Significance
Large parasites are a persistent source of morbidity and mortality in humans, domesticated animals, and wildlife. Hosts are subject to strong natural selection to eliminate or tolerate these parasite infections. Here, we document the recent evolution of a striking form of resistance by a vertebrate host (threespine stickleback) against its cestode parasite (Schistocephalus solidus). After the Pleistocene glacial retreat, marine stickleback colonized freshwater lakes, encountered Schistocephalus, and evolved varying levels of resistance to it. We show that heavily and rarely infected populations of stickleback can similarly resist Schistocephalus colonization, but rarely infected fish suppress parasite growth by orders of magnitude. These populations represent ends of a natural continuum of cestode growth suppression which is associated with reduced infection prevalence.
Keywords: immune evolution, helminth, resistance, Schistocephalus solidus, threespine stickleback
Abstract
Parasites can be a major cause of natural selection on hosts, which consequently evolve a variety of strategies to avoid, eliminate, or tolerate infection. When ecologically similar host populations present disparate infection loads, this natural variation can reveal immunological strategies underlying adaptation to infection and population divergence. For instance, the tapeworm Schistocephalus solidus persistently infects 0–80% of threespine stickleback (Gasterosteus aculeatus) in lakes on Vancouver Island. To test whether these heterogeneous infection rates result from evolved differences in immunity, we experimentally exposed laboratory-reared fish from ecologically similar high-infection and no-infection populations to controlled doses of Schistocephalus. We observed heritable between-population differences in several immune traits: Fish from the naturally uninfected population initiated a stronger granulocyte response to Schistocephalus infection, and their granulocytes constitutively generate threefold more reactive oxygen species in cell culture. Despite these immunological differences, Schistocephalus was equally successful at establishing initial infections in both host populations. However, the no-infection fish dramatically suppressed tapeworm growth relative to high-infection fish, and parasite size was intermediate in F1 hybrid hosts. Our results show that stickleback recently evolved heritable variation in their capacity to suppress helminth growth by two orders of magnitude. Data from many natural populations indicate that growth suppression is widespread but not universal and, when present, is associated with reduced infection prevalence. Host suppression of helminth somatic growth may be an important immune strategy that aids in parasite clearance or in mitigating the fitness costs of persistent infection.
Helminth parasites (trematodes, nematodes, and cestodes) currently infect ∼24% of humanity (1), undermine agricultural productivity (2, 3), and threaten conservation of some wild populations (4). These social, economic, and environmental costs motivate substantial interest in how vertebrate hosts combat helminth infection. Helminths often severely reduce their hosts’ fitness and therefore represent a strong source of natural selection in host populations (5–7). In response, vertebrates have evolved a complex repertoire of innate and adaptive immune responses that serve to detect and eliminate infections or to promote tolerance of successful infections.
Despite vertebrates’ sophisticated immune systems, helminths remain common and persistent, often establishing infections that last months or years. Parasites’ continued success may be attributed to (i) hosts’ failure to evolve effective resistance because of insufficient time or genetic diversity (8), (ii) trade-offs that constrain hosts’ ability to evolve costly immune traits (9–12), or (iii) parasites’ counter adaptations to undermine host immunity (13). These factors may apply unequally across host populations because of differences in host–parasite encounter rates, host genetic diversity, coevolutionary time, ecological costs of immunity, or parasite genotypes (14). As a result, some hosts may be substantially more resistant or tolerant than others.
Natural variation in host immunity provides an opportunity to elucidate vertebrates’ evolving strategies to limit helminth infections. This evaluation can be done by surveying diverse natural populations to identify instances of exceptionally high or low parasite prevalence and testing whether these differently infected populations vary with respect to immune phenotypes. Finally, we can evaluate whether genetic differences in resistance are responsible for the natural variation in infection rates. This approach is exciting, because it can identify immune strategies that natural selection has favored to mitigate parasite infections. Unlike traditional immunogenetics, which uses mutagenesis to create phenotypic aberrations [frequently involving loss-of-function mutations (15)] or forward genetic approaches to map causative loci (16), natural selection scans entire populations for many generations to find beneficial resistance traits.
Here, we document wide-ranging variation in parasite abundance (from 0 to 80% prevalence) in wild populations and show that this variation is associated with heritable differences in immune response and the recent evolutionary gain of an underappreciated form of resistance: suppression of parasite size. Threespine stickleback (Gasterosteus aculeatus) inhabit brackish and freshwater habitats throughout coastal north temperate regions. After the Pleistocene deglaciation (∼12,000 y ago), marine stickleback colonized many replicate freshwater lakes on Vancouver Island and elsewhere. This colonization brought marine fish into contact with a freshwater parasite, the cestode Schistocephalus solidus. Because S. solidus eggs do not hatch in brackish water (17), marine stickleback are rarely infected by this cestode and therefore have not evolved effective resistance (18). Newly established freshwater populations encountered S. solidus at a higher rate, resulting in reduced survival and fecundity (19, 20) and selection for increased resistance. Independently colonized lake populations evolved parallel gain of resistance (18). Prior studies showed this resistance entails both reduced infection establishment rates (18, 21) and slight suppression of parasite growth (21, 22). Here, we show that this parallel evolution is incomplete: Lake populations differ with respect to immune responses and parasite resistance, because some populations evolved to suppress cestode growth by two orders of magnitude.
Results
Infection Prevalence Differs Among Natural Stickleback Populations.
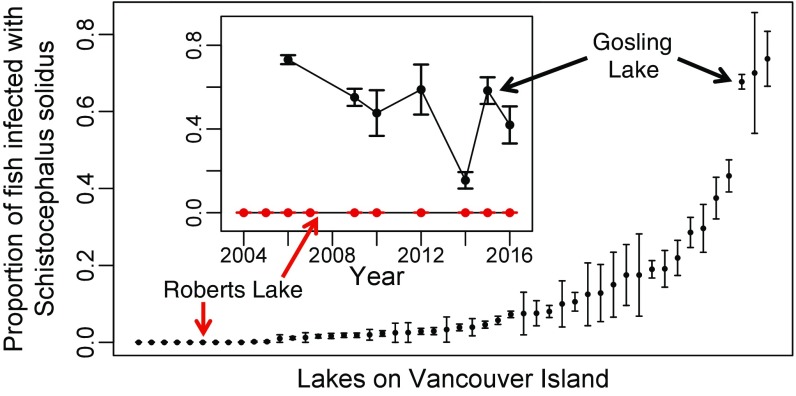
Samples of stickleback from 50 lakes on Vancouver Island (Table S1) revealed among-population variation in S. solidus infection prevalence spanning at least two orders of magnitude and persisting for a decade (χ2 = 4,629, df = 49, P < 0.0001) (Fig. 1). To test experimentally for variation in stickleback resistance to S. solidus, we focused on two lakes that bracket the range of infection prevalence. Gosling Lake stickleback (GOS) had the third highest infection prevalence in our sample (50–80% across years). In contrast, a decade of sampling revealed no infections in Roberts Lake stickleback (ROB) (Fig. 1; n = 1,480 fish).
Table S1.
Number of fish sampled in each lake between 2001 and 2016
| Lake | UTM North | UTM East | UTM zone | 2001 | 2002 | 2004 | 2005 | 2006 | 2007 | 2009 | 2010 | 2012 | 2013 | 2014 | 2015 | 2016 |
| Amor Lake | 5,559,399 | 315,779 | 10U | 0 | 0 | 0 | 101 | 0 | 0 | 60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Beaver Lake | 5,606,759 | 619,565 | 09U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 44 | 0 | 0 | 0 |
| Blackwater Lake | 5,561,779 | 315,242 | 10U | 0 | 0 | 70 | 498 | 0 | 43 | 82 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bob Lake | 5,575,549 | 319,979 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 80 | 0 | 0 | 0 | 0 | 0 | 0 |
| Boot Lake | 5,548,421 | 318,359 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 93 | 0 | 0 | 40 | 0 | 0 | 0 |
| Brewster Lake | 5,552,090 | 315,614 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 63 | 0 | 0 | 0 | 0 | 0 | 0 |
| Browns Bay Lake | 5,558,988 | 327,438 | 10U | 0 | 0 | 50 | 0 | 0 | 0 | 29 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cecil Lake | 5,567,980 | 318,586 | 10U | 0 | 344 | 0 | 0 | 435 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 |
| Cedar Lake | 5,564,339 | 316,861 | 10U | 0 | 0 | 0 | 243 | 0 | 0 | 98 | 0 | 0 | 0 | 0 | 0 | 0 |
| Comida Lake | 5,558,379 | 319,367 | 10U | 0 | 0 | 0 | 161 | 0 | 81 | 5 | 70 | 0 | 40 | 0 | 0 | 0 |
| Cranberry Lake | 5,551,340 | 324,365 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 66 | 0 | 0 | 0 | 0 | 0 | 0 |
| Dugout Lake | 5,561,994 | 319,853 | 10U | 0 | 279 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Echo Lake | 5,539,718 | 327,353 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 68 | 0 | 0 | 0 | 0 | 0 | 0 |
| Farewell Lake | 5,564,245 | 315,400 | 10U | 0 | 301 | 0 | 297 | 0 | 243 | 94 | 0 | 0 | 0 | 0 | 0 | 0 |
| First Lake | 5,548,119 | 300,414 | 10U | 0 | 0 | 0 | 470 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Frederick Lake | 5,413,636 | 351,540 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Fry Lake | 5,544,508 | 315,779 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 101 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gosling Lake | 5,547,004 | 320,855 | 10U | 0 | 0 | 0 | 0 | 435 | 0 | 147 | 21 | 17 | 0 | 84 | 60 | 31 |
| Gray Lake | 5,548,001 | 314,224 | 10U | 0 | 0 | 66 | 0 | 435 | 0 | 75 | 0 | 0 | 0 | 0 | 0 | 0 |
| Higgens Lake | 5,549,207 | 320,469 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 81 | 60 | 0 | 0 | 0 | 0 | 0 |
| Joe Lake | 5,609,028 | 607,255 | 09U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Kennedy Lake | 5,441,197 | 311,220 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Lawson Lake | 5,545,844 | 315,317 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 82 | 0 | 0 | 0 | 0 | 0 | 0 |
| Little Goose Lake | 5,559,765 | 322,236 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 76 | 0 | 0 | 0 | 0 | 0 | 0 |
| Little Mud Lake | 5,564,752 | 318,009 | 10U | 0 | 0 | 65 | 0 | 400 | 0 | 60 | 11 | 0 | 0 | 0 | 0 | 0 |
| Little Woss Lake | 5,561,258 | 670,547 | 10U | 0 | 0 | 0 | 300 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lower Campbell Lake | 5,545,324 | 322,686 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 |
| Lower Stella Lake | 5,576,289 | 318,433 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 104 | 0 | 0 | 0 | 0 | 0 | 0 |
| McCreight Lake | 5,578,601 | 312,181 | 10U | 0 | 0 | 0 | 499 | 0 | 33 | 60 | 0 | 0 | 0 | 0 | 0 | 0 |
| McNair Lake | 5,567,169 | 316,386 | 10U | 0 | 0 | 0 | 0 | 428 | 0 | 60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Merril Lake | 5,548,351 | 316,999 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 38 | 0 | 0 | 0 | 0 | 0 | 0 |
| Misty Lake | 5,607,337 | 622,517 | 09U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Mohun Lake | 5,559,827 | 322,238 | 10U | 0 | 0 | 0 | 0 | 414 | 0 | 60 | 68 | 0 | 0 | 0 | 0 | 0 |
| Moore Lake | 5,601,505 | 564,884 | 09U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Muchalat Lake | 5,528,556 | 703,714 | 09U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Mud Lake | 5,562,558 | 317,676 | 10U | 0 | 0 | 55 | 0 | 428 | 0 | 60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muskeg Lake | 5,563,937 | 315,816 | 10U | 349 | 0 | 0 | 0 | 0 | 0 | 104 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northy Lake | 5,520,804 | 344,487 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 39 | 0 | 0 | 0 |
| Ormund Lake | 5,561,643 | 319,602 | 10U | 0 | 0 | 0 | 0 | 40 | 0 | 60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pachena Lake | 5,411,771 | 350,856 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Pye Lake | 5,576,770 | 317,451 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 42 | 0 | 0 | 0 |
| Roberts Lake | 5,565,502 | 318,883 | 10U | 0 | 0 | 55 | 531 | 427 | 296 | 101 | 20 | 100 | 50 | 25 | 20 | 25 |
| Second Lake | 5,548,662 | 300,767 | 10U | 0 | 0 | 0 | 0 | 430 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Snow Lake | 5,576,619 | 315,338 | 10U | 0 | 0 | 0 | 479 | 0 | 0 | 17 | 0 | 0 | 0 | 0 | 0 | 0 |
| Stella Lake | 5,574,926 | 320,709 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 60 | 0 | 0 | 0 | 0 | 0 | 0 |
| Swan Lake | 5,613,899 | 562,476 | 09U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Thiemer Lake | 5,598,153 | 642,916 | 09U | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 40 | 0 | 0 | 0 |
| Upper Campbell Lake | 5,541,303 | 314,796 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 105 | 0 | 0 | 0 | 0 | 0 | 0 |
| Village Bay Lake | 5,559,913 | 343,769 | 10U | 0 | 0 | 0 | 0 | 0 | 0 | 49 | 0 | 0 | 40 | 0 | 0 | 0 |
| Woss Lake | 5,561,804 | 669,641 | 09U | 0 | 0 | 0 | 54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
UTM denotes GPS coordinates using the Universal Transverse Mercator system. UTM North indicates latitude, UTM East indicates longitude, and UTM zone indicates the global grid square. The number and letter of the UTM zone respectively define the longitudinal and latitudinal position of one square on the global grid, within which the coordinates are positioned.
Fig. 1.
Natural variation in S. solidus prevalence among 50 lakes on Vancouver Island, British Columbia. Lake names, sample year, sample size, and location details are given in Table S1. Error bars indicate SEs of each sample. The Inset presents data from two focal populations, Roberts and Gosling Lakes, over a roughly 10-y time span.
Ecological differences between the two lakes are minimal. Gosling and Roberts Lakes are only 17 km apart, both are moderately large (60 and 160 ha, respectively) and are similarly deep (maximum depth, 40 and 53 m, respectively). Both lakes contain large pelagic zones in which stickleback forage on copepods (S. solidus’ first host) at similar rates [averaging 0.444 and 0.457 cyclopoid copepods per fish per day, respectively [Poisson generalized linear model (GLM) P = 0.9707 for n = 27 and 35 fish] (23). Both lakes host at least one breeding pair of loons (a terminal host for S. solidus) per summer. The Roberts Lake loons make regular foraging trips to nearby lakes where S. solidus is common. Consequently, there is no currently known difference in stickleback’s risk of exposure to S. solidus in Gosling and Roberts Lakes.
Divergence in Immune Responses.
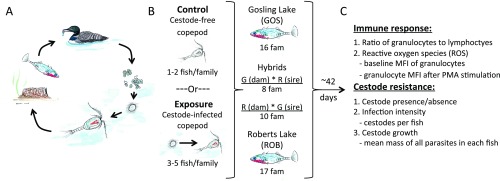
We used a common-garden breeding experiment to test whether GOS and ROB stickleback evolved divergent immune phenotypes. We crossed wild-caught adults from GOS and ROB to generate 51 families, which included both pure-population fish and F1 hybrids with GOS or ROB dams (G×R or R×G, respectively). The eggs were hatched and reared to adulthood in laboratory aquaria. We also bred S. solidus plerocercoids obtained from infected fish from three lakes in British Columbia. Approximately five fish per family were fed copepods containing infective S. solidus procercoids (Fig. S1). One to two siblings per family were fed uninfected copepods as a control (Fig. S1). Approximately 42 d postexposure, we measured host immune phenotypes to test for constitutive and infection-induced differences between stickleback populations. Here, we focus on two traits previously associated with defense against macroparasites: the ratio of granulocytes to lymphocytes in head kidney (HK) primary cell cultures and the magnitude of reactive oxygen species (ROS) produced by granulocytes (9, 24–28).
Fig. S1.
Experimental cestode infections. (A) The life cycle of S. solidus involves hatching from eggs in freshwater, a brief free-swimming period, copepod infection, infection of threespine stickleback, infection of piscivorous birds and sexual reproduction (outcrossing or selfing) in this final host, followed by fecal excretion of eggs into water. (B) We fed uninfected or cestode-infected copepods to numerous laboratory-reared stickleback families (indicated as “fam”) to test whether fish from naturally high- and low-infection populations (and their hybrids) differ heritably in immune responses and parasite resistance. (C) A summary of immune traits and resistance traits measured on the control, exposed, and infected fish.
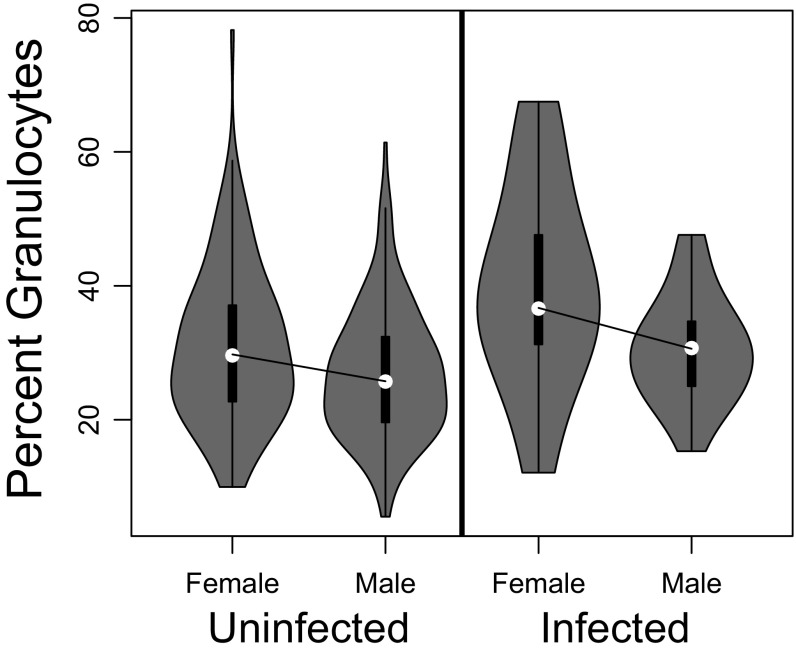
The relative abundance of granulocytes differed between the sexes (4.7% less in males; t = 4.03, P = 8.4e-5) (Fig. S2) and also varied among stickleback families within the GOS and ROB populations (random family effect, Table S2). This family-level variation could be caused either by segregating genetic variation within populations or by transgeneration effects of environmental differences among the natural-caught parents (29). Granulocyte relative abundance was independent of cestode genotype (Table S2) and showed no statistically significant main effect of experimental exposure to or infection by S. solidus (Fig. S3).
Fig. S2.
There is a significant but weak tendency for male stickleback to have relatively fewer granulocytes (or, equivalently, more lymphocytes) than females. Because sex does not interact with fish or parasite genotype, here we lump all host genotypes together to focus on the sex effect in uninfected and infected fish, respectively.
Table S2.
AIC scores for all statistical models comparing GOS and ROB fish
| Response | Model parameters | AIC | ΔAIC with best model |
| Infection presence | Null | 140.4 | — |
| Family | 142.3 | 1.9 | |
| Sex | 142.3 | 1.9 | |
| Host-pop | 140.1 | −0.3 | |
| Worm-pop | 143.9 | 3.5 | |
| Sex × host-pop | 143.7 | 3.3 | |
| Sex × worm-pop | 148.8 | 8.4 | |
| Host-pop × worm-pop | 142.8 | 2.4 | |
| Host-pop × worm-pop × sex | 148.5 | 8.1 | |
| Infection intensity | Null | 81.8 | — |
| Family | 83.7 | 1.9 | |
| Sex | 83.8 | 2.0 | |
| Host-pop | 83.8 | 2.0 | |
| Worm-pop | 84.4 | 2.6 | |
| Sex × host-pop | 87.6 | 5.8 | |
| Sex × worm-pop | 83.6 | 1.8 | |
| Host-pop × worm-pop | 85.4 | 3.6 | |
| Host-pop × worm-pop × sex | 87.9 | 6.1 | |
| Granulocyte: lymphocyte (all fish) | Null | 1,541.1 | 73.2 |
| Family | 1,505.9 | 38.0 | |
| Family + sex | 1,496.6 | 28.7 | |
| Family + host-pop | 1,504.6 | 36.7 | |
| Family + exposure | 1,504.8 | 36.9 | |
| Family + infection | 1,492.5 | 24.6 | |
| Family + parasite load | 1,500 | 32.1 | |
| Family + sex + infection | 1,480.7 | 12.8 | |
| Family + sex × host-pop | 1,496.1 | 28.2 | |
| Family + sex × exposure | 1,498.1 | 30.2 | |
| Family + sex × infection | 1,481.2 | 13.3 | |
| Family + host-pop × exposure | 1,504.7 | 36.8 | |
| Family + host-pop × infection | 1,481.4 | 13.5 | |
| Family + sex + host-pop × exposure | 1,496.2 | 28.3 | |
| Family + sex + host-pop × infection | 1,467.9 | — | |
| Family + sex × host-pop × exposure | 1,497.5 | 29.6 | |
| Family + sex × host-pop × infection | 1,471.4 | 3.5 | |
| Granulocyte: lymphocyte (exposed fish) | Null | 1,092.3 | 36.5 |
| Family | 1,080.9 | 25.1 | |
| Family + sex | 1,077.5 | 21.7 | |
| Family + host-pop | 1,080.8 | 25.0 | |
| Family + infection | 1,069.8 | 14.0 | |
| Family + parasite load | 1,076.1 | 20.3 | |
| Family + worm-pop | 1,078.1 | 22.3 | |
| Family + sex + worm-pop | 1,064.1 | 8.3 | |
| Family + sex × infection | 1,064 | 8.2 | |
| Family + sex × host-pop | 1,076 | 20.2 | |
| Family + sex × worm-pop | 1,077.9 | 22.1 | |
| Family + host-pop × worm-pop | 1,082.4 | 26.6 | |
| Family + host-pop × infection | 1,062.9 | 7.1 | |
| Family + worm-pop × infection | 1,062.9 | 7.1 | |
| Family + sex + worm-pop × infection | 1,057.5 | 1.7 | |
| Family + sex + host-pop × infection | 1,055.8 | — | |
| Family + host-pop × worm-pop × infection | 1,064.1 | 8.3 | |
| Family + sex + host-pop × worm-pop × infection | 1,058.7 | 2.9 | |
| Family + host-pop × worm-pop × sex | 1,084.1 | 28.3 | |
| Family + host-pop × infection × sex | 1,056.4 | 0.6 | |
| Granulocyte: lymphocyte (infected fish) | Null | 180.4 | 4.6 |
| Family | 182.1 | 6.3 | |
| Sex | 175.8 | — | |
| Host-pop | 180.2 | 4.4 | |
| Worm-pop | 182.3 | 6.5 | |
| Parasite load | 182.1 | 6.3 | |
| Sex × host-pop | 176.3 | 0.5 | |
| Sex × worm-pop | 179.4 | 3.6 | |
| Host-pop × worm-pop | 183.9 | 8.1 | |
| Sex + host-pop × worm-pop | 179.7 | 3.9 | |
| Sex × host-pop × worm-pop | 184.7 | 8.9 | |
| P_Gadj_MFI (all fish) | Null | 392.8 | 171.1 |
| Family | 253.4 | 31.7 | |
| Family + sex | 255.3 | 33.6 | |
| Family + host-pop | 221.7 | — | |
| Family + exposure | 254.8 | 33.1 | |
| Family + infection | 254.9 | 33.2 | |
| Family + parasite load | 254.2 | 32.5 | |
| Family + sex × host-pop | 225.6 | 3.9 | |
| Family + sex × exposure | 258.5 | 36.8 | |
| Family + sex × infection | 255.9 | 34.2 | |
| Family + host-pop × exposure | 225.1 | 3.4 | |
| Family + host-pop × infection | 224.5 | 2.8 | |
| Family + host-pop × parasite load | 224.4 | 2.7 | |
| Family + host-pop + sex × infection | 224.1 | 2.4 | |
| Family + sex × host-pop × exposure | 232.7 | 11.0 | |
| Family + sex × host-pop × infection | 228.8 | 7.1 | |
| P_Gadj_MFI (exposed fish) | Null | 271.3 | 108.8 |
| Family | 193.7 | 31.2 | |
| Family + sex | 195 | 32.5 | |
| Family + host-pop | 162.5 | — | |
| Family + infection | 195.2 | 32.7 | |
| Family + parasite load | 193.9 | 31.4 | |
| Family + worm-pop | 197.2 | 34.7 | |
| Family + sex × infection | 195.1 | 32.6 | |
| Family + sex × host-pop | 166.1 | 3.6 | |
| Family + sex × worm-pop | 201.8 | 39.3 | |
| Family + host-pop × worm-pop | 163.4 | 0.9 | |
| Family + host-pop × infection | 164.4 | 1.9 | |
| Family + host-pop × parasite load | 164.6 | 2.1 | |
| Family + worm-pop × infection | 201.6 | 39.1 | |
| Family + host-pop × worm-pop × infection | 168.3 | 5.8 | |
| Family + host-pop × worm-pop × sex | 173.8 | 11.3 | |
| Family + host-pop × infection × sex | 168 | 5.5 | |
| Family + sex × infection × host-pop × worm-pop | 184.6 | 22.1 | |
| P_Gadj_MFI (infected fish) | Null | 48.8 | 31.7 |
| Family | 47.9 | 30.8 | |
| Sex | 50 | 32.9 | |
| Host-pop | 28.3 | 11.2 | |
| Parasite load | 50.5 | 33.4 | |
| Worm-pop | 47.1 | 30.0 | |
| Host-pop + worm-pop | 27.8 | 10.7 | |
| Sex × host-pop | 28.5 | 11.4 | |
| Sex × worm-pop | 48.9 | 31.8 | |
| Host-pop × worm-pop | 17.1 | — | |
| Sex × host-pop × worm-pop | 19.1 | 2.0 | |
| BG_Gadj_MFI (all fish) | Null | 118.9 | 89.0 |
| Family | 42.2 | 12.3 | |
| Family + sex | 34.8 | 4.9 | |
| Family + host-pop | 41.1 | 11.2 | |
| Family + exposure | 36.3 | 6.4 | |
| Family + infection | 40.9 | 11.0 | |
| Family + parasite load | 41.4 | 11.5 | |
| Family + sex + exposure | 29.9 | — | |
| Family + sex + exposure + host-pop | 29.1 | −0.8 | |
| Family + sex + exposure + infection | 29.7 | −0.2 | |
| Family + sex × host-pop | 32.8 | 2.9 | |
| Family + sex × exposure | 30.6 | 0.7 | |
| Family + sex × infection | 34.5 | 4.6 | |
| Family + host-pop × exposure | 36.9 | 7.0 | |
| Family + host-pop × infection | 41.6 | 11.7 | |
| Family + sex × host-pop × exposure | 31.7 | 1.8 | |
| Family + sex × host-pop × infection | 36.3 | 6.4 | |
| BG_Gadj_MFI (exposed fish) | Null | 65.6 | 72.1 |
| Family | 4.2 | 10.7 | |
| Family + sex | 2.6 | 9.1 | |
| Family + host-pop | 4 | 10.5 | |
| Family + infection | 4.8 | 11.3 | |
| Family + parasite load | 5.4 | 11.9 | |
| Family + worm-pop | −0.8 | 5.7 | |
| Family + sex + worm-pop | −4 | 2.5 | |
| Family + sex × infection | 4.3 | 10.8 | |
| Family + sex × host-pop | −0.6 | 5.9 | |
| Family + sex × worm-pop | −2.2 | 4.3 | |
| Family + host-pop × worm-pop | 1 | 7.5 | |
| Family + host-pop × infection | 4.5 | 11.0 | |
| Family + worm-pop × infection | 3.8 | 10.3 | |
| Family + host-pop × worm-pop × infection | 8.9 | 15.4 | |
| Family + sex × host-pop × worm-pop | −6.5 | — | |
| Family + sex × worm-pop × infection | 7.2 | 13.7 | |
| Family + sex × host-pop × infection | 2.1 | 8.6 | |
| Family + sex × host-pop × worm-pop × infection | 5 | 11.5 | |
| BG_Gadj_MFI (infected fish) | Null | 0.9 | 9.4 |
| Family | −4.5 | 4.0 | |
| Sex | −2.9 | 5.6 | |
| Host-pop | −8 | 0.5 | |
| Parasite load | −3.8 | 4.7 | |
| Worm-pop | −8.5 | — | |
| Host-pop + worm-pop | −10 | −1.5 | |
| Sex × host-pop | −4.7 | 3.8 | |
| Sex × worm-pop | −3.3 | 5.2 | |
| Host-pop × worm-pop | −10 | −1.5 | |
| Sex × host-pop × worm-pop | −3 | 5.5 | |
| Δ_MFI unstimulated vs. PMA-stimulated (all fish) | Null | 810.4 | 10.7 |
| Family | 803.2 | 3.5 | |
| Family + sex | 802.6 | 2.9 | |
| Family + host-pop | 799.7 | — | |
| Family + exposure | 804.6 | 4.9 | |
| Family + infection | 805.2 | 5.5 | |
| Family + parasite load | 805.1 | 5.4 | |
| Family + sex × host-pop | 799.7 | 0.0 | |
| Family + sex × exposure | 806.2 | 6.5 | |
| Family + sex × infection | 806.6 | 6.9 | |
| Family + host-pop × exposure | 803 | 3.3 | |
| Family + host-pop × infection | 803.1 | 3.4 | |
| Family + host-pop × parasite load | 803.2 | 3.5 | |
| Family + sex × host-pop × exposure | 807.3 | 7.6 | |
| Family + sex × host-pop × infection | 806.2 | 6.5 | |
| Δ_MFI unstimulated vs. PMA-stimulated (exposed fish) | Null | 532.8 | 12.5 |
| Family | 523.7 | 3.4 | |
| Family + sex | 522.8 | 2.5 | |
| Family + host-pop | 520.3 | — | |
| Family + infection | 525.7 | 5.4 | |
| Family + parasite load | 525.6 | 5.3 | |
| Family + worm-pop | 526 | 5.7 | |
| Family + sex + host-pop | 519.9 | −0.4 | |
| Family + sex × infection | 526.8 | 6.5 | |
| Family + sex × host-pop | 520.5 | 0.2 | |
| Family + sex × worm-pop | 524.4 | 4.1 | |
| Family + host-pop × worm-pop | 526.6 | 6.3 | |
| Family + host-pop × infection | 523.8 | 3.5 | |
| Family + host-pop × parasite load | 524.1 | 3.8 | |
| Family + worm-pop × infection | 531 | 10.7 | |
| Family + host-pop × worm-pop × infection | 536.2 | 15.9 | |
| Family + sex × host-pop × worm-pop | 525.6 | 5.3 | |
| Family + sex × worm-pop × infection | 535.3 | 15.0 | |
| Family + sex × host-pop × infection | 527.1 | 6.8 | |
| Family + sex × host-pop × worm-pop × infection | 540.8 | 20.5 | |
| Δ_MFI unstimulated vs. PMA-stimulated (infected fish) | Null | 59.8 | 27.3 |
| Family | 59.6 | 27.1 | |
| Sex | 60.9 | 28.4 | |
| Host-pop | 41.7 | 9.2 | |
| Parasite load | 61.6 | 28.1 | |
| Worm-pop | 60.1 | 27.6 | |
| Sex × host-pop | 41.9 | 9.4 | |
| Sex × worm-pop | 61.9 | 29.4 | |
| Host-pop × worm-pop | 32.5 | — | |
| Host-pop × parasite load | 43.7 | 11.2 | |
| Sex × host-pop × worm-pop | 35.4 | 2.9 | |
| log(Avg cestode mass) (infected fish) | Null | 116.2 | 32.7 |
| Family | 100.3 | 16.8 | |
| Family + sex | 102.1 | 18.6 | |
| Family + host-pop | 86.8 | 3.3 | |
| Family + worm-pop | 104.1 | 20.6 | |
| Family + parasite load | 98.1 | 14.6 | |
| Family + host mass | 96.9 | 13.4 | |
| Family + host length | 102.1 | 18.6 | |
| Family + host condition | 98.4 | 14.9 | |
| Family + host-pop + host mass | 83.5 | — | |
| Family + host-pop + parasite load | 85.2 | 1.7 | |
| Family + sex × host-pop | 90.6 | 7.1 | |
| Family + sex × worm-pop | 104.7 | 21.2 | |
| Family + host-pop × parasite load | 87.0 | 3.5 | |
| Family + host-pop × worm-pop | 91.3 | 7.8 | |
| Family + host-pop × host mass | 83.7 | 0.2 | |
| Family + sex × host-pop × worm-pop | 95.2 | 11.7 |
Bold italic text indicates the best-fit model (lowest AIC score). ΔAIC scores are in relation to the best-fit model.
Fig. S3.
The percentage of granulocytes in HK cell culture as a function of fish genotype (GOS, reciprocal F1s, and ROB) and experimental infection status. Here, we distinguish between three infection states: unexposed control fish that did not encounter S. solidus (fed a copepod only), fish that were exposed but did not have a detectable infection 42 d later, and fish that were infected 42 d postexposure. We observe no significant difference between unexposed and exposed-but-uninfected fish, so for the figures and results in the main text we combined these groups into a single uninfected category.
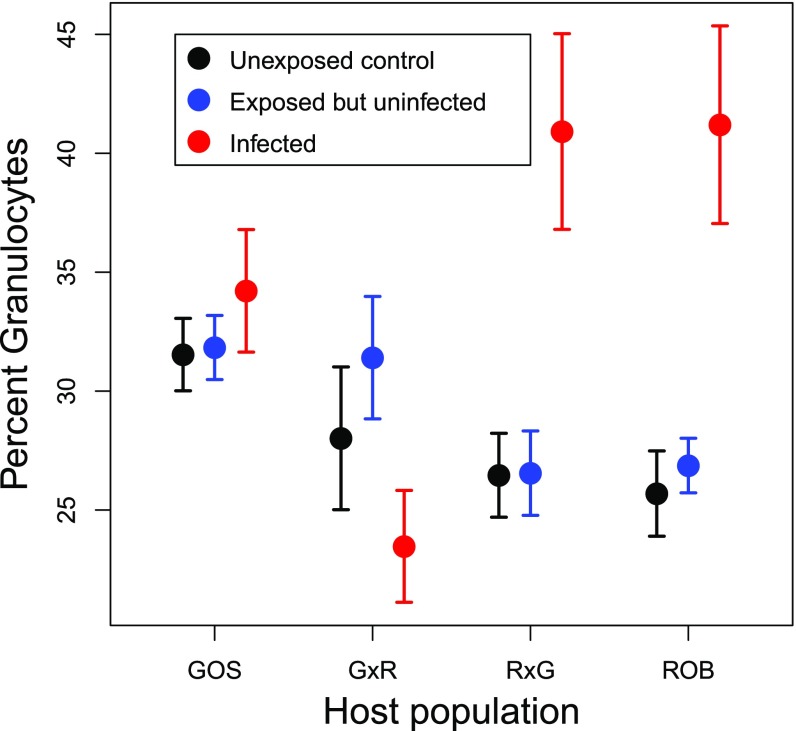
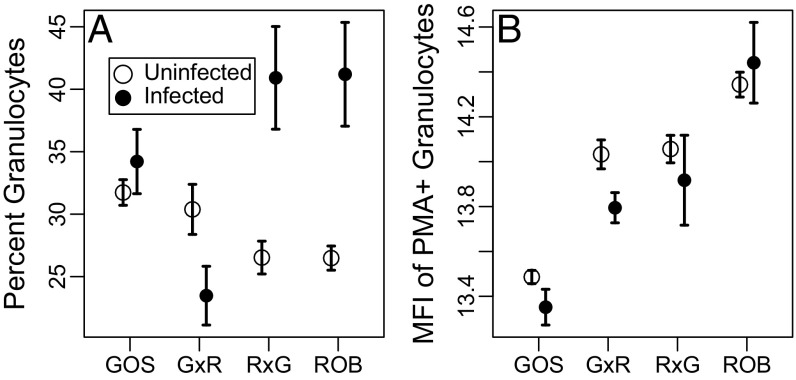
After accounting for family effects and averaging over sex, granulocytes constituted a lower proportion of HK cells in ROB fish (5.4% less than in GOS fish; t = 2.06, P = 0.05). Despite this lower initial frequency, ROB fish responded to S. solidus infection by increasing granulocyte proportions (16.3% higher than uninfected ROB fish; t = 5.13, P < 0.0001) (Fig. 2A). Infection had no effect on GOS fish, resulting in an interaction between fish population and infection status (Table S2). An interaction also occurred in F1 hybrids: Infection did not affect granulocyte frequency in G×R hybrids, but granulocytes increased 11.4% in infected R×G fish (t = 2.17, P = 0.03) (Fig. 2A). This asymmetry in response to infection by reciprocal F1 hybrids strongly suggests that a maternal effect in ROB stickleback drives the observed phenotype.
Fig. 2.
Variation in stickleback immunity, as measured by (A) the percentage of granulocytes in HK primary cell culture and (B) the MFI of PMA-stimulated granulocytes. Error bars indicate one SE interval above and below the mean. Sample sizes of uninfected and infected fish in each population: GOS, 95 and 16; G×R, 43 and 10; R×G, 57 and 7; ROB, 105 and 9, respectively.
The production of ROS was quantified by measuring the median fluorescence intensity (MFI) of HK cells stained with Dihydrorhodamine-123 (DHR). DHR is a cell-permeable fluorophore that fluoresces in the presence of peroxide and peroxynitrite (two forms of ROS). For each fish we measured (i) baseline ROS production in unstimulated granulocytes and (ii) ROS production of cells exposed to Phorbol 12-myristate 13-acetate (PMA), which is a potent granulocyte stimulant. Here, we focus on the second metric (Fig. S4 for baseline ROS). Similar to granulocyte frequency, ROS production varied among sibships (random family effect, Table S2). Neither fish sex nor cestode genotype had detectable effects on granulocyte ROS production. After accounting for family differences, PMA-stimulated ROB cells generated 2.4-fold greater MFI than GOS cells (post hoc pairwise Tukey tests on ln-transformed MFI, t = 7.89, P < 0.0001) (Fig. 2B). ROS production by F1 hybrids’ granulocytes was intermediate between ROB and GOS stickleback (Fig. 2B). G×R and R×G cells generated equivalent ROS levels (P = 0.988), less ROS than ROB fish (P = 0.032 and 0.061, respectively), and more ROS than GOS fish (both P ≤ 0.0034). This trait did not differ between infected and uninfected (control or experimental) stickleback, nor was there an interaction between fish population and infection status (Table S2). Because ROS was unaffected by infection status, we infer that ROS production differs constitutively between GOS and ROB fish. Overall, we conclude that GOS and ROB stickleback display heritable differences in immune responses. Some differences (i.e., ROS levels) are constitutive and are consistent with genetic effects, whereas others (i.e., the percentage of granulocytes) are induced by infection and could be caused by either environmental or genetic mechanisms.
Fig. S4.
The effects of S. solidus exposure or infection and host genotype on ROS production as measured by the MFI of granulocytes in HK primary cell culture. As in Fig. S3, we distinguish between unexposed control, exposed but uninfected, and infected fish. We measure three ROS variables here: (A) background MFI in resting-state granulocytes (PMA−); (B) MFI following PMA stimulation; and (C) the difference between stimulated versus background MFI (ΔMFI). We observe no significant effect of S. solidus infection status, but there is a systematic trend for ROB genotypes to produce more ROS. Higher ROS in ROB fish is observed both for baseline ROS (A) and a stronger induced response to PMA stimulation of cell culture granulocytes (B and C). Reciprocal F1 hybrids are intermediate between the parental types for both baseline and PMA-stimulated MFI, suggesting an additive genetic basis of this between-population difference. Baseline MFI varied significantly among families within populations.
Recently Evolved Differences in Resistance.
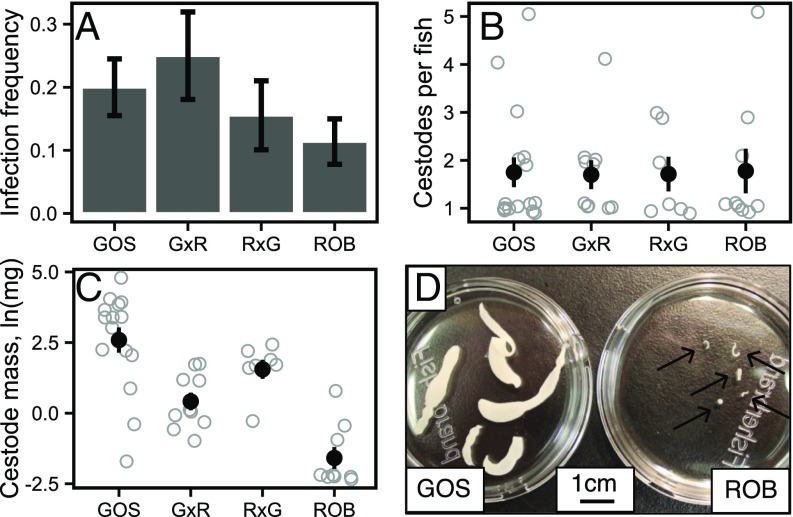
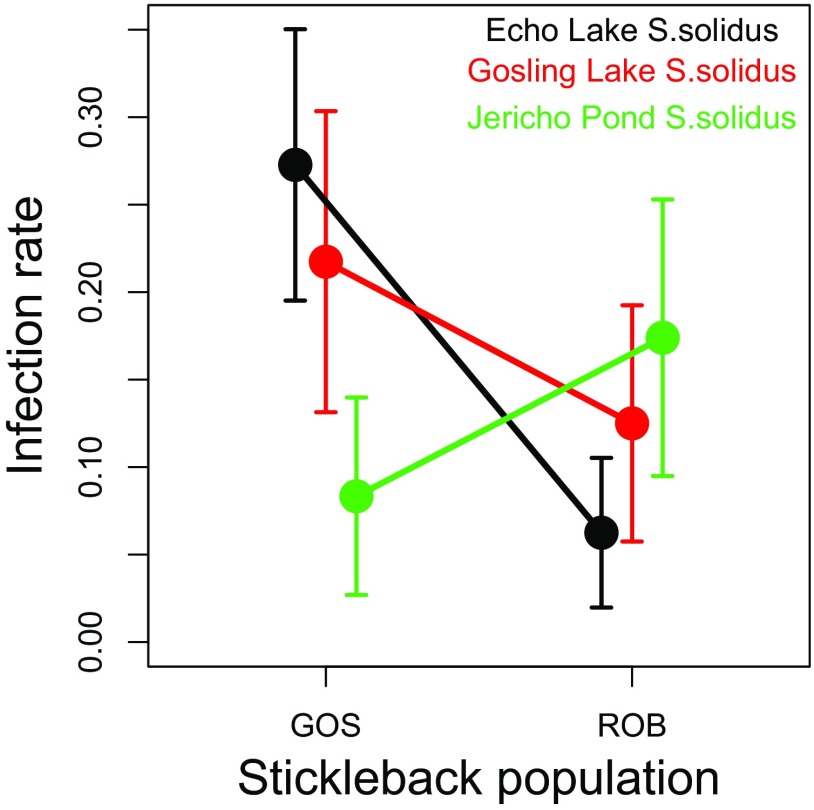
GOS and ROB stickleback did not differ significantly in the frequency or intensity of laboratory infections (Fig. 3 A and B). Infection rates in GOS and ROB fish were 20.0 and 11.4% (with SEs of 4.5 and 3.6%), respectively (binomial GLM, Z = −1.47, P = 0.14). Including random effects of host family did not improve model fit [Akaike information criterion (AIC) = 142.3 and 140.4 with and without family, respectively]. Although parasite genotype did not affect the infection success rate significantly (P = 0.81), there was a marginal interaction between host and parasite genotype (P = 0.09) (Fig. S5). Infected GOS and ROB fish carried equivalent numbers of cestodes (1.75 versus 1.78 out of five cestodes provided per fish; binomial GLM, Z = 0.06, P = 0.95). We conclude that the absence of S. solidus from wild ROB fish is not attributable to any greater resistance to colonization by S. solidus. Fish from both populations raised and infected in a common-garden laboratory setting have higher resistance than laboratory-raised marine fish (the putative ancestral form), indicating that resistance is heritable and recently evolved. This conclusion implies that the separately colonized lake populations independently evolved quantitatively similar resistance to S. solidus establishment, relative to their highly susceptible marine ancestors (18). Thus we provide a rarely documented instance of convergently evolved helminth immunity in a wild vertebrate.
Fig. 3.
Infection success as measured by (A) infection frequency (the proportion of stickleback with S. solidus), (B) mean abundance of S. solidus per fish, and (C) log-transformed cestode mass (averaged across all cestodes in each fish). Error bars indicate SEs. (D) Full-sibling cestodes removed from GOS and ROB stickleback 42 d postexposure. Fig. S7 presents cestode masses from marine genotypes and F1 crosses between marine fish and GOS or ROB fish. Number of experimental fish per population in A: GOS, 80; G×R, 40; R×G, 45; ROB, 79. The number of infected fish per population is noted in Fig. 2.
Fig. S5.
The effects of host and parasite genotypes on infection success rates. We observed a marginally nonsignificant interaction (P = 0.09) between host and parasite genotypes on the infection rates among experimentally exposed hosts. Here, we plot the infection rate as a function of host genotype (GOS or ROB) and cestode population. The geographically more distant cestodes, from Jericho Pond on the University of British Columbia campus, trended toward higher infection success in the otherwise more resistant ROB fish, whereas both local cestode populations had somewhat higher success rate in GOS fish. Although no single contrast is significant, the different slopes for Jericho versus Echo or Gosling cestodes generate the marginally nonsignificant interaction.
Although infection rate and intensity were equivalent, age-matched cestodes grew dramatically larger in laboratory-raised GOS than in ROB stickleback (post hoc test, t = 5.79, P < 0.0001) (Fig. 3C). After controlling for effects of fish family and host mass on cestode size (parasite mass increased by ∼0.2 mg for every 0.1-g increase in host mass; t = 2.81, P = 0.007), parasites from GOS fish were on average 34-fold larger than those from ROB fish. The mean mass of each S. solidus removed from the high-infection population (GOS) was 10.2 mg, whereas cestodes from ROB fish averaged 0.3 mg. There was no effect of cestode origin (Table S2). After controlling for host mass, cestodes grew to intermediate size in G×R and R×G hybrids (means of 2.1 mg and 3.8 mg, respectively). Although S. solidus sizes did not differ between the reciprocal hybrids (P = 0.88), these cestodes did differ from those removed from the pure host populations. Cestodes from GOS fish were 5.3-fold larger than those from G×R hybrids (P = 0.11) and were 2.9-fold larger than those from R×G hybrids (P = 0.53). Conversely, cestodes from R×G hybrids were 12.7-fold larger (P = 0.006) and those from G×R hybrids were sevenfold larger (P = 0.02) than those from pure ROB fish. Cestode mass was independent of host length, condition (mass/total length), sex, and infection intensity (Table S2). Note, however, that the influence of host size may change in older cestodes that are larger and more space-limited (30, 31). These results demonstrate there are heritable and thus evolved differences between the ROB and GOS stickleback, given differences in laboratory-raised sticklebacks’ ability to suppress cestode growth. The intermediate size of parasites in hybrid fish also strongly suggests an additive genetic basis for this evolutionary difference.
Because most S. solidus growth occurs in stickleback, egg production is correlated with the adult parasite’s dry weight, and because S. solidus must reach a minimum size (∼10–50 mg) to establish infections in its terminal host (32, 33), growth suppression effectively constrains the parasite’s reproductive potential. Arguably, this measure of parasite success represents an extended phenotype (34) of the stickleback, in the sense that the host’s genotype alters the parasite’s phenotype.
To determine whether reduced parasite growth is an ancestral or derived trait, we measured S. solidus growth in susceptible marine fish. These cestodes grew quickly, reaching up to 320 mg in 2.5 mo (mean = 130 mg) (Fig. S6). This size is 4.5-fold larger than the mean size of S. solidus in GOS fish, possibly because the cestodes from marine fish were older (75–83 d for marine, 41–51 d for GOS). We then compared cestodes from pure marine fish with cestodes from marine × freshwater F1 hybrids (ROB×M and M×GOS, all assayed 2.5 mo postexposure). Consistent with the results reported above, cestodes from M×GOS fish (n = 2) were 77.7-fold heavier than cestodes from the ROB×M fish (n = 3; t test, P = 0.001; Wilcoxon signed-rank test, P = 0.2). Although this sample is small, the effect size is massive and is quantitatively consistent with the fold-difference between cestodes from pure GOS and ROB fish. These data demonstrate that the growth-suppressive phenotype observed in ROB fish is an evolutionary gain of immune function from a susceptible and growth-permissive ancestral marine population, which has evolved rapidly during the ∼12,000 y since Roberts Lake was likely colonized by marine fish. The magnitude of cestode growth suppression by ROB fish is also extreme compared with previous stickleback–S. solidus studies. Kalbe et al. (21) found that S. solidus from Germany grew to a mean mass of 144 mg in local stickleback from a brackish habitat (infection duration of 84 d). Notably, this parasite mass is very similar to the mass we observed in marine fish over the same infection duration. In contrast, the same German parasites only grew to ∼50 mg in a population of Norwegian freshwater stickleback (21). Our study differed from the last experiment in several respects, making it difficult to compare results precisely. However, quadrupling the mean mass of ROB parasites (i.e., to 1.2 mg) should adequately account for differences in infection duration between the two studies (especially considering that ROB fish were approximately twofold larger than the Norwegian fish). These results indicate that the magnitude of cestode growth suppression by ROB stickleback is more than 40-fold greater than previously reported.
Fig. S6.
Differences in ln-transformed cestode mass between laboratory-raised Sayward Estuary marine stickleback (black points) and cestodes from F1 hybrids resulting from crosses of marine × GOS fish (M×G; dark blue) or marine × ROB fish (M×R; dark red). These parasites were measured after a longer infection period (75–83 d postinfection) than those in Fig. 3. Each data point represents an individual cestode, several of which come from an individual fish (e.g., the lower cluster of black points are 10 cestodes from a single host and are correspondingly smaller than their less-crowded siblings). The squares represent genotype average cestode mass with 95% CI bars.
Growth Suppression in Wild Populations.
We hypothesize that the extreme growth suppression observed in laboratory-raised ROB fish may explain the absence of observed infections in wild ROB fish. To test this hypothesis, we predicted that stickleback populations that suppress S. solidus growth more effectively would have correspondingly fewer observable infections. As expected, we found a positive correlation across populations between the mean size of S. solidus and the parasite’s abundance. Focusing first on variation among fish within populations, we confirmed a well-established crowding effect (30, 31). Controlling for a random effect of lake, individual stickleback with more S. solidus have correspondingly smaller S. solidus (mixed-model linear regression P < 0.0001, controlling for host mass) (Fig. 4A). However, we observed a counter gradient trend across populations: Lakes with higher prevalence of S. solidus on average had larger cestodes (P = 0.0058) (Fig. 4B). GOS and ROB stickleback sit at opposite ends of this continuum, ROB growth suppression being so extreme that we could not locate infections in wild fish to measure cestode mass. This counter gradient trend lends additional support to our inference that stickleback populations differ in their ability to suppress parasite growth and that growth suppression is a repeatedly evolved trait that reduces S. solidus prevalence. An important caveat, however, is that, because these are natural rather than controlled infections, the age of each parasite is a potentially important but unknown covariate.
Fig. 4.
Cestode mass is correlated with infection abundance in the wild, but the effect direction is reversed when comparing individual fish within each of many populations (A) versus averages across populations (B). In A, points represent individuals within each of many populations. Within-population trend lines are shown in black, and the consensus effect is shown in blue (from a mixed-model GLM). In B, we plot population mean cestode abundance versus mean cestode mass with lakes as the level of replication.
Discussion
Stickleback are well known for their parallel evolution of adaptations to freshwater habitats (35, 36). These transitions include repeated independent evolution of increased resistance to freshwater-specialized parasites (18). However, as shown here, this immune evolution is not entirely parallel: ROB and GOS stickleback live in ecologically similar lakes, but, unlike GOS stickleback, ROB stickleback evolved an ability to suppress S. solidus growth by two orders of magnitude. We infer that growth suppression by ROB fish explains the apparent absence of S. solidus in wild-caught fish from Roberts Lake. In laboratory trials, these fish are just as susceptible to infection as GOS fish. However, growth-suppressed cestodes can be so small that extant infections in wild-caught fish could easily be overlooked. Our survey of wild-caught fish suggests that growth suppression may be a widespread phenomenon; lakes with smaller S. solidus tended to also have lower parasite abundance.
This work identifies a number of heritable differences between stickleback populations related to parasite resistance and immunity, despite parallel evolution of reduced infection rates relative to marine ancestors. However, why the lake populations evolved such divergent phenotypes is unknown. Evolutionary theory suggests that selection should optimize the intensity of immune responses to balance marginal costs and benefits (37), which depend on ecological variables such as parasite exposure rates, nutritional state, and predation risk, as well as behavioral differences among populations of stickleback (38–41). The populations studied here consume copepods (the first host of S. solidus) at roughly comparable rates (23) and thus should have similar exposure risks. However, these populations’ diets differ in other respects. Future ecological measurements (e.g., natural rates of copepod infections and exposure rates) and experiments (e.g., transplants and perturbation of immune traits) are necessary to address this issue.
An important caveat is that we exposed stickleback to a one-time dose of five tapeworms. This exposure is probably lower than natural exposure rates in some populations, which may be drawn out over an extended period (we observed some wild fish infected with >20 S. solidus). Nevertheless, we believe our results are ecologically relevant. We found large tapeworms not only in laboratory-reared GOS fish with low parasite exposures and loads but also in naturally caught GOS fish with high parasite loads. Fast growth of cestodes in GOS fish means that wild individuals will tend to harbor at least some large and successful cestodes, regardless of what happens to later exposures. How these fast-growing cestodes impact later arrivals through direct competition for host resources or through indirect effects via host immune suppression or activation is an interesting question. Negligible growth of cestodes in our laboratory-raised ROB fish suggests that infections will typically fail (or go undetected). Even if wild ROB fish experience high exposure rates, the absence of detectable cestodes indicates that this exposure does not influence parasite size or establishment. Finally, although parasite load was not a significant predictor in any of our statistical models, high rates or multiple bouts of exposure may lead to more complex immune dynamics than those reported here.
Previous laboratory-based infection experiments found differences in tapeworm size among stickleback populations (21, 22). However, the extreme magnitude of growth suppression (>40-fold stronger than previous reports), ancestral context for divergence, rapid evolution, and tremendous natural variation in parasite size that we report here considerably extend the implications of this extended phenotype. Several lines of evidence suggest that growth suppression may be common in other vertebrates as well. There is pervasive heritability for resistance-related traits in ruminant livestock (42). Most livestock studies measure helminth burden and resistance using noninvasive fecal egg counts (FECs). However, helminth length has very high heritability among hosts and likely contributes to variation in FEC (43, 44). Even humans harbor heritable variation in helminth resistance and size (45). Notably, a study on hookworm weight in Papua New Guinea villagers suggests that humans vary in their ability to regulate parasite size (46).
Bishop elegantly defined resistance as a host’s ability “to exert control over the parasite life cycle,” even in persistent infections (e.g., heritable variation for lower FEC) (47, 48). Using this definition, stickleback control of cestode growth represents an effective form of resistance. In populations such as GOS that lack growth suppression, individual cestodes can grow to be up to 72% of the parasite-free mass of their host. Such large parasites can impose severe fitness costs by limiting a host’s ability to forage, grow, disperse, evade predators, and breed (19, 20, 49). To the extent that these effects depend on parasite size, growth suppression may partially or wholly rescue the fitness of ROB fish by facilitating survival or reproduction despite infection.
Although tapeworm growth suppression could also be viewed as a form of tolerance, this interpretation conflicts with both theoretical expectations and our natural data. Tolerance is expected to increase infection prevalence within populations and to alleviate antagonistic host–parasite interactions (50). However, we find that infection prevalence is lowest in populations with small parasites. Moreover, size reduction is almost certainly costly to the parasite and is likely to have ancillary effects on parasite epidemiology. Smaller cestodes should be less fecund (33) and also may be less effective at manipulating host behavior in ways that may facilitate transfer to the terminal bird host (51, 52). Last, suppressing cestode growth may help the host clear the parasite infection. We occasionally observed small S. solidus encased in cysts (Fig. S7) within ROB (but not GOS) hosts. Some of these cysts contained degraded cestodes. We infer that growth suppression may aid in cyst formation (smaller parasites being easier to envelop), which in turn facilitates cestode killing.
Fig. S7.

An S. solidus cestode encased in a cyst (white structures within the green box), within the body cavity of a stickleback (head to left of image). Some cysts found in experimentally infected stickleback contained dead S. solidus, suggesting that small cestodes trapped in a cyst could be eliminated.
We also found heritable between-population differences in constitutive and infection-induced immune traits (granulocyte ROS production and granulocyte frequency, respectively). Both traits have been linked to macroparasite killing by vertebrates (9, 24–28). However, we were unable to find reports of their influence on macroparasite growth, and our study is not designed to test this association formally. The infection-associated increase in HK granulocytes in ROB and R×G fish is consistent with the eosinophilia observed with helminthiasis in other vertebrate species (53). However, two observations suggest this increase is insufficient to explain variation in cestode growth. First, R×G hybrids had larger cestodes than ROB fish despite exhibiting comparable granulocyte abundance. Second, R×G hybrids had slightly larger parasites than G×R hybrids, despite the former’s stronger granulocyte response. Although granulocyte ROS levels negatively correlated with cestode size (Figs. 2B and 3C), this trend is confounded with overall population effects and does not hold among families within populations. The interactions between granulocyte-generated ROS and cestode growth warrant further investigation.
Although the proximate cause of cestode growth suppression remains unknown, our results offer several insights into stickleback immune evolution. First, even though GOS and ROB fish evolved to resist initial establishment by S. solidus equally, other immune traits exhibited nonparallel evolution. Relatively little is known about the extent of parallel (or nonparallel) evolution of immune phenotypes across evolutionary replicate populations, in contrast to more easily measured morphological traits. We suggest that parallel and nonparallel changes in immunity will prove to be useful in studying the context dependency of immune evolution and redundancy in immune function.
Second, we found that ROS production (both pre- and post-PMA stimulation) was largely unresponsive to S. solidus infection. This result is contrary to several previous studies, which are themselves conflicting. One study suggested that ROS response increases in early-stage S. solidus infections but is eventually suppressed (27), and another suggested that granulocyte proportions fluctuate throughout infection but ROS levels increase in only late-stage infection (54). These different results may be a function of the particular populations studied (here Canadian populations versus the European stickleback and parasites used in prior studies) or of the variation in postexposure time points of immune assays. Given the differences in granulocyte response between geographically nearby lakes (Gosling and Roberts Lakes), it is quite plausible that host–parasite interactions on different continents may follow very different kinetics. Differences in ROS measurement and in vitro assays may also play a role. For example, infection-associated ROS differences may be hard to detect if only a small subset of cells contacts or indirectly responds to parasite presence. This possibility could be addressed by experimentally exposing cell cultures to parasite antigens before assaying ROS (55).
Finally, the maternally inherited increase in relative granulocyte abundance is an intriguing response that warrants additional attention. ROB but not GOS fish initiated a strong shift toward granulocytes following S. solidus infection, and F1 hybrids matched the response of their maternal parent. A recent stickleback study also found that establishment of S. solidus seems to depend on an interaction between maternal effect and parasite origin (21). However, we found no significant maternal effects on cestode success in our study. Many immunological maternal effects in birds, fish, and mammals are related to antibody transmission between mother and offspring (56). These antibody-based effects tend to be relatively short-lived. Because fish in the present study were not exposed to S. solidus until adulthood, it is possible that a different mechanism may be involved.
Debate continues about the heritability of human immune responses (57, 58), but our results show that genetic variation for immunity segregates both within and among wild populations and that immune differences influence parasite infection. Identifying the underlying mechanisms of variation in natural infection will provide important contributions to this debate. Natural selection in disparate wild populations provides a powerful genetic scan to locate adaptive genetic variation involved in parasite resistance and immune function (59).
Materials and Methods
Estimating Natural Infection Prevalence.
We used unbaited minnow traps to sample threespine stickleback (Scientific Fish Collection permit NA12-77018 and NA12-84188) (Table S1). The majority of sites were sampled once in 2009 or in 2013, but some were sampled repeatedly over multiple years (Table S1). All animals were killed by immersion in MS-222, fixed in 10% neutral-buffered formalin, and then dissected to evaluate the presence of S. solidus.
Breeding Wild-Caught Stickleback.
We generated crosses between wild-caught anadromous marine stickleback from Sayward Estuary to generate pure marine families (M genotype). We also generated one M×R cross and one G×M cross. All crosses were performed in June 2012, and fertilized eggs were sent to The University of Texas at Austin (Transfer License NA12-76852), where all animals were reared in freshwater conditions and housed with full-siblings (9–39 animals per family) in either 40-L or 10-L tanks. This experiment was approved by The University of Texas Institutional Animal Care and Use Committee as part of AUP-2010-00024.
S. solidus Collection and Experimental Exposures.
S. solidus were harvested from stickleback captured at three sites in British Columbia, Canada (Gosling Lake, Echo Lake, and Jericho Pond). The parasites were bred to obtain eggs from full-sibling families, using medium and methods described in refs. 18 and 60. Juvenile copepods (Macrocyclops albidus) were exposed to one or two freshly hatched tapeworm coracidia in 24-well plates. Two weeks after exposure we screened copepods to identify singly and doubly infected individuals, which were fed to fish between 21–26 d postexposure [when S. solidus coracidia are developmentally capable of infecting stickleback (61)]. Similarly aged uninfected copepods were used for control exposures.
Stickleback exposures began by isolating five to seven fish per family and clipping either the first or second dorsal spine to distinguish control (one or two fish per family) from experimental animals. At least 12 d after clipping, we moved individual fish into 1-L containers and withheld food for 24 h. Experimental and control fish were fed four or five infected or uninfected copepods, respectively. The following day, after confirming that all copepods were consumed, we combined control and experimental animals from each family into a single tank. We used one tank per family. At 41–51 d postexposure (41–44 d for all but two families), we dissected fish, recorded fish metrics (length, mass, sex), noted the presence or absence of tapeworm infection, and performed immune assays. All fish exposed to S. solidus were between 15 and 23 mo of age.
Measurement of Immune Traits via Flow Cytometry.
We dissected HKs from control and experimental fish and immediately placed organs in cold HK medium [0.9× Roswell Park Memorial Institute (RPMI) medium containing 10% FCS, 100 μM nonessential amino acids (NEAA), 100 U/mL penicillin, 100 μg/mL streptomycin, and 55 μM β-mercaptoethanol]. HK were manually disrupted and filtered by grinding against a cell strainer (35-μm mesh; Falcon 352235) and rinsing with 4 mL of cold HK medium. Cell suspensions were centrifuged at 300 × g, 4 °C for 10 min; then the supernatant was removed, and the cell pellet was resuspended in the remaining medium (∼200 μL). Live HK cells were counted using a hemacytometer (Hausser Scientific 3520) based on Trypan blue exclusion (Corning 25-900-CI). For each fish, HK cells were divided into three treatment groups: (i) medium-only control, (ii) DHR-123–stained, and (iii) DHR-123–stained and PMA-stimulated.
For each treatment group 2 × 105 HK cells were plated in 200 μL of HK medium (group 1) or HK medium containing DHR-123 (2 μg/mL) (Sigma D1054) (groups 2 and 3). All samples were incubated in a 96-well plate at 18 °C, 3% CO2 for 10 min. Next, PMA (130 ng/mL; Sigma P8139) was added to group 3, and groups 1 and 2 received equivalent volumes of plain HK medium. After cells were incubated for an additional 20 min at 18 °C, 3% CO2, all samples were run on an Accuri C6 Sampler and analyzed using FlowJo (TreeStar). Granulocyte:lymphocyte ratios were calculated from the medium-only controls (Fig. S8). The magnitude of ROS production was determined by comparing the median fluorescence of unstimulated and PMA-stimulated cells. Fig. S8 contains additional information on gating strategies.
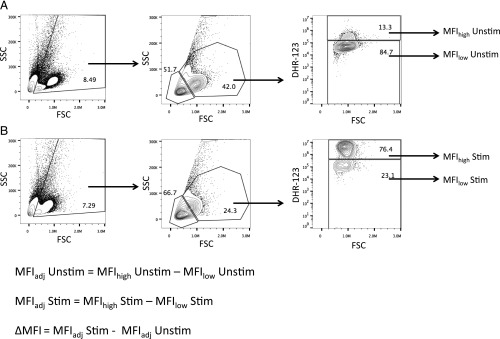
Fig. S8.
Calculation of MFI of DHR-123–stained HK granulocytes. The frequencies of HK leukocytes were determined via flow cytometry based on forward-scatter (FSC) and side-scatter (SSC) parameters. Granulocytes are FSChigh SSChigh, whereas lymphocytes are FSClow SSClow. The intensity of ROS production for each treatment group was determined based on the MFI of DHR-stained cells. To account for well-to-well and experiment-to-experiment variation in DHR staining intensity, we subtracted the background DHR staining intensity (MFIlow population) from that of the DHR+ cells (MFIhigh) to obtain the adjusted MFI (MFIadj) for each sample. We calculated the background MFI of resting, unstimulated (MFIadjUnstim) (A) and PMA-stimulated (MFIadjStim) (B) granulocytes. The total change in ROS intensity (ΔMFI) was calculated by subtracting MFIadjUnstim from MFIadjStim.
Statistical Analyses.
All statistical tests were performed in the R software environment (V3.3-1) (62). For models without random effects, we performed Tukey’s honestly significant difference (HSD)–corrected post hoc comparisons using the multcomp package (V1.4-5) (63). We used the lme4 package (V1.1-12) (64) to construct random effect models and used maximum likelihood to calculate AIC scores for each model (Table S1). We used the lsmeans package (V2.23-5) (65) to perform Tukey’s HSD-corrected post hoc comparisons on mixed models.
Acknowledgments
We thank L. Ma for assistance with laboratory work. Wenfei Tong produced the illustrations in Fig. S1. Douglas Emlen provided comments on earlier drafts. This research was funded by the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. E.J.P. is a guest editor invited by the Editorial Board.
Data deposition: Data reported in this paper are available on the Open Science Framework repository, https://osf.io/e53y4.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620095114/-/DCSupplemental.
References
- 1. World Health Organization (2017) Fact sheet: Soil-transmitted helminth infections. Available at www.who.int/mediacentre/factsheets/fs366/en/. Accessed May 23, 2017.
- 2.Charlier J, et al. Decision making on helminths in cattle: Diagnostics, economics and human behaviour. Ir Vet J. 2016;69:14. doi: 10.1186/s13620-016-0073-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry BD, Randolph TF. Improving the assessment of the economic impact of parasitic diseases and of their control in production animals. Vet Parasitol. 1999;84:145–168. doi: 10.1016/s0304-4017(99)00040-0. [DOI] [PubMed] [Google Scholar]
- 4.Thompson RC, Lymbery AJ, Smith A. Parasites, emerging disease and wildlife conservation. Int J Parasitol. 2010;40:1163–1170. doi: 10.1016/j.ijpara.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Fumagalli M, et al. The landscape of human genes involved in the immune response to parasitic worms. BMC Evol Biol. 2010;10:264. doi: 10.1186/1471-2148-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fumagalli M, et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206:1395–1408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayward AD, et al. Natural selection on individual variation in tolerance of gastrointestinal nematode infection. PLoS Biol. 2014;12:e1001917. doi: 10.1371/journal.pbio.1001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King KC, Lively CM. Does genetic diversity limit disease spread in natural host populations? Heredity (Edinb) 2012;109:199–203. doi: 10.1038/hdy.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashley NT, Weil ZM, Nelson RJ. Inflammation: Mechanisms, costs, and natural variation. Annu Rev Ecol Evol Syst. 2012;43:385–406. [Google Scholar]
- 10.Auld SK, et al. Variation in costs of parasite resistance among natural host populations. J Evol Biol. 2013;26:2479–2486. doi: 10.1111/jeb.12243. [DOI] [PubMed] [Google Scholar]
- 11.Sandland GJ, Minchella DJ. Costs of immune defense: An enigma wrapped in an environmental cloak? Trends Parasitol. 2003;19:571–574. doi: 10.1016/j.pt.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Sorci G, Faivre B. Inflammation and oxidative stress in vertebrate host-parasite systems. Philos Trans R Soc Lond B Biol Sci. 2009;364:71–83. doi: 10.1098/rstb.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maizels RM, et al. Helminth parasites–masters of regulation. Immunol Rev. 2004;201:89–116. doi: 10.1111/j.0105-2896.2004.00191.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson PTJ, de Roode JC, Fenton A. Why infectious disease research needs community ecology. Science. 2015;349:1259504. doi: 10.1126/science.1259504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Housden BE, et al. Loss-of-function genetic tools for animal models: Cross-species and cross-platform differences. Nat Rev Genet. 2017;18:24–40. doi: 10.1038/nrg.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appleby MW, Ramsdell F. A forward-genetic approach for analysis of the immune system. Nat Rev Immunol. 2003;3:463–471. doi: 10.1038/nri1109. [DOI] [PubMed] [Google Scholar]
- 17.Simmonds NE, Barber I. The effect of salinity on egg development and viability of Schistocephalus solidus (Cestoda: Diphyllobothriidea) J Parasitol. 2016;102:42–46. doi: 10.1645/14-701. [DOI] [PubMed] [Google Scholar]
- 18.Weber JN, et al. Resist globally, infect locally: A transcontinental test of adaptation by stickleback and their tapeworm parasite. Am Nat. 2017;189:43–57. doi: 10.1086/689597. [DOI] [PubMed] [Google Scholar]
- 19.Bagamian KH, Heins DC, Baker JA. Body condition and reproductive capacity of three-spined stickleback infected with the cestode Schistocephalus solidus. J Fish Biol. 2004;64:1568–1576. [Google Scholar]
- 20.Heins DC, Baker JA. Fecundity compensation and fecundity reduction among populations of the three-spined stickleback infected by Schistocephalus solidus in Alaska. Parasitology. 2014;141:1088–1096. doi: 10.1017/S0031182014000535. [DOI] [PubMed] [Google Scholar]
- 21.Kalbe M, Eizaguirre C, Scharsack JP, Jakobsen PJ. Reciprocal cross infection of sticklebacks with the diphyllobothriidean cestode Schistocephalus solidus reveals consistent population differences in parasite growth and host resistance. Parasit Vectors. 2016;9:130. doi: 10.1186/s13071-016-1419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurtz J, et al. Major histocompatibility complex diversity influences parasite resistance and innate immunity in sticklebacks. Proc Biol Sci. 2004;271:197–204. doi: 10.1098/rspb.2003.2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snowberg LK, Hendrix KM, Bolnick DI. Covarying variances: More morphologically variable populations also exhibit more diet variation. Oecologia. 2015;178:89–101. doi: 10.1007/s00442-014-3200-7. [DOI] [PubMed] [Google Scholar]
- 24.Allen JE, Maizels RM. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 25.Maizels RM, Hewitson JP, Smith KA. Susceptibility and immunity to helminth parasites. Curr Opin Immunol. 2012;24:459–466. doi: 10.1016/j.coi.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moreau E, Chauvin A. Immunity against helminths: Interactions with the host and the intercurrent infections. J Biomed Biotechnol. 2010;2010:428593. doi: 10.1155/2010/428593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharsack JP, Kalbe M, Derner R, Kurtz J, Milinski M. Modulation of granulocyte responses in three-spined sticklebacks Gasterosteus aculeatus infected with the tapeworm Schistocephalus solidus. Dis Aquat Organ. 2004;59:141–150. doi: 10.3354/dao059141. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Pellitero P. Fish immunity and parasite infections: From innate immunity to immunoprophylactic prospects. Vet Immunol Immunopathol. 2008;126:171–198. doi: 10.1016/j.vetimm.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Kaufmann J, Lenz TL, Milinski M, Eizaguirre C. Experimental parasite infection reveals costs and benefits of paternal effects. Ecol Lett. 2014;17:1409–1417. doi: 10.1111/ele.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heins DC, Baker JA, Martin HC. The “crowding effect” in the cestode Schistocephalus solidus: Density-dependent effects on plerocercoid size and infectivity. J Parasitol. 2002;88:302–307. doi: 10.1645/0022-3395(2002)088[0302:TCEITC]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Read CP. The “crowding effect” in tapeworm infections. J Parasitol. 1951;37:174–178. [PubMed] [Google Scholar]
- 32.Hopkins CA, McCAIG MLO. Studies on Schistocephalus solidus. I. The correlation of development in the plerocercoid with infectivity to the definitive host. Exp Parasitol. 1963;13:235–243. doi: 10.1016/0014-4894(63)90075-4. [DOI] [PubMed] [Google Scholar]
- 33.Tierney JF, Crompton DWT. Infectivity of plerocercoids of Schistocephalus solidus (Cestoda: Ligulidae) and fecundity of the adults in an experimental definitive host, Gallus gallus. J Parasitol. 1992;78:1049–1054. [PubMed] [Google Scholar]
- 34.Dawkins R. 1999. The Extended Phenotype: The Long Reach of the Gene (Oxford Univ Press, New York) Revised edition, p 313.
- 35.Colosimo PF, et al. Widespread parallel evolution in sticklebacks by repeated fixation of Ectodysplasin alleles. Science. 2005;307:1928–1933. doi: 10.1126/science.1107239. [DOI] [PubMed] [Google Scholar]
- 36.Jones FC, et al. Broad Institute Genome Sequencing Platform and Whole Genome Assembly Team The genomic basis of adaptive evolution in threespine sticklebacks. Nature. 2012;484:55–61. doi: 10.1038/nature10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viney ME, Riley EM, Buchanan KL. Optimal immune responses: Immunocompetence revisited. Trends Ecol Evol. 2005;20:665–669. doi: 10.1016/j.tree.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 38.Bell AM, Sih A. Exposure to predation generates personality in threespined sticklebacks (Gasterosteus aculeatus) Ecol Lett. 2007;10:828–834. doi: 10.1111/j.1461-0248.2007.01081.x. [DOI] [PubMed] [Google Scholar]
- 39.Ingram T, et al. Intraguild predation drives evolutionary niche shift in threespine stickleback. Evolution. 2012;66:1819–1832. doi: 10.1111/j.1558-5646.2011.01545.x. [DOI] [PubMed] [Google Scholar]
- 40.Jolles JW, Manica A, Boogert NJ. Food intake rates of inactive fish are positively linked to boldness in three-spined sticklebacks Gasterosteus aculeatus. J Fish Biol. 2016;88:1661–1668. doi: 10.1111/jfb.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Svanbäck R, Bolnick DI. Intraspecific competition drives increased resource use diversity within a natural population. Proc Biol Sci. 2007;274:839–844. doi: 10.1098/rspb.2006.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop SC, Morris CA. Genetics of disease resistance in sheep and goats. Small Rumin Res. 2007;70:48–59. [Google Scholar]
- 43.Stear MJ, et al. How hosts control worms. Nature. 1997;389:27. doi: 10.1038/37895. [DOI] [PubMed] [Google Scholar]
- 44.Stear MJ, Boag B, Cattadori I, Murphy L. Genetic variation in resistance to mixed, predominantly Teladorsagia circumcincta nematode infections of sheep: From heritabilities to gene identification. Parasite Immunol. 2009;31:274–282. doi: 10.1111/j.1365-3024.2009.01105.x. [DOI] [PubMed] [Google Scholar]
- 45.Quinnell RJ. Genetics of susceptibility to human helminth infection. Int J Parasitol. 2003;33:1219–1231. doi: 10.1016/s0020-7519(03)00175-9. [DOI] [PubMed] [Google Scholar]
- 46.Quinnell RJ, Griffin J, Nowell MA, Raiko A, Pritchard DI. Predisposition to hookworm infection in Papua New Guinea. Trans R Soc Trop Med Hyg. 2001;95:139–142. doi: 10.1016/s0035-9203(01)90138-5. [DOI] [PubMed] [Google Scholar]
- 47.Bishop SC. A consideration of resistance and tolerance for ruminant nematode infections. Front Genet. 2012;3:168. doi: 10.3389/fgene.2012.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bishop SC, Stear MJ. Modeling of host genetics and resistance to infectious diseases: Understanding and controlling nematode infections. Vet Parasitol. 2003;115:147–166. doi: 10.1016/s0304-4017(03)00204-8. [DOI] [PubMed] [Google Scholar]
- 49.Barber I, Huntingford FA. The effect of Schistocephalus solidus (Cestoda: Pseudophyllidea) on the foraging and shoaling behaviour of three-spined sticklebacks, Gasterosteus aculeatus. Behaviour. 1995;132:1223–1240. [Google Scholar]
- 50.Best A, White A, Boots M. The coevolutionary implications of host tolerance. Evolution. 2014;68:1426–1435. doi: 10.1111/evo.12368. [DOI] [PubMed] [Google Scholar]
- 51.Giles N. Behavioural effects of the parasite Schistocephalus solidus (Cestoda) on an intermediate host, the three-spined stickleback, Gasterosteus aculeatus L. Anim Behav. 1983;31:1192–1194. [Google Scholar]
- 52.Milinski M. Risk of predation of parasitized sticklebacks (Gasterosteus aculeatus L.) under competition for food. Behaviour. 1985;93:203–216. [Google Scholar]
- 53.Shin MH, Lee YA, Min D-Y. Eosinophil-mediated tissue inflammatory responses in helminth infection. Korean J Parasitol. 2009;47:S125–S131. doi: 10.3347/kjp.2009.47.S.S125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scharsack JP, Koch K, Hammerschmidt K. Who is in control of the stickleback immune system: Interactions between Schistocephalus solidus and its specific vertebrate host. Proc Biol Sci. 2007;274:3151–3158. doi: 10.1098/rspb.2007.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Franke F, et al. In vitro leukocyte response of three-spined sticklebacks (Gasterosteus aculeatus) to helminth parasite antigens. Fish Shellfish Immunol. 2014;36:130–140. doi: 10.1016/j.fsi.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 56.Grindstaff JL, Brodie ED, 3rd, Ketterson ED. Immune function across generations: Integrating mechanism and evolutionary process in maternal antibody transmission. Proc Biol Sci. 2003;270:2309–2319. doi: 10.1098/rspb.2003.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brodin P, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell. 2015;160:37–47. doi: 10.1016/j.cell.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, et al. Inter-individual variability and genetic influences on cytokine responses to bacteria and fungi. Nat Med. 2016;22:952–960. doi: 10.1038/nm.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Downs CJ, Adelman JS, Demas GE. Mechanisms and methods in ecoimmunology: Integrating within-organism and between-organism processes. Integr Comp Biol. 2014;54:340–352. doi: 10.1093/icb/icu082. [DOI] [PubMed] [Google Scholar]
- 60.Jakobsen PJ, et al. In vitro transition of Schistocephalus solidus (Cestoda) from coracidium to procercoid and from procercoid to plerocercoid. Exp Parasitol. 2012;130:267–273. doi: 10.1016/j.exppara.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Benesh DP, Hafer N. Growth and ontogeny of the tapeworm Schistocephalus solidus in its copepod first host affects performance in its stickleback second intermediate host. Parasit Vectors. 2012;5:90. doi: 10.1186/1756-3305-5-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2016. [Google Scholar]
- 63.Hothorn T, Bretz F, Westfall P. Simultaneous inference in general parametric models. Biom J. 2008;50:346–363. doi: 10.1002/bimj.200810425. [DOI] [PubMed] [Google Scholar]
- 64.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. [Google Scholar]
- 65.Lenth RV. Least-squares means: The R package lsmeans. J Stat Softw. 2016;69:1–33. [Google Scholar]