Abstract
A major hurdle in the development of effective treatments for amyotrophic lateral sclerosis (ALS) has been the lack of robust biomarkers for use as clinical trial endpoints. Neurochemical profiles obtained in vivo by high field proton magnetic resonance spectroscopy (1H-MRS) can potentially provide biomarkers of cerebral pathology in ALS. However, previous 1H-MRS studies in ALS have produced conflicting findings regarding alterations in the levels of neurochemical markers such as glutamate (Glu) and myo-inositol (mIns). Furthermore, very few studies have investigated the neurochemical abnormalities associated with ALS early in its course. In this study, we measured neurochemical profiles using single-voxel 1H-MRS at 7 tesla (T) and glutathione (GSH) levels using edited MRS at 3 T in 19 subjects with ALS who had relatively high functional status (ALS Functional Rating Scale-Revised mean ± SD = 39.8 ± 5.6) and 17 healthy controls. We observed significantly lower total N-acetylaspartate over mIns (tNAA/mIns) ratio in the motor cortex and pons of subjects with ALS versus healthy controls. No group differences were detected in GSH at 3 and 7 T. In subjects with ALS, the levels of tNAA, mIns, and Glu in the motor cortex were dependent on the extent of disease represented by El Escorial diagnostic subcategories. Specifically, combined probable/definite ALS had lower tNAA than possible ALS and controls (both p = 0.03), higher mIns than controls (p < 0.01), and lower Glu than possible ALS (p < 0.01). The effect of disease stage on MRS-measured metabolite levels may account for dissimilar findings among previous 1H-MRS studies in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal adult-onset motor neuron disease characterized by progressive and irreversible loss of voluntary muscle function. Affected persons generally die within two to four years after symptom onset, and riluzole – the only FDA-approved drug for ALS — has provided only a modest benefit in prolonging survival [1,2]. On post-mortem exam, ALS is distinguished from other motor neuron diseases by the selective degeneration of both the upper motor neurons (UMNs) that descend from the primary motor cortex to the brainstem and spinal cord and the lower motor neurons (LMNs) that project peripherally to the musculature. The diagnosis of ALS currently depends upon the clinician’s ability to detect signs of UMN and LMN dysfunction through physical examination. While electrophysiological testing can provide additional supportive evidence of LMN dysfunction, the reliability of tools for quantitatively and objectively measuring UMN degeneration in ALS has not been established. Such tools could help identify biomarkers of disease activity in the brain that are critical for evaluating treatment efficacy in therapeutic trials for ALS [3].
Single-voxel proton magnetic resonance spectroscopy (1H-MRS) is an advanced magnetic resonance imaging (MRI) technique for examining the neurochemistry of the brain. Whereas conventional brain MRI is unremarkable in ALS [4], 1H-MRS has revealed various neurochemical abnormalities that may indicate underlying disease processes, including gliosis, glutamate excitotoxicity, and oxidative stress. 1H-MRS studies over the years in humans with ALS have suggested abnormal levels of the following brain metabolites: N-acetylaspartate (NAA, marker of neuronal integrity) [5–7]; myo-inositol (mIns, putative marker of glial cells) [8,9]; glutamate (Glu, excitatory neurotransmitter) [10]; γ-aminobutyric acid (GABA, inhibitory neurotransmitter) [5]; and glutathione (GSH, thiol antioxidant) [11]. Nearly all of these studies included the primary motor cortex and its underlying white matter as a region of investigation.
The most commonly reported finding across 1H-MRS studies of ALS to date is lower levels of NAA in the motor cortex, indicating neuronal loss and/or dysfunction. However, apart from this finding, alterations in the levels of other metabolites have been reported inconsistently in ALS literature. For example, only a few studies reported a significant elevation in motor cortex mIns levels [8,9], while the majority did not. In addition, it remains unclear as to whether motor cortex Glu levels are higher or lower in ALS, as differences from controls have been reported in both directions [5,9,10,12]. This discrepancy may stem from the difficulty in quantifying Glu at low field strengths due to its considerable overlap with glutamine (Gln), resulting in the reporting of Glu and Gln as a sum termed Glx. Furthermore, in two relatively recent studies, lower GABA and GSH levels were observed in the motor cortex of patients using edited 1H-MRS techniques at 3 T [5,11]. GABA and GSH may mark states of cortical hyperexcitability and chronic oxidative stress, respectively, in ALS [13,14] and require further investigation with advanced 1H-MRS methods.
Neurochemical profiles that consist of 10–15 metabolites can be reliably quantified with 1H-MRS at 7 T [15,16]. A major advantage of increasing the magnetic field to 7 T is improved resolution of the overlapping J-coupled resonances of Gln, Glu, GSH, and GABA, resulting in quantification of these metabolites with excellent test-retest reproducibility [16,17] and without the need for metabolite-specific spectral editing. The purpose of the present study was to utilize our advanced 7 T 1H-MRS protocol to establish the neurochemical profiles of an early-stage ALS cohort and compare them with a cohort of matched healthy controls. Specifically, the study was designed to (1) clarify discrepancies in the literature regarding metabolite concentrations in ALS by using state-of-the-art 1H-MRS technology at 7 T and (2) investigate the dependence of neurochemical abnormalities on clinical measures of functional status, upper motor neuron disease burden, and diagnostic classification. We selected volumes-of-interest (VOI) in the motor cortex and pons, which are regions that show histopathologic evidence of neurodegeneration in ALS [18,19]. On the same participants, we also performed edited 1H-MRS at 3 T for measuring GSH levels in the motor cortex, which were recently reported to be lower in ALS versus controls [11]. Finally, we examined the relationships between brain metabolite levels and measures of clinical status, including ALS Functional Rating Scale-Revised (ALSFRS-R) scores, upper motor neuron (UMN) burden scores, and El Escorial diagnostic criteria.
Materials and Methods
Study Participants and Design
A total of 38 volunteers (20 individuals with ALS and 18 healthy controls, matched by age-range and sex-ratio) were enrolled in the study after giving written informed consent using procedures approved by the Institutional Review Board: Human Subjects Committee of the University of Minnesota. All volunteers with ALS were recruited from the ALS Association Certified Treatment Centers of Excellence at the University of Minnesota and Hennepin County Medical Center and fulfilled the revised El Escorial Criteria [20] for clinically possible (N = 8), probable (N = 8), or definite (N = 4) ALS. Two volunteers were withdrawn from the study after enrollment: 1 control volunteer with evidence of neurologic disease and 1 individual with ALS who was unable to undergo scanning due to claustrophobia.
Participants underwent both 3 and 7 T MR scanning no more than one week apart and a neuromuscular examination by a board-certified neurologist on the day of the 3 T scan (19 subjects with ALS, 17 healthy controls total). At 7 T, MR spectra were acquired from two VOIs: primary motor cortex and pons. At 3 T, spectra were acquired from the primary motor cortex only. In subjects with ALS, the motor cortex VOI was selected in the hemisphere contralateral to the more clinically affected side of the body. In controls, the hemispheres were chosen so that the cohorts would have similar proportions of left- and right-sided scans. The neuromuscular exam was used to determine the clinical characteristics of subjects with ALS, including the more affected side of the body, the El Escorial diagnosis, and a score on a novel upper motor neuron (UMN) burden scale. Active riluzole use at the time of the exam was also recorded in order to account for the drug’s potential effect on metabolite concentrations [5]. Disease duration and site of onset were obtained from patient medical records. Disease duration was calculated as the time from the first symptom occurrence to the date of the MR exam. The UMN burden score was based on neurologic exam signs and was used to measure the extent of UMN involvement in subjects with ALS. This score was generated by assigning point values of “1” to the presence of upper motor neuron signs in the cranial, cervical, and lumbosacral cord segments. Specifically, six signs were used in the rating: 1) pathologic jaw jerk, 2) slow tongue coordination, 3) abnormal upper limb reflexes (increased biceps reflex, triceps reflex, brachioradialis reflex, and/or finger flexors), 4) upper limb spasticity, 5) abnormal lower limb reflexes (increased patellar reflex, Achilles reflex, and/or extensor plantar response), 6) lower limb spasticity. Summing the points resulted in a total scale ranging from 0 to 6 (a higher score indicating greater UMN burden). All subjects with ALS were also evaluated with the ALS Functional Rating Scale-Revised (ALSFRS-R) as a measure of global disease severity [21]. The ALSFRS-R consists of 12 physical function queries that address bulbar, respiratory, gross motor, and fine motor domains. It yields a composite score ranging from 0 (most severe) to 48 (no disability). Participant characteristics are summarized in Table 1.
Table 1.
Clinical and 1H-MRS (7 T) data characteristics of scanned subjects by group and brain region examined
| Motor Cortex | Pons | |||||
|---|---|---|---|---|---|---|
| Clinical parameters | ALS(N = 19) | Controls (N = 17) | P-values | ALS(N = 15) | Controls (N = 15) | P-values |
| Sex ratio, male:female | 10:9 | 10:7 | 0.75* | 8:7 | 8:7 | 1* |
| Age [range], years | 57 ± 9 [31–74] | 57 ± 9 [30–69] | 1 | 56 ± 10 [31–70] | 56 ± 9 [30–69] | 0.92 |
| Brain hemisphere scanned, left:right | 8:11 | 8:9 | 1* | N/A | N/A | |
| Disease duration [range], months | 40.2 ± 43.0 [3.5–147.8] | N/A | 37.0 ± 40.2 [3.5–147.8] | N/A | ||
| Site of onset, limb:bulbar | 15:4 | N/A | 12:3 | N/A | ||
| El Escorial Diagnosis (no. of patients) | Possible (7) Probable (8) Definite (4) |
N/A | Possible (6) Probable (7) Definite (2) |
N/A | ||
| Current riluzole use, yes:no | 8:11 | N/A | 6:9 | N/A | ||
| ALSFRS-R score [range] | 39.8 ± 5.6 [26–45] | 48 ± 0 | <0.001 | 40.6 ± 5.2 [26–45] | 48 ± 0 | <0.001 |
| UMN score [range] | 2.5 ± 1.3 [1–6] | N/A | 2.3 ± 1.1 [1–5] | N/A | ||
| 7 T MRS quality parameters | ||||||
| Signal-to-noise ratio | 205 ± 37 | 201 ± 35 | 0.72 | 45 ± 9 | 53 ± 17 | 0.11 |
| Water linewidth, Hz | 10 ± 2 | 11 ± 2 | 0.53 | 16 ± 2 | 15 ± 2 | 0.15 |
| % CSF | 11.3 ± 5.3 | 10.2 ± 3.9 | 0.55 | 0.9 ± 0.3 | 1.1 ± 0.3 | 0.26 |
Data are given as mean ± SD or counts.
denotes P-values that are from Fisher’s exact tests.
Other P-values are from unpaired, two-tailed Student’s t-tests.
Data Acquisition
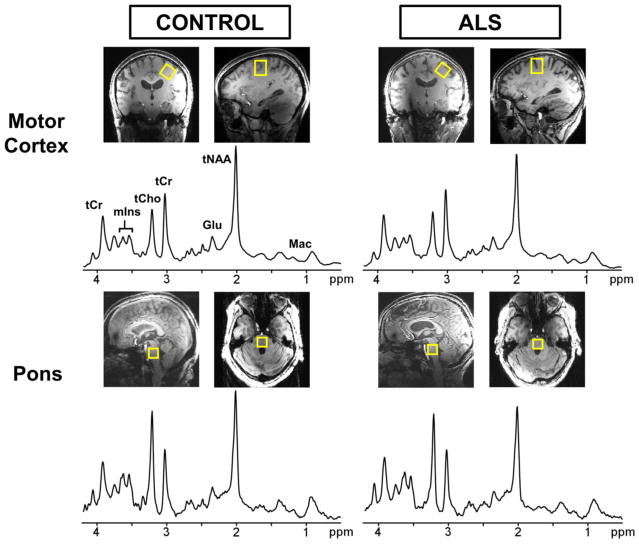
Proton MRS studies were performed on a 7 T whole body Siemens MAGNETOM scanner and a 3 T whole body Siemens Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany). At 7 T, a 16-channel transceiver array coil [22] allowed B1+ shimming as described previously [23]. T1-weighted MPRAGE images were acquired to position the VOI for MRS using the following parameters: repetition time (TR) = 2500 ms, echo time (TE) = 2.4 ms, flip angle = 5°, slice thickness = 1 mm, number of slices = 176, field of view = 232 × 256 mm2, and matrix size = 232 × 256. VOI placement was based on anatomical landmarks. For the primary motor cortex, a 2.2 × 2.2 × 2.2 cm3 voxel was placed in the area representing the upper limb and aligned parallel to the dura mater in the coronal orientation in order to maximize inclusion of the cortical surface (Fig. 1). The upper limb portion of the motor homunculus was specifically selected because a large majority of subjects with ALS were predicted to have some degree of upper limb involvement during the time of scanning. For the pons, a 1.6 × 1.6 × 1.6 cm3 voxel was aligned parallel to the pontomedullary junction in the sagittal orientation to maximize inclusion of pons tissue (Fig. 1).
Fig. 1.
Localized proton spectra obtained from the motor cortex (top) and pons (bottom) at 7 T using semi-LASER (TE = 26 ms, TR = 5 s, 64 averages). A 2.2 × 2.2 × 2.2 cm3 voxel (shown on T1-weighted images) was selected in the motor cortex and angulated parallel to the slope of the dural surface in the coronal orientation. A 1.6 × 1.6 × 1.6 cm3 voxel was selected to cover nearly the entire pons region. The spectra are shown with 1-Hz exponential and 5-Hz Gaussian weighting. Left: healthy control (68 year-old female); right: subject with ALS (64 year-old female)
After B1+ shimming and VOI selection, first- and second-order B0 shims were adjusted in the VOI using FASTMAP (a fast, automatic shimming technique by mapping along projections) with an echo planar imaging readout [24]. Then, B1 levels required for localization and water suppression were adjusted, as described previously [16]. Metabolite and water reference spectra were acquired using a modified semi-LASER sequence [25] (TR = 5 s, TE = 26 ms, 64 averages). In addition, fully relaxed unsuppressed water signals were acquired at TEs ranging from 28 to 4000 ms (TR = 15 s) to estimate the cerebrospinal fluid (CSF) contribution to the VOI [26].
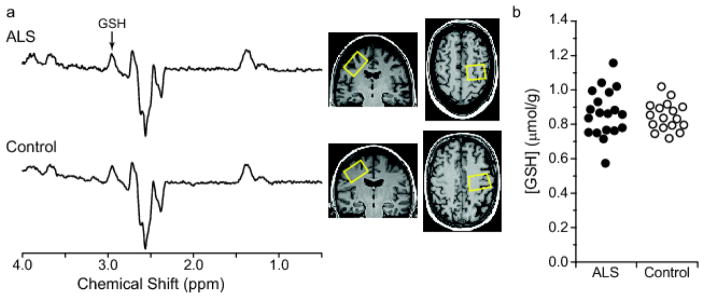
At 3 T, radiofrequency transmission was performed with the built-in body coil, and signal was received with a 32-channel receive-only head coil. T1-weighted MPRAGE images were obtained for VOI positioning (TR = 2.6 s, TE = 2.43 ms, flip angle = 7°, slice thickness = 1 mm, number of slices = 224, field of view = 256 × 256 mm2, and matrix size = 256 × 256). Spectra were acquired from a 3.5 × 2.5 × 2.3 cm3 VOI placed in the motor cortex using the MEGA-PRESS sequence [27,28] (TR = 2 s, TE = 68 ms, 512 averages) with editing pulse (180° Shinnar-Le Roux; duration, 22.7 ms; bandwidth, 52 Hz) applied at 4.56 ppm and 7.5 ppm. The larger VOI relative to that selected at 7 T included areas of the motor cortex corresponding to the face and lips, in addition to upper limb (Fig. 2).
Fig. 2.
GSH-edited spectra and GSH concentrations from the motor cortex of subjects with ALS and healthy controls using MEGA-PRESS at 3 T (TE = 68 ms, TR = 2 s, 512 averages). (a) Representative difference spectra acquired in a subject with ALS (top) and healthy control (bottom). Motor cortex voxel placement (3.5 × 2.5 × 2.3 cm3) is shown on T1-weighted images. Spectra are shown with 1-Hz exponential line broadening and with vertical scale adjusted using NAA resonance. (b) GSH concentrations quantified using LCModel in subjects with ALS and controls; p = 0.6 from ANOVA adjusting for brain hemisphere scanned
Data Post-processing and Quality Control
For 7 T data, single-shot metabolite spectra were corrected for frequency and phase fluctuations as well as residual eddy currents before summing [29]. Summed spectra were then visually inspected for the presence of unwanted coherences. Signal-to-noise ratio (SNR) measurements were made on the raw spectra by taking the ratio of the NAA methyl resonance at 2.02 ppm and root mean square of the noise measured from −4 to −2 ppm. Linewidth was measured as the full-width at half-maximum of the unsuppressed water reference spectrum. Spectra were excluded from further analysis if SNR < 25. Based on this criterion, three spectra from the pons were excluded.
For 3 T data, edit-off and edit-on spectra were corrected for phase and frequency fluctuations before subtracting to generate difference spectra. Edit-off and edit-on spectra were visually inspected for unwanted coherences. Difference spectra were also inspected for small frequency shifts between edit-off and edit-on spectra.
Metabolite Quantification
Summed 7 T spectra were analyzed with LCModel (version 6.3-0G) [30]. The basis set for LCModel contained 19 metabolites that were simulated using a density matrix approach [31] based on previously reported chemical shifts and coupling constants [32,33]: alanine (Ala), aspartate (Asp), ascorbate (Asc), glycerophosphocholine (GPC), phosphocholine (PCho), creatine (Cr), phosphocreatine (PCr), γ-aminobutyric acid (GABA), glucose (Glc), glutamine (Gln), glutamate (Glu), glutathione (GSH), myo-inositol (mIns), lactate (Lac), N-acetylaspartate (NAA), N-acetylaspartylglutamate (NAAG), phosphoethanolamine (PE), scyllo-inositol (sIns), and taurine (Tau). The basis set also included a macromolecule spectrum that was measured previously using an inversion recovery technique in 5 healthy subjects (TR = 2 s, inversion time = 685 ms, 640 averages total, occipital cortex) [15,34]. Absolute metabolite concentrations were determined by utilizing the unsuppressed water spectrum as an internal reference and correcting for tissue water and CSF content [16]. A tissue water content of 82% was assumed for the motor cortex and pons [35]. The T2 relaxation of tissue water was taken into account in the LCModel fitting by assuming values of 68 ms for the motor cortex and 74 ms for the pons. These assumptions were determined by acquiring a series of unsuppressed water signals at different echo times (TE = 28–4000 ms, TR = 15 s) in the respective VOI in ten healthy control subjects and fitting their integrals with a biexponential function. For the biexponential fit, the T2 of CSF was fixed at 565 ms, which was measured using the same sequence in the lateral ventricles of four healthy subjects (TE = 28–4000 ms, TR = 15 s, VOI = 0.125–0.360 mL). The measured free precession T2 values of tissue water in the motor cortex (45.4 ± 2.5 ms) and pons (48.9 ± 0.9 ms) were multiplied by a factor of 1.5 assuming Carr-Purcell conditions [29]. The % CSF contribution to each VOI was also obtained by fitting the integrals of the unsuppressed water spectra acquired in each VOI at different TE values with a biexponential decay function [26].
The 3 T MEGA-PRESS edit-off and difference spectra were analyzed with LCModel (version 6.3-1J) using a protocol nearly identical to that described above. However, NAA was separated into singlet and multiplet, and tCr was separated into CH3 and CH2 groups. The difference spectra were analyzed with the basis set that contained simulated information for NAA and GSH using previously described fitting parameters [36]. For edit-off data, absolute metabolite concentrations were obtained by utilizing an unsuppressed water reference spectrum and correcting for tissue water and CSF content. The CSF content was determined by segmenting the MPRAGE images using the SPM8 software package and then applying iterative threshold selection to obtain within-VOI fractions of gray matter, white matter, and CSF, as described previously [37]. A tissue water content of 82% was assumed and the T1 of tissue water of 1.08 s and T2 of 95 ms were used [38,39].
For each region, only metabolites quantified with mean Cramér-Rao Lower Bounds (CRLB) ≤ 20% were selected for analysis. In addition, metabolites were reported only as sums if they correlated strongly with each other (r < −0.7), as in the cases of total creatine (tCr; Cr+PCr) and total choline (tCho; GPC+PCho). Additionally, total NAA (tNAA; NAA+NAAG) and metabolite ratios (i.e., mIns/tCr, tNAA/tCr, NAA/mIns, tNAA/mIns) were reported in order to compare with prior work performed at lower field. The sums Glc+Tau and Glu+Gln (Glx) were also reported if any metabolite within each pair did not meet mean CRLB ≤ 20% criterion. Lac at 7 T was not quantified in the motor cortex of 6 subjects with ALS and 5 healthy controls due to the presence of unwanted coherences near 1.3 ppm.
Statistical Analysis
Except for left/right hemisphere and male/female scan counts, all measures were compared between the two cohorts using unpaired, two-tailed Student’s t-tests. Group comparisons of the count data were made using Fisher’s exact tests. Neurochemical levels were compared between the cohorts using t-tests and then two-way ANOVA to adjust for hemisphere scanned (motor cortex data only). Age and sex were not included as covariates in the models because they were similar between the ALS and control groups. The neurochemical levels were also compared pairwise across El Escorial diagnostic subgroups and controls using one-tailed t-tests (with the direction pre-specified according to disease severity in the diagnostic subgroups); a step-down Bonferroni procedure was used to correct type I error for multiple comparisons [40]. Comparisons in tNAA levels assumed that Definite ALS < Probable ALS < Possible ALS < Controls because these levels were expected, based on literature, to become lower as more disease signs appear [7,41]. Conversely, mIns levels were expected to be higher in disease and comparisons assumed that Definite ALS > Probable ALS > Possible ALS > Controls [8,9]. For Glu level comparisons, two-tailed t-tests were performed between subgroups and controls because there was no obvious expectation that Glu levels are altered in one direction as opposed to the other. Neurochemical levels were also compared across riluzole-treatment subgroups using pairwise two-tailed t-tests between riluzole-users, riluzole-non-users, and control groups, while again applying the step-down Bonferroni correction. Pearson’s correlation tests were used to examine possible linear relationships between neurochemical levels and clinical status measures.
Results
Cohort characteristics
Motor cortex 1H-MRS data were obtained at both 3 T and 7 T for all 19 subjects with ALS and 17 controls. Three subjects were unable to complete the exam’s second half, which was allotted for pons data acquisition. An additional three subjects had larger heads that resulted in increased coil loading. For these subjects, pons spectra were acquired with subadiabatic RF transmit power and were excluded due to low SNR. Thus, usable pons 1H-MRS data were acquired from a total of 15 subjects with ALS and 15 controls.
The ALS and control cohorts were matched on average for age and sex for both regions examined (Table 1). For motor cortex data, the cohorts were also approximately matched for brain hemisphere scanned. In the ALS cohort, 4 out of 19 subjects had bulbar-onset ALS, and of these, only one did not exhibit signs of upper limb involvement during the time of scanning. Compared to most prior studies [5,8,42], our ALS cohort had a higher mean ALSFRS-R score, indicating that the subjects as a group had milder disease. Scores on the UMN burden scale also indicated that they had mild upper motor neuron involvement on average. Lastly, seven subjects within the scanning cohort were classified by El Escorial criteria as having possible ALS. Subjects within this particular subgroup had the fewest disease signs, and their ALSFRS-R scores were significantly higher than the subjects with probable or definite ALS (Supplemental Table 1). Notably, the subjects with probable and definite ALS also had mean ALSFRS-R scores higher than those commonly reported in literature.
Spectral quality in subjects with ALS and controls
High-SNR metabolite spectra were obtained at 7 T from the motor cortex and pons of subjects with ALS and healthy controls (Fig. 1). No differences in spectral quality were apparent between the groups, while differences were observed between the two regions as expected. Pons spectra had lower SNR and broader linewidths than motor cortex spectra (Table 1). The spectral patterns were also distinct between the regions and noted to be characteristic of the motor cortex and pons. Similarly, comparable spectral quality was obtained in ALS and control groups at 3 T (Fig. 2).
The CSF content within the motor cortex and pons VOIs was not significantly different between subjects with ALS and controls; therefore there was no indication of atrophy in these regions due to disease. In the motor cortex at 7 T, eleven metabolites had mean CRLB ≤ 20%: Gln, Glu, GSH, Lac, mIns, NAA, NAAG, tCho, tCr, tNAA, and Glc+Tau. Notably, the major metabolites (tNAA, tCho, tCr, mIns) as well as Glu, Gln, and GSH were each quantified with mean CRLB less than 10%, consistent with prior findings at 7 T using semi-LASER [16]. In the pons, the CRLB criteria were fulfilled by the same metabolites as above, with the exception of GSH and Gln; the sum Glx (Glu+Gln) was therefore reported. Asc measurements in the pons also passed the mean CRLB ≤ 20% criterion.
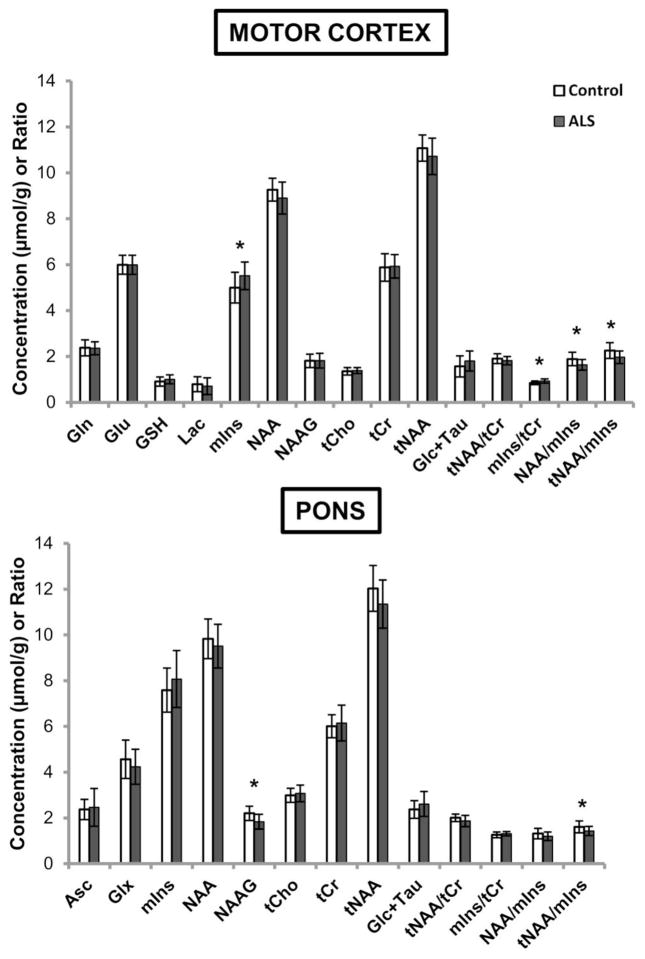
Neurochemical alterations in ALS
Metabolite concentrations from the motor cortex and pons of subjects with ALS and controls were quantified at 7 T (Fig. 3, Supplemental Table 2). In the motor cortex, the greatest group differences were seen in the levels of mIns. Subjects with ALS had significantly higher mIns levels and mIns/tCr ratio than controls (both p < 0.01). In addition, participants with ALS had significantly lower NAA/mIns and tNAA/mIns ratios than controls (both p < 0.01). In the pons, subjects with ALS had significantly lower NAAG and tNAA/mIns ratio compared to controls (p < 0.01 and p = 0.04, respectively). Analysis of the edit-off data acquired at 3 T from the larger motor cortex VOI (Fig. 2) produced similar findings (data not shown); significantly lower NAA/mIns and tNAA/mIns ratios (both p < 0.01) were observed in subjects with ALS versus controls.
Fig. 3.
Neurochemical profiles of healthy controls and subjects with ALS obtained at 7 T (shown as mean concentration and standard deviation bars). Only neurochemicals that were quantified with mean CRLB ≤ 20% are plotted. Motor cortex: ALS (N = 19), controls (N = 17). Pons: ALS (N = 15), controls (N = 15). Asc, ascorbate; Glc+Tau, glucose+taurine; Gln, glutamine; Glu, glutamate; Glx, glutamate+glutamine; GSH, glutathione; Lac, lactate; mIns, myo-inositol; NAA, N-acetylaspartate; NAAG, N-acetylaspartylglutamate; tCho, phosphocholine+glycerophosphocholine; tCr, creatine+phosphocreatine; tNAA, N-acetylaspartate+N-acetylaspartylglutamate. *p < 0.05 from ANOVAs adjusting for brain hemisphere scanned
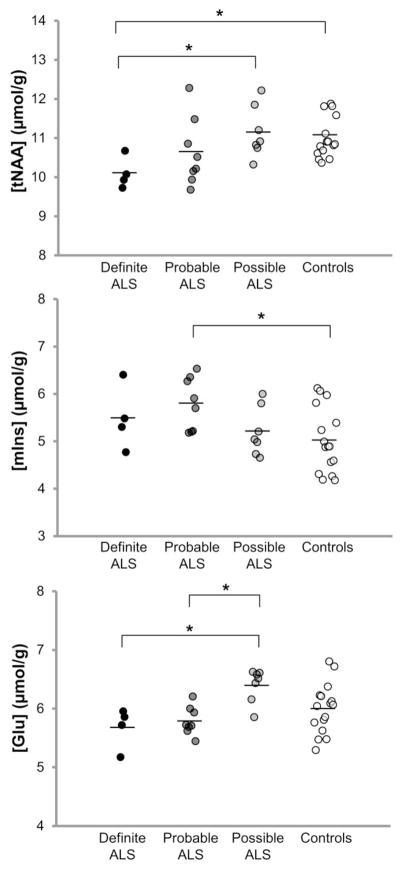
In order to investigate whether neurochemical levels were different for different diagnostic categories, the ALS cohort was separated into El Escorial diagnostic classifications (Fig. 4, Supplemental Table 1). Total NAA levels in the motor cortex were the lowest in subjects with definite ALS and were significantly different in these subjects compared to those with possible ALS (p = 0.04) and healthy controls (p = 0.04). Myo-inositol levels in the motor cortex were significantly higher in subjects with probable ALS than in controls (p = 0.02). Finally, Glu levels in subjects with either probable or definite ALS were significantly lower compared to those with possible ALS (both p = 0.01). In addition, since most prior 1H-MRS studies only enrolled subjects with definite and probable ALS and reported their results from whole cohort comparisons versus controls, we also pooled these two subgroups (N = 12; ALSFRS-R mean ± SD = 37.3 ± 5.7) and compared their neurochemical levels to those of subjects with possible ALS (ALSFRS-R mean ± SD = 44.1 ± 0.7) and healthy controls. In the motor cortex, combined probable/definite ALS had lower tNAA than possible ALS and controls (both p = 0.03), higher mIns than controls (p < 0.01), and lower Glu than possible ALS (p < 0.01).
Fig. 4.
Motor cortex NAA, mIns, and Glu levels differ between healthy controls and ALS subgroups classified by El Escorial diagnostic criteria. Controls (N = 17); ALS diagnostic subgroups: definite (N = 4), probable (N = 8), possible (N = 7). For tNAA and mIns comparisons, one-tailed Student’s t-tests were performed because the expected group differences are unidirectional. For the Glu comparison, two-tailed t-tests were performed as no such assumption was made. See Methods for details. *indicates p < 0.05
Unlike their dependence on El Escorial classification, neurochemical levels did not vary in relation to the severity of UMN dysfunction. Subjects with ALS were separated into subgroups consisting of those with higher burden (UMN score ≥ 3) and those with lower burden (UMN score < 3); no differences in the levels of metabolites were observed between these subgroups.
We also investigated the potential effects of riluzole treatment on neurochemical levels (Supplemental Table 3). In the motor cortex, no significant metabolite differences were observed between 8 subjects with ALS who were using riluzole at the time of MR scanning and 11 subjects who were not. When compared with healthy controls, riluzole-non-users had lower tNAA/mIns (p = 0.04). In the pons, no significant differences were observed among riluzole-users, riluzole-non-users, and healthy controls.
Finally, we observed no differences in GSH levels between the ALS and healthy control cohorts using either non-edited 1H-MRS at 7 T or edited 1H-MRS at 3 T (Fig. 2, 3).
Correlations with functional status
Metabolite levels and ratios in the motor cortex significantly correlated with ALSFRS-R scores, but not with UMN scores or disease duration. Specifically, moderate positive correlations were observed between ALSFRS-R and ratios of tNAA/tCr (r = 0.61; p < 0.01) and tNAA/mIns (r = 0.56; p = 0.01). Weaker correlations were seen between ALSFRS-R and absolute concentrations of tNAA (r = 0.46; p = 0.05), Glu (r = 0.48; p = 0.04), and mIns (r = −0.42; p = 0.07). Metabolite levels in the pons did not correlate with any of the clinical measures.
Discussion
To date, only a limited number of studies have utilized in vivo 1H-MRS at ultra-high field (7 T) for investigating neurochemical alterations associated with neurological diseases. These few studies have reported abnormalities in Parkinson’s disease, Huntington’s disease, adrenoleukodystrophy, and schizophrenia [43–47], while many other conditions have not been explored with 1H-MRS at 7 T. Aside from research abstracts [42,48], this is the first report of neurochemical alterations in ALS determined via 1H-MRS at 7 T. In addition, only two other studies have reported 1H-MRS data from subjects with early-stage ALS [49,50]. Consistent with previous reports, our data at 7 T indicate that people with ALS have metabolite alterations in the motor cortex and pons (Fig. 3). In particular, the tNAA/mIns ratio was significantly lower in these areas in affected subjects compared to healthy controls and suggests neurodegenerative changes [8,51]. Importantly, we also observed that the levels of NAA, mIns, and Glu depend on diagnostic categories as defined by El Escorial criteria (Fig. 4).
Using an optimized semi-LASER protocol at 7 T, we acquired proton spectra with high SNR and resolution from subjects with ALS and healthy controls. Relative to the motor cortex, the lower spectral quality obtained from the pons was expected and due to multiple factors, including smaller voxel size, deeper brain location, and increased intrinsic field inhomogeneities [25]. The increased sensitivity and spectral dispersion at 7 T allowed us to quantify a greater number of metabolites compared to previous 1H-MRS studies of ALS at lower fields. However, a few metabolites with low abundance (i.e., GABA, Asp, PE) did not pass the mean CRLB ≤ 20% criterion, which was unlike recent data on the test-retest reproducibility of 7 T 1H-MRS in the cerebellum and cingulate cortex [16]. Thus, the detectability of metabolites by 1H-MRS depends on brain region in addition to data acquisition methodology and magnetic field strength.
This study was partly motivated by a recent report that described lower levels of GSH in the motor cortex of subjects with ALS using spectral editing at 3 T [11]. In addition to our advanced 1H-MRS protocol at 7 T, we performed the previously-described protocol on the same subjects at 3 T in order to compare GSH quantitation in ALS across these two methods. The results were the same using both methods, with no differences observed in GSH levels and significant differences observed in tNAA/mIns ratio in the motor cortex between groups. The discrepancy between our GSH findings and those of the previous study is likely not explained by differences in methodology. Both studies used similar edited 1H-MRS protocols at 3 T, but in our study the size of the motor cortex VOI was larger (20.1 versus 12.5 mL) [11]. Furthermore, our smaller motor cortex VOI at 7 T (10.6 mL) also did not reveal a GSH difference between ALS and controls. Instead, differences in the characteristics of the studies’ cohorts may be important. Our study had a larger sample size and a potentially earlier-stage ALS cohort. The mean ALSFRS-R score of our cohort was among the highest of all ALS cohorts studied to date using 1H-MRS (ALSFRS-R mean ± SD = 39.8 ± 5.6). Notably, while the previous study reported a mean ALSFRS-R score similar to ours, it was calculated using data from only half of their cohort (N = 6). Thus, a difference between the cohorts’ disease severities may explain the difference in results. Our data show that GSH levels are not different from controls in people with ALS who possess relatively high functional status. Whether these levels change as ALS progresses needs to be examined further through longitudinal investigations.
Previous studies also reported alterations in the levels of NAA and Glx in the motor cortex and pons of humans with ALS [5,10]. The present study suggests that the levels of these metabolites are not significantly altered in these regions early in ALS. In particular, no differences in these metabolite levels were observed when comparing the entire early-stage ALS cohort with healthy controls (Fig. 3). Rather, group differences in motor cortex NAA and Glu levels became apparent only when performing comparisons with a subgroup of individuals with greater disease burden (i.e., subjects with probable/definite ALS [ALSFRS-R mean ± SD: 37.3 ± 5.7]). This finding is remarkable in the context of previous literature, in which many studies restricted their enrollment to probable or definite ALS. These studies commonly reported lower motor cortex NAA in subjects with ALS than in controls, suggesting neuronal loss and/or dysfunction [5,7,8]. Notably, the few studies that did not observe alterations in NAA included 1) a study of an early-stage cohort [50] and 2) a study of possible/suspected ALS [49]. Thus, motor cortex NAA levels appear dependent on disease stage, which likely explains differences in findings.
This is one of the first 1H-MRS studies of ALS to report absolute Glu levels in the motor cortex. Due to lower sensitivity and spectral resolution, previous studies at lower field strengths only reported Glx, a sum of Glu and Gln. Multiple contradictory reports have indicated either higher or lower levels of Glx in subjects with ALS [5,9,10,12], and therefore the absolute quantification of Glu is essential for determining the true direction of Glu alteration in ALS. Recently, Ratai et al. described lower motor cortex Glu levels in 12 subjects with ALS compared to 12 healthy controls [48]. In the present study, we analyzed a larger sample of subjects and did not observe differences in Glu levels between the cohorts. However, the El Escorial subgroup comparison suggests that motor cortex Glu levels also depend on disease stage in ALS. Lower Glu levels in subjects with probable/definite ALS than in those with possible ALS suggest glutamatergic neuron loss/dysfunction with progressing disease. This is further supported by earlier work in post-mortem human tissue that demonstrated neuronal loss as well as markedly lower Glu levels in the motor cortex in end-stage ALS [19,52].
Due to its potential as a glial cell marker [53,54], mIns has been quantified in the brain extensively using in vivo 1H-MRS. Elevated mIns levels have been reported for several neurodegenerative diseases, including Alzheimer’s disease [55], Huntington’s disease [56], hereditary ataxias [57,58], and ALS [5,8]. In these cases, investigators interpreted the elevated levels as an indication of gliosis, a proliferative response by glial cell types to neuronal injury. In ALS, gliosis is commonly seen on post-mortem exam and includes increased numbers of reactive astrocytes and activated microglia in the motor cortex [19,59]. Prior work in ALS using a positron emission tomography (PET) tracer that binds specifically to these cells has shown evidence of gliosis in the motor cortex in vivo [60–62]. This PET biomarker also positively correlates with mIns levels, according to a recent pilot investigation [63]. Consistent with these previous studies, we observed higher mIns levels in the motor cortex in subjects with ALS than in healthy controls. Importantly, this group difference was primarily driven by higher mIns levels in those with probable ALS. Similar to tNAA and Glu, mIns levels in subjects with possible ALS were not significantly different from controls (Fig. 4). Thus, mIns levels appear to increase with progressing disease in ALS. Notably, a prior study that enrolled subjects at a similarly early disease stage (ALSFRS-R mean ± SD = 40.1 ± 4.3) reported no differences in mIns levels [50].
One of the limitations of this study is the relatively small sample sizes of each El Escorial subgroup. In particular, the pons data were acquired from fewer subjects, which may have impacted our ability to detect subgroup differences in neurochemical levels in this region. Secondly, while the El Escorial classifications provide information on disease extent according to the number of regions affected by ALS, they were designed as a tool for providing diagnostic certainty rather than an indicator of disease stage. Furthermore, the El Escorial criteria require counting both UMN and LMN clinical signs in order to determine the classification. Therefore, novel classification schemes based only on clinical signs of UMN dysfunction may be better for assessing the extent of disease within the brain. In this study, there were no associations between our novel UMN burden score and neurochemical levels in subjects with ALS. There were also no differences in neurochemical levels between those with greater burden (UMN score ≥3) and those with lesser burden (score <3). Incorporating additional components into our rating scale that are recognized as highly sensitive to UMN dysfunction, such as finger- and foot-tapping speed, may provide a better means of separating disease cohorts according to the severity of UMN burden.
In conclusion, the data from this study suggest that differences between disease cohorts, particularly in subjects’ functional status and El Escorial classification, may be responsible for the discrepancies in the literature regarding the concentrations of MRS-measured metabolites in ALS. In addition, alterations in MRS-measured metabolites, namely NAA, mIns, and Glu, are restricted to individuals with ALSFRS-R scores lower than 44. Notably, these three metabolites show weak-to-moderate correlations with ALSFRS-R scores, suggesting that their levels change with disease severity. Longitudinal studies to monitor this ALS cohort, particularly those individuals with possible ALS, will be critical for evaluating the sensitivity of these and additional metabolites to disease progression in ALS.
Supplementary Material
Acknowledgments
We thank the research coordinators Susan Rolandelli, Valerie Ferment, and Pamela Droberg, and Georgios Manousakis, M.D., and Gaurav Guliani, M.D., at the University of Minnesota and Hennepin County Medical Center ALS Clinics for their help in the recruitment of human subjects, and Edward J. Auerbach, Ph.D., for the implementation of MEGA-PRESS and FASTMAP on the Siemens platforms. This work was supported by the Bob Allison Ataxia Research Center, the University of Minnesota Foundation, and the National Institute of Neurological Disorders and Stroke (NINDS) grant R01 NS080816. The Center for Magnetic Resonance Research is supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant P41 EB015894 and the Institutional Center Cores for Advanced Neuroimaging award P30 NS076408. Research reported in this publication was also supported by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–55. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Bensimon G, Lacomblez L, Meininger V. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N Engl J Med. 1994;330(9):585–91. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 3.Turner MR, Kiernan MC, Leigh PN, Talbot K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 2009;8(1):94–109. doi: 10.1016/S1474-4422(08)70293-X. [DOI] [PubMed] [Google Scholar]
- 4.Turner MR, Verstraete E. What does imaging reveal about the pathology of amyotrophic lateral sclerosis? Curr Neurol Neurosci Rep. 2015;15(7):45. doi: 10.1007/s11910-015-0569-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foerster BR, Pomper MG, Callaghan BC, Petrou M, Edden RA, Mohamed MA, Welsh RC, Carlos RC, Barker PB, Feldman EL. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol. 2013;70(8):1009–16. doi: 10.1001/jamaneurol.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gredal O, Rosenbaum S, Topp S, Karlsborg M, Strange P, Werdelin L. Quantification of brain metabolites in amyotrophic lateral sclerosis by localized proton magnetic resonance spectroscopy. Neurology. 1997;48(4):878–81. doi: 10.1212/wnl.48.4.878. [DOI] [PubMed] [Google Scholar]
- 7.Unrath A, Ludolph AC, Kassubek J. Brain metabolites in definite amyotrophic lateral sclerosis. A longitudinal proton magnetic resonance spectroscopy study. J Neurol. 2007;254(8):1099–106. doi: 10.1007/s00415-006-0495-2. [DOI] [PubMed] [Google Scholar]
- 8.Kalra S, Hanstock CC, Martin WR, Allen PS, Johnston WS. Detection of cerebral degeneration in amyotrophic lateral sclerosis using high-field magnetic resonance spectroscopy. Arch Neurol. 2006;63(8):1144–8. doi: 10.1001/archneur.63.8.1144. [DOI] [PubMed] [Google Scholar]
- 9.Bowen BC, Pattany PM, Bradley WG, Murdoch JB, Rotta F, Younis AA, Duncan RC, Quencer RM. MR imaging and localized proton spectroscopy of the precentral gyrus in amyotrophic lateral sclerosis. AJNR Am J Neuroradiol. 2000;21(4):647–58. [PMC free article] [PubMed] [Google Scholar]
- 10.Han J, Ma L. Study of the features of proton MR spectroscopy (1H-MRS) on amyotrophic lateral sclerosis. J Magn Reson Imaging. 2010;31(2):305–8. doi: 10.1002/jmri.22053. [DOI] [PubMed] [Google Scholar]
- 11.Weiduschat N, Mao X, Hupf J, Armstrong N, Kang G, Lange DJ, Mitsumoto H, Shungu DC. Motor cortex glutathione deficit in ALS measured in vivo with the J-editing technique. Neurosci Lett. 2014;570:102–7. doi: 10.1016/j.neulet.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 12.Kalra S, Seres P, Choi C. In vivo quantification of excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis. Proceedings of the 21st Annual Meeting of the International Society for Magnetic Resonance in Medicine; Salt Lake City, UT, USA. 2013. p. 1017. [Google Scholar]
- 13.Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic Biol Med. 2010;48(5):629–41. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Bae JS, Simon NG, Menon P, Vucic S, Kiernan MC. The puzzling case of hyperexcitability in amyotrophic lateral sclerosis. J Clin Neurol. 2013;9(2):65–74. doi: 10.3988/jcn.2013.9.2.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tkáč I, Öz G, Adriany G, Ugurbil K, Gruetter R. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med. 2009;62(4):868–79. doi: 10.1002/mrm.22086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terpstra M, Cheong I, Lyu T, Deelchand DK, Emir UE, Bednarik P, Eberly LE, Öz G. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn Reson Med. 2016;76(4):1083–91. doi: 10.1002/mrm.26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van de Bank BL, Emir UE, Boer VO, van Asten JJ, Maas MC, Wijnen JP, Kan HE, Öz G, Klomp DW, Scheenen TW. Multi-center reproducibility of neurochemical profiles in the human brain at 7 T. NMR Biomed. 2015;28(3):306–16. doi: 10.1002/nbm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith MC. Nerve Fibre Degeneration in the Brain in Amyotrophic Lateral Sclerosis. J Neurol Neurosurg Psychiatry. 1960;23(4):269–82. doi: 10.1136/jnnp.23.4.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Udaka F, Kameyama M, Tomonaga M. Degeneration of Betz cells in motor neuron disease. A Golgi study. Acta Neuropathol. 1986;70(3–4):289–95. doi: 10.1007/BF00686086. [DOI] [PubMed] [Google Scholar]
- 20.Brooks BR, Miller RG, Swash M, Munsat TL World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1(5):293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 21.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) J Neurol Sci. 1999;169(1–2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 22.Adriany G, Van de Moortele PF, Ritter J, Moeller S, Auerbach EJ, Akgun C, Snyder CJ, Vaughan T, Ugurbil K. A geometrically adjustable 16-channel transmit/receive transmission line array for improved RF efficiency and parallel imaging performance at 7 Tesla. Magn Reson Med. 2008;59(3):590–7. doi: 10.1002/mrm.21488. [DOI] [PubMed] [Google Scholar]
- 23.Emir UE, Auerbach EJ, Van De Moortele PF, Marjańska M, Ugurbil K, Terpstra M, Tkáč I, Öz G. Regional neurochemical profiles in the human brain measured by 1H MRS at 7 T using local B1 shimming. NMR Biomed. 2012;25(1):152–60. doi: 10.1002/nbm.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319–23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Öz G, Tkáč I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. Magn Reson Med. 2011;65(4):901–10. doi: 10.1002/mrm.22708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ernst TKR, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson. 1993;102(1):1–8. [Google Scholar]
- 27.Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR Biomed. 1998;11(6):266–72. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 28.Marjańska M, Lehericy S, Valabregue R, Popa T, Worbe Y, Russo M, Auerbach EJ, Grabli D, Bonnet C, Gallea C, Coudert M, Yahia-Cherif L, Vidailhet M, Meunier S. Brain dynamic neurochemical changes in dystonic patients: a magnetic resonance spectroscopy study. Mov Disord. 2013;28(2):201–9. doi: 10.1002/mds.25279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deelchand DK, Adanyeguh IM, Emir UE, Nguyen TM, Valabregue R, Henry PG, Mochel F, Öz G. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn Reson Med. 2015;73(5):1718–1725. doi: 10.1002/mrm.25295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 31.Deelchand DK, Henry PG, Ugurbil K, Marjańska M. Measurement of transverse relaxation times of J-coupled metabolites in the human visual cortex at 4 T. Magn Reson Med. 2012;67(4):891–7. doi: 10.1002/mrm.23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13(3):129–53. doi: 10.1002/1099-1492(200005)13:3<129::aid-nbm619>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 33.Tkáč I. Refinement of Simulated Basis Set for LCModel Analysis. Proceedings of the 16th Annual Meeting of the International Society for Magnetic Resonance in Medicine; Toronto, Ontario, Canada. 2008. p. 1624. [Google Scholar]
- 34.Schaller B, Xin L, Gruetter R. Is the macromolecule signal tissue-specific in healthy human brain? A 1H MRS study at 7 Tesla in the occipital lobe. Magn Reson Med. 2014;72(4):934–40. doi: 10.1002/mrm.24995. [DOI] [PubMed] [Google Scholar]
- 35.Siegel GJ. Basic Neurochemistry: Molecular, Cellular, and Medical Aspects. 6. Philadelphia, PA, USA: Lippincott-Raven Publishers; 1999. [Google Scholar]
- 36.Emir UE, Deelchand D, Henry PG, Terpstra M. Noninvasive quantification of T2 and concentrations of ascorbate and glutathione in the human brain from the same double-edited spectra. NMR Biomed. 2011;24(3):263–9. doi: 10.1002/nbm.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bednarik P, Moheet A, Deelchand DK, Emir UE, Eberly LE, Bares M, Seaquist ER, Öz G. Feasibility and reproducibility of neurochemical profile quantification in the human hippocampus at 3 T. NMR Biomed. 2015;28(6):685–93. doi: 10.1002/nbm.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rooney WD, Johnson G, Li X, Cohen ER, Kim SG, Ugurbil K, Springer CS., Jr Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med. 2007;57(2):308–18. doi: 10.1002/mrm.21122. [DOI] [PubMed] [Google Scholar]
- 39.Wansapura JP, Holland SK, Dunn RS, Ball WS., Jr NMR relaxation times in the human brain at 3.0 tesla. J Magn Reson Imaging. 1999;9(4):531–8. doi: 10.1002/(sici)1522-2586(199904)9:4<531::aid-jmri4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Holm S. A Simple Sequentially Rejective Bonferroni Test Procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 41.Mitsumoto H, Ulug AM, Pullman SL, Gooch CL, Chan S, Tang MX, Mao X, Hays AP, Floyd AG, Battista V, Montes J, Hayes S, Dashnaw S, Kaufmann P, Gordon PH, Hirsch J, Levin B, Rowland LP, Shungu DC. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology. 2007;68(17):1402–10. doi: 10.1212/01.wnl.0000260065.57832.87. [DOI] [PubMed] [Google Scholar]
- 42.Atassi N, Triantanfyllou C, Keil B, Lawson R, Kaplan L, Dheel C, Murphy A, Berry J, Salibi N, Seethamraju R, Rosen B, Cudkowicz M, Ratai E. Ultra High-Field (7T) Magnetic Resonance Spectroscopy (MRS) in People with Amyotrophic Lateral Sclerosis (ALS). Proceedings of the 21st Annual Meeting of the International Society for Magnetic Resonance in Medicine; Salt Lake City, UT, USA. 2013. p. 1018. [Google Scholar]
- 43.Emir UE, Tuite PJ, Öz G. Elevated pontine and putamenal GABA levels in mild-moderate Parkinson disease detected by 7 tesla proton MRS. PLoS One. 2012;7(1):e30918. doi: 10.1371/journal.pone.0030918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratai E, Kok T, Wiggins C, Wiggins G, Grant E, Gagoski B, O’Neill G, Adalsteinsson E, Eichler F. Seven-Tesla proton magnetic resonance spectroscopic imaging in adult X-linked adrenoleukodystrophy. Arch Neurol. 2008;65(11):1488–94. doi: 10.1001/archneur.65.11.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Unschuld PG, Edden RA, Carass A, Liu X, Shanahan M, Wang X, Oishi K, Brandt J, Bassett SS, Redgrave GW, Margolis RL, van Zijl PC, Barker PB, Ross CA. Brain metabolite alterations and cognitive dysfunction in early Huntington’s disease. Mov Disord. 2012;27(7):895–902. doi: 10.1002/mds.25010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brandt AS, Unschuld PG, Pradhan S, Lim IA, Churchill G, Harris AD, Hua J, Barker PB, Ross CA, van Zijl PC, Edden RA, Margolis RL. Age-related changes in anterior cingulate cortex glutamate in schizophrenia: A 1H MRS Study at 7 Tesla. Schizophr Res. 2016;172(1–3):101–5. doi: 10.1016/j.schres.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van den Bogaard SJ, Dumas EM, Teeuwisse WM, Kan HE, Webb A, van Buchem MA, Roos RA, van der Grond J. Longitudinal metabolite changes in Huntington’s disease during disease onset. J Huntingtons Dis. 2014;3(4):377–86. doi: 10.3233/JHD-140117. [DOI] [PubMed] [Google Scholar]
- 48.Atassi N, Xu M, Triantanfyllou C, Keil B, Long C, Lawson R, Cernasov P, Ratti E, Paganoni S, Salibi N, Seethamraju R, Rosen B, Cudkowicz M, Ratai E. Biochemical Characteristics in Amyotrophic Lateral Sclerosis Detected by 7T MR Spectroscopy. Proceedings of the 24th Annual Meeting of the International Society for Magnetic Resonance in Medicine; Singapore. 2016. p. 2408. [Google Scholar]
- 49.Rule RR, Suhy J, Schuff N, Gelinas DF, Miller RG, Weiner MW. Reduced NAA in motor and non-motor brain regions in amyotrophic lateral sclerosis: a cross-sectional and longitudinal study. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(3):141–9. doi: 10.1080/14660820410017109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.van der Graaff MM, Lavini C, Akkerman EM, Majoie Ch B, Nederveen AJ, Zwinderman AH, Brugman F, van den Berg LH, de Jong JM, de Visser M. MR spectroscopy findings in early stages of motor neuron disease. AJNR Am J Neuroradiol. 2010;31(10):1799–806. doi: 10.3174/ajnr.A2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray ME, Przybelski SA, Lesnick TG, Liesinger AM, Spychalla A, Zhang B, Gunter JL, Parisi JE, Boeve BF, Knopman DS, Petersen RC, Jack CR, Jr, Dickson DW, Kantarci K. Early Alzheimer’s disease neuropathology detected by proton MR spectroscopy. J Neurosci. 2014;34(49):16247–55. doi: 10.1523/JNEUROSCI.2027-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai GC, Stauch-Slusher B, Sim L, Hedreen JC, Rothstein JD, Kuncl R, Coyle JT. Reductions in acidic amino acids and N-acetylaspartylglutamate in amyotrophic lateral sclerosis CNS. Brain Res. 1991;556(1):151–6. doi: 10.1016/0006-8993(91)90560-i. [DOI] [PubMed] [Google Scholar]
- 53.Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci. 1993;15(3–5):289–98. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- 54.Glanville NT, Byers DM, Cook HW, Spence MW, Palmer FB. Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochim Biophys Acta. 1989;1004(2):169–79. doi: 10.1016/0005-2760(89)90265-8. [DOI] [PubMed] [Google Scholar]
- 55.Voevodskaya O, Sundgren PC, Strandberg O, Zetterberg H, Minthon L, Blennow K, Wahlund LO, Westman E, Hansson O Swedish BioFINDER study group. Myo-inositol changes precede amyloid pathology and relate to APOE genotype in Alzheimer disease. Neurology. 2016;86(19):1754–61. doi: 10.1212/WNL.0000000000002672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sturrock A, Laule C, Wyper K, Milner RA, Decolongon J, Dar Santos R, Coleman AJ, Carter K, Creighton S, Bechtel N, Bohlen S, Reilmann R, Johnson HJ, Hayden MR, Tabrizi SJ, Mackay AL, Leavitt BR. A longitudinal study of magnetic resonance spectroscopy Huntington’s disease biomarkers. Mov Disord. 2015;30(3):393–401. doi: 10.1002/mds.26118. [DOI] [PubMed] [Google Scholar]
- 57.Iltis I, Hutter D, Bushara KO, Clark HB, Gross M, Eberly LE, Gomez CM, Öz G. 1H MR spectroscopy in Friedreich’s ataxia and ataxia with oculomotor apraxia type 2. Brain Res. 2010;1358:200–10. doi: 10.1016/j.brainres.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Öz G, Hutter D, Tkáč I, Clark HB, Gross MD, Jiang H, Eberly LE, Bushara KO, Gomez CM. Neurochemical alterations in spinocerebellar ataxia type 1 and their correlations with clinical status. Mov Disord. 2010;25(9):1253–61. doi: 10.1002/mds.23067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brettschneider J, Toledo JB, Van Deerlin VM, Elman L, McCluskey L, Lee VM, Trojanowski JQ. Microglial activation correlates with disease progression and upper motor neuron clinical symptoms in amyotrophic lateral sclerosis. PLoS One. 2012;7(6):e39216. doi: 10.1371/journal.pone.0039216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alshikho MJ, Zürcher NR, Loggia ML, Cernasov P, Chonde DB, Izquierdo Garcia D, Yasek JE, Akeju O, Catana C, Rosen BR, Cudkowicz ME, Hooker JM, Atassi N. Glial activation colocalizes with structural abnormalities in amyotrophic lateral sclerosis. Neurology. 2016;87(24):2554–2561. doi: 10.1212/WNL.0000000000003427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lavisse S, Guillermier M, Herard AS, Petit F, Delahaye M, Van Camp N, Ben Haim L, Lebon V, Remy P, Dolle F, Delzescaux T, Bonvento G, Hantraye P, Escartin C. Reactive astrocytes overexpress TSPO and are detected by TSPO positron emission tomography imaging. J Neurosci. 2012;32(32):10809–18. doi: 10.1523/JNEUROSCI.1487-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zürcher NR, Loggia ML, Lawson R, Chonde DB, Izquierdo-Garcia D, Yasek JE, Akeju O, Catana C, Rosen BR, Cudkowicz ME, Hooker JM, Atassi N. Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [11C]-PBR28. Neuroimage Clin. 2015;7:409–14. doi: 10.1016/j.nicl.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ratai E, Alshikho M, Zürcher N, Loggia M, Cernasov P, Fish J, Seth R, Paganoni S, Rosen B, Cudkowicz M, Hooker J, Atassi N. Glial Activation Measured by [11C]-PBR28 PET Correlates with 1H-MRS Brain Metabolites in Amyotrophic Lateral Sclerosis. 27th International Symposium on ALS/MND; Dublin, Ireland. 2016. p. 60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.