Significance
A key feature in the evolution of all vernalization systems is a cold-regulated component. In pooid grasses, up-regulation of the flowering promoter VERNALIZATION1 (VRN1) by prolonged cold is a key feature of vernalization, although little is known about the genes that repress VRN1 prior to cold exposure or activate it afterward. Here, we report the identification of REPRESSOR OF VERNALIZATION1 (RVR1), a repressor of VRN1 that is involved in creating a vernalization requirement in Brachypodium distachyon. RVR1 is present in all sequenced flowering plant genomes but is not found outside the plant kingdom. This report describes a role for the RVR1 class of genes in plants and an upstream component of the VRN1 regulatory system.
Keywords: vernalization, Brachypodium, flowering, VRN1, RVR1
Abstract
A requirement for vernalization, the process by which prolonged cold exposure provides competence to flower, is an important adaptation to temperate climates that ensures flowering does not occur before the onset of winter. In temperate grasses, vernalization results in the up-regulation of VERNALIZATION1 (VRN1) to establish competence to flower; however, little is known about the mechanism underlying repression of VRN1 in the fall season, which is necessary to establish a vernalization requirement. Here, we report that a plant-specific gene containing a bromo-adjacent homology and transcriptional elongation factor S-II domain, which we named REPRESSOR OF VERNALIZATION1 (RVR1), represses VRN1 before vernalization in Brachypodium distachyon. That RVR1 is upstream of VRN1 is supported by the observations that VRN1 is precociously elevated in an rvr1 mutant, resulting in rapid flowering without cold exposure, and the rapid-flowering rvr1 phenotype is dependent on VRN1. The precocious VRN1 expression in rvr1 is associated with reduced levels of the repressive chromatin modification H3K27me3 at VRN1, which is similar to the reduced VRN1 H3K27me3 in vernalized plants. Furthermore, the transcriptome of vernalized wild-type plants overlaps with that of nonvernalized rvr1 plants, indicating loss of rvr1 is similar to the vernalized state at a molecular level. However, loss of rvr1 results in more differentially expressed genes than does vernalization, indicating that RVR1 may be involved in processes other than vernalization despite a lack of any obvious pleiotropy in the rvr1 mutant. This study provides an example of a role for this class of plant-specific genes.
A common adaptation for optimal timing of flowering in temperate climates is the evolution of a vernalization requirement. Vernalization is the process by which plants become competent to flower after prolonged exposure to the cold temperatures of winter (1). Cold exposure alone, however, is typically not sufficient to induce flowering; rather, it often must be followed by an inductive photoperiod, such as the increasing day lengths of spring. The combination of a requirement for a prolonged period of cold followed by a requirement for increasing day lengths has the adaptive value of preventing flowering in the fall season before the onset of winter, which would likely compromise reproductive success (2).
To date, information about the vernalization response in grasses has largely been derived from studies of existing allelic variation in wheat and barley (for reviews, see refs. 3–5). Wheat and barley varieties can be classified as either spring or winter types. Spring varieties do not require vernalization to flower rapidly in inductive long days (LD), whereas vernalization enables rapid flowering of winter varieties (5, 6). A current molecular model of vernalization in temperate grasses consists of a regulatory loop including the genes VERNALIZATION1 (VRN1), VERNALIZATION2 (VRN2), and VERNALIZATION3 (VRN3) (6, 7). VRN3 is orthologous to FLOWERING LOCUS T (FT), which encodes a small, mobile, flowering-inducing protein also known as florigen (8, 9); hereafter, VRN3 will be referred to as FT1 (10). The regulatory loop is thought to exist in leaves where all three genes are expressed (10–12). In the fall, before cold exposure, high levels of VRN2 repress FT1 to prevent flowering (12, 13). VRN2 contains a putative zinc finger and a CONSTANS, CONSTANS-LIKE, and TIMING OF CAB1 domain and is part of the type VI CO-like gene family (12, 14). Before cold exposure, both VRN1 and FT1 are expressed at low levels (6). However, during the cold of winter, VRN1 is up-regulated proportionately to the length of cold experienced, and in turn, higher VRN1 levels are thought to cause a decrease of VRN2 expression (11, 15, 16). VRN1 encodes a MADS box transcription factor related to the APETALA1/FRUITFULL (AP1/FUL)-like class of floral homeotic genes in Arabidopsis thaliana (17, 18). Like FUL in A. thaliana, VRN1 expression is not restricted to the meristem, but it is also expressed in leaves where, in pooid grasses, it participates in the vernalization regulatory loop (19). VRN1 enables FT1 expression in leaves by turning off VRN2. VRN1 has been shown to bind to the promoter of VRN2 so the repression may be direct (16, 20, 21). Vernalization-mediated activation of VRN1 expression is accompanied by changes in chromatin modifications in a presumed regulatory region of its first intron; specifically, there is a decrease in repressive H3K27 methylation and an increase in the activating H3K4 methylation (22). However, no changes in chromatin modifications have been observed around the VRN2 locus during or after cold (22). Upon repression of VRN2, increasing FT1 expression contributes to the up-regulation of VRN1 expression in leaves, creating a positive feedback loop ensuring the inductive flowering signal is maintained (10, 20, 23).
The temperate grass model Brachypodium distachyon (Bd) (24) contains orthologs of the three vernalization genes VRN1, VRN2, and FT1, which are important in regulating flowering in wheat and barley (25; for review see ref. 26). Furthermore, allelic variation at these genes likely contributes to natural variation in flowering responses in B. distachyon because they colocalize under quantitative trait loci controlling flowering-time variation (27, 28). Similar to their role in wheat and barley, FT1 and VRN1 are promoters of flowering in B. distachyon (29). Overexpression of FT1 and VRN1 results in rapid flowering in B. distachyon (29) and knockdown of FT1 and VRN1 results in delayed flowering (30, 31). As in wheat and barley, BdVRN1 mRNA levels increase quantitatively during increasing lengths of cold treatment and remain elevated after cold (29, 32, 33). In addition, overexpression of VRN1 results in elevated expression of FT1 and overexpression of FT1 results in elevated VRN1 expression, consistent with the positive feedback loop suggested from studies in wheat and barley (7, 29). Unlike wheat and barley, however, BdVRN2 is not suppressed by cold exposure (in fact, BdVRN2 is induced during the cold) and its expression is not regulated by VRN1 despite being a LD repressor of flowering (31).
From a screen of ethyl methane sulfonate-treated plants for mutants that flower rapidly without vernalization, we identified REPRESSOR OF VERNALIZATION1 (RVR1), a plant-specific gene containing a bromo-adjacent homology (BAH) and transcriptional elongation factor S-II (TFIIS) domain that is involved in establishing a vernalization requirement in B. distachyon by repressing VRN1 before cold exposure.
Results and Discussion
Isolation of Rapid-Flowering Mutants in B. distachyon.
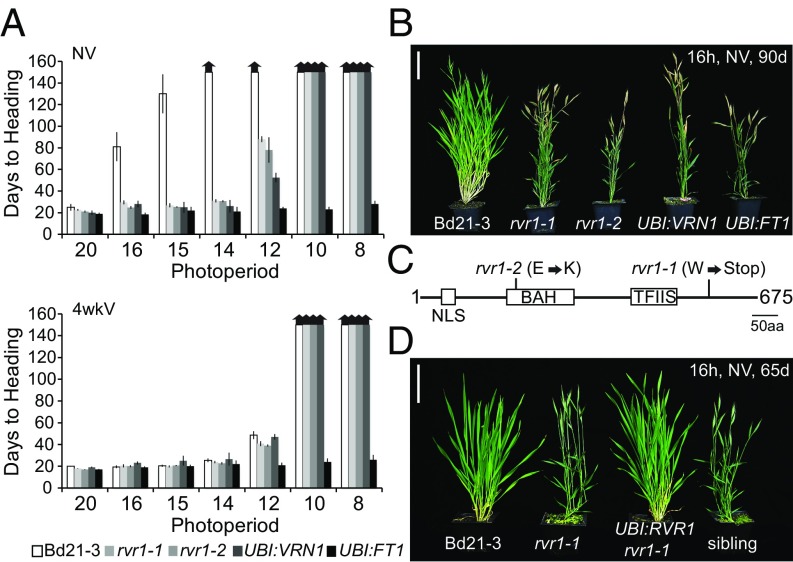
To identify genes that are necessary to create a vernalization requirement in temperate grasses, we screened for mutants in B. distachyon that flower rapidly without vernalization. From this screen, we identified many rapid-flowering mutants, two of which are allelic (Fig. 1C). These two mutants flower rapidly in 16-h daylengths at ∼20 d after germination, with six leaves on the parent culm similar to vernalized wild-type parental Bd21-3 plants, whereas nonvernalized (NV) wild-type [which has a facultative vernalization requirement (29)] flower at around 80–90 d, with greater than 16 leaves on the parent culm (Fig. 1A). Interestingly, in 16-h days without vernalization these mutants flower as rapidly as lines constitutively expressing either of two critical floral promoters BdVRN1 or BdFT1 (Fig. 1 A and B). There is no difference in the rate of leaf initiation between the mutant and wild-type in LD or short days (SD). Other than rapid flowering, no other visible mutant phenotypes were observed. Thus, rvr1 mutants behave as vernalized plants.
Fig. 1.
A gene with a BAH and TFIIS domain (RVR1) represses flowering time. (A) Flowering times of Bd21-3 wild-type, rvr1-1, rvr1-2, UBI:VRN1, and UBI:FT1 (VRN1 and FT1 cDNA under control of the maize ubiquitin promoter) grown in 20, 16, 15, 14, 12, 10, and 8 h of light either without cold exposure (NV) and with cold exposure (V) for 4 wk (4wkV). Bars represent the average of 12 plants ± standard deviation. The experiment was repeated with similar results. Arrows above bars indicate that none of the plants flowered at the end of the experiment (150 d). (B) Representative image of Bd21-3 wild-type, the two alleles of rvr1, UBI:VRN1, and UBI:FT1 grown in 16-h of light for 90 d after germination without vernalization (NV). (Scale bar, 5 cm.) (C) Domain structure of the 675-aa-long RVR1 protein showing the location and corresponding amino acid changes of the ethyl methane sulfonate-induced mutations of the two mutant alleles rvr1-1 and rvr1-2. Domain structure of RVR1 includes a putative nuclear localization signal (NLS), BAH, and TFIIS domains. (D) Image of representative T1 plants grown in a 16-h photoperiod. Bd21-3, rvr1-1, and segregating nontransgenic sibling plants are controls for the effect of overexpression of the wild-type RVR1 cDNA in the rvr1-1 background (UBI:RVR1::rvr1-1). (Scale bar, 5 cm.)
Identification of the RVR1 Gene.
To identify the causative lesion in these mutants, we made F2 mapping populations by crossing the mutants, which are in the Bd21-3 background, to the polymorphic mapping partner Bd3-1 [we have previously shown that an F2 derived from crossing the wild-type parents does not segregate for flowering time (34)]. Both mutants were mapped, using previously developed insertion–deletion (indel) markers (34), to the same 400-kb interval around 40 mb on chromosome 2, indicating that they might be allelic. To identify the causal mutation in each mutant line, we carried out whole-genome sequencing on DNA pooled from approximately 100 mutants from the F2 mapping population that segregated for the rapid flowering rvr1 phenotype. Sequencing reads were analyzed using the CloudMap pipeline (35) as optimized for B. distachyon (Fig. S1) (34). The CloudMap results were consistent with our initial indel marker mapping data (Fig. S1), and analysis of the sequence data revealed only one gene in which both mutants contain a lesion. The first allele (rvr1-1) contains a C-to-T mutation converting a Trp codon into a premature stop in the last exon in a previously uncharacterized gene (Bradi2g40147), annotated as a gene with a nuclear-localization signal, BAH, and TFIIS domains (Fig. 1C). We named this gene RVR1. The second allele (rvr1-2) contains a C-to-T mutation creating a Glu-to-Lys missense mutation located within the BAH domain (Fig. 1C). BAH domains appear to mediate protein–protein interactions in protein complexes involved in chromatin modification or complexes that “read” chromatin states (36–38). The TFIIS domain is associated with transcriptional regulation by interacting with RNA polymerase II in transcriptional elongation (39). qRT-PCR in rvr1-1 plants revealed a reduction in the abundance of the RVR1 transcript by nearly 80% compared with wild-type plants, perhaps through nonsense-mediated decay as a result of the presence of the premature stop codon (Fig. S2A). rvr1-2 behaves as a semidominant mutation (for mapping purposes the homozygous mutants are more rapid flowering than heterozygotes). Overexpressing the RVR1 cDNA using the maize ubiquitin promoter (UBI:RVR1) in either rvr1-1 or rvr1-2 rescued the mutant phenotype (Fig. 1D and Fig. S2B).
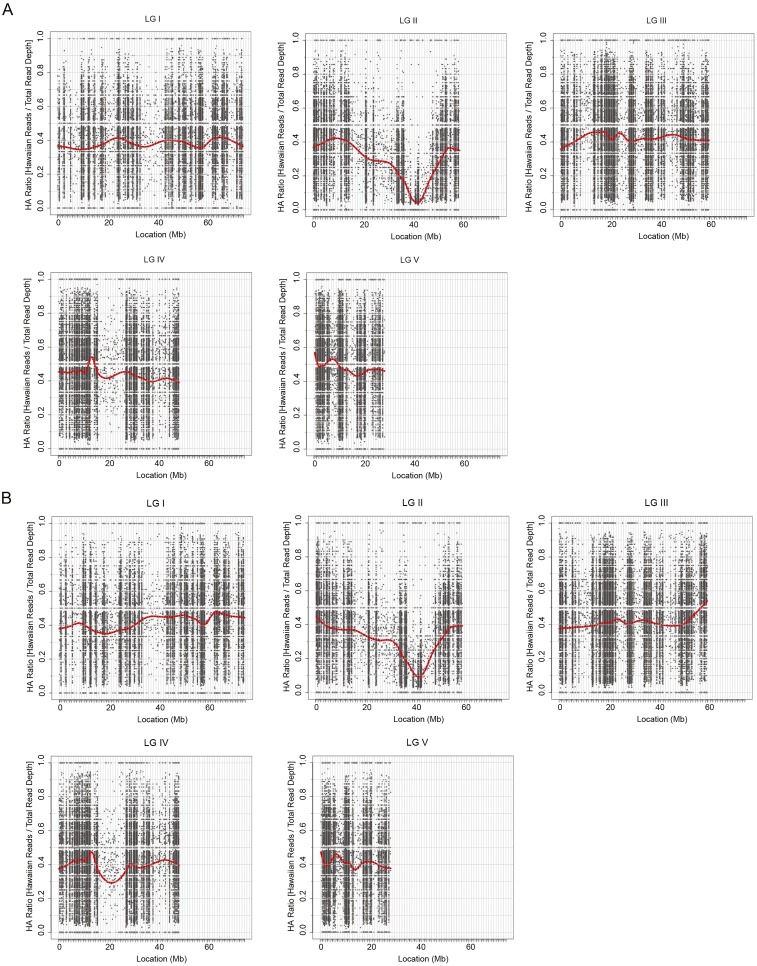
Fig. S1.
Mapping frequencies of the five chromosomes in Brachypodium showing a skew toward the Bd21-3 genotype around 40 mb on chromosome 2: (A) rvr1-1, (B) rvr1-2. HA, Hawaiian; LG, linkage group.
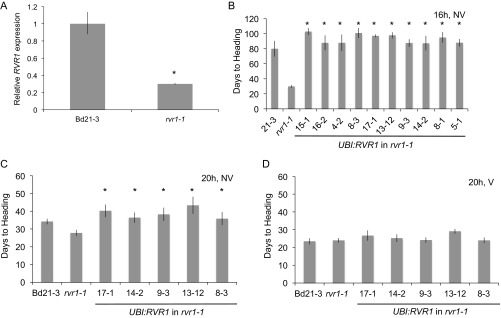
Fig. S2.
(A) RVR1 gene expression in Bd21-3 and rvr1-1 grown in 1× 6-h days NV. Newly emerged third leaf was harvested; data relative to Bd21-3. (B) Days to heading in Bd21-3, rvr1-1, and 10 transgenic (rescue) lines overexpressing the RVR1 cDNA from Bd21-3 in the rvr1-1 background grown in 16-h NV. (C and D) Subset of the transgenic lines in A grown in 20-h days NV and V. In B–D, values represent the average of six plants ± standard deviation. *P < 0.01.
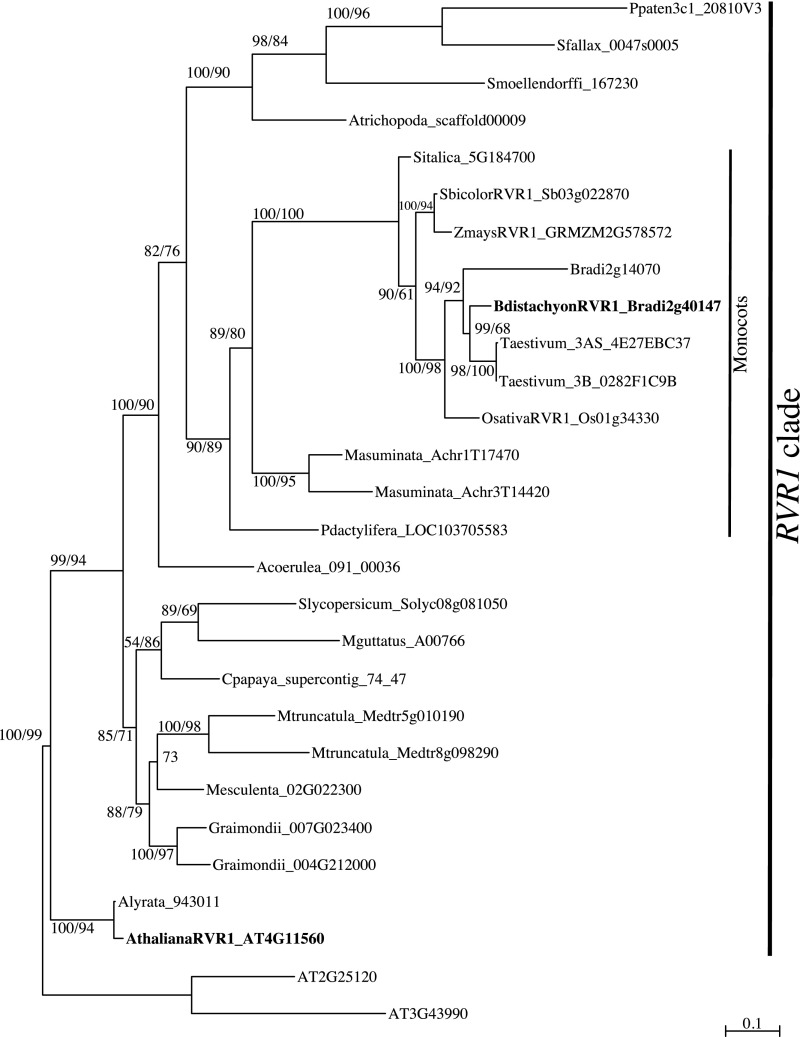
Phylogenetic analyses of RVR1 estimate a well-supported clade containing species that span land plant diversification; however, no identifiable orthologs are present outside of the plant kingdom (Fig. S3). Interestingly, RVR1 does not appear to be part of a gene family; it is found in single copy throughout land plant diversification in the plant genomes queried for this analysis, except in B. distachyon, where there is another related gene, Bradi2g14070, which also has a BAH and TFIIS domain.
Fig. S3.
Maximum-likelihood inference of the phylogenetic relationships among RVR1 genes based on a nucleotide alignment of the conserved BAH domain. Maximum-likelihood bootstrap support values (Right) and Bayesian posterior probabilities (Left) are indicated at each branch. Scale bar indicates substitutions per site. Focal species are labeled in boldface font.
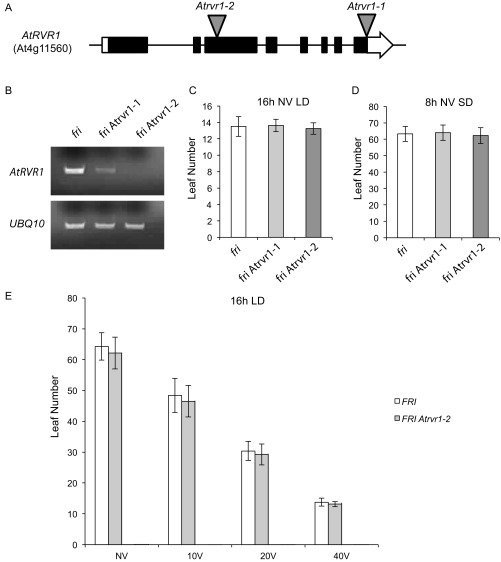
To test if the RVR1 ortholog in A. thaliana (AtRVR1; At4g11560) affects flowering as it does in B. distachyon, we evaluated the phenotype of lines carrying T-DNA insertions in Atrvr1-1 [Atrvr1-1 (Salk_017758) and Atrvr1-2 (Sail_1246_E10)] (Fig. S4A). The T-DNA alleles are likely to result in loss-of-function because they are within the coding region of the gene, and semiquantitative PCR revealed the expression of AtRVR1 was abolished in Atrvr1-2 (Fig. S4B). The mutants flowered the same as wild-type in both 16-h LD and 8-h SD (Fig. S4 C and D), and we did not observe any visible mutant phenotypes. Additionally, Atrvr1-2 did not affect flowering after vernalization in a “winter” A. thaliana accession that contains a functional FRIGIDA (FRI) allele (Fig. S4E). In A. thaliana, FRI is required for the activation of the potent floral repressor, FLOWERING LOCUS C (FLC) that is repressed by prolonged cold exposure (40, 41). That mutations in RVR1 do not affect flowering time in A. thaliana is not surprising because A. thaliana and grass vernalization systems evolved independently (42) and, as discussed below, in B. distachyon RVR1 acts as a repressor of VRN1, but the orthologs of B. distachyon VRN1 in A. thaliana (AP1/FUL) do not play a role in A. thaliana vernalization. Whether a role of RVR1 in VRN1 repression is specific to grass vernalization systems or is present more broadly in monocots will be interesting to determine.
Fig. S4.
Mutant characterization of the RVR1 ortholog in Arabidopsis thaliana Atrvr1 (At4g11560). (A) Gene diagram of RVR1 from Arabidopsis with the location of the two T-DNAs analyzed, Atrvr1-1 (Salk_017758) and Atrvr1-2 (Sail_1246_E10). (B) Semiquantitative PCR of AtRVR1 in frigida (fri, wild-type; Columbia ecotype) and the two T-DNA alleles confirming the presence of the given T-DNA. UBQ10 used as a loading control. (C and D) Flowering time of the T-DNAs as reported via leaf counts in LD and SD NV. (E) Flowering times after a vernalization time course of Atrvr1-2 introgressed within the FRI background grown in 16-h LD. Imbibed seeds were vernalized at 5 °C for either 10, 20, or 40 d.
Comparison of the rvr1 Phenotype with Constitutive Expression of VRN1 and FT1.
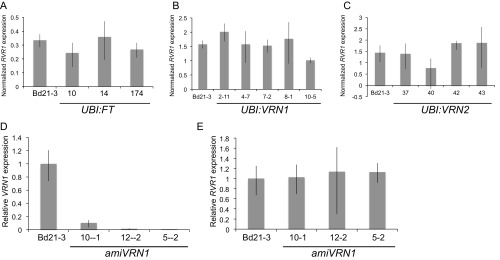
To further characterize the rvr1 mutant phenotype, we grew wild-type, rvr1-1, rvr1-2, UBI:FT1, and UBI:VRN1 in different photoperiods (20-, 16-, 15-, 14-, 12-, 10-, 8-h days) either without vernalization (NV) or after a saturating 4-wk vernalization treatment (V) (Fig. 1A). NV plants were planted at the end of the vernalization treatment so that V and NV plants were grown at the same time and were developmentally matched. We have previously shown that 12-h daylengths are the shortest daylengths inductive for flowering; shorter daylengths do not permit flowering even in V or UBI:VRN1 plants (31). Similar to UBI:VRN1 and V wild-type plants, rvr1 fails to flower in daylengths less than 12-h. However, 12-h UBI:VRN1 plants flower ∼30–40 d earlier then rvr1 (Fig. 1A). In contrast, UBI:FT1 plants flower around 20 d with or without vernalization in all of the tested photoperiods, consistent with FT1 acting downstream of photoperiod sensing (Fig. 1A). Thus, rvr1 is similar phenotypically to V plants and NV plants constitutively expressing VRN1.
VRN1 and FT1 Expression in rvr1.
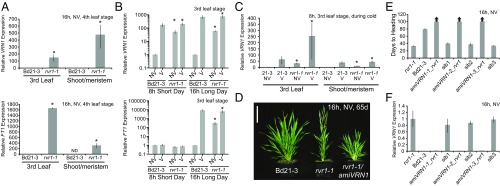
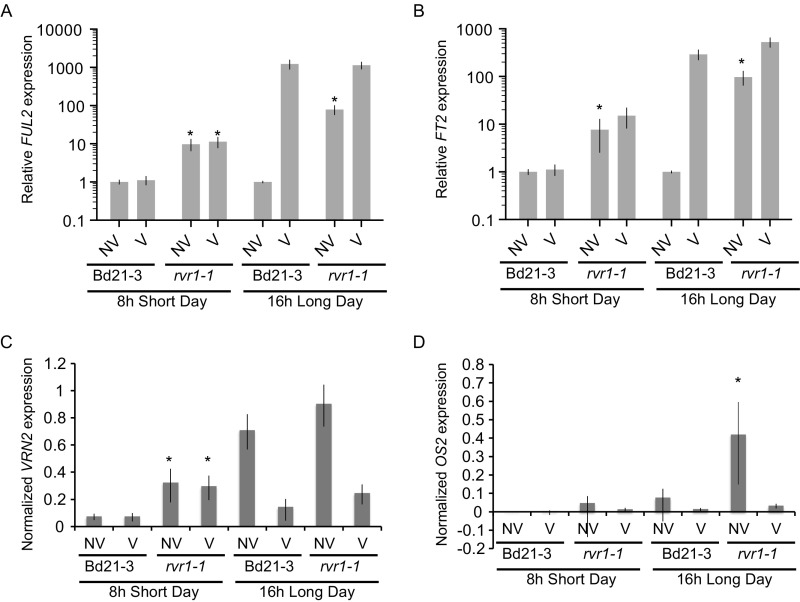
As discussed above, rvr1 mutants phenocopy constitutive VRN1 expression in most daylengths, but in SD-lengths rvr1 mutants do not phenocopy constitutive FT1 expression. This finding is consistent with loss of RVR1 leading to constitutive VRN1 expression in all daylengths, but not to FT1 expression in SD. To evaluate this model, we examined VRN1 and FT1 mRNA levels in rvr1 compared with NV wild-type. Indeed, in LD VRN1 and FT1 mRNA levels were significantly elevated in rvr1-1 compared with NV wild-type in both leaves and in shoot tissue enriched for the shoot apical meristem (Fig. 2A). Furthermore, when rvr1 is grown in 8-h SD—a condition in which rvr1 does not flower rapidly—VRN1 mRNA expression levels remain elevated despite FT1 expression being nearly undetectable (Fig. 2B). This demonstrates that the up-regulation of VRN1 in rvr1 can occur independently of FT1 expression. This is important to note because a positive feedback loop exists between VRN1 and FT1 in B. distachyon (29, 32), similar to that reported in wheat and barley (7, 11).
Fig. 2.
RVR1 represses VRN1 expression and the rvr1-1 mutant phenotype is dependent upon VRN1. (A) qRT-PCR data from samples of the third-leaf or meristem-enriched apex (shoot/meristem) of NV Bd21-3 and rvr1-1 grown in a 16-h photoperiod to the fourth-leaf stage. VRN1 expression (Upper) and FT1 expression (Lower). Bars represent the average of three biological replicates ± standard deviation. (B) qRT-PCR of VRN1 expression (Upper) and FT1 expression (Lower). Third leaf harvested at the third-leaf stage of Bd21-3 and rvr1-1 grown in 8-h SD or 16-h LD. V plants were exposed to 4 wk of cold as an imbibed seed and the NV plants were planted at the end of the vernalization treatment. (C) qRT-PCR of VRN1 expression of leaf three or meristem-enriched shoot apex from Bd21-3 and rvr1-1 plants either NV or from plants during cold exposure. (D) Representative image of Bd21-3 wild-type, rvr1-1, and rvr1-1/amiVRN1 grown in 16-h days NV. Image taken 65 d postgermination. (Scale bar, 5 cm.) (E) Days to heading of rvr1-1, Bd21-3, three independent T1 amiVRN1/rvr1-1 transgenic plants, and nontransgenic sibling plants segregated grown in 16-h days NV. Arrows above bars indicate that none of the plants flowered at the end of the experiment. (F) qRT-PCR of VRN1 expression in amiVRN1/rvr1-1 confirming the knock-down of VRN1 expression. Asterisks above bars indicate statistically significant contrasts between rvr1 and Bd21-3 NV (*P < 0.01).
Growth of rvr1 plants in 12-h days reveals a difference in flowering time between rvr1 and UBI:VRN1 (Fig. 1A). Specifically, NV UBI:VRN1 flower ∼30–40 d earlier than rvr1 mutants (Fig. 1A). However, this flowering difference is eliminated after vernalization (Fig. 1A). One possible explanation for this difference is that the VRN1 threshold needed to activate flowering is higher in 12-h days versus longer photoperiods; thus, the increased VRN1 activity when driven by the ubiquitin promoter may achieve that threshold in 12-h daylengths, but loss of RVR1 may not. Indeed, expression of VRN1 in UBI:VRN1 transgenic lines is over 100-fold higher compared with that in rvr1-1. Because there is no flowering-time difference between rvr1 and UBI:VRN1 after vernalization, we were interested in determining if VRN1 levels increase in rvr1 during and after vernalization (Fig. 2 B and C). We measured VRN1 mRNA levels during cold exposure of seedlings and after vernalization of imbibed seeds (Fig. 2 B and C). Indeed, VRN1 mRNA levels in the rvr1 mutant were elevated by several hundred-fold during and after vernalization (Fig. 2 B and C). These data are consistent with a threshold model in which UBI:VRN1 transgenics flower more rapidly than rvr1-1 in 12-h daylengths because of more highly elevated VRN1 mRNA levels.
The rvr1 Rapid-Flowering Phenotype Is Dependent upon VRN1.
To determine whether the alteration of VRN1 expression is required for the rvr1 phenotype, we knocked-down VRN1 expression using an artificial microRNA that is specific to VRN1 and does not affect the expression of the closely related FUL2 paralog (31) (amiVRN1) in the rvr1-1 background (Fig. 2 D and E). amiVRN1 delays flowering in wild-type Bd21-3 even after vernalization (31). The amiVRN1 suppressed the rvr1-1 phenotype in all 33 T0 transgenic plants we generated; these transgenic lines took longer than 200 d to flower, even under highly inductive 20-h days (29). Furthermore, we also characterized the progeny of three independent T0 lines; those with the amiVRN1 construct were delayed in flowering, whereas sibling plants lacking the transgene exhibited the rapid flowering rvr1 phenotype (Fig. 2E). qRT-PCR confirmed that the delayed-flowering phenotype was associated with reduced VRN1 expression in the rvr1-1 background because VRN1 expression in amiVRN1/rvr1 was significantly lower than in sibling plants lacking the transgene (Fig. 2F). Thus, the rapid flowering rvr1 mutant is dependent upon VRN1.
Expression of Other Putative Flowering Genes in rvr1.
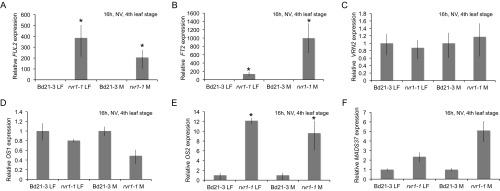
Expression of other putative flowering-time genes are also elevated in rvr1. The FT1 and VRN1 paralogs, FUL2 and FT2, are up-regulated in the rvr1-1 background compared with wild-type (Figs. S5 A and B and S6 A and B). No significant expression differences of the flowering-time repressor VRN2 were observed between wild-type and rvr1-1 in leaf and meristem tissues (Fig. S5); however, under 8-h SD VRN2 mRNA levels were somewhat elevated in rvr1-1 compared with wild-type (Fig. S6).
Fig. S5.
(A–F) qRT-PCR data of leaf three or shoot/meristem from fourth-leaf stage Bd21-3 and rvr1-1 plants grown NV in a 16-h photoperiod. Bars represent the average of three biological replicates ± standard deviation. Expression is relative to Bd21-3. *P < 0.01.
Fig. S6.
Third leaf harvested at the third-leaf stage of Bd21-3 and rvr1-1 grown in 8-h SD or 16-h LD. V plants were treated with 4 wk of cold as an imbibed seed and the NV were planted at end of vernalization treatment. Expression of (A) FUL2, (B) FT2, (C) VRN2, (D) OS2. In A and B expression was relative to Bd21-3 NV. In C and D expression was normalized to UBC18. *P < 0.01.
mRNA levels of two FLC-like genes, OS2 and MADS37 (43), were also elevated in rvr1-1 compared with wild-type (Fig. S5 E and F). As noted above, in A. thaliana FLC is a potent flowering repressor that confers a vernalization requirement in Brassicaceae and is repressed by cold (2). The up-regulation of OS2 and MADS37 expression in rvr1-1 is not likely to be a result of cold-mediated VRN1 expression because in lines overexpressing VRN1, MADS37, and OS2 mRNA levels do not change (31).
RVR1 Is Constitutively Expressed and Its Overexpression Does Not Suppress the Vernalization Response.
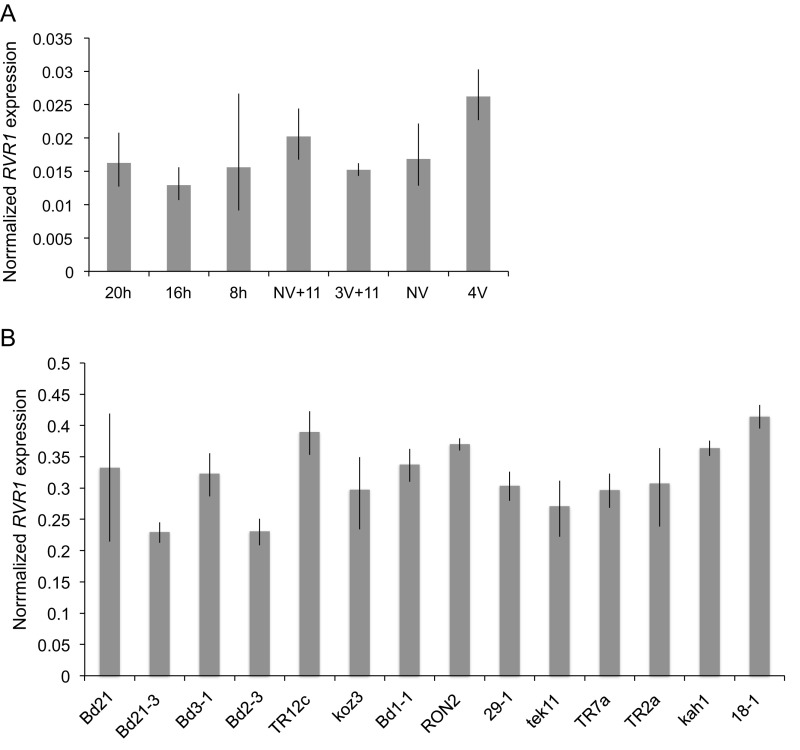
Because RVR1 is involved in the repression of VRN1 before vernalization, we evaluated whether or not the promotion of flowering by vernalization might involve modulation of RVR1 expression. We found, RVR1 transcript levels were not affected by vernalization (Fig. S7). Additionally, we tested if RVR1 mRNA levels are affected by photoperiod and found that RVR1 mRNA levels were not significantly different under 8-, 16-, or 20-h photoperiods (Fig. S7).
Fig. S7.
(A) RVR1 gene expression in leaves of Bd21-3 grown in a variety of different conditions. All plants are the third-leaf stage and the newly formed third leaf was harvested. NV, 4 wk V (4V), NV+11 d after cold developmental control for 3V+11 (NV+11), 3 wk V+11 d after cold (3V+11). Expression normalized to UBC18 as done in Ream et al. (29). (B) RVR1 expression in the third leaf of different B. distachyon accessions grown in 20-h days NV. In A and B, values represent the average of three biological replicates ± standard deviation.
We also analyzed RVR1 mRNA levels in different accessions to determine if there was a correlation with flowering time in a 20-h photoperiod (a condition in which rapid-flowering accessions have elevated VRN1 expression compared with delayed-flowering accessions). RVR1 expression varied among accessions by less than twofold and there was no consistent pattern of RVR1 expression level between the rapid flowering or the delayed flowering accessions (Fig. S7).
The rapid-flowering phenotype of the rvr1 mutant indicates that RVR1 acts as a repressor of flowering. To test if increased expression of RVR1 might delay flowering or prevent vernalization, we grew NV and V UBI:RVR1 transgenics in a 20-h photoperiod and measured days to heading (Fig. S2). UBI:RVR1 transgenic plants flowered approximately at the same time as wild-type, with or without vernalization (Fig. S2). Thus, the RVR1 expression level is not likely to be limiting in flowering repression in B. distachyon.
Modulation of VRN1, FT1, and VRN2 Expression Does Not Affect RVR1 mRNA Levels.
To test if VRN1, FT1, or VRN2 affects RVR1 expression, we measured RVR1 mRNA levels in UBI:VRN1, UBI:FT1, UBI:VRN2, amiVRN2, and amiVRN1 transgenic plants. In all cases, RVR1 expression was not significantly affected in these different genetic backgrounds (Fig. S8). Thus, these genes do not appear to be involved in a feedback loop with RVR1, and this is consistent with RVR1 being upstream of these genes in the vernalization pathway (Fig. S8).
Fig. S8.
(A–C) RVR1 expression in the third leaf grown NV in 16-h days. RVR1 expression in (A) UBI:FT, (B) UBI:VRN1, (C) UBI:VRN2. (D and E) VRN1 expression (D) and RVR1 expression (E) in the third leaf of plants grown NV in 20-h days.
The rvr1-1 Mutant Exhibits Reduced Levels of H3K27me3 Around the VRN1 Locus Similar to Vernalized Samples of Bd21-3.
In barley, induction of HvVRN1 by vernalization correlates with a reduction of the repressive chromatin modification trimethylation at lysine 27 of histone 3 (H3K27me3) (22). Specifically, repression of HvVRN1 before vernalization is associated with high levels of H3K27me3, and during vernalization the level of H3K27me3 decreases close to the transcription start site of HvVRN1 and within its first intron (22). The stable reduction of H3K27me3 after vernalization at the HvVRN1 locus has been suggested to contribute to the stable transcriptional “on state” of HvVRN1 after vernalization (22), and this stable on state may contribute to the memory of a vernalized state in cereals (22, 32). Indeed, that BdVRN1 expression in B. distachyon is also maintained after cold exposure in SD conditions, in which FT1 is not expressed and flowering does not occur, indicates that vernalization is likely to cause a stable BdVRN1 on state more broadly throughout Pooideae (32).
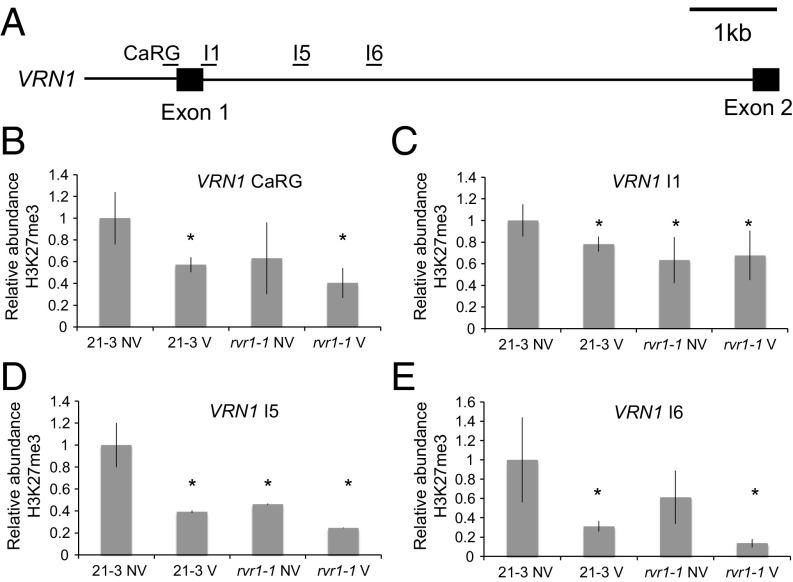
We find that in NV, wild-type Bd21-3 control seedlings grown in SD, the levels of H3K27me3 are reduced at BdVRN1 after vernalization as in barley (Fig. 3), and this correlates with the stable induction of BdVRN1 expression observed after vernalization (32). The precocious VRN1 expression observed in NV rvr1-1 mutants (Fig. 2 A and B) is also associated with decreased levels of H3K27me3 relative to NV Bd21-3 control samples (Fig. 3 B–E). Interestingly, at all four sites tested within the VRN1 locus, H3K27me3 levels in NV rvr1-1 are similar to those in V Bd21-3 samples (Fig. 3 B–E). Thus, RVR1 is required to achieve the levels of H3K27me3 associated with the repressed state of BdVRN1 in wild-type before vernalization.
Fig. 3.
The effect of vernalization on the histone modification, H3K27me3 at BdVRN1 in Bd21-3 and rvr1-1 seedlings. (A) Diagram of the 5′ end of BdVRN1 showing the regions analyzed by ChIP, followed by qRT-PCR. (B–E) Relative abundance of H3K27me3 at BdVRN1 in NV and V seedlings from Bd21-3 and rvr1-1. Data represent the mean ± SEM from three biological replicate experiments. An asterisk (*) indicates significant differences compared with 21-3 NV (*P < 0.05). Primer sequences are provided in Table S1.
Transcriptomics in the rvr1 Mutant and Vernalized Plants.
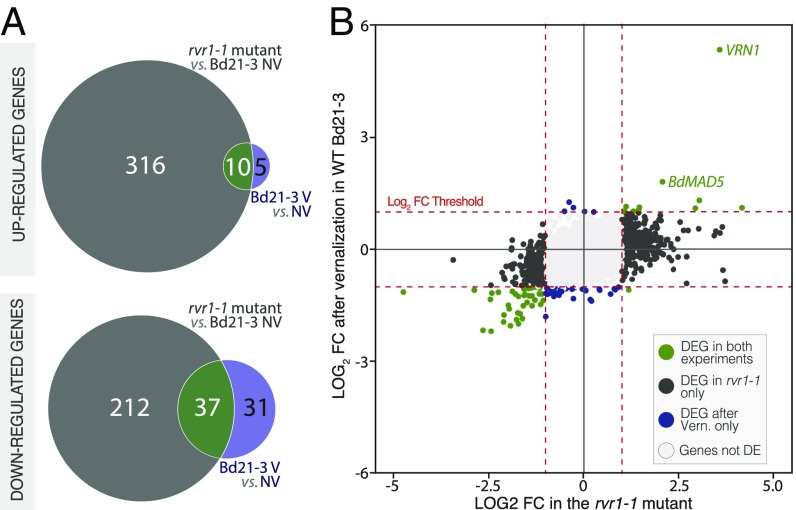
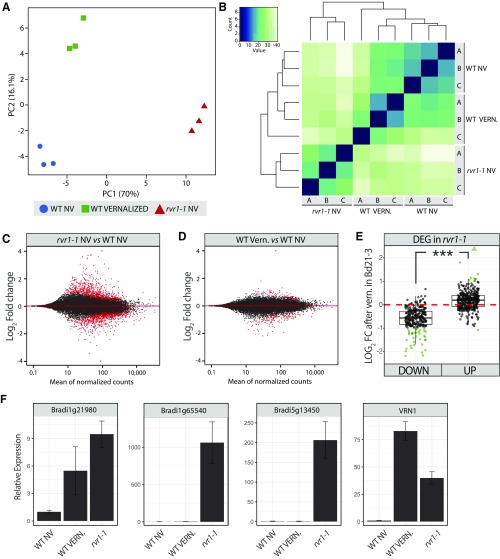
To explore the specificity of RVR1 in establishing a vernalization requirement, we performed RNA sequencing followed by transcriptomic analyses in the NV rvr1-1 mutant versus V and NV wild-type plants. To avoid the confounding effects of flowering, RNA was prepared from plants that were grown in 8-h SD. To focus on genes that were stably affected by vernalization, RNA was prepared from V plants 3 wk after cold exposure. We found 575 genes differentially expressed in newly expanded third-leaf tissue in NV rvr1-1 compared with NV wild-type plants [fold-change (FC) > 2; P < 0.001] (Fig. 4). The enrichment of up-regulated genes in rvr1-1 (58%) is consistent with an RVR1 repressor function (Fig. 4A). In V plants, 83 genes were differentially expressed compared with NV controls (FC > 2; P < 0.001) (Fig. 4). VRN1 was one of most highly up-regulated genes in rvr1-1 and the most highly up-regulated gene in V plants (Fig. 4B and Fig. S9). The strong and stable induction of VRN1 by cold supports the important role of VRN1 in the maintenance of the vernalized state (32). Overall, 10 of the 15 (66%) up-regulated genes and 37 of the 68 (54%) down-regulated genes in V plants overlap with genes up or down-regulated in rvr1 mutants, consistent with loss of RVR1 creating at least a partial mimic of a V state at a molecular level (Fig. 4). Furthermore, many other differentially expressed genes that did not exceed the FC threshold discussed above also showed a similar trend in V plants (Fig. 4B and Fig. S9). However, 528 additional genes are differentially expressed in the rvr1-1 mutant compared with the V transcriptome, indicating that RVR1 is a more general regulator not specific to vernalization (Fig. 4A). Although a broader role for RVR1 might predict that loss of RVR1 activity would deleteriously affect other plant processes, we did not observe any visible phenotypes other than an effect on flowering in the rvr1 mutant. However, the plants were well-fertilized and grown in a growth chamber, conditions in which other phenotypes might not be apparent.
Fig. 4.
Effects of rvr1-1 mutation and vernalization on the leaf transcriptome. (A) Overlap between the transcriptomic changes observed in rvr1-1 seedling and Bd21-3 wild-type plants postvernalization (FC > 2; P < 0.001). (Upper) Number of genes up-regulated; (Lower) number of genes down-regulated. (B) Comparison of the FC observed in the nonvernalized rvr1-1 mutant and in vernalized Bd21-3 seedlings. Red dotted lines indicate fold-change thresholds used for the identification of differentially expressed genes (DEG).
Fig. S9.
Analysis of RNA-seq data. (A) Principal component analysis computed using the 500 genes showing the highest mean expression levels across studied conditions (three replicates per condition). (B) Heatmap showing the clustering of RNA-seq replicates in the different experimental conditions. The heatmap was computed using all of the detected genes. (C and D) MA plots highlighting the differentially genes for (C) the rvr1-1 mutant vs. NV wild-type plants and (D) V vs. NV wild-type plants. (E) Log2 of the FC in V vs. NV wild-type plants. Genes are categorized according to their down- or up-regulation in the rvr1-1 vs. NV wild-type plants analysis. Green dots indicate genes differentially regulated in both experiments and triangles show genes out of the scale. ***P < 0.001. (F) qRT-PCR verification of the RNA-seq data. Data were normalized using a geometric mean of three reference genes: SamDC, ACT2, and UBC18. The relative expression of the wild-type NV sample was set to one. DEG, differentially expressed genes; VERN, vernalization.
Concluding Remarks
RVR1 is required for the repression of VRN1 before cold. Thus, RVR1 plays a role in establishing a vernalization requirement in B. distachyon and is likely to play the same role in other vernalization-requiring pooid grasses. It is also interesting that although RVR1 is a plant-specific gene that is conserved across the plant kingdom, this work is a unique reported example of a phenotype due to loss of RVR1.
There are several possibilities as to how RVR1-mediated repression of VRN1 is overcome by exposure to prolonged cold, enabling the achievement of a vernalized state. One possibility is that the capability for RVR1 repression is maintained after vernalization, but VRN1 activators are induced by cold that outcompete RVR1-mediated repression. Another possibility is that RVR1 activity is abolished by cold. RVR1 is constitutively expressed, and its expression is not influenced by cold or daylength; however, a vernalization-mediated direct inactivation of RVR1 could occur via a posttranscriptional mechanism, such as differential translation, protein modification, or protein turnover. However, a direct inactivation may be unlikely because transcriptional profiling reveals that RVR1 affects the expression of many more genes than does vernalization: that is, RVR1 appears to have a broader role than simply to create a vernalization requirement, and thus loss of RVR1 activity might deleteriously affect other plant processes. Alternatively, RVR1 could be part of a general complex with different permutations, and loss of a flowering-specific component of a VRN1-repressing permutation after prolonged cold exposure could abolish VRN1 repression. An example in which a specific permutation of a general chromatin-modifying complex provides flowering specificity exists in A. thaliana. The polycomb repressive complex 2 (PRC2) is involved in the repression of many A. thaliana genes, but during vernalization a subset of the PRC2s acquires the vernalization-specific component VERNALIZATION INSENSITIVE 3, which is necessary for PRC2 to regulate FLC and achieve a vernalized state (44, 45).
Materials and Methods
Mutant Screen and RVR1 Gene Identification.
The mutant screen was conducted as previously described (34). Mutagenized plants were imbibed for 3 d at 5 °C before being placed into soil and grown under 16-h days at 21 °C. rvr1-1 was backcrossed three times to Bd21-3 to reduce background mutations and the backcrossed material was used in all experiments. The rvr1 mutation was mapped using the CloudMap pipeline optimized for B. distachyon, sequence data can be found at the National Center for Biotechnology Information (NCBI) Sequence Read Archive rvr1-1, SRX2568123; rvr1-2, SRX2568122 (34).
Plant Growth and Flowering-Time Measurements.
Growth and scoring of plants were done as described in Ream et al. (29). Differences in heading date and gene expression between wild-type, nontransgenic, and transgenic plants were assessed using the Student’s t test and deemed significant if P < 0.05.
Phylogenetic Analysis.
Phylogenetic analyses were performed using both the full-length BdRVR1 gene and the individual BAH or TFIIS domains as seed sequences for BLAST searches using Phytozome, CoGe, and the NCBI (46). Handling of sequence and Bayesian trees were generated as described by Woods et al. (46). Maximum-likelihood analyses were conducted using SeaView v4.5.4 (47).
qRT-PCR.
RNA extraction and expression analysis was done as detailed in Ream et al. (29).
Generation of UBI:RVR1.
For generation of UBI:RVR1, see SI Materials and Methods.
Generation of amiVRN1/rvr1-1 Transgenic Lines.
The amiVRN1 construct used to knock-down VRN1 expression in rvr1-1 was previously published (31). Transformation of embryonic tissue was conducted as done in Ream et al. (29).
ChIP:H3K27me3.
Wild-type and rvr1-1 were grown to the third-leaf stage in 8-h SD. Eight grams of above-ground seedling tissue (four replicates) were harvested into liquid nitrogen in the middle of the photoperiod and stored at −80 °C. The remaining seedlings were moved to a 5 °C cold chamber set for 8-h SD and were vernalized for 4 wk, which is a saturating vernalization treatment for Bd21-3 (29). After 4 wk of cold exposure, the seedlings were still at the third-leaf stage and 8 g of above-ground portion of the seedling were harvested into liquid nitrogen during the middle of the photoperiod and stored at −80 °C. For isolation of chromatin and ChIP details, see SI Materials and Methods.
RNA-seq Analysis.
See SI Materials and Methods for plant growth and RNA-seq analysis details. Also see Dataset S1 for a complete list of differentially expressed genes, which shows the FC in a logarithmic scale [log2(FC)]. Note that genes were considered as differentially expressed when FC > 2 [i.e., log2(FC) > 1] and adjusted P < 0.001.
For RNA-seq validation, the expression of four differentially expressed genes from the RNA-seq analysis were randomly chosen for qPCR analysis to independently validate the RNA-seq analysis (Fig. S9). Plant growth conditions and harvesting procedure were the same as described above; however, the validation was conducted with a distinct set of biological replicates. The expression of all of the genes tested was consistent with the RNA-seq analysis (Fig. S9).
SI Materials and Methods
ChlP:H3K27me3.
Wild-type and rvr1-1 were grown to the third-leaf stage in 8-h SD. Eight grams of above-ground seedling tissue (four replicates) were harvested into liquid nitrogen in the middle of the photoperiod and stored at −80 °C. The remaining seedlings were moved to a 5 °C cold chamber set for 8-h SD and were vernalized for 4 wk, which is a saturating vernalization treatment for Bd21-3 (29). After 4 wk of cold exposure, the seedlings were still at the third-leaf stage and 8 g of above-ground portion of the seedling were harvested into liquid nitrogen during the middle of the photoperiod and stored at −80 °C. For isolation of chromatin, 2 g of tissue per replicate were ground in liquid nitrogen and 25 mL nuclear isolation buffer was added to the tissue powder [10 mM Hepes pH 8.0, 1 M Sucrose, 5 mM KCl, 5 mM MgCl2, 5 mM EDTA, 0.6% Triton X- 100, 0.4 mM PMSF, Pepstatin A, and Protease Inhibitor Complete (Roche)]. The PMSF, pepstatin A, and Protease inhibitor were added just before use of the nuclear isolation buffer. Samples were incubated at room temperature for 15 min before being cross-linked with 37% formaldehyde with gentle shaking for 20 min at room temperature.
Cross-linking was stopped with 2 M glycine and samples were filtered through miracloth. Samples were pelleted and resuspended with 1 mL of extraction buffer plus inhibitors [0.25 M sucrose, 10 mM Tris pH 8.0, 10 mM MgCl2, 1% Triton X-100, 1 mM EDTA, 5 mM β-mercaptoethanol, 0.1 mM PMSF, Protease Inhibitor Complete (Roche)]. Samples were pelleted again and resuspended in 300 μL of nuclei lysis buffer [50 mM Tris pH 8.0, 10 mM EDTA, 1% SDS, 0.1 mM PMSF, Protease Inhibitor Complete (Roche)] and 700 μL ChIP dilution before [Triton X-100, 1.2 mM EDTA, 16.7 mM Tris pH 8.0, 167 mM NaCL, 0.1 mM PMSF, Protease Inhibitor Complete (Roche)]. Samples were sonicated to shear DNA into fragments of roughly 500 bp. After sonication, an additional 2 mL of ChIP dilution buffer was added and then pelleted. A 150-μL aliquot of the supernatant was removed and saved for input. The remaining samples were used for immunopreciptation with either anti-H3 (Abcam) or anti-H3K27me3 (Millipore) antibodies. Five micrograms of antibody was added and incubated at 4 °C for 1 h with rotation. Dynabeads (Invitrogen) were prepared and added to samples containing antibodies and incubated at 4 °C with rotation overnight. The beads were then washed with 2× low-salt buffer, 1× high-salt buffer, and 1× LiCl buffer. An additional 2× TE wash was performed. Immunopreciptates were eluted from the beads with 2 × 250-μL incubations with 1% SDS and 0.1 M NaHCO3 on a rotary shaker for 10 min at 65 °C. Next, 5 M NaCl was added to the samples and incubated at 65 °C overnight. The samples were then treated with 0.5 M EDTA, 1 M Tris pH 6.8, 2 μL Proteinase K for 1 h at 42 °C. A phenol chloroform extraction was done and then samples were precipitated overnight in ethanol, sodium acetate, and glycogen before being pelleted. DNA was then used for quantitative real-time PCR following the guidelines from Haring et al. (48). For each primer pair, the amount of DNA precipitated using anti-H3K27me3 antibodies was normalized to the amount precipitated by an anti-H3 antibody from the same sample to correct for differences in ChIP input. Input and no-antibody control reactions were performed in parallel with each antibody reaction to verify that the immunopreciptated DNA was enriched. The data presented represent the mean of three biological replicates ± SEM. Primer sequence are provided in Table S1.
Table S1.
Primers and sequences
| Primer | Sequence |
| Bradi2g40147 qPCR F1 | TGCACCAGATGTGGTTGTTTCG |
| Bradi2g40147 qPCR R1 | TGCGCAGTACAGGGCTATTCTTG |
| Bradi2g40147 CDS F | caccATGGGAAGGCGCCGCCGGTTC |
| Bradi2g40147 CDS R stop | TTACTCATGATCAGCTGAACGTGC |
| VRN1_I1_F | TACGCACGCCTACGCTTAAG |
| VRN1_I1_R | GAAATGGAGCAGACAGGCAAG |
| VRN1_I5_F | GCACGGACGTGTAGGTTAAAGT |
| VRN1_I5_R | CACTGCCTGTGTGCATCTTC |
| VRN1_I6_F | GCAGGCAGCAAATAGGAGAAG |
| VRN1_I6_R | GAGCCAGTAGTAGCAAGGTGAGC |
| VRN1_CArG_F | CGACAACGGATATGCTCCAGACC |
| VRN1_CArG_R | GAAGAGAGCCGGAGAGTGGGT |
RNA-seq Analysis.
Plant growth.
To obtain V and NV plants that were developmentally matched, the V samples were vernalized as imbibed seed in soil in SD [although at the imbibed seed stage there is no effect of daylength on flowering during cold exposure (29)]. At the end of the cold treatment, NV samples were planted and all samples were then grown to the third-leaf stage in 8-h SD and the fully expanded third leaf was harvested in the middle of the photoperiod. Plants were grown in an 8-h photoperiod to eliminate any changes in gene expression that might be a result of floral induction. A total of four leaves were harvested per biological replicate and RNA extracted as previously described (29). RNA quality was verified using an Agilent bioanalyzer and quantified using Qubit.
Library creation.
Stranded RNA-Seq libraries were created and quantified by qPCR. Sequencing was performed using an Illumina HiSeq instrument.
RAW fastq files uploaded to Short Read Archive at NCBI.
The RAW fastq files uploaded to the Short Read Archive at the NCBI are: rvr1 rep1 SRX2574346; rvr1 rep2 SRX2574345; rvr1 rep3 SRX2574344; Bd213_NV_rep1 SRX2574343; Bd213_NV_rep2 SRX2574342; Bd213_NV_rep3 SRX2574341; Bd213_V_rep1 SRX2574340; Bd213_V_rep2 SRX2574339; Bd213_V_rep3 SRX2574338.
Read preprocessing, read alignment, and counting.
RNA-Seq samples were processed using CLC Genomics Workbench v8.5 (https://www.qiagenbioinformatics.com/products/clc-genomics-workbench/). The Trim Sequences tool was used to remove adapter sequence and low quality regions (quality limit: 0.05). The RNA-Seq Analysis tool was used to map reads against Brachypodium distachyon 283 v2.0 (length fraction: 0.8; similarity fraction: 0.8) and quantify read alignments over gene regions. Total read counts were used to call differentially expressed genes.
Differential gene expression.
DESeq2 (v1.14.0) (49) was subsequently used to determine which genes were differentially expressed between pairs of conditions. After verifying log-transformed data [rlog() function] by heatmap clustering (Fig. S9), we determined differentially expressed genes using the DESeq() function with default parameters for each contrast. Genes were considered as differentially expressed when the P < 0.001 and FC > 2 (Fig. S9).
Generation of UBI:RVR1.
RVR1 cDNAs were amplified from Bd21-3 cDNA using a forward primer at the start codon and a reverse primer containing a stop codon. cDNAs were gel-extracted (Qiagen) and were cloned into pENTR-d-TOPO (Life Technologies) using the manufacturer’s protocol. Cloning of RVR1 into pANIC10a was done as described in Ream et al. (29). Primer pairs used to clone each cDNA are listed in Table S1. The UBI:VRN1 and UBI:FT lines were previously published (29).
Phylogenetic Analysis.
Phylogenetic analyses were performed using both the full-length BdRVR1 gene and the individual BAH or TFIIS domains as seed sequences for BLAST searches using Phytozome, CoGe, and NCBI. The handling of sequence is described in Woods et al. (31) and Bayesian trees were generated as described previously (13). Maximum-likelihood analyses were conducted using SeaView 4.5.4 (47).
Supplementary Material
Acknowledgments
We thank undergraduate students Ryland Bednarek, Gerald Weiss, Jane Lee, Mary Kojima, and Leah Varner for assistance in the forward genetic screen; Jill Mahoy and Heidi Kaeppler for their continued support of our research by providing transgenic material in Brachypodium distachyon; Dean Sanders and Xuehua Zhong from the University of Wisconsin–Madison for guidance conducting ChIP experiments; the University of Wisconsin Biotechnology Center DNA sequencing facility for providing whole-genome sequencing of both mutant alleles of rvr1; and the Joint Genome Institute, specifically Kerrie Berry, Anna Lipzen, and Erika Lindquist, for RNA sequencing. This work was funded in part by National Science Foundation Grant IOS-1258126 (to R.M.A.); the Department of Energy Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FCO2-07ER64494) (to R.M.A. and C.W.); a National Institutes of Health-sponsored predoctoral training fellowship to the University of Wisconsin Genetics Training program (to D.P.W.); the Gordon and Betty Moore Foundation and the Life Sciences Research Foundation for their postdoctoral fellowship (T.S.R.); and Wallonie–Bruxelles International fellowships (to F.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequences reported in this paper have been deposited in the NCBI Sequence Read Archive, https://www.ncbi.nlm.nih.gov/sra (accession nos. SRX2568123, SRX2568122; rvr1 rep1, SRX2574346; rvr1 rep2, SRX2574345; rvr1 rep3, SRX2574344; Bd213_NV_rep1, SRX2574343; Bd213_NV_rep2, SRX2574342; Bd213_NV_rep3, SRX2574341; Bd213_V_rep1, SRX2574340; Bd213_V_rep2, SRX2574339; and Bd213_V_rep3, SRX2574338).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700536114/-/DCSupplemental.
References
- 1.Chouard P. Vernalization and its relations to dormancy. Annu Rev Plant Physiol. 1960;11:191–238. [Google Scholar]
- 2.Amasino R. Seasonal and developmental timing of flowering. Plant J. 2010;61:1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- 3.Shrestha R, Gómez-Ariza J, Brambilla V, Fornara F. Molecular control of seasonal flowering in rice, Arabidopsis and temperate cereals. Ann Bot (Lond) 2014;114:1445–1458. doi: 10.1093/aob/mcu032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fjellheim S, Boden S, Trevaskis B. The role of seasonal flowering responses in adaptation of grasses to temperate climates. Front Plant Sci. 2014;5:431. doi: 10.3389/fpls.2014.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Distelfeld A, Li C, Dubcovsky J. Regulation of flowering in temperate cereals. Curr Opin Plant Biol. 2009;12:178–184. doi: 10.1016/j.pbi.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Greenup A, Peacock WJ, Dennis ES, Trevaskis B. The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann Bot (Lond) 2009;103:1165–1172. doi: 10.1093/aob/mcp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Distelfeld A, Dubcovsky J. Characterization of the maintained vegetative phase deletions from diploid wheat and their effect on VRN2 and FT transcript levels. Mol Genet Genomics. 2010;283:223–232. doi: 10.1007/s00438-009-0510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbesier L, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007;316:1030–1033. doi: 10.1126/science.1141752. [DOI] [PubMed] [Google Scholar]
- 9.Zeevaart JA. Leaf-produced floral signals. Curr Opin Plant Biol. 2008;11:541–547. doi: 10.1016/j.pbi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Yan L, et al. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proc Natl Acad Sci USA. 2006;103:19581–19586. doi: 10.1073/pnas.0607142103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sasani S, et al. The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare) J Exp Bot. 2009;60:2169–2178. doi: 10.1093/jxb/erp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yan L, et al. The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science. 2004;303:1640–1644. doi: 10.1126/science.1094305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemming MN, Peacock WJ, Dennis ES, Trevaskis B. Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiol. 2008;147:355–366. doi: 10.1104/pp.108.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffiths S, Dunford RP, Coupland G, Laurie DA. The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol. 2003;131:1855–1867. doi: 10.1104/pp.102.016188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubcovsky J, et al. Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Mol Biol. 2006;60:469–480. doi: 10.1007/s11103-005-4814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen A, Dubcovsky J. Wheat TILLING mutants show that the vernalization gene VRN1 down-regulates the flowering repressor VRN2 in leaves but is not essential for flowering. PLoS Genet. 2012;8:e1003134. doi: 10.1371/journal.pgen.1003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan L, et al. Positional cloning of the wheat vernalization gene VRN1. Proc Natl Acad Sci USA. 2003;100:6263–6268. doi: 10.1073/pnas.0937399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preston JC, Kellogg EA. Reconstructing the evolutionary history of paralogous APETALA1/FRUITFULL-like genes in grasses (Poaceae) Genetics. 2006;174:421–437. doi: 10.1534/genetics.106.057125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teper-Bamnolker P, Samach A. The flowering integrator FT regulates SEPALLATA3 and FRUITFULL accumulation in Arabidopsis leaves. Plant Cell. 2005;17:2661–2675. doi: 10.1105/tpc.105.035766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimada S, et al. A genetic network of flowering-time genes in wheat leaves, in which an APETALA1/FRUITFULL-like gene, VRN1, is upstream of FLOWERING LOCUS T. Plant J. 2009;58:668–681. doi: 10.1111/j.1365-313X.2009.03806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng W, et al. Direct links between the vernalization response and other key traits of cereal crops. Nat Commun. 2015;6:5882. doi: 10.1038/ncomms6882. [DOI] [PubMed] [Google Scholar]
- 22.Oliver SN, Finnegan EJ, Dennis ES, Peacock WJ, Trevaskis B. Vernalization-induced flowering in cereals is associated with changes in histone methylation at the VERNALIZATION1 gene. Proc Natl Acad Sci USA. 2009;106:8386–8391. doi: 10.1073/pnas.0903566106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Distelfeld A, Tranquilli G, Li C, Yan L, Dubcovsky J. Genetic and molecular characterization of the VRN2 loci in tetraploid wheat. Plant Physiol. 2009;149:245–257. doi: 10.1104/pp.108.129353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brutnell TP, Bennetzen JL, Vogel JP. Brachypodium distachyon and Setaria viridis: Model genetic systems for the grasses. Annu Rev Plant Biol. 2015;66:465–485. doi: 10.1146/annurev-arplant-042811-105528. [DOI] [PubMed] [Google Scholar]
- 25.Higgins JA, Bailey PC, Laurie DA. Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. PLoS One. 2010;5:e10065. doi: 10.1371/journal.pone.0010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woods DP, Amasino RM. Dissecting the control of flowering time in grasses using Brachypodium distachyon. In: Vogel PJ, editor. Genetics and Genomics of Brachypodium. Springer International; Cham, Switzerland: 2015. pp. 259–273. [Google Scholar]
- 27.Woods DP, et al. Genetic architecture of flowering-time variation in Brachypodium distachyon. Plant Physiol. 2017;173:269–279. doi: 10.1104/pp.16.01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bettgenhaeuser J, et al. Natural variation in Brachypodium links vernalization and flowering time loci as major flowering determinants. Plant Physiol. 2017;173:256–268. doi: 10.1104/pp.16.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ream TS, et al. Interaction of photoperiod and vernalization determines flowering time of Brachypodium distachyon. Plant Physiol. 2014;164:694–709. doi: 10.1104/pp.113.232678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lv B, et al. Characterization of FLOWERING LOCUS T1 (FT1) gene in Brachypodium and wheat. PLoS One. 2014;9:e94171. doi: 10.1371/journal.pone.0094171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woods DP, McKeown MA, Dong Y, Preston JC, Amasino RM. Evolution of VRN2/Ghd7-like genes in vernalization-mediated repression of grass flowering. Plant Physiol. 2016;170:2124–2135. doi: 10.1104/pp.15.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods DP, Ream TS, Amasino RM. Memory of the vernalized state in plants including the model grass Brachypodium distachyon. Front Plant Sci. 2014;5:99. doi: 10.3389/fpls.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ream TS, Woods DP, Amasino RM. The molecular basis of vernalization in different plant groups. Cold Spring Harb Symp Quant Biol. 2012;77:105–115. doi: 10.1101/sqb.2013.77.014449. [DOI] [PubMed] [Google Scholar]
- 34.Woods DP, Ream TS, Minevich G, Hobert O, Amasino RM. PHYTOCHROME C is an essential light receptor for photoperiodic flowering in the temperate grass, Brachypodium distachyon. Genetics. 2014;198:397–408. doi: 10.1534/genetics.114.166785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minevich G, Park DS, Blankenberg D, Poole RJ, Hobert O. CloudMap: A cloud-based pipeline for analysis of mutant genome sequences. Genetics. 2012;192:1249–1269. doi: 10.1534/genetics.112.144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Callebaut I, Courvalin JC, Mornon JP. The BAH (bromo-adjacent homology) domain: A link between DNA methylation, replication and transcriptional regulation. FEBS Lett. 1999;446:189–193. doi: 10.1016/s0014-5793(99)00132-5. [DOI] [PubMed] [Google Scholar]
- 37.Su Z, Denu JM. Reading the combinatorial histone language. ACS Chem Biol. 2016;1:564–574. doi: 10.1021/acschembio.5b00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang N, Xu R-M. Structure and function of the BAH domain in chromatin biology. Crit Rev Biochem Mol Biol. 2013;48:211–221. doi: 10.3109/10409238.2012.742035. [DOI] [PubMed] [Google Scholar]
- 39.Kim B, et al. The transcription elongation factor TFIIS is a component of RNA polymerase II preinitiation complexes. Proc Natl Acad Sci USA. 2007;104:16068–16073. doi: 10.1073/pnas.0704573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johanson U, et al. Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science. 2000;290:344–347. doi: 10.1126/science.290.5490.344. [DOI] [PubMed] [Google Scholar]
- 41.Michaels SD, Amasino RM. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouché F, Woods DP, Amasino RM. Winter memory throughout the plant kingdom: Different paths to flowering. Plant Physiol. 2017;173:27–35. doi: 10.1104/pp.16.01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruelens P, et al. FLOWERING LOCUS C in monocots and the tandem origin of angiosperm-specific MADS-box genes. Nat Commun. 2013;4:2280. doi: 10.1038/ncomms3280. [DOI] [PubMed] [Google Scholar]
- 44.Wood CC, et al. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc Natl Acad Sci USA. 2006;103:14631–14636. doi: 10.1073/pnas.0606385103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Lucia F, Crevillen P, Jones AME, Greb T, Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci USA. 2008;105:16831–16836. doi: 10.1073/pnas.0808687105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woods DP, Hope CL, Malcomber ST. Phylogenomic analyses of the BARREN STALK1/LAX PANICLE1 (BA1/LAX1) genes and evidence for their roles during axillary meristem development. Mol Biol Evol. 2011;28:2147–2159. doi: 10.1093/molbev/msr036. [DOI] [PubMed] [Google Scholar]
- 47.Gouy M, Guindon S, Gascuel O. SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 48.Haring M, et al. Chromatin immunopreciptation: Optimization, quantitative analysis and data normalization. Plant Methods. 2007;3:11. doi: 10.1186/1746-4811-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.