Significance
Communication is central to group living: Individuals use signals to identify each other and coordinate tasks. Surprisingly, little is known about how these communication systems change as social behavior evolves, particularly during the transition between solitary and group living. This study shows that, as sociality is gained and lost in halictid bees, convergent changes arise in the sensory systems and chemical signals of these groups. Solitary species show a repeated reduction of hair-like sensilla on the antennae, and social and solitary forms of the same species differ in their chemical signals. These results suggest that changes in group complexity are closely linked to changes in communication and that social bees invest more in these systems than their solitary counterparts.
Keywords: social behavior, communication, comparative methods, halictid bees
Abstract
Social animals must communicate to define group membership and coordinate social organization. For social insects, communication is predominantly mediated through chemical signals, and as social complexity increases, so does the requirement for a greater diversity of signals. This relationship is particularly true for advanced eusocial insects, including ants, bees, and wasps, whose chemical communication systems have been well-characterized. However, we know surprisingly little about how these communication systems evolve during the transition between solitary and group living. Here, we demonstrate that the sensory systems associated with signal perception are evolutionarily labile. In particular, we show that differences in signal production and perception are tightly associated with changes in social behavior in halictid bees. Our results suggest that social species require a greater investment in communication than their solitary counterparts and that species that have reverted from eusociality to solitary living have repeatedly reduced investment in these potentially costly sensory perception systems.
Communication is crucial for social life because individuals require signals to mediate many aspects of colony organization. More complex social organizations require a greater diversity of signals to accommodate the correspondingly complex coordination of activities, including task allocation, care of offspring, reproductive status, nest defense, and food acquisition (1). For social insects, this information is typically conveyed by chemical signals, including pheromones, which can vary in both the molecular composition and the relative proportions of the constituent chemical compounds; their interpretation is often context-dependent (2). The chemical composition and function of these pheromones have been extensively characterized in many of the advanced social insect species, including honey bees and ants (3–7). Perhaps as a result of their social complexity, the chemical communication systems of these species are among the most elaborate yet described (8).
Despite the detailed characterization of the chemical communication systems in a few highly social species, we know surprisingly little about how these systems change during transitions between solitary and group living (9, 10). In highly social insects, queens produce a pheromone that signals their reproductive status and also inhibits worker reproduction (11–15). Some of these pheromones contain compounds that can be highly conserved across species, suggesting that there may be some aspects of these blends that have been coopted from the mating or defense signals of their solitary ancestors (16). Although several comparative studies have examined the evolution of these compounds across social insect species (17–20), the focus has been primarily on their role as either reliable indicators of reproductive status or a mechanism of queen manipulation to render workers sterile (21, 22).
Even fewer studies have examined the complementary role of signal perception in chemical communication, despite the fact that the sensory systems will be subject to significant selective pressures (23–25). Insects detect chemical signals with sensilla lining the antennae and legs. Each olfactory sensillum houses one or more sensory neurons, each of which expresses a single olfactory receptor and coreceptor. These receptors bind odor molecules and activate the corresponding sensory neurons, which project to specialized brain regions for their decoding (26, 27).
Sensilla are not trivial structures: Each requires the development and maintenance of a variety of sensory neurons and supporting glial cells (28, 29). Antennal sensilla are varied in function although chemo- and mechanosensory sensilla dominate (30). Poor diets during development can negatively impact sensilla production (31). Differences in sensilla numbers and types are associated with differences in foraging ecology, nestmate recognition, caste, and sex in the social insects (32–36). Importantly, the number and density of antennal sensilla influence the perception of social signals. For example, workers of Oecophylla ants with fewer sensilla are less aggressive to nonnestmates (37). Similarly, antennal sensilla density is positively correlated with increased sensitivity to chemical signals and with body size in some bee species (38, 39).
In addition to sensilla, several genetic studies have examined the expression and evolution of the olfactory receptor genes in social insect genomes. These studies have found that these genes evolve rapidly among species and that some olfactory receptors show lineage-specific signatures of positive selection. Interestingly, the frequency of positive selection on these genes seems to be greater in eusocial species than in their solitary ancestors (40), suggesting that chemical sensory systems play an important role in social evolution.
Halictid bees are an ideal group for investigating how communication systems change as social complexity evolves because they encompass the full range of social behaviors, from solitary to eusocial (41). Solitary females produce only reproductive offspring whereas eusocial females first produce a worker brood that help to rear the subsequent, reproductive generation. Within halictids, there have been two to three gains of eusociality and at least 12 reversions to solitary behavior (42). These repeated gains and losses allow the identification of common changes in communication systems against phylogenetically independent changes in social structure. Furthermore, the same variation in social and solitary life histories occurs within some species among geographically distinct populations. In the socially polymorphic species, Lasioglossum albipes, this variation has a genetic component (43), where genes associated with the production and perception of chemical cues might diverge among behavioral forms (44).
Here, we explored covariation in signal and receiver components of chemical communication in this socially diverse group of bees and asked whether evolutionary transitions in social behavior correlate with differential investments in communication systems within and among species. We used a phylogenetic comparative study across 36 halictid species to examine how changes in peripheral sensory systems correlate with sociality, and then we focused on an intraspecific comparison of a single, socially polymorphic species (L. albipes) to determine whether changes in social behavior are correlated with changes in both peripheral detection and signal production.
Results
Sensilla Density in Halictidae.
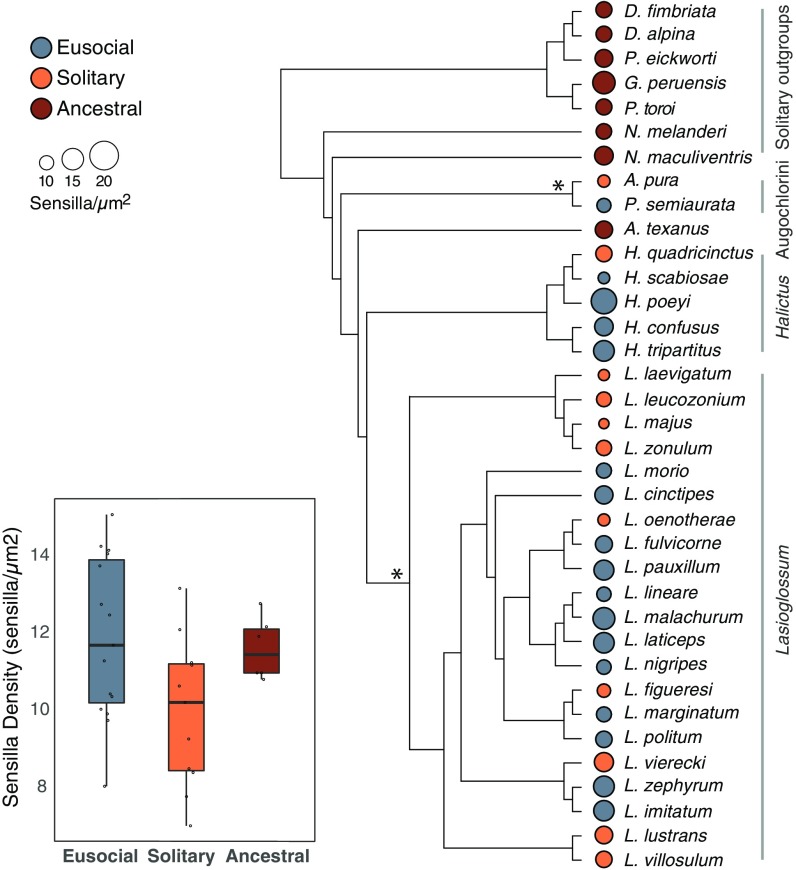
Life history changes, such as social behavior, may require adjustments to investment in mechanisms of signal perception, which is likely to be reflected in antennal morphology, where many of the sensilla lining the antennae receive chemical signals. We used a phylogenetic generalized least squares (PGLS) analysis to test for differences in the densities of hair-like sensilla across 36 halictid bee species. We classified species into three behavioral types: ancestral solitary, eusocial, or secondarily solitary (representing a loss of social behavior). Our results revealed significantly higher densities of hair-like sensilla in social species compared with secondarily solitary species (P = 0.001) (Fig. 1). Ancestral solitary species were not significantly different from social species (P = 0.867) and had sensilla densities significantly higher than the secondarily solitary species (P = 0.015). The maximum likelihood value for λ was λ = 0 (consistent with no phylogenetic structure in the measured traits), with an upper bound for the 95% confidence interval estimated at 0.409. There were no significant differences in pore-plate sensilla between social and solitary species (Fig. S1). Density of all sensilla types increased distally along the length of the antennae and was not significantly correlated with body size (Fig. S2). To exclude the possibility that differences in sensilla density were driven solely by host specialization, we reran the analysis including only polylectic (i.e., generalist) bee species. There was still a significant correlation between social behavior and sensilla density (PGLS, R2 = 0.218, P = 0.04).
Fig. 1.
Phylogeny of halictid bees used in this study. Halictids encompass the full range of social behavior, from solitary to eusocial. Social behavior in this group has originated at least twice independently (indicated with asterisks). Solitary species are depicted in orange, social species in blue; and the size of the circle is relative to the mean sensilla density for each species. PGLS analysis revealed a strong trend of correlated evolution in antennal traits, independent of the species’ shared phylogenetic history (R2 = 0.2125), F(2, 33) = 4.45, P = 0.019. This difference is driven primarily by the decrease in sensilla density in secondarily solitary species that have reverted to a solitary life history compared with their eusocial ancestors (P = 0.0096). Ancestrally solitary species have densities intermediate to eusocial and secondarily solitary forms, which is perhaps driven by oligolecty in the ancestral, solitary lineages.
Fig. S1.
The density of pore-plate sensilla does not vary with social behavior. There was no correlation between the density of pore-plate sensilla and social behavior: PGLS, R2 = 0.001; F(1, 23) = 0.003, P = 0.956.
Fig. S2.
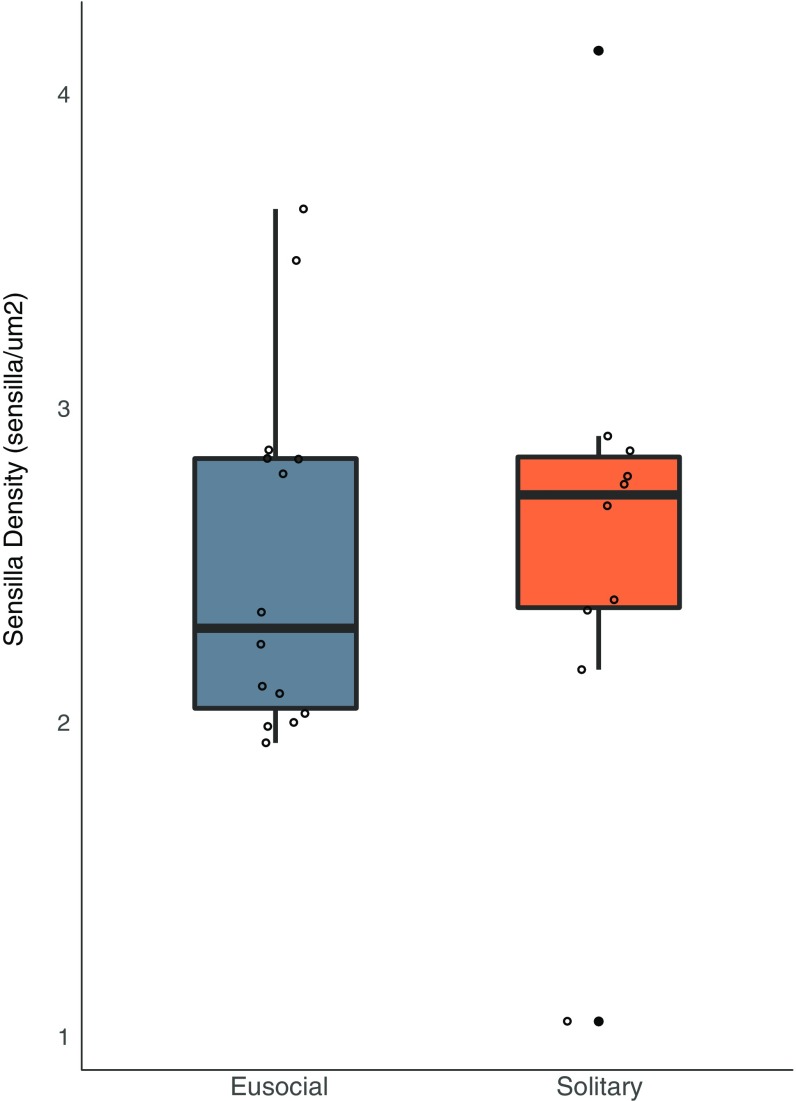
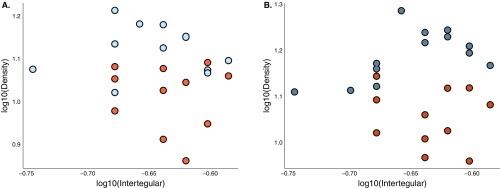
Body size and sensilla density are not correlated across halictid species. (A) There was no correlation between body size (as measured by intertegular distance) on segment 9 (r = 0.36, n = 28, P ≥ 0.05) or (B) segment 10 (r = 0.22, n = 28, P ≥ 0.25) of antennae across halictid species.
Sensilla Density in L. albipes.
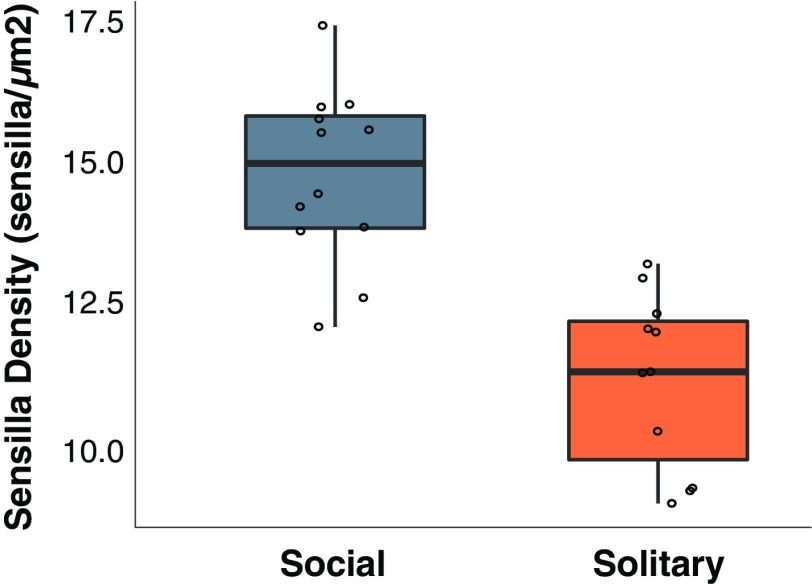
The intraspecific comparisons among social and solitary populations of L. albipes yielded similar patterns to those found across the halictids, with significantly greater density of sensilla in females from social populations (Fig. 2). The different types of sensilla were similarly distributed across the entire antenna as described in other halictids (39). Again, we found no significant relationship between body size and sensilla density (Fig. S3). We assigned individuals to castes based on the timing and location of their collection. To confirm these groupings, we measured five physiological traits commonly used for caste assignments (ovary development, mandible wear, wing wear, fat body stores, and mating status) (45, 46) and used these in a linear discriminant analysis (LDA). The LDA was able to significantly discriminate among castes (dimension 1, 66.0%; dimension 2, 34.0%; Wilk’s lambda, P = 0.032), and cross-validation was able to correctly assign individuals to their respective groups 83% of the time.
Fig. 2.
Differential investment in antennal sensilla in social and solitary populations of L. albipes. Boxplot of antennal sensilla density across social and solitary populations of L. albipes. There is a significant difference of mean sensilla density among the two behavioral morphs (F1,21 = 29.9, P = 2.00e-05).
Fig. S3.
Body size and sensilla density are not correlated in L. albipes. (A) There was no correlation between body size (as measured by intertegular distance) and sensilla density on segment 9 (r = 0.14, n = 23, P ≥ 0.54) or (B) segment 10 (r = 0.0497, n = 24, P ≥ 0.82) of antennae among social forms of L. albipes. Social forms in blue, solitary in orange.
Signaling Chemistry in L. albipes.
Changes in social behavior are correlated with changes in signaling chemistry in L. albipes (42). In halictids, two major sources of chemical signals have been described: the cuticle and the Dufour’s gland. The insect cuticle is covered in cuticular hydrocarbons (CHCs) that have two major functions: CHCs form a waxy layer on the insect cuticle that is important for preventing desiccation (47, 48) and can also play an important role in nestmate recognition and mating in social insects (10). The role of the Dufour’s gland in the production of social signals is significant and varied. The gland produces chemical secretions that contain sex pheromones and other compounds used in colony signaling (49, 50). These secretions are also used to create a hydrophobic cell lining (51) and to mark the nest entrance (52) of in-ground nests. We thus characterized the chemical profiles of both the cuticle (Table S1) and the Dufour’s gland (Table S2) in L. albipes.
Table S1.
Differences in relative abundances of chemicals from cuticular extracts
| Compound | Social populations | Solitary populations- reproductive | FDR P value | Best-fit model | |||
| Foundress | Worker | Sociality | Caste | Behavior | Caste | ||
| 13-Methyl pentacosane | 0.116 | 0.622 | 0.042 | 0.10 | 0.09 | X | |
| 13-Methyl heptacosane | 0.059 | 0.159 | 0.197 | 0.65 | 0.40 | ||
| Dodecanoic acid | 0.002 | 0.049 | 0.023 | 0.97 | 0.23 | ||
| Tetradecanoic acid | 0.189 | 0.256 | 0.133 | 0.11 | 0.17 | X | X |
| 14-Tetradecanolide | 0.026 | 0.010 | 0.006 | 0.60 | 0.20 | ||
| 16-Hexadecanolide | 28.328 | 24.163 | 51.628 | 0.14 | 0.20 | ||
| Hexadecenoic acid | 0.272 | 0.047 | 0.050 | 0.76 | 0.78 | ||
| 16-Hexadecenolide | 6.914 | 10.721 | 2.426 | 0.11 | 0.17 | X | |
| Octadecanal | 0.224 | 0.071 | 0.036 | 0.14 | 0.16 | ||
| 18-Octadecanolide | 9.094 | 3.807 | 7.526 | 0.20 | 0.20 | X | X |
| 18-Octadecenolide | 26.567 | 30.006 | 18.135 | 0.78 | 0.50 | ||
| Ethyl hexadecanoate | 3.491 | 4.334 | 1.843 | 0.19 | 0.24 | ||
| 20-Eicosanolide | 0.678 | 0.944 | 1.161 | 0.60 | 0.76 | ||
| Heneicosane | 3.427 | 0.165 | 0.061 | 0.14 | 0.23 | ||
| Tricosane | 4.756 | 3.241 | 2.946 | 0.87 | 0.45 | ||
| Tricosene | 0.246 | 0.101 | 0.088 | 0.38 | 0.45 | X | X |
| Tricosadiene | 0.063 | 0.052 | 0.006 | 0.10 | 0.16 | X | X |
| Tetracosane | 0.151 | 0.082 | 0.065 | 0.32 | 0.45 | ||
| Tetracosene | 0.308 | 0.244 | 0.069 | 0.14 | 0.20 | ||
| Pentacosane | 1.050 | 2.485 | 2.252 | 0.33 | 0.44 | ||
| Pentacosene | 0.216 | 2.184 | 0.509 | 0.31 | 0.19 | ||
| Pentacosadiene | 1.076 | 0.150 | 0.055 | 0.14 | 0.16 | X | X |
| Hexacosane | 0.073 | 0.083 | 0.048 | 0.14 | 0.23 | X | X |
| Hexacosene | 0.229 | 0.180 | 0.217 | 0.11 | 0.19 | ||
| Heptacosane | 0.977 | 0.746 | 0.624 | 0.19 | 0.29 | ||
| Heptacosene | 0.681 | 4.787 | 0.231 | 0.10 | 0.05 | X | X |
| Heptacosadiene | 0.577 | 0.524 | 0.515 | 0.16 | 0.13 | X | |
| Octacosene | 0.107 | 0.067 | 0.018 | 0.14 | 0.23 | ||
| Nonacosane | 0.201 | 0.041 | 0.123 | 0.87 | 0.55 | ||
| Nonacosene | 0.728 | 1.373 | 0.386 | 0.14 | 0.22 | ||
| Hentriacontene | 0.115 | 0.243 | 0.083 | 0.92 | 0.23 | ||
| Dimethyl 16-hexadecanolide | 0.153 | 0.133 | 0.184 | 0.33 | 0.45 | ||
| Dimethyl 18-octadecanolide | 0.199 | 0.027 | 0.082 | 0.78 | 0.20 | ||
| Dimethyl 16-hexadecenolide | 0.103 | 0.055 | 0.128 | 0.78 | 0.72 | ||
| Hentriacontadiene | 0.182 | 0.059 | 0.072 | 0.87 | 0.28 | ||
| Nonacosadiene | 0.133 | 0.131 | 0.025 | 0.14 | 0.20 | ||
| Octadecanoic acid | 0.920 | 1.292 | 0.617 | 0.78 | 0.89 | ||
| Octadecenoic acid | 7.177 | 6.610 | 7.665 | 0.60 | 0.44 | ||
| Tritriacontadiene | 0.184 | 0.040 | 0.139 | 0.39 | 0.54 | X | |
| Tritriacontene | 0.012 | 0.041 | 0.051 | 0.24 | 0.40 | X | X |
FDR-corrected P values were generated by fitting a standard least squares ANOVA model with compound abundance as the dependent variable and social behavior as a fixed effect for both sociality (solitary vs. eusocial) and caste. The best-fit model columns indicate whether the compounds were included in the elastic net model with the lasso penalty (alpha = 1).
Table S2.
Differences in relative abundances of chemicals from Dufour’s gland extracts
| Compound | Social populations | Solitary populations- reproductive | FDR P value | Best-fit model | |||
| Foundress | Worker | Sociality | Caste | Behavior | Caste | ||
| Tritriacontene | 0.060 | 0.016 | 0.004 | 0.40 | 0.07 | ||
| 14-Tetradecanolide | 0.179 | 0.047 | 0.047 | 0.58 | 0.30 | ||
| 15 Pentadecanolide | 0.068 | 0.026 | 0.029 | 0.40 | 0.06 | ||
| 22-Docosanolide | 0.191 | 0.002 | 0.003 | 0.40 | 0.04 | ||
| 24-Tetracosanolide | 2.155 | 0.123 | 0.079 | 0.43 | 0.31 | ||
| 26 Hexadecanolide | 0.090 | 0.030 | 0.013 | 0.46 | 0.21 | ||
| Methyl oleate | 0.113 | 0.028 | 0.024 | 0.51 | 0.31 | ||
| Hexadecanoic acid | 0.268 | 0.024 | 0.010 | 0.58 | 0.06 | X | X |
| 16-Hexadecanolide | 24.642 | 58.905 | 51.516 | 0.40 | 0.43 | ||
| 16-Hexadecenolide | 2.143 | 0.698 | 9.834 | 0.40 | 0.21 | X | X |
| 18-Octadecanolide | 26.717 | 17.484 | 15.863 | 0.51 | 0.58 | ||
| 18-Octadecenolide | 13.316 | 14.624 | 15.447 | 0.40 | 0.04 | ||
| Eicosanamide | 0.234 | 0.028 | 0.014 | 0.40 | 0.10 | ||
| 20-Eicosanolide | 3.636 | 0.100 | 0.049 | 0.74 | 0.50 | ||
| Docosane | 0.009 | 0.001 | 0.006 | 0.40 | 0.04 | ||
| Docosanamide | 4.178 | 0.615 | 0.260 | 0.59 | 0.17 | X | X |
| Tricosane | 4.144 | 1.085 | 1.582 | 0.64 | 0.12 | X | |
| Tricosene | 0.083 | 0.008 | 0.027 | 0.46 | 0.17 | ||
| Tetracosane | 1.827 | 0.048 | 0.071 | 0.40 | 0.12 | ||
| Pentacosane | 6.758 | 1.208 | 0.987 | 0.40 | 0.07 | ||
| Hexacosane | 0.384 | 0.058 | 0.037 | 0.40 | 0.14 | ||
| Heptacosane | 1.647 | 0.702 | 0.325 | 0.40 | 0.04 | ||
| Heptacosene | 1.131 | 0.248 | 0.054 | 0.40 | 0.06 | X | X |
| Nonacosene (first isomer) | 0.338 | 0.045 | 0.053 | 0.40 | 0.04 | ||
| Nonacosene (second isomer) | 0.850 | 0.095 | 0.058 | 0.50 | 0.30 | ||
| Dimethyl-16-hexadecanolide | 0.914 | 0.206 | 0.158 | 0.40 | 0.30 | ||
| Dimethyl-16-hexadecenolide | 0.056 | 0.076 | 0.110 | 0.40 | 0.16 | X | |
| Dimethyl 18-octadecanolide | 0.360 | 0.073 | 0.046 | 0.46 | 0.43 | ||
| Ethyl hexadecanoate | 0.004 | 0.000 | 0.005 | 0.61 | 0.50 | ||
| Ethyl oleate | 0.041 | 0.003 | 0.037 | 0.61 | 0.12 | X | X |
| Heneicosane | 0.027 | 0.005 | 0.009 | 0.40 | 0.04 | ||
| Hentriacontane | 0.160 | 0.012 | 0.018 | 0.40 | 0.04 | ||
| Hentriacontadiene | 0.544 | 0.024 | 0.034 | 0.65 | 0.43 | ||
| Methyl 15-pentadecanolide | 0.006 | 0.021 | 0.019 | 0.84 | 0.35 | ||
| Methyl 18-octadecanolide | 0.028 | 0.000 | 0.015 | 0.43 | 0.46 | X | X |
| Methyl 19-nonadecanolide | 0.862 | 1.258 | 1.568 | 0.62 | 0.30 | X | |
| Methyl 16-hexadecanolide | 0.029 | 0.000 | 0.005 | 0.40 | 0.47 | ||
| Octadecanal | 0.005 | 0.000 | 0.013 | 0.99 | 1.00 | ||
| Octadecanoic acid | 0.008 | 0.008 | 0.008 | 0.87 | 0.77 | ||
| Octadecenoic acid | 0.192 | 1.818 | 1.437 | 0.51 | 0.35 | ||
| Pentacosene (first isomer) | 0.2700 | 0.0322 | 0.0124 | 0.40 | 0.12 | ||
| Pentacosene (second isomer) | 0.7701 | 0.1893 | 0.0898 | 0.40 | 0.04 | X | X |
| Triacontane | 0.1287 | 0.0165 | 0.0149 | 0.40 | 0.21 | ||
| Tritriacontene | 0.4341 | 0.0063 | 0.0105 | 0.40 | 0.08 | ||
FDR-corrected P values were generated by fitting a standard least squares ANOVA model with compound abundance as the dependent variable and social behavior as a fixed effect for both sociality (solitary vs. eusocial) and caste. The best-fit model columns indicate whether the compounds were included in the elastic net model with the lasso penalty (alpha = 1).
We found that there were measurable differences in both the cuticular (Fig. 3) and Dufour’s gland (Fig. 4) chemical profiles of solitary and social L. albipes populations, and our linear discriminant analysis accurately differentiated between behavioral forms (Figs. 3A and 4A), as well as among different castes (Figs. 3B and 4B). These differences are unlikely to be caused by genetic or geographic variation alone: The profiles of females from social populations seemed more similar to each other than did those of bees from solitary populations even though the two social populations sampled were the most geographically distant from each other (Fig. S4).
Fig. 3.
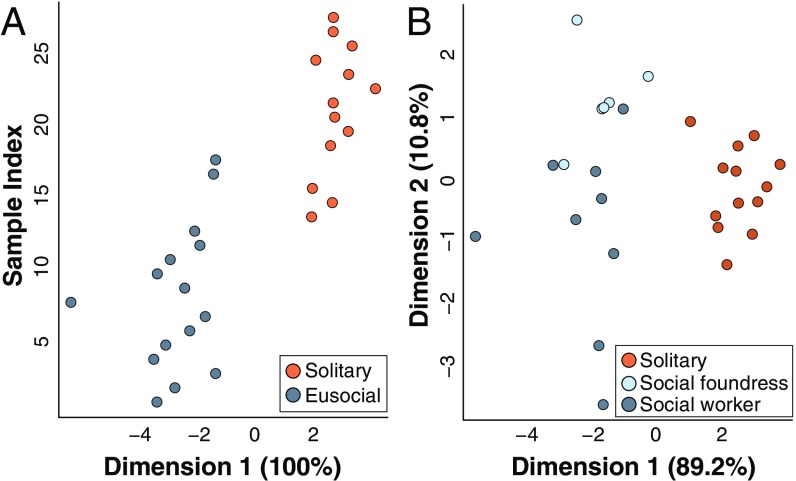
L. albipes females have distinguishable cuticular chemical profiles as exhibited both by behavior (solitary versus social) and by caste (workers, social foundresses, and solitary reproductives). (A) The LDA accurately differentiated social vs. solitary forms of L. albipes: dimension 1, 100%; Wilk’s lambda, P = 1.901e−05. Cross-validation was able to correctly classify 100% of the samples. (B) The LDA was able to accurately discriminate individuals by caste (dimension 1, 89.2%; dimension 2, 10.8%; Wilk’s lambda, P = 0.001), and cross-validation correctly classified 93.8% of the samples.
Fig. 4.
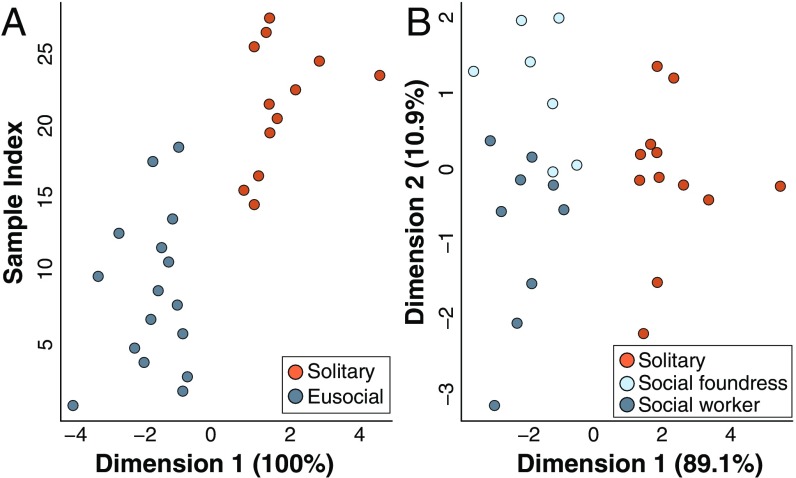
L. albipes females have distinguishable Dufour’s gland chemical profiles as exhibited both by behavior (solitary versus social) and by caste (workers, social foundresses, and solitary reproductives). (A) The LDA accurately differentiated social vs. solitary forms of L. albipes: canonical 1, 100%; Wilk’s lambda, P = 2.169e-05. Cross-validation was able to correctly classify 100% of the samples. (B) The LDA was able to accurately place individuals by caste (dimension 1, 89.1%; dimension 2, 10.9%; Wilk’s lambda, P = 0.0074), and cross-validation correctly classified samples 96.4% of the time.
Fig. S4.
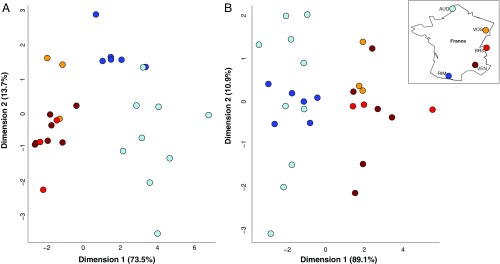
LDA by population. Solitary populations are shades of red, and social populations are shades of blue. All populations are approximately equally geographically and genetically divergent from each other regardless of social behavior, suggesting that the observed chemical differences between social forms cannot be due to population-level variation alone. (A) The LDA was moderately successful at differentiating populations of L. albipes based on cuticular hydrocarbons (dimension 1, 75.8%; dimension 2, 12.8%; Wilk’s lambda, P = 0.024). (B) However, the LDA did not successfully differentiate populations of L. albipes based on Dufour’s glands: dimension 1, 74.9%; dimension 2, 16.0%; Wilk’s lambda, P = 0.082.
CHCs.
A relatively small number of the cuticular compounds sampled were required for accurate discrimination among social forms and castes in this species. For social forms, the best-fitting model included 11 of 40 compounds, and cross-validation could accurately predict group membership in 100% of individuals (Fig. 3A). For caste-level differences, 9 of 40 were included in the best-fitting model, and cross-validation correctly classified 93.8% of individuals.
Dufour’s glands.
We also found differences in the relative abundances of a number of chemical compounds in the Dufour’s gland between social and solitary forms of L. albipes. Seven of 44 compounds were included in the best-fitting model, and they could correctly classify all our specimens as social or solitary with cross-validation (Fig. 4A). Ten of 44 compounds were included in the best-fitting model for caste-related differences (Fig. 4B), and cross-validation correctly assigned the caste of individuals 96.4% of the time.
In both the cuticle and the Dufour’s glands, the compounds that were most informative to caste and social form were not the most abundant compounds, but rather those found at intermediate levels (Tables S1 and S2). The differences in the cuticular compounds were primarily in the relative abundances of long-chain hydrocarbons, and, in the Dufour’s glands, differences were found in macrocyclic lactones, hydrocarbons, and ethyl esters. Macrocyclic lactones are considered a hallmark of halictine Dufour’s gland secretions and show remarkable variation within and among species (50). Ethyl esters have been documented in closely related halictid species, and the relative abundances of similar ethyl esters (including ethyl octadecanoate) differ between virgin and ovipositing queens (53).
Discussion
We documented evidence that evolutionary transitions between solitary and social living are associated with complimentary changes in signaling chemistry and antennal morphology. We observed consistent changes in sensilla density as social behavior changed within this group. Moreover, signal production and perception changed rapidly within a single, socially polymorphic species (L. albipes), where both the chemical profiles as well as antennal morphology differed between social and solitary behavioral forms. Taken together, these results suggest that there is a strong link between the evolution of social behavior and investment in communication.
Interestingly, we did not observe an increase in sensilla density as social behavior is gained. Rather, the ancestrally solitary halictids seemed to have sensilla densities similar to eusocial species. Importantly, most of the ancestrally solitary species included in this study were from the subfamily Rophitinae, and most species in this group are oligolectic and specialize on pollen collection from a small number of closely related plants (54). It is possible that this specialization selects for increased antennal receptors to accurately identify host species. Another possibility is that a higher sensilla density may be an important precursor to the evolution of social behavior and could help to explain the repeated gains of eusociality in this group of bees.
We observed consistent decreases in sensilla density as social behavior is lost. This pattern was observed both across halictid species as well as within a single, socially polymorphic species. The reduction in sensilla numbers in secondarily solitary species relative to their social ancestors may indicate a functional redundancy in antennal sensilla in halictids. A similar reduction in sensory systems occurs in cave-dwelling animals, where their visual system is greatly reduced and individuals are often blind (55). Importantly, the reduction we observed in this group is not a complete vestigilization. Instead, it represents a decreased investment in antennal sensilla, perhaps in the absence of complex communication associated with group living.
There were, however, some interesting outliers in both the social and the solitary species. For example, Halictus scabiosae has one of the lowest measures of sensilla density among all species examined. Recent behavioral studies may shed some light on this observation. Although this species is eusocial, individuals also show a large degree of drifting among nests, and females will often leave their natal nests to reproduce in nests of unrelated individuals (56, 57). Many halictids mark nests with chemical cues, and these odors are used by guards to distinguish nestmates and nonnestmates (58, 59). It is possible that the high degree of drifting in this species could be associated with a decrease in olfactory cues or perception although work is needed to determine whether this is indeed the case. Lasioglossum vierecki had the highest density of antennal sensilla among the solitary halictids and exceeded the observed densities of several of the eusocial species. Although nest excavations have suggested that it is indeed solitary (60, 61), little else is known about the nesting biology of this species.
A large proportion of filiform sensilla are associated with the detection of chemical odors. Although both solitary and social individuals use chemical odors to perceive signals and cues (e.g., in mate finding and foraging), social bees must also perceive social signals, including those used to identify nestmates and infer information about social organization. A greater number of antennal sensilla may confer two functional benefits specific to the needs of social living. The first is an increase in surface area, which could increase the speed of discrimination. Previous work with ants supports this prediction: Workers with a greater density of sensilla more accurately recognized nonnestmates and responded with greater levels of aggression than workers with fewer sensilla (37). The extra sensilla found in social species may also represent an increase in the variety of chemoreceptors present on the antennae without compromising sensitivity and could accommodate more precise or complex signals used in social interactions. In fact, several studies have suggested that the evolution of eusociality is correlated with an expansion of the chemoreceptor gene families (40, 62). Similarly, the genomic data available for L. albipes suggest that several olfactory receptors are subject to positive selection in this lineage, compared with other social insect species (40), and initial analyses of divergence between social and solitary forms within this species have identified differences accumulating in a putative olfactory receptor (63).
In addition to chemoreception, filiform sensilla can also have mechanosensory functions. We were unable to distinguish between these different sensory functions, and it is possible that differences in sensilla density could similarly be associated with differences in the mechanosensory abilities of social and solitary bees. These differences could also be explained by differences in the requirements of social interactions because, along with chemical cues, social hierarchies in halictids are also frequently maintained through physical interactions between the dominant female and workers (64, 65). In eusocial colonies, queens can use aggressive behavior, such as nudging or ramming workers, to elicit subordinate behavior and maintain a reproductive division of labor in the group (66).
Nevertheless, the differences in the chemical profiles of social and solitary L. albipes individuals suggest that the variation observed in sensilla density is likely to have a chemosensory component. Mirroring the patterns in sensilla density, differences in the chemical composition of the cuticle and Dufour’s gland could accurately discriminate among different castes and social forms within this species. This variation in signal production was driven by changes in the relative abundances of these compounds rather than differences in the total number or absolute amounts of the chemicals produced. These observations are consistent with other studies that have documented intraspecific variation in chemical production, where changes in relative abundances of particular compounds are associated with differences in sex pheromones and attractiveness to mates (67–69), colony membership and nestmate recognition (33, 70), caste, and task allocation (71–73). For example, in one halictid species, Lasioglossum malachurum, changes in both the total amounts and relative abundances of volatiles on the cuticle surface and in the Dufour’s gland correlate with the reproductive status of gynes and old foundresses (53), and the cuticular extracts seem to contain the compounds most attractive to males during mating (74). Solitary bees may not require such dynamic or detailed chemical odors as social females. For example, it may be sufficient for solitary bees to identify self and nonself and to recognize potential mates whereas social bees may require a greater degree of signal lability to further distinguish additional social signals, such as nestmates from nonnestmates and/or signals associated with a social hierarchy.
A relatively consistent subset of the compounds examined were associated with social behavior in L. albipes. Cuticular differences associated with behavioral types were largely driven by variation in long-chain hydrocarbons, primarily alkanes and alkenes. Caste- and behavior-related differences in the Dufour’s glands were largely associated with differences in the relative amounts of macrocyclic lactones, a key component of halictid Dufour’s secretions (75). Two compounds were included in all of the best-fitting models constructed: 16-hexadecenolide and heptacosene. 16-Hexadecenolide is a lactone often associated with the subgenus Eyvlaeus and with L. albipes in particular (76) although little is known of its function. Heptacosene is a long-chain hydrocarbon that is associated with differences in mating status in the closely-related L. malachurum (74).
In conclusion, the rapid changes in chemical signaling within a single, socially polymorphic species, coupled with the repeated reduction in features associated with signal perception within and between species, suggest that there are trade-offs associated with communication in social groups. By investing in higher numbers of sensilla, social bees may increase the number of receptors available and thereby increase the speed and accuracy of their response to social signals. In contrast, solitary females can still recognize relevant stimuli but no longer require dense, costly sensilla to perceive complex social signals. This pattern of divestment in sensory systems as group living is lost highlights the tight links between communication systems and social behavior.
Materials and Methods
If changes in sensory systems are a common feature of changes in social structure, then they should be evident among the independent gains and losses of social behavior within the Halictidae. We imaged antennae of adult females from 36 focal species that span all of the gains and a large proportion of the losses of social behavior in this clade (Fig. 1). No socially polymorphic species were included in the interspecific comparisons. We analyzed the data with a PGLS model and used existing, molecular data for Halictidae (77–81) to generate a maximum-likelihood tree for our focal species with RAxML using a mixed/partition model with GTRGAMMA for bootstrapping with autoMRE. The majority-rule consensus tree was used for all downstream analyses. This tree was nearly identical to previously published phylogenies (77–81).
Antennal Imaging and Sensilla Quantification.
We used environmental scanning electron microscopy (eSEM) to quantify sensilla density on the 9th and 10th segments of antennae across 36 halictid species (solitary, n = 19; social, n = 17) representing all origins of eusociality in this group, as well as the majority of reversals to solitary living (Fig. 1) (81). Sensilla density increases distally across the length of the antenna (39), and the last segments are most likely to come into contact with other workers and be responsible for detecting social signals. Given this functional importance, this study focused on sensilla density of the 9th and 10th segments only. To evaluate whether or not these changes can also occur within a single species, we also compared sensilla density of individuals sampled from two social and two solitary populations of L. albipes.
Specimens used for eSEM were pinned specimens borrowed from the American Museum of Natural History and the Harvard Museum of Comparative Zoology. L. albipes samples were obtained from Cecile Plateaux-Quénu, Audresselles, France; these samples were collected midsummer from established social and solitary populations. Antennal morphology was imaged using an environmental SEM (Zeiss EVO 55) with an Everhart–Thornley SE detector operating in variable pressure mode. Samples were previously preserved dry, and no additional preparation was necessary. Antennae were selected at random from each individual.
Image analysis was carried out in ImageJ v1.49 (82) with the Cell Counter package (83). Analysts were blind with respect to species and social behavior. Density of hair-like sensilla was measured across three randomly placed quadrats and averaged to give a density figure for each antennomere. Density results were subsequently adjusted to account for image resolution differences.
Statistical Tests for Sensilla Density.
PGLS analysis was performed in R version 3.3.0 (84), using the package “caper” (85). Pagel’s λ was estimated concurrently by maximum likelihood.
Compound Identification and Characterization in L. albipes.
Females were hand-netted during foraging bouts. Social females were sampled at two time points during the field season: during nest initiation in April to May 2014 and midseason in June to July 2014. Solitary females were sampled throughout June to July 2014; these populations initiate their nests later in the season and were not active before this time. See Caste Assignment for more details.
Females were flash-frozen in liquid nitrogen and stored at −80C until they were processed for the chemical analyses. Insect cuticles and Dufour’s glands were dissected and placed into glass vials with 100 μL of hexane. Extracts were then processed using GC-MS and GC-flame ionization detection (FID). Samples were first analyzed by combined gas chromatography/mass spectrometry (GC-MS) (GC 7890A, MS 5975C; Agilent) using an HP-5MS capillary column, with temperature programmed from 60 °C to 300 °C at 10 °C /min. Compounds were identified by their mass fragmentation and retention times compared with synthetic standards when available. Compound quantification across samples was thereafter performed by gas chromatography with flame ionization detection (GC-FID) (CP 3800; Varian) using a DB-1 fused silica capillary column (30 m × 0.25 mm i.d.), temperature programmed as above, using peak integration. Analysts were blind with respect to sample identity and social behavior. Peaks quantifiable in fewer than nine samples were removed from downstream analysis. Known contaminants were also excluded from subsequent analyses. All remaining peaks were used in a multivariate analysis, and jack-knife values were calculated to identify outliers. Forty compounds from cuticular extracts and 44 compounds from Dufour’s gland extracts remained for subsequent analysis. Finally, the relative proportions of each compound were then transformed by taking the centered log ratio of the relative proportions to better approximate a normal distribution for subsequent statistical tests (86).
Statistical Tests for Chemical Analyses.
One-way ANOVAs were performed on the centered, log ratio-transformed data for each compound to assess differences between social forms (social vs. solitary) and castes (solitary reproductive, social foundress, and social worker). To account for multiple testing, a false discovery rate (FDR) correction was applied to the results of each model across all tested compounds. To assess the best-fitting models for the chemical datasets, we used the glmnet package in R to use an elastic net (87). We then used these best-fitting models to conduct linear discriminant analyses for each behavioral group using the lda function in R. Last, we conducted cross-validation to assess model performance. Stepwise, linear discriminant analyses and penalized LDA were also implemented in R; in all instances, behavioral and caste groupings were successfully distinguished.
Caste Assignment.
Behavioral morphs of L. albipes are allopatric: Solitary populations are found in eastern France and eusocial nests in western France. This difference seems to be genetic: When individual females from these populations were reared in the laboratory under common and reciprocal conditions, they maintained the social behaviors observed in the field (43). We determined caste based on the timing and location of our field collections. Social reproductives were collected in the spring (April to May), and social workers were collected in the summer (June to July). Solitary reproductives were also collected in summer from the solitary populations. During dissection, a number of physiological traits were recorded, including ovarian development, fat body size, mandible wear, and wing wear. These traits are often used as biomarkers to assign caste based on field-collected specimens (45, 46), and an LDA was able to categorize our collected females successfully into each of three groups: foundress, social worker, or solitary reproductive, based on these four traits alone.
Acknowledgments
We thank Jason Gibbs for sharing the tree files for his 2012 phylogeny; and Carolyn Marks (Center for Nanoscale Systems, Harvard University) and John Schreiber (Princeton Institute for Science and Technology of Materials) for advice and guidance with eSEM imaging. This work was supported by Holsworth Research Wildlife funding (to B.W.), by NSF Grant IOS-1257543 (to N.E.P. and S.D.K.), by Australian Research Council Grants DP0987360 and DP120100162 (to M.A.E.), by The Norman and Rose Lederer Chair of Biology (A.H.), and by Princeton University.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 6424.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620780114/-/DCSupplemental.
References
- 1.Blum MS. Semiochemical parsimony in the Arthropoda. Annu Rev Entomol. 1996;41:353–374. doi: 10.1146/annurev.en.41.010196.002033. [DOI] [PubMed] [Google Scholar]
- 2.Smith AA, Millar JG, Suarez AV. A social insect fertility signal is dependent on chemical context. Biol Lett. 2015;11:20140947. doi: 10.1098/rsbl.2014.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum MS, Brand JM. Social insect pheromones: Their chemistry and function. Am Zool. 1972;12:553–576. [Google Scholar]
- 4.Blum M. Pheromonal sociality in the Hymenoptera. In: Birch M, editor. Pheromones. Vol 32. North-Holland; Amsterdam: 1974. pp. 222–249. [Google Scholar]
- 5.Le Conte Y, Hefetz A. Primer pheromones in social hymenoptera. Annu Rev Entomol. 2008;53:523–542. doi: 10.1146/annurev.ento.52.110405.091434. [DOI] [PubMed] [Google Scholar]
- 6.van Wilgenburg E, Symonds MR, Elgar MA. Evolution of cuticular hydrocarbon diversity in ants. J Evol Biol. 2011;24:1188–1198. doi: 10.1111/j.1420-9101.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- 7.Grüter C, Keller L. Inter-caste communication in social insects. Curr Opin Neurobiol. 2016;38:6–11. doi: 10.1016/j.conb.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Wyatt TD. Pheromones and Animal Behavior: Chemical Signals and Signatures. 2nd Ed Cambridge Univ Press; Cambridge, MA: 2014. [Google Scholar]
- 9.Elgar MA. Integrating insights across diverse taxa: Challenges for understanding social evolution. Front Ecol Evol. 2015;3:1–8. [Google Scholar]
- 10.Leonhardt SD, Menzel F, Nehring V, Schmitt T. Ecology and evolution of communication in social insects. Cell. 2016;164:1277–1287. doi: 10.1016/j.cell.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Hoover SER, Keeling CI, Winston ML, Slessor KN. The effect of queen pheromones on worker honey bee ovary development. Naturwissenschaften. 2003;90:477–480. doi: 10.1007/s00114-003-0462-z. [DOI] [PubMed] [Google Scholar]
- 12.Holman L, Jørgensen CG, Nielsen J, d’Ettorre P. Identification of an ant queen pheromone regulating worker sterility. Proc Biol Sci. 2010;277:3793–3800. doi: 10.1098/rspb.2010.0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuura K, et al. Identification of a pheromone regulating caste differentiation in termites. Proc Natl Acad Sci USA. 2010;107:12963–12968. doi: 10.1073/pnas.1004675107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holman L, Lanfear R, d’Ettorre P. The evolution of queen pheromones in the ant genus Lasius. J Evol Biol. 2013;26:1549–1558. doi: 10.1111/jeb.12162. [DOI] [PubMed] [Google Scholar]
- 15.Nunes TM, et al. Queen signals in a stingless bee: Suppression of worker ovary activation and spatial distribution of active compounds. Sci Rep. 2014;4:7449. doi: 10.1038/srep07449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blum MS. Alarm pheromones. Annu Rev Entomol. 1969;14:57–80. [Google Scholar]
- 17.Van Oystaeyen A, et al. Conserved class of queen pheromones stops social insect workers from reproducing. Science. 2014;343:287–290. doi: 10.1126/science.1244899. [DOI] [PubMed] [Google Scholar]
- 18.Oliveira RC, et al. The origin and evolution of queen and fertility signals in Corbiculate bees. BMC Evol Biol. 2015;15:254. doi: 10.1186/s12862-015-0509-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oi C A, et al. Dual effect of wasp queen pheromone in regulating insect sociality. Curr Biol. 2015;25:1638–1640. doi: 10.1016/j.cub.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 20.Kather R, Martin SJ. Evolution of cuticular hydrocarbons in the hymenoptera: A meta-analysis. J Chem Ecol. 2015;41:871–883. doi: 10.1007/s10886-015-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peso M, Elgar MA, Barron AB. Pheromonal control: Reconciling physiological mechanism with signalling theory. Biol Rev Camb Philos Soc. 2015;90:542–559. doi: 10.1111/brv.12123. [DOI] [PubMed] [Google Scholar]
- 22.Kocher SD, Grozinger CM. Cooperation, conflict, and the evolution of queen pheromones. J Chem Ecol. 2011;37:1263–1275. doi: 10.1007/s10886-011-0036-z. [DOI] [PubMed] [Google Scholar]
- 23.Greenfield MD. Signalers and Receivers: Mechanisms and Evolution of Arthropod Communication. Oxford Univ Press; Oxford: 2002. [Google Scholar]
- 24.Symonds MR, Elgar MA. The evolution of pheromone diversity. Trends Ecol Evol. 2008;23:220–228. doi: 10.1016/j.tree.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Endler JA. Some general comments on the evolution and design of animal communication systems. Philos Trans R Soc Lond B Biol Sci. 1993;340:215–225. doi: 10.1098/rstb.1993.0060. [DOI] [PubMed] [Google Scholar]
- 26.Kaissling K-E. Insect olfaction. In: Beidler LM, editor. Handbook of Sensory Physiology. Vol IV. Springer; Heidelberg: 1971. pp. 351–431. [Google Scholar]
- 27.Hildebrand JG. Analysis of chemical signals by nervous systems. Proc Natl Acad Sci USA. 1995;92:67–74. doi: 10.1073/pnas.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanes JR, Hildebrand JG. Structure and development of antennae in a moth, Manduca sexta. Dev Biol. 1976;51:280–299. [PubMed] [Google Scholar]
- 29.Sanes JR, Hildebrand JG. Origin and morphogenesis of sensory neurons in an insect antenna. Dev Biol. 1976;51:300–319. doi: 10.1016/0012-1606(76)90145-7. [DOI] [PubMed] [Google Scholar]
- 30.Chapman R. The Insects: Structure and Function. Cambridge Univ Press; Cambridge, UK: 2013. [Google Scholar]
- 31.Bernays E, Chapman R. Phenotypic plasticity in numbers of antennal chemoreceptors in a grasshopper: Effects of food. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1998;183:69–76. [Google Scholar]
- 32.Renthal R, Velasquez D, Olmos D, Hampton J, Wergin WP. Structure and distribution of antennal sensilla of the red imported fire ant. Micron. 2003;34:405–413. doi: 10.1016/S0968-4328(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 33.Ozaki M, et al. Ant nestmate and non-nestmate discrimination by a chemosensory sensillum. Science. 2005;309:311–314. doi: 10.1126/science.1105244. [DOI] [PubMed] [Google Scholar]
- 34.Babu MJ, Ankolekar SM, Rajashekhar K. Castes of the weaver ant Oecophylla smaragdina (Fabricius) differ in the organization of sensilla on their antennae and mouthparts. Curr Sci. 2011;101:755–764. [Google Scholar]
- 35.Ravaiano SV, Ferreira RdeP, Campos LA, Martins GF. The antennal sensilla of Melipona quadrifasciata (Hymenoptera: Apidae: Meliponini): A study of different sexes and castes. Naturwissenschaften. 2014;101:603–611. doi: 10.1007/s00114-014-1184-0. [DOI] [PubMed] [Google Scholar]
- 36.Fialho Md CQ, Guss-Matiello CP, Zanuncio JC, Campos LAO, Serrão JE. A comparative study of the antennal sensilla in corbiculate bees. J Apic Res. 2014;53:392–403. [Google Scholar]
- 37.Gill KP, van Wilgenburg E, Macmillan DL, Elgar MA. Density of antennal sensilla influences efficacy of communication in a social insect. Am Nat. 2013;182:834–840. doi: 10.1086/673712. [DOI] [PubMed] [Google Scholar]
- 38.Spaethe J, Brockmann A, Halbig C, Tautz J. Size determines antennal sensitivity and behavioral threshold to odors in bumblebee workers. Naturwissenschaften. 2007;94:733–739. doi: 10.1007/s00114-007-0251-1. [DOI] [PubMed] [Google Scholar]
- 39.Wcislo WT. Sensilla numbers and antennal morphology of parasitic and nonparasitic bees (Hymenoptera, Apoidea) Int J Insect Morphol Embryol. 1995;24:63–81. [Google Scholar]
- 40.Zhou X, et al. Chemoreceptor evolution in hymenoptera and its implications for the evolution of eusociality. Genome Biol Evol. 2015;7:2407–2416. doi: 10.1093/gbe/evv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kocher SD, Paxton RJ. Comparative methods offer powerful insights into social evolution in bees. Apidologie (Celle) 2014;45:289–305. [Google Scholar]
- 42.Danforth BN, Cardinal S, Praz C, Almeida EAB, Michez D. The impact of molecular data on our understanding of bee phylogeny and evolution. Ann Rev Entomol. 2013;58:57–78. doi: 10.1146/annurev-ento-120811-153633. [DOI] [PubMed] [Google Scholar]
- 43.Plateaux-Quénu C, Plateaux L, Packer L. Population-typical behaviours are retained when eusocial and non-eusocial forms of Evylaeus albipes (F.)(Hymenoptera, Halictidae) are reared simultaneously in the laboratory. Insectes Soc. 2000;47:263–270. [Google Scholar]
- 44.Trabalon M, Plateaux-Quenu C, Plateaux L. Comparaison des secretions de la glande de dufour chez differentes populations d'especes proches parentes d'abeilles halictines: Evylaeus albipes (f.), Evylaeus calceatus (scop.) Bulletin des Académie et Société Lorraines des Sciences. 1996;35:171–176. [Google Scholar]
- 45.Richards M, Vickruck J, Rehan S. Colony social organisation of Halictus confusus in southern Ontario, with comments on sociality in the subgenus H. (Seladonia) J Hymenopt Res. 2010;19:144–158. [Google Scholar]
- 46.Plateaux-Quénu C. Comparative biological data in two closely related eusocial species: Evylaeus calceatus (Scop.) and Evylaeus albipes (F.)(Hym., Halictinae) Insectes Soc. 1992;39:351–364. [Google Scholar]
- 47.Hadley NF. Cuticle: Ecological significance. In: Bereiter-Hahn J, Matoltsy AG, Richards KS, editors. Biology of the Integument: Invertebrates. Vol 1. Springer; Berlin: 1984. pp. 685–693. [Google Scholar]
- 48.Bereiter-Hahn J, Matoltsy AG, Richards KS, editors. Biology of the Integument: Invertebrates. Vol 1 Springer; Berlin: 1984. [Google Scholar]
- 49.Hefetz A. The role of Dufour’s gland secretions in bees. Physiol Entomol. 1987;12:243–253. [Google Scholar]
- 50.Hefetz A, Bergström G, Tengö J. Species, individual and kin specific blends in Dufour’s gland secretions of halictine bees: Chemical evidence. J Chem Ecol. 1986;12:197–208. doi: 10.1007/BF01045603. [DOI] [PubMed] [Google Scholar]
- 51.Brooks RW, Cane JH. Origin and chemistry of the secreted nest entrance lining of Halictus hesperus (Hymenoptera: Apoidea) J Kans Entomol Soc. 1984;57:161–165. [Google Scholar]
- 52.Hefetz A. Individual badges and specific messages in multicomponent pheromones of bees (Hymenoptera: Apidae) Entomol Gen. 1990;15:103–113. [Google Scholar]
- 53.Ayasse M, Engels W, Hefetz A, Lübke G, Francke W. Ontogenetic patterns in amounts and proportions of Dufour’s gland volatile secretions in virgin and nesting queens of Lasioglossum malachurum (Hymenoptera: Halictidae) Z Naturforsch C. 1990;45:709–714. [Google Scholar]
- 54.Michener CD. The Bees of the World. John Hopkins Univ Press; Baltimore: 2007. [Google Scholar]
- 55.Fong DW, Kane TC, Culver DC. Vestigialization and loss of nonfunctional characters. Annu Rev Ecol Syst. 1995;26:249–268. [Google Scholar]
- 56.Ulrich Y, Perrin N, Chapuisat M. Flexible social organization and high incidence of drifting in the sweat bee, Halictus scabiosae. Mol Ecol. 2009;18:1791–1800. doi: 10.1111/j.1365-294X.2009.04154.x. [DOI] [PubMed] [Google Scholar]
- 57.Brand N, Chapuisat M. Low relatedness and frequent inter-nest movements in a eusocial sweat bee. Insectes Soc. 2016;63:249–256. [Google Scholar]
- 58.Barrows EM, Bell WJ, Michener CD. Individual odor differences and their social functions in insects. Proc Natl Acad Sci USA. 1975;72:2824–2828. doi: 10.1073/pnas.72.7.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wcislo WT. Social interactions and behavioral context in a largely solitary bee, Lasioglossum (Dialictus) figueresi (Hymenoptera, Halictidae) Insectes Soc. 1997;44:199–208. [Google Scholar]
- 60.Knerer G. Synergistic evolution of halictine nest architecture and social behavior. Can J Zool. 1969;47:925–930. [Google Scholar]
- 61.Michener CD. Bees of a limited area in southern Mississippi (Hymenoptera; Apoidea) Am Midl Nat. 1947;38:443–455. [Google Scholar]
- 62.Kapheim KM, et al. Genomic signatures of evolutionary transitions from solitary to group living. Science. 2015;348:1139–1143. doi: 10.1126/science.aaa4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kocher SD, et al. The draft genome of a socially polymorphic halictid bee, Lasioglossum albipes. Genome Biol. 2013;14:R142. doi: 10.1186/gb-2013-14-12-r142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pabalan N, Davey K, Packer L. Escalation of aggressive interactions during staged encounters in Halictus ligatus Say (Hymenoptera: Halictidae), with a comparison of circle tube behaviors with other Halictine species’. J Insect Behav. 2000;13:627–650. [Google Scholar]
- 65.Arneson L, Wcislo WT. Dominant-subordinate relationships in a facultatively social, nocturnal bee, Megalopta genalis (Hymenoptera: Halictidae) J Kans Entomol Soc. 2003;76:183–193. [Google Scholar]
- 66.Michener CD, Brothers DJ. Were workers of eusocial hymenoptera initially altruistic or oppressed? Proc Natl Acad Sci USA. 1974;71:671–674. doi: 10.1073/pnas.71.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paulmier I, et al. Alkenes as a sexual pheromone in the alfalfa leaf-cutter bee Megachile rotundata. J Chem Ecol. 1999;25:471–490. [Google Scholar]
- 68.Liebig J, Peeters C, Oldham NJ, Markstädter C, Hölldobler B. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc Natl Acad Sci USA. 2000;97:4124–4131. doi: 10.1073/pnas.97.8.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cuvillier-Hot V, Cobb M, Malosse C, Peeters C. Sex, age and ovarian activity affect cuticular hydrocarbons in Diacamma ceylonense, a queenless ant. J Insect Physiol. 2001;47:485–493. doi: 10.1016/s0022-1910(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 70.Martin SJ, Vitikainen E, Helanterä H, Drijfhout FP. Chemical basis of nest-mate discrimination in the ant Formica exsecta. Proc Biol Sci. 2008;275:1271–1278. doi: 10.1098/rspb.2007.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wagner D, et al. Task-related differences in the cuticular hydrocarbon composition of harvester ants, Pogonomyrmex barbatus. J Chem Ecol. 1998;24:2021–2037. doi: 10.1023/a:1010408725464. [DOI] [PubMed] [Google Scholar]
- 72.Martin SJ, Drijfhout FP. Nestmate and task cues are influenced and encoded differently within ant cuticular hydrocarbon profiles. J Chem Ecol. 2009;35:368–374. doi: 10.1007/s10886-009-9612-x. [DOI] [PubMed] [Google Scholar]
- 73.Tentschert J, Bestmann H, Heinze J. Cuticular compounds of workers and queens in two Leptothorax ant species: A comparison of results obtained by solvent extraction, solid sampling, and SPME. Chemoecology. 2002;12:15–21. [Google Scholar]
- 74.Ayasse M, Engels W, Lübke G, Taghizadeh T, Francke W. Mating expenditures reduced via female sex pheromone modulation in the primitively eusocial halictine bee, Lasioglossum (Evylaeus) malachurum (Hymenoptera: Halictidae) Behav Ecol Sociobiol. 1999;45:95–106. [Google Scholar]
- 75.Duffield RM, Fernandes A, Lamb C, Wheeler JW, Eickwort GC. Macrocyclic lactones and isopentenyl esters in the Dufour’s gland secretion of halictine bees (Hymenoptera: Halictidae) J Chem Ecol. 1981;7:319–331. doi: 10.1007/BF00995755. [DOI] [PubMed] [Google Scholar]
- 76.Johansson I, Svensson BG, Tengö J, Bergström G. Systematic relationship of halictinae bees based on the pattern of macrocyclic lactones in the Dufour gland secretion. Insect Biochem. 1982;12:161–170. [Google Scholar]
- 77.Brady SG, Sipes S, Pearson A, Danforth BN. Recent and simultaneous origins of eusociality in halictid bees. Proc Biol Sci. 2006;273:1643–1649. doi: 10.1098/rspb.2006.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Danforth BN. Phylogeny of the bee genus Lasioglossum (Hymenoptera: Halictidae) based on mitochondrial COI sequence data. Syst Entomol. 1999;24:377–393. [Google Scholar]
- 79.Danforth BN, Brady SG, Sipes SD, Pearson A. Single-copy nuclear genes recover cretaceous-age divergences in bees. Syst Biol. 2004;53:309–326. doi: 10.1080/10635150490423737. [DOI] [PubMed] [Google Scholar]
- 80.Danforth BN, et al. Phylogeny of Halictidae with an emphasis on endemic African Halictinae. Apidologie (Celle) 2008;39:86–101. [Google Scholar]
- 81.Gibbs J, Brady SG, Kanda K, Danforth BN. Phylogeny of halictine bees supports a shared origin of eusociality for Halictus and Lasioglossum (Apoidea: Anthophila: Halictidae) Mol Phylogenet Evol. 2012;65:926–939. doi: 10.1016/j.ympev.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 82.Abràmoff MD, Magalhães PJ, Ram SJ. Image processing with ImageJ. Biophoton Int. 2004;11:36–42. [Google Scholar]
- 83.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.R Core Team 2016 R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at https://www.r-project.org/. Accessed March 16, 2017.
- 85.Orme D. 2013. The Caper Package: Comparative Analysis of Phylogenetics and Evolution in R, R package version 0.5.2. Available at https://cran.r-project.org/web/packages/caper/vignettes/caper.pdf. Accessed November 26, 2016.
- 86.Aitchison J. The Statistical Analysis of Compositional Data. Chapman and Hall; London: 1986. [Google Scholar]
- 87.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33:1–22. [PMC free article] [PubMed] [Google Scholar]