Significance
Food intake is essential for survival in all species for meeting energetic demands. However, food intake also modulates various biochemical processes underlying cognition. Across two studies, we showed that different macronutrient compositions in standard European meals affect plasma neurotransmitter precursor levels, and these in turn influence social decision making. Our results provide evidence that variations in the macronutrient content of a normal European meal exert a significant impact on high-level human cognition. This study opens perspectives on nutrition-driven cognition modulation. The results have implications for education, economics, and public policy by emphasizing the importance of a balanced diet on fundamental expressions of cognition.
Keywords: nutrition, social decision, tyrosine, decision making, ultimatum game
Abstract
Food intake is essential for maintaining homeostasis, which is necessary for survival in all species. However, food intake also impacts multiple biochemical processes that influence our behavior. Here, we investigate the causal relationship between macronutrient composition, its bodily biochemical impact, and a modulation of human social decision making. Across two studies, we show that breakfasts with different macronutrient compositions modulated human social behavior. Breakfasts with a high-carbohydrate/protein ratio increased social punishment behavior in response to norm violations compared with that in response to a low carbohydrate/protein meal. We show that these macronutrient-induced behavioral changes in social decision making are causally related to a lowering of plasma tyrosine levels. The findings indicate that, in a limited sense, “we are what we eat” and provide a perspective on a nutrition-driven modulation of cognition. The findings have implications for education, economics, and public policy, and emphasize that the importance of a balanced diet may extend beyond the mere physical benefits of adequate nutrition.
Food intake is a fundamental basis for survival in all living organisms insofar as an adequate daily food intake secures energy levels (1). Each meal contains a different macronutrient composition that in turn influences a variety of biochemical processes (2, 3). In addition to supplying the body with nutrients, these biochemical processes also influence brain processes, including higher-level cognition, such as social decision making (4, 5). Therefore, it is not only whether and when we eat that is important, but equally what we eat.
Macronutrient composition, the relation of fat, protein, and carbohydrates, is known to modify endocrine signals (6–8). More specifically, large neutral amino acids (LNAAs) (9) can act to modulate brain neurotransmitter dynamics (10). Specifically, consuming protein-rich food has been shown to alter blood tyrosine levels (a LNAA and precursor of dopamine) (2), whereas the intake of carbohydrate-rich food increases blood tryptophan levels (a LNAA and precursor of serotonin) (2). Furthermore, this change in peripheral LNAA levels has been linked to brain dopamine and serotonin synthesis (Food and LNAAs) (11–13).
On the other hand, social decisions, such as helping, trusting, or social punishment [usually assessed as rejection rates in the ultimatum game (UG)] are susceptible to influences from hormonal and neurotransmitter states (3, 5, 14). Human studies on neuromodulation typically induce supraphysiological effects, for example, through pharmacological manipulations that are beyond that induced by regular food intake (e.g., ref. 2 vs. ref. 4). Other studies have attempted to manipulate blood glucose concentrations either by drinks containing glucose or through cognitive exercises thought to deplete glucose resources (3, 15). In contrast to highly selective pharmacological interventions or the impact of glucose-containing drinks, a balanced meal contains a mixture of proteins and carbohydrates, leading to physiological fluctuations evident across a range of metabolic parameters. However, it is unknown whether the macronutrient composition of a simple Western-style meal is sufficient to change metabolic parameters that in turn impact social decision making.
In this study, we investigated the impact of the macronutrient composition of a typical Western-style meal on social decision making. First, we tested whether the macronutrient composition of breakfast influences social decision-making behavior. In a second study, we experimentally manipulated the macronutrient composition of a standardized breakfast and monitored metabolic parameters while assessing social decisions. As a primary outcome, we assessed rejection rates (as a proxy for social punishment) that target nonnorm-compliant behavior in a UG (16). We hypothesized that a meal with a high-carbohydrate/protein (carb/protein) ratio would induce distinct metabolic and hormonal changes that translated into different social punishment rates. We predicted that blood glucose concentrations, as well as tryptophan levels, would be higher in a high-carb/protein compared with a low-carb/protein condition. In contrast, we predicted tyrosine levels would be lower in a high-carb/protein compared with a low-carb/protein condition. For all other parameters we did not have an a priori hypotheses.
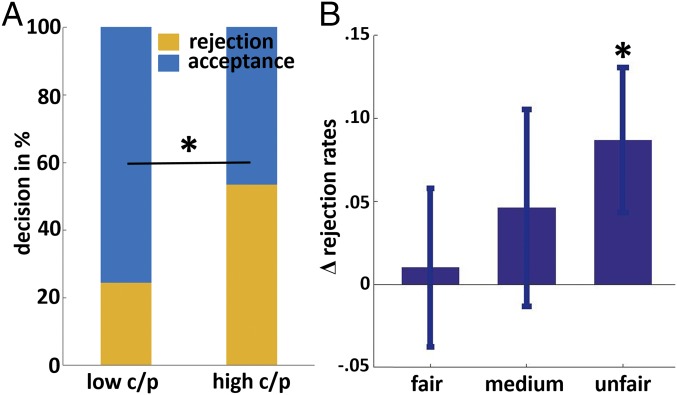
In study 1, we assessed the relationship between the subject’s breakfast macronutrient composition and social rejection rates after the subject’s natural breakfast intake. Before lunch (between 1100 hours and 1300 hours), subjects submitted a detailed food list of their breakfast on that day. In addition, they played a one-shot UG, in which they could punish a norm violator who had made an unfair offer. We computed each individual breakfast’s carbohydrate and protein ratio for group subjects as high-carb/protein ratio or low-carb/protein ratio based on a median split. When comparing rejection rates, we observed a significant difference between groups [χ2 (1) = 6.926, P = 0.011, ϕ = 0.302) (Fig. 1A). Within the low-carb/protein group, 24% of subjects decided to reject unfair offers. In contrast, 53% of the high-carb/protein group decided to reject unfair offers. A point–biserial correlation analysis confirmed this finding by indicating a positive correlation between the carb/protein ratio and rejection rates (rpb = 0.22, P = 0.03). Of note, there were no differences between the groups for age, gender, body-mass index (BMI), or total energy of breakfast (Additional Results, Study 1 for more results).
Fig. 1.
Decision making depends on breakfast carb/protein ratio. (A) In study 1, subjects were grouped in low- vs. high-carb/protein groups depending on the macronutrient composition of their breakfast on that morning. Yellow bars indicate the fraction of subjects who decided to reject. Subjects with high-carb/protein ratio breakfasts showed significantly more rejection behavior (*P < 0.05). (B) Differences (high-carb/protein minus low-carb/protein condition) in rejection rates separated for fairness categories (fair, medium, unfair) during the UG in the intervention experiment (study 2). Subjects showed an increase in rejection rates after a high-carb/protein ratio breakfast compared with after a low-carb/protein ratio breakfast. The values indicated are mean changes (±SEM, *P < 0.05).
Motivated by the findings of study 1, we designed a randomized, balanced, cross-over intervention experiment with a double-blind design that enabled us to test for a causal relationship between the macronutrient composition of a controlled meal and rejection rates (study 2). Because our aim was to induce physiological fluctuations in metabolic parameters because of food consumption, we offered subjects breakfasts that differed in macronutrient composition, albeit within a range present in real-life Western-style breakfasts. This approach enabled us to assess the impact of a controlled low- (50%/25%) vs. high- (80%/10%) carb/protein ratio of a standard breakfast meal on subsequent rejection decisions and individual metabolic parameters.
In line with predictions from study 1, we hypothesized higher rejection rates following a high-carb/protein breakfast compared with a low-carb/protein breakfast. To be considered as a candidate metabolic parameter for explaining food-related behavior modifications, we required that two criteria must be met. First, the metabolic parameter should show a significant change depending on the breakfast’s macronutrient composition. Second, the significant parameter change between breakfast conditions should predict significant behavioral changes across the conditions.
Confirming the finding from study 1 in a controlled experimental setting, in study 2 the macronutrient composition of breakfast significantly modulated rejection rates in response to unfair offers. The results indicated a main effect for both breakfast [F(1, 2,090) = 7.77, P = 0.005] and fairness categories [F(2, 2,090) = 209.859, P < 0.001]. Rejection rates were significantly higher in the high-carb/protein condition (mean = 0.69) compared with the low-carb/protein condition (mean = 0.60) for unfair offers [t(2,090) = 2.82, P = 0.005] (Fig. 1B). Thus, subjects rejected unfair offers more often after a high-carb/protein breakfast.
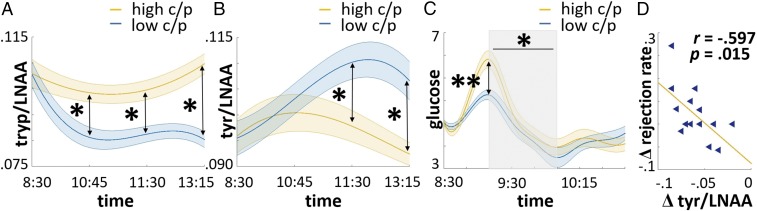
We then analyzed whether the macronutrient composition of breakfast had an influence on postprandial tryptophan and tyrosine levels. Ingesting the high-carb/protein breakfast significantly increased plasma tryptophan [t(15) = 4.873, P < 0.001] (Fig. 2A and Additional Results, Study 2) and significantly lowered plasma tyrosine levels [t(15) = 2.13, P = 0.025] (Fig. 2B and Additional Results, Study 2) compared with the low-carb/protein breakfast. Furthermore, peak blood glucose concentrations were significantly higher in the high-carb/protein condition [t(21) = 4.675, P < 0.001]. Additionally, we documented a steeper decline after a high-carb/protein breakfast [t(21) = 2.26, P = 0.035] (Fig. 2C and Additional Results, Study 2).
Fig. 2.
Macronutrient composition-dependent changes in postprandial tryptophan, tyrosine, and glucose and the correlation of tyrosine with rejection rates. Blue lines indicate low- and yellow lines indicate high-carb/protein condition for (A) tryptophan/LNAA, (B) tyrosine/LNAA, and (C) glucose values (±SEM in shadowed area, *P < 0.05 and **P < 0.001). For visualization purposes, the data points were interpolated. (C) Shadowed area represents the time window of glucose decline, which significantly differs between the conditions. (D) Triangles indicate single data points for change in rejection rates and change in tyrosine/LNAA values od high- vs. low-carb/protein conditions.
No other metabolic parameters [insulin, testosterone, cortisol, adrenocorticotropic hormone (ACTH), and leptin] were significantly modulated by the different breakfasts (Table S1). Postprandial tyrosine, tryptophan, and glucose thus all fulfill the first criteria for candidate parameter underlying food-related changes in behavior.
Table S1.
ANOVA results for metabolic parameters
| Parameters | F (condition) | P (condition) | F (time) | P (time) | F (interaction) | P (interaction) |
| Cortisol | 0.045 | 0.834 | 65.994 | <0.001** | 1.324 | 0.275 |
| ACTH | 0.757 | 0.394 | 30.781 | <0.001** | 0.134 | 0.868 |
| Insulin | 2.224 | 0.151 | 21.723 | <0.001** | 1.185 | 0.323 |
| FAI | 0.183 | 0.673 | 8.390 | <0.001** | 0.751 | 0.629 |
| Leptin | 0.786 | 0.385 | 2.434 | 0.093 | 0.244 | 0.974 |
*P < 0.05; **P < 0.01; F indicates the F value and P the corresponding P value.
To test whether any metabolic parameter fulfilled the second criteria—that is, to predict behavioral changes across conditions—a regression model was implemented that included the differences in metabolic parameters between conditions as predictors of change in rejection rates. In detail, the differences in areas under the curve (AUCs; 0830–1045 hours) for cortisol, ACTH, insulin, leptin, and free androgen index (FAI), the differences in AUCs (0830–1315 hours) for tryptophan and tyrosine/LNAA ratios, and the difference in glucose slope between 0915 and 1000 hours were used as predictors. The analysis revealed tyrosine, insulin, and cortisol as significant negative predictors and ACTH as a significant positive predictor for rejection rates in the UG (model: R2 = 0.648, f2 = 1.99; tyrosine: P = 0.012; insulin: P = 0.023; cortisol: P = 0.021; ACTH: P = 0.044) (Table S2). Thus, differences in tyrosine, insulin, cortisol, and ACTH predict changes in rejection rates between both conditions, thereby fulfilling the second criteria for a candidate mechanism underlying food-related changes in behavior. Strikingly, only tyrosine fulfilled both criteria because its levels were significantly modulated by the breakfasts and this difference significantly predicted changes in rejection rates (Fig. 2D; the correlation between changes in tyrosine and rejection rate is shown).
Table S2.
Regression analysis
| Parameters | B | SE B | β |
| Tryptophan/LNAA ratio | −0.530 | 1.247 | −0.091 |
| Tyrosine/LNAA ratio | −5.799 | 1.726 | −0.955* |
| Glucose | −0.171 | 0.122 | −0.312 |
| Insulin | −0.002 | 0.001 | −0.785* |
| Testosterone | −0.001 | 0.001 | −0.346 |
| Cortisol | −0.011 | 0.004 | −0.989* |
| ACTH | 0.003 | 0.001 | 0.592* |
| Leptin | 0.006 | 0.005 | 0.305 |
R2 = 0.648; *P < 0.05; B, unstandardized coefficient; SE B, SE of unstandardized coefficient; β, standardized coefficient; glucose slope and AUC for the other parameters were used as variables; dependent variable and predictors depict the change between breakfast conditions (high-car/protein ratio minus low-carb/protein ratio). Tyrosine remains a significant predictor of the change in rejection rates even by excluding potential outlier.
Finally, we checked for changes in mood across conditions to rule out the possibility that induced differences in rejection behavior are explained as a secondary consequence of a change in mood. We did not observe any statistically significant differences in mood between the conditions [positive affect/negative affect scale (PANAS) negative: P = 0.99; PANAS positive: P = 0.156; SHS; P = 0.163)]. Moreover, the change in rejection rates was not influenced by personality traits (Table S3).
Table S3.
Correlation analysis of questionnaire data with change in rejection rates
| Personality measurements | r | P |
| SVO egoist | −0.019S | 0.934 |
| SVO competitor | 0.227S | 0.309 |
| SVO altruist | 0.005S | 0.983 |
| BFI extraversion | 0.082S | 0.716 |
| BFI agreeableness | −0.319S | 0.148 |
| BFI conscientiousness | −0.091S | 0.687 |
| BFI neuroticism | 0.122S | 0.590 |
| BFI openness | 0.108S | 0.632 |
| IRI perspective taking | 0.044P | 0.845 |
| IRI fantasy | −0.104P | 0.646 |
| IRI empathic concern | −0.053P | 0.814 |
| IRI personal distress | 0.155P | 0.492 |
| BIS | −0.104P | 0.645 |
| BAS | −0.157P | 0.484 |
| STAI | 0.215P | 0.336 |
BAS, behavioral approach system; BFI, Big Five Inventory; BIS, behavioral inhibitory system; IRI, interpersonal reactivity index; P, P value; p, Pearson correlation; r, correlation coefficient; s, Spearman correlation; STAI, state-trait anxiety inventory; SVO, social value orientation. The Bonferroni-corrected significance threshold is 0.003.
We provided converging evidence from two studies showing that a relatively small variation in breakfast’s macronutrient composition has a striking impact on social decisions. In study 1, we showed a significant behavioral difference depending on the carb/protein ratio of a subject’s natural breakfast, with subjects reporting a higher-carb/protein breakfast intake exhibiting higher rejection rates in a subsequent UG.
A limitation of study 1 is its restricted explanatory power concerning the effect of time and the exact macronutrient composition. Some subjects reported more than one breakfast time, because they consumed snacks after their actual breakfast. Furthermore, we only have the information about when subjects started the online questionnaire, but not when exactly they performed the UG. Additionally, not only was the computation of the macronutrient composition based on self-report, there was also a large individual difference in reported macronutrient composition. These considerations motivated us to conduct study 2, where we could control both the timing of ingestion and its exact macronutrient composition.
In study 2, we were able to go one step further than was possible in study 1 by testing the direct causality of distinct macronutrient compositions on rejection rates while controlling time and macronutrient composition. This approach also allowed us to characterize the specific metabolic dynamics underlying a change in decisions. We again replicated the results from study 1 by showing that a high-carb/protein breakfast increases rejection behavior, and this effect is explained as arising out of a decrease in plasma tyrosine.
Across both studies, rejection rates in the UG were higher after a high-carb/protein breakfast. An equicaloric breakfast with a higher-carb/protein ratio led to markedly different postprandial blood glucose and neurotransmitter precursor levels. Specifically, a high-carb/protein meal caused lower tyrosine levels, higher tryptophan levels, and a steeper decline in postprandial blood glucose. No other blood parameters differed significantly between conditions. The observed changes in rejection decisions correlated with several metabolic parameters, but only tyrosine fulfilled both defined criteria to be an underlying factor driving the change in behavior. First, tyrosine levels were significantly different between conditions: that is, being lower after the high-carb/protein breakfast. Second, changes in tyrosine levels significantly predicted changes in rejection rates.
The observed macronutrient-driven changes in glucose, tyrosine, and tryptophan levels are in line with the literature (1, 12). Furthermore, our data shed new light on previous findings on food-related changes in metabolic and hormonal parameters and their impact on behavior. So far, food-related changes in behavior have often been explained by the ego-depletion theory, implying that an overall energy (glucose) decline below optimal levels changes behavior by decreasing self-control (3, 17). However, it is important to highlight that in most studies, blood glucose was not assessed, and recent evidence indicates rather inconsistent results (18). Although we observed a significantly steeper glucose decline in the high- compared with the low-carb/protein condition, we did not find a direct link between glucose decline and social decisions. Altered neurotransmitter concentrations after food intake have been previously demonstrated by measuring differences in brain tryptophan and tyrosine levels engendered by food consumption (2, 9). However, a possible impact on behavior was not assessed.
Previous studies have already shown that brain tyrosine and its neurotransmitter product dopamine are involved in a variety of social decisions (19). Genetic studies indicate a link between the dopamine system and social punishment (20), suggesting a dose-dependent dopamine effect. Furthermore, fMRI studies have suggested a role for mesolimbic dopamine in social decisions (21). Dopamine neurons encode reward prediction errors: that is, deviations between predicted and experienced reward. The UG induces robust reward prediction errors as a consequence of unexpected unfair offers (22). Thus, differences in tyrosine levels might alter rejection rates via an influence on this dopamine prediction error signaling.
Although suggested by previous studies, we did not find a direct link between meal-induced changes in tryptophan on subsequent punishment rates. Previous studies reported higher rejection rates after tryptophan depletion and lower rates after pharmacological increase (4, 23). Of note, in these studies tryptophan concentrations, although associated with punishment rates, failed to significantly predict the rejection rates (4). Thus, it is difficult to rule out a possibility that tryptophan changes were not the causal underlying factor. An alternative explanation is that the significant changes in blood tryptophan levels in our study might not have been sufficient to cause changes in behavior.
One limitation of study 2 is its constrained generalizability. Because previous studies have shown gender differences in metabolism (24), only male subjects were included in study 2. The reported results thus only apply for men. Furthermore, the present results only apply for a very specific macronutrient composition. Although findings of study 1 suggest that results might be similar for women and for varying macronutrient compositions, the exact impact of varying macronutrient compositions as well as their effect on female metabolism and behavior need to be the focus of future studies.
In this study, we demonstrated that the macronutrient composition of food acutely influences our social decisions, showing a modulation in the dopamine precursor as the underlying mechanism. Our results shed new light on the striking relevance of food intake. This opens new perspectives on problems, such as antisocial behavior as well as the global problem of poor nutrition. The latter may not only have negative consequences on physical health but also on social decisions. With that background, popular diets—as for example low-carb diets—might be treated with caution. Independent of a diet’s effectiveness for losing weight, it could have potential side effects on people’s social behavior. By emphasizing the importance of educational and support campaigns to establish a balanced diet, our results have implications for society, economics, and policy formation. Specifically, the nature of large-scale food distribution—such as in kindergarten, schools, and the military—would merit reconsideration. Finally, our results hint at possibilities inherent in targeted food interventions as possible additional treatments in the clinical context that might support established behavioral modification programs.
Food and LNAAs
Protein containing food normally includes tyrosine (11) and differences in protein content can influence tyrosine and brain dopamine levels (11). The ratio between tyrosine and the remaining large neutral amino acids as determined from peripheral blood samples is used to index brain tyrosine levels in humans (37, 38). Because all LNAAs enter the brain via a blood–brain barrier transport, the concentration of a single LNAA in relation to the remaining circulating LNAAs determines its competitive transport into the brain and function as a precursor for dopamine (38).
Additional Results
Study 1.
Effect of time.
The effect of time in study 1 was analyzed by taking the difference between the first indicated eating time and the time the subject opened the questionnaire, and using a point–biserial correlation to test whether this time correlates with rejection rates. Results indicate that time does not have an influence on rejection rates (rpb = 0.10, P = 0.38). However, this result is difficult to interpret because some subjects indicated several eating time points and we only know when subjects opened the online questionnaire, but not when exactly they performed the UG. For these reasons, we conducted study two and controlled the timing.
Study 2.
Tryptophan results.
In regards to ingestion, any breakfast significantly changed the postprandial time course of tryptophan [F(1, 15) = 13.61, P = 0.002], showing a borderline significance for the interaction with the respective macronutrient condition [F(2, 30) = 2.73, P = 0.084]. Post hoc analysis revealed higher tryptophan levels after the high-carb/protein breakfast for all postprandial samples [1045 hours: t(17) = 2.99, P = 0.024; 1130 hours: t(17) = 3.47, P = 0.009; 1315 hours: t(19) = 4.13, P = 0.003].
Tyrosine results.
When analyzing individual time points, there was a main effect of condition [F(1, 15) = 11.66, P = 0.004] and time [F(2, 30) = 4.79, P = 0.019], and a trend for an interaction between the two factors [F(2, 30) = 3.87, P = 0.053]. Tyrosine levels were significantly lower in the high-carb/protein condition at 1130 hours [t(17) = 3.762, P = 0.006] and 1315 hours [t(19) = 3.77, P = 0.003], showing a later rise after the breakfast.
Glucose results.
The individual time course of blood glucose was significantly modulated by the respective macronutrient condition [interaction effect between time and condition; F(8, 168) = 3.195, P = 0.002]. Blood glucose levels were significantly higher in the high-carb/protein condition (mean = 5.91) compared with the low-carb/protein condition (mean = 5.13) at peak (0915 hours; t = 4.675, P < 0.001).
Materials and Methods
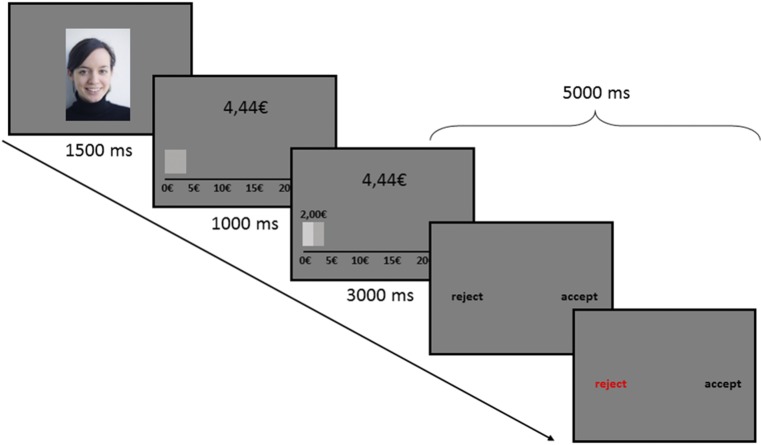
The Ultimatum Game (UG) is a two person game in which one person (the proposer) suggests how to share a sum of money with another player (the receiver; see Fig. S1). If the receiver accepts the proposed offer, both players are paid accordingly. However, if the receiver rejects the offer, neither receives any payment. Studies show that receivers usually reject unfair offers, which is interpreted as a form of social punishment (16).
Fig. S1.
UG procedure. A picture of the proposer is shown for 1,500 ms, subsequently the endowment of the corresponding proposer is presented (1,000 ms), and then his or her offer (3,000 ms). Subjects can decide whether they accept or to reject the offer within 5,000 ms and their decision is highlighted on the screen.
Study 1
Subjects.
Eighty-seven subjects (54 woman; mean age = 23.74 y, SD = 4.40; mean BMI = 22.28, SD = 2.98) participated in an online survey using the online platform Soci Survey (©2006–2015 SoSci Survey GmbH). Subjects were students of the University of Lübeck recruited via an internal mailing list. Before participation, all subjects were informed about the procedure and data handling, that they could stop the questionnaire at any time point, and that they gave their consent for participation. The study was approved by the local Medical Ethical Commission of the University of Lübeck.
Procedure.
The online survey was accessible only between 1100 and 1300 hours, and subjects were instructed beforehand to complete the survey before lunch. First, subjects received instructions about the UG and were informed that the decisions of 20 randomly selected subjects would be paid accordingly. Subsequently, subjects played a one-shot version of the UG. Here, subjects were in the role of the receiver. All subjects were told that the proposer, who participated in the online study before them, was endowed with 10€ and that the proposer decided to offer 2€ to the subject. Thus, all subjects received an unfair offer of 2€ and could decide whether to accept or reject this offer.
After the UG, subjects submitted a detailed description of all food items they consumed previously on that day (breakfast and snacks). The ratio of carbohydrates and proteins (carb/protein) as a percentage of total energy intake was calculated using the DGExpert algorithm (25).
Data Analysis.
Eleven subjects were excluded from the analyses because they indicated that they had no breakfast on the corresponding day. Therefore, subsequent analyses included the data of 76 subjects. To test whether there is an association between a subject’s macronutrient composition and rejection rates in the UG, we compared the rejection behavior of subjects who had a high-carb/protein ratio with those who had a low ratio (groups were determined by median split). A χ2 test was applied to compare rejection rates between groups. Additional tests were conducted to control whether groups differed concerning age, BMI, gender, or total energy intake. Because age, BMI, and total energy intake were not normally distributed, Mann–Whitney U tests were used. To compare the gender distribution between the groups, a χ2 test was applied. Furthermore, a point–biserial correlation was applied to determine the relationship between carb/protein ratio and punishment and to support the median-split analysis. For this analysis, three outliers (mean ± 2 SD) were excluded and one-sided P values are reported.
Study 2
Subjects.
Twenty-four male subjects participated in the experimental study (mean age = 24.64 y, SD = 4.06; mean BMI = 22.59, SD = 1.82). The sample size was chosen based on previous metabolic studies (4, 26–28). Because other studies have shown gender differences in metabolism, only male subjects were included (24). Before experimental participation, every subject underwent a medical screening with a special focus on metabolic diseases. The medical screening consisted of a blood sample, a questionnaire, and a complete physical examination, including a visual examination of the mouth, eyes, and skin; manual palpation of the lymph nodes, thyroid gland, and abdomen; manual tapping of the spinal column and kidneys; auscultation of the heart, lungs, and abdomen; measurement of body weight, blood pressure, and heart rate; and an electrocardiogram. The following blood parameters were examined: full blood count, glucose, liver enzymes, thyroid function, kidney function, electrolytes, and blood lipids. Exclusion criteria were any abnormalities in the blood results or physical examination, any physical or psychological disease, shifted day/night rhythm, being a high-performance athlete, BMI under 18 kg/m2 or above 25 kg/m2, smoking, or food allergies. Before participation, all subjects gave written informed consent according to the Declaration of Helsinki. The study was approved by the local Medical Ethical Commission of the University of Lübeck.
Experimental Procedure.
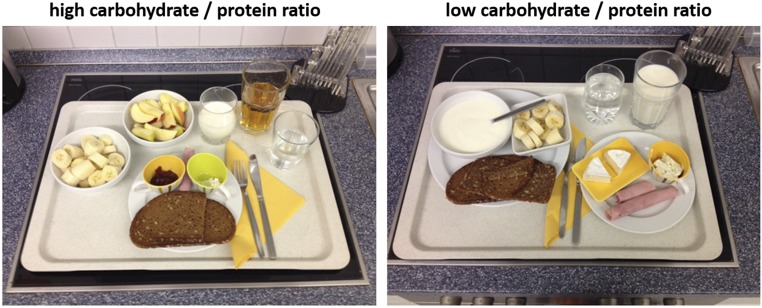
Subjects were tested in a randomized, balanced, within-subject design during two sessions separated by at least 7 days (maximum 9 days). Both sessions were identical except for the macronutrient composition in the breakfast subjects had. On one day, subjects received a breakfast with a low-carbohydrate and high-protein content (low-carb/protein condition), and on the other day they received a breakfast with a high-carbohydrate and low-protein content (high-carb/protein condition). In the low-carb/protein condition the breakfast contained 50% carbohydrates, 25% lipids, and 25% proteins, and the high-carb/protein condition was 80% carbohydrates, 10% lipids, and 10% proteins (Fig. S2). In detail, the high-carb/protein breakfast contained: 88-g “Vital-Fit” whole-grain bread, 20-g ham, 5-g cream cheese, 30-g strawberry marmalade, 130-mL milk, 200-mL apple juice, 110-mL water, 225-g banana, and 225-g apple. The low-carb/protein breakfast contained: 70-g sunflower seed bread, 70-g Vital-Fit whole-grain bread, 40-g ham, 30-g “Bresso” (cream cheese), 40-g Camembert, 240-mL milk, 200-mL water, 250-mL yogurt, and 120-g banana. Both breakfasts had the same total amount of calories (850 kcal) and subjects had to consume the whole breakfast.
Fig. S2.
Breakfast study 2. According to the experimental condition, either a high-carb/protein breakfast (Left) or low-carb/protein breakfast (Right) was served.
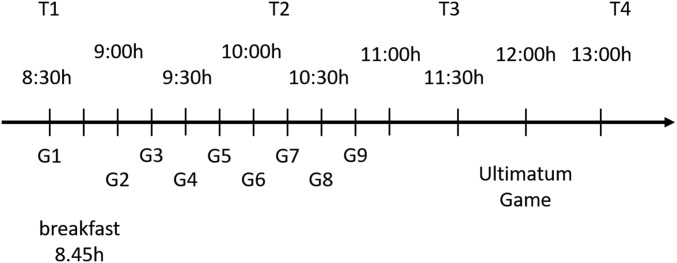
After arrival at the research unit at 0800 hours, an intravenous catheter was inserted into a vein of the participant’s nondominant distal forearm and at 0830 hours the first blood sample was obtained. At 0845 hours subjects received breakfast in a single room according to the respective high-carb/protein or low-carb/protein condition. From 0900 hours until 1045 hours, blood samples were drawn in 15-min intervals with additional blood samples at 1130 and 1315 hours (Fig. S3). During the whole procedure subjects could either lie in bed or sit on a chair but were not allowed to leave their room or perform any type of physical exercise.
Fig. S3.
Timeline study 2. Subjects attended the research unit at 0800 hours and were prepared for the experiments. At 0845 hours subjects received breakfast according to the respective experimental condition. Blood was drawn at 11 time points with a 15-min interval between 0845 hours and 1045 hours, with additional samples at 1130 and 1315 hours. T1–T4 indicates blood samples used for measurement of tryptophan and tyrosine and G1–G9 indicates blood samples used for measurement of glucose, cortisol, ACTH, insulin, testosterone, and SHBG.
At 1200 hours, subjects were guided to a different room where they completed a test battery including the UG, the interpersonal reactivity index (29), the social value orientation (30), the PANAS (31), the subjective happiness scale (SHS) (32), the state-trait anxiety inventory (33), and the behavioral inhibitory system/behavioral approach system (34). These assessments were performed at 1215 hours because the maximum difference in neurotransmitter precursor concentrations between conditions was expected to be present 3.5–4 h after the food intake (2, 4). Subjects first received written instructions on the UG and were asked to answer a set of comprehension questions before starting the game.
In study 2 all subjects were in the role of the receiver and played 48 trials of the UG with 48 different proposers via a computer interface. In each trial, subjects sequentially observed the picture of the proposer (1,500 ms), the endowment of the proposer (1,500 ms), and the offer of the proposer (3,000 ms). By pressing one of two buttons, subjects could indicate whether they accepted or rejected the offer. Their response was highlighted on the screen (Fig. S1). Proposer pictures were randomly matched with the offers. Eight “fair,” eight “medium,” and eight “unfair” offers were all presented twice in a randomized order. The fair offers ranged between 40% and 50% of the proposer’s endowment, the medium offers between 27% and 33%, and the unfair between 18% and 22%. Thus, in different trials the same amount could either be a fair or an unfair offer, depending on the proposer’s endowment. This way we could investigate fairness independent of monetary reward. The dependent variable was the punishment decision (yes or no) with respect to rejected fair, medium, and unfair offers. Subjects were told that the proposers in the picture previously participated in the experiment and that they would receive their money according to the receiver’s decisions. Each trial started with a picture of the alleged proposer. Half of the pictures showed male and the other half showed female faces.
After completing the UG, subjects again filled in the respective questionnaires and were guided back to their room where the last blood sample was obtained (1315 hours). Subjects received a fee for their participation in the whole study. They were told that, in addition, one of all trials of the test battery of both sessions would randomly be picked and payed out after the second session.
Blood Samples.
Twenty-two plasma amino acids were determined from blood samples drawn at 0830, 1045, 1130, and 1315 hours. From the blood samples drawn at 0830, 0900, 0915, 0930, 0945, 1000, 1015, 1030, and 1045 hours, the following parameters were determined: glucose, testosterone, sex hormone-binding globulin (SHGB), cortisol, ACTH, leptin, and insulin. All blood samples were immediately centrifuged at 4 °C and stored at −80 °C until analysis. Measurement of plasma amino acids was performed according to the method of Harder et al. (35). Harder et al. combine precipitation, derivatization, and chromatographic separation to determine all proteinogenic amino acids, citrulline, and ornithine. For a detailed description, see ref. 35.
Blood glucose was measured by an enzymatic-amperometric method (EKF Diagnostics) [coefficient of variability (CV) ≤ 1.5%]. Leptin concentrations were assessed by a radioimmunoassay (RIA Kit, EMD Millipore) (within-CV < 8.3%, between-CV < 6.2%). Insulin, cortisol, ACTH, and SHGB were assessed by immunoassays (Immulite 2000, Siemend Healthcare Diagnostics). The assessed within- and between-assay variations were as follows: insulin within-CV 3.3–3.9%, between-CV 4.1–5.0%; SHGB within-CV 2.5–2.7%, between-CV < 5.2%; ACTH within-CV < 8.7%, between-CV < 10.0%; cortisol within-CV < 5.2%, between-CV < 6.8% and testosterone within-CV 8.3–7.2%, between-CV 9.1–8.2%.
Data Analysis.
Two subjects were excluded from the UG analyses, one because of technical recording problems and one because he was aware of the study design and hypothesis before participating, yielding a final sample size of 22 subjects.
First, we tested for a difference in rejection rates between the two breakfast conditions using a mixed logistic model to analyze the effect of macronutrient content on UG decisions. Rejection rates were used as dependent variable, breakfast (high carb/protein and low carb/protein) and fairness category (fair, medium, and unfair) were used as fixed effects, and a random intercept was allowed for each subject. Corresponding post hoc tests were applied to investigate the exact relationship between the factors.
Second, the influence of breakfast’s macronutrient composition on tryptophan and tyrosine levels was examined. Ratios between plasma concentrations of tryptophan and tyrosine and the other LNAAs were used as a proxy for brain tryptophan and tyrosine levels and ultimately brain serotonin and dopamine levels (2, 9). One subject was excluded from this analysis because of technical problems. Furthermore, blood data of five participants could not be analyzed for every time point. Differences in AUC values (0830–1315 hours) of tryptophan- or tyrosine/LNAA ratios were tested using dependent sample t tests (one-sided tested according to a priori hypothesis). To check at which time points the values differed, repeated-measures ANOVA was conducted with the factor’s time (1045–1315 hours) and condition as within-subject factors and either tryptophan- or tyrosine/LNAA ratio (corrected for baseline) as the dependent variable. Corresponding post hoc tests were applied to investigate the exact relationship between factors.
Next, we tested for differences in glucose drop between conditions. Because the decline in blood glucose was shown to be a better predictor for hypoglycemia symptoms (36), we used a dependent sample t test to test for differences in glucose decline between 0915 and 1000 hours. A repeated-measures ANOVA with the within-subject factor’s time (0830–1045 hours) and condition (respective breakfast condition) was used to address differences in blood glucose over time. Corresponding post hoc tests were performed to investigate the exact relationship between the factors.
Moreover, we analyzed the effect of the respective condition on cortisol, ACTH, insulin, leptin concentrations, and FAI. FAI as the ratio of total testosterone and sex hormone-binding globulin (SHBG) × 100 is used as a proxy of free (bioactive) testosterone. Differences in AUC (0830–1045 hours) of cortisol, ACTH, insulin, leptin, and FAI were tested using dependent sample t tests. To test at which time points values differed between the breakfast conditions, a repeated-measures ANOVA was conducted with the factor’s time (0845–1045 hours) and condition as within-subject factors and either cortisol, ACTH, insulin, or FAI (corrected for baseline) as the dependent variable.
To test if any of the parameters had an influence on rejection rates, a regression model including all blood parameters as predictors and rejection rates as the dependent variable was applied. In detail, changes in the parameter values (differences in AUCs; 0830–1045 hours) for cortisol, ACTH, insulin, leptin, and FAI; AUCs for tryptophan- and tyrosine/LNAA ratios (0830–1315 hours); glucose slope between 0915 and 1000 hours, and rejection rates between both breakfast conditions (high carb/protein minus low carb/protein) were used for this analysis. Furthermore, we tested whether mood was influenced by the different conditions when comparing the PANAS (because it is not normally distributed, a Wilcoxon test for dependent samples was used) and SHS (t test for dependent samples) scores. All questionnaire results were corrected for multiple comparisons, resulting in a Bonferroni-corrected significance threshold of 0.003.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grants INST 392/125-1 and PA 2682/1-1 (to S.Q.P.) and European Research Council Advanced Grant META-GROWTH (ERC-2012-AdG 322605).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. M.D.L. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620245114/-/DCSupplemental.
References
- 1.Wolever TM, Jenkins DJ. The use of the glycemic index in predicting the blood glucose response to mixed meals. Am J Clin Nutr. 1986;43:167–172. doi: 10.1093/ajcn/43.1.167. [DOI] [PubMed] [Google Scholar]
- 2.Wurtman RJ, et al. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr. 2003;77:128–132. doi: 10.1093/ajcn/77.1.128. [DOI] [PubMed] [Google Scholar]
- 3.Gailliot MT, et al. Self-control relies on glucose as a limited energy source: Willpower is more than a metaphor. J Pers Soc Psychol. 2007;92:325–336. doi: 10.1037/0022-3514.92.2.325. [DOI] [PubMed] [Google Scholar]
- 4.Crockett MJ, Clark L, Tabibnia G, Lieberman MD, Robbins TW. Serotonin modulates behavioral reactions to unfairness. Science. 2008;320:1739. doi: 10.1126/science.1155577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colzato LS, et al. Tryptophan promotes interpersonal trust. Psychol Sci. 2013;24:2575–2577. doi: 10.1177/0956797613500795. [DOI] [PubMed] [Google Scholar]
- 6.Hartmann S, Lacorn M, Steinhart H. Natural occurrence of steroid hormones in food. Food Chem. 1998;62:7–20. [Google Scholar]
- 7.Knerr I, Gröschl M, Rascher W, Rauh M. Endocrine effects of food intake: Insulin, ghrelin, and leptin responses to a single bolus of essential amino acids in humans. Ann Nutr Metab. 2003;47:312–318. doi: 10.1159/000072405. [DOI] [PubMed] [Google Scholar]
- 8.Markus R, Panhuysen G, Tuiten A, Koppeschaar H. Effects of food on cortisol and mood in vulnerable subjects under controllable and uncontrollable stress. Physiol Behav. 2000;70:333–342. doi: 10.1016/s0031-9384(00)00265-1. [DOI] [PubMed] [Google Scholar]
- 9.Fernstrom JD, Wurtman RJ. Brain serotonin content: Physiological dependence on plasma tryptophan levels. Science. 1971;173:149–152. doi: 10.1126/science.173.3992.149. [DOI] [PubMed] [Google Scholar]
- 10.Lehnert H, Wurtman RJ. Amino acid control of neurotransmitter synthesis and release: Physiological and clinical implications. Psychother Psychosom. 1993;60:18–32. doi: 10.1159/000288676. [DOI] [PubMed] [Google Scholar]
- 11.Wurtman RJ, Fernstrom JD. Control of brain neurotransmitter synthesis by precursor availability and nutritional state. Biochem Pharmacol. 1976;25:1691–1696. doi: 10.1016/0006-2952(76)90400-7. [DOI] [PubMed] [Google Scholar]
- 12.Acworth IN, During MJ, Wurtman RJ. Tyrosine: Effects on catecholamine release. Brain Res Bull. 1988;21:473–477. doi: 10.1016/0361-9230(88)90161-x. [DOI] [PubMed] [Google Scholar]
- 13.Lehnert H, Reinstein DK, Strowbridge BW, Wurtman RJ. Neurochemical and behavioral consequences of acute, uncontrollable stress: Effects of dietary tyrosine. Brain Res. 1984;303:215–223. doi: 10.1016/0006-8993(84)91207-1. [DOI] [PubMed] [Google Scholar]
- 14.Margittai Z, et al. Exogenous cortisol causes a shift from deliberative to intuitive thinking. Psychoneuroendocrinology. 2016;64:131–135. doi: 10.1016/j.psyneuen.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 15.Halali E, Bereby-Meyer Y, Ockenfels A. Is it all about the self? The effect of self-control depletion on ultimatum game proposers. Front Hum Neurosci. 2013;7:240. doi: 10.3389/fnhum.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strang S, Park SQ. Human cooperation and its underlying mechanisms. Curr Top Behav Neurosic. 2016;30:223–239. doi: 10.1007/7854_2016_445. [DOI] [PubMed] [Google Scholar]
- 17.Masicampo EJ, Baumeister RF. Toward a physiology of dual-process reasoning and judgment: Lemonade, willpower, and expensive rule-based analysis. Psychol Sci. 2008;19:255–260. doi: 10.1111/j.1467-9280.2008.02077.x. [DOI] [PubMed] [Google Scholar]
- 18.Vadillo MA, Gold N, Osman M. The bitter truth about sugar and willpower: The limited evidential value of the glucose model of ego depletion. Psychol Sci. 2016;27:1207–1214. doi: 10.1177/0956797616654911. [DOI] [PubMed] [Google Scholar]
- 19.Rutledge RB, Skandali N, Dayan P, Dolan RJ. Dopaminergic modulation of decision making and subjective well-being. J Neurosci. 2015;35:9811–9822. doi: 10.1523/JNEUROSCI.0702-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reuter M, et al. The influence of dopaminergic gene variants on decision making in the ultimatum game. Front Hum Neurosci. 2013;7:242. doi: 10.3389/fnhum.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rilling JK, King-Casas B, Sanfey AG. The neurobiology of social decision-making. Curr Opin Neurobiol. 2008;18:159–165. doi: 10.1016/j.conb.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Pessiglione M, Seymour B, Flandin G, Dolan RJ, Frith CD. Dopamine-dependent prediction errors underpin reward-seeking behaviour in humans. Nature. 2006;442:1042–1045. doi: 10.1038/nature05051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crockett MJ, Clark L, Lieberman MD, Tabibnia G, Robbins TW. Impulsive choice and altruistic punishment are correlated and increase in tandem with serotonin depletion. Emotion. 2010;10:855–862. doi: 10.1037/a0019861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dionne I, Després JP, Bouchard C, Tremblay A. Gender difference in the effect of body composition on energy metabolism. Int J Obes Relat Metab Disord. 1999;23:312–319. doi: 10.1038/sj.ijo.0800820. [DOI] [PubMed] [Google Scholar]
- 25.Deutsche Gesellschaft für Ernährung 2015. DGExpert algorithm (Deutsche Gesellschaft für Ernährung, Bonn), Version 1.7.4.1.26.
- 26.Akhavan T, Luhovyy BL, Brown PH, Cho CE, Anderson GH. Effect of premeal consumption of whey protein and its hydrolysate on food intake and postmeal glycemia and insulin responses in young adults. Am J Clin Nutr. 2010;91:966–975. doi: 10.3945/ajcn.2009.28406. [DOI] [PubMed] [Google Scholar]
- 27.Beulens JWJ, Bindels JG, de Graaf C, Alles MS, Wouters-Wesseling W. Alpha-lactalbumin combined with a regular diet increases plasma Trp-LNAA ratio. Physiol Behav. 2004;81:585–593. doi: 10.1016/j.physbeh.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 28.Pal S, Ellis V. The acute effects of four protein meals on insulin, glucose, appetite and energy intake in lean men. Br J Nutr. 2010;104:1241–1248. doi: 10.1017/S0007114510001911. [DOI] [PubMed] [Google Scholar]
- 29.Davis MH. A multidimensional approach to individual differences in empathy. JSAS Catalogue of Selected Documents in Psychology. 1980;10:85. [Google Scholar]
- 30.Van Lange P. The pursuit of joint outcomes and equality in outcomes: An integrative model of social value orientation. J Pers Soc Psychol. 1999;77:337–349. [Google Scholar]
- 31.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 32.Lyubomirsky S, Lepper HS. A measure of subjective happiness: Preliminary reliability and construct validation. Soc Indic Res. 1999;46:137–155. [Google Scholar]
- 33.Spielberger CD, Gorsuch RL, Lushene RE. State-Trait Anxiety Inventory. Consulting Psychologists; Palo Alto, CA: 1970. [Google Scholar]
- 34.Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. J Pesonality Soc Psychol. 1994;67:319–333. [Google Scholar]
- 35.Harder U, Koletzko B, Peissner W. Quantification of 22 plasma amino acids combining derivatization and ion-pair LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:495–504. doi: 10.1016/j.jchromb.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 36.Hadji-georgopoulos A, Schmidt I, Kowarski AA. Elevated hypoglycemic index and late hyperinsulinism. J Clin Endocrinol Metab. 2015;50:371–376. doi: 10.1210/jcem-50-2-371. [DOI] [PubMed] [Google Scholar]
- 37.Fernstrom JD. Large neutral amino acids: Dietary effects on brain neurochemistry and function. Amino Acids. 2013;45:419–430. doi: 10.1007/s00726-012-1330-y. [DOI] [PubMed] [Google Scholar]
- 38.Pardridge WM, Oldendorf WH. Kinetic analysis of blood-brain barrier transport of amino acids. Biochim Biophys Acta. 1975;401:128–136. doi: 10.1016/0005-2736(75)90347-8. [DOI] [PubMed] [Google Scholar]