Abstract
Pseudoaneurysms (PSAs) of the hepatic and/or cystic artery are a rare complication following a laparoscopic cholecystectomy (LC). Generally, PSA cases present with haemobilia several weeks following the procedure. Transarterial embolisation (TAE) is considered the optimal management approach. We report a 70-year-old woman who presented to the Sultan Qaboos University Hospital, Muscat, Oman, in 2016 with massive hemoperitoneum two weeks after undergoing a LC procedure in another hospital. She was successfully managed using coil TAE. An extensive literature review revealed 101 cases of hepatic or cystic artery PSAs following a LC procedure. Haemobilia was the main presentation (85.1%) and the mean time of postoperative presentation was 36 days. The hepatic artery was involved in most cases (88.1%), followed by the cystic artery (7.9%) and a combination of both (4.0%). Most cases were managed with TAE (72.3%), with a 94.5% success rate. The overall mortality rate was 2.0%.
Keywords: Hepatic Artery, Pseudoaneurysm, Hemoperitoneum, Therapeutic Embolization, Laparoscopic Cholecystectomy
A laparoscopic cholecystectomy (LC) is the gold standard of treatment for symptomatic cholelithiasis due to its distinct advantages of reduced pain, early discharge and reduced wound complications.1,2 However, it is rarely associated with major complications, such as vascular complications (0.25%).1,2 These include intraoperative bleeding following injury to the cystic/hepatic artery or the portal vein as well as postoperative pseudoaneurysms (PSAs) of the cystic or hepatic arteries.2–5 The mechanisms involved in the formation of a PSA may be multifactorial and include excessive use of cauterisation during the dissection of Calot’s triangle, particularly if there are any abnormal anatomical variations of the vasculature.2,6 Patients who present postoperatively with PSAs may develop hemoperitoneum or haemobilia. 3–9 This article describes a patient with a PSA of the hepatic artery originating in the cystic artery and then presents an extensive review of the scientific literature regarding the aetiopathogenesis, presentation, diagnosis and management of PSAs following LC procedures.
Case Report
A 70-year-old female with known hypertension presented to the Emergency Medicine Department of the Sultan Qaboos University Hospital, Muscat, Oman, in 2016 after collapsing due to sudden-onset abdominal pain. She had undergone a LC at another hospital 13 days previously and had had a prolonged four-day admission following the procedure for reasons unknown. The abdominal pain was diffuse, colicky in nature and associated with nausea and vomiting. There was no associated fever, haematemesis or melena.
At presentation, the patient was hypotensive but responded to fluid resuscitation. She appeared pale and in severe pain. A physical examination revealed tenderness on the right side of the abdomen with abdominal guarding. Laboratory investigations revealed a haemoglobin level of 6.7 g/dL, haematocrit level of 0.26 L/L and raised lactate levels, with all other measurements within normal limits. A computed tomography (CT) scan of the abdomen with intravenous contrast showed multiple collections of fluid in the abdomen and pelvis with some reactionary changes to the bowel wall and no evidence of mesenteric ischaemia [Figures 1A and B]. After receiving a blood transfusion, the patient was transferred to the operating room for a diagnostic laparoscopy, which revealed 1,500 mL of blood and clots with small bleeding points in the liver bed around the clips from the initial LC surgery. These bleeding points were subsequently clipped. There was no evidence of bile leakage.
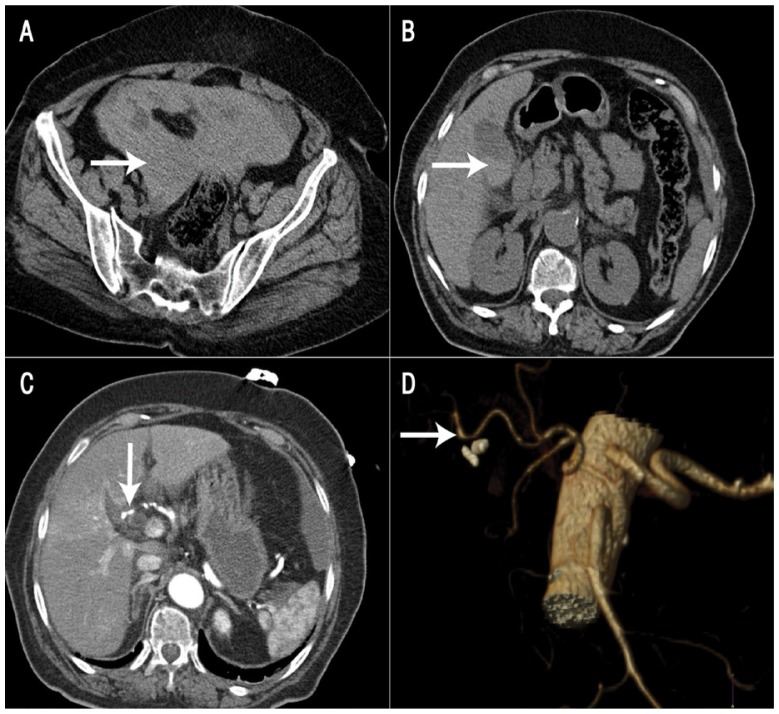
Figure 1.
Computed tomography (CT) images of a 70-year-old woman two weeks after a laparoscopic cholecystectomy. A: Non-contrast CT scan of the pelvis showing hyper-dense pelvic fluid (arrow). B: Non-contrast CT scan of the upper abdomen showing a small collection of fluid in the gallbladder fossa with layering hyper-dense contents (arrow). C: Arterial-phase CT scan showing a slight irregularity (arrow) in the right hepatic artery (RHA). D: Three-dimensional CT reconstruction confirming the irregularity and focal dilatation of the RHA (arrow).
Following the procedure, detailed examination of the CT scans suggested a PSA of the right hepatic artery (RHA) at the point of origin of the cystic artery [Figures 1C and D]. Conventional angiography confirmed these findings and coil embolisation of the PSA in the RHA was carried out [Figure 2]. The patient’s liver function test findings remained normal during the postoperative period. However, the postoperative course was complicated by a hernia at the umbilical port site for which she underwent an open repair. She was discharged on the 12th postoperative day. Six weeks later, during a follow-up appointment in the outpatient clinic, she appeared to be doing well.
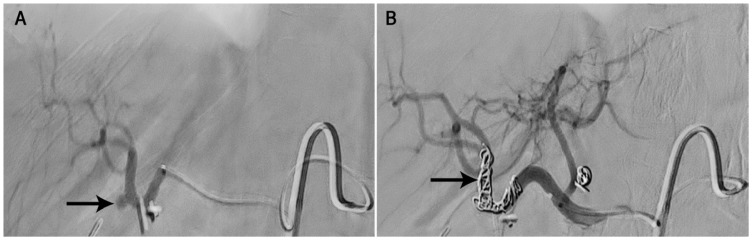
Figure 2.
Selective angiograms of the hepatic artery of a 70-year-old woman two weeks after a laparoscopic cholecystectomy showing (A) focal outpouching from the right hepatic artery (RHA) at the site of origin of the cystic artery (arrow) and (B) complete obliteration of the pseudoaneurysm (arrow) following coil embolisation of the RHA.
Literature Review
METHODS
A literature search was carried out using the MEDLINE® database (National Library of Medicine, Bethesda, Maryland, USA) for all English-language articles on post-LC hepatic or cystic artery PSAs. In addition, references in articles were cross-checked to further identify potential articles on this topic. The inclusion criteria for articles encompassed all article types (including case reports and case series) published before April 2016. However, articles with inadequate data were excluded.
RESULTS
A total of 60 articles were identified during the literature search, including 101 cases of post-LC hepatic or cystic artery PSAs [Table 1].3–62 Among the cases which specified the gender or age of the patient, 45.2% were male and the age ranged from 12–81 years with a mean age of 49.8 years.4,5,9–13,15,17–21,24–31,33–35,37–43,46–48,51,57,58,60,62 The time of presentation following the LC procedure ranged from six days to five years with a mean of 36 days.3–5,7–13,15,17–21,23,24,26,28–31,33–44,46–53,55–62 The most common presentation was haemobilia (85.1%), followed by haematemesis (10.9%), jaundice (9.9%), abdominal pain (9.9%) anaemia (5.0%) and melena (5.0%).3–62
Table 1.
Literature review of cases of pseudoaneurysm of the cystic and/or hepatic arteries following a laparoscopic cholecystectomy3–62
| Author and year of article | N | Age in years/gender | Vessel involved | Details of cholecystectomy | Time of presentation | Presentation | Treatment | Complications | Patient outcome |
|---|---|---|---|---|---|---|---|---|---|
| Feng et al.16 2017* | 14 | - | RHA (n = 14) | - | - | •HMB | TAE (coils) | Post-embolisation syndrome and hepatic ischaemia (n = 9) | Good |
| Hsiao et al.17 2015 | 1 | 40/M | RHA (33 mm) | - | 2 weeks | •JN •Ab pain |
TAE (coils and PTCD) | None | Good |
| Bin Traiki et al.18 2015 | 1 | 64/M | RHA arising from a replaced SMA and CBD injury | - | 4 weeks | •Fever •Pain •JN |
Stent followed by a repeat stent and TAE (gel foam) | Bleeding from the LHA after placement of the initial stent | Good |
| Abdalla et al.19 2015 | 1 | 40/M | RHA | Single-port LC | 2 weeks | •JN •Ab pain |
TAE (coil) | - | Good |
| Kumar et al.6 2014 | 1 | - | CA branch (20 mm) | - | - | •HMB | TAE (coils) followed by percutaneous TI | Failed TAE | - |
| Rencuzogullari et al.20 2014 | 1 | 43/M | RHA (ant/post bifurcation) | - | 3 weeks | •HMB •Pain |
RHA ligation and T-tube placement | - | - |
| Panda et al.21 2014 | 1 | 29/M | RHA (right post sectoral artery PSA) | CA was divided using a harmonic scalpel | 2 weeks | •Ab pain •AN |
Laparoscopic repair (Pringle manoeuvre); TAE was not carried out as the aneurysm was close to the RHA from the sectoral branch | - | Good |
| Murugesan et al.22 2014 | 3 | - | RHA (n = 3) | - | - | •HMB | TAE | - | Good |
| Kirschberg et al.23 2013 | 1 | - | RHA | - | 4 months | •HMB •Melena •Dizziness •Sweating |
TAE (microcoil) | - | Good |
| Mate et al.24 2013 | 1 | 45/M | RHA | - | 15 days | •HMB •Dizziness |
RHA ligation and loop colostomy | - | Good |
| Petrou et al.15 2012 | 1 | 34/F | CA (13 mm) | - | 3 months | •HMB •Haematemesis •Ab pain |
TAE (microcoil) followed by RHA ligation | Bleeding after a month following TAE as multiple previous coils affected the bifurcation | Good |
| Butet et al.26 2012 | 1 | 79 | CA and RHA (35 mm) | - | 3 months | •JN •Ab pain |
RHA ligation and PSA excision | Immediate decompression of CBD | Good |
| Sansonna et al.31 2011 | 1 | 55/F | RHA arising from a replaced SMA (20 mm) and bile leakage from the segment V biliary branch | - | 3 weeks | •HMB •Hypotension •Recurrent melena •Hb level of 8 g/dL |
TAE (microcoils) and nasobiliary drainage for bile leakage | None | Good |
| Hadj et al.30 2011 | 1 | 32/F | RHA (40mm) | Difficult LC procedure with some bleeding near the cystic duct which was controlled with clips | 5 months | •HMB | TAE (microcoils) and ERCP removal of blood clots | None | Good |
| Khan et al.28 2011 | 1 | 54/F | RHA | - | 5 months | •HMB •Haematemesis •Hb level of 5 g/dL |
TAE (microcoils) | None | Good |
| Kamani et al.29 2011 | 1 | 60/M | Accessory RHA | - | 4 months | •HMB | TAE (microcoils) | None | Good |
| Gandhi et al.25 2011 | 3 | 39†/16:6‡ | RHA (n = 3) | - | - | •HMB | TAE (microcoils) | None | Good |
| Caminitiet al.27 2011 | 1 | - | RHA | - | - | •HMB | TAE (microcoils) | None | Good |
| Hylton et al.33 2010 | 1 | 53/F | RHA (32 × 20 mm) | - | 1 year | •HMB •Stenosis of RHA with PSA (90%) |
Stent cover | None | Good |
| Yao et al.34 2010 | 1 | 54/M | RHA | - | 6 weeks | •Ab pain •AN (Hb level of 7.6 g/dL) •Gangrenous cholecystitis |
TAE (microcoils) | None | Good |
| Malik et al.57 2010 | 1 | 35/F | RHA (35 × 35 mm) and mass-effect partial CBD compression | - | 1 month | •Fever •Ab pain •Vomiting •AN (Hb level of 7.5 g/dL) |
Exploratory laparotomy and RHA transfixation ligature | Hypotension and collapse upon manipulation on the operating table; patient was given 7 units of blood in the ICU for a month | Good |
| Boddy et al.32 2010 | 1 | - | RHA | - | - | •HMB | Stent and TI for small residual PSA | None | Good |
| Chen et al.35 2009 | 1 | 67/M | RHA | - | 2 months | •HMB •Haematemesis •Hb level of 9.3 g/dL |
TAE | None | Good |
| Moses et al.58 2008 | 1 | 26/M | CA stump PSA with no active bleeding | - | 3 months | •Upper GI HMB | TAE (microcoils) | None | Good |
| Masannat et al.41 2008 | 1 | 71/F | Multiple RHA PSAs and CA adhesions ant to the common HA | The LC was converted to an open procedure | 6 days | •Ab pain •Drop in Hb level (9 gm/dL) |
TAE (microcoil) | None | Good |
| Srinivasaiah et al.39 2008 | 1 | 57/F | RHA (69 mm) | - | 4 weeks | •HMB •Haematemesis •Acute cholecystitis |
TAE (microcoil) | None | Good |
| Sebastián et al.40 2008 | 1 | 66/M | RHA | - | 1 month | •HMB •Haematemesis •Formation of a fistula from the RHA to the duodenum |
Laparostomy and RHA suturing followed by a repeat laparostomy due to bleeding | Severe haemorrhagic relapse | Patient death |
| Nakase et al.37 2008 | 1 | 63/F | CA (30 mm) | Monopolar hook dissection | 11 days | •HMB •Melena •Haematemesis (Hb level of 7.4 g/dL) •Acute cholecystitis |
TAE with NBCA (normal angiogram) followed by microcoils to treat the other PSA | Liver abscess and a second PSA seen on repeat CT 27 days after the first surgery | Good |
| Kumar et al.36 2008 | 6 | - | •RHA (n = 3) •Ant branch RHA (n = 1) •RHA with common hepatic duct injury (n = 2) |
- | 18–98 days | • HMB | TAE (microcoils) | None | Good |
| Madanur et al.4 2007 | 4 | 57–64;3:1‡ | •RHA (n = 3; 19–30 mm) •CA (n = 1) •Common hepatic duct injury (n = 4) |
- | 2–5 weeks | •HMB (n = 4) •Haematemesis (n = 2) •HMB and melena (n = 2) |
•TAE with microcoils (n = 3) •Stent (n = 1) •RHA ligation (n = 1) •Hepaticojejunostomy (n = 3) |
Intra-abdominal bile collection (n = 1) | Good |
| Roche-Nagle et al.42 2006 | 1 | 58/F | RHA branch (30 mm) | - | 5 days | •Intraperitoneal bleeding (Hb level of 6.1 g/dL) •Ab pain •Fever •Acute cholecystitis |
TAE (multiple coils) followed by an exploratory laparotomy, ligation of feeding vessel into PSA and closure of sac | Failed TAE with growth of PSA over 2 months | Good |
| De Molla Neto et al.38 2006 | 1 | 31/F | CA | - | 50 days | •Pale •JN |
RHA ligation as stump was inflammatory | None | Good |
| Heyn et al.62 2006 | 1 | 78/M | CA | - | 1 year | •HMB •Recurrent melena •Haematemesis |
Exploratory laparotomy, PSA resection of PSA, CBD repair and T-tube placement | None | Good |
| Journé et al.14 2004 | 1 | - | RHA and CA | - | - | •HMB | TAE (coil/nasobiliary drainage) | None | Good |
| Chigot et al.43 2003 | 1 | 12/F | RHA ant branch (20 × 20 mm) and bleeding from inferior RHA branch | Difficult LC due to oedema and inflammation | 28 days | •HMB •Ab pain |
TAE (cyanoacrylate) via transhepatic route under fluoroscopy | None | Good |
| Yahchouchy-Chouillard et al.13 2003 | 2 | 68/F 81/F |
•RHA (200 mm) •RHA |
- | •3 weeks •3 months |
•HMB, fever, AN, nausea and Hb levels of 8.2 g/dL •JN, pain, melena and Hb levels of 8.4 g/dL |
TAE (microcoil; n = 2) | None | Good |
| Saldinger et al.9 2002 | 1 | 50/F | Interconnected RHA and CA | - | 3 weeks | •HMB •Haematemesis •Melena |
TAE (microcoils) | None | Good |
| Ozkan et al.45 2002 | 1 | - | RHA | - | - | •HMB | TAE (Guglielmi detachable coil) | Erosion of coil into CBD presenting as pancreatitis 2 years later | Good |
| Bulut et al.7 2002 | 3 | - | •RHA (small PSA) •RHA (intrahepatic PSA) with CBD/PV injury •RHA with rupture into liver parenchyma |
- | Weeks–5 years | •HMB | •TAE (n = 1) •Surgical ligation (n = 1) •TAE followed by surgery (n = 1) |
Failed TAE | •Good •Good •Patient death |
| Jain et al.44 2002 | 2 | - | •HA •GDA |
- | •1 month •2 months |
•HMB | TAE with homemade coils (n = 2) | None | Good |
| Nicholson et al.3 1999 | 9 | - | RHA (n = 9) | - | 9–43 days | •HMB | TAE | Candida liver abscess | Good |
| Halbe et al.46 1999 | 1 | 32/F | RHA | - | 3 weeks | •HMB •Melena •Haematemesis |
TAE (microcoil) | None | Good |
| Kwauk et al.47 1998 | 1 | 35/F | RHA | - | 3 weeks | •HMB | TAE | None | Good |
| Balasara et al.8 1998 | 2 | - | RHA (n = 2) | - | •7 days •4 months |
•Hemoperitoneum and bile leakage •JN and HMB |
Exploratory laparotomy and ligation (n = 2) | None | Good |
| Ribeiro et al.48 1998 | 1 | 57/F | RHA | - | 13 months | •HMB | Exploratory laparotomy and ligation | None | Good |
| England et al.59 1998 | 1 | - | RHA | - | 4 weeks | •HMB | TAE | None | Good |
| Doctor et al.60 1998 | 1 | 49/F | RHA | - | 6 weeks | •Recurrent HMB | TAE | None | Good |
| Ibrarullah et al.12 1997 | 1 | - | RHA | - | 2 weeks | •HMB •Massive haematemesis |
Laparotomy and RHA ligation | None | Good |
| Kapoor et al.50 1997 | 1 | - | RHA | - | 7 weeks | •HMB | TAE | None | Good |
| Halpern49 1997 | 1 | - | RHA | - | 6 weeks | •HMB | TAE | None | Good |
| Yelle et al.51 1996 | 1 | 49/F | RHA and right hepatic duct injury | - | 3 weeks | •HMB •Bile fistula |
Exploratory laparotomy and suturing of RHA tear followed by repeat laparotomy and suturing | Recurrence of bleeding 3 weeks later | Good |
| Siablis et al.11 1996 | 1 | 29/M | RHA | - | 1 month | •HMB •Pain •JN |
TAE | None | Good |
| Rivitz et al.53 1996 | 1 | - | RHA | - | 1 month | •HMB | TAE | None | Good |
| Porte et al.52 1996 | 1 | - | RHA | - | 11 days | •HMB | TAE | None | Good |
| Stewart et al.54 1995 | 4 | - | RHA ( n = 4) | - | - | •HMB | TAE (n = 4) | None | Good |
| Bergey et al.10 1995 | 1 | 39/M | RHA | - | 3 weeks | •HMB | TAE | None | Good |
| Zilberstein et al.61 1994 | 1 | - | CA | - | Weeks–4 months | •HMB | TAE | None | Good |
| Moran et al.56 1994 | 1 | - | RHA | - | 3–5 days | •HMB | TAE | None | Good |
| Genyk et al.5 1994 | 1 | 57/F | RHA | - | 2 weeks | •HMB •JN •Ab pain •Upper GI bleeding |
TAE and direct TI to PSA | None | Good |
| Bloch et al.55 1994 | 1 | - | RHA | - | 28 days | •HMB | TAE (platinum coils) | None | Good |
| Total | 101 | 49.8†/19:23‡ |
RHA: 88 CA: 8 Combined RHA/CA: 4 GDA: 1 |
- | 32 days† |
HMB: 86 Haematemesis: 11 Ab pain: 10 JN: 10 AN: 5 Melena: 5 Fever: 4 Pain: 4 Other: 25 |
TAE: 73 Laparotomy: 12 Ligation: 8 Stent: 4 TI: 4 Other: 16 |
None: 47 Liver abscess: 10 Post-embolisation syndrome and hepatic ischaemia: 9 Bleeding: 4 PSA recurrence: 2 Other: 8 |
Good: 97 Patient death: 2 Unknown: 2 |
RHA = right hepatic artery; HMB = haemobilia; TAE = transarterial embolisation; M = male; JN = jaundice; Ab = abdominal; PTCD = percutaneous transhepatic cholangiography and drainage; SMA = superior mesenteric artery; CBD = common bile duct; LHA = left hepatic artery; LC = laparoscopic cholecystectomy; CA = cystic artery; TI = thrombin injection; ant = anterior; post = posterior; PSA = pseudoaneurysm; AN = anaemia; F = female; Hb = haemoglobin; ERCP = endoscopic retrograde cholangiopancreatography; ICU = intensive care unit; GI = gastrointestinal; HA = hepatic artery; NBCA = N-butyl cyanoacrylate; CT = computed tomography; PV = portal vein; GDA = gastroduodenal artery.
First published online in March 2016.
Mean.
Male-to-female ratio.
The commonest vessel involved was the RHA (n = 88; 87.1%), the cystic artery (n = 8; 7.9%), both the cystic and hepatic arteries (n = 4; 4.0%) and the gastroduodenal artery (n = 1; 1.0%).3–62 In two cases, the RHA arose from a replaced superior mesenteric artery and, in one case, it was an accessory artery.18,29,31 The size of the PSAs ranged from 13–200 mm, with a mean size of 43 mm.4,6,13,15,17,26,30,31,33,37,39,42,43,57 The modality of management was transarterial embolisation (TAE) in 72.3%, endovascular stent placement in 4.0%, exploratory laparotomy and ligation of the hepatic artery in 5.0% and thrombin injection in 4.0% of cases.3–62 The TAE procedure alone failed to control bleeding in four cases (5.5%), resulting in a 94.5% success rate.6,7,15,42 One of these cases was managed further using a percutaneous thrombin injection, while two cases were managed by exploratory laparotomy and ligation of vessels.
Reasons for TAE failure included continuous growth of the PSA over a period of two months, persistent bleeding and other associated injuries, including to the portal vein.6,7,42 Among the cases treated with TAE, complications included liver abscesses (n = 10; 13.7%), post-embolisation syndrome and hepatic ischaemia (n = 9; 12.3%), postoperative bleeding for a month (n = 1; 1.4%) and erosion of the stent into the common bile duct (CBD; n = 1; 1.4%).3,15,16,37,45 The overall mortality rate was 2.0% (n = 2).7,40 One fatality was due to severe haemorrhagic relapse following repair of the PSA, resulting in irreversible hypovolaemic shock despite re-exploration and ligation.40 In the second case, the patient died from a concomitant injury to the portal vein.7
Discussion
INCIDENCE
The true incidence of PSAs in the cystic/hepatic arteries is difficult to determine as many cases are asymptomatic and thus never detected.57 Moreover, small subclinical PSAs may thrombose spontaneously or be too small to be observed, even on imaging.57 The reported incidence of PSAs involving the cystic or hepatic arteries following an LC procedure ranges from 0.06–0.6%.3,63 The incidence of haemobilia following an emergency LC for acute cholecystitis (within 72 hours) has been reported to be 0.001%, while it has been observed to be 0.0003% for those undergoing an elective LC.31 A higher incidence of PSAs involving the hepatic artery has been reported in patients with a concomitant bile duct injury (4.5%).4 However, the RHA is usually the most commonly involved vessel.28,30,32,33,39–41,57 Other involved sites include the cystic artery and, in some cases, the common hepatic and gastroduodenal arteries.4,9,37,38,44,47,58
AETIOPATHOGENESIS
A PSA may arise due to multiple mechanisms of injury, including laceration, transection or occlusion of the blood vessels following a mechanical or thermal injury to the artery.6,37,51 This may occur when inappropriate energy (thermal/mechanical) is applied during dissection of the Calot’s triangle or following dissection with a laser.5,37 A thermal injury could result from the direct transfer of heat to the vessel by cautery or indirectly via a metal clip in contact with the artery.3,18,31 Injury of the vessel could also be due to clip intrusion, bile leakage or infection.4,9,31 In numerous cases, the PSA has been found directly adjacent to a clip.31,43,46,51 Bile acids are powerful solubilisers of membrane lipids due to their cytotoxic and amphipathic properties, causing cell death in patients with bile leaks; this has been postulated to cause direct weakening and erosion of the vascular wall, leading to a PSA.4,18 In patients with bilomas, the resulting secondary infection may precipitate the development of a PSA.4 Associations have also been reported between PSAs and intraoperative adhesions and anatomical variations of the ducts and cystic artery.16,41,57 Major anatomical variations include a doubled cystic artery (20%), a “caterpillar hump” formation of the RHA and the unusual course of the cystic artery anterior to the CBD or common hepatic artery.41,57
During a LC procedure, vessel injury can be avoided by adhering to basic surgical tenets, including performing the dissection in the plane closest to the gallbladder wall, retaining adequate view of the cystic artery and the duct entering the gallbladder before applying an energy source, freeing the infundibulum inferiorly to widen Calot’s triangle (i.e. pediculisation) and ensuring meticulous dissection without bleeding so as to identify structures before applying the clips.1,57 In the event of bleeding, the blind application of clips or use of cautery may also enhance the risk of vascular injury.1 Some researchers have recommended the use of bipolar cautery or ultrasonic dissection for a thick-walled gallbladder, particularly when the dissection penetrates deep into the liver—as in a buried gallbladder— because the potential carbonisation with the use of these tools is minimal in comparison to laser or monopolar cautery.31 The risk of a PSA rupture is related to its size, with a greater than 10-fold risk when the aneurysm is more than 5 cm.7 In cases of rupture, bleeding can be either minimal or heavy, particularly in those presenting late.7,15 In such cases, a LC carried out for acute cholecystitis or concomitant ductal injury may be a difficult and prolonged procedure.4,6,18,30,31,37,41
PRESENTATION
A PSA may present either in the initial days following a cholecystectomy or be delayed over weeks, months or, on rare occasions, years.31,41 A delay in presentation following a thermal injury could be due to charring of a vessel which gets detached weeks later, particularly in the presence of bile.31 Patients may present with gastrointestinal (GI) bleeding (i.e. haemobilia) and/or hemoperitoneum, particularly when the PSA is large; in contrast, smaller PSAs may occasionally present with minimal abdominal discomfort.7 Haemobilia has been reported to be the most frequent presentation (90%), while abdominal pain (70%) and jaundice (60%) are other common presentations.3,31 The classical presentation of Quincke’s triad (jaundice, abdominal pain and GI bleeding) may be seen in less than 40% of haemobilia cases.64 Rarely, an abdominal mass with a bruit may be noted.42 GI bleeding may present as haematemesis or melena, based on the rate of bleeding.63
During its natural course, a PSA will progressively grow in size before rupturing in 21–80% of cases.30 The rupture of a PSA into the peritoneal cavity may present with hypovolaemic shock or may be contained temporarily by the surrounding tissue—often referred to as “double rupture phenomenon”, as the initial contained bleeding may be followed by secondary bleeding which is more severe.7 Erosion of a PSA into the cystic duct stump or GI tract forming a fistula between the two structures has been previously reported.5,9,23,24,35,40 This could involve the duodenum, leading to haematemesis, the jejunum, for example in a patient presenting with lower GI bleeding who had previously undergone Roux-en-Y reconstruction following a stomach carcinoma, or hepatic flexure in a patient presenting with hematochezia.23,24,35,40 In most patients (80%), the PSA usually presents approximately one month following the LC surgery; however, delayed PSA presentation as late as five years after the surgery has been reported.6,7
DIAGNOSTIC AND THERAPEUTIC INVESTIGATIONS
The diagnosis of a PSA can be confirmed by a combination of history-taking (i.e. checking the patient’s surgical history), an upper GI endoscopy, regular or Doppler ultrasonography, a contrast CT scan and/or an angiogram.6,9,13,18,20,23,29,33–35,37,53 Ultrasonography may lead to the detection of an aneurysm or fluid consistent with haemorrhage; however, the diagnostic accuracy of this option is directly related to the size of the PSA and the operator’s experience.13,16,25 Three-dimensional (3D) CT reconstruction or catheter angiography can also help to define the aneurysm and its feeding vessels. With the advent of multidetector CT scans and advancements in 3D imaging software in the form of volume determination, CT is the preferred technique for primary vascular imaging among patients with suspected PSAs.20 Recent reports have discussed features of haemobilia on a CT scan, including the presence of a haematoma within the abdominal cavity and gallbladder fossae, high attenuation of blood clots within the bile duct, biliary dilatation, a PSA of the RHA, contrast extravasation, enhancement of the bile duct wall and hypoperfusion of the right lobe of the liver.16,65
Catheter angiography can be both diagnostic and therapeutic.18,20,29,31 Indications of a dilated cystic artery stump on an angiogram are considered ominous and may warrant embolisation, despite a lack of active bleeding during other investigations.58 Nevertheless, angiography has certain diagnostic limitations which may be influenced by variable flow rate, intermittent bleeding at the time of investigation and hepatic artery abnormalities.15 Previous research has indicated that coeliac angiography can fail to identify PSAs subsequently detected via selective RHA angiography.6,18 However, angiography has been reported to detect vascular abnormalities in 90% of patients with significant haemobilia.66
TREATMENT
A diagnosis of PSA is considered an acute emergency and requires immediate and aggressive intervention, as a rupture could lead to life-threatening exsanguination; the rupture of a PSA in the hepatic artery has been associated with a mortality rate of 21–43%.17,18,22,33 Historically, PSAs were principally managed via surgery, which involved resectioning of the PSA and ligation of the cystic artery stump or RHA.6 However, due to significant advances in catheter-based therapies, PSAs are now primarily treated with TAE by occluding the sac or the feeding vessel with a variety of embolic agents, including gel foam, coils, N-butyl cyanoacrylate and thrombin, before ideally embolising the vessel distal and proximal to the PSA in order to prevent collateral filling of the PSA.5,6,15–19,22,23,25,37,43 When coils are used, they can induce thrombosis; hence, in patients with significant coagulopathies, the vessel may still remain patent despite embolisation and the procedure may be ineffective in controlling bleeding. In small PSAs, glue may be used instead as the adhesive conforms to the shape of PSA.43,64 In addition, coil placement may be difficult in patients with a small PSA.25 In some cases, both approaches may be used.25
Distinct advantages of TAE include the reduced risk of parenchymal injury, the use of local rather than general anaesthesia and rapid patient recovery following the procedure. However, in some instances, TAE may fail initially and the patient may require a further attempt at embolisation.6 Failure may be due to the difficulty in approaching the PSA in the presence of a thrombosed proximal vessel, such as the coeliac or tortuous hepatic arteries.5,11,24,36,48,55 In patients where cannulation is feasible, embolisation failure could be related to an inability to isolate the bleeding vessel, misidentification of the bleeding vessel or incomplete occlusion despite the disappearance of the sac or feeding vessel on post-embolisation angiography. 6,22 In addition, smaller vessels may feed the PSA and contribute to rebleeding, which may not be easily detected during angiography.42 Moreover, loosely packed coils can lead to recanalisation of the aneurysm.6 Failure to successfully embolise a PSA may require nonselective embolisation of the RHA or direct surgical exploration of the PSA. Nonselective embolisation of the RHA may also be required if there is bleeding from multiple sites.39
Recently, several researchers have reported the successful management of PSAs by injecting thrombin directly into the hepatic artery aneurysm.5,6,32 However, embolisation using this technique may be nonselective, resulting in potentially serious complications such as liver and bowel infarctions; injecting small aliquots of thrombin with real-time ultrasound and Doppler guidance may reduce this risk.6 On the other hand, angiographic embolisation may be associated with serious risks, including rupture of the PSA during coil embolisation, an extension of the thrombosis in the RHA, hepatobiliary necrosis, bleeding, abscess formation and CBD stricture due to ischaemia.3,4,22,37 A recent report found that post-embolisation syndrome occurred in nine out of 14 patients and was associated with the age of the patient and the time interval between the LC and TAE.16 In patients with liver cirrhosis or abscesses, some researchers have recommended assessment of adequate arterial and collateral flow prior to hepatic artery embolisation to avoid the risk of ischaemia.37 Others have advised use of a covered stent while treating the PSA in order to maintain blood flow to the liver and prevent complications related to reduced flow.33
Stents may also be used for patients with concomitant hepatic artery stenosis and PSAs.33 For small visceral vessels, the choice of stent is limited; one option is the JOSTENT® GraftMaster® graft (Abbott Vascular Inc., Chicago, Illinois, USA), which is 3–5 mm in diameter and 12–26 mm long. The placement of a stent for a PSA of the RHA is considered technically challenging due its distant location, smaller diameter and often complex or altered anatomy.33 The patency of the graft is generally better in arteries with larger diameters and high flow rates. Even though the hepatic artery has a smaller diameter, its high flow rate ensures good patency in most cases.33 In patients where an intra-arterial approach to the hepatic artery is not feasible, successful embolisation via a transhepatic approach has been reported.43 In one case, erosion of the TAE coil into the CBD was observed in a patient who presented two years after the LC procedure with acute pancreatitis.45
Surgical intervention is required if embolisation fails, for patients who lack the necessary haemodynamic stability to undergo a TAE procedure and for those with concomitant bile duct compression or injuries.4,15,22,57 The latter patient group may also require simultaneous biliodigestive anastomosis, although surgical repair can generally be delayed if the duct injury is detected later.2,8,12,19 Surgical intervention may also be mandatory when endovascular intervention facilities are not available and the patient is not stable enough to be transferred to a tertiary hospital.25,40 In cases whereby the PSA has continued to expand after initial control with TAE, subsequent surgical control has been reported in which an exploratory laparotomy and ligation of the feeding vessel resulted in a complete recovery.42
Surgical interventions for PSAs may involve excision of the PSA and ligation of the RHA, removal of a biliary obstruction or, in exceptional cases, a hepatectomy.4,6,20 Excision ensures that a PSA will not grow due to sustained arterial blood pressure; moreover, failure to excise the PSA may lead to its rupture as the aneurysm is often infected. Infection could also lead to a high risk of vascular suture rupture following ligation of the artery; in one report, a patient died 48 hours after having undergone surgical repair of a PSA due to fatal GI bleeding.40 Although in most cases a PSA is surgically treated using an open method, a recent report described the successful laparoscopic suture ligation of a PSA in the right posterior sector of the hepatic artery.21 The initial inspection and manipulation of the PSA led to severe bleeding, which was adequately controlled with a vascular clamp using the laparoscopic Pringle manoeuvre. With a clearer view of the surrounding anatomy, laparoscopic ligation was performed by taking some of the periarterial tissue to prevent ligature slippage.21
Following ligation of the RHA, the risk of a liver infarction is generally limited as blood flow is maintained by portal circulation.26 In addition, revascularisation of the right liver lobe occurs due to the rapid development within the hilar plate of an omega-shaped collateral arterial circulatory system, originating from the preserved branch of the hepatic artery.26 Some patients who present with haemobilia and obstructive jaundice may also require endoscopic retrograde cholangiopancreatography, transhepatic biliary drainage or CBD exploration to evacuate the clot if the jaundice does not improve.17,30,64 Embolisation has been reported as generally successful in 82% of cases, with surgery required for the remaining 18% of patients.7 Surgical management of massive haemobilia is reportedly successful for 90% of patients, with rebleeding and mortality rates of <5% and 10%, respectively.22 However, there have been reports of higher operative mortality rates (27–50%), particularly among haemodynamically unstable patients.36,67,68
Conclusion
A hepatic and/or cystic artery PSA following a LC procedure is an uncommon yet potentially life-threatening complication. The often delayed presentation of this condition, which can occur weeks or months after the surgery, and its common manifestation with GI bleeding can easily result in misdiagnosis or delayed treatment. Hence, a high index of clinical suspicion is required for patients with unexplained GI bleeding following a LC procedure. A contrast CT scan or angiogram usually confirms the diagnosis and TAE is considered the gold standard of management, with a high success rate. However, surgical intervention is required for cases in which TAE is unfeasible or fails. Precautions should be taken to avoid vascular injury while performing a LC in order to reduce the risk of a PSA, particularly when the cholecystectomy procedure is difficult.
References
- 1.Machado NO. Biliary complications post laparoscopic cholecystectomy: Mechanism, preventive measures, and approach to management - A review. Diagn Ther Endosc. 2011;2011:967017. doi: 10.1155/2011/967017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strasberg SM, Helton WS. An analytical review of vasculobiliary injury in laparoscopic and open cholecystectomy. HPB (Oxford) 2011;13:1–14. doi: 10.1111/j.1477-2574.2010.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicholson T, Travis S, Ettles D, Dyet J, Sedman P, Wedgewood K, et al. Hepatic artery angiography and embolization for hemobilia following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol. 1999;22:20–4. doi: 10.1007/s002709900323. [DOI] [PubMed] [Google Scholar]
- 4.Madanur MA, Battula N, Sethi H, Deshpande R, Heaton N, Rela M. Pseudoaneurysm following laparoscopic cholecystectomy. Hepatobiliary Pancreat Dis Int. 2007;6:294–8. [PubMed] [Google Scholar]
- 5.Genyk YS, Keller FS, Halpern NB. Hepatic artery pseudoaneurysm and hemobilia following laser laparoscopic cholecystectomy: A case report. Surg Endosc. 1994;8:201–4. doi: 10.1007/BF00591830. [DOI] [PubMed] [Google Scholar]
- 6.Kumar A, Sheikh A, Partyka L, Contractor S. Cystic artery pseudoaneurysm presenting as a complication of laparoscopic cholecystectomy treated with percutaneous thrombin injection. Clin Imaging. 2014;38:522–5. doi: 10.1016/j.clinimag.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Bulut T, Yamaner S, Bugra D, Akyuz A, Acarli K, Poyanli A. False aneurysm of the hepatic artery after laparoscopic cholecystectomy. Acta Chir Belg. 2002;102:459–63. doi: 10.1080/00015458.2002.11679352. [DOI] [PubMed] [Google Scholar]
- 8.Balsara KP, Dubash C, Shah CR. Pseudoaneurysm of the hepatic artery along with common bile duct injury following laparoscopic cholecystectomy: A report of two cases. Surg Endosc. 1998;12:276–7. doi: 10.1007/s004649900651. [DOI] [PubMed] [Google Scholar]
- 9.Saldinger PF, Wang JY, Boyd C, Lang E. Cystic artery stump pseudoaneurysm following laparoscopic cholecystectomy. Surgery. 2002;131:585–6. doi: 10.1067/msy.2002.115353. [DOI] [PubMed] [Google Scholar]
- 10.Bergey E, Einstein DM, Herts BR. Cystic artery pseudoaneurysm as a complication of laparoscopic cholecystectomy. Abdom Imaging. 1995;20:75–7. doi: 10.1007/BF00199652. [DOI] [PubMed] [Google Scholar]
- 11.Siablis D, Tepetes K, Vasiou K, Karnabatidis D, Perifanos S, Tzorakoleftherakis E. Hepatic artery pseudoaneurysm following laparoscopic cholecystectomy: Transcatheter intraarterial embolization. Hepatogastroenterology. 1996;43:1343–6. [PubMed] [Google Scholar]
- 12.Ibrarullah M, Singh B, Mehrotra P, Kaushik SP. Right hepatic artery pseudoaneurysm after laparoscopic cholecystectomy. Am J Gastroenterol. 1997;92:528–9. [PubMed] [Google Scholar]
- 13.Yahchouchy-Chouillard E, Limot O, Ghiles E, Etienne JC, De Baer T, Picone O, et al. Embolization for right hepatic artery pseudoaneurysm following laparoscopic cholecystectomy. ANZ J Surg. 2003;73:82–4. doi: 10.1046/j.1445-2197.2003.02625.x. [DOI] [PubMed] [Google Scholar]
- 14.Journé S, De Simone P, Laureys M, Le Moine O, Gelin M, Closset J. Right hepatic artery pseudoaneurysm and cystic duct leak after laparoscopic cholecystectomy. Surg Endosc. 2004;18:554–6. doi: 10.1007/s00464-003-4262-5. [DOI] [PubMed] [Google Scholar]
- 15.Petrou A, Brennan N, Soonawalla Z, Silva MA. Hemobilia due to cystic artery stump pseudoaneurysm following laparoscopic cholecystectomy: Case presentation and literature review. Int Surg. 2012;97:140–4. doi: 10.9738/CC52.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng W, Yue D, ZaiMing L, ZhaoYu L, Wei L, Qiyong G. Hemobilia following laparoscopic cholecystectomy: Computed tomography findings and clinical outcome of transcatheter arterial embolization. Acta Radiol. 2017;58:46–52. doi: 10.1177/0284185116638570. [DOI] [PubMed] [Google Scholar]
- 17.Hsiao CY, Kuo TC, Lai HS, Yang CY, Tien YW. Obstructive jaundice as a complication of a right hepatic artery pseudoaneurysm after laparoscopic cholecystectomy. J Minim Access Surg. 2015;11:163–4. doi: 10.4103/0972-9941.144097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bin Traiki TA, Madkhali AA, Hassanain MM. Hemobilia post laparoscopic cholecystectomy. J Surg Case Rep. 2015;2015:rju159. doi: 10.1093/jscr/rju159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdalla S, Thome A, Reslinger V, Atanasiu C, Pellerin O, Sapoval M, et al. Compressive hematoma due to pseudoaneurysm of the right hepatic artery: A rare cause of obstructive jaundice after single-port cholecystectomy. Surg Laparosc Endosc Percutan Tech. 2015;25:e42–4. doi: 10.1097/SLE.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 20.Rencuzogullari A, Okoh AK, Akcam TA, Roach EC, Dalci K, Ulku A. Hemobilia as a result of right hepatic artery pseudoaneurysm rupture: An unusual complication of laparoscopic cholecystectomy. Int J Surg Case Rep. 2014;5:142–4. doi: 10.1016/j.ijscr.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panda N, Narasimhan M, Gunaraj A, Ardhanari R. Laparoscopic management of post-cholecystectomy sectoral artery pseudoaneurysm. J Minim Access Surg. 2014;10:37–9. doi: 10.4103/0972-9941.124471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murugesan SD, Sathyanesan J, Lakshmanan A, Ramaswami S, Perumal S, Perumal SU, et al. Massive hemobilia: A diagnostic and therapeutic challenge. World J Surg. 2014;38:1755–62. doi: 10.1007/s00268-013-2435-5. [DOI] [PubMed] [Google Scholar]
- 23.Kirschberg O, Scheding A, Saers T, Krakamp B. Detection and treatment of an aneurysma spurium of the arteria hepatica dextra after laparoscopic cholecystectomy. BMC Gastroenterol. 2013;13:121. doi: 10.1186/1471-230X-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mate AD, Surnare KR, Deolekar SS, Gvalani AK. Lower gastrointestinal bleeding due to hepatic artery pseudoaneurysm following laparoscopic cholecystectomy. J Minim Access Surg. 2013;9:31–3. doi: 10.4103/0972-9941.107135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gandhi V, Doctor N, Marar S, Nagral A, Nagral S. Major hemobilia: Experience from a specialist unit in a developing country. Trop Gastroenterol. 2011;32:214–18. [PubMed] [Google Scholar]
- 26.Butet Y, Bouras AF, Truant S, Pruvot FR. Pseudoaneurysm of the cystic artery as a complication of laparoscopic cholecystectomy. Dig Liver Dis. 2012;44:449–50. doi: 10.1016/j.dld.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Caminiti R, Rossitto M, Ciccolo A. Pseudoaneurysm of the hepatic artery and hemobilia: A rare complication of laparoscopic cholecystectomy - Clinical case and literature review. Acta Chir Belg. 2011;111:400–3. doi: 10.1080/00015458.2011.11680782. [DOI] [PubMed] [Google Scholar]
- 28.Khan MR, Raza R, Salam B. Leaking pseudoaneurysm of hepatic artery: A potentially life-threatening complication of a common procedure. J Pak Med Assoc. 2011;61:504–6. [PubMed] [Google Scholar]
- 29.Kamani L, Mosharraf SM, Tanveer-ul-Haq, Shah HA. Haemobilia: A rare cause of gastrointestinal bleeding. J Coll Physicians Surg Pak. 2011;21:766–8. [PubMed] [Google Scholar]
- 30.Hadj AK, Goodwin M, Schwalb H, Nikfarjam M. Pseudoaneurysm of the hepatic artery. J Gastrointest Surg. 2011;15:1899–901. doi: 10.1007/s11605-011-1535-5. [DOI] [PubMed] [Google Scholar]
- 31.Sansonna F, Boati S, Sguinzi R, Migliorisi C, Pugliese F, Pugliese R. Severe hemobilia from hepatic artery pseudoaneurysm. Case Rep Gastrointest Med. 2011;2011:925142. doi: 10.1155/2011/925142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boddy A, Macanovic M, Thompson J, Watkinson A. Use of an endovascular stent graft and percutaneous thrombin injection to treat an iatrogenic hepatic artery pseudoaneurysm. Ann R Coll Surg Engl. 2010 doi: 10.1308/147870810X12822015504806. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Hylton JR, Pevec WC. Successful treatment of an iatrogenic right hepatic artery pseudoaneurysm and stenosis with a stent graft. J Vasc Surg. 2010;51:1510–13. doi: 10.1016/j.jvs.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 34.Yao CA, Arnell TD. Hepatic artery pseudoaneurysm following laparoscopic cholecystectomy. Am J Surg. 2010;199:e10–11. doi: 10.1016/j.amjsurg.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 35.Chen CC, Chen BB, Wang HP. Upper gastrointestinal bleeding owing to right hepatic artery pseudoaneurysm after laparoscopic cholecystectomy. Gastroenterology. 2009;137:e5–6. doi: 10.1053/j.gastro.2009.02.083. [DOI] [PubMed] [Google Scholar]
- 36.Kumar M, Gupta S, Soin A, Nundy S. Management of massive haemobilia in an Indian hospital. Indian J Surg. 2008;70:288–95. doi: 10.1007/s12262-008-0085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakase Y, Takagi T, Fukumoto K, Kassai K, Yamagami T, Itani K, et al. Hemobilia and cystic artery stump pseudoaneurysm associated with liver abscess after laparoscopic cholecystectomy: Report of a case. Surg Today. 2008;38:567–71. doi: 10.1007/s00595-007-3663-9. [DOI] [PubMed] [Google Scholar]
- 38.De Molla Neto OL, Ribeiro MA, Saad WA. Pseudoaneurysm of cystic artery after laparoscopic cholecystectomy. HPB (Oxford) 2006;8:318–19. doi: 10.1080/13651820600869628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Srinivasaiah N, Bhojak M, Jackson R, Woodcock S. Vascular emergencies in cholelithiasis and cholecystectomy: Our experience with two cases and literature review. Hepatobiliary Pancreat Dis Int. 2008;7:217–20. [PubMed] [Google Scholar]
- 40.Sebastián JJ, Peña E, Blas JM, Ceña G. Fatal upper gastrointestinal bleeding due to hepatic artery pseudoaneurysm diagnosed by endoscopy. Dig Dis Sci. 2008;53:1152–3. doi: 10.1007/s10620-007-9971-5. [DOI] [PubMed] [Google Scholar]
- 41.Masannat YA, Al-Naser S, Al-Tal Y, Al-Koteesh J, Sharaf UI. A rare complication of a common operation: Hepatic artery pseudo aneurysm following cholecystectomy report of a case. Ir J Med Sci. 2008;177:397–8. doi: 10.1007/s11845-007-0053-7. [DOI] [PubMed] [Google Scholar]
- 42.Roche-Nagle G, Maceneaney, Harte P. Pseudo-aneurysm of the hepatic artery after laparoscopic cholecystectomy: A case report. J Minim Access Surg. 2006;2:73–5. doi: 10.4103/0972-9941.26652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chigot V, Lallier M, Alvarez F, Dubois J. Hepatic artery pseudoaneurysm following laparoscopic cholecystectomy. Pediatr Radiol. 2003;33:24–6. doi: 10.1007/s00247-002-0772-x. [DOI] [PubMed] [Google Scholar]
- 44.Jain R, Batra Y, Acharya SK. Post cholecystectomy hemobilia: Transcatheter embolization of pseudoaneurysms with homemade steel coils. Indian J Gastroenterol. 2002;21:161–2. [PubMed] [Google Scholar]
- 45.Ozkan OS, Walser EM, Akinci D, Nealon W, Goodacre B. Guglielmi detachable coil erosion into the common bile duct after embolization of iatrogenic hepatic artery pseudoaneurysm. J Vasc Interv Radiol. 2002;13:935–8. doi: 10.1016/S1051-0443(07)61778-3. [DOI] [PubMed] [Google Scholar]
- 46.Halbe S, Ahmed NI, Sundar K, Sathyakumar C. Pseudoaneurysm in gall bladder fossa following laparoscopic cholecystectomy. Indian J Gastroenterol. 1999;18:122. [PubMed] [Google Scholar]
- 47.Kwauk ST, Cameron R, Burbridge B, Keith RG. Traumatic pseudoaneurysm of the hepatic artery after percutaneous liver biopsy and laparoscopic cholecystectomy in a patient with biliary cirrhosis: A case report. Can J Surg. 1998;41:316–20. [PMC free article] [PubMed] [Google Scholar]
- 48.Ribeiro A, Williams H, May G, Fulmer JT, Spivey JR. Hemobilia due to hepatic artery pseudoaneurysm thirteen months after laparoscopic cholecystectomy. J Clin Gastroenterol. 1998;26:50–3. doi: 10.1097/00004836-199801000-00013. [DOI] [PubMed] [Google Scholar]
- 49.Halpern NB. False aneurysm of a hepatic artery branch. Surg Endosc. 1997;11:402–3. doi: 10.1007/s004649900375. [DOI] [PubMed] [Google Scholar]
- 50.Kapoor R, Agarwal S, Calton R, Pawar G. Hepatic artery pseudoaneurysm and hemobilia following laparoscopic cholecystectomy. Indian J Gastroenterol. 1997;16:32–3. [PubMed] [Google Scholar]
- 51.Yelle JD, Fairfull-Smith R, Rasuli P, Lorimer JW. Hemobilia complicating elective laparoscopic cholecystectomy: A case report. Can J Surg. 1996;39:240–2. [PMC free article] [PubMed] [Google Scholar]
- 52.Porte RJ, Coerkamp EG, Koumans RK. False aneurysm of a hepatic artery branch and a recurrent subphrenic abscess: Two unusual complications after laparoscopic cholecystectomy. Surg Endosc. 1996;10:161–3. doi: 10.1007/BF00188363. [DOI] [PubMed] [Google Scholar]
- 53.Rivitz SM, Waltman AC, Kelsey PB. Embolisation of an hepatic artery pseudoaneurysm following laparoscopic cholecystectomy. Cardiovasc Intervent Radiol. 1996;19:43–6. doi: 10.1007/BF02560147. [DOI] [PubMed] [Google Scholar]
- 54.Stewart BT, Abraham RJ, Thomson KR, Collier NA. Postcholecystectomy haemobilia: Enjoying a renaissance in the laparoscopic era? Aust N Z J Surg. 1995;65:185–8. doi: 10.1111/j.1445-2197.1995.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 55.Bloch P, Modiano P, Foster D, Bouhot F, Gompel H. Recurrent hemobilia after laparoscopic cholecystectomy. Surg Laparosc Endosc. 1994;4:375–7. [PubMed] [Google Scholar]
- 56.Moran J, Del Grosso E, Wills JS, Hagy JA, Baker R. Laparoscopic cholecystectomy: Imaging of complications and normal postoperative CT appearance. Abdom Imaging. 1994;19:143–6. doi: 10.1007/BF00203489. [DOI] [PubMed] [Google Scholar]
- 57.Malik KA, Muneeb MD, Jawaid M, Khan ML. Post laparoscopic cholecystectomy hepatic artery pseudo-aneurysm. Pak J Surg. 2010;26:89–91. [Google Scholar]
- 58.Moses V, Keshava SN, Wann VC, Joseph P, Sitaram V. Cystic artery pseudoaneurysm after laparoscopic cholecystectomy presenting as haemobilia: A case report. Trop Gastroenetrol. 2008;29:107–9. [PubMed] [Google Scholar]
- 59.England RE, Marsh PJ, Ashleigh R, Martin DF. Case report: Pseudoaneurysm of the cystic artery - A rare cause of haemobilia. Clin Radiol. 1998;53:72–5. doi: 10.1016/S0009-9260(98)80041-X. [DOI] [PubMed] [Google Scholar]
- 60.Doctor N, Dooley JS, Dick R, Watkinson A, Rolles K, Davidson BR. Multidisciplinary approach to biliary complications of laparoscopic cholecystectomy. Br J Surg. 1998;85:627–32. doi: 10.1046/j.1365-2168.1998.00662.x. [DOI] [PubMed] [Google Scholar]
- 61.Zilberstein B, Cecconello I, Ramos AC, Sallet JA, Pinheiro EA. Hemobilia as a complication of laparoscopic cholecystectomy. Surg Laparosc Endosc. 1994;4:301–3. [PubMed] [Google Scholar]
- 62.Heyn J, Sommerey S, Schmid R, Hallfeldt K, Schmidbauer S. Fistula between cystic artery pseudoaneurysm and cystic bile duct cause of acute anemia one year after laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2006;16:609–12. doi: 10.1089/lap.2006.16.609. [DOI] [PubMed] [Google Scholar]
- 63.Deziel DJ, Millikan KW, Economou SG, Doolas A, Ko ST, Airan MC. Complications of laparoscopic cholecystectomy: A national survey of 4,292 hospitals and an analysis of 77,604 cases. Am J Surg. 1993;165:9–14. doi: 10.1016/S0002-9610(05)80397-6. [DOI] [PubMed] [Google Scholar]
- 64.Machado NO. Hemobilia post liver biopsy: Mechanism, presentation, complications and management. J Gastroenterol Pancreatol Liver Disord. 2015;2:1–14. doi: 10.15226/2374-815x/2/3/00142. [DOI] [Google Scholar]
- 65.Wen F, Dong Y, Lu ZM, Liu ZY, Li W, Guo QY. Hemobilia after laparoscopic cholecystectomy: Imaging features and management of an unusual complication. Surg Laparosc Endosc Percutan Tech. 2016;26:e18–24. doi: 10.1097/SLE.0000000000000241. [DOI] [PubMed] [Google Scholar]
- 66.Merrell SW, Schneider PD. Hemobilia: Evolution of current diagnosis and treatment. West J Med. 1991;155:621–5. [PMC free article] [PubMed] [Google Scholar]
- 67.Hsu KL, Ko SF, Chou FF, Sheen-Chen SM, Lee TY. Massive hemobilia. Hepatogastroenterology. 2002;49:306–10. [PubMed] [Google Scholar]
- 68.Mohil R, Narayan N, Narayan A, Kerketta Z, Jain S, Bhatnagar D. Life-threatening lower gastrointestinal haemorrhage from an aneurysm of the right hepatic artery: A rare case presentation due to delayed rupture. Internet J Surg. 2009;24:1. [Google Scholar]