Abstract
Objectives
Imbalances in effector T cell functioning have been associated with a number of autoimmune diseases, including Hashimoto’s thyroiditis (HT). Differentiation of effector T helper (Th) 1, Th2, Th17 and regulatory T cell (Treg) lymphocytes is regulated by transcription factors, including Th1-specific T box (T-bet), GATA binding protein-3 (GATA3), retinoid-related orphan receptor (ROR)-α and forkhead box P3 (FOXP3). This study aimed to investigate Th1/Th2, Th1/Treg, Th2/Treg and Th17/Treg balances at the level of these transcription factors.
Methods
This study took place between October 2015 and August 2016. Peripheral blood mononuclear cells were collected from a control group of 40 healthy women recruited from the Zahedan University of Medical Sciences, Zahedan, Iran, and a patient group of 40 women with HT referred to the Hazrat Ali Asghar Hospital, Zahedan. Total ribonucleic acid extraction was performed and the gene expression of transcription factors was quantitated using a real-time polymerase chain reaction technique.
Results
Expression of T-bet and GATA3 was significantly elevated, while FOXP3 expression was significantly diminished among HT patients in comparison with the controls (P = 0.03, 0.01 and 0.05, respectively). Expression of RORα was higher among HT patients, although this difference was not significant (P = 0.15). Expression of T-bet/FOXP3, GATA3/FOXP3 and RORα/FOXP3 ratios were increased among HT patients in comparison with the controls (P <0.02, <0.01 and <0.01, respectively).
Conclusion
These results indicate that HT patients have imbalances in Th1/Treg, Th2/Treg and Th17/Treg lymphocytes at the level of the transcription factors, deviating towards Th1, Th2 and Th17 cells. Correction of these imbalances may therefore be therapeutic.
Keywords: Hashimoto Thyroiditis, Transcription Factors, Lymphocytes, Th1 Cells, Th2 Cells, Th17 Cells, Regulatory T Cells
Advances in Knowledge
- The results of the current study indicate that patients with Hashimoto’s thyroiditis (HT) have T helper (Th) 1/regulatory T cell (Treg), Th2/Treg and Th17/Treg cell imbalances at the level of the transcription factors, with a deviation towards Th1, Th2 and Th17 cells.
- According to these findings, a combination of Th1, Th2 and Th17 cell-related immune responses may synergistically contribute to the pathogenesis of HT.
Application to Patient Care
- As HT patients in the current study were found to have Th1/Treg, Th2/Treg and Th17/Treg imbalances, correction of these imbalances may provide immunotherapeutic benefits for these patients.
- Since Th1-specific T box (T-bet), retinoid-related orphan receptor (ROR)-α and forkhead box P3 (FOXP3) are the specific transcription factors for Th1, Th17 and Treg cells, respectively, the expression of T-bet/FOXP3, GATA binding protein-3/FOXP3 and RORα/FOXP3 messenger ribonucleic acid ratios may be a useful surrogate parameter to determine Th1/Treg, Th2/Treg and Th17/Treg cell status among HT patients.
Hashimoto’s thyroiditis (ht) is one of the most prevalent organ-specific and T cell-mediated disorders worldwide and predominantly affects women, with approximately 4–10 women developing the disease for every one man.1 Pathologically, HT is characterised by the homing of a number of leukocytes—such as cluster of differentiation (CD) 4+ T cells, cytotoxic T lymphocytes, B cells and natural killer cells—to the thyroid gland.2 These leukocytes then proceed to kill thyroid follicular cells via antibody-dependent cytotoxicity and/or apoptosis.2 Serologically, anti-thyroid antibodies, especially anti-thyroglobulin (TG) and anti-thyroperoxidase (TPO), have been positively associated with more severe thyroid inflammation and the development of hypothyroidism.3 Many immunological abnormalities have been reported in patients with hypothyroidism.4 Autoreactive CD4+ T helper (Th) lymphocytes play a major role in the pathogenesis of HT.3 The antigenic determinants of thyroglobulin are present on the surface of antigen-presenting cells and are recognised by specific CD4+ T cells.5 Following antigenic stimulation, different effector CD4+ T cells are differentiated from naïve T cells, including Th1, Th2, Th17 or regulatory T cell (Treg) lymphocytes, which produce different cytokines.5,6
Among patients with HT, Th1 lymphocytes are the predominant cells in thyroid tissue.3 Th1 cells mainly produce interferon (IFN)-γ, which triggers cell-mediated immunity.7 The master transcription factor of Th1 cells is Th1-specific T box (T-bet).6 Th2 cells produce interleukin (IL)-4, IL-5, IL-6 and IL-13, which are involved in the induction of humoral immune responses.7,8 For Th2 cells, the master transcription factor is GATA binding protein-3 (GATA3).6 Th2 lymphocytes may contribute to thyroiditis through the stimulation of B cells, which produce antibodies against thyroid antigens.3 Th17 cells secrete a number of cytokines, particularly IL-17 (also known as IL-17A), IL-17F, IL-21, IL-22, tumour necrosis factor-α and granulocyte macrophage colony-stimulating factor.9,10 Th17 cells are involved in the induction of inflammatory responses.11,12 Retinoid-related orphan receptor (ROR)-γt and RORα serve as the transcription factors of Th17 cells.12,13 Elevated numbers of Th17 cells have been indicated in patients with HT.3 Treg cells play a major role in the establishment of immune tolerance through the secretion of immunoregulatory cytokines such as transforming growth factor (TGF)-β, IL-10 and IL-35.14 Treg cell functions depend on the expression of forkhead box P3 (FOXP3).14,15 Treg cells play a protective role against thyroid autoimmune diseases, with Treg cell depletion shown to promote experimental autoimmune thyroiditis in mice.16,17
A balanced ratio of Th1/Th2, Th1/Treg, Th2/Treg and Th17/Treg cells is required for normal immune function; as such, imbalances in effector T cell functioning have been associated with a number of autoimmune diseases.12,13,18 Functionally, Th1 and Th2 cells inhibit each other at the level of the transcription factors, with Th17 and Treg cells similarly inhibiting one another.19,20 Although a few studies have investigated transcription factors among HT patients, few have measured Th1/Th2, Th1/Treg, Th2/Treg and Th17/ Treg cell balances at the level of these transcription factors.21,22 This study therefore aimed to determine the balance of effector T cells—specifically, Th1, Th2, Th17 and Treg cells—at the level of transcription factors by measuring the expression of their master transcription factors—T-bet, GATA3, RORα/RORγt and FOXP3, respectively—and determining their ratios in the peripheral blood mononuclear cells (PBMCs) of HT patients in comparison to healthy individuals.
Methods
This study was conducted from October 2015 to August 2016 and included both HT patients and healthy individuals. The patient group consisted of 40 women with HT referred to the Hazrat Ali Asghar Hospital, Zahedan, Iran. A diagnosis of HT was made by expert endocrinologists based on commonly accepted clinical and laboratory criteria, including positive results for anti-TG and/or anti-TPO antibodies at high titres and evidence of thyroid dysfunction on a sonogram.23 At the time of the study, none of the patients were receiving antithyroid drugs, thyroid hormone replacement therapy or any immunomodulatory or anti-inflammatory treatments. The control group comprised 40 healthy women who were recruited from members of staff at the Zahedan University of Medical Sciences, Zahedan. None of the healthy controls had thyroiditis or other relevant diseases or were currently suffering from any acute or chronic illnesses.
A total of 10 mL of peripheral blood was taken from each subject and stored at −20 °C. Thyroid hormones and autoantibodies were measured by assessing serum concentrations of triiodothyronine (T3), thyroxine (T4) and thyroid-stimulating hormone (TSH) using a commercial radioimmunoassay kit (Kavoshyar Iran Co., Tehran, Iran). Normal ranges were considered to be 80.0–190.0 ng/dL, 4.5–12.0 μg/dL and 0.3–4.0 μU/mL for T3, T4 and TSH, respectively.24 Anti-TG and anti-TPO antibodies were measured using a rapid enzyme-linked immunosorbent assay (Genesis Diagnostics Ltd., Ely, UK). Positive anti-TG and anti-TPO concentrations were defined as >100.0 IU/mL and >75.0 IU/mL, respectively.24
The peripheral blood samples were then heparinised and loaded into a lymphocyte separation media (Lymphosep®, Biosera, Labtech International Ltd., Uckfield, UK). Subsequently, the PBMCs were separated by gradient centrifugation and the total ribonucleic acid (RNA) extracted using a reagent (TRIzol®, ThermoFisher Scientific Inc., Waltham, Massachusetts, USA), according to the manufacturer’s guidelines. Electrophoresis in ethidium bromide pretreated agarose gel was used to determine the quality of the extracted RNA; purity was determined by measuring absorption using a spectrophotometer to achieve a ratio of 260:280. In order to prepare the extracted RNA for quantification using a real-time polymerase chain reaction (PCR) technique, it was first converted to a complementary DNA template using a synthesis kit (Bioneer Corp., Daejeon, South Korea) containing both oligo-deoxythymidine and random hexamer primers. The reverse transcription protocol involved 10 minutes at 70 °C, then one minute at 20 °C to cool, followed by the addition of the reverse transcription enzyme, incubation at 42 °C for 60 minutes and finally incubation at 95 °C for 10 minutes to halt activation of the reverse transcription enzyme.
Real-time PCR was carried out three times with a thermal cycler (Corbett Rotor-Gene 6000, ThermoFisher Scientific Inc.) using a PCR master mix (SYBR Green I, Bioneer Corp.) that was mixed with 200 ng of complementary DNA and 2 μL of each primer (5 pmol/μL) [Table 1]. The thermal cycling conditions entailed 15 minutes at 95 °C as an initial denaturing step, 40 cycles for 30 seconds at 95 °C as a secondary denaturing step, 40 cycles for 30 seconds at 60 °C for annealing and extension and finally 40 cycles for 30 seconds at 72 °C. A housekeeping gene (β-actin) was applied to normalise the data. The quantity of expression of the transcription factors was calculated using the 2-ΔΔCt formula and expressed as units relative to the amount of the β-actin gene expression. The dissociation process, melting curves and data quantitation were carried out using the thermal cycler software (Corbett Rotor-Gene 6000, ThermoFisher Scientific Inc.).
Table 1.
Primers for gene expressions
| Gene | Primer | |
|---|---|---|
| Forward | Reverse | |
| T-bet | 5-GATGTTTGTGGACGTGGTCTTG-3 | 5-CTTTCCACACTGCACCCACTT-3 |
| GATA3 | 5-AGCCAGGAGAGCAGGGACG-3 | 5-CTGTTAATATTGTGAAGCTTGTAGTAGAG-3 |
| RORα | 5-GTGCGACTTCATTTTCCTCCAT-3 | 5-GCTTAGGTGATAACATTTACCCATCA-3 |
| FOXP3 | 5-GAACGCCATCCGCCACAACCTGA-3 | 5-CCCTGCCCCCACCACCTCTGC-3 |
| β-actin | 5-GCCGGGACCTGACTGACTAC-3 | 5-TTCTCCTTAATGTCACGCACGAT-3 |
T-bet = Th1-specific T box; GATA3 = GATA binding protein-3; RORα = retinoid-related orphan receptor-α; FOXP3 = forkhead box P3.
Gene expression amounts were presented as means ± standard error. The data were analysed statistically using a t-test. A P value of <0.05 was considered statistically significant. The Ethical Committee of the Kerman University of Medical Sciences, Kerman, Iran, approved this study (#K-93-418). Moreover, informed written consent was obtained from all subjects prior to their participation.
Results
The mean ages of the HT patients and healthy controls were 38.6 ± 10.6 years and 36.1 ± 11.2 years, respectively (P = 0.32). Mean serum concentrations of T3, T4 and TSH were 25.7 ± 5.6 ng/dL, 2.8 ± 0.7 μg/dL and 7.2 ± 1.2 μU/mL, respectively, among HT patients and 105.0 ± 23.0 ng/dL, 8.2 ± 1.9 μg/dL and 1.8 ± 1.1 μU/mL, respectively, among healthy controls (P <0.01 each). All of the HT patients had hypothyroidism. The mean serum levels of anti-TG and anti-TPO were 543.1 ± 212.6 IU/mL and 283.6 ± 194.4 IU/mL, respectively, among HT patients and 16.7 ± 6.5 IU/mL and 12.8 ± 4.5 IU/mL, respectively, among healthy controls (P <0.01 each).
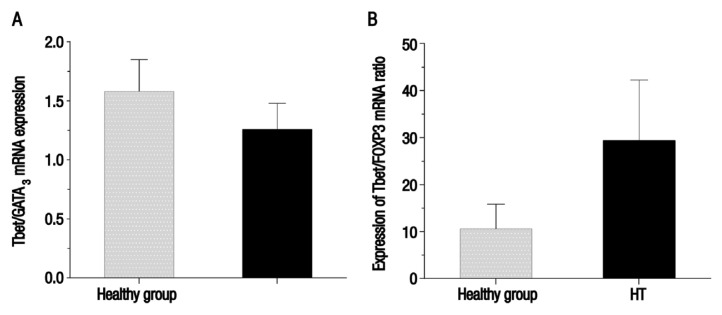
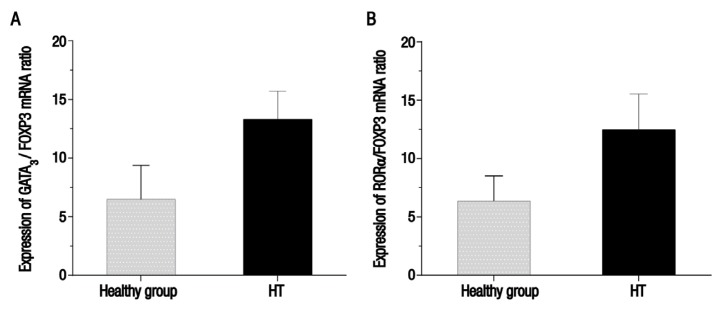
Fold changes in the expression of the T-bet, GATA3, RORα and FOXP3 transcription factors and their ratios are shown in Table 2. Expression of T-bet and GATA3 was significantly higher among patients with HT in comparison to the control group (P = 0.03 and 0.01, respectively). Although RORα expression was increased in the patient group, the difference between HT patients and the healthy control group was not significant (P = 0.15). Expression of FOXP3 among HT patients was significantly downregulated as compared with the control group (P = 0.05). No significant difference was observed between HT patients and the control group regarding the mean messenger RNA (mRNA) expression of the T-bet/GATA3 ratio [Figure 1A]. However, mean mRNA expression of the T-bet/FOXP3, GATA3/FOXP3 and RORα/FOXP3 ratios was significantly higher among patients with HT in comparison with the healthy control group (P <0.02, <0.01 and <0.01, respectively) [Figures 1B and 2].
Table 2.
Messenger ribonucleic acid expression of effector T cell-specific transcription factors using the peripheral blood mononuclear cells of female healthy controls and patients with Hashimoto’s thyroiditis from Zahedan, Iran (N = 80)
| Transcription factor | Mean mRNA expression ± SE | P value | |
|---|---|---|---|
| Healthy controls (n = 40) | HT patients (n = 40) | ||
| T-bet | 1.06 ± 0.18 | 2.08 ± 0.43 | 0.03 |
| GATA3 | 1.02 ± 0.15 | 1.94 ± 0.32 | 0.01 |
| RORα | 1.00 ± 0.16 | 1.39 ± 0.24 | 0.15 |
| FOXP3 | 1.00 ± 0.38 | 0.35 ± 0.09 | 0.05 |
| T-bet/GATA3 | 1.58 ± 0.27 | 1.26 ± 0.22 | 0.36 |
| GATA3/FOXP3 | 6.48 ± 2.90 | 13.30 ± 2.42 | 0.01 |
| T-bet/FOXP3 | 10.59 ± 5.26 | 29.37 ± 12.87 | 0.02 |
| RORα/FOXP3 | 6.36 ± 2.15 | 12.46 ± 3.06 | 0.01 |
mRNA = messenger ribonucleic acid; SE = standard error; HT = Hashimoto’s thyroiditis; T-bet = Th1-specific T box; GATA3 = GATA binding protein-3; RORα = retinoid-related orphan receptor-α; FOXP3 = forkhead box P3.
Figure 1.
Charts showing the mean messenger ribonucleic acid (mRNA) expression of the (A) Th1-specific T box (T-bet)/GATA binding protein-3 (GATA3) and (B) T-bet/forkhead box P3 (FOXP3) ratios using the peripheral blood mononuclear cells of female healthy controls and patients with Hashimoto’s thyroiditis (HT) from Zahedan, Iran (N = 80). The mRNA expression of the T-bet/GATA3 ratio was lower among the HT patients than the healthy controls; however, this difference was not significant (P = 0.36). In comparison, the mRNA expression of the T-bet/FOXP3 ratio was significantly higher among HT patients than the healthy controls (P <0.02).
T-bet = Th1-specific T box; GATA3 = GATA binding protein-3; mRNA = messenger ribonucleic acid; HT = Hashimoto’s thyroiditis; FOXP3 = forkhead box P3.
Figure 2.
Charts showing the mean messenger ribonucleic acid (mRNA) expression of the (A) GATA binding protein-3 (GATA3)/forkhead box P3 (FOXP3) and (B) retinoid-related orphan receptor (ROR)-α/FOXP3 ratios using the peripheral blood mononuclear cells of female healthy controls and patients with Hashimoto’s thyroiditis (HT) from Zahedan, Iran (N = 80). The mRNA expression of both the GATA3/FOXP3 and RORα/FOXP3 ratios was significantly higher among HT patients than the healthy controls (P <0.01 each).
GATA3 = GATA binding protein-3; FOXP3 = forkhead box P3; mRNA = messenger ribonucleic acid; HT = Hashimoto’s thyroiditis; RORα = retinoid-related orphan receptor-α.
Discussion
A number of studies have demonstrated alterations in the frequency and/or function of effector CD4+ T cells among HT patients.3,25,26 However, the molecular mechanisms of these changes remain unclear. The results of the current study show that expression of T-bet, the Th1 transcription factor, was significantly higher among HT patients than in the healthy control group. Th1 cells are induced in response to IFN-γ and IL-12, which are secreted primarily by dendritic and natural killer cells, respectively.6 IFN-γ- and IL-12-related signal pathways are mediated through signal transducer and activator of transcription (STAT) 1 and STAT4, which cause T-bet expression; T-bet then induces IFN-γ production, strengthening Th1 polarisation and forming a positive feedback loop.6 Higher levels of IFN-γ and IL-12 have been reported among patients with HT, which may account for the increased expression of T-bet in these patients.27,28 It seems that HT is a Th1-dependent process in that the IFN-γ-secreting Th1 cells activate cytotoxic T lymphocytes and macrophages, killing thyroid follicular cells.28
The current study also demonstrated that expression of GATA3, the Th2 transcription factor, was significantly increased among HT patients as compared to the healthy controls. Differentiation of Th2 cells is induced by IL-4, which is secreted primarily by mast cells and basophils.8 The IL-4-related signal pathway is mediated through STAT6, which causes GATA3 expression, thus inducing the production of Th2-type cytokines and self-activation in a self-reinforcing cycle.6 The elevated expression of IL-4 has been previously reported among patients with HT, which may explain the increased expression of GATA3 among these patients.29 However, no significant difference was noted between HT patients and the control group in the current study regarding expression of the T-bet/GATA3 ratio; this suggests that both Th1 and Th2 cell-related immune responses may contribute to the pathogenesis of HT. Qin et al. demonstrated increased expression of Th1-related cytokines in both the thyroid gland and PBMCs of HT patients.30 Moreover, increased expression of Th1-related cytokines has been associated with elevated titres of anti-TPO antibodies, the destruction of thyroxisomes and the induction of apoptosis in thyroid follicular cells.31–33 However, the importance of the role of Th2 cells in the pathogenesis of HT is still debatable.
Expression of FOXP3, the Treg cell transcription factor, was significantly reduced among patients with HT in comparison with the healthy control group in the current study. Treg cells are divided into two subgroups—natural Treg and inducible Treg cells.14,15 The former are generated from precursor cells in the thymus, whereas antigenic stimulation in the presence of TGF-β and IL-2 causes differentiation of naïve CD4+ T cells into the latter cells in the peripheral lymphoid organs.14,15 Indeed, lower levels of TGF-β have been reported among patients with HT, potentially accounting for their decreased FOXP3 expression.34 The findings of the present study suggest that the downregulation of Treg cell-related functions may contribute to HT pathogenesis. Moreover, diminished numbers of Treg cells and low gene expression of FOXP3 in PBMCs has previously been reported among HT patients.35,36 Certain Treg cell-related cytokines (TGF-β, IL-10 and IL-35) have been associated with low-grade thyroid inflammation and an improvement in HT symptoms.26 However, another study demonstrated FOXP3 upregulation in the T cells of peripheral blood samples from patients with HT.21 This discrepancy is perhaps attributable to differing inclusion criteria used in these studies.21,35,36
In the current study, expression of the T-bet/FOXP3, GATA3/FOXP3 and RORα/FOXP3 ratios was significantly increased among patients with HT in comparison with the healthy control group; however, while the expression of T-bet, GATA3 and RORα alone was increased, FOXP3 expression alone was reduced among HT patients. Therefore, it is logical that expression of the T-bet/FOXP3, GATA3/FOXP3 and RORα/FOXP3 ratios was significantly increased among the HT patients. Xue et al. reported that the Treg/Th17 ratio among HT patients was significantly decreased when compared with a euthyroid control group.35 These findings indicate that imbalances in Th1/Treg, Th2/Treg and Th17/Treg cells at the level of transcription factors may contribute to the pathogenesis of HT, with a tendency towards Th1, Th2 and Th17 cells. As such, the correction of these imbalances may be of immunotherapeutic benefit for affected patients. As STAT5 and STAT3 regulate the differentiation of Treg and Th17 cells, respectively, the suppression of STAT3 by small interfering RNA molecules has been found to significantly reduce Th17 cells and increase Treg cells in the CD4+ T cells of patients with rheumatoid arthritis.13 Similar immunotherapeutic strategies may be considered for the treatment of HT. Additionally, T-bet/FOXP, GATA3/FOXP3 and RORα/FOXP3 ratios may be useful surrogate parameters to determine the status of Th1/Treg, Th2/Treg and Th17/Treg cells in affected patients.
Increased IL-6 expression has been reported among patients with HT.37 In the presence of IL-6 and TGF-β, the antigenic induction of naïve CD4+ T cells results in expression of RORγt and RORα (transcription factors of Th17 cells) via STAT3.11,13 Pyzik et al. also observed increased numbers of Th17 cells and elevated Th17-related cytokines among patients with HT, highlighting the role of Th17 in HT pathogenesis.3 In the present study, there was no significant difference between HT patients and the healthy group regarding RORα expression, although expression was higher among HT patients. No study has yet investigated expression of RORα in patients with HT; however, Shi et al. reported increased RORγt among HT patients.22 It is possible that RORα plays a less important role than RORγt in the development of Th17 cells. Noack et al. demonstrated that knocking out the RORα and RORγt genes disturbed differentiation of naïve CD4+ T cells to Th17 lymphocytes; however, knocking out the RORγt gene exhibited more powerful consequences, suggesting that the RORγt gene plays a more important role in this process.13
Initially, HT was believed to be a Th1-mediated disease; however, recent data suggest that Th17 cells may play a more prominent role in HT pathogenesis.3,25 The results of the current study suggest that a combination of Th1, Th2 and Th17 cell-related immune responses may synergistically contribute to the pathogenesis of HT. However, further research is needed to determine which Th subset plays a more important role in the development of HT. Recently, increased expression of FOXP3 and T-bet was demonstrated among HT patients who were severely affected but not euthyroid.21 Thus, it seems that different immunopathological mechanisms may be involved in the various expressions of the spectrum of HT-associated diseases. Moreover, thyroid function may also influence the expression of T cell-related transcription factors in HT patients.21
Conclusion
The results of the current study indicate that HT patients have imbalances in Th1/Treg, Th2/Treg and Th17/Treg cells at the level of transcription factors, with a deviation towards Th1, Th2 and Th17 cells. Since T-bet, RORα and FOXP3 are specific transcription factors for Th1, Th17 and Treg cells, respectively, the expression of T-bet/FOXP, GATA3/FOXP3 and RORα/FOXP3 mRNA ratios may be considered useful surrogate parameters to determine the status of Th1/Treg, Th2/Treg and Th17/Treg cells in such patients.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
FUNDING
This study was funded by the Kerman University of Medical Sciences (grant #93-321).
References
- 1.Antonelli A, Ferrari SM, Corrado A, Di Domenicantonio A, Fallahi P. Autoimmune thyroid disorders. Autoimmun Rev. 2015;14:174–80. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 2.Ajjan RA, Weetman AP. The pathogenesis of Hashimoto’s thyroiditis: Further developments in our understanding. Horm Metab Res. 2015;47:702–10. doi: 10.1055/s-0035-1548832. [DOI] [PubMed] [Google Scholar]
- 3.Pyzik A, Grywalska E, Matyjaszek-Matuszek B, Roliński J. Immune disorders in Hashimoto’s thyroiditis: What do we know so far? J Immunol Res. 2015;2015:979167. doi: 10.1155/2015/979167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafarzadeh A, Poorgholami M, Izadi N, Nemati M, Rezayati M. Immunological and hematological changes in patients with hyperthyroidism or hypothyroidism. Clin Invest Med. 2010;33:E271–9. doi: 10.25011/cim.v33i5.14352. [DOI] [PubMed] [Google Scholar]
- 5.Cogni G, Chiovato L. An overview of the pathogenesis of thyroid autoimmunity. Hormones (Athens) 2013;12:19–29. doi: 10.1007/BF03401283. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y, Zhang Y, Gu W, Sun B. TH1/TH2 cell differentiation and molecular signals. Adv Exp Med Biol. 2014;841:15–44. doi: 10.1007/978-94-017-9487-9_2. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Zhang Y, Gu W, He L, Sun B. Th1/Th2 cell’s function in immune system. Adv Exp Med Biol. 2014;841:45–65. doi: 10.1007/978-94-017-9487-9_3. [DOI] [PubMed] [Google Scholar]
- 8.Na H, Cho M, Chung Y. Regulation of Th2 cell immunity by dendritic cells. Immune Netw. 2016;16:1–12. doi: 10.4110/in.2016.16.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu R, Hatton RD, Weaver CT. The Th17 family: Flexibility follows function. Immunol Rev. 2013;252:89–103. doi: 10.1111/imr.12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rathore JS, Wang Y. Protective role of Th17 cells in pulmonary infection. Vaccine. 2016;34:1504–14. doi: 10.1016/j.vaccine.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Volpe E, Battistini L, Borsellino G. Advances in T helper 17 cell biology: Pathogenic role and potential therapy in multiple sclerosis. Mediators Inflamm. 2015;2015:475158. doi: 10.1155/2015/475158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Etesam Z, Nemati M, Ebrahimizadeh MA, Ebrahimi HA, Hajghani H, Khalili T, et al. Altered expression of specific transcription factors of Th17 (RORγt, RORα) and Treg lymphocytes (FOXP3) by peripheral blood mononuclear cells from patients with multiple sclerosis. J Mol Neurosci. 2016;60:94–101. doi: 10.1007/s12031-016-0789-5. [DOI] [PubMed] [Google Scholar]
- 13.Noack M, Miossec P. Th17 and regulatory T cell balance in autoimmune and inflammatory diseases. Autoimmun Rev. 2014;13:668–77. doi: 10.1016/j.autrev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 14.Jafarzadeh A, Jamali M, Mahdavi R, Ebrahimi HA, Hajghani H, Khosravimashizi A, et al. Circulating levels of interleukin-35 in patients with multiple sclerosis: Evaluation of the influences of FOXP3 gene polymorphism and treatment program. J Mol Neurosci. 2015;55:891–7. doi: 10.1007/s12031-014-0443-z. [DOI] [PubMed] [Google Scholar]
- 15.Nie J, Li YY, Zheng SG, Tsun A, Li B. FOXP3(+) Treg cells and gender bias in autoimmune diseases. Front Immunol. 2015;6:493. doi: 10.3389/fimmu.2015.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González-Amaro R, Marazuela M. T regulatory (Treg) and T helper 17 (Th17) lymphocytes in thyroid autoimmunity. Endocrine. 2016;52:30–8. doi: 10.1007/s12020-015-0759-7. [DOI] [PubMed] [Google Scholar]
- 17.Horie I, Abiru N, Sakamoto H, Iwakura Y, Nagayama Y. Induction of autoimmune thyroiditis by depletion of CD4+CD25+ regulatory T cells in thyroiditis-resistant IL-17, but not interferon-gamma receptor, knockout nonobese diabetic-H2h4 mice. Endocrinology. 2011;152:4448–54. doi: 10.1210/en.2011-1356. [DOI] [PubMed] [Google Scholar]
- 18.Jafarzadeh A, Shokri F. TH1 and TH2 responses are influenced by HLA antigens in healthy neonates vaccinated with recombinant hepatitis B vaccine. Iran J Allergy Asthma Immunol. 2012;11:308–15. [PubMed] [Google Scholar]
- 19.Evans CM, Jenner RG. Transcription factor interplay in T helper cell differentiation. Brief Funct Genomics. 2013;12:499–511. doi: 10.1093/bfgp/elt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ichiyama K, Yoshida H, Wakabayashi Y, Chinen T, Saeki K, Nakaya M, et al. FOXP3 inhibits RORgammat-mediated IL-17A mRNA transcription through direct interaction with RORgammat. J Biol Chem. 2008;283:17003–8. doi: 10.1074/jbc.M801286200. [DOI] [PubMed] [Google Scholar]
- 21.Tokić S, Štefanić M, Glavaš-Obrovac L, Jaman S, Novosadová E, Petrkova J, et al. The expression of T cell FOXP3 and T-bet is upregulated in severe but not euthyroid Hashimoto’s thyroiditis. Mediators Inflamm. 2016;2016:3687420. doi: 10.1155/2016/3687420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Y, Wang H, Su Z, Chen J, Xue Y, Wang S, et al. Differentiation imbalance of Th1/Th17 in peripheral blood mononuclear cells might contribute to pathogenesis of Hashimoto’s thyroiditis. Scand J Immunol. 2010;72:250–5. doi: 10.1111/j.1365-3083.2010.02425.x. [DOI] [PubMed] [Google Scholar]
- 23.Caturegli P, De Remigis A, Rose NR. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun Rev. 2014;13:391–7. doi: 10.1016/j.autrev.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 24.Aminorroaya A, Amini M, Hovsepian S. Prevalence of goitre in Isfahan, Iran, fifteen years after initiation of universal salt iodization. J Health Popul Nutr. 2010;28:351–8. doi: 10.3329/jhpn.v28i4.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konca Degertekin C, Aktas Yilmaz B, Balos Toruner F, Kalkanci A, Turhan Iyidir O, Fidan I, et al. Circulating Th17 cytokine levels are altered in Hashimoto’s thyroiditis. Cytokine. 2016;80:13–17. doi: 10.1016/j.cyto.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz H, Cakmak M, Ceydilek B, Demir C, Aktas A. Role of interlekin-35 as a biomarker in patients with newly diagnosed Hashimoto’s thyroiditis. Endocr Regul. 2016;50:55–61. doi: 10.1515/enr-2016-0009. [DOI] [PubMed] [Google Scholar]
- 27.Phenekos C, Vryonidou A, Gritzapis AD, Baxevanis CN, Goula M, Papamichail M. Th1 and Th2 serum cytokine profiles characterize patients with Hashimoto’s thyroiditis (Th1) and Graves’ disease (Th2) Neuroimmunomodulation. 2004;11:209–13. doi: 10.1159/000078438. [DOI] [PubMed] [Google Scholar]
- 28.Khan FA, Al-Jameil N, Khan MF, Al-Rashid M, Tabassum H. Thyroid dysfunction: An autoimmune aspect. Int J Clin Exp Med. 2015;8:6677–81. [PMC free article] [PubMed] [Google Scholar]
- 29.Bossowski A, Harasymczuk J, Moniuszko A, Bossowska A, Hilczer M, Ratomski K. Cytometric evaluation of intracellular IFN-γ and IL-4 levels in thyroid follicular cells from patients with autoimmune thyroid diseases. Thyroid Res. 2011;4:13. doi: 10.1186/1756-6614-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qin Q, Liu P, Liu L, Wang R, Yan N, Yang J, et al. The increased but non-predominant expression of Th17- and Th1-specific cytokines in Hashimoto’s thyroiditis but not in Graves’ disease. Braz J Med Biol Res. 2012;45:1202–8. doi: 10.1590/S0100-879X2012007500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karanikas G, Schuetz M, Wahl K, Paul M, Kontur S, Pietschmann P, et al. Relation of anti-TPO autoantibody titre and T-lymphocyte cytokine production patterns in Hashimoto’s thyroiditis. Clin Endocrinol (Oxf) 2005;63:191–6. doi: 10.1111/j.1365-2265.2005.02324.x. [DOI] [PubMed] [Google Scholar]
- 32.Marique L, Van Regemorter V, Gérard AC, Craps J, Senou M, Marbaix E, et al. The expression of dual oxidase, thyroid peroxidase, and caveolin-1 differs according to the type of immune response (TH1/TH2) involved in thyroid autoimmune disorders. J Clin Endocrinol Metab. 2014;99:1722–32. doi: 10.1210/jc.2013-3469. [DOI] [PubMed] [Google Scholar]
- 33.Wang SH, Bretz JD, Phelps E, Mezosi E, Arscott PL, Utsugi S, et al. A unique combination of inflammatory cytokines enhances apoptosis of thyroid follicular cells and transforms nondestructive to destructive thyroiditis in experimental autoimmune thyroiditis. J Immunol. 2002;168:2470–4. doi: 10.4049/jimmunol.168.5.2470. [DOI] [PubMed] [Google Scholar]
- 34.Manolova I, Gerenova J, Ivanova M. Serum levels of transforming growth factor-β1 (TGF-β1) in patients with systemic lupus erythematosus and Hashimoto’s thyroiditis. Eur Cytokine Netw. 2013;24:69–74. doi: 10.1684/ecn.2013.0331. [DOI] [PubMed] [Google Scholar]
- 35.Xue H, Yu X, Ma L, Song S, Li Y, Zhang L, et al. The possible role of CD4+CD25(high)Foxp3+/CD4+IL-17A+ cell imbalance in the autoimmunity of patients with Hashimoto thyroiditis. Endocrine. 2015;50:665–73. doi: 10.1007/s12020-015-0569-y. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Tang X, Tian J, Zhu C, Peng H, Rui K, et al. Th17/Treg cells imbalance and GITRL profile in patients with Hashimoto’s thyroiditis. Int J Mol Sci. 2014;15:21674–86. doi: 10.3390/ijms151221674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, You R, Yu N, Gong Y, Qu C, Zhang Y, et al. Increased proportions of Tc17 cells and NK cells may be risk factors for disease progression in Hashimoto’s thyroiditis. Int Immunopharmacol. 2016;40:332–8. doi: 10.1016/j.intimp.2016.09.016. [DOI] [PubMed] [Google Scholar]