Abstract
Context:
Individuals with an acute knee-injury history are 4 times more likely to develop knee osteoarthritis than those without a prior knee injury, and it is unknown why. Individuals with an injury history may exhibit aberrant changes in tissue turnover after physical activity (eg, running), which could lead to osteoarthritis, but this has yet to be determined among young, physically active individuals.
Objective:
To determine collagen degradation and synthesis and inflammatory biomarker concentration levels before exercise and changes in response to an acute running bout in injured participants compared with healthy control participants.
Design:
Cohort study.
Setting:
Research laboratory.
Patients or Other Participants:
A total of 22 physically active individuals between 18 and 25 years of age were recruited for the study: 11 injured participants (knee injury within 4 years of the study) who were medically cleared for physical activity and 11 matched healthy control participants.
Main Outcome Measure(s):
The independent variable was group (injured or control). Dependent variables were serum biomarker concentrations for cartilage oligomeric matrix protein, matrix metalloproteinase-13, proinflammatory marker interleukin-1β, c-terminal cross-linking telopeptide of type II collagen, and type II collagen synthesis marker. Each participant provided prerun and postrun blood samples for biomarker-concentration analysis.
Results:
No group differences existed in serum biomarker concentrations before exercise or in serum biomarker changes from pre-exercise to postexercise.
Conclusions:
After an acute bout of moderate-intensity running, young, active individuals in a high-risk postinjury population had similar biochemical responses as matched healthy controls. However, the external generalizability of these findings to other exercises and populations has yet to be determined.
Key Words: osteoarthritis, biological markers, collagen, anterior cruciate ligament, serum
Acute knee injuries involving the anterior cruciate ligament (ACL) or meniscus are relatively common within the physically active and general populations1 and result in a 4 times greater likelihood of developing knee osteoarthritis (OA) than if there is no history of knee injury.2,3 The acutely injured knee is exposed to biomechanical changes and new loading patterns4 that increase OA risk, regardless of treatment (ie, surgical, nonsurgical) for the injured ACL5,6 and meniscus.7 It is particularly concerning that evident radiographic changes (eg, sclerotic and subchondral bone remodeling, osteophyte formation) can occur within 3 years postinjury,8 and radiographic OA will occur in 25% of individuals within 5 years of knee injury5 and in 50% of those within 10 to 20 years of knee injury.9 Because it may be too late to reverse the course of knee OA once radiographic structural changes have occurred,10 it is important to understand why these patients develop early-onset knee OA.
Mechanical alterations (eg, increased focal loading4) and biochemical alterations (eg, increased inflammatory11–13 and collagen-turnover markers14,15) occur immediately after an acute knee injury and can persist for prolonged periods of time.9,16–18 Nearly all physically active individuals (eg, college-aged athletes) with an ACL or meniscal injury (or both) return to preinjury weight-bearing activities within 1 year despite the persistence of biomechanical and biochemical abnormalities.19 Compounding concerns during this time period are the simultaneous changes in structural integrity, joint loading, and biochemical concentrations that may indicate an active healing process and joint tissues that might not be ready to effectively respond or successfully adapt to the biomechanical loads (eg, intensities, levels) to which they are being exposed.
One mechanosensitive biomarker that may reflect a compromised response to physical activity in the knee joint is serum cartilage oligomeric matrix protein (COMP). Serum COMP concentration levels generally increase in an apparent dose-dependent response after various physical activity tasks such as walking20,21 in older healthy and osteoarthritic populations as well as after running22–25 in healthy populations. Changes in COMP concentrations from prewalk to postwalk have predicted cartilage loss,21 but resting serum COMP levels have not. Cartilage oligomeric matrix protein changes have been seen immediately postexercise in both healthy and osteoarthritic populations.22–25 Whereas some investigators22–24 have investigated baseline biomarker differences with and cartilage biomarker responses to activity in the healthy population, we are unaware of any published articles regarding this response specifically in a young and physically active group with an injury history. Moreover, no research has been published involving other biomarkers (eg, bone, inflammation, collagen) within this population, making our study novel, given the multi-tissue nature of OA. Furthermore, biomarker responses to an acute running bout could provide information about the underlying pathophysiology of early-onset OA and may serve as an indicator of impending, subclinical OA in at-risk populations such as those with a history of injury, but this is yet to be determined. The purpose of our study was to determine biomarker concentrations pre-exercise and in response to an acute running bout in participants with an injury history compared with healthy control participants.
METHODS
Research Design
A 2-group pretest-posttest cohort study design was used to determine the biomarker response to an acute running bout in participants with an injury history compared with matched, healthy controls. The independent variables were group (injured and control) and time (pre-exercise and postexercise). The dependent variables were the biomarker concentrations. Our primary biomarker was a collagen-turnover biomarker (ie, COMP), and our secondary, exploratory biomarkers were for collagen degradation (ie, matrix metalloproteinase [MMP]-13, c-terminal cross-linking telopeptide of type II collagen [CTX-II]), collagen synthesis (ie, type II collagen fragments [CPII]), degradation-to-synthesis ratios (ie, CTX-II : CPII), and inflammation (ie, interleukin [IL]-1β).
Participants
We determined that a sample size of 7 to 11 per group was needed on the basis of an a priori power (0.80) analysis with an α level of .05 and a medium effect size (Cohen effect size [d] = 0.30) for the primary aim (COMP biomarker concentration). This computation was based on research conducted by Niehoff et al,24 who used the same running protocol and demonstrated significant COMP increases of 31% in 14 healthy individuals. A total of 22 physically active individuals (11 injured and 11 controls) between 18 and 25 years of age were recruited to participate in the study. Participants were recruited from an area in the Philadelphia metropolitan region that was within a 1-hour drive of the study laboratory and were required to be regularly involved in moderate physical activity a minimum of 3 times per week. Potential participants were recruited through e-mail, posted flyers, and word of mouth.
Injured participants had been diagnosed with an ACL or meniscal tear (or both) that was confirmed with a magnetic resonance imaging report and at the time of surgery. Inclusion criteria were an acute knee injury within 4 years of the study and medical clearance from the attending physician for unrestricted physical activity within 1 year of their acute knee injury or surgery. Exclusion criteria were self-reported, physician-diagnosed arthritis (eg, rheumatoid), or a self-reported history of intra-articular injection within 30 days of the study. No imaging was conducted to assess OA status during this study. Control participants were recruited and matched to injured participants by age (±2 years), sex (same), height (±5 cm), mass (±6 kg), and sport or physical activity level (same level of impact and risk of injury [low, medium, or high]).26 Exclusion criteria for control participants were prior significant lower extremity injury (ie, missed physical activity for >2 weeks, use of crutches for >1 week) or any self-reported, physician-diagnosed arthritis (eg, OA, rheumatoid). Two university institutional review boards approved the study; however, all research was conducted at the same research site. All participants reported to the same laboratory and completed and signed the approved informed consent and Health Insurance Portability and Accountability Act forms before the study.
Pre-Exercise Protocol
Participants were scheduled during the morning hours (from 7:00 to 9:00 am) to allow for biomarker normalization. The participant sat in a semirecumbent position (supine with a bolster behind the back) for 30 minutes, while completing study paperwork, to allow for biomarker normalization from activities of daily living. Paperwork consisted of consent and confidentiality forms as well as a health history questionnaire. Participants also self-reported their average amount of exercise per week in the month leading up to the study. During this time, the participant was fitted with a heart-rate monitor (Polar, Lake Success, NY) and continued to sit in a semirecumbent position for the remainder of the 30-minute period. Then the participant's blood was drawn. Blood samples were collected first thing in the morning on the day of study participation because COMP levels have been shown to return to baseline levels in a dose-dependent fashion ranging from 1 to 24 hours postactivity.22–24
Biospecimen Acquisition
A certified phlebotomist performed antecubital venous blood draws (7 mL) per standard certified venous access specialist protocol to determine serum biomarker concentrations. The phlebotomist vigorously cleaned the needle insertion site with isopropyl alcohol and then inserted the needle to draw whole blood into a chilled 7-mL ethylenediaminetetraacetic acid (EDTA) vacutainer tube. Each blood sample was coded with a unique participant identity code, placed on ice, and transported to the laboratory for centrifugation. The whole blood sample was then centrifuged at 1000 rpm for 20 minutes at 4°C. The serum was transferred to cryovials and stored at −80°C for analysis.
Exercise Protocol
The exercise protocol for our study, which was replicated from a previous study24 on COMP, consisted of the participant running at 2.2 m/s for 30 minutes. Given those findings of increased COMP in a healthy sedentary population, we hypothesized that serum COMP would increase. Heart rate and rate of perceived exertion were monitored during the running task every 5 minutes to ensure participant safety. Participants were familiar with and adapted to running: They all indicated they had been involved in physical activity (including running) a minimum of 3 times per week for at least 30 minutes. No participant required exercise termination due to excessive heart rate or rate of perceived exertion (ie, 90% of his or her estimated maximal heart rate or a rate of perceived exertion of 9 or higher27 or both) during the exercise bout.
Postexercise Assessment
After completing the running bout, the participant returned to the research laboratory for the postexercise blood draw. The blood draw was performed within 10 minutes after exercise ended and was prepared for storage and analysis as previously described. The COMP changes have been seen within this time frame in healthy and osteoarthritic populations.22–25
Biomarker Analysis
Once data collection was complete for all participants, we thawed and analyzed the serum samples. The following commercially available enzyme-linked immunosorbent serologic assay (ELISA) kits were used to quantify the serum concentrations: for COMP and CTX-II: MyBioSource (San Diego, CA); for IL-1β and MMP-13, Abcam Inc (Cambridge, MA); and for CPII, IBEX Pharmaceuticals (Montreal, Quebec, Canada). The sensitivity concentrations and mean intra-assay coefficient of variance percentages were as follows: COMP, <1.0 pg/mL and <9%, respectively; CTX-II, <3.7 ng/mL and <15%, respectively; IL-1β, <1.0 pg/mL and <10%, respectively; MMP-13, <4.8 pg/mL and <10%, respectively; and CPII, <11 ng/mL and <5%, respectively.
Statistical Analyses
Statistical analyses consisted of descriptive and inferential statistics. Descriptive variables were analyzed parametrically with independent Student t tests to determine whether significant differences existed between the injured and control groups. We used Pearson correlations to determine the relationships between biomarker concentration and time since injury.
Data were analyzed for normality and to ensure that appropriate assumptions for the parametric statistics were met. A Shapiro-Wilk test and a visual inspection of Q-Q and box plots showed that serum biomarker concentrations were not normally distributed for the injured and control groups, which is common in serum biomarker research.22 Accordingly, the central-tendency median and appropriate nonparametric statistical tests (ie, Wilcoxon signed rank tests, Spearman correlations) were used for analyses. Paired analyses were chosen to statistically control for potential confounding variables28 on which participants were matched: sex, age, height, weight, body mass index, and sport or activity impact risk level. The signed rank sum test is preferred in this case because it permits a comparison between matched individuals.29 Ultimately, these statistical analyses allowed for between-groups comparisons while controlling for potentially confounding variables that were used to match participants. Matched participants were also divided into 3 categories to assess the number of participants with meaningful biomarker changes: (1) the injured participant had a 20% greater change in a biomarker than the matched control, (2) the injured participant had a biomarker change within 20% of the matched control, or (3) the healthy participant had a 20% greater change in a biomarker than the matched injured participant. Data were analyzed using SPSS for Windows statistical program (version 21.0; IBM Corporation, Armonk, NY). Statistical significance was defined a priori as P ≤ .05.
Key Points
The biochemical effects of activity have not been investigated in a young, postinjury population that is at risk for early osteoarthritis.
Young, active individuals with or without a history of knee injury had similar biochemical responses after a moderate-intensity run.
These biochemical responses to activity may be activity specific, dose specific, or affected by other variables.
RESULTS
No differences existed between the injured and control groups for age, height, weight, body mass index, impact risk level, activity level, or activity minutes per week. We previously reported30 that injured participants had poorer Knee Osteoarthritis Outcomes Scores in all subscales (ie, symptoms, stiffness, pain, daily living function, and sports/recreational activity function) in comparison with matched healthy control participants. Descriptive data for all 22 participants are presented in Table 1. All 11 injured participants had knee surgery, were cleared by their physician for activity within 1 year of surgery, and provided supporting medical documentation. Detailed injury characteristics for the 11 injured participants are presented in Table 2. Potential relationships between biomarker concentrations and time since injury were analyzed for the injury group using Spearman correlations, which revealed no significant values (rs = −0.31 to 0.30; P = .36 to .83).
Table 1. .
Demographic Variables for Injured Participants and Matched Healthy Controls
| Variable |
Group |
t Value |
P Value |
|
| Injured (n = 11) |
Control (n = 11) |
|||
| Sex, males/femalesa | 5/6 | 5/6 | ||
| Age, ya | 20.09 ± 1.04 | 19.91 ± 1.64 | −0.310 | .760 |
| Height, ma | 1.74 ± 0.13 | 1.73 ± 0.11 | −0.094 | .926 |
| Mass, kga | 74.38 ± 13.98 | 73.35 ± 14.42 | −0.171 | .866 |
| Body mass index, kg/m2a | 24.45 ± 2.83 | 24.19 ± 2.83 | −0.214 | .833 |
| Impact risk level, high/mediuma | 10/1 | 10/1 | 0.000 | 1.000 |
| Tegner Activity Scale score | 6.9 ± 1.5 | 6.9 ± 1.8 | 0.000 | 1.000 |
| Activity (min/wk) | 429.09 ± 151.24 | 325.45 ± 148.15 | −1.623 | .120 |
| Knee Osteoarthritis Outcome Score | ||||
| Subscale | ||||
| Pain | 90.40 ± 8.27 | 98.23 ± 5.86 | 2.560 | .020b |
| Symptoms | 79.22 ± 15.63 | 98.05 ± 4.62 | 3.831 | .001b |
| Activities of Daily Living | 95.32 ± 7.94 | 99.33 ± 2.21 | 1.613 | .122 |
| Sport and Recreation Activity | 79.55 ± 20.79 | 96.82 ± 10.55 | 2.457 | .001b |
| Quality of Life | 74.43 ± 19.05 | 98.86 ± 3.77 | 4.173 | .001b |
Matched criteria.
P ≤ .05. Data are presented as mean ± SD.
Table 2. .
Detailed Injury Characteristics for Injured Participantsa
| Participant |
Knee |
Injured Structure(s) |
Time Since Injury, y |
|||||
| Anterior Cruciate Ligament |
Medial Collateral Ligament |
Lateral Collateral Ligament |
Posterior Cruciate Ligament |
Medial Meniscus |
Lateral Meniscus |
|||
| 1 | Left | X | X | X | 2 | |||
| 2 | Right | X | X | X | 3 | |||
| 3 | Left | X | X | X | X | 2 | ||
| 4 | Left | X | 4 | |||||
| 5 | Left | X | X | 3 | ||||
| 6 | Left | X | X | 1 | ||||
| 7 | Right | X | X | 2 | ||||
| 8 | Left | X | X | X | 2 | |||
| 9 | Left | X | 1 | |||||
| 10 | Right | X | 2 | |||||
| 11 | Right | X | 3 | |||||
All participants had surgery. All anterior cruciate ligament tears were reconstructed, and all meniscal tears were resected.
Biomarker Concentrations
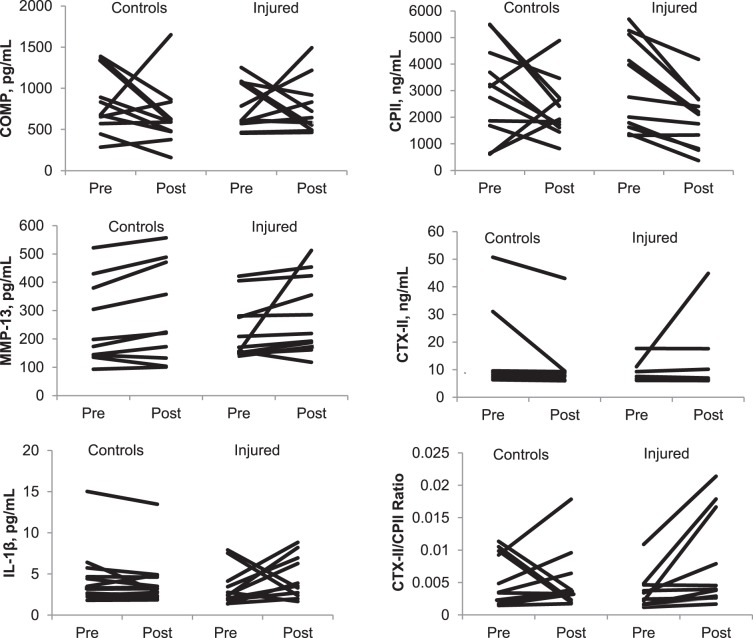
Serum biomarker-concentration measures and statistics are reported in Table 3. We explored the influence of all biomarker-concentration outliers greater than 2 standard deviations from the mean, and their removal did not affect the results. Accordingly, we left all participants in the analysis. No differences existed in serum biomarker concentrations pre-exercise or in the change from pre-exercise to postexercise between the injured and control groups. No differences were noted over time in serum biomarker concentrations from pre-exercise to postexercise. Serum biomarker concentrations for each participant by group are presented in the Figure. The COMP increases occurred in 45% of the participants, whereas COMP decreases occurred in the remaining 55%. However, only 32% of the participants had increases that were greater than the 10% intra-assay kit variation. By group, 55% of those with an injury history had COMP increases, whereas only 36% of healthy controls had COMP increases. Of 11 matched pairs, 5 had similar COMP changes after running (within 20% of one another). The injured participant had greater COMP changes among 4 matched pairs, and the healthy control participant had greater COMP changes among the remaining 2 matched pairs. Clinically meaningful percentage changes (ie, greater than 20%) by pairs are presented in Table 4.
Table 3. .
Biomarker-Concentration Comparisons for Injured and Control Groupsa
| Biomarker |
Measurement |
Group, Mean ± SD |
Z Value |
P Value |
|
| Injured |
Control |
||||
| Cartilage oligomeric matrix protein, pg/mL | Pretest | 777.33 ± 287.25 | 830.32 ± 381.30 | −0.800 | .424 |
| Posttest | 764.55 ± 331.33 | 660.93 ± 382.33 | −0.356 | .722 | |
| Difference | −12.78 ± 439.72 | −169.39 ± 484.94 | −0.978 | .328 | |
| Type II collagen fragments, ng/mL | Pretest | 3188.19 ± 1688.87 | 3002.89 ± 1708.65 | −0.455 | .657 |
| Posttest | 1936.90 ± 1091.87 | 2320.23 ± 1115.23 | −0.889 | .374 | |
| Difference | −1251.29 ± 953.93 | −682.66 ± 1750.35 | −0.622 | .534 | |
| Matrix metalloproteinase 13, pg/mL | Pretest | 228.25 ± 104.01 | 269.19 ± 150.39 | −0.445 | .657 |
| Posttest | 279.78 ± 135.19 | 268.04 ± 170.04 | −0.311 | .756 | |
| Difference | 51.54 ± 105.22 | −1.15 ± 109.85 | −0.089 | .929 | |
| Cross-linked c-terminal telopeptide of type II collagen, ng/mL | Pretest | 8.19 ± 3.52 | 13.43 ± 14.33 | −0.800 | .424 |
| Posttest | 11.24 ± 11.67 | 10.62 ± 10.83 | −0.089 | .929 | |
| Difference | 3.05 ± 10.19 | −2.82 ± 6.62 | −1.067 | .286 | |
| Interleukin-1β, pg/mL | Pretest | 4.74 ± 3.72 | 3.39 ± 2.29 | −1.690 | .091 |
| Posttest | 4.52 ± 2.57 | 4.23 ± 3.25 | −0.622 | .534 | |
| Difference | 1.13 ± 3.56 | −0.51 ± 1.26 | −1.334 | .182 | |
| Cross-linked c-terminal telopeptide of type II collagen: type II collagen fragments ratio | Pretest | 0.01 ± 0.01 | 0.01 ± 0.01 | −1.600 | .110 |
| Posttest | 0.01 ± 0.01 | 0.01 ± 0.01 | −1.423 | .155 | |
| Difference | 0.01 ± 0.01 | −0.01 ± 0.01 | −1.334 | .182 | |
No group differences (P ≥ .05).
Figure.
Biomarker concentrations by participant within group pre- and postexercise. Abbreviations: COMP, cartilage oligomeric matrix protein; CPII, type II collagen fragments; CTX-II, c-terminal cross-linking telopeptide of type II collagen; IL, interleukin-1β; MMP, matrix metalloproteinase-13.
Table 4. .
Clinically Meaningful Percentage Changes by Pairs in Injured and Healthy Participantsa
| Pair |
Changes |
||||
| Cartilage Oligomeric Matrix Protein |
Type II Collagen Fragments |
Matrix Metalloproteinase 13 |
Cross-Linked C Telopeptide of Type II Collagen |
Interleukin-1β |
|
| 1 | > | < | > | > | > |
| 2 | = | < | = | > | > |
| 3 | = | = | = | = | < |
| 4 | = | < | = | = | < |
| 5 | > | = | > | = | > |
| 6 | > | > | = | = | > |
| 7 | = | = | = | = | > |
| 8 | < | < | = | = | < |
| 9 | = | = | = | = | > |
| 10 | < | = | = | = | > |
| 11 | > | = | = | = | > |
| Pairs with equivalent change | 5/11 | 6/11 | 9/11 | 9/11 | 0/11 |
| Injured higher | 4/11 | 1/11 | 2/11 | 2/11 | 7/11 |
| Healthy higher | 2/11 | 4/11 | 0/11 | 0/11 | 4/11 |
Table key: > indicates injured had higher concentrations than healthy (greater than 20% change); < indicates healthy had higher concentration than injured (greater than 20% change); = indicates percentage change difference between injured and healthy was within 20%.
DISCUSSION
To our knowledge, this is the first study to investigate serum biomarker concentrations in response to an acute exercise bout in physically active, college-aged participants with or without an injury history. We are also the first to conduct an exploratory investigation of serum biomarker concentrations other than COMP, including markers of collagen degradation, collagen synthesis, and inflammation in this population. The biomarkers assessed in this study were not different on the basis of injury-history status pre-exercise and did not change after 30 minutes of running. Understanding the serum biomarker response to physical activity may be important to understanding early OA pathophysiology and determining viable interventions before structural changes occur. Our findings showed that an acute bout of moderate-intensity running elicited a similar biochemical response in a high-risk population with a history of injury when compared with matched healthy controls. Although these findings are novel, the external generalizability is yet to be determined. All the knee injuries were treated surgically and most participants underwent ACL reconstruction; however, we did not exclude or statistically control for the multiple structures concomitantly injured during the primary knee trauma.
The COMP Response to Activity
Median serum COMP concentrations increased slightly in injured participants in response to an acute running exercise, whereas mean COMP concentrations remained close to baseline values, in comparison with healthy participants who showed an overall decrease in COMP concentrations. However, the COMP response within participants as well as between groups was heterogeneous. The lack of significant findings may be driven by the heterogeneous COMP baseline and change concentrations, both within participants and between groups. These heterogeneous findings are similar to the findings of some researchers20,23 but contradict the homogeneous findings of others.31,32 The pre-exercise to postexercise changes were not statistically significant, which is similar to the findings of Erhart-Hledik et al,21 who reported an insignificant COMP trend toward an increase after a 30-minute walking task in OA participants. Contradictory to these findings, serum COMP concentration increased after walking at a self-selected pace in OA and healthy populations20,33 and increased even more after various running intensities and durations in a healthy population.22–25
We found a lesser overall COMP response in injured participants in comparison with healthy control participants after the 30-minute running bout; however, these findings were also insignificant. The lack of a significant difference between the groups is similar to the findings of Mundermann et al,20 who reported no significant differences in serum COMP changes between OA and healthy control participants after a 30-minute walking task. Whereas the lack of group differences may indicate a possible lack of diagnostic value to serum biomarker analysis, it may be interesting to prospectively study whether there is a prognostic value to serum biomarker analysis in a population at risk for OA development and progression.
Factors potentially affecting the nonsignificant biomarker-concentration findings in the current study include age and physical activity level. The mean age of participants in the current study (20 ± 1.4 years) was lower than in the previous studies in which mean serum COMP concentration increased postexercise: 23.1 ± 2 years,25 26 ± 2 years,23 31.6 ± 8 years,24 31.9 ± 6 years,20 and 66.1 ± 11 years.32 The lack of significant COMP changes in this study may reflect the younger participants because age is a known OA-progression risk factor; however, we have highlighted a unique age group in whom no research has been conducted.
Our participants also reported higher physical activity levels than those in earlier research.24 Participants were involved in at least 30 minutes of moderate physical activity 3 times per week, averaging 6.2 hours per week. In contrast, Niehoff et al24 investigated COMP changes in a sedentary population who reported participating in occasional leisure physical activity, averaging 1.1 hours per week. The current study population exceeded the definition of moderately physically active of 150 minutes of moderate-intensity physical activity per week27; they were involved in an average of 360 minutes of moderate-intensity physical activity per week. Another investigatory group31 found COMP changes in sedentary collegiate men after a 10-week running program. This finding further highlights the lack of research in highly physically active populations, including women and girls, as well as those with a knee-injury history. Accordingly, the lower age and higher physical activity level of our study population may have contributed to the insignificant biomarker differences by group.
Other Biomarker Comparisons in Response to Physical Activity
We also analyzed exploratory biomarkers consisting of CPII, MMP-13, CTX-II, IL-1β, and the CTX-II : CPII ratio. These biomarkers have the potential to respond to an acute exercise bout and have not previously been studied within this population. Similar to the COMP findings, the exploratory biomarkers did not differ between the injured and control groups. Although not significantly different, median CPII decreased in both groups in response to exercise, with a larger decrease in injured participants (median CPII change = −3025) than in healthy control participants (median CPII change = −963). This biomarker indicates type II collagen synthesis, and it may be particularly important to consider in future research because lower overall CPII serum concentrations have been associated with a greater risk for multiple-site OA.34
Baseline Biomarker Comparison
Biomarker-concentration differences were not found between the injured and healthy control groups pre-exercise in the current study. These results do not support those of previous investigators who reported increased CPII up to 2 years after an acute knee injury,15 increased CTX-II within 1 year of an acute knee injury,16 and abnormal CTX-II : CPII ratios up to 4 years after an acute knee injury.18 The injuries of our participants occurred within all of these time frames; the average was 2 years (range, 4 to 44 months) post–knee injury. The previous studies that demonstrated biomarker abnormalities were prospective cohort in design, allowing for a comparison of biomarker concentrations immediately after injury and at various follow-up time points. This was not the case in our study, which was cross sectional. Accordingly, it may be more valuable to follow participants at multiple time points, conducting serial biomarker analyses to determine their prognostic value for early OA development and progression.
LIMITATIONS AND DIRECTIONS FOR FUTURE RESEARCH
The current findings must be interpreted within the context of several methodologic limitations. An important consideration is that the exercise bout may have been at an insufficient intensity to affect the serum biomarker response postexercise within the target population. Although previous investigators23–25 have shown significant effects of exercise on serum biomarker responses, participants in these studies were older and less physically active than ours. More research needs to be conducted on the college-aged population, which is often highly physically active. Within this group, it may also be important to incorporate the dose, load, and various types of exercise in which they typically engage.
Another limitation is that we did not measure absolute joint-surface forces and other biomechanical properties. Biomechanical35,36 and biochemical12,16,17,19 adaptations occur after a knee injury, and more knowledge about these changes postinjury would be gained by measuring ground reaction forces or joint forces. The premise of our study was that loading from the running protocol may cause an exacerbated biochemical response that may be related to altered joint metabolism within the target population. Biomechanical adaptations may have occurred and resulted in less (or more) loading, which would have implications for biochemical-marker concentration levels.
An additional limitation is that the healthy control participants were self-reported to be healthy, and diagnostic imaging was not performed for confirmation. Furthermore, no physical activity restrictions were placed on participants in the days leading up to the study. If an individual was extremely physically active the day before the study, this may have had an unintended effect on baseline biomarker-concentration levels.
The restricted panel of biomarkers analyzed is another limitation of the study. All of the biomarkers analyzed are largely related to type II collagen. A broader range of biomarkers would encompass more metabolic processes and may more accurately reflect the multi-tissue pathophysiology of OA. For example, the addition of more bone (type I collagen), synovium (type III collagen), inflammatory, and angiogenic biomarkers would help to identify other metabolic processes that may be occurring, such as osteophyte formation or subchondral bone changes.
The sample size of the study and concomitant injured structures also constituted limitations. Injury type could not be statistically controlled with 11 participants in the injured group. Furthermore, no information was available regarding concomitant articular cartilage or synovial injuries. On the basis of prior COMP biomarker research24 using the same running protocol, we estimated that 7 to 11 participants per group were needed to detect a moderate effect size. The participants in the current study were much more physically active than the cohort in the study used to determine a priori sample size,24 which resulted in a lower than expected effect size. Post hoc power analysis for COMP revealed an effect size of 0.34 with a power level of 0.32. However, the mean COMP change differences between groups were within the intra-assay standard of error of the COMP assay kit (<10%); therefore, they are not likely to be clinically meaningful. An efficacious activity load (eg, intensity, frequency, duration, type) for this highly physically active population may be needed to elicit biomarker responses and to determine whether statistically significant group differences exist.
CONCLUSIONS
An acute bout of moderate-intensity running elicited similar biochemical responses in a high-risk injury history population and in matched healthy controls. However, these findings should be interpreted with caution because it is yet to be determined whether a higher exercise dose or intensity (or both), as well as other types of exercise, elicit adverse biomarker changes in this population. The external generalizability of these study findings to other populations (eg, older ages) is also yet to be determined. Postinjury pathophysiology is complex, and although unique variables may accelerate OA onset, they may interact to accelerate and propagate OA. Further investigation is needed.
REFERENCES
- 1. Hootman JM, Dick R, Marshall S, Agel J. . An evaluation of select rule and policy changes on injury rates in 3 NCAA sports. Med Sci Sports Exerc. 2008; 40 5: S233– S233. [Google Scholar]
- 2. Muthuri SG, McWilliams DF, Doherty M, Zhang W. . History of knee injuries and knee osteoarthritis: a meta-analysis of observational studies. Osteoarthritis Cartilage. 2011; 19 11: 1286– 1293. [DOI] [PubMed] [Google Scholar]
- 3. Richmond SA, Fukuchi RK, Ezzat A, Schneider K, Schneider G, Emery CA. . Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J Orthop Sports Phys Ther. 2013; 43 8: 515– 519. [DOI] [PubMed] [Google Scholar]
- 4. Ristanis S, Stergiou N, Patras K, Vasiliadis HS, Giakas G, Georgoulis AD. . Excessive tibial rotation during high-demand activities is not restored by anterior cruciate ligament reconstruction. Arthroscopy. 2005; 21 11: 1323– 1329. [DOI] [PubMed] [Google Scholar]
- 5. Frobell RB, Roos HP, Roos EM, Roemer FW, Ranstam J, Lohmander LS. . Treatment for acute anterior cruciate ligament tear: five year outcome of randomised trial. Br J Sports Med. 2015; 49 10: 700. [DOI] [PubMed] [Google Scholar]
- 6. Harris KP, Driban JB, Sitler MR, Cattano NM, Balasubramanian E. . Tibiofemoral osteoarthritis after surgical or nonsurgical treatment of anterior cruciate ligament rupture: a systematic review. J Athl Train. 2017; 52 6: 507– 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sturinieks DL, Besier TF, Mills PM, et al. Knee joint biomechanics following arthroscopic partial meniscectomy. J Orthop Res. 2008; 26 8: 1075– 1080. [DOI] [PubMed] [Google Scholar]
- 8. Buckland-Wright JC, Lynch JA, Dave B. . Early radiographic features in patients with anterior cruciate ligament rupture. Ann Rheum Dis. 2000; 59 8: 641– 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lohmander LS, Ostenberg A, Englund M, Roos H. . High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004; 50 10: 3145– 3152. [DOI] [PubMed] [Google Scholar]
- 10. Aigner T, Fundel K, Saas J, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006; 54 11: 3533– 3544. [DOI] [PubMed] [Google Scholar]
- 11. Cuellar JM, Scuderi GJ, Cuellar VG, Golish SR, Yeomans DC. . Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J Bone Joint Surg Am. 2009; 91 10: 2313– 2320. [DOI] [PubMed] [Google Scholar]
- 12. Cuellar VG, Cuellar JM, Golish SR, Yeomans DC, Scuderi GJ. . Cytokine profiling in acute anterior cruciate ligament injury. Arthroscopy. 2010; 26 10: 1296– 1301. [DOI] [PubMed] [Google Scholar]
- 13. Scanzello CR, McKeon B, Swaim B, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 2011; 63 2: 391– 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. . The role of synovial macrophages and macrophage-produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther. 2006; 8 6: R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Catterall JB, Stabler TV, Flannery CR, Kraus VB. . Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther. 2010; 12 6: R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tchetverikov I, Lohmander LS, Verzijl N, et al. MMP protein and activity levels in synovial fluid from patients with joint injury, inflammatory arthritis, and osteoarthritis. Ann Rheum Dis. 2005; 64 5: 694– 698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Svoboda SJ, Harvey TM, Owens BD, Brechue WF, Tarwater PM, Cameron KL. . Changes in serum biomarkers of cartilage turnover after anterior cruciate ligament injury. Am J Sports Med. 2013; 41 9: 2108– 2116. [DOI] [PubMed] [Google Scholar]
- 18. Tourville TW, Johnson RJ, Slauterbeck JR, Naud S, Beynnon BD. . Assessment of early tibiofemoral joint space width changes after anterior cruciate ligament injury and reconstruction: a matched case-control study. Am J Sports Med. 2013; 41 4: 769– 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beynnon BD, Uh BS, Johnson RJ, et al. Rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind comparison of programs administered over 2 different time intervals. Am J Sports Med. 2005; 33 3: 347– 359. [DOI] [PubMed] [Google Scholar]
- 20. Mundermann A, King KB, Smith RL, Andriacchi TP. . Change in serum COMP concentration due to ambulatory load is not related to knee OA status. J Orthop Res. 2009; 27 11: 1408– 1413. [DOI] [PubMed] [Google Scholar]
- 21. Erhart-Hledik JC, Favre J, Asay JL, et al. A relationship between mechanically-induced changes in serum cartilage oligomeric matrix protein (COMP) and changes in cartilage thickness after 5 years. Osteoarthritis Cartilage. 2012; 20 11: 1309– 1315. [DOI] [PubMed] [Google Scholar]
- 22. Neidhart M, Muller-Ladner U, Frey W, et al. Increased serum levels of non-collagenous matrix proteins (cartilage oligomeric matrix protein and melanoma inhibitory activity) in marathon runners. Osteoarthritis Cartilage. 2000; 8 3: 222– 229. [DOI] [PubMed] [Google Scholar]
- 23. Niehoff A, Kersting UG, Helling S, et al. Different mechanical loading protocols influence serum cartilage oligomeric matrix protein levels in young healthy humans. Eur J Appl Physiol. 2010; 110 3: 651– 657. [DOI] [PubMed] [Google Scholar]
- 24. Niehoff A, Muller M, Bruggemann L, et al. Deformational behaviour of knee cartilage and changes in serum cartilage oligomeric matrix protein (COMP) after running and drop landing. Osteoarthritis Cartilage. 2011; 19 8: 1003– 1010. [DOI] [PubMed] [Google Scholar]
- 25. Kersting UG, Stubendorff JJ, Schmidt MC, Bruggeman GP. . Changes in knee cartilage volume and serum COMP concentration after running exercise. Osteoarthitis Cartilage. 2005; 13 10: 925– 934. [DOI] [PubMed] [Google Scholar]
- 26. Driban JB, Hootman JM, Sitler MR, Harris KP, Cattano NM. . Is participation in certain sports associated with knee osteoarthritis? A systematic review. J Athl Train. 2017; 52 6: 497– 506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American College of Sports Medicine. ACSM's Guidelines for Exercise Testing and Prescription. 9th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins Health; 2013. [Google Scholar]
- 28. Balasubramanian R, Houseman EA, Coull BA, et al. Variable importance in matched case-control studies in settings of high dimensional data. J R Stat Soc Ser C Appl Stat. 2014; 63 4: 639– 655. [Google Scholar]
- 29. Rothman K. . Modern Epidemiology. Boston, MA: Little Brown & Co Inc; 1986. [Google Scholar]
- 30. Cattano NM, Driban JB, Barbe MF, Amin M, Tierney R, Sitler MR. . Physical activity and quality of life relate to changes in collagen turnover and inflammation after a running bout. Osteoarthritis Cartilage. 2015; 23 suppl 2: A28– A29. [Google Scholar]
- 31. Celik O, Salci Y, Ak E, Kalaci A, Korkusuz F. . Serum cartilage oligomeric matrix protein accumulation decreases significantly after 12 weeks of running but not swimming and cycling training: a randomised controlled trial. Knee. 2013; 20 1: 19– 25. [DOI] [PubMed] [Google Scholar]
- 32. Hunt M, Pollock C, Kraus VB, et al. Relationships amongst osteoarthritis biomarkers, dynamic knee joint load, and exercise: results from a randomized controlled pilot study. BMC Musculoskelet Disord. 2013; 14: 115– 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Subburaj K, Souza RB, Stehling C, et al. Association of MR relaxation and cartilage deformation in knee osteoarthritis. J Orthop Res. 2012; 30 6: 919– 926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Conrozier T, Poole AR, Ferrand F, et al. Serum concentrations of type II collagen biomarkers (C2C, C1, 2C, and CPII) suggest different pathophysiologies in patients with hip osteoarthritis. Clin Exp Rheumatol. 2008; 26 3: 430– 435. [PubMed] [Google Scholar]
- 35. Kuenze C, Hertel J, Weltman A, Diduch DR, Saliba S, Hart JM. . Jogging biomechanics after exercise in individuals with ACL-reconstructed knees. Med Sci Sports Exerc. 2014; 46(6)1067–1076. [DOI] [PubMed]
- 36. Swärd P, Fridén T, Boegård T, Kostogiannis I, Neuman P, Roos H. . Association between varus alignment and post-traumatic osteoarthritis after anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2013; 21 9: 2040– 2047. [DOI] [PubMed] [Google Scholar]