Abstract
Context:
Understanding the factors associated with thicker cartilage in a healthy population is important when developing strategies aimed at minimizing the cartilage thinning associated with knee osteoarthritis progression. Thicker articular cartilage is commonly thought to be healthier cartilage, but whether the sagittal-plane biomechanics important to gait are related to cartilage thickness is unknown.
Objective:
To determine the relationship of a weight-bearing region of the medial femoral condyle's cartilage thickness to sagittal gait biomechanics in healthy individuals.
Design:
Descriptive laboratory study.
Setting:
Laboratory.
Patients or Other Participants:
Twenty-eight healthy participants (15 women: age = 21.1 ± 2.1 years, height = 1.63 ± 0.07 m, weight = 64.6 ± 9.9 kg; 13 men: age = 22.1 ± 2.9 years, height = 1.79 ± 0.05 m, weight = 75.2 ± 9.6 kg).
Main Outcome Measure(s):
Tibiofemoral angle (°) was obtained via goniometric assessment, thickness of the medial femoral condyle cartilage (mm) was obtained via ultrasound imaging, and peak internal knee-extensor moment (% body weight · height) was measured during 10 trials of over-ground walking at a self-selected pace. We used linear regression to examine the extent to which peak internal knee-extensor moment predicted cartilage thickness after accounting for tibiofemoral angle and sex.
Results:
Sex and tibiofemoral angle (12.3° ± 3.2°) were entered in the initial step as control factors (R2 = 0.01, P = .872). In the final step, internal knee-extensor moment (1.5% ± 1.3% body weight · height) was entered, which resulted in greater knee-extensor moment being related to greater cartilage thickness (2.0 ± 0.3 mm; R2Δ = 0.31, PΔ = .003).
Conclusion:
Individuals who walked with a greater peak internal knee-extensor moment during gait had a cartilage structure that is generally considered beneficial in a healthy population. Our study offers promising findings that a potentially modifiable biomechanical factor is associated with cartilage status in a healthy population. Establishing these baseline relationships in uninjured populations may help us to better understand potential factors related to maladaptive gait patterns that predispose a person to adverse changes in the cartilage environment.
Key Words: ultrasound, osteoarthritis, biomechanics
Key Points
Individuals who walked with a greater peak internal knee-extensor moment during gait had a cartilage structure that is generally considered beneficial in a healthy population.
Establishing baseline relationships in uninjured populations may allow us to better understand the gait patterns that may be beneficial to long-term joint health.
By the time individuals become symptomatic and seek medical care for knee osteoarthritis (OA), irreversible damage to the articular cartilage has occurred.1 Thus, establishing the clinical factors associated with thicker cartilage in a healthy population is a critical step in designing protocols that seek to reduce the risk of OA development. Cartilage thickness is 1 important measure in describing both OA development and progression.2,3 Although the very earliest OA stages may result in an increase in cartilage thickness,4,5 the development and progression of clinical OA are commonly characterized by structural changes including erosion and loss of articular cartilage. Individuals with established knee OA have less tibiofemoral cartilage than healthy individuals6 and lose significant articular knee cartilage annually.7 It has been suggested that the central medial femoral cartilage be assessed for changes in cartilage morphology associated with early signs of knee OA.8
Normal chondrocyte loading is critical in the development and maintenance of healthy articular cartilage.9 Patterns of knee-joint loading during walking influence cartilage thickness, as healthy knee medial femoral compartment cartilage is thickest in primary weight-bearing areas.10 Additionally, thinner knee cartilage was found in people with spinal cord injuries compared with healthy control participants and is correlated with the length of limb disuse.11 Repetitive, long-term loading, such as walking, likely increases thickness in the areas of greatest loading.10 This suggests that healthy cartilage adapts to local demands and that thickening of healthy cartilage is a positive physiological factor.
Knee-joint moments are a common proxy for knee-joint loading.12 Medial tibiofemoral loading is often considered in the health of the articular cartilage.13 Although the external knee-adduction moment accounted for 63% of the variance in medial knee-compartment loading, the peak external knee-flexion moment (equivalent to an internal extensor moment) added a significant 22% variance to the prediction of medial loading.14 In a model of joint compressive force, greater internal knee-extensor moments were associated with greater quadriceps forces.15 These greater quadriceps forces, along with greater hamstrings and gastrocnemius forces in turn, were related to greater joint compressive forces.15 Thus, increased knee-extensor moments during gait in uninjured populations may contribute to greater compressive loading of the articular knee cartilage, which may result in healthier or thicker articular cartilage.
Relative frontal-plane knee-joint–loading patterns during walking have been associated with articular cartilage thickness patterns,16 but the relationships between cartilage thickness and sagittal knee-loading patterns during gait in a healthy population are not well understood. Further, the authors of a recent meta-analysis17 suggested that sagittal-plane biomechanics, rather than the commonly reported knee-adduction moment, appeared to be more relevant in a population at risk of early OA development. Understanding the relationship of sagittal-plane biomechanics to cartilage health is important, as approximately 85% of the work during gait occurs in the sagittal plane.18 Establishing these baseline relationships may help us to better understand potential ambulation factors that may predispose a person to or protect a person from future OA development.
For assessing articular cartilage thickness, the criterion standard for measurement has been magnetic resonance imaging (MRI).19 However, this measure is expensive and not easily accessible. As an alternative, diagnostic ultrasound is a valid and reliable means of assessing cartilage thickness in injured populations.20,21 Compared with MRI, ultrasound is less expensive and much more clinically available. Thus, the purpose of our study was to determine the relationship of the cartilage thickness of a weight-bearing region of the medial femoral condyle, as assessed by ultrasound, to sagittal gait biomechanics in healthy individuals, while controlling for the potentially confounding factors of sex and tibiofemoral angle. Given that medial compartment OA is more prevalent than lateral compartment OA,22 we chose to focus on the medial aspect.
METHODS
Experimental Protocol
Participants attended 1 laboratory testing session. Height, weight, and tibiofemoral angle (TFA) were obtained; the medial femoral knee cartilage was assessed using ultrasound; and biomechanical testing of the lower extremity during gait was performed. Sex was noted and TFA was obtained to control for previously reported relationships of sex and knee cartilage volume23 and TFA and medial knee cartilage volume and thickness.24,25
Participants
Twenty-eight healthy participants (15 women: age = 21.1 ± 2.1 years, height = 1.63 ± 0.07 m, weight = 64.6 ± 9.9 kg; 13 men: age = 22.1 ± 2.9 years, height = 1.79 ± 0.05 m, weight = 75.2 ± 9.6 kg) who were at least recreationally active (2 hours of physical activity per week) and had no current orthopaedic injury or history of significant injury or surgery in the left limb were recruited from a general university population. Before data collection began, all participants reviewed and signed an informed consent form approved by the university's institutional review board (which also approved the study) and the rights of all participants were protected. On the testing day, participants were instructed to avoid vigorous physical activity before arriving at the laboratory.
Procedures
Using a transverse approach previously established in the literature,26 a single examiner performed ultrasound imaging of the left knee medial femoral articular cartilage with a 13-MHz linear ultrasound transducer (SonoSite, Inc, Bothell, WA). After 10 minutes of quiet sitting, participants were instructed to sit on a table with the left knee in 90° of flexion. The bony medial femoral condyle was palpated and outlined with a washable marker. The transducer head was then placed transversely over the medial femoral condyle to measure the cartilage thickness over the midportion (the area bearing the most weight). Three adjacent sections (approximately 2 mm each) of cartilage were measured from a thin hyperechoic line at the soft tissue-cartilage interface to the hyperechoic line at the cartilage-bone interface (Figure 1). These 3 values were averaged to obtain a single value that represented the cartilage thickness of the middle medial femoral condyle. Before the study, the ultrasound examiner established between-days intratester reliability and precision by assessing 12 participants using the same inclusion and exclusion criteria as in the current investigation; the intraclass correlation coefficient (2,3) ± SEM for measurements separated by 2 to 7 days was 0.91 ± 0.1 mm. Additionally, in a pilot test of 10 participants, the transverse ultrasound thickness and average thickness of the central weight-bearing region as assessed via MRI were significantly positively related (r = .75, P = .012).27
Figure 1. .
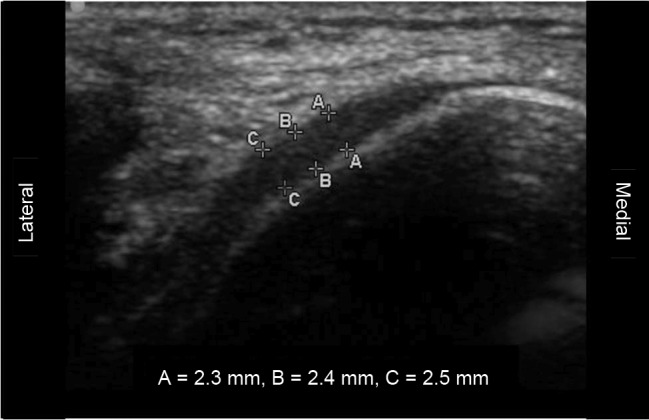
Representative ultrasound image demonstrating medial femoral cartilage thicknesses at 3 adjacent sites (A, B, C) that were averaged for analyses.
The TFA represented the angle formed by the anatomic axis of the femur and tibia in the frontal plane28 in bilateral stance using a standard goniometer (increased value = greater valgus) as measured by a single investigator. The goniometer was placed over the knee center with the stationary arm aligned to a proximal landmark (midpoint between the anterior-superior iliac spine and the most prominent aspect of the greater trochanter), and the movable arm was aligned to the ankle-joint center.29 The average of 3 measures was used for analyses. Before data collection, the TFA examiner established between-days intratester reliability and precision by assessing 14 healthy, uninjured participants at intervals of 2 to 4 days; the intraclass correlation coefficient (2,3) ± SEM = 0.99° ± 0.4°.
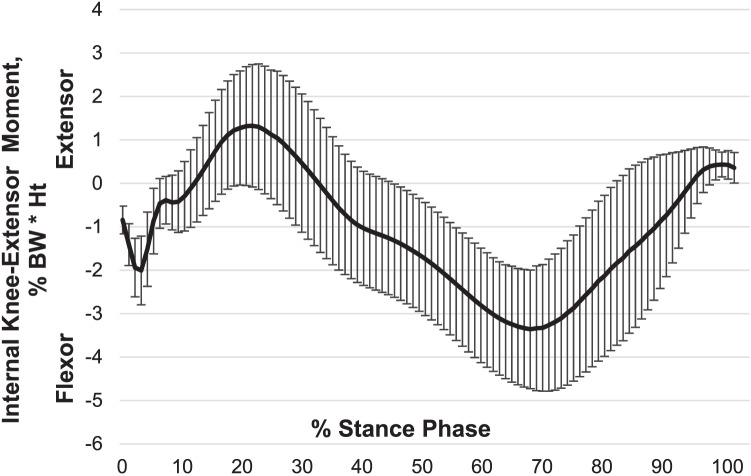
Gait biomechanics were collected during 10 walking trials at a self-selected pace across a 6-m runway. Although gait speed can greatly influence knee-extensor moment,30 we allowed each person to self-select gait speed to best represent individual function. Participants were instrumented using 4 optical light-emitting diode markers on each segment (foot, shank, thigh, and pelvis), and kinematic data were collected with an 8-camera optical light-emitting diode system (model Impulse; PhaseSpace, San Leandro, CA). Participants were allowed adequate practice to ensure full foot contact with the force platform (model 4060-NC; Bertec Corporation, Columbus, OH) during their self-selected pace. Kinematic and kinetic data were collected at 240 Hz and 1000 Hz, respectively. Pace was monitored using center-of-mass velocity. If the participant did not maintain ±5% of the self-selected pace used in practice trials, the trial was repeated. All kinematic and kinetic data were processed and calculated using MotionMonitor (model 8.2; Innovative Sports Training, Chicago, IL). Biomechanical kinematic and kinetic data were synchronized and processed using a fourth-order, zero-lag, low-pass Butterworth filter at 12 Hz. Intersegmental motions were calculated via Euler equations; intersegmental forces and internal moments were calculated using inverse-dynamics equations.31 Internal knee-extensor moment (extension was defined as positive) for the stance phase was normalized to 100 points, normalized to percentage of body weight and height (% body weight · height) and then ensemble averaged over the 10 trials. Stance phase (heel contact to toe off) was defined as the time when the vertical ground reaction force exceeded 15 N (Figure 2).
Figure 2. .
Internal knee-extensor moment (mean ± standard deviation) across the stance phase of gait. Abbreviations: BW, body weight; Ht, height.
Statistical Analysis
We used a forward stepwise linear regression to predict medial femoral cartilage thickness. Sex and TFA were entered in the first step to control for previously reported relationships of sex and knee cartilage volume23 and TFA and medial knee cartilage volume and thickness.24,25 In the second step, internal knee-extensor moment was entered.
RESULTS
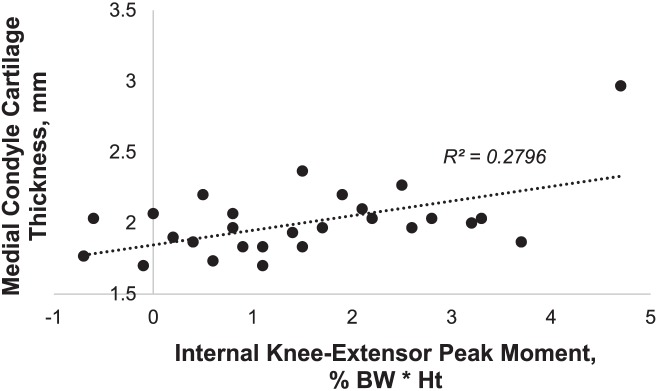
Descriptive data by sex are presented in Table 1. After accounting for sex and TFA (R2 = .01, P = .872), greater internal knee-extensor moment (R2Δ = 0.31, PΔ = .003) was related to greater medial femoral cartilage thickness (Table 2). The final regression model coefficients and correlations are shown in Table 3. A scatterplot of internal knee-extensor moment and medial femoral cartilage thickness is illustrated in Figure 3.
Table 1. .
Participants' Characteristics
| Characteristic |
Mean ± SD |
P Value |
|
| Women |
Men |
||
| Tibiofemoral angle, ° | 13.4 ± 3.5 | 11.0 ± 2.3 | .043 |
| Knee-extensor peak moment, % body weight · height | 1.53 ± 1.3 | 1.43 ± 1.4 | .847 |
| Medial femoral condyle cartilage thickness, mm | 1.97 ± 0.1 | 2.02 ± 0.4 | .600 |
Table 2. .
Stepwise Regression Results
| Model |
R |
R2 |
R2Δ |
Significance FΔ |
| 1 | 0.104a | 0.011 | 0.011 | .872 |
| 2 | 0.564b | 0.318 | 0.307 | .003 |
Model 1 variables: constant, sex, tibiofemoral angle.
Model 2 variables: constant, sex, tibiofemoral angle, internal knee-extensor moment.
Table 3. .
Final Regression Model Coefficients and Correlations
| Coefficient |
Unstandardized β |
Standardized β |
t Value |
P Value |
Correlations |
||
| Zero Order |
Partial |
Semipartial |
|||||
| Constant | 2.00 | 7.38 | .000 | ||||
| Tibiofemoral angle | –0.015 | –0.190 | –0.99 | .334 | –0.027 | –0.197 | –0.166 |
| Sex | 0.027 | 0.053 | 0.29 | .776 | 0.104 | 0.059 | 0.048 |
| Internal knee-extensor moment | 0.115 | 0.586 | 3.29 | .003 | 0.524 | 0.557 | 0.554 |
Figure 3. .
Relationship of medial femoral condyle thickness to peak internal knee-extensor moment. Abbreviations: BW, body weight; Ht, height.
DISCUSSION
The knee's mechanical environment during walking has been suggested to influence the health and the breakdown of knee articular cartilage.32 Thus, we undertook a study to better understand the role of sagittal-plane loading during gait in a surrogate measure of knee cartilage health. Our primary finding was that a larger peak internal knee-extensor moment was significantly positively correlated with medial femoral cartilage thickness in healthy individuals. In the following discussion, we will address the mechanistic relationship of knee loading to cartilage thickness and the potential role of the current findings in the future of knee OA prevention and care.
Our results suggest that individuals who use a gait pattern with greater internal knee-extensor magnitude have a cartilage structure that is generally considered healthier in an uninjured population. Although biomechanical correlates of thicker cartilage in a healthy population are not commonly reported in the literature, such knowledge may serve as the basis for programs promoting joint health. In young, healthy individuals examined during hopping, greater sagittal knee moments were associated with decreased cartilage quantitative MRI values, which indicate a denser cartilage matrix (higher proteoglycan and collagen content).33 During gait, participants with severe OA walked with the knee less extended and with smaller knee-extension moments than younger asymptomatic participants and older participants with moderate OA.34 Additionally, participants in both OA groups had smaller extension moments than the younger, healthy participants.34 Although limited by a lack of longitudinal data, these studies provide supporting evidence that larger internal knee-extension moments are associated with healthier knee articular cartilage.
It is commonly understood that the quadriceps muscles contribute to sagittal-plane control of the knee during gait by contributing to the internal-extensor moment that helps to counteract the magnitude of the external-flexion moment. Further, quadriceps muscle strength may protect against knee-joint damage and progression of existing OA.35 Supporting this premise, greater lower limb muscle mass has been positively related to medial tibial cartilage volume,36 and baseline quadriceps strength has been positively related to 2-year total cartilage volume changes in a healthy adult population.12 Although reducing the hamstrings contribution during the stance phase of gait could aid in resisting the external-flexion moment, the quadriceps muscles contribute more than the hamstrings in controlling the knee during the stance phase of gait.37 Given the importance of the quadriceps in resisting the external-flexion moment by producing an internal-extensor moment, it is plausible that increasing the internal knee-extension moment, thereby generating a greater quadriceps contribution during gait, may be beneficial to cartilage health.
Reasonable evidence suggests that a complex relationship may exist between healthy cartilage and loading. Although we did not assess the associations between activity levels and joint compressive loading, this related literature may help to explain our finding of increased internal knee-extensor moment being related to greater cartilage thickness in healthy individuals. Moderate joint loading increased cartilage thickness in the knee joints of growing canines, whereas intensive loading resulted in either no change or a decrease in thickness.38 Similarly, in a cross-sectional study,39 strenuous exercise was positively correlated with increased cartilage volume in growing children. In a cross-sectional study40 of adults, cartilage thickness between triathletes and inactive individuals did not differ, but the triathletes had more joint surface. Taken together, the evidence indicates that exercise has the potential to affect cartilage and joint morphology. Although evidence is limited with respect to dosage amounts, exercise that includes a greater internal knee-extensor moment may positively affect joint health.
The literature surrounding anterior cruciate ligament (ACL) injury and joint health may offer additional insights into the role of knee-extensor moments in joint health. A systematic review41 demonstrated that patients with ACL-deficient or ACL-reconstructed limbs had decreased knee-extensor moments. Quadriceps weakness has been associated with reduced knee moments during gait in ACL-deficient patients.42 Also, a decrease in internal knee-extension moment may result from inadequate quadriceps activation.43 Given the relatively high incidence of OA development in the ACL-injured population,44 this would suggest that increasing the internal knee-extensor moment during gait may be beneficial for joint health.
Our finding that TFA had no relationship to cartilage thickness in healthy individuals was somewhat unexpected. The varus-valgus angulation of the knee has been associated with mediolateral distribution of loads on the knee's articular structures.45 Minor increases in varus alignment (approximately 5%) have the potential to greatly increase (approximately 20%) medial knee loading.45 Further, participant-specific 3-dimensional finite element models of a knee with varus alignment had the largest stresses in the medial compartment compared with the participants who had normal or valgus alignment.46 However, a 2009 systematic review13 demonstrated limited evidence (because few investigators studied incident OA) for the role of alignment in OA development. Thus, further work is needed to understand the role of frontal-plane knee angle in the maintenance of cartilage health in a population without OA.
Due to greater clinical incidence of medial knee OA—the ratio of medial to lateral cartilage disease is roughly 5 to 1 among women and 8–9 to 1 among men22—our study was limited to assessment of the medial femoral condyle. Additionally, the assessment of knee cartilage was limited to ultrasound, even though the criterion standard is MRI. Although the validity of ultrasound assessment has previously been established in OA populations,47,48 a pilot validation of ultrasound measures has been performed.27 Current work is underway to fully validate ultrasound assessment of femoral cartilage thickness in a healthy population. Also, the use of ultrasound limits the ability to detect the established subtle regional differences in cartilage thickness10 because of the difficulty in assessing the same exact condylar subregions among participants. Furthermore, we did not quantify the physical activity of our participants. A range of physical activity levels in our population may have confounded the results. Finally, whether this same relationship of knee-extensor moment to cartilage thickness would exist in individuals at higher risk of OA development, such as a person with an ACL injury, is unknown.
CONCLUSIONS
Individuals who walk with a greater peak internal knee-extensor moment during gait have a cartilage structure that is generally considered beneficial in a healthy population. Helping to establish baseline relationships in uninjured populations may allow us to better understand gait patterns that may be beneficial to long-term joint health. Our findings are promising in that a potentially modifiable biomechanical factor is a determinant of cartilage thickness in healthy individuals. Future researchers should focus on better understanding the role of other biomechanical factors in knee cartilage health. Identifying the biomechanical variables that are associated with healthier cartilage could ultimately direct the creation of strategies to delay both the onset of clinical signs and symptoms related to OA development and the need for more costly solutions.
REFERENCES
- 1. Dillon CF, Rasch EK, Gu Q, Hirsch R. . Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006; 33 11: 2271– 2279. [PubMed] [Google Scholar]
- 2. Eckstein F, Cicuttini F, Raynauld JP, Waterton JC, Peterfy C. . Magnetic resonance imaging (MRI) of articular cartilage in knee osteoarthritis (OA): morphological assessment. Osteoarthritis Cartilage. 2006; 14 suppl A: A46– A75. [DOI] [PubMed] [Google Scholar]
- 3. Buck RJ, Wyman BT, Le Graverand MP, Hudelmaier M, Wirth W, Eckstein F. . Osteoarthritis may not be a one-way-road of cartilage loss—comparison of spatial patterns of cartilage change between osteoarthritic and healthy knees. Osteoarthritis Cartilage. 2010; 18 3: 329– 335. [DOI] [PubMed] [Google Scholar]
- 4. Frobell RB, Nevitt MC, Hudelmaier M, et al. Femorotibial subchondral bone area and regional cartilage thickness: a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1,003 knees from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2010; 62 11: 1612– 1623. [DOI] [PubMed] [Google Scholar]
- 5. Cotofana S, Buck R, Wirth W, et al. Cartilage thickening in early radiographic knee osteoarthritis: a within-person, between-knee comparison. Arthritis Care Res. 2012; 64 11: 1681– 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cicuttini FM, Wluka AE, Stuckey SL. . Tibial and femoral cartilage changes in knee osteoarthritis. Ann Rheum Dis. 2001; 60 10: 977– 980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wluka AE, Stuckey S, Snaddon J, Cicuttini FM. . The determinants of change in tibial cartilage volume in osteoarthritic knees. Arthritis Rheum. 2002; 46 8: 2065– 2072. [DOI] [PubMed] [Google Scholar]
- 8. Frobell RB, Le Graverand MP, Buck R, et al. The acutely ACL injured knee assessed by MRI: changes in joint fluid, bone marrow lesions, and cartilage during the first year. Osteoarthritis Cartilage. 2009; 17 2: 161– 167. [DOI] [PubMed] [Google Scholar]
- 9. Beaupre GS, Stevens SS, Carter DR. . Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000; 37 2: 145– 151. [PubMed] [Google Scholar]
- 10. Andriacchi TP, Koo S, Scanlan SF. . Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J Bone Joint Surg Am. 2009; 91 suppl 1: 95– 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kara M, Tiftik T, Oken O, Akkaya N, Tunc H, Ozcakar L. . Ultrasonographic measurement of femoral cartilage thickness in patients with spinal cord injury. J Rehabil Med. 2013; 45 2: 145– 148. [DOI] [PubMed] [Google Scholar]
- 12. Foley S, Ding C, Cicuttini F, Jones G. . Physical activity and knee structural change: a longitudinal study using MRI. Med Sci Sports Exerc. 2007; 39 3: 426– 434. [DOI] [PubMed] [Google Scholar]
- 13. Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. . Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis Rheum. 2009; 61 4: 459– 467. [DOI] [PubMed] [Google Scholar]
- 14. Manal K, Gardinier E, Buchanan TS, Snyder-Mackler L. . A more informed evaluation of medial compartment loading: the combined use of the knee adduction and flexor moments. Osteoarthritis Cartilage. 2015; 23 7: 1107– 1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Messier SP, Legault C, Loeser RF, et al. Does high weight loss in older adults with knee osteoarthritis affect bone-on-bone joint loads and muscle forces during walking? Osteoarthritis Cartilage. 2011; 19 3: 272– 280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koo S, Andriacchi TP. . A comparison of the influence of global functional loads vs. local contact anatomy on articular cartilage thickness at the knee. J Biomech. 2007; 40 13: 2961– 2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hart HF, Culvenor AG, Collins NJ, et al. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2016; 50 10: 597– 612. [DOI] [PubMed] [Google Scholar]
- 18. Eng JJ, Winter DA. . Kinetic analysis of the lower limbs during walking: what information can be gained from a three-dimensional model? J Biomech. 1995; 28 6: 753– 758. [DOI] [PubMed] [Google Scholar]
- 19. Stammberger T, Eckstein F, Englmeier KH, Reiser M. . Determination of 3D cartilage thickness data from MR imaging: computational method and reproducibility in the living. Magn Reson Med. 1999; 41 3: 529– 536. [DOI] [PubMed] [Google Scholar]
- 20. Pradsgaard DO, Fiirgaard B, Spannow AH, Heuck C, Herlin T. . Cartilage thickness of the knee joint in juvenile idiopathic arthritis: comparative assessment by ultrasonography and magnetic resonance imaging. J Rheumatol. 2015; 42 3: 534– 540. [DOI] [PubMed] [Google Scholar]
- 21. Naredo E, Acebes C, Moller I, et al. Ultrasound validity in the measurement of knee cartilage thickness. Ann Rheum Dis. 2009; 68 8: 1322– 1327. [DOI] [PubMed] [Google Scholar]
- 22. Felson DT, Nevitt MC, Zhang Y, et al. High prevalence of lateral knee osteoarthritis in Beijing Chinese compared with Framingham Caucasian subjects. Arthritis Rheum. 2002; 46 5: 1217– 1222. [DOI] [PubMed] [Google Scholar]
- 23. Ding C, Cicuttini F, Scott F, Glisson M, Jones G. . Sex differences in knee cartilage volume in adults: role of body and bone size, age and physical activity. Rheumatology (Oxford). 2003; 42 11: 1317– 1323. [DOI] [PubMed] [Google Scholar]
- 24. Cicuttini F, Wluka A, Hankin J, Wang Y. . Longitudinal study of the relationship between knee angle and tibiofemoral cartilage volume in subjects with knee osteoarthritis. Rheumatology (Oxford). 2004; 43 3: 321– 324. [DOI] [PubMed] [Google Scholar]
- 25. Sharma L, Eckstein F, Song J, et al. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum. 2008; 58 6: 1716– 1726. [DOI] [PubMed] [Google Scholar]
- 26. Abraham AM, Goff I, Pearce MS, Francis RM, Birrell F. . Reliability and validity of ultrasound imaging of features of knee osteoarthritis in the community. BMC Musculoskelet Dis. 2011; 12: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmitz RJ, Wang HM, Polprasert DR, Kraft RA. . Relationships of ultrasound based knee cartilage thickness measures to a MRI-based gold standard [abstract]. J Athl Train. 2016; 51 suppl 6: S39– S40. [Google Scholar]
- 28. Moreland JR, Bassett LW, Hanker GJ. . Radiographic analysis of the axial alignment of the lower extremity. J Bone Joint Surg Am. 1987; 69 5: 745– 749. [PubMed] [Google Scholar]
- 29. Nguyen AD, Shultz SJ. . Sex differences in clinical measures of lower extremity alignment. J Orthop Sports Phys Ther. 2007; 37 7: 389– 398. [DOI] [PubMed] [Google Scholar]
- 30. Lelas JL, Merriman GJ, Riley PO, Kerrigan DC. . Predicting peak kinematic and kinetic parameters from gait speed. Gait Posture. 2003; 17 2: 106– 112. [DOI] [PubMed] [Google Scholar]
- 31. Gagnon D, Gagnon M. . The influence of dynamic factors on triaxial net muscular moments at the L5/S1 joint during asymmetrical lifting and lowering. J Biomech. 1992; 25 8: 891– 901. [DOI] [PubMed] [Google Scholar]
- 32. Andriacchi TP, Mundermann A. . The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr Opin Rheumatol. 2006; 18 5: 514– 518. [DOI] [PubMed] [Google Scholar]
- 33. Souza RB, Fang C, Luke A, Wu S, Li X, Majumdar S. . Relationship between knee kinetics during jumping tasks and knee articular cartilage MRI T1rho and T2 relaxation times. Clin Biomech (Bristol, Avon). 2012; 27 4: 403– 408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Favre J, Erhart-Hledik JC, Andriacchi TP. . Age-related differences in sagittal-plane knee function at heel-strike of walking are increased in osteoarthritic patients. Osteoarthritis Cartilage. 2014; 22 3: 464– 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sharma L, Dunlop DD, Cahue S, Song J, Hayes KW. . Quadriceps strength and osteoarthritis progression in malaligned and lax knees. Ann Intern Med. 2003; 138 8: 613– 619. [DOI] [PubMed] [Google Scholar]
- 36. Cicuttini FM, Teichtahl AJ, Wluka AE, Davis S, Strauss BJ, Ebeling PR. . The relationship between body composition and knee cartilage volume in healthy, middle-aged subjects. Arthritis Rheum. 2005; 52 2: 461– 467. [DOI] [PubMed] [Google Scholar]
- 37. Arnold AS, Anderson FC, Pandy MG, Delp SL. . Muscular contributions to hip and knee extension during the single limb stance phase of normal gait: a framework for investigating the causes of crouch gait. J Biomech. 2005; 38 11: 2181– 2189. [DOI] [PubMed] [Google Scholar]
- 38. Kiviranta I, Tammi M, Jurvelin J, Arokoski J, Saamanen AM, Helminen HJ. . Articular cartilage thickness and glycosaminoglycan distribution in the canine knee joint after strenuous running exercise. Clin Orthop Relat Res. 1992; 283: 302– 308. [PubMed] [Google Scholar]
- 39. Jones G, Glisson M, Hynes K, Cicuttini F. . Sex and site differences in cartilage development: a possible explanation for variations in knee osteoarthritis in later life. Arthritis Rheum. 2000; 43 11: 2543– 2549. [DOI] [PubMed] [Google Scholar]
- 40. Eckstein F, Faber S, Muhlbauer R, et al. Functional adaptation of human joints to mechanical stimuli. Osteoarthritis Cartilage. 2002; 10 1: 44– 50. [DOI] [PubMed] [Google Scholar]
- 41. Hart JM, Ko JW, Konold T, Pietrosimone B. . Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: a systematic review. Clini Biomech (Bristol, Avon). 2010; 25 4: 277– 283. [DOI] [PubMed] [Google Scholar]
- 42. Patel RR, Hurwitz DE, Bush-Joseph CA, Bach BR Jr Andriacchi TP. . Comparison of clinical and dynamic knee function in patients with anterior cruciate ligament deficiency. Am J Sports Med. 2003; 31 1: 68– 74. [DOI] [PubMed] [Google Scholar]
- 43. Palmieri-Smith RM, Thomas AC. . A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009; 37 3: 147– 153. [DOI] [PubMed] [Google Scholar]
- 44. Luc B, Gribble PA, Pietrosimone BG. . Osteoarthritis prevalence following anterior cruciate ligament reconstruction: a systematic review and numbers-needed-to-treat analysis. J Athl Train. 2014; 49 6: 806– 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schipplein OD, Andriacchi TP. . Interaction between active and passive knee stabilizers during level walking. J Orthop Res. 1991; 9 1: 113– 119. [DOI] [PubMed] [Google Scholar]
- 46. Yang NH, Nayeb-Hashemi H, Canavan PK, Vaziri A. . Effect of frontal plane tibiofemoral angle on the stress and strain at the knee cartilage during the stance phase of gait. J Orthop Res. 2010; 28 12: 1539– 1547. [DOI] [PubMed] [Google Scholar]
- 47. Ostergaard M, Court-Payen M, Gideon P, et al. Ultrasonography in arthritis of the knee. A comparison with MR imaging. Acta Radiol. 1995; 36 1: 19– 26. [PubMed] [Google Scholar]
- 48. Yoon CH, Kim HS, Ju JH, Jee WH, Park SH, Kim HY. . Validity of the sonographic longitudinal sagittal image for assessment of the cartilage thickness in the knee osteoarthritis. Clin Rheum. 2008; 27 12: 1507– 1516. [DOI] [PubMed] [Google Scholar]