement protein C1q, a collectin protein, is a key component of the classical pathway of C1 complex. Ultimately, this cascade can lead to formation of the membrane-attack complex, which forms pores in the lipid bilayer membrane, responsible for disruption of the proton gradient, thereby killing the pathogen (Endo et al., 1998). Wu et al showed that SP-A and SP-D could increase cell membrane permeability of the Escherichia coli K12 strain independently of macrophage activity or pathogen aggregation (Wu et al., 2003). However, Kuzmenko and colleagues demonstrated that oxidative stress inhibits the antibacterial activity of SP-A by a mechanism that includes oxidative modification and functional inactivation of the protein (Kuzmenko et al., 2005). This is a primary concern in lung pathologies that require the delivery of high fractions of supplemental oxygen than the room air oxygen tension of the regular environment.
8. Interaction of Molecules with Collectins- Ligands and Receptors
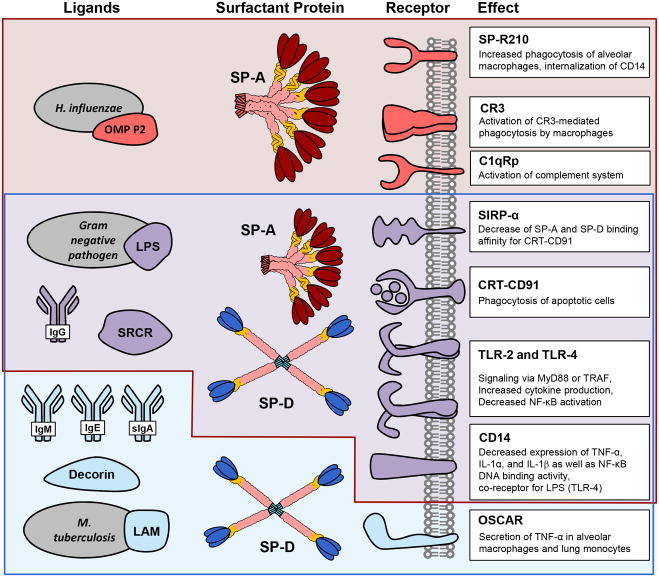
The age-old question still remains: are SP-A and SP-D pro- or anti-inflammatory molecules? The simple answer is that they are both. However, the true nature of their activity is far more complex. These collectins may be activated by upstream ligands, while they themselves are known to bind to a multitude of receptors leading to an activation of cell immunity signaling pathways. Rather, it is important to understand that inflammation is a dynamic and important process in the defense against invading microbes, but, that these very same reactions may overwhelm the host, leading to catastrophic cytokine release or induction of systemic inflammation that overwhelms the host. Therefore, it is important to categorize the various ligands that may bind to or activate SP-A and SP-D and then characterize the downstream receptors to which these collectins can also bind. The various ligands and receptors of SP-A and SP-D are comprehensively illustrated in Figure 4.
Figure 4.
Ligands and receptors of SP-A and SP-D. Common ligands and Receptors for SP-A are in red, for SP-D in blue and the receptors which may bind to both are in purple. The function of the ligand-receptor activation is also noted for each receptor.
8.1 Ligands or Protein Acceptors
As immune response is the primary function of collectins, specific ligands are needed to for SP-A and SP-D to fulfill their downstream effects. Specific ligand classes are reviewed in the following sections.
a) Pathogenic Organisms
LPS is a Gram-negative outer membrane toxin that is known to elicit major inflammatory reactions once bound to either SP-A or SP-D (McIntosh et al., 1996). Kuan et al demonstrated that bacterial LPS is a major ligand for SP-D on Escherichia coli. They showed that LPS from rough strain mutants, but not by smooth which suggested that core terminal glucose and/or heptose residues in SP-D bind to LPS. The ability of SP-D to interact with species of LPSs that are deficient in terminal O-polysaccharide is important because the majority of clinical Gram-negative isolates and isolates of Gram-negative bacteria from the gut of healthy humans express O-polysaccharides and demonstrate a “smooth” phenotype. Enteric bacteria that lack O-specific polysaccharides are usually nonpathogenic and rapidly cleared. However, many non-enteric organisms do not express terminal O-polysaccharide, such as the organisms that colonize the upper aerodigestive tract. Of note, isolates of Pseudomonas aeruginosa from patients with chronic lung infections in the setting of cystic fibrosis are typically deficient in O-polysaccharides (Hancock et al., 1983). Curiously, there is a report in the literature that Pseudomonas aeruginosa can develop resistance to the binding capacity of surfactant proteins by glycosylation of the type IV pilus in the lung. This could explain why this is a common pathogen in patients with chronic lung diseases such as cystic fibrosis (Tan et al., 2015).
The P2 outer membrane protein (OMP P2) of Haemophilus influenzae was initially found to bind to SP-A (McNeely and Coonrod, 1994). Similarly, lipoarabino-mannan (LAM) of the Mycobacterium tuberculosis binds to SP-D (Ferguson et al., 1999).
b) Immunoglobulins
Most classes of immunoglobulins have been shown to bind or aggregate with SP-A and/or SP-D. Immunoglobulins have two functional regions, the fragment of antigen recognition (Fab) and fragment crystallizable region (Fc). The first region, as the name implies, recognizes antigens such as bacteria and viruses. The second is recognized by immune cells such as collectins and complement (Tenner et al., 1989). This concept implies that the surfactant proteins not only participate in the innate immune system, but also in the adaptive immune system. It has been demonstrated that SP-D interacts with IgG, IgE, IgM and secretory IgA, but not serum IgA. This interaction occurs via either the Fab or Fc regions (Nadesalingam et al., 2005). SP-A, on the other hand, has been demonstrated only to bind with IgG and that, too, only via the Fc region (Lin and Wright, 2006; Wofford and Wright, 2007).
c) Scavenger Receptor Cysteine Rich
Another class of ligands known to interact with both SP-A and SP-D is the innate immunity, scavenger receptor cysteine rich (SRCR) domain. Glycoprotein-340 (GP-340), Salivary Agglutinin (SAG) and Deleted in Malignant Brain Tumor 1 (DMBT1) are the same protein with different names, as they were all initially identified in various organ systems, but later found to have sequence homology. In particular, there appears to be an abundance of this protein in salivary glands and in the oral mucosa (Ligtenberg et al., 2001). It has been shown to interact with a broad range of pathogens, such as streptococci and Helicobacter pylori, influenza viruses and HIV, but also with mucosal defense proteins, such as IgA and surfactant proteins. Stimulation of alveolar macrophage migration, suppression of neutrophil oxidative burst and activation of the complement cascade are also implicated as important immune functions of this protein (Ligtenberg et al., 2007). GP-340 has been shown to be present in the soluble form as well as in alveolar macrophages; however it was only demonstrated to bind to the surfactant proteins in the soluble form. Soluble SCRC has been found to be a regulator of the lectin pathway of complement. The authors, however, believed that there is a dual physiological role; i.e. it acts as an activator of complement when it is bound to a surface, but, when free in the fluid phase, it acts as an inhibitor of the lectin pathway of complement. The lectin pathway inhibition is likely due to formation of similar soluble complexes with MBL (Reichhardt et al., 2012).
d) Defensins
Several ligands described in the literature appear to have only affinity to SP-D. Defensins from neutrophils were demonstrated to have high affinity for SP-D, which decreases the infectivity of enveloped viruses (Hartshorn et al., 1997; Hartshorn et al., 2006). Decorin, a proteoglycan present in the amniotic fluid and in the extracellular matrix, can also interact with SP-D (Nadesalingam et al., 2003). This protein is a component of connective tissue, binds to type I collagen fibrils, and plays a role in matrix assembly. Since SP-D has a collagen domain, it was found to interact with decorin in the same manner as collagens fibrils (Nadesalingam et al., 2003; Salinas and Anseth, 2009).
8.2 Protein Receptors
Cell-surface receptors or trans-membrane proteins are necessary to enable the broad downstream immune function of SP-A and SP-D. These are described below.
a) Calreticulin
The structural similarity between the collagenous-like region of C1q and collectins makes the possibility, that they share a common receptor, an attractive one. The first common receptor described for C1q, MBL, conglutinin and SP-A was calreticulin (CRT), which binds to these extracellular proteins via the collagen domain (Eggleton et al., 1994). Curiously, CRT is an intracellular protein present in the endoplasmic reticulum. It is bound to trans membrane protein CD91, an important receptor for heat shock proteins (HSP's) (Pawaria and Binder, 2011). HSP's stabilize newly transcribed proteins by ensuring correct folding or by helping to refold proteins that have been damaged by cell stress (Balchin et al., 2016). This is when collectin proteins are likely to have highest expression in tissues. Specifically, to SP-A and SP-D, Vandivier et al demonstrated that the CRT-CD91 complex are one of the mechanisms used by surfactant proteins to drive phagocytosis of apoptotic cells (Vandivier et al., 2006).
b) C1q receptor which modulates phagocytosis (C1qRp)
This receptor is expressed on cells of myeloid lineage and is also known as CD93. It was initially described as an important receptor for C1q, MBL and SP-A since monoclonal antibodies against collectin proteins decrease the phagocytosis (Nepomuceno et al., 1997). However, later it was shown that collectins have no enhanced binding to CD93 and CD93 knockout mice show conflicting results of phagocytosis in vitro versus in vivo (McGreal et al., 2002; Norsworthy et al., 2004).
C1qRp was shown to mediate enhancement of phagocytosis in monocytes and was suggested to be a receptor of C1q. Steinberger and colleagues showed that cells expressing CD93 had enhanced capacity to bind C1q (Steinberger et al., 2002).
c) CD14
Another important receptor of SP-A and SP-D is CD14, present on macrophages and tissue monocytes. The Cluster Differentiation 14 receptor acts as a co-receptor with TLR-4 for LPS from Gram-negative bacteria leading to a significant inflammatory response. This interaction is dependent in the presence of lipopolysaccharide binding protein (LBP) (Remer et al., 2003). Both SP-A and SP-D modulate LPS/CD14 interaction. The SP-A neck domain recognizes a peptide component of CD14 whereas the SP-D lectin domain recognizes a carbohydrate moiety of CD14. This disparity is evidence of a divergence in modulation of LPS/CD14 interaction. SP-D has been shown to decrease CD14 binding to both smooth and rough LPS. SP-A, on the other hand, enhances CD14 binding to rough LPS and inhibits binding to smooth (Sano et al., 2000). When SP-A and D interact as ligands with CD14, the result is decreased expression of TNF-α, IL-1α, and IL-1β as well as NF-κB DNA binding activity, proving that surfactant collectins have important modulatory effects during Gram-negative infection (Alcorn and Wright, 2004; Senft et al., 2005).
d) SIRPα
Another demonstration of the anti-inflammatory effect of surfactant collectins is the ability to interact with Signal-Inhibitory Regulatory Protein alpha (SIRPα). Interestingly, surfactant collectins can alter their engagement depending on the presence of pathogens or debris in the extracellular content. SP-A and D can bind to SIRPα via the lectin domain, thereby decreasing bind to CRT/CD91. On the other hand, in the presence of a pathogen, both proteins can bind to those through the lectin domain and the collagen domain activates immune cells through CRT/CD91 (Gardai et al., 2003).
e) Toll-like receptors
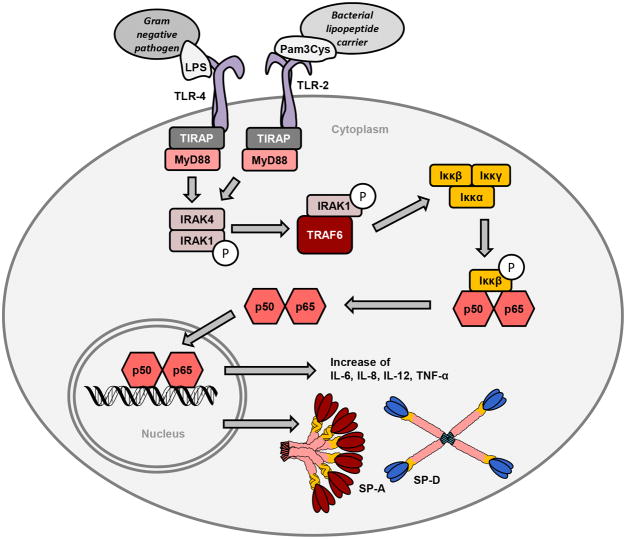
TLR's are important receptors in the innate immune system. They are expressed on immune cells such as macrophages and dendritic cells which recognize microbial substrates (Kaisho and Akira, 2000). SP-A and SP-D are ligands for both TLR-2 and TLR-4. Both receptors are present on the cell surface and modulate intracellular signaling either via MyD88 or TRAF adaptors, ultimately increasing protein transcription of cytokines (Akira et al., 2000; Chuang et al., 2011; Wu et al., 2015). The TLR signaling pathway, in association with ligands and expression of cytokines, is depicted in Figure 5. Other common TLR-2 ligands are bacterial peptidoglycan from gram-positive organisms and LPS for TLR-4 (Chuang et al., 2011; Ohya et al., 2006). Sato and his colleagues showed that SP-A attenuates cytokine production by TLR-2 due to directly binding to the receptor when exposed to pro-inflammatory substances (Sato et al., 2003). A direct interaction of SP-A with TLR-2 has also been shown to alter peptidoglycan-induced cell signaling, thereby modulating inflammatory responses to bacterial components. Yamada et al also demonstrated a similar effect of SP-A on the TLR4 and MD-2 receptors decreasing NF-κB activation (Yamada et al., 2006). SP-D has also been shown to have a similar function as a ligand for TLR-2 and TLR-4 as was previously demonstrated for SP-A. Ohya and colleagues demonstrated that natural and recombinant SP-D's exhibit specific binding to the extracellular domains of the soluble forms of recombinant TLR-2 and TLR-4. Binding was both concentration and Ca2+-dependent via CRD (Ohya et al., 2006).
Figure 5.
TLR-2 and TLR-4 activation by peptide ligands from microbial agents leads to activation of the myeloid differentiation primary response gene 88 (MyD88). This must occur in the presence of the Toll-Interleukin 1 Receptor (TIR) Domain Containing Adaptor Protein, or TIRAP, which is necessary to recruit MyD88 to the TLR's. MyD88 then signals through the Interleukin receptor-associated kinase (IRAK) family leading to phosphorylation of the NFκB. After multiple steps, NFκB then enters the nucleus, resulting in transcription of a variety of cytokines as well as SP-A and SP-D.
Interestingly, SP's themselves, can be regulated by other TLR ligands. LPS from Gram-negative bacteria has been show to induce expression of both SP-A and SP-D (Herbein and Wright, 2001; Saka et al., 2016; Vayrynen et al., 2002) Similarly, our research group has shown that human retinal Müller cells, when exposed to the TLR-2 ligand (PamCy3) and TLR-4 ligand (LPS), showed an up regulation of SP-A protein expression (Bhatti et al., 2015).
f) CR3 or CD11 b
Also known as Macrophage-1 antigen (or integrin αMβ2 or macrophage integrin or Mac-1) CR3 is a complement receptor, consisting of CD11b and CD18. These receptors are important for macrophage phagocytosis. Gil and colleagues demonstrated that SP-A modulates cell expression of CR3. SP-A knockout mice had reduced levels of CR3, however, after endotracheal administration of SP-A, translocation of CR3 to the cell surface was observed (Gil et al., 2009). This is important, because SP-A augmented CR3-mediated phagocytosis in a manner that was attenuated by N-glycanase or collagenase treatment of SP-A, implicating the N-linked sugar and collagen-like domains in phagocytosis. The binding of CR3 to SP-A was calcium dependent. N-linked sugars were more critical than the collagen-like domain on the extent of oligomeric assembly. Therefore, the authors concluded that SP-A modulates the cell surface expression of CR3 on alveolar macrophages, binds to CR3, and enhances CR3-mediated phagocytosis. This has implications for various bacterial infections. Inhalational pneumonic tularemia, caused by Francisella tularensis, is lethal in humans. It has been demonstrated that human macrophages phagocytose more F. tularensis than monocytes and that this phagocytosis was dependent on CR3, Fc gamma receptors, the mannose receptor, and SP-A present in lung alveoli (Balagopal et al., 2006).
g) SP-R210
Macrophages have two receptors that have been shown to interact only with SP-A. SPR-210 which is found on type II alveolar cells, seems to play an active role in Mycobacterium bovis uptake (Weikert et al., 1997). SP-R210 is another SP-A receptor, with two different isoforms, SP-R210L and SP-R210S that result from alternative splicing of the gene. SP-R210 has been shown to increase phagocytosis (Sever-Chroneos et al., 2011). Using WT alveolar macrophages, it was demonstrated that active functioning macrophages are represented by expression of SP-R210 (L). Furthermore, this receptor was important in decreasing susceptibility to the bacteria, Staphylococcus aureus. The lungs of susceptible mice generated abnormal inflammatory responses that were associated with impaired killing and persistence of S. aureus infection in the lung. This SP-A receptor also regulates internalization of CD14 via distinct macro pinocytosis-like mechanisms, thereby enhancing the response to LPS (Yang et al., 2015).
h) OSCAR
One of the more recently described receptors, is an osteoclast-associated receptor (OSCAR) which binds to the collagenous domain of SP-D (Barrow et al., 2015). OSCAR receptors are present in the alveolar macrophage, interstitial lung and blood CCR2+ inflammatory monocyte. These cells secrete TNF-α when exposed to SP-D in an OSCAR-dependent fashion.
9 Environmental Exposure and Epigenetics
Over the last several decades, the role of epigenetics in mediating genetic and physiological responses to a variety of environmental insults has come to the forefront of medicine. Epigenetics refers to the alteration of gene expression without a change to the DNA sequence itself (Holliday, 1989, 1994; Laird and Jaenisch, 1996). The primary mechanisms include structural changes in chromatin, resulting in DNA methylation of promoter regions of CG dinucleotide repeats (inhibition of translation), called CpG sites. Another mechanism is the acetylation of histone proteins that participate in the enveloping of DNA. Lastly, post-transcriptional gene regulation represented by microRNA-mediated repression of gene expression and chromatin-mediated regulation of alternative splicing of proteins (Weinhold, 2006).
Surfactant proteins appear to be susceptible to environmental factors, which can modify their function. Mikerov et al demonstrated that oxidation, by in vivo and in vitro exposure to ozone, can reduce the ability of SP-A to enhance macrophage phagocytosis (Mikerov et al., 2008). Similarly, SP-D exposed to metabolites from alcohol and smoking, known as malondialdehyde-acetaldehyde-adducted proteins (MAA adducts), may result in a pro- inflammatory response via up regulation of TNF-α and IL-6 in pulmonary macrophages (Sapkota et al., 2016).
Lin et al. were able to identify DNA methylation of CpG sites for SP-A and D in patients with lung cancer by using universal bead arrays (Lin et al., 2007). Similarly, histone acetylation at regulatory regions of the SP-A gene promoters have been shown to affect SP-A expression in lung cells during development and also in conditions such as hypoxia and glucocorticoid exposure (Islam and Mendelson, 2006) (Islam and Mendelson, 2008), (Benlhabib and Mendelson, 2011). These seemingly different responses to environmental factors and epigenetic mechanisms, demonstrate that the surfactant collectins can have heterogeneous expression depending on the exposure suffered by a particular individual.
10 Expression of Surfactant Proteins in Bacteria
In 2013, Bräuer and colleagues made the first known determination of expression of all four SP's by bacteria (Brauer et al., 2013) in the bacterial strains Staphylococcus aureus and Pseudomonas aeruginosa. For all four SP's, genomic DNA was identified by PCR, mRNA transcripts identified by RT-PCR, protein expression was detected by western blotting and quantified by ELISA. For S. aureus, the concentration of each SP was significantly increased in case of anaerobic cultivation. SP levels in P. aeruginosa also rose after anaerobic cultivation, but not to a statistically significant degree. In order to study sub-cellular localization, they used immunofluorescent and immunogold labeling in electron microscopy. In both bacterial strains, antibody reactivity was present mainly on the surface of the microorganisms, but also in the cytosol to a lesser degree. The authors speculated that the ability of bacteria to produce SP's may have evolved in an adaptive capacity. This exciting discovery further adds to the complexity of the known immune modulating mechanisms and the resistance factors utilized by microbial agents to escape destruction.
11 Anti-Inflammatory Effects of Surfactant Collectins
In recent years, new data suggest the importance of SP's in regulating inflammation to reduce injury of tissues. Acute pancreatic injury is a common finding in sepsis and one of the organs to suffer during an exaggerated inflammatory response. Liu et. al using a mouse model of acute pancreatic injury demonstrated that wild type mice had decreased apoptosis and neutrophil infiltration when compared to SP-D knockout mice, demonstrating the protective effect of SP-D (Liu et al., 2015). Saka et al established the protective effect of SP-D in the devastating neonatal intestinal disease, necrotizing enterocolitis (NEC), by establishing that SP-D could attenuate TLR-4 over-expression in immature embryonal intestine cells (Saka et al., 2016). Furthermore, human purified SP-A, given orally to mice in an experimental model of neonatal NEC, reduced intestinal levels of pro-inflammatory cytokines and TLR-4 proteins ameliorating adverse outcomes associated with this often fatal clinical condition (Quintanilla et al., 2015). These results give further credence to the idea that modulation of these surfactant proteins has the potential to be used as targeted therapies in a variety of pathological conditions.
12 Review of The Localization and Known Functions of Extra-Pulmonary SP-A AND SP-D
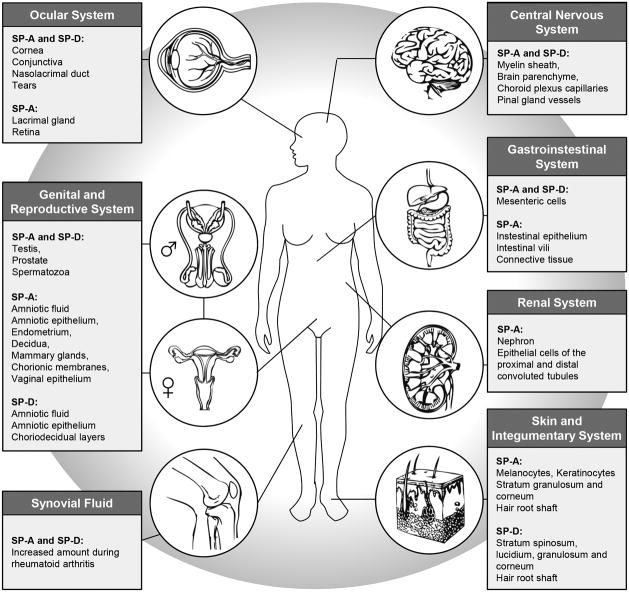
Now that we have discussed the underlying mechanisms and signaling pathways that collectin SP's are involved in, it will allow us to understand the wide array of organ and tissue distribution that these SP's have been reported in. The range of collectins is systematically depicted in Figure 6. Many of the roles of SP's in particular organ systems are still a subject of intense study. Nonetheless, the information known to date bears review.
Figure 6.
SP-A and SP-D in extra pulmonary tissues and cells as reported in the literature. The references regarding the role of these proteins are noted in the text.
12.1 Central Nervous System
A study in 2004 systemically mapped SP-A protein in various organs of the rat, by immunohistochemistry. SP-A was detected in the myelin sheath of the rat brain using a polyclonal antibody (Luo et al., 2004). In 2013, Schob and colleagues conducted an extensive study on post mortem human brain tissue in which they gave a very comprehensive analysis of the presence of SP-A, B, C and D using immunohistochemistry (IHC) and RT-PCR (Schob et al., 2013). Utilizing IHC, both SP-A and D proteins were detected in the tissue surrounding the microvasculature of the brain parenchyma, the capillaries of choroid plexus stroma and the small vessels at the base of the pineal gland. These proteins were present in vascular tissue as well as in the adjacent brain tissue. They speculated that the localization of these proteins at the blood brain barrier aided in the protection of brain matter from invading microorganisms. Furthermore, subjects who had autoimmune disease had higher expression of SP-A than controls, whereas subjects with cerebral infarctions and cerebral infections had lower levels compared to controls. Expression of SP-D, however, was decreased in all subjects with any inflammatory, ischemic or infectious pathology as compared to controls. When comparing the concentration of brain SP's to that in the lung, the concentration in brain tissue was measured to be approximately twenty five percent of the surfactant protein concentration in the lungs. The only exception was the choroid plexus. SP's in the cerebellum, the sub ventricular cortex and the brainstem were in an equivalent range, whereas the tissue concentration of all SPs in the choroid plexus was approximately twofold higher compared to the concentration in brainstem, cerebellum and sub ventricular cortex. Again, this speaks to the importance of protective role these proteins may play in highly vascular choroid and periventricular area that separates cerebrospinal fluid (CSF) from brain tissue.
In a subsequent paper, the same group of researchers presented further evidence of the role of SP's in CNS physiology when they reported that SP's were co-localized in neonatal and adult rat brains with use of astrocyte, a neuronal and endothelial cell markers (Schob et al., 2016). They found that SP-A was expressed in the CNS of rats during early embryonic age whereas SP-D was found in the adult only. The SP's were expressed in cells adjacent to CSF spaces, which the authors concluded, was likely important in influencing and maintaining physiological CSF flow.
12.2 Ocular System
SP-D mRNA was first detected in human lacrimal gland tissue in 2000 by Madsen et al (Madsen et al., 2000) followed by its detection in mice by Akiyama and colleagues in 2002 (Akiyama et al., 2002). In 2007, Bräuer et al performed an in-depth analysis of the distribution of SP-A and SP-D on the ocular surface and in the lacrimal system. SP-A and D mRNA and protein levels were demonstrated in healthy lacrimal gland, conjunctiva, cornea, and nasolacrimal duct samples. Moreover, both proteins were present in tears, but were absent in aqueous humor. IHC revealed the production of both peptides by acinar epithelial cells of the lacrimal gland and epithelial cells of the conjunctiva and nasolacrimal ducts, whereas goblet cells revealed no reactivity. Healthy cornea revealed weak reactivity on epithelial surface cells only. In contrast, SP-A and SP-D revealed strong reactivity in patients with herpetic keratitis and corneal ulceration surrounding lesions and in several immigrated defense cells. Reactivity in corneal epithelium and endothelium was also seen in patients with keratoconus. Cell culture experiments revealed that SP-A and SP-D are produced by both epithelial cell lines without and after stimulation with cytokines and bacterial components.
Our lab group studies the association of the collectins, SP-A and D with retinopathy of prematurity and pathological neovascularization (NV). NV is also a hallmark of diabetic retinopathy and sometimes with wet age related macular degeneration. We recently showed that SP-A is present in the mouse retina and appears to be associated with the vasculature of both choroidal and retinal artery distribution (Bhatti et al., 2015). Up-regulation of SP-A protein was seen in both retinal tissues as well in human retinal Müller cells grown in culture. Utilizing SP-A knockout mice, we furthermore showed that neovascularization was significantly decreased in the absence of SP-A in the oxygen-induced retinopathy model. This leads us to believe that collectins in general and SP-A in particular may be up-regulated during systemic inflammation in the retina and that this leads to an increase in pro-inflammatory cytokines leading to a disruption of the normal growth of blood vessels in the developing retina. Unpublished data from our lab also shows that when neonatal mice are subjected to systemic inflammation induced by LPS, there is an up-regulation of SP-A protein in the retina, which is also associated with activation of retinal microglial cells.
We conclude that SP-A and possibly SP-D may be important in regulating normal angiogenesis in the developing retina. We furthermore believe that retinal SP-A is up regulated during both local and systemic inflammation leading to activation of retinal microglia. These activated cells further potentiate aberrant vascular signaling and expression of local cytokines.
12.3 Gastrointestinal System
Chailley-Heu et al first reported the expression of surfactant proteins in the GI tract in 1997 (Chailley-Heu et al., 1997). They showed that SP-A and SP-D are expressed by mesenteric cells and are of embryonic origin (mesodermal), which differentiates them from SP's in the lung and digestive tract (endodermal origin). In 2001, Bourbon et al reported that an antibody raised against mammalian lung SP-A binds to proteins present in intestine and swim bladder of fish (Bourbon and Chailley-Heu, 2001). They concluded that these proteins, immunologically identical to SP-A found in humans, are present in modern fish that evolved from ancestors that never possessed lungs. Moreover, the finding of an SP-A-like protein in intestine and swim bladder of actinopterygian fish implies that the ancestral form of the protein was already present before the emergence of lung structures. From a developmental standpoint, this is an important observation as it points to an independent embryological process to equip the gastrointestinal tract with a first line of defense against ingested microbial and inflammatory insults.
In 2008, Luo and colleagues reported the expression and distribution of SP-A in intestinal tissue using immunohistochemistry in pathological specimens derived from patients with Crohn's disease and ulcerative colitis (Luo et al., 2008). SP-A was identified in intestinal epithelium, the surface of intestinal villi, and in association with the connective tissue of blood vessels, and in association with inflammatory cells and macrophages. Macrophages expressing SP-A and being CD68 positive were dramatically increased in tissue that was inflamed when compared to adjacent non-inflammatory tissue.
Definitive evidence supporting the role of SP-A in mediating intestinal host defense was reported by Gowdy et al in 2012 (Gowdy et al., 2012). SP-A-/- as well as control mice were engrafted with allogenic bone marrow transplants. After four weeks, SP-A-/- mice had significant weight loss and graft vs. host disease. Intestinal inflammation scores were increased, as was the expression of TNF-α, IL-1 β, IL-6, and IFN-γ mRNA when compared to control tissues.
More recently, the therapeutic value of SP-A protein was studied in an experimental rat model of neonatal necrotizing enterocolitis (NEC), a potentially fatal infectious/inflammatory pathology of the intestine seen in premature infants (Quintanilla et al., 2015). They found that oral administration of purified human SP-A protein reduced intestinal levels of pro-inflammatory cytokines and TLR-4, as measured by ELISA and western blotting. Furthermore, it reduced intestinal pathology scores and mortality in rats with NEC treated with SP-A as compared to placebo.
These seemingly conflicting data may perhaps be a function of the immaturity of the intestinal tract in premature infants as compared to adults. In the context of low gestational age and NEC, SP-A may perhaps be functionally or quantifiably limited. Therefore, replacement therapy may balance the inflammatory milieu of the intestine reducing inflammation and improving mortality. This needs further study to define the precise mechanisms by which this occurs.
12.4 Renal System
Utilizing human kidney samples from patients with rejected renal transplants, Kankavi in 2003, showed that SP-A and SP-D were both present in kidney specimens using western blotting (Kankavi, 2003). Discrete bands were observed using mouse monoclonal antibody against SP-A and a rabbit polyclonal antibody against SP-D.
Liu and colleagues were the first to report a clinical association between SP-A polymorphisms and urinary tract infections (UTI's) in a Chinese cohort with recurrent UTI's (rUTI) (Liu et al., 2010). Looking for 11 single nucleotide polymorphisms (SNP's) for SP-A1, SP-A2 and SP-D, they found that the 19Ala allele of SP-A1 gene and the 223Gln allele of SP-A2 gene are risk factors for r-UTI.
The specific localization of SP-A in the nephron of human renal tissue and in human renal epithelial cells (HK-2) was performed by the same group in 2013 (Liu et al., 2013). SP-A was seen in the renal tubular epithelial cells of the proximal and distal convoluted tubules by IHC. SP-A-specific cDNA products (a primer pair common to SP-A1 and SP-A2, 439 bp) were amplified from the renal tissues and HK-2 cells. LPS was also found to induce SP-A1 and SP-A2 mRNA and protein syntheses in cultured HK-2 cells. The same group in 2014 studied the expression of SP-A in HK-2 cells treated with LPS (Liu et al., 2014). They found that SP-A1, SP-A2 and TNF-α expression were significantly increased in HK-2 cells after treatment with LPS. They concluded that SP-A plays an important role in protecting renal tubular cells against sepsis-induced acute kidney injury by inhibiting NF-κB activity to modulate LPS-induced increase in TNF-α expression.
12.5 Male Genital and Reproductive Systems
The earliest report of SP-A in the male genital tract was published in 2000 when Madsen et al, performed RT-PCR analysis of SP-D in various organs of the human body (Madsen et al., 2000). They found high expression of SP-D mRNA in the testis, prostate, and placenta. Low expression was found in the uterus and mammary gland. In 2008, Kankavi et al analyzed the spermatozoa from healthy male volunteers by performing IHC and western blot analysis using an SP-A polyclonal antibody (Kankavi et al., 2008). They reported that SP-A was found in the tail, the mid piece and sometimes at the equatorial region of human spermatozoa by IHC. SP-A protein was detected in spermatozoa by western blotting as well. The polyclonal antibody detected a single band corresponding to the molecular weight of 34 kDa in spermatozoa. A monoclonal SP-D antibody showed the band at 43 kDa in sperm. The same group then followed with an in depth study to determine how surfactant proteins are altered in prostate adenocarcinomas (Kankavi et al., 2014). Protein expressions were tested by IHC and Western blots. Immunoreactivity was detected in the cytoplasm from both basal cells and secretory epithelial cells in malignant and non-malignant areas. SP-A and SP-D reacted with 34 kDa (SP-A) and 43 kDa (SP-D) immunoreactive single bands, but these were decreased in tumor tissues. They concluded that the development of prostate cancer might be related to decreased levels of surfactant protein A and D. In 2015, Beileke et al described all four SP-A, B, C and D in testicular tissue from male cadavers and male subjects biopsied for detection of testicular cancer (Beileke et al., 2015). They reported PCR products of the expected sizes for human SP-A and SP-D in human healthy testis with no significant alterations in peritumoral samples. Healthy testicular tissue, neoplastic testicular tissue, prostate tissue, spermatozoa and supernatant of spermatozoal secretions expressed SP-A bands on Western blotting along with lung. However, while the healthy and diseased testes, prostate, spermatozoa and lung had three bands at 26, 38 and 60 kDa, spermatozoal secretions expressed only two bands at 26 and 60 kDa. All tested tissue had a band expressed for SP-D at 43 kDa except for the spermatozoa which showed no band. The authors concluded that both proteins could be components of the immune system of the male reproductive system, and may play a role in defense against pathogens within the urogenital tract.
12.6 Female Genital and Reproductive Systems
In 1988, Snyder and colleagues reported that the concentration of surfactant apoprotein in amniotic fluid increased as a function of gestational age and that an increase in the concentration of the surfactant apoprotein in amniotic fluid was first observed at about 32 weeks of gestation (Snyder et al., 1988). They also found that the amniotic fluid SP-A concentration was decreased in diabetic, normotensive women when compared to that in a matched control group of non-diabetic women. Miyumura and his colleagues performed a more in depth analysis of SP-A and SP-D localization in fetal membranes in 1994 (Miyamura et al, 1994). They found that both SP-A and SP-D were detected by IHC in amniotic fluid as early as 26 weeks gestation. SP-A levels rose sharply from 32 weeks towards term, but that SP-D levels in the same samples rose only moderately. IHC of fetal membranes, revealed both SP-A and SP-D in the amniotic epithelium and chorio-decidual layers. They concluded that SP-A and SP-D might play a role in the antibody-independent recognition and clearance of pathogens in the amniotic fluid.
Further confirmation was found in 2011, when Snegovskikh et al demonstrated that SP-A localized to endometrium and decidua. (Snegovskikh et al, 2011). Term decidual stromal cells were treated with high-dose SP-A, inhibiting PGF2α, but the production of other inflammatory mediators was not affected. Furthermore, they demonstrated that decidual SP-A expression decreased significantly with initiation of labor.
Macneill and Floros also looked at vaginal tissue and fluid of premenopausal women and found that SP-A was present in the vaginal epithelium, in particular in the cytoplasm of cells in the deep portion of the intermediate layer adjacent to the parabasal layer; and in the superficial layer (MacNeill et al., 2004). They also noted that there were no differences in localization or intensity of the protein as a function of the ovarian cycle. Further supporting evidence for the importance of SP-A in the female reproductive tract was seen when Han and colleagues reported that SP-A is also increased in the chorionic membranes of women suffering from chorioamniotis (Han et al., 2007). Quantitative real-time RT-PCR demonstrated expression of SP-A1 mRNA, which was 17.4-fold higher in patients delivering preterm with chorioamniotis. Several studies have also established that SP-A is an important marker that increases during the intricate signaling that mediates parturition (Breuiller-Fouche et al., 2010; Chaiworapongsa et al., 2008; Garcia-Verdugo et al., 2010; Leong et al., 2008; Salminen et al., 2008; Snegovskikh et al., 2011; Yadav et al., 2011). Most recently, statistically significant delay in the time to parturition was evident in SP-A and D doubly deficient mice (Montalbano et al., 2013). It was also found that delayed parturition was associated with reduced inflammatory and contraction-associated protein gene expression (IL-1β and IL-6) in myometrial tissues.
Most recently in 2016, Kay and colleagues examined the role of SP-D on the female uterine structure, function and reproductive cycle (Kay et al., 2016). Using SP-D knockout mice, they demonstrated that mice deficient in SP-D had extended estrous cycles, and altered ovarian profiles. They observed higher levels of serum progesterone in proestrous and estrous phases of SP-D knockout mice. These mice had significantly lower levels of serum estradiol in diestrous and proestrous phases of the cycle. Using Real-time PCR, they also showed 7-fold and 6-fold higher transcript levels of estrogen and progesterone receptors respectively, in SP-D knockout mice uteri (estrous phase). SP-D knockout mice also had smaller uterine sizes and smaller litter sizes when compared to controls. Most interestingly, they observed that the uteri of knockout mice had an increase in pro-inflammatory cytokines TNF-α, IL-1, IL-17A, CXCL1 and CCL5 with a modest increase in the anti-inflammatory cytokine IL-10 as compared to wild type mice. This indicated an altered inflammatory cytokine favoring inflammation. There was also a significant infiltration of M1 monocytes/macrophages in the uteri of knockout mice
Taken together, these data suggest that collectins modulate not only development of the female reproductive system, but are also important in the initiation of parturition. Furthermore, they maintain homeostasis and an anti-inflammatory environment in the uterus, cervix and vaginal cavities/fluids.
12.7 Skin and integumentary System
Because of their roles as the first line of defense on an array of mucosal surfaces, it was intuitive to examine the skin as the primary physical barrier of the body to the environment and foreign antigens. Mo and colleagues performed an in-depth analysis of the distribution and possible role of surfactant proteins in various dermatological cell types (Mo et al., 2007). Utilizing commercially available adult and fetal skin cDNA, they showed that the SP-A PCR signal was observed for both adult and fetal skin cDNA. Next, they examined human skin lines and used RT-PCR to detect the mRNA signal. The genetic message for SP-D was detected in all skin cell types examined as well as the skin cell lines (HaCaT and Colo16). SP-A expression following nested PCR was observed only in melanocyte and in two keratinocyte cell lines. They next examined human skin samples and found that RT-PCR analysis of individual skin samples and control lung showed SP-D expression in all donors, but that SP-A expression was observed only in two abdominal, but not breast skin donors. Although of similar sizes to those from human lung, SP-A expression in skin was markedly lower than compared to lung. IHC analysis of human skin showed strong staining of SP-A in the epidermis of human skin and throughout the layers of the stratum granulosum and stratum corneum to the skin surface. SP-D was observed in epidermal layers including the stratum spinosum, stratum lucidium, and stratum granulosum, and was visible on the outer surface of the stratum corneum. In addition, SP-D reactivity was also noticed in the dermis, although the structures responsible for this staining have not been identified. Staining for SPs was also observed around the hair follicle shaft, although a definitive pattern of distribution could not be obtained. SP-A stained quite intensely throughout the outer and inner root sheath, whereas SP-D seemed to have a much lower and diffuse staining through these structures. In surface tension studies, skin SP-A and SP-D did not affect the organization of monolayers of artificial sebum. They concluded that the distribution of these surfactants may be associated with defense against local infections and other skin diseases.
This premise was further supported by the findings of Aiad et al in 2012 (Aiad et al., 2012), when they studied the skin of patients with psoriasis and demonstrated that in unaffected skin, SP-A was restricted to the basal layer; however, in psoriatic skin, it predominantly appeared in suprabasal layers. Dermal inflammatory cells showed SP-A in about half the cases studied. Absence of SP-A staining in suprabasal layers after NB-UVB therapy was correlated to better response to therapy and shorter duration of treatment. This again supports the pro-inflammatory role of SP-A in a primarily inflammation driven disease process.
12.8 Synovial Fluid
Several studies have looked at the presence of surfactant proteins A and D in synovial fluid. The earliest report is from 1994 when Dobbie and colleagues demonstrated the presence of lamellar bodies in synovial fluid from patients with rheumatoid arthritis. SP-A immunoblotting showed strong reactivity in area, intensity and density of cells, which paralleled the frequency and degree of pathological involvement characteristic of rheumatoid disease. More recently, Kankavi et al performed a more in depth analysis to determine the concentrations of SP-A and SP-D in synovial fluid samples from patients with rheumatoid arthritis (RA) and healthy controls. They demonstrated that patients with RA had increased concentrations of proteins and lipids in the synovial fluid, which correlated with a moderate increase in SP-A and a significant increase in SP-D concentrations. Total protein and phospholipid content in synovial fluid samples from patients with rheumatoid arthritis was increased in comparison with samples of healthy synovial fluid. They suggested that SP-A and SP-D may play role in the initiation of immune system and joint inflammation, and may be an integral component of synovial fluid.
13. Conclusions
This comprehensive review of the surfactant proteins A and D shows both pro-inflammatory as well as anti-inflammatory roles for these proteins. More importantly, they contribute to the host defense of a variety of tissues and organ systems throughout life. In the context of the neonate, collectins appear to drive homeostasis needed for developmental and embryological processes culminating in the initiation of parturition and uterine contractions, expulsion and birth. Postnatally, these collectins are important motors of the inflammatory and stress responses of the neonate (especially in prematurity) and may be impaired both in form of a deficiency related to gestational age as well as post-natal up regulation during infection or stress. In the adult, a multitude of inflammatory disease conditions, which are related to abnormalities in first line defense mechanisms, may be attributed to abnormalities in SP-A/D expression and function. In short, these systemic proteins are crucial mediators of downstream pathways related to both the up regulation of cytokines as well as macrophage monocyte activation. Therefore, it is imperative that the role and functions of these unique collectin proteins be thoroughly examined to identify pathways that have the potential to be targeted in infection and stress.
Acknowledgments
We acknowledge the assistance and support of the NEI/DMEI Cellular Imaging Core Facility at OUHSC (NIH: P30EY021725-Center Core Grant for Vision Research); the Histology Core Facility; Mark Dittmar, Manager, Animal Facilities at Dean McGee Eye Institute, University of Oklahoma Health Sciences Center.
This work was partially supported by grant funding to F.B. through a Knights Templar Eye Foundation Pediatric Ophthalmology Career Grant and US National Institutes of Health COBRE P20 RR017703-10.
Abbreviations
- SP-A
Surfactant Protein A
- SP-D
Surfactant Protein D
- SP's
Surfactant Proteins
- MBL
Mannose binding lectin
- UTR
Untranslated region
- LPS
Lipopolysaccharide
- GP
Glycoprotein
- RSV
Respiratory syncytial virus
- RDS
Respiratory distress syndrome
- BPD
Bronchopulmonary dysplasia
- CRD
Carbohydrate recognition domain
- TLR
Toll like receptor
- CRT
Calreticulin
- C1qRp
C1q receptor which modulates phagocytosis
- IHC
Immunohistochemistry
- CSF
Cerebrospinal fluid
- NEC
Necrotizing enterocolitis
- RT-PCR
Reverse transcription polymerase chain reaction
- HSP
Heat shock proteins
- NV
Neovascularization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Aiad HA, El-Farargy SM, Soliman MM, El-Wahed Gaber MA, El-Aziz Othman SA. Immunohistochemical staining of surfactant proteins A and B in skin of psoriatic patients before and after narrow-band UVB phototherapy. American journal of clinical dermatology. 2012;13:341–348. doi: 10.2165/11630720-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Akira S, Hoshino K, Kaisho T. The role of Toll-like receptors and MyD88 in innate immune responses. J Endotoxin Res. 2000;6:383–387. [PubMed] [Google Scholar]
- Akiyama J, Hoffman A, Brown C, Allen L, Edmondson J, Poulain F, Hawgood S. Tissue distribution of surfactant proteins A and D in the mouse. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society. 2002;50:993–996. doi: 10.1177/002215540205000713. [DOI] [PubMed] [Google Scholar]
- Akiyama J, Volik SV, Plajzer-Frick I, Prince A, Sago H, Weier HU, Vanderbilt JN, Hawgood S, Poulain FR. Characterization of the mouse collectin gene locus. American journal of respiratory cell and molecular biology. 1999;21:193–199. doi: 10.1165/ajrcmb.21.2.3681. [DOI] [PubMed] [Google Scholar]
- Alcorn JF, Wright JR. Surfactant protein A inhibits alveolar macrophage cytokine production by CD14-independent pathway. American journal of physiology. Lung cellular and molecular physiology. 2004;286:L129–136. doi: 10.1152/ajplung.00427.2002. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Brown K, King C, Awasthi V, Bondugula R. A TLR4-interacting Surfactant Protein-A-derived Peptide Suppresses TNF-alpha release from Mouse JAWS II Dendritic Cells. J Pharmacol Exp Ther. doi: 10.1124/jpet.110.173765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi S, Coalson JJ, Yoder BA, Crouch E, King RJ. Deficiencies in lung surfactant proteins A and D are associated with lung infection in very premature neonatal baboons. Am J Respir Crit Care Med. 2001;163:389–397. doi: 10.1164/ajrccm.163.2.2004168. [DOI] [PubMed] [Google Scholar]
- Awasthi S, Madhusoodhanan R, Wolf R. Surfactant protein-A and tolllike receptor-4 modulate immune functions of preterm baboon lung dendritic cell precursor cells. Cell Immunol. 2011;268:87–96. doi: 10.1016/j.cellimm.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagopal A, MacFarlane AS, Mohapatra N, Soni S, Gunn JS, Schlesinger LS. Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect Immun. 2006;74:5114–5125. doi: 10.1128/IAI.00795-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balchin D, Hayer-Hartl M, Hartl FU. In vivo aspects of protein folding and quality control. Science. 2016;353:aac4354. doi: 10.1126/science.aac4354. [DOI] [PubMed] [Google Scholar]
- Barrow AD, Palarasah Y, Bugatti M, Holehouse AS, Byers DE, Holtzman MJ, Vermi W, Skjodt K, Crouch E, Colonna M. OSCAR is a receptor for surfactant protein D that activates TNF-alpha release from human CCR2+ inflammatory monocytes. Journal of immunology. 2015;194:3317–3326. doi: 10.4049/jimmunol.1402289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baughman RP, Sternberg RI, Hull W, Buchsbaum JA, Whitsett J. Decreased surfactant protein A in patients with bacterial pneumonia. Am Rev Respir Dis. 1993;147:653–657. doi: 10.1164/ajrccm/147.3.653. [DOI] [PubMed] [Google Scholar]
- Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX, Schlesinger LS. Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. Journal of immunology. 2002;169:3565–3573. doi: 10.4049/jimmunol.169.7.3565. [DOI] [PubMed] [Google Scholar]
- Beileke S, Claassen H, Wagner W, Matthies C, Ruf C, Hartmann A, Garreis F, Paulsen F, Schicht M, Brauer L. Expression and Localization of Lung Surfactant Proteins in Human Testis. PLoS One. 2015;10:e0143058. doi: 10.1371/journal.pone.0143058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlhabib H, Mendelson CR. Epigenetic regulation of surfactant protein A gene (SP-A) expression in fetal lung reveals a critical role for Suv39h methyltransferases during development and hypoxia. Mol Cell Biol. 2011;31:1949–1958. doi: 10.1128/MCB.01063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson B, Hawgood S, Schilling J, Clements J, Damm D, Cordell B, White RT. Structure of canine pulmonary surfactant apoprotein: cDNA and complete amino acid sequence. Proc Natl Acad Sci U S A. 1985;82:6379–6383. doi: 10.1073/pnas.82.19.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti F, Ball G, Hobbs R, Linens A, Munzar S, Akram R, Barber AJ, Anderson M, Elliott M, Edwards M. Pulmonary surfactant protein a is expressed in mouse retina by Muller cells and impacts neovascularization in oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2015;56:232–242. doi: 10.1167/iovs.13-13652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggaram V, Mendelson CR. Transcriptional regulation of the gene encoding the major surfactant protein (SP-A) in rabbit fetal lung. J Biol Chem. 1988;263:19060–19065. [PubMed] [Google Scholar]
- Bourbon JR, Chailley-Heu B. Surfactant proteins in the digestive tract, mesentery, and other organs: evolutionary significance. Comparative biochemistry and physiology. Part A, Molecular & integrative physiology. 2001;129:151–161. doi: 10.1016/s1095-6433(01)00312-9. [DOI] [PubMed] [Google Scholar]
- Brauer L, Schicht M, Worlitzsch D, Bensel T, Sawers RG, Paulsen F. Staphylococcus aureus and Pseudomonas aeruginosa express and secrete human surfactant proteins. PLoS One. 2013;8:e53705. doi: 10.1371/journal.pone.0053705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuiller-Fouche M, Dubois O, Sediki M, Garcia-Verdugo I, Palaniyar N, Tanfin Z, Chissey A, Cabrol D, Charpigny G, Mehats C. Secreted surfactant protein A from fetal membranes induces stress fibers in cultured human myometrial cells. American journal of physiology, Endocrinology and metabolism. 2010;298:E1188–1197. doi: 10.1152/ajpendo.00746.2009. [DOI] [PubMed] [Google Scholar]
- Brodsky-Doyle B, Leonard KR, Reid KB. Circular-dichroism and electron-microscopy studies of human subcomponent C1q before and after limited proteolysis by pepsin. Biochem J. 1976;159:279–286. doi: 10.1042/bj1590279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chailley-Heu B, Rubio S, Rougier JP, Ducroc R, Barlier-Mur AM, Ronco P, Bourbon JR. Expression of hydrophilic surfactant proteins by mesentery cells in rat and man. Biochem J. 1997;328(Pt 1):251–256. doi: 10.1042/bj3280251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiworapongsa T, Hong JS, Hull WM, Kim CJ, Gomez R, Mazor M, Romero R, Whitsett JA. The concentration of surfactant protein-A in amniotic fluid decreases in spontaneous human parturition at term. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2008;21:652–659. doi: 10.1080/14767050802215193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CY, Chen TG, Tai YT, Chen TL, Lin YH, Tsai CH, Chen RM. Toll-like receptor 2-mediated sequential activation of MyD88 and MAPKs contributes to lipopolysaccharide-induced sp-a gene expression in human alveolar epithelial cells. Immunobiology. 2011;216:707–714. doi: 10.1016/j.imbio.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Clements JA. Surface tension of lung extracts. Proc Soc Exp Biol Med. 1957;95:170–172. doi: 10.3181/00379727-95-23156. [DOI] [PubMed] [Google Scholar]
- Crouch E, Rust K, Veile R, Donis-Keller H, Grosso L. Genomic organization of human surfactant protein D (SP-D). SP-D is encoded on chromosome 10q22.2-23.1. J Biol Chem. 1993;268:2976–2983. [PubMed] [Google Scholar]
- Drickamer K, Dordal MS, Reynolds L. Mannose-binding proteins isolated from rat liver contain carbohydrate-recognition domains linked to collagenous tails. Complete primary structures and homology with pulmonary surfactant apoprotein. J Biol Chem. 1986;261:6878–6887. [PubMed] [Google Scholar]
- Eggleton P, Lieu TS, Zappi EG, Sastry K, Coburn J, Zaner KS, Sontheimer RD, Capra JD, Ghebrehiwet B, Tauber AI. Calreticulin is released from activated neutrophils and binds to C1q and mannan-binding protein. Clin Immunol Immunopathol. 1994;72:405–409. doi: 10.1006/clin.1994.1160. [DOI] [PubMed] [Google Scholar]
- Endo M, Ohi H, Ohsawa I, Fujita T, Matsushita M, Fujita T. Glomerular deposition of mannose-binding lectin (MBL) indicates a novel mechanism of complement activation in IgA nephropathy. Nephrol Dial Transplant. 1998;13:1984–1990. doi: 10.1093/ndt/13.8.1984. [DOI] [PubMed] [Google Scholar]
- Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS. Surfactant protein D binds to Mycobacterium tuberculosis bacilli and lipoarabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. Journal of immunology. 1999;163:312–321. [PubMed] [Google Scholar]
- Fisher JH, Emrie PA, Shannon J, Sano K, Hattler B, Mason RJ. Rat pulmonary surfactant protein A is expressed as two differently sized mRNA species which arise from differential polyadenylation of one transcript. Biochim Biophys Acta. 1988;950:338–345. doi: 10.1016/0167-4781(88)90130-3. [DOI] [PubMed] [Google Scholar]
- Floros J, Steinbrink R, Jacobs K, Phelps D, Kriz R, Recny M, Sultzman L, Jones S, Taeusch HW, Frank HA, et al. Isolation and characterization of cDNA clones for the 35-kDa pulmonary surfactant-associated protein. J Biol Chem. 1986;261:9029–9033. [PubMed] [Google Scholar]
- Floros J, Wang G, Mikerov AN. Genetic complexity of the human innate host defense molecules, surfactant protein A1 (SP-A1) and SP-A2--impact on function. Crit Rev Eukaryot Gene Expr. 2009;19:125–137. doi: 10.1615/critreveukargeneexpr.v19.i2.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao E, Wang Y, McCormick SM, Li J, Seidner SR, Mendelson CR. Characterization of two baboon surfactant protein A genes. Am J Physiol. 1996;271:L617–630. doi: 10.1152/ajplung.1996.271.4.L617. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo I, Tanfin Z, Breuiller-Fouche M. Surfactant protein A: an immunoregulatory molecule involved in female reproductive biology. The international journal of biochemistry & cell biology. 2010;42:1779–1783. doi: 10.1016/j.biocel.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR, Greene KE, Henson PM. By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell. 2003;115:13–23. doi: 10.1016/s0092-8674(03)00758-x. [DOI] [PubMed] [Google Scholar]
- Gerdes JS, Abbasi S, Karp K, Hull W, Whitsett JA. Surfactant protein-A in bronchoalveolar lavage fluid from neonates with RDS on conventional and high-frequency oscillatory ventilation. Pediatr Pulmonol. 1990;9:166–169. doi: 10.1002/ppul.1950090308. [DOI] [PubMed] [Google Scholar]
- Ghildyal R, Hartley C, Varrasso A, Meanger J, Voelker DR, Anders EM, Mills J. Surfactant protein A binds to the fusion glycoprotein of respiratory syncytial virus and neutralizes virion infectivity. J Infect Dis. 1999;180:2009–2013. doi: 10.1086/315134. [DOI] [PubMed] [Google Scholar]
- Gil M, McCormack FX, Levine AM. Surfactant protein A modulates cell surface expression of CR3 on alveolar macrophages and enhances CR3-mediated phagocytosis. J Biol Chem. 2009;284:7495–7504. doi: 10.1074/jbc.M808643200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowdy KM, Cardona DM, Nugent JL, Giamberardino C, Thomas JM, Mukherjee S, Martinu T, Foster WM, Plevy SE, Pastva AM, Wright JR, Palmer SM. Novel role for surfactant protein A in gastrointestinal graft-versus-host disease. Journal of immunology. 2012;188:4897–4905. doi: 10.4049/jimmunol.1103558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsman HP, Hawgood S, Sargeant T, Buckley D, White RT, Drickamer K, Benson BJ. The major lung surfactant protein, SP 28-36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem. 1987;262:13877–13880. [PubMed] [Google Scholar]
- Haagsman HP, White RT, Schilling J, Lau K, Benson BJ, Golden J, Hawgood S, Clements JA. Studies of the structure of lung surfactant protein SP-A. Am J Physiol. 1989;257:L421–429. doi: 10.1152/ajplung.1989.257.6.L421. [DOI] [PubMed] [Google Scholar]
- Haataja R, Ramet M, Marttila R, Hallman M. Surfactant proteins A and B as interactive genetic determinants of neonatal respiratory distress syndrome. Hum Mol Genet. 2000;9:2751–2760. doi: 10.1093/hmg/9.18.2751. [DOI] [PubMed] [Google Scholar]
- Hallman M, Haataja R. Surfactant protein polymorphisms and neonatal lung disease. Semin Perinatol. 2006;30:350–361. doi: 10.1053/j.semperi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Han YM, Romero R, Kim YM, Kim JS, Richani K, Friel LA, Kusanovic JP, Jeanty C, Vitale S, Nien JK, Espinoza J, Kim CJ. Surfactant protein-A mRNA expression by human fetal membranes is increased in histological chorioamnionitis but not in spontaneous labour at term. The Journal of pathology. 2007;211:489–496. doi: 10.1002/path.2131. [DOI] [PubMed] [Google Scholar]
- Hancock RE, Mutharia LM, Chan L, Darveau RP, Speert DP, Pier GB. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun. 1983;42:170–177. doi: 10.1128/iai.42.1.170-177.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartshorn K, Chang D, Rust K, White M, Heuser J, Crouch E. Interactions of recombinant human pulmonary surfactant protein D and SP-D multimers with influenza A. Am J Physiol. 1996;271:L753–762. doi: 10.1152/ajplung.1996.271.5.L753. [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, White MR, Shepherd V, Reid K, Jensenius JC, Crouch EC. Mechanisms of anti-influenza activity of surfactant proteins A and D: comparison with serum collectins. Am J Physiol. 1997;273:L1156–1166. doi: 10.1152/ajplung.1997.273.6.L1156. [DOI] [PubMed] [Google Scholar]
- Hartshorn KL, White MR, Tecle T, Holmskov U, Crouch EC. Innate defense against influenza A virus: activity of human neutrophil defensins and interactions of defensins with surfactant protein D. Journal of immunology. 2006;176:6962–6972. doi: 10.4049/jimmunol.176.11.6962. [DOI] [PubMed] [Google Scholar]
- Hawgood S, Clements JA. Pulmonary surfactant and its apoproteins. J Clin Invest. 1990;86:1–6. doi: 10.1172/JCI114670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbein JF, Wright JR. Enhanced clearance of surfactant protein D during LPS-induced acute inflammation in rat lung. American journal of physiology. Lung cellular and molecular physiology. 2001;281:L268–277. doi: 10.1152/ajplung.2001.281.1.L268. [DOI] [PubMed] [Google Scholar]
- Herias MV, Hogenkamp A, van Asten AJ, Tersteeg MH, van Eijk M, Haagsman HP. Expression sites of the collectin SP-D suggest its importance in first line host defence: power of combining in situ hybridisation, RT-PCR and immunohistochemistry. Mol Immunol. 2007;44:3324–3332. doi: 10.1016/j.molimm.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Hickling TP, Bright H, Wing K, Gower D, Martin SL, Sim RB, Malhotra R. A recombinant trimeric surfactant protein D carbohydrate recognition domain inhibits respiratory syncytial virus infection in vitro and in vivo. Eur J Immunol. 1999;29:3478–3484. doi: 10.1002/(SICI)1521-4141(199911)29:11<3478::AID-IMMU3478>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Holliday R. DNA methylation and epigenetic mechanisms. Cell biophysics. 1989;15:15–20. doi: 10.1007/BF02991575. [DOI] [PubMed] [Google Scholar]
- Holliday R. Epigenetics: an overview. Developmental genetics. 1994;15:453–457. doi: 10.1002/dvg.1020150602. [DOI] [PubMed] [Google Scholar]
- Holmskov U, Lawson P, Teisner B, Tornoe I, Willis AC, Morgan C, Koch C, Reid KB. Isolation and characterization of a new member of the scavenger receptor superfamily, glycoprotein-340 (gp-340), as a lung surfactant protein-D binding molecule. J Biol Chem. 1997;272:13743–13749. doi: 10.1074/jbc.272.21.13743. [DOI] [PubMed] [Google Scholar]
- Hoppe HJ, Reid KB. Collectins--soluble proteins containing collagenous regions and lectin domains--and their roles in innate immunity. Protein Sci. 1994;3:1143–1158. doi: 10.1002/pro.5560030801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz S, Watkins RH, Auten RL, Jr, Mercier CE, Cheng ER. Differential accumulation of surfactant protein A, B, and C mRNAs in two epithelial cell types of hyperoxic lung. Am J Respir Cell Mol Biol. 1991;5:511–515. doi: 10.1165/ajrcmb/5.6.511. [DOI] [PubMed] [Google Scholar]
- Islam KN, Mendelson CR. Permissive effects of oxygen on cyclic AMP and interleukin-1 stimulation of surfactant protein A gene expression are mediated by epigenetic mechanisms. Mol Cell Biol. 2006;26:2901–2912. doi: 10.1128/MCB.26.8.2901-2912.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam KN, Mendelson CR. Glucocorticoid/glucocorticoid receptor inhibition of surfactant protein-A (SP-A) gene expression in lung type II cells is mediated by repressive changes in histone modification at the SP-A promoter. Mol Endocrinol. 2008;22:585–596. doi: 10.1210/me.2007-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaisho T, Akira S. Critical roles of Toll-like receptors in host defense. Crit Rev Immunol. 2000;20:393–405. [PubMed] [Google Scholar]
- Kankavi O. Immunodetection of surfactant proteins in human organ of Corti, Eustachian tube and kidney. Acta biochimica Polonica. 2003;50:1057–1064. [PubMed] [Google Scholar]
- Kankavi O, Ata A, Celik-Ozenci C, Sati L, Ciftcioglu MA, Demir R, Baykara M. Presence and subcellular localizations of surfactant proteins A and D in human spermatozoa. Fertil Steril. 2008;90:1904–1909. doi: 10.1016/j.fertnstert.2007.09.064. [DOI] [PubMed] [Google Scholar]
- Kankavi O, Baykara M, Eren Karanis MI, Bassorgun CI, Ergin H, Ciftcioglu MA. Evidence of surfactant protein A and D expression decrement and their localizations in human prostate adenocarcinomas. Renal failure. 2014;36:258–265. doi: 10.3109/0886022X.2013.846831. [DOI] [PubMed] [Google Scholar]
- Karinch AM, Floros J. Translation in vivo of 5′ untranslated-region splice variants of human surfactant protein-A. Biochem J. 1995;307(Pt 2):327–330. doi: 10.1042/bj3070327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katyal SL, Singh G, Locker J. Characterization of a second human pulmonary surfactant-associated protein SP-A gene. Am J Respir Cell Mol Biol. 1992;6:446–452. doi: 10.1165/ajrcmb/6.4.446. [DOI] [PubMed] [Google Scholar]
- Kay S, Madan T, Yadav AK, Chaudhari H, Shah PK, Madan T, Sotiriadis G, Dodagatta-Marri E, Kouser L, Alhamlan FS, Kishore U, Karteris E. Fertility defects in Surfactant associated protein D knockout female mice: altered ovarian hormone profile. Mol Immunol. 2016;71:87–97. doi: 10.1016/j.molimm.2016.01.002. Expression and localization of collectins in feto-maternal tissues of human first trimester spontaneous abortion and abortion prone mouse model Surfactant Proteins SP-A and SP-D Modulate Uterine Contractile Events in ULTR Myometrial Cell Line. [DOI] [PubMed] [Google Scholar]
- Kishore U, Bernal AL, Kamran MF, Saxena S, Singh M, Sarma PU, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D in human health and disease. Arch Immunol Ther Exp (Warsz) 2005;53:399–417. [PubMed] [Google Scholar]
- Kishore U, Greenhough TJ, Waters P, Shrive AK, Ghai R, Kamran MF, Bernal AL, Reid KB, Madan T, Chakraborty T. Surfactant proteins SP-A and SP-D: structure, function and receptors. Mol Immunol. 2006;43:1293–1315. doi: 10.1016/j.molimm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Kolble K, Reid KB. The genomics of soluble proteins with collagenous domains: C1q, MBL, SP-A, SP-D, conglutinin, and CL-43. Behring Inst Mitt. 1993:81–86. [PubMed] [Google Scholar]
- Korfhagen TR. Surfactant protein A (SP-A)-mediated bacterial clearance: SP-A and cystic fibrosis. Am J Respir Cell Mol Biol. 2001;25:668–672. doi: 10.1165/ajrcmb.25.6.f221. [DOI] [PubMed] [Google Scholar]
- Korfhagen TR, Bruno MD, Glasser SW, Ciraolo PJ, Whitsett JA, Lattier DL, Wikenheiser KA, Clark JC. Murine pulmonary surfactant SP-A gene: cloning, sequence, and transcriptional activity. The American journal of physiology. 1992;263:L546–554. doi: 10.1152/ajplung.1992.263.5.L546. [DOI] [PubMed] [Google Scholar]
- Korfhagen TR, Glasser SW, Bruno MD, McMahan MJ, Whitsett JA. A portion of the human surfactant protein A (SP-A) gene locus consists of a pseudogene. Am J Respir Cell Mol Biol. 1991;4:463–469. doi: 10.1165/ajrcmb/4.5.463. [DOI] [PubMed] [Google Scholar]
- Kudo K, Sano H, Takahashi H, Kuronuma K, Yokota S, Fujii N, Shimada K, Yano I, Kumazawa Y, Voelker DR, Abe S, Kuroki Y. Pulmonary collectins enhance phagocytosis of Mycobacterium avium through increased activity of mannose receptor. Journal of immunology. 2004;172:7592–7602. doi: 10.4049/jimmunol.172.12.7592. [DOI] [PubMed] [Google Scholar]
- Kuroki Y, Tsutahara S, Shijubo N, Takahashi H, Shiratori M, Hattori A, Honda Y, Abe S, Akino T. Elevated levels of lung surfactant protein A in sera from patients with idiopathic pulmonary fibrosis and pulmonary alveolar proteinosis. Am Rev Respir Dis. 1993;147:723–729. doi: 10.1164/ajrccm/147.3.723. [DOI] [PubMed] [Google Scholar]
- Kuzmenko AI, Wu H, Wan S, McCormack FX. Surfactant protein A is a principal and oxidation-sensitive microbial permeabilizing factor in the alveolar lining fluid. J Biol Chem. 2005;280:25913–25919. doi: 10.1074/jbc.M411344200. [DOI] [PubMed] [Google Scholar]
- Lahti M, Lofgren J, Marttila R, Renko M, Klaavuniemi T, Haataja R, Ramet M, Hallman M. Surfactant protein D gene polymorphism associated with severe respiratory syncytial virus infection. Pediatr Res. 2002;51:696–699. doi: 10.1203/00006450-200206000-00006. [DOI] [PubMed] [Google Scholar]
- Laird PW, Jaenisch R. The role of DNA methylation in cancer genetic and epigenetics. Annu Rev Genet. 1996;30:441–464. doi: 10.1146/annurev.genet.30.1.441. [DOI] [PubMed] [Google Scholar]
- Leong AS, Norman JE, Smith R. Reproductive sciences. Vol. 15. Thousand Oaks, Calif: 2008. Vascular and myometrial changes in the human uterus at term; pp. 59–65. [DOI] [PubMed] [Google Scholar]
- Li J, Gao E, Seidner SR, Mendelson CR. Differential regulation of baboon SP-A1 and SP-A2 genes: structural and functional analysis of 5′-flanking DNA. Am J Physiol. 1998;275:L1078–1088. doi: 10.1152/ajplung.1998.275.6.L1078. [DOI] [PubMed] [Google Scholar]
- Ligtenberg AJ, Veerman EC, Nieuw Amerongen AV, Mollenhauer J. Salivary agglutinin/glycoprotein-340/DMBT1: a single molecule with variable composition and with different functions in infection, inflammation and cancer. Biol Chem. 2007;388:1275–1289. doi: 10.1515/BC.2007.158. [DOI] [PubMed] [Google Scholar]
- Ligtenberg TJ, Bikker FJ, Groenink J, Tornoe I, Leth-Larsen R, Veerman EC, Nieuw Amerongen AV, Holmskov U. Human salivary agglutinin binds to lung surfactant protein-D and is identical with scavenger receptor protein gp-340. Biochem J. 2001;359:243–248. doi: 10.1042/0264-6021:3590243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin PM, Wright JR. Surfactant protein A binds to IgG and enhances phagocytosis of IgG-opsonized erythrocytes. American journal of physiology. Lung cellular and molecular physiology. 2006;291:L1199–1206. doi: 10.1152/ajplung.00188.2006. [DOI] [PubMed] [Google Scholar]
- Lin Z, Pearson C, Chinchilli V, Pietschmann SM, Luo J, Pison U, Floros J. Polymorphisms of human SP-A, SP-B, and SP-D genes: association of SP-B Thr131Ile with ARDS. Clin Genet. 2000;58:181–191. doi: 10.1034/j.1399-0004.2000.580305.x. [DOI] [PubMed] [Google Scholar]
- Lin Z, Thomas NJ, Bibikova M, Seifart C, Wang Y, Guo X, Wang G, Vollmer E, Goldmann T, Garcia EW, Zhou L, Fan JB, Floros J. DNA methylation markers of surfactant proteins in lung cancer. Int J Oncol. 2007;31:181–191. [PubMed] [Google Scholar]
- Liu J, Hu F, Liang W, Wang G, Singhal PC, Ding G. Polymorphisms in the surfactant protein a gene are associated with the susceptibility to recurrent urinary tract infection in chinese women. The Tohoku journal of experimental medicine. 2010;221:35–42. doi: 10.1620/tjem.221.35. [DOI] [PubMed] [Google Scholar]
- Liu J, Hu F, Wang G, Zhou Q, Ding G. Lipopolysaccharide-induced expression of surfactant proteins A1 and A2 in human renal tubular epithelial cells. Journal of inflammation (London, England) 2013;10:2. doi: 10.1186/1476-9255-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liu Z, Feng L, Ding G, Chen D, Zhou Q. Effect of surfactant protein A on lipopolysaccharide-induced tumor necrosis factor-alpha expression in human proximal tubular epithelial cells. Chinese medical journal. 2014;127:343–347. [PubMed] [Google Scholar]
- Liu Z, Shi Q, Liu J, Abdel-Razek O, Xu Y, Cooney RN, Wang G. Innate Immune Molecule Surfactant Protein D Attenuates Sepsis-induced Acute Pancreatic Injury through Modulating Apoptosis and NF-kappaB-mediated Inflammation. Sci Rep. 2015;5:17798. doi: 10.1038/srep17798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lofgren J, Ramet M, Renko M, Marttila R, Hallman M. Association between surfactant protein A gene locus and severe respiratory syncytial virus infection in infants. J Infect Dis. 2002;185:283–289. doi: 10.1086/338473. [DOI] [PubMed] [Google Scholar]
- Lu J, Willis AC, Reid KB. Purification, characterization and cDNA cloning of human lung surfactant protein D. Biochem J. 1992;284(Pt 3):795–802. doi: 10.1042/bj2840795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo JM, Liu ZQ, Eugene CY. Overexpression of pulmonary surfactant protein A like molecules in inflammatory bowel disease tissues. Zhong nan da xue xue bao. Yi xue ban = Journal of Central South University Medical sciences. 2008;33:979–986. [PubMed] [Google Scholar]
- Luo JM, Wan YS, Liu ZQ, Wang GR, Floros J, Zhou HH. Regularity of distribution of immunoreactive pulmonary surfactant protein A in rat tissues. International journal of molecular medicine. 2004;14:343–351. [PubMed] [Google Scholar]
- Macklin CC. The pulmonary alveolar mucoid film and the pneumonocytes. Lancet. 1954;266:1099–1104. doi: 10.1016/s0140-6736(54)92154-6. [DOI] [PubMed] [Google Scholar]
- MacNeill C, Umstead TM, Phelps DS, Lin Z, Floros J, Shearer DA, Weisz J. Surfactant protein A, an innate immune factor, is expressed in the vaginal mucosa and is present in vaginal lavage fluid. Immunology. 2004;111:91–99. doi: 10.1111/j.1365-2567.2003.01782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan T, Reid KB, Clark H, Singh M, Nayak A, Sarma PU, Hawgood S, Kishore U. Susceptibility of mice genetically deficient in SP-A or SP-D gene to invasive pulmonary aspergillosis. Mol Immunol. 47:1923–1930. doi: 10.1016/j.molimm.2010.02.027. [DOI] [PubMed] [Google Scholar]
- Madsen J, Kliem A, Tornoe I, Skjodt K, Koch C, Holmskov U. Localization of lung surfactant protein D on mucosal surfaces in human tissues. Journal of immunology. 2000;164:5866–5870. doi: 10.4049/jimmunol.164.11.5866. [DOI] [PubMed] [Google Scholar]
- Marttila R, Haataja R, Guttentag S, Hallman M. Surfactant protein A and B genetic variants in respiratory distress syndrome in singletons and twins. Am J Respir Crit Care Med. 2003a;168:1216–1222. doi: 10.1164/rccm.200304-524OC. [DOI] [PubMed] [Google Scholar]
- Marttila R, Haataja R, Ramet M, Lofgren J, Hallman M. Surfactant protein B polymorphism and respiratory distress syndrome in premature twins. Hum Genet. 2003b;112:18–23. doi: 10.1007/s00439-002-0835-y. [DOI] [PubMed] [Google Scholar]
- Mayer S, Raulf MK, Lepenies B. C-type lectins: their network and roles in pathogen recognition and immunity. Histochem Cell Biol. 2017;147:223–237. doi: 10.1007/s00418-016-1523-7. [DOI] [PubMed] [Google Scholar]
- McCormack FX, Damodarasamy M, Elhalwagi BM. Deletion mapping of N-terminal domains of surfactant protein A. The N-terminal segment is required for phospholipid aggregation and specific inhibition of surfactant secretion. J Biol Chem. 1999;274:3173–3181. doi: 10.1074/jbc.274.5.3173. [DOI] [PubMed] [Google Scholar]
- McCormack FX, King TE, Jr, Voelker DR, Robinson PC, Mason RJ. Idiopathic pulmonary fibrosis. Abnormalities in the bronchoalveolar lavage content of surfactant protein A. Am Rev Respir Dis. 1991;144:160–166. doi: 10.1164/ajrccm/144.1.160. [DOI] [PubMed] [Google Scholar]
- McCormack FX, Pattanajitvilai S, Stewart J, Possmayer F, Inchley K, Voelker DR. The Cys6 intermolecular disulfide bond and the collagenlike region of rat SP-A play critical roles in interactions with alveolar type II cells and surfactant lipids. J Biol Chem. 1997;272:27971–27979. doi: 10.1074/jbc.272.44.27971. [DOI] [PubMed] [Google Scholar]
- McGreal EP, Ikewaki N, Akatsu H, Morgan BP, Gasque P. Human C1qRp is identical with CD93 and the mNI-11 antigen but does not bind C1q. Journal of immunology. 2002;168:5222–5232. doi: 10.4049/jimmunol.168.10.5222. [DOI] [PubMed] [Google Scholar]
- McIntosh JC, Swyers AH, Fisher JH, Wright JR. Surfactant proteins A and D increase in response to intratracheal lipopolysaccharide. Am J Respir Cell Mol Biol. 1996;15:509–519. doi: 10.1165/ajrcmb.15.4.8879185. [DOI] [PubMed] [Google Scholar]
- McNeely TB, Coonrod JD. Aggregation and opsonization of type A but not type B Hemophilus influenzae by surfactant protein A. Am J Respir Cell Mol Biol. 1994;11:114–122. doi: 10.1165/ajrcmb.11.1.8018334. [DOI] [PubMed] [Google Scholar]
- Mikerov AN, Haque R, Gan X, Guo X, Phelps DS, Floros J. Ablation of SP-A has a negative impact on the susceptibility of mice to Klebsiella pneumoniae infection after ozone exposure: sex differences. Respir Res. 2008;9:77. doi: 10.1186/1465-9921-9-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamura K, Malhotra R, Hoppe HJ, Reid KB, Phizackerley PJ, Macpherson P, Lopez Bernal A. Surfactant proteins A (SP-A) and D (SP-D): levels in human amniotic fluid and localization in the fetal membranes. Biochim Biophys Acta. 1994;1210:303–307. doi: 10.1016/0005-2760(94)90233-x. [DOI] [PubMed] [Google Scholar]
- Mo YK, Kankavi O, Masci PP, Mellick GD, Whitehouse MW, Boyle GM, Parsons PG, Roberts MS, Cross SE. Surfactant protein expression in human skin: evidence and implications. The Journal of investigative dermatology. 2007;127:381–386. doi: 10.1038/sj.jid.5700561. [DOI] [PubMed] [Google Scholar]
- Montalbano AP, Hawgood S, Mendelson CR. Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2 manifest delayed parturition and decreased expression of inflammatory and contractile genes. Endocrinology. 2013;154:483–498. doi: 10.1210/en.2012-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motwani M, White RA, Guo N, Dowler LL, Tauber AI, Sastry KN. Mouse surfactant protein-D. cDNA cloning, characterization, and gene localization to chromosome 14. Journal of immunology. 1995;155:5671–5677. [PubMed] [Google Scholar]
- Nadesalingam J, Bernal AL, Dodds AW, Willis AC, Mahoney DJ, Day AJ, Reid KB, Palaniyar N. Identification and characterization of a novel interaction between pulmonary surfactant protein D and decorin. J Biol Chem. 2003;278:25678–25687. doi: 10.1074/jbc.M210186200. [DOI] [PubMed] [Google Scholar]
- Nadesalingam J, Reid KB, Palaniyar N. Collectin surfactant protein D binds antibodies and interlinks innate and adaptive immune systems. FEBS letters. 2005;579:4449–4453. doi: 10.1016/j.febslet.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Nayak A, Dodagatta-Marri E, Tsolaki AG, Kishore U. An Insight into the Diverse Roles of Surfactant Proteins, SP-A and SP-D in Innate and Adaptive Immunity. Front Immunol. 2012;3:131. doi: 10.3389/fimmu.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepomuceno RR, Henschen-Edman AH, Burgess WH, Tenner AJ. cDNA cloning and primary structure analysis of C1qR (P), the human C1q/MBL/SPA receptor that mediates enhanced phagocytosis in vitro. Immunity. 1997;6:119–129. doi: 10.1016/s1074-7613(00)80419-7. [DOI] [PubMed] [Google Scholar]
- Norsworthy PJ, Fossati-Jimack L, Cortes-Hernandez J, Taylor PR, Bygrave AE, Thompson RD, Nourshargh S, Walport MJ, Botto M. Murine CD93 (C1qRp) contributes to the removal of apoptotic cells in vivo but is not required for C1q-mediated enhancement of phagocytosis. Journal of immunology. 2004;172:3406–3414. doi: 10.4049/jimmunol.172.6.3406. [DOI] [PubMed] [Google Scholar]
- Ohya M, Nishitani C, Sano H, Yamada C, Mitsuzawa H, Shimizu T, Saito T, Smith K, Crouch E, Kuroki Y. Human pulmonary surfactant protein D binds the extracellular domains of Toll-like receptors 2 and 4 through the carbohydrate recognition domain by a mechanism different from its binding to phosphatidylinositol and lipopolysaccharide. Biochemistry. 2006;45:8657–8664. doi: 10.1021/bi060176z. [DOI] [PubMed] [Google Scholar]
- Pavlovic J, Papagaroufalis C, Xanthou M, Liu W, Fan R, Thomas NJ, Apostolidou I, Papathoma E, Megaloyianni E, DiAngelo S, Floros J. Genetic variants of surfactant proteins A, B, C, and D in bronchopulmonary dysplasia. Dis Markers. 2006;22:277–291. doi: 10.1155/2006/817805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawaria S, Binder RJ. CD91-dependent programming of T-helper cell responses following heat shock protein immunization. Nat Commun. 2011;2:521. doi: 10.1038/ncomms1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson A, Chang D, Crouch E. Surfactant protein D is a divalent cation-dependent carbohydrate-binding protein. J Biol Chem. 1990;265:5755–5760. [PubMed] [Google Scholar]
- Petersen SV, Thiel S, Jensenius JC. The mannan-binding lectin pathway of complement activation: biology and disease association. Mol Immunol. 2001;38:133–149. doi: 10.1016/s0161-5890(01)00038-4. [DOI] [PubMed] [Google Scholar]
- Quintanilla HD, Liu Y, Fatheree NY, Atkins CL, Hashmi SS, Floros J, McCormack FX, Rhoads JM, Alcorn JL. Oral administration of surfactant protein-a reduces pathology in an experimental model of necrotizing enterocolitis. J Pediatr Gastroenterol Nutr. 2015;60:613–620. doi: 10.1097/MPG.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rausch F, Schicht M, Paulsen F, Ngueya I, Brauer L, Brandt W. “SP-G”, a putative new surfactant protein--tissue localization and 3D structure. PloS one. 2012;7:e47789. doi: 10.1371/journal.pone.0047789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichhardt MP, Loimaranta V, Thiel S, Finne J, Meri S, Jarva H. The salivary scavenger and agglutinin binds MBL and regulates the lectin pathway of complement in solution and on surfaces. Front Immunol. 2012;3:205. doi: 10.3389/fimmu.2012.00205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid KB. Functional roles of the lung surfactant proteins SP-A and SP-D in innate immunity. Immunobiology. 1998;199:200–207. doi: 10.1016/S0171-2985(98)80027-2. [DOI] [PubMed] [Google Scholar]
- Remer KA, Brcic M, Jungi TW. Toll-like receptor-4 is involved in eliciting an LPS-induced oxidative burst in neutrophils. Immunol Lett. 2003;85:75–80. doi: 10.1016/s0165-2478(02)00210-9. [DOI] [PubMed] [Google Scholar]
- Saka R, Wakimoto T, Nishiumi F, Sasaki T, Nose S, Fukuzawa M, Oue T, Yanagihara I, Okuyama H. Surfactant protein-D attenuates the lipopolysaccharide-induced inflammation in human intestinal cells overexpressing toll-like receptor 4. Pediatr Surg Int. 2016;32:59–63. doi: 10.1007/s00383-015-3812-y. [DOI] [PubMed] [Google Scholar]
- Salinas CN, Anseth KS. Decorin moieties tethered into PEG networks induce chondrogenesis of human mesenchymal stem cells. J Biomed Mater Res A. 2009;90:456–464. doi: 10.1002/jbm.a.32112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Paananen R, Vuolteenaho R, Metsola J, Ojaniemi M, Autio-Harmainen H, Hallman M. Maternal endotoxin-induced preterm birth in mice: fetal responses in toll-like receptors, collectins, and cytokines. Pediatr Res. 2008;63:280–286. doi: 10.1203/PDR.0b013e318163a8b2. [DOI] [PubMed] [Google Scholar]
- Sano H, Chiba H, Iwaki D, Sohma H, Voelker DR, Kuroki Y. Surfactant proteins A and D bind CD14 by different mechanisms. J Biol Chem. 2000;275:22442–22451. doi: 10.1074/jbc.M001107200. [DOI] [PubMed] [Google Scholar]
- Sapkota M, Kharbanda KK, Wyatt TA. Malondialdehyde-Acetaldehyde-Adducted Surfactant Protein Alters Macrophage Functions Through Scavenger Receptor A. Alcohol Clin Exp Res. 2016;40:2563–2572. doi: 10.1111/acer.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Sano H, Iwaki D, Kudo K, Konishi M, Takahashi H, Takahashi T, Imaizumi H, Asai Y, Kuroki Y. Direct binding of Toll-like receptor 2 to zymosan, and zymosan-induced NF-kappa B activation and TNF-alpha secretion are down-regulated by lung collectin surfactant protein A. Journal of immunology. 2003;171:417–425. doi: 10.4049/jimmunol.171.1.417. [DOI] [PubMed] [Google Scholar]
- Schicht M, Rausch F, Finotto S, Mathews M, Mattil A, Schubert M, Koch B, Traxdorf M, Bohr C, Worlitzsch D, Brandt W, Garreis F, Sel S, Paulsen F, Brauer L. SFTA3, a novel protein of the lung: three-dimensional structure, characterisation and immune activation. The European respiratory journal. 2014;44:447–456. doi: 10.1183/09031936.00179813. [DOI] [PubMed] [Google Scholar]
- Schob S, Dieckow J, Fehrenbach M, Peukert N, Weiss A, Kluth D, Thome U, Quaschling U, Lacher M, Preuss M. Occurrence and colocalization of surfactant proteins A, B, C and D in the developing and adult rat brain. Ann Anat. 2016 doi: 10.1016/j.aanat.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Schob S, Schicht M, Sel S, Stiller D, Kekule AS, Paulsen F, Maronde E, Brauer L. The detection of surfactant proteins A, B, C and D in the human brain and their regulation in cerebral infarction, autoimmune conditions and infections of the CNS. PLoS ONE. 2013;8:e74412. doi: 10.1371/journal.pone.0074412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senft AP, Korfhagen TR, Whitsett JA, Shapiro SD, LeVine AM. Surfactant protein-D regulates soluble CD14 through matrix metalloproteinase-12. Journal of immunology. 2005;174:4953–4959. doi: 10.4049/jimmunol.174.8.4953. [DOI] [PubMed] [Google Scholar]
- Sever-Chroneos Z, Krupa A, Davis J, Hasan M, Yang CH, Szeliga J, Herrmann M, Hussain M, Geisbrecht BV, Kobzik L, Chroneos ZC. Surfactant protein A (SP-A)-mediated clearance of Staphylococcus aureus involves binding of SP-A to the staphylococcal adhesin eap and the macrophage receptors SP-A receptor 210 and scavenger receptor class A. J Biol Chem. 2011;286:4854–4870. doi: 10.1074/jbc.M110.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfarth J, Garred P, Madsen HO. The ‘involution’ of mannose-binding lectin. Hum Mol Genet. 2005;14:2859–2869. doi: 10.1093/hmg/ddi318. [DOI] [PubMed] [Google Scholar]
- Smith LM, Qureshi N, Chao CR. Effects of single and multiple courses of antenatal glucocorticoids in preterm newborns less than 30 weeks' gestation. J Matern Fetal Med. 2000;9:131–135. doi: 10.1002/(SICI)1520-6661(200003/04)9:2<131::AID-MFM9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Snegovskikh VV, Bhandari V, Wright JR, Tadesse S, Morgan T, Macneill C, Foyouzi N, Park JS, Wang Y, Norwitz ER. Surfactant protein-A (SP-A) selectively inhibits prostaglandin F2alpha (PGF2alpha) production in term decidua: implications for the onset of labor. The Journal of clinical endocrinology and metabolism. 2011;96:E624–632. doi: 10.1210/jc.2010-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JM, Kwun JE, O'rien JA, Rosenfeld CR, Odom MJ. The concentration of the 35-kDa surfactant apoprotein in amniotic fluid from normal and diabetic pregnancies. Pediatr Res. 1988;24:728–734. doi: 10.1203/00006450-198812000-00016. [DOI] [PubMed] [Google Scholar]
- Steinberger P, Szekeres A, Wille S, Stockl J, Selenko N, Prager E, Staffler G, Madic O, Stockinger H, Knapp W. Identification of human CD93 as the phagocytic C1q receptor (C1qRp) by expression cloning. J Leukoc Biol. 2002;71:133–140. [PubMed] [Google Scholar]
- Tan RM, Kuang Z, Hao Y, Lee F, Lee T, Lee RJ, Lau GW. Type IV pilus glycosylation mediates resistance of Pseudomonas aeruginosa to opsonic activities of the pulmonary surfactant protein A. Infect Immun. 2015;83:1339–1346. doi: 10.1128/IAI.02874-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner AJ, Robinson SL, Borchelt J, Wright JR. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem. 1989;264:13923–13928. [PubMed] [Google Scholar]
- van Iwaarden F, Welmers B, Verhoef J, Haagsman HP, van Golde LM. Pulmonary surfactant protein A enhances the host-defense mechanism of rat alveolar macrophages. Am J Respir Cell Mol Biol. 1990;2:91–98. doi: 10.1165/ajrcmb/2.1.91. [DOI] [PubMed] [Google Scholar]
- Vandivier RW, Henson PM, Douglas IS. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- Vayrynen O, Glumoff V, Hallman M. Regulation of surfactant proteins by LPS and proinflammatory cytokines in fetal and newborn lung. American journal of physiology. Lung cellular and molecular physiology. 2002;282:L803–810. doi: 10.1152/ajplung.00274.2001. [DOI] [PubMed] [Google Scholar]
- Von Neergaard K. Neue Auffassungen uber einen Grundbegriff der Atemmechanik- Die Retraktionskraft der Lunge, abhängig von der Oberfl ächenspannung in den Alveolen. Z Gesamt Exp Med. 1929;66:373–394. [Google Scholar]
- Voss T, Eistetter H, Schafer KP, Engel J. Macromolecular organization of natural and recombinant lung surfactant protein SP 28-36. Structural homology with the complement factor C1q. J Mol Biol. 1988;201:219–227. doi: 10.1016/0022-2836(88)90448-2. [DOI] [PubMed] [Google Scholar]
- Voss T, Melchers K, Scheirle G, Schafer KP. Structural comparison of recombinant pulmonary surfactant protein SP-A derived from two human coding sequences: implications for the chain composition of natural human SP-A. Am J Respir Cell Mol Biol. 1991;4:88–94. doi: 10.1165/ajrcmb/4.1.88. [DOI] [PubMed] [Google Scholar]
- Weikert LF, Edwards K, Chroneos ZC, Hager C, Hoffman L, Shepherd VL. SP-A enhances uptake of bacillus Calmette-Guerin by macrophages through a specific SP-A receptor. Am J Physiol. 1997;272:L989–995. doi: 10.1152/ajplung.1997.272.5.L989. [DOI] [PubMed] [Google Scholar]
- Weinhold B. Epigenetics: the science of change. Environ Health Perspect. 2006;114:A160–167. doi: 10.1289/ehp.114-a160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis WI, Drickamer K, Hendrickson WA. Structure of a C-type mannose-binding protein complexed with an oligosaccharide. Nature. 1992;360:127–134. doi: 10.1038/360127a0. [DOI] [PubMed] [Google Scholar]
- White RA, Dowler LL, Adkison LR, Ezekowitz RA, Sastry KN. The murine mannose-binding protein genes (Mbl 1 and Mbl 2) localize to chromosomes 14 and 19. Mamm Genome. 1994;5:807–809. doi: 10.1007/BF00292020. [DOI] [PubMed] [Google Scholar]
- Wofford JA, Wright JR. Surfactant protein A regulates IgG-mediated phagocytosis in inflammatory neutrophils. American journal of physiology. Lung cellular and molecular physiology. 2007;293:L1437–1443. doi: 10.1152/ajplung.00239.2007. [DOI] [PubMed] [Google Scholar]
- Wong CJ, Akiyama J, Allen L, Hawgood S. Localization and developmental expression of surfactant proteins D and A in the respiratory tract of the mouse. Pediatr Res. 1996;39:930–937. doi: 10.1203/00006450-199606000-00002. [DOI] [PubMed] [Google Scholar]
- Wright JR. Host defense functions of pulmonary surfactant. Biol Neonate. 2004;85:326–332. doi: 10.1159/000078172. [DOI] [PubMed] [Google Scholar]
- Wu H, Kuzmenko A, Wan S, Schaffer L, Weiss A, Fisher JH, Kim KS, McCormack FX. Surfactant proteins A and D inhibit the growth of Gram-negative bacteria by increasing membrane permeability. J Clin Invest. 2003;111:1589–1602. doi: 10.1172/JCI16889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Zhao G, Lin J, Jiang N, Li C, Hu L, Peng X, Xu Q, Wang Q, Li H, Zhang Y. The production mechanism and immunosuppression effect of pulmonary surfactant protein D via toll like receptor 4 signaling pathway in human corneal epithelial cells during Aspergillus fumigatus infection. Int Immunopharmacol. 2015;29:433–439. doi: 10.1016/j.intimp.2015.10.018. [DOI] [PubMed] [Google Scholar]
- Yadav AK, Madan T, Bernal AL. Surfactant proteins A and D in pregnancy and parturition. Frontiers in bioscience (Elite edition) 2011;3:291–300. doi: 10.2741/e244. [DOI] [PubMed] [Google Scholar]
- Yamada C, Sano H, Shimizu T, Mitsuzawa H, Nishitani C, Himi T, Kuroki Y. Surfactant protein A directly interacts with TLR4 and MD-2 and regulates inflammatory cellular response. Importance of supratrimeric oligomerization. J Biol Chem. 2006;281:21771–21780. doi: 10.1074/jbc.M513041200. [DOI] [PubMed] [Google Scholar]
- Yamada M, Oritani K, Kaisho T, Ishikawa J, Yoshida H, Takahashi I, Kawamoto S, Ishida N, Ujiie H, Masaie H, Botto M, Tomiyama Y, Matsuzawa Y. Complement C1q regulates LPS-induced cytokine production in bone marrow-derived dendritic cells. Eur J Immunol. 2004;34:221–230. doi: 10.1002/eji.200324026. [DOI] [PubMed] [Google Scholar]
- Yang L, Carrillo M, Wu YM, DiAngelo SL, Silveyra P, Umstead TM, Halstead ES, Davies ML, Hu S, Floros J, McCormack FX, Christensen ND, Chroneos ZC. SP-R210 (Myo18A) Isoforms as Intrinsic Modulators of Macrophage Priming and Activation. PLoS One. 2015;10:e0126576. doi: 10.1371/journal.pone.0126576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ikegami M, Crouch EC, Korfhagen TR, Whitsett JA. Activity of pulmonary surfactant protein-D (SP-D) in vivo is dependent on oligomeric structure. J Biol Chem. 2001;276:19214–19219. doi: 10.1074/jbc.M010191200. [DOI] [PubMed] [Google Scholar]