Significance
Acute traumatic stress increases the sensitivity to develop epileptic seizures in certain people. It is therefore important to discover physiological mechanisms that avoid epilepsy. To test if rapidly inducible microRNAs (miRs) could mediate such protection, we combined mouse engineering, RNA sequencing, electric recording of brain activity, and learning tests. We discovered that miR-211, originating from an epilepsy-related genomic locus, may be involved, and therefore engineered mice produce a drug-suppressible excess of brain miR-211. In these mice, suppressing miR-211 excess to the original expression levels in normal brains led to electrically recorded epilepsy and hypersensitivity to epilepsy-inducing compounds; it also modified acetylcholine receptor composition. The functional impact of miR-211 dynamics on seizure threshold may enable future development of miR-211–directed therapeutics.
Keywords: acetylcholinesterase, cholinergic, EEG, epilepsy, microRNA
Abstract
Epilepsy is a common neurological disease, manifested in unprovoked recurrent seizures. Epileptogenesis may develop due to genetic or pharmacological origins or following injury, but it remains unclear how the unaffected brain escapes this susceptibility to seizures. Here, we report that dynamic changes in forebrain microRNA (miR)-211 in the mouse brain shift the threshold for spontaneous and pharmacologically induced seizures alongside changes in the cholinergic pathway genes, implicating this miR in the avoidance of seizures. We identified miR-211 as a putative attenuator of cholinergic-mediated seizures by intersecting forebrain miR profiles that were Argonaute precipitated, synaptic vesicle target enriched, or differentially expressed under pilocarpine-induced seizures, and validated TGFBR2 and the nicotinic antiinflammatory acetylcholine receptor nAChRa7 as murine and human miR-211 targets, respectively. To explore the link between miR-211 and epilepsy, we engineered dTg-211 mice with doxycycline-suppressible forebrain overexpression of miR-211. These mice reacted to doxycycline exposure by spontaneous electrocorticography-documented nonconvulsive seizures, accompanied by forebrain accumulation of the convulsive seizures mediating miR-134. RNA sequencing demonstrated in doxycycline-treated dTg-211 cortices overrepresentation of synaptic activity, Ca2+ transmembrane transport, TGFBR2 signaling, and cholinergic synapse pathways. Additionally, a cholinergic dysregulated mouse model overexpressing a miR refractory acetylcholinesterase-R splice variant showed a parallel propensity for convulsions, miR-211 decreases, and miR-134 elevation. Our findings demonstrate that in mice, dynamic miR-211 decreases induce hypersynchronization and nonconvulsive and convulsive seizures, accompanied by expression changes in cholinergic and TGFBR2 pathways as well as in miR-134. Realizing the importance of miR-211 dynamics opens new venues for translational diagnosis of and interference with epilepsy.
Epileptic seizures are generated when excitation/inhibition imbalances or abnormal afferent signals to a brain region lead to excessive or parallel nonspecific neuronal activation (1). Epilepsy has a wide range of manifestations, from brief loss of consciousness to overt convulsions. All mammalian brains, including healthy human brains, are prone to the generation of seizures, and diverse brain insults (ischemic, traumatic, infectious) reduce the threshold to seizures (2, 3). However, why some brains undergo epileptogenesis and develop recurrent unprovoked seizures following insults while others escape this pathophysiology remains unknown (4). Specifically, genes whose modulation mitigates these processes, and the neurotransmission and/or growth factor pathways involved, remain largely unexplored.
Recent reports point at microRNAs (miRs) as functional regulators of epileptogenesis. MiRs are small noncoding RNA molecules that regulate the expression levels of most protein coding genes in mammals and may orchestrate whole transcriptional pathways (5). Recent studies on status epilepticus (SE) animal models of epilepsy identified seizure-characteristic miR changes (6). Also, different miRs, including miR-132 (7), miR-34a (8), and miR-146a (9) emerged as actively involved in the pathogenesis of epilepsy. Of growing interest is miR-134, whose silencing exerts protective effects both in reducing the in vivo severity of SE (10, 11) and in in vitro-cultured hippocampal neurons (12), accompanied by numerous links to human epilepsy (13).
Disrupted ACh signaling and malfunctioning of the TGF-β pathway may both lead to excessive neuronal activation and seizures, and underlie some cases of epilepsy (14, 15). However, the molecular mechanisms linking these two pathways with epileptogenesis are only partially known. Disrupted ACh signaling leads to seizures due to the modulatory function of ACh over mammalian brain neurotransmitters such as glutamate or GABA, and affects synaptic transmission in large cortical networks. Cholinergic receptors include muscarinic metabotropic receptors, which function via G proteins, and nicotinic cation-channel receptors, which are exclusively excitatory (17); modulating the action of both receptor types results in seizure activity (18). In autosomal dominant nocturnal frontal lobe epilepsy, missense mutations in either the nAChRα4 or β2 nicotinic receptor subunits interrupt cholinergic regulation, leading to seizures (19). Also, focal hypercholinergic activity suffices as an initial event in generating network hyperexcitability and seizures (20). Additionally, hippocampal induction of hyperexcitability by cholinesterase inhibitors induces epileptogenesis in rodent models of epilepsy, and injecting the cholinergic agonist pilocarpine initiates epileptic events, seizures, or full SE (21). Nevertheless, mechanisms underlying the control of such modulatory changes and avoidance of epileptogenesis in the healthy brain are still sought after.
Cholinergic-mediated control of excitability may also target nonneuronal components of the neurovascular unit. ACh modulates inflammatory signaling in both the periphery and the brain, which together with blood–brain barrier integrity may critically control network excitability and seizure threshold. For example, brain insults such as trauma may lead to increased permeability of the microvasculature to serum albumin, which then binds TGFβR2 (TGFBR2) receptors on astrocytes, inducing a chain of epileptogenic events. Such events include activation and phosphorylation of Smad2 and downstream transcriptional effects, entailing neuroinflammation, excitatory synaptogenesis, and reduced neuronal inhibitory ques, all leading to chronic epilepsy (22). Concordantly, local or systemic application of losartan, a potential TGF-β–signaling blocker, can reduce albumin-derived TGF-β–signaling and thus block epileptogenesis (23).
Numerous genetic epileptic syndromes are hallmarked by cholinergic dysfunctions that control neuronal excitability and seizure threshold (18, 20). Therefore, we predicted that miR modulators of cholinergic activities may avoid neuronal hypersynchronization by performing coordinated surveillance over signaling networks that keep synchronized firing in check, and sought miRs that may play functional role(s) in inducing and/or suppressing seizure-related cholinergic signaling by affecting synaptic targets. For this purpose, we generated and tested transgenic mouse models, used continuous electrocorticography recordings to detect changes in neocortical activity, and used RNA sequencing (RNA-seq), bioinformatics analyses, and corresponding validations, all of which identified miR-211 as an attenuator of coregulated cholinergic and TGFBR2-associated seizures.
Results
Neuronal miR-211 Controls Cholinergic Synapse Transcripts in the Epileptogenic Brain.
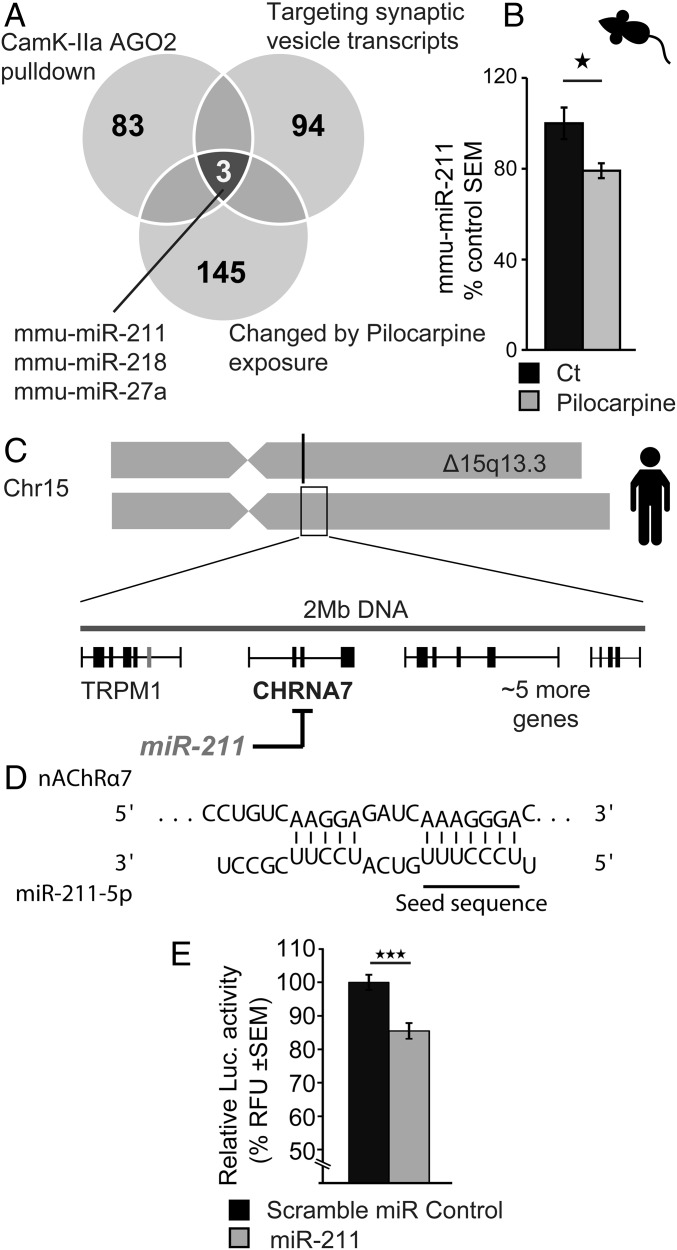
To perform a nonbiased search for neuronal miRs regulating synaptic processes and responding to cholinergic seizure-related cues, we intersected publicly available transcriptional profiles of miRs regulating synaptic vesicle transcripts (24), overrepresented in healthy forebrain immune precipitates of neuronal Argonaute 2 (25) and differentially expressed following pilocarpine injection, which induces cholinergic-mediated seizures (26). Of the three miRs that emerged in all three profiles (miR-211-5p, -218-5p, and -27a-3p; Fig. 1A), we experimentally observed hippocampal miR-211 to be down-regulated in acute pilocarpine-injected model mice, compared with saline-injected controls (48 h following injection; Fig. 1B).
Fig. 1.
Identifying MiR-211 as a synaptic candidate associated with cholinergic signaling-induced seizures. (A) Three candidate miRs (miR-211, -218, and -27a) emerged by intersecting rodent miRs whose levels modify following exposure to the cholinergic facilitator pilocarpine (145 miRs) (6); interact with the RNA-induced silencing complex (RISC) protein Argonaute 2 (AGO2) in CamK2a-expressing cells (83 miRs) (25); and target synaptic vesicle transcripts (94 miRs) (75), predicting involvement in cholinergic-related epileptic seizures. (B) qRT-PCR measurements show mmu-miR-211 decline in hippocampal RNA 24 h following exposure to pilocarpine. (C) Human MiR-211, as well as its in silico target, nicotinic nAChRα7, and five other genes localize to a 15q13.3 chromosomal region where heterozygote deletions entail cognitive impairments with recurrent seizures. (D) The seed domain of hsa-miR-211-5p shows sequence complementarity with the inflammation-regulating nicotinic nAChRα7. (E) Luciferase assay validated direct targeting by miR-211 of nAChRα7 in human embryonic kidney cells. Results were considered significant at *P < 0.05, ***P < 0.001, after correction for multiple testing when applicable.
Notably, miR-211 is a conserved intragenic miR located within the TRPM1 calcium channel gene (5), itself within the 15q13.3 locus where heterozygote microdeletions [Online Mendelian Inheritance in Man (OMIM) no. 612001] associate with mental retardation and recurrent epileptic seizures (27, 28), and homozygous deletions associate with severe neurodevelopmental problems, including epileptic encephalopathy (29). In proximity to the TRPM1 gene and within the 15q13.3 locus is the nicotinic receptor nAChRα7 (30) (Fig. 1C), a gain-of-function mutation that results in nicotine-induced seizures (31). In silico miR–target interaction analysis (32, 33) predicted that Homo sapiens (hsa)-miR-211 targets nAChRα7 via a 7-mer miR response element (Fig. 1D). Validating this prediction, miR-211 expression in cultured HEK293 cells directly down-regulated a luciferase expression construct containing the nAChRα7 3′ UTR (Fig. 1E); taken together, this indicated that miR-211 is both affected by and may control cholinergic transmission in an epilepsy-relevant manner.
DTg-211 Mice Present Spontaneous Emergence of Nonconvulsive Seizures upon miR-211 Suppression.
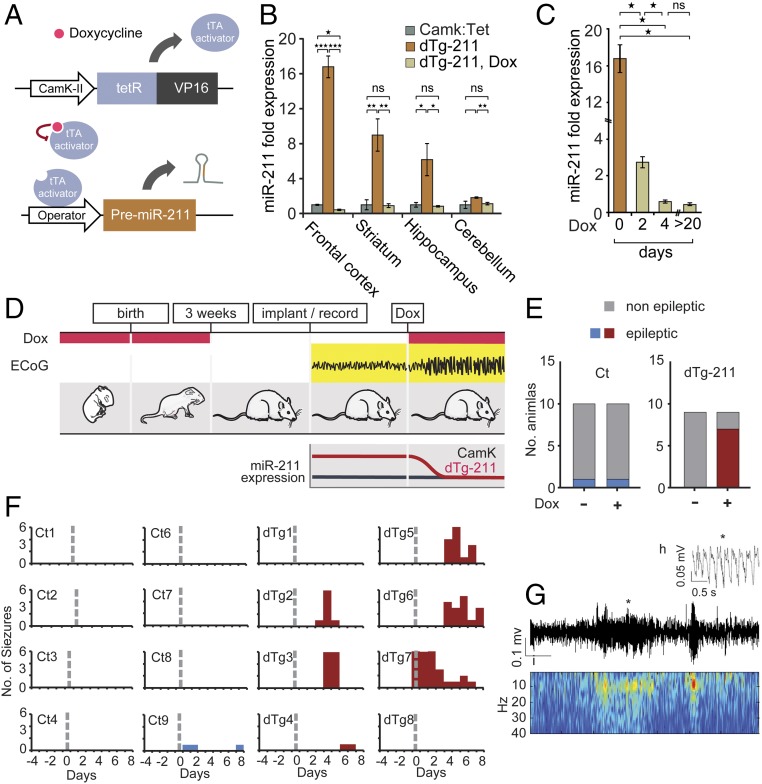
In the retina, the half-life of miR-211 is regulated via active degradation by light and neuronal activity (34). Therefore, using a constitutive, single time point expression system would have missed the dynamic dimension of its in vivo demise. To explore the in vivo impact of miR-211 decline on cholinergic signaling and seizure susceptibility, we used a double-transgenic tetracycline-repressible (Tet-Off) system, where engineered mice exclusively express miR-211 from the TRE insertion in CamK2a–expressing cells (i.e., forebrain neurons) and only in the absence of doxycycline (Dox), allowing temporal follow-up of the effects of introducing and removing overexpression. To overcome the possibility of phenotypic consequences due to the expression of the Tet activator, rather than miR-211 in CamK2a-expressing cells, we used littermate CamK mice (which express the transgenic inducer in the same cells and contexts, but with no miR-211 overexpression) as controls throughout the study. Briefly, we cloned the murine premiR-211 sequence under control of the tetracycline regulatory region (using a pTRETight plasmid system), and established TRE-miR-211 mouse lines. These were crossed with Tet-Off CaMK2-transactivator (tTA) mice (referred to as CamK mice; see Table S1 and Methods). Progeny double-transgenic mice (dTg-211), unlike littermate CamK controls, overexpressed miR-211 in forebrain tissues, but only in the absence of Dox (scheme in Fig. 2A). When administered with Dox in drinking water for 6–8 wk, dTg-211 mice exhibited normally low forebrain miR-211 expression levels, indistinguishable from those in control mice (Fig. 2B). In contrast, Dox withdrawal induced miR-211 accumulation in the frontal cortex, hippocampus, and striatum, but not in the cerebellum (Fig. 2B), essentially as reported for other CamK:Tet mouse lines (35). When readministered with Dox, adult (2-mo-old) dTg-211 mice showed miR-211 decline to basal levels in the frontal cortex within 4 d (Fig. 2C), supporting the applicability of these mice for evaluating temporal attributes of miR-211 decreases in the forebrain.
Table S1.
Primers for PCR-based genotyping
| No. | Gene | Primer | Sequence |
| 1 | PTRE-miR-211 | PTRE-miR-211-F | GTGTACGGTGGGAGGCCTAT |
| 2 | PTRE-miR-212 | PTRE-miR-211-R | GGGAGGTGTGGGAGGTTTT |
| 3 | CamK:tTA | oIMR8746 | CGCTGTGGGGCATTTTACTTTAG |
| 4 | CamK:tTA | oIMR8747 | CATGTCCAGATCGAAATCGTC |
Fig. 2.
dTg-211 mice develop spontaneous nonconvulsive seizures following Dox-induced reduction of forebrain miR-211 excess. (A) DTg-211 mice carry the CamK2a promoter, followed by a tTA coding sequence and a pTRE-transgene inducing Dox-suppressible expression of mmu-miR-211 in forebrain neurons. (B) MiR-211 overexpression in the mouse forebrain but not cerebellum is Dox suppressible. Expression normalized to CamK controls. (C) Dox-suppressed miR-211 levels decline to basal levels within days. (D) DTg-211 mice were administered Dox before and after birth, preventing transgene overexpression during development. ECoG recordings in dTg-211, but not control, mice showed synchronous neuronal cortical activity after Dox treatment, parallel to declined miR-211 levels. (E) DTg-211 mice presented ECoG-recorded seizures exclusively after Dox administration. (F) ECoG plots showing number of seizures per day in single dTg-211 mice and controls (red, blue). Dashed gray line marks initiation of Dox administration. (G) Representative ECoG recording plot shows a seizure of a Dox-exposed dTg-211 mouse; corresponding heat map shows representative higher-power seizure of low-frequency oscillations (∼5 Hz) at the same time window. (H) Magnification of a single event (marked by asterisk in G), presenting an enlarged section of the seizure activity, with spike and wave form. Results were considered significant at *P < 0.05, **P < 0.01, ***P < 0.001, after correction for multiple testing when applicable. ns, not significant.
To directly test if miR-211 decline affects cortical neuronal hyperexcitability, we performed electrocorticography (ECoG) measurements on the mice before and following Dox-mediated miR-211 reduction. In this paradigm, both CamK controls and dTg-211 mice received Dox from conception, to prevent developmental effects due to miR-211 overexpression; Dox was later withdrawn to allow miR-211 accumulation in the forebrain. At 12 wk of age, ECoG recordings were initiated and 4–6 d after, Dox was readministered, once again reducing the elevated miR-211 levels (Fig. 2 C and D). During the subsequent 6 d, ECoG recordings demonstrated spontaneous nonconvulsive seizures in six of eight dTg-211 mice but in none of nine control CamK mice receiving similar Dox treatment (Fig. 2E). Seizures mostly initiated by the third or fourth day after Dox administration (Fig. 2F), parallel to the decline in miR-211 overexpression (Fig. 2C). Identified seizures showed a pattern of low frequency (∼5 Hz) and sharp activity (Fig. 2 G and H), without observed motor convulsions. Notably, similarly slow hypersynchronous cortical activity is reminiscent of several human syndromes manifested in epilepsy (36), altogether raising the possibility that forebrain miR-211 elevation may be protective against spontaneous nonconvulsive seizures, whereas its reduction induces them.
Forebrain miR-211 Suppression Exacerbates Long-Lasting Pentylenetetrazole-Induced Seizures and TGF-β Signaling.
The spontaneous seizures induced following Dox-suppression in dTg-211 mice suggested that miR-211 decline may also entail a sustained susceptibility to convulsions. To test such an effect, we treated dTg-211 mice with Dox for 6 d, and challenged them and matched controls with the seizure-provoking pentylenetetrazole (PTZ) agent 5 d after Dox has been removed, when the levels of cortical miR-211 are again elevated (Fig. 3A). Tested dTg-211 mice showed increased susceptibility to convulsive stimuli (Fig. S1A), and ECoG recording of consequent epileptiform spikes in dTg-211 mice showed an increase in spike counts and total numbers (Fig. 3 B and C), as well as seizure numbers and latency to first spike and to first seizure compared with CamK control mice (Fig. 3 D–F), where miR-211 levels were conspicuously lower (Fig. 3G), all pointing to long-lasting epileptiform impact of Dox in dTg-211 mice.
Fig. 3.
Dox-treated dTg-211 mice show sustained susceptibility to PTZ-induced convulsions alongside TGFBR-associated gene changes in RNA sequence. (A) Scheme of PTZ injection 4 d after 5-d Dox administration, aimed to examine long-term susceptibility to this convulsant. See Fig. S3 for increased manual convulsions index scores in dTg-211 mice. (B) ECoG recording shows larger spikes per minute counts, reflecting seizure susceptibility in PTZ-exposed dTg-211 mice compared with CamK controls. (C) Number of seizures. (D) Latency to first spike. (E) Number of seizure events by neuronal networks analysis. (F) Latency to first seizure. (G) DTg-211 mice regained miR-211 overexpression after Dox removal, at the time of PTZ test. (H) Luciferase validation tests of miR-211 targeting of the murine TGFBR2 3′-UTR but not a control sequence. (I) Reduced TGFBR2 protein concentration (twofold) in dTg-211 frontal cortex (ELISA, n = 7 + 7, P < 0.001). (J) Increased TGFBR2 mRNA levels following Dox administration. (K) Fold-change volcano plot differences for dTg-211 with/without Dox (Right) compared with dTg-211/CamK brains (Left). Dots represent genes, with positive or negative twofold change (orange), passing cutoff threshold for significance (red), both (green), or unmodified (black). (L) ECDF plots show differential expression (P values) following Dox of reduced (orange) but not elevated genes (green) in dTg-211 cortices or in all genes (gray). (M) Cortical genes up-regulated in dTg-211 are reduced (red) following Dox compared with (N) all genes. (O) Per-gene fold changes following Dox for TGF-β–signaling genes modified 12 h following status epilepticus (38). Results were considered significant at *P < 0.05, **P < 0.01, ***P < 0.001, after correction for multiple testing when applicable. ns, not significant.
Fig. S1.
dTg-211 mice with reduced forebrain miR-211 levels show higher susceptibility to PTZ-mediated seizures. (A) dTg-211 mice showed higher convulsive scores following PTZ injection, after a Dox administration paradigm. (B) Sequence complementarity of the murine TGFBR2 3′-UTR and the seed sequence of mmu-miR-211-5p, in a conserved 8-mer binding site (TargetScan, PCT = 0.46). (C) Frontal cortex samples of non–Dox-treated dTg-211 showed reduction of TGFBR2 transcript levels by RT-qPCR. ***P < 0.001.
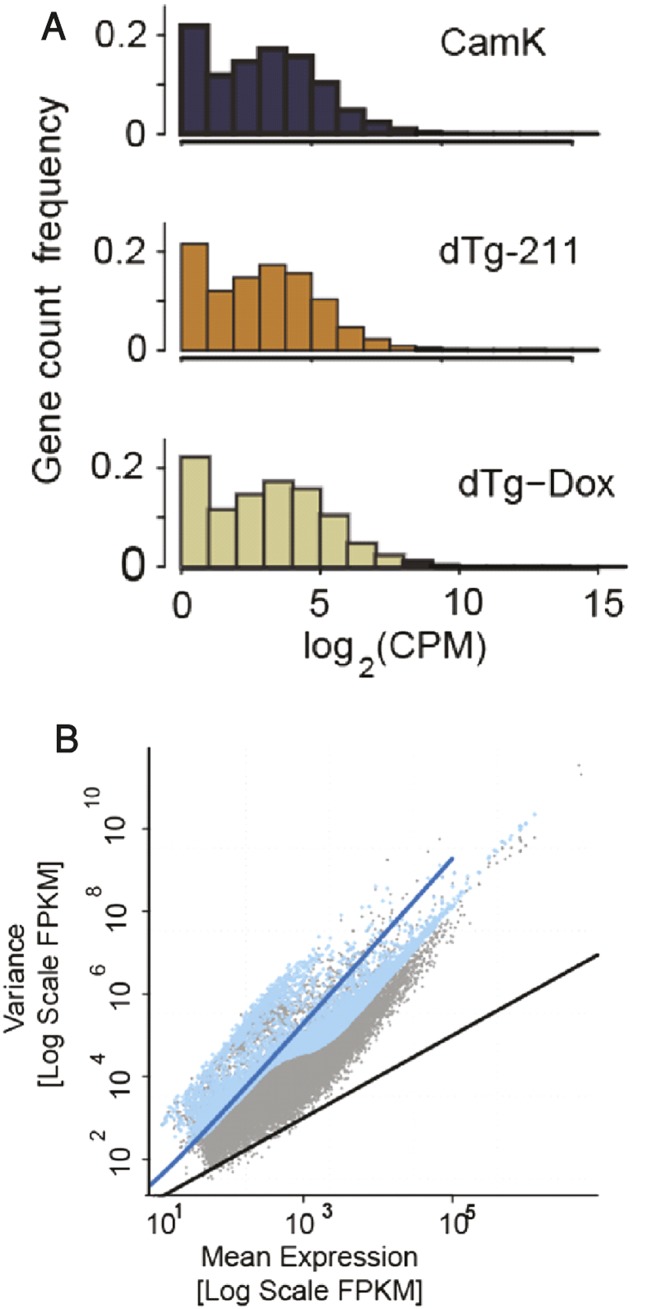
Given the reported role of TGFβR signaling in epileptogenesis, we next examined if the TGFBR pathway genes that change following status epilepticus were modified in the epilepsy-susceptible dTg-211 mice following Dox. The 3′-UTR of the murine TGFBR2 gene shows seed sequence complementarity with the mature Mus musuculus (mmu)-miR-211 (Fig. S1B), and an in vitro psiCHECK assay showed direct down-regulation of the murine TGFBR2 3′-UTR reporter by mmu-miR-211, validating this miR–target link (Fig. 3H). Correspondingly, non–Dox-treated frontal dTg-211 cortices showed twofold lower TGFBR2 transcript levels compared with controls (Fig. S2B), alongside ∼40% reduced TGFBR2 protein (Fig. 3I; ELISA). Reciprocally, administration of Dox induced a step-wise fourfold increase in TGFBR2 mRNA within 4 d (Fig. 3J). To explore if TGFBR2 pathway genes are globally changed, we turned to unbiased RNA-seq of dTg-211 cortical tissue RNAs (without and with Dox suppression of miR-211 overexpression) compared with matched control tissues. The cDNA libraries showed overall similar sequencing reads distribution across expression level and interrelated tag-wise normalized variance and expression levels (Fig. S2 A and B). Nevertheless, comparing dTg-211 brains before and after 5 d of Dox administration showed substantially higher numbers of differentially expressed genes than comparing naïve dTg-211 brains to CamK controls (Fig. 3K), suggesting that Dox-induced suppression of miR-211 overexpression may entail an extensive physiological change. Notably, grouped miR-211 target transcripts, as predicted in silico by the TargetScan algorithm (37), showed significant, albeit mild, increases and decreases following Dox administration [empirical cumulative distribution function (ECDF) plots] (Fig. S3). We conclude that the bulk of transcriptome changes induced by miR-211 perturbations occurred in secondarily affected transcripts and that the transcriptional footprint of miR-211 reduction was greater than that of its sustained overexpression.
Fig. S2.
RNA sequencing quality checks. (A) Illumina-compatible cDNA libraries show overall similar sequencing depth and distribution across expression level. (B) Tag-wise normalized variance predictably correlated to expression levels.
Fig. S3.
Forebrain miR-211 targets are preferentially modulated under miR-211 decline. Target transcripts of miR-211, as predicted in silico by the TargetScan algorithm (37) and grouped by MRE site type, showed mild albeit significant modification under miR-211 decline, with increased probability depending on site length following Dox administration; ECDF plot.
Predictably, genes that were down-regulated in dTg-211 forebrains compared with controls were more commonly differentially expressed following Dox administration, in contrast to all genes or all up-regulated genes (Fig. 3L; ECDF, Kolmogorov–Smirnov test, P < 0.001). Also, those genes that were down-regulated in dTg-211 brains showed preferred post-Dox up-regulation (Fig. 3 M and N). Numerous TGF-β pathway genes that were modified 12 h following SE (38) were also changed following Dox, either by up- or down-regulation (Fig. 3O), including the Myc protooncogene protein (MyC), the Chordin (chrd), inhibitor of DNA binding 2, HLH protein (id2), and SMAD family members 1 and 9 (smad1, smad9). Together, these findings suggested that the Dox-induced release of TGFBR2 from miR-211–mediated suppression impacted forebrain TGF-β signaling. Murine miR-211 thus emerged as a modulator of epilepsy, whose forebrain suppression induces long-lasting hyperexcitability and epileptiform activity alongside TGF-β pathway alterations.
MiR-211 Decline Induces Transcriptome Changes of Endothelial, Synaptic, and Cholinergic Functions.
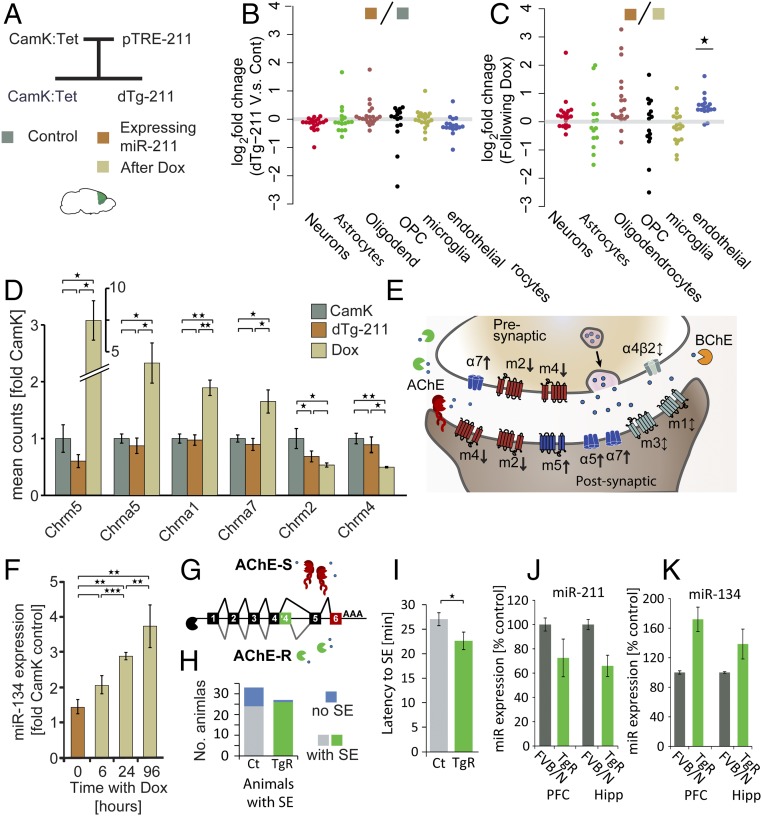
To explore the global transcriptional changes following miR-211 suppression, and test if they relate to specific brain cell types, we compared dTg-211 brains’ transcripts to controls. Specifically, we tested highly expressed genes characteristic of neurons, astrocytes, oligodendrocytes and their progenitor cells (OPCs), microglia, and endothelial cells (39) (Fig. 4 A and B). None of the cell type marker groups showed changes in dTg-211 brains compared with controls. However, comparing dTg-211 transcripts in brains with and without Dox (Fig. 4C) demonstrated that 19 of 21 endothelial cell markers, but none of the other cell type markers showed an increase following Dox administration (P < 0.05, perturbation analysis; Methods). Thus, miR-211 reaction to Dox appeared to potentiate endothelial gene expression, predicting functional relevance for neurovascular unit activities.
Fig. 4.
MiR suppression in dTg-211 mice alters cell type marker genes and cholinergic receptors, and a cholinergic mouse model shows concordant miR changes alongside increased seizure susceptibility. (A) Experimental setup: CamK:TtA mice bred with Tg-pTRE-211 mice generated dTg-211 mice and littermate CamK controls. Illumina-compatible libraries from frontal cortex RNA of mice before or under doxycycline (color-coded squares) were sequenced. (B) Sustained cell-type marker (39) in dTg brains. (C) Elevated endothelial marker genes following Dox. (D) Modified muscarinic and nicotinic cholinergic receptors in Dox-treated dTg-211 brains. (E) Scheme of cholinergic receptors and regulators (shown in D) in brain cholinergic synapses. Note Dox-induced down-regulation of cholinergic receptors suppressing synaptic transmission: CHRM2 and CHRM4 (m2 and m4); and up-regulation of facilitators CHRM5 (m5), CHRNA5, and CHRNA7 (α5 and α7). (F) MiR-134 up-regulation in dTg-211 mice following Dox administration parallels the time frame of seizure induction in this model. (G) Scheme of the synaptic and nonsynaptic AChE transcript variants and corresponding protein forms. (H) Mice overexpressing the nonsynaptic cholinergic enzyme AChE-R (TgR) (76) show higher propensity with (I) shorter latency for status epilepticus event following pilocarpine injection, alongside (J) miR-211 reduction and (K) miR-134 elevation in prefrontal cortex (PFC) and hippocampus (Hipp) of TgR mice. Results were considered significant at *P < 0.05, **P < 0.01, ***P < 0.001, after correction for multiple testing when applicable.
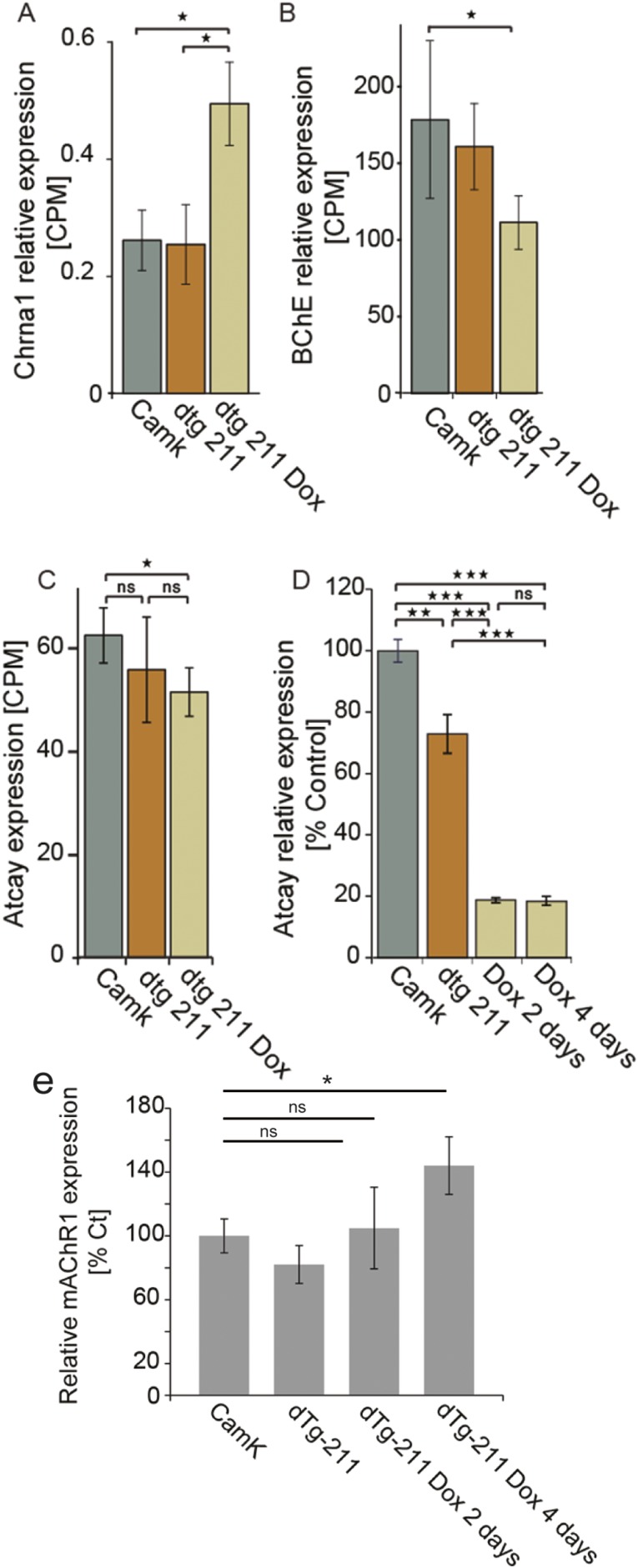
Next, we searched for Dox-induced changes in the expression of cholinergic receptor genes. The excitatory muscarinic ACh receptor-5 (30), a positive effector of cholinergic synaptic transmission (31), was elevated by fourfold (Fig. 4D). Likewise, the excitatory nAChRα-1 neuronal nicotinic receptor and α-5 nicotinic receptor (40, 41), and the ionotropic α-7 nicotinic receptor, responsible for post- and presynaptic excitation (17) and blocker of inflammation (42), were all elevated. In contrast, the metabotropic muscarinic ACh receptors 4 and 2 (mAChR4, mAChR2) were both twofold reduced following Dox administration (Fig. 4D and scheme in Fig. 4E). MAChR4 is located on both pre- and postsynaptic sites in brain cholinergic synapses, and exerts inhibitory effects on synaptic firing (43) with a role in locomotion. Additionally, we noted increases in butyrylcholinesterase (BChE; Fig. S4B), which hydrolyzes ACh in the brain alongside AChE and is elevated in AD brains (44). In contrast, we noted decrease of ATCAY/BNIP-H (Fig. S4 C and D), an ataxia-related brain-specific scaffold protein, which was recently found to recruit choline acetyltransferase (ChAT) to neurite terminals, and promote cholinergic signaling (45, 46). Furthermore, within 4 d following Dox administration, dTg-211 mice presented fourfold increases in the forebrain levels of miR-134 (Fig. 4F), known to be causally involved with the induction of convulsive seizures (7, 10, 11, 47).
Fig. S4.
Bidirectional cholinergic gene changes under forebrain miR-211 decline. (A) Increased alpha-1 neuronal nicotinic receptor (Chrna1) and (B) BChE following Dox administration in the forebrain of dTg-211 mice. (C and D) Decrease of ATCAY/BNIP-H expression with Dox administration (RNA-seq and qRT-PCR, respectively). (E) Muscarinic acetylcholine receptor M1 (mAChR1) is elevated in dTg-211 mice 4 d following Dox administration. Results were considered significant at *P < 0.05, **P < 0.01, ***P < 0.001, after correction for multiple testing when applicable. ns, not significant.
To examine how extrasynaptic cholinergic imbalance would affect miR-211 expression and the risk of epilepsy, we used transgenic AChE-R (TgR) mice overexpressing the soluble, nonsynaptic stress-induced splice variant of AChE from which the 3′-untranslated region (3′-UTR), which contains the miR regulatory element, had been deleted (Fig. 4G, scheme). TgR mice, which constitutively overexpress AChE-R that catalyzes ACh breakdown in extrasynaptic sites and show chronic stress behaviors, are hypersensitized to nicotine administration (48). Intriguingly, these mice also experienced higher susceptibility to seizures, manifested as larger fraction of mice presenting full SE after pilocarpine injection (Fig. 4H); this was accompanied by shorter latency until SE was observed (Fig. 4I; P < 0.05), reduced miR-211 expression in the hippocampus and frontal cortex compared with controls (Fig. 4J), and overexpression of miR-134 in the prefrontal cortex and hippocampus (Fig. 4K), possibly in relation to their hypersynchronous state. Thus, modified cholinergic regulation in TgR mice reciprocally modified forebrain miR-211 and miR-134 levels in a similar fashion as well as exacerbated susceptibility to epileptic seizures.
Dox-Induced Decline of Forebrain miR-211 Impairs Numerous Signaling Pathways.
To test for phenotypic outputs that may contribute to the synergic effects of transcript changes in functionally related proteins, we established protein–protein interaction (PPI) networks (49). We used genes that were both highly (top 3,000 genes) and differentially expressed [false discovery rate (FDR) < 0.01] following Dox to generate a network of PPI-annotated partners defined by 61 core proteins, and that included first neighbor-interacting proteins of 515 edges and 434 nodes (Fig. 5A). Enriched up-regulated and down-regulated genes and pathways within this network included numerous synaptic vesicle cycle genes (e.g., reduced Vamp2, Snap25; Fig. 5B). Intriguingly, 19 of the 61 core proteins within this network related to the cholinergic synapse KEGG (Kyoto Encyclopedia of Genes and Genomes) pathway 04725 (P < 10-8; Fig. 5C). Also, numerous neuronal-related pathways were either up- or down-regulated (Fig. 5 D and E; see Table S2 for full list of pathways), including calcium-signaling (KEGG 04020) and synaptic vesicle cycle (KEGG 04721) pathways.
Fig. 5.
Protein–protein interaction network of Dox-induced differentially expressed synaptic vesicle and cholinergic genes. (A) Protein–protein interaction-based interconnected network of stringently defined 134 differentially expressed node genes and overall 427 genes. (B) Fold changes ± SEM of the synaptic vesicle cycle pathway genes within the network. (C) Fold changes ± SEM of the cholinergic synapse genes within the network. (D and E) Enriched biological process GO terms for PPI networks for genes differentially expressed following Dox, either down or up. (Fold enrichment, asterisks denote significance P value based on permutation analysis. Results were considered significant at *P < 0.05, **P < 0.01, ***P < 0.001, after correction for multiple testing when applicable.)
Table S2.
Full list of up- or down-regulated KEGG pathways in the PPI network of differentially expressed genes following Dox administration
| Pathway | Total | Expected | Hits | Ratio enrichment | FDR | P value |
| Cholinergic synapse | 97 | 3.01 | 8 | 2.7 | 0.045 | 0.010 |
| Oocyte meiosis | 109 | 3.38 | 9 | 2.7 | 0.031 | 0.006 |
| Fc gamma R-mediated phagocytosis | 92 | 2.85 | 8 | 2.8 | 0.035 | 0.007 |
| Epstein–Barr virus infection | 114 | 3.54 | 10 | 2.8 | 0.015 | 0.003 |
| Natural killer cell-mediated cytotoxicity | 123 | 3.82 | 11 | 2.9 | 0.009 | 0.001 |
| Focal adhesion | 199 | 6.17 | 18 | 2.9 | 0.000 | 0.000 |
| Measles | 99 | 3.07 | 9 | 2.9 | 0.019 | 0.003 |
| Cell cycle | 127 | 3.94 | 12 | 3.0 | 0.004 | 0.001 |
| TGF-β–signaling pathway | 84 | 2.61 | 8 | 3.1 | 0.022 | 0.004 |
| Renal cell carcinoma | 61 | 1.89 | 6 | 3.2 | 0.048 | 0.011 |
| Leukocyte transendothelial migration | 111 | 3.44 | 11 | 3.2 | 0.004 | 0.001 |
| Osteoclast differentiation | 110 | 3.41 | 11 | 3.2 | 0.004 | 0.001 |
| MAPK-signaling pathway | 265 | 8.22 | 27 | 3.3 | 0.000 | 0.000 |
| Salmonella infection | 68 | 2.11 | 7 | 3.3 | 0.024 | 0.005 |
| HTLV-I infection | 223 | 6.92 | 23 | 3.3 | 0.000 | 0.000 |
| Prostate cancer | 87 | 2.70 | 9 | 3.3 | 0.009 | 0.001 |
| Herpes simplex infection | 105 | 3.26 | 11 | 3.4 | 0.003 | 0.000 |
| Acute myeloid leukemia | 57 | 1.77 | 6 | 3.4 | 0.037 | 0.008 |
| Dopaminergic synapse | 129 | 4.00 | 14 | 3.5 | 0.000 | 0.000 |
| GnRH-signaling pathway | 92 | 2.85 | 10 | 3.5 | 0.004 | 0.001 |
| Insulin-signaling pathway | 137 | 4.25 | 15 | 3.5 | 0.000 | 0.000 |
| Pathways in cancer | 306 | 9.49 | 34 | 3.6 | 0.000 | 0.000 |
| Gap junction | 88 | 2.73 | 10 | 3.7 | 0.003 | 0.000 |
| Cocaine addiction | 43 | 1.33 | 5 | 3.8 | 0.045 | 0.010 |
| Notch-signaling pathway | 49 | 1.52 | 6 | 3.9 | 0.020 | 0.004 |
| Salivary secretion | 49 | 1.52 | 6 | 3.9 | 0.020 | 0.004 |
| Bacterial invasion of epithelial cells | 57 | 1.77 | 7 | 4.0 | 0.010 | 0.002 |
| Gastric acid secretion | 55 | 1.71 | 7 | 4.1 | 0.009 | 0.001 |
| Pancreatic cancer | 70 | 2.17 | 9 | 4.1 | 0.003 | 0.000 |
| Long-term potentiation | 69 | 2.14 | 9 | 4.2 | 0.003 | 0.000 |
| Apoptosis | 82 | 2.54 | 11 | 4.3 | 0.000 | 0.000 |
| Wnt-signaling pathway | 147 | 4.56 | 20 | 4.4 | 0.000 | 0.000 |
| Endocrine and other factor-regulated calcium reabsorption | 44 | 1.36 | 6 | 4.4 | 0.013 | 0.002 |
| ErbB-signaling pathway | 87 | 2.70 | 12 | 4.4 | 0.000 | 0.000 |
| Melanogenesis | 100 | 3.10 | 14 | 4.5 | 0.000 | 0.000 |
| Neurotrophin-signaling pathway | 126 | 3.91 | 18 | 4.6 | 0.000 | 0.000 |
| Basal cell carcinoma | 47 | 1.46 | 7 | 4.8 | 0.004 | 0.001 |
| Endometrial cancer | 44 | 1.36 | 7 | 5.1 | 0.003 | 0.000 |
| Glioma | 66 | 2.05 | 11 | 5.4 | 0.000 | 0.000 |
| Viral myocarditis | 29 | 0.90 | 5 | 5.6 | 0.010 | 0.002 |
| Thyroid cancer | 28 | 0.87 | 5 | 5.8 | 0.010 | 0.001 |
| Chronic myeloid leukemia | 74 | 2.30 | 15 | 6.5 | 0.000 | 0.000 |
| Adherens junction | 71 | 2.20 | 15 | 6.8 | 0.000 | 0.000 |
| Colorectal cancer | 49 | 1.52 | 11 | 7.2 | 0.000 | 0.000 |
| Amphetamine addiction | 64 | 1.99 | 15 | 7.5 | 0.000 | 0.000 |
| Synaptic vesicle cycle | 17 | 0.53 | 4 | 7.6 | 0.010 | 0.002 |
| African trypanosomiasis | 20 | 0.62 | 5 | 8.1 | 0.003 | 0.000 |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 12 | 0.37 | 5 | 13.4 | 0.000 | 0.000 |
| Prion diseases | 20 | 0.62 | 9 | 14.5 | 0.000 | 0.000 |
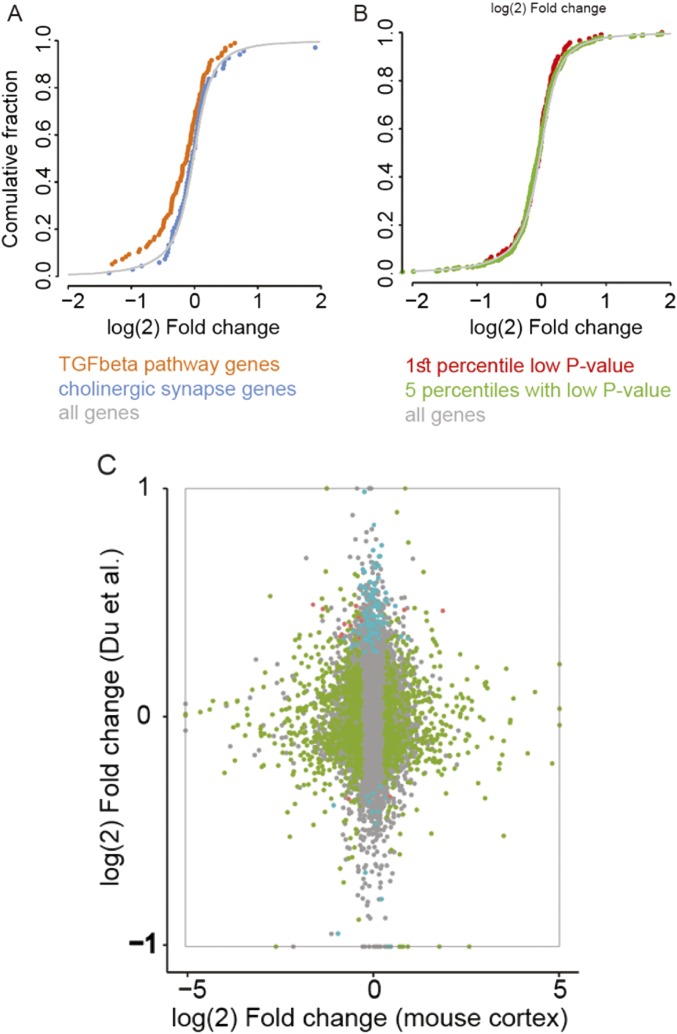
A possible drawback for examining transcriptional effects using Tet-Off mice is that Dox administration may itself impact the transcriptional profile. To test for this possibility, we examined if cholinergic synapse and TGF-β pathway genes change in publically available transcriptional data of other Dox administration models (Fig. S5). The outcome profile was unique and nonoverlapping, indicating that miR-211 decline underlines diverse, rapid changes in forebrain cholinergic synapses of dTg-211 mice that are unrelated to the effect of Dox itself. To conclude, miR-211 suppression led to selective transcriptional changes in specific functional groups that control cholinergic synaptic transmission and/or affecting hyperexcitability.
Fig. S5.
MiR-211 decline exerts distinct RNA-seq changes from those of Dox administration. (A) In cultured cells (84) TGF-β pathway genes (red), but not cholinergic synapse genes (green), show down-regulation following Dox administration. (B) In the frontal cortex of dTg-211 mice following Dox administration, genes most differentially expressed in the Du et al. (84) dataset [first (red) and five upper (green) percentiles low P value in ref. 84] show similar distribution of up- and down-regulation as all genes. (C) Genes modified in cultured cells following Dox are unchanged in the forebrain of dTg-211 mice administered with Dox. Shown is a scatterplot of per-gene fold change following Dox in both experiments. Dots represent genes: gray, unmodified; green, modified in the mouse forebrain; blue, modified in cells; red, modified in both. Note very few genes along the diagonal, and few genes to be modified in both experiments.
MiR-211 Excess Associates with Impaired Learning Strategy in dTg-211 Mice.
Cholinergic transmission in the mammalian brain has important roles in learning and memory through numerous circuits feeding into the cortex (17). Concordantly, enrichment analysis using the RNA sequencing data identified functional Dox-induced outputs in the dTg-211 cortical datasets. Gene Ontology (GO) (24) showed 166 genes with log fold change less than 0.5 or greater than 2, FDR-corrected P value < 0.01, and absolute expression levels greater than log cpm of 6.2 (Dataset S1). GO annotation enrichment analysis using PANTHER (49, 50) highlighted mostly neural-related pathways (Fig. 5E; see Table S3 for full list), with top GO annotations consisting of processes responsible for synaptic events (four terms); neurotransmitter functioning (six); higher brain functions (two); transporter activity (three), and learning/memory (Fig. 6A), predicting cognitive miR-211–mediated transcript differences.
Table S3.
List of GO annotation enrichment analysis using PANTHER
| GO biological process complete | mmu | No. of genes | Expected no. of genes | Overrepresented | Fold enrichment | Enrichment P value |
| Regulation of synaptic vesicle priming (GO:0010807) | 9 | 4 | 0.06 | + | >5 | 0.004 |
| Negative regulation of microtubule polymerization or depolymerization (GO:0031111) | 36 | 5 | 0.25 | + | >5 | 0.0421 |
| Regulation of microtubule polymerization or depolymerization (GO:0031110) | 59 | 6 | 0.4 | + | >5 | 0.0282 |
| Regulation of calcium ion transmembrane transport (GO:1903169) | 61 | 6 | 0.42 | + | > 5 | 0.0341 |
| Regulation of neurotransmitter transport (GO:0051588) | 65 | 6 | 0.44 | + | >5 | 0.0488 |
| Neurotransmitter secretion (GO:0007269) | 88 | 7 | 0.6 | + | >5 | 0.0216 |
| Regulation of synaptic plasticity (GO:0048167) | 136 | 10 | 0.93 | + | >5 | 0.00032 |
| Regulation of exocytosis (GO:0017157) | 165 | 10 | 1.12 | + | >5 | 0.00187 |
| Regulation of ion transmembrane transporter activity (GO:0032412) | 167 | 10 | 1.14 | + | >5 | 0.00208 |
| Regulation of transmembrane transporter activity (GO:0022898) | 172 | 10 | 1.17 | + | >5 | 0.00272 |
| Synaptic transmission (GO:0007268) | 320 | 18 | 2.18 | + | >5 | 8.03E-08 |
| Regulation of transporter activity (GO:0032409) | 182 | 10 | 1.24 | + | >5 | 0.00452 |
| Regulation of synapse structure or activity (GO:0050803) | 237 | 13 | 1.61 | + | >5 | 8.98E-05 |
| Exocytosis (GO:0006887) | 11 | 1.44 | + | >5 | 0.00204 | |
| Modulation of synaptic transmission (GO:0050804) | 289 | 14 | 1.97 | + | >5 | 0.000113 |
| Regulation of protein complex assembly (GO:0043254) | 301 | 14 | 2.05 | + | >5 | 0.000186 |
| Learning or memory (GO:0007611) | 223 | 10 | 1.52 | + | >5 | 0.0272 |
| Cognition (GO:0050890) | 250 | 11 | 1.7 | + | >5 | 0.0105 |
| Regulation of vesicle-mediated transport (GO:0060627) | 396 | 16 | 2.7 | + | >5 | 0.000125 |
| Single-organism behavior (GO:0044708) | 418 | 16 | 2.85 | + | >5 | 0.000262 |
| Cell-cell signaling (GO:0007267) | 537 | 20 | 3.66 | + | >5 | 7.17E-06 |
| Secretion by cell (GO:0032940) | 359 | 13 | 2.44 | + | >5 | 0.00979 |
| Secretion (GO:0046903) | 451 | 16 | 3.07 | + | >5 | 0.00073 |
| Behavior (GO:0007610) | 564 | 20 | 3.84 | + | >5 | 1.64E-05 |
| Positive regulation of secretion (GO:0051047) | 376 | 13 | 2.56 | + | >5 | 0.0162 |
| Regulation of ion transmembrane transport (GO:0034765) | 380 | 13 | 2.59 | + | >5 | 0.0182 |
| Regulation of transmembrane transport (GO:0034762) | 397 | 13 | 2.7 | + | 4.81 | 0.0291 |
| Regulation of neuron projection development (GO:0010975) | 400 | 13 | 2.72 | + | 4.77 | 0.0315 |
| Regulation of cell projection organization (GO:0031344) | 508 | 16 | 3.46 | + | 4.62 | 0.00352 |
| Regulation of cellular component biogenesis (GO:0044087) | 637 | 20 | 4.34 | + | 4.61 | 0.000123 |
| Regulation of ion transport (GO:0043269) | 612 | 19 | 4.17 | + | 4.56 | 0.000344 |
| Regulation of secretion by cell (GO:1903530) | 629 | 17 | 4.28 | + | 3.97 | 0.0122 |
| Regulation of secretion (GO:0051046) | 680 | 18 | 4.63 | + | 3.89 | 0.00789 |
| Neuron development (GO:0048666) | 654 | 17 | 4.45 | + | 3.82 | 0.0204 |
| Cell migration (GO:0016477) | 658 | 17 | 4.48 | + | 3.79 | 0.0221 |
| Cytoskeleton organization (GO:0007010) | 779 | 20 | 5.31 | + | 3.77 | 0.00305 |
| Generation of neurons (GO:0048699) | 1,291 | 33 | 8.79 | + | 3.75 | 3.03E-07 |
| Single-organism cellular localization (GO:1902580) | 669 | 17 | 4.56 | + | 3.73 | 0.0275 |
| Regulation of neurogenesis (GO:0050767) | 683 | 17 | 4.65 | + | 3.65 | 0.0361 |
| Positive regulation of transport (GO:0051050) | 889 | 22 | 6.05 | + | 3.63 | 0.00141 |
| Localization of cell (GO:0051674) | 731 | 18 | 4.98 | + | 3.62 | 0.0216 |
| Cell motility (GO:0048870) | 731 | 18 | 4.98 | + | 3.62 | 0.0216 |
| Locomotion (GO:0040011) | 940 | 23 | 6.4 | + | 3.59 | 0.000882 |
| Neuron differentiation (GO:0030182) | 821 | 20 | 5.59 | + | 3.58 | 0.00684 |
| Regulation of nervous system development (GO:0051960) | 781 | 19 | 5.32 | + | 3.57 | 0.0134 |
| Neurogenesis (GO:0022008) | 1,372 | 33 | 9.34 | + | 3.53 | 1.45E-06 |
| Regulation of cell development (GO:0060284) | 851 | 20 | 5.8 | + | 3.45 | 0.0118 |
| Regulation of transport (GO:0051049) | 1,676 | 39 | 11.41 | + | 3.42 | 5.74E-08 |
| Nervous system development (GO:0007399) | 1,800 | 41 | 12.26 | + | 3.34 | 2.8E-08 |
| Negative regulation of multicellular organismal process (GO:0051241) | 977 | 21 | 6.65 | + | 3.16 | 0.0255 |
| Movement of cell or subcellular component (GO:0006928) | 1,029 | 22 | 7.01 | + | 3.14 | 0.0158 |
| Regulation of cellular localization (GO:0060341) | 1,153 | 24 | 7.85 | + | 3.06 | 0.00823 |
| Regulation of cellular component organization (GO:0051128) | 2,095 | 41 | 14.27 | + | 2.87 | 2.99E-06 |
| Regulation of localization (GO:0032879) | 2,246 | 43 | 15.3 | + | 2.81 | 1.92E-06 |
| Positive regulation of signaling (GO:0023056) | 1,326 | 25 | 9.03 | + | 2.77 | 0.0277 |
| Positive regulation of cell communication (GO:0010647) | 1,446 | 27 | 9.85 | + | 2.74 | 0.0128 |
| Regulation of biological quality (GO:0065008) | 2,643 | 47 | 18 | + | 2.61 | 2.47E-06 |
| Regulation of signaling (GO:0023051) | 2,486 | 42 | 16.93 | + | 2.48 | 0.000135 |
| Regulation of cell communication (GO:0010646) | 2,620 | 44 | 17.84 | + | 2.47 | 6.48E-05 |
| Single-organism localization (GO:1902578) | 2,664 | 44 | 18.14 | + | 2.43 | 0.000107 |
| Regulation of molecular function (GO:0065009) | 2,260 | 35 | 15.39 | + | 2.27 | 0.0215 |
| Single-organism transport (GO:0044765) | 2,467 | 38 | 16.8 | + | 2.26 | 0.00811 |
| Localization (GO:0051179) | 4,100 | 59 | 27.92 | + | 2.11 | 2.24E-05 |
| System development (GO:0048731) | 3,499 | 49 | 23.83 | + | 2.06 | 0.00226 |
| Establishment of localization (GO:0051234) | 3,295 | 45 | 22.44 | + | 2.01 | 0.017 |
| Positive regulation of biological process (GO:0048518) | 4,910 | 67 | 33.44 | + | 2 | 8.53E-06 |
| Transport (GO:0006810) | 3,170 | 43 | 21.59 | + | 1.99 | 0.0372 |
| Negative regulation of cellular process (GO:0048523) | 3,811 | 49 | 25.95 | + | 1.89 | 0.0283 |
| Positive regulation of cellular process (GO:0048522) | 4,363 | 56 | 29.71 | + | 1.88 | 0.00394 |
| Cellular component organization or biogenesis (GO:0071840) | 4,260 | 54 | 29.01 | + | 1.86 | 0.0106 |
| Anatomical structure development (GO:0048856) | 4,183 | 53 | 28.49 | + | 1.86 | 0.0143 |
| Multicellular organismal development (GO:0007275) | 4,036 | 51 | 27.49 | + | 1.86 | 0.027 |
| Cellular component organization (GO:0016043) | 4,121 | 51 | 28.06 | + | 1.82 | 0.0497 |
| Regulation of cellular process (GO:0050794) | 9,809 | 102 | 66.8 | + | 1.53 | 5.42E-05 |
| Biological regulation (GO:0065007) | 10,699 | 109 | 72.86 | + | 1.5 | 1.74E-05 |
| Regulation of biological process (GO:0050789) | 10,256 | 103 | 69.84 | + | 1.47 | 0.000353 |
| Cellular process (GO:0009987) | 13,249 | 122 | 90.23 | + | 1.35 | 0.000246 |
| Single-organism process (GO:0044699) | 12,188 | 111 | 83 | + | 1.34 | 0.0171 |
| Unclassified (UNCLASSIFIED) | 1,652 | 8 | 11.25 | − | 0.71 | 0 |
Fig. 6.
Mmu-miR-211–expressing mice show reduced memory abilities in the Morris water maze, and hsa-miR-211 is overexpressed in Alzheimer’s disease patient brains. (A) PANTHER classification of GO shows Dox-induced enrichment of differentially expressed gene groups, mainly regulation (Reg.) of neuron-related pathways in the dTg-211 brain transcripts (Methods). (B) Time to reach platform in the Morris water maze shows reduced learning ability in the first and second training days for dTg-211 mice. (C) Search strategy scores divided by trials and days for individual dTg-211 and CamK mice. Fewer trials of dTg-211 mice in the last days showed focal or directed strategy. (D) Loss of preference of the platform quadrant, reflecting impaired reference memory for dTg-211 compared with CamK mice in probe trials. (E) Higher miR-211 levels (∼twofold) in postmortem Alzheimer’s entorhinal cortices (73) compared with nondemented controls, n = 7 each, P < 0.05, Student’s t test. Results were considered significant at *P < 0.05, **P < 0.01, ***P < 0.001, after correction for multiple testing when applicable. ns, not significant.
To examine whether miR-211 excess perturbs learning functions, we used a recent Morris water maze protocol. In the first and second day of trials, dTg-211 mice expressing miR-211 took longer than littermate CamK controls to reach a submerged platform, a mild underperformance that was mitigated in the latter 2 d of trials (Fig. 6B). The search strategy of dTg mice in these trials was also affected, with slower strategy progression toward directed platform search over days (Fig. 6C). In a probe trial examining reference memory with the platform removed, CamK controls showed clear quadrant preference but dTg-211 mice lost the capacity to locate the platform quadrant (Fig. 6D), possibly indicating short memory duration even once the task had been learned. In contrast, dTg-211 mice performed similarly to CamK controls in locomotion-based tests for anxiety and exploratory behavior, such as the elevated plus maze (Fig. S6 A and B) and the open field test (Fig. S6 C and D). Thus, dTg-211 mice presented a specific functional phenotype of impaired allocentric navigation, a disability attributed to functional impairments in the hippocampus, entorhinal cortex, and some surrounding structures, the functioning of which is strongly modulated by the cholinergic system (51, 52).
Fig. S6.
DTg-211 mice do not show elevated anxiety. (A) No difference in time spent in open and closed arms of the elevated plus maze for dTg-211 compared with controls. (B) No difference in the fraction of time spent moving. (C) In the open field test, dTg mice showed no difference in time spent in border of the maze or (D) overall distance moved. (E) Temporal lobe samples from Alzheimer’s disease (AD) patients show hsa-miR-211 levels comparable to those of individuals without AD symptoms (CNT) or nondemented individuals with increased postmortem-observed BRAAK indexes (PATH nonD).
Notably, both memory loss and perturbed cholinergic function are reminiscent of Alzheimer’s disease (53). Also, miR-211 levels are elevated in epileptic human brain tissues (47), and epileptic events are arguably common in the AD brain (54). Therefore, the possibility of a miR-211 involvement in this condition should be explored. Indeed, postmortem entorhinal cortices from AD patients showed twofold increases in miR-211 levels compared with matched controls (Fig. 6E; P < 0.05). Of note, in temporal lobe samples of a discrete cohort of AD patients, no significant change in miR-211 levels was noted (Fig. S6B). Together, these findings may indicate multileveled roles of miR-211 dynamics in cholinergic-mediated neuronal functions.
Discussion
Our study implicated miR-211 as functionally involved in cholinergic surveillance that may limit the risk of neuronal hypersynchronization in the mammalian brain. We combined forebrain RNA-seq analyses with electrocorticography tests of engineered mice presenting conditional miR-211 excess, or overexpressing a miR refractory AChE-R. This exploration linked forebrain miR-211 decreases with spontaneous ECoG-detected nonconvulsive seizures, exacerbated susceptibility to PTZ-induced seizures and increased miR-134 levels, accompanied by comodified synaptic, cholinergic, and TGFBR2 pathways, with a selective impact on endothelial brain cells, indicating involvement of the neurovascular unit (55). The changes associated with miR-211 decline may all add to the risks of convulsive seizures in a long-lasting manner, together attributing several hallmarks of the dynamics and efficacy of cholinergic-mediated susceptibility for seizures in the adult mammalian brain to miR-211 suppression.
ACh-related mechanisms are widely involved in, and sufficient for, generating epileptic seizures (56), with cholinergic imbalances hallmarking several epileptic syndromes (57) and cholinergic agonists inducing limbic seizures (58). Recent independent reports implicated miRs in both initiating and changing the response to seizure-inducing agents (59), but did not explore the putative regulatory role of miRs controlling neuronal cholinergic transmission (57) in avoiding such seizures and affecting the susceptibility to epilepsy. To address these questions, we intersected datasets for miRs targeting synaptic functions (24), experimentally found in neurons (25) and modified following pilocarpine injection (26). This search identified miRs that are directly or consequentially affected by epileptic perturbations, and that potentially function in the synapse. Of these miRs, we focused on miR-211, which was significantly reduced in our hands, either directly or consequentially by pilocarpine injection, and is one of those few genes that are lost in the 15q13.3 deletion syndrome (27, 60) (OMIM no. 612001), which is commonly manifested in epilepsy (27, 28). We also demonstrated miR-211 regulation over the human nAChRα7 nicotinic receptor, mapped within this genomic locus (61), and whose gain-of-function mutation associates with seizure induction in patients (29). Additionally, miR-211 targets the angiopoietin-1 (ANGPT1) gene, where a single nucleotide variation disrupting its binding site associates with elevated risk of ischemic stroke (62), a condition with diverse links to epileptic seizures (4). The miR-211 candidate thus emerged as putatively linked to epilepsy in several ways.
Notably, our work differs from many other miR-focused reports, which depict functional regulation by miRs by assessing their steady-state levels. Rather, we observed limited transcriptional changes in dTg-211 forebrains overexpressing the miR compared with controls, yet found dramatic transcriptional and electrophysiological changes in the brain following the dynamic reduction of miR-211 to its basal levels. That the decline of miR-211 has a more extensive effect than its steady-state level suggests that the function of this miR can be only partially assessed by single time-point measurements. Thus, Dox-induced suppression of the miR-211 transgene in dTg-211 mice induced both nonconvulsive seizures and entailed increased susceptibility to convulsive seizures while elevating the TGFBR2 transcript and protein levels. Because these ECoG-documented changes were absent in control mice, they unlikely reflect the mere impact of Dox, but rather point to a sustained epileptiform effect of miR-211 down-regulation, with possible relevance to human epileptogenic conditions. Additionally, RNA-seq showed a selective elevation in 19 of 21 epithelial marker genes, indicating cellular composition changes of the microvasculature (63), reported to hallmark and contribute to epileptogenesis, with possible gross changes in the epithelial transcriptional landscape.
Intriguingly, the observed seizures were characterized by sharp 5-Hz activity oscillations, with similarity to spike and wave seizures reminiscent of those of absence epilepsy patients. These human syndromes and their corresponding mouse models are manifested in epilepsy, which affects GABAergic modulatory neurotransmission but lacks observed motor convulsions (36, 64). Also, we noted expression changes concordant with systematic facilitation of cholinergic synaptic activation following Dox; specifically, this involved simultaneous elevation in the excitatory nicotinic receptors 5 and 7 and muscarinic receptor 5, and down-regulation of the inhibitory muscarinic receptors 2 and 4. We hypothesized that the muscarinic receptor 1 (mAChR1) will be concordantly modified; supporting this notion, qRT-PCR assays showed significant mAChR1 reduction in dTg-211 mice, and elevation following Dox suppression (Fig. S4E). That we did not observe a parallel change in the sequencing experiment may reflect mRNA editing of this gene’s transcripts (15), and for which alignment of sequencing reads is sensitive. We also observed elevation of the ataxia-related scaffold protein ATCAY/BNIP-H gene, which affects shuttling of the ACh synthesizing enzyme ChAT to neurite terminals (45, 46), and mutations in which associate with variable psychomotor retardation phenotypes and cerebellar dysfunction, together indicating multileveled exacerbation of cholinergic signaling.
After Dox has been removed and forebrain miR-211 levels were reelevated, we observed increased susceptibility of dTg-211 mice to PTZ-induced convulsions, suggesting an apparent link between miR-211 expression dynamics to convulsions. Alternative interpretations may, however, exist. For example, abrupt miR-211 reduction may function as a priming event for broad neurological changes, including susceptibility for convulsions, such as those implied by the selective elevation of epithelial marker genes. Alternatively, or in addition, sustained miR-211 elevation may have a supportive or even a merely ancillary role in the complex genetic makeup protecting the neuronal network from hyperexcitability, whereas its perturbation is functionally potent enough to disrupt that balance. Further work would be required to explore these possibilities.
At the cellular level, miR-211 reacts to endoplasmic reticulum stress by targeting the proximal promoter of the proapoptotic transcription factor chop/gadd153, which attenuates apoptotic processes, allowing the cell to reestablish homeostasis and avoid cell death (65); this may indicate attenuation by miR-211 of stressful cellular signals, inversely proposing that its down-regulation may exacerbate such stress-induced reactions. If indeed the observed miR-211 elevation in the Alzheimer’s entorhinal cortex relates to the disease pathophysiology, it may suggest an explanation to the greater risk for unprovoked seizures in early onset patients and those with more severe disease (66), where miR-211 increases would prove insufficient in avoiding seizures. This expression change, not seen in temporal lobe samples, may relate to the prominent cholinergic innervation of the entorhinal cortex by the medial septum.
Neuronal networks operate over a wide range of activity levels, and their imbalance underlies many neurological diseases, including epilepsy. Our study indicates that miR-211–induced transcription changes may be involved. Concordantly, PPI network analysis showed modified cholinergic synapse pathway genes (KEGG 04725) under miR-211 decline. Additionally, this decline affected protein kinase C gamma and inositol 1,4,5-trisphosphate receptor type 1, which modulate ion metabolism at the cholinergic synapse, and are linked to ataxia (67, 68), indicating globally modified synaptic composition and function under reduced miR-211 expression. Supporting this notion, a Caenorhabditis elegans gain-of-function mutation in a neuronal ACh receptor, acr-2(gf), causes an epileptic-like convulsion behavior, and the behavioral and physiological effects of acr-2(gf) require the activity of the TRPM1-similar channel GTL-2 (69). Thus, the functional link among ACh, the miR-211 host gene TRPM1, and epilepsy spans many species.
TgR mice overexpressing the nonsynaptic AChE-R splice variant show both enhanced seizure susceptibility and miR-211 reduction, which agrees with the role of miR-211 as both a regulator of and affected by cholinergic signaling-mediated seizures. Compatible with this notion, we identified a time-dependent induction of miR-134 in both TgR mice and within the 4 d following Dox administration to dTg-211 mice, alongside the susceptibility to seizures. Notably, miR-134 levels increase in temporal lobe samples from temporal lobe epilepsy patients and from mice 24 h after induction of status epilepticus (10). Moreover, antagomir silencing of miR-134 reduces recurrent seizures and development of epileptogenesis, and miR-134 affects long-term depression via the Pumilio-2/Polo-like kinase 2 (Pum2/Plk2) pathway, leading to inhibition due to altered subunit composition of plasma membrane-localized AMPA receptors (70), perhaps indicating reversibility of miR-211 decreases.
Pathway analysis performed on all genes that were differentially expressed in dTg-211 cortices following Dox indicated modified cognition, learning, and memory-related GO terms, suggesting that mice expressing miR-211 may have altered memory abilities, possibly due to the role of synaptic cholinergic signaling in memory (71). In the Morris water maze, dTg-211 mice presented delayed progression in search strategy and reduced reference memory abilities in probe trials. The impaired allocentric navigation phenotype may be attributed to impaired hippocampal, entorhinal cortex and some surrounding structures, the functioning of which is strongly modulated by the cholinergic system (51, 52, 72).
In conclusion, we show that dynamic reduction of miR-211 overexpression in forebrain neurons leads to major transcriptional change, including elevated levels of endothelial marker genes and enriched GO pathways relating to synapse genes, neurotransmitter functioning, higher brain functions, and transporter activity. MiR-211 decreases further induce overall potentiation of cholinergic synapses, and spontaneous ECoG documented nonconvulsive seizures in the brains of tested mice. Our findings present the dynamic changes in miR-211 and miRs at large as possible mediators and attenuators of seizures induced by cholinergic transmission imbalances. The long-lasting functional effects of miR-211 on the process of epileptogenesis and its implications for diagnostic and therapeutic avenues thus require further research.
Methods
Transgenic Mice Generation and Experiments.
We generated pTRE-miR-211 mice by cloning the premmu-miR-211 sequence into a pTRE- tight vector, followed by pronuclear injection at the Weizmann Institute of Science Animals Facility. Mice were held in specific pathogen-free conditions at the Hebrew University, an Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International accredited institute. All procedures, including animal tests, were approved by the Institutional Ethics Committee of The Hebrew University of Jerusalem (ethics no. NS-16-14729-4), with concordance to AAALAC International guidelines. tTA-CamKa strain was acquired, backcrossed, and crossed and housed as in SI Methods. dTg mice and CamK littermates were housed together and administered Dox in parallel. The cholinergic muscarinic agonist pilocarpine (Sigma; 290–340 mg/kg) was i.p. injected to mice as in ref. 73. Behavioral tests, including Morris water maze, elevated plus maze, and open field tests were performed as described in SI Methods.
Human-Derived Samples.
Postmortem samples of entorhinal cortex from AD and age-matched controls were obtained from the Netherlands Brain Bank (NBB) at the Netherlands Institute for Neuroscience (73). Samples were collected following written informed consent for a brain autopsy by the NBB.
Statistical Analyses.
Differential expression in sequencing experiments was derived from adjusted P values in DESeq (74) on R platform, after FDR correction. For specific genes presented, fold change and ratio values are shown as mean ± SEM. For cholinergic receptors, t test was used on normalized count data. For enrichment of cell type marker genes, P value was defined based on permutation analysis. Box and whisker plots show second and third quintiles for box, and 1.5 quantile distances from median for whiskers, as by convention. Results were considered significant if *P < 0.05, **P < 0.01, ***P < 0.001, after correction for multiple testing when applicable. For ECoG seizure and spike observations, a Mann–Whitney test was used.
Additional methods are described in SI Methods.
SI Methods
Transgenic Mice.
For genotyping, tail-tip PCR was used (sequences given in Table S1). B6;CBA-Tg(Camk2a-tTA)1Mmay/J (CamK mice) were purchased from the Jackson Laboratory. Mice were backcrossed to FVB/N background for three generations and further crossed to generate double-transgenic mice and littermate controls.
Luciferase Assay.
MiR-211-5p direct targeting was predicted in silico and experimentally assessed using in vitro luciferase measurement with the Dual-Luciferase Kit (Promega) 48 h after transfection. Specifically, a 3′-UTR fragment of human nAChRα7 OR murine TGFBR2 transcripts (both similar in mouse and human), was cloned into a luciferase reporter vector. Reporter and miR-211 expression vector (Genecopia; MmiR3291-MR04) were transfected into HEK293-T cells (ATCC) using polyethylenimine, and luciferase activity measured and normalized according to manufacturer’s instructions.
ECoG Recordings and Analysis.
ECoG recordings were performed as previously described (77). Briefly, mice were placed in a stereotaxic frame under deep isoflurane anesthesia (1–3%), after shaving and disinfecting the dorsal aspect of the head; the skull was exposed by a longitudinal incision. Holes were drilled in coordinates 3 mm caudal and 2 mm lateral relative to bregma. Stainless steel screws were fixed to the holes. After placing a wireless transmitter (Data Science International) within a pocket formed s.c. in the dorsal aspect of the body, the electrodes were connected to the screws and isolated with bone cement. Before termination of anesthesia, buprenorphine was administered (i.p., 0.05 mg/kg). Following recovery, animals were moved to a behavior room with 12-h light/dark cycle and had access to food and water ad libitum. After 4 d of habituation we began continuous ECoG recording using a homemade Matlab-based program that allows reliable unbiased detection of seizures (23). The results were revised manually and blindly. ECoG spikes were implemented via the wavelet transform algorithm (78) following a bandpass filtration of the ECoG signal between 1 and 45 Hz. After automated detection and clustering using the Matlab program, blind human revision was performed to reassure the results.
PTZ-Induced Seizures.
Animals were placed individually in Plexiglas boxes, and seizure behavior was observed for 30 min following PTZ injection (50 mg/kg, s.c.). Seizure intensity was evaluated (as in ref. 79). Parallel ECoG recording was analyzed as mentioned above.
RNA Samples Collection for RT-qPCR and Sequencing-Compatible Libraries.
Mice were anesthetized with isoflurane before cervical dislocation, and their brain regions, including frontal cortices, were dissected and collected in liquid nitrogen. For RNA sequencing, RNA was extracted by miRNeasy kit (QIAGEN); RNA quality determined with RNA 6000 Nano Bioanalyzer (Agilent), and samples with RIN values ranged between 8 and 8.9 were used. RNA was extracted for qRT-PCR validation analysis using TRIzol Reagent (Invitrogen), as reported (80). cDNA and PCR were as described herein.
High-Throughput RNA Sequencing.
Sequencing-compatible poly(A)-terminated single-end libraries were generated using an RNA Library prep kit (NEBNext Multiplex, E7330S; New England BioLabs) following manufacturer’s instructions, with 12 amplification rounds. Libraries were barcoded and sequenced on a NextSeq Series Sequencing System (HUJ Center for Genomic Technologies) using two Illumina flow cells (Illumina 500 NextSeq High Output v2 Kit, FC-404-2005; Illumina). Raw cluster densities for samples ranged between 170 and 189 K/mm2. Reads were aligned (90% mapping) to the mouse transcriptome (TopHat2) (81), and expression analysis was performed using the DESeq (74) software via R platform (82). Libraries from all tissues were overall similar in depth, with a similar distribution of transcript numbers per expression level and tag-wise normalized variance predictably correlated to expression levels (Fig. S2). A 6.2 log cpm value for PPI thresholding represents the upper 10 percentiles of 18,570 genes in datasets.
Bioinformatics, Pathway Analysis, and Luciferase Assays.
Cell type marker genes (39) were selected as mouse orthologs name-wise and their levels plotted as fold change; P value presented after Bonferroni correction and permutation analysis were used to determine significance per marker. ECDF plots were generated in R via the stats package (82) after thresholding genes by counts [mean log(cpm) > 1] and subsetting by significance of differential expression. PANTHER (Protein ANalysis THrough Evolutionary Relationships) (50) version 10, GO version 1.2, annotated June 22, 2016, was used for GO of differentially expressed genes for the KEGG database.
ELISA.
Mice frontal cortex samples were used for ELISA using an EIAab kit (for murine TGFBR2, catalog no. E9935m). Cortices were homogenized in radioimmunoprecipitation assay buffer containing protease inhibitor (1:200) and centrifuged. Sample protein content was measured with the Lowry assay (Thermo Scientific). Samples were further diluted 1:3 in PBS and used for quantifying TGFBR2 by EIAab kit (catalog no. E9935m) as per manufacturer’s instructions.
Morris Water Maze.
Mice were subjected to a Morris water maze to assess learning and memory. Briefly, mice were released to search for a submerged platform in a fiberglass water tank, 1.2 m in diameter. Water temperature was fixed, and colored white for opaqueness. Lighting remained fixed; platform directions were queued with visible marks to allow for allocentric navigation, and systematic release directions. Each mouse was tested in four trials a day for 4 d, followed by a single day of probe trial, in which mice search for the platform that had been removed. Experiments were performed blind, using alias numbers per mouse, recorded from a monochromatic ceiling-fixed video camera, and automatic movement tracing and quantitative data analysis was performed using EthoVision (83) XT (Noldus version 8; Information Technology).
cDNA and qRT-PCR.
A qScript Kit (Quanta) was used for reverse transcription. Real-time PCR performed on a CFX-96 machine (Bio-Rad), and quantification performed using the ΔΔCt method, with snoRD47 as a loading control for miRs and β-actin for long transcripts.
Supplementary Material
Acknowledgments
The authors thank the Netherlands Brain Bank for human-derived samples. This research was supported by European Research Council Advanced Award 321501 (to H.S.); European Union’s Seventh Framework Program FP7/2007–2013 Grant 602102, EPITARGET (to A.F.); Israeli Ministry of Science, Technology and Space Grant 53140; Legacy Heritage Science Initiative of the Israel Science Foundation Grants 817/13 (to H.S.) and 717/15 (to A.F.); The Hebrew University of Jerusalem Planning and Budgeting Committee and the Edmond and Lily Safra Center for Brain Sciences postdoctoral fellowship (to N.M.); and the Howard and Diana Wendy Predoctoral Fellowship (to U.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE99307).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1701201114/-/DCSupplemental.
References
- 1.Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: New advances. Lancet. 2015;385:884–898. doi: 10.1016/S0140-6736(14)60456-6. [DOI] [PubMed] [Google Scholar]
- 2.Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. Nat Med. 2013;19:1584–1596. doi: 10.1038/nm.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pitkänen A, et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016;15:843–856. doi: 10.1016/S1474-4422(16)00112-5. [DOI] [PubMed] [Google Scholar]
- 4.Pitkänen A, Roivainen R, Lukasiuk K. 2015. Development of epilepsy after ischaemic stroke. Lancet Neurol, 10.1016/S1474-4422(15)00248-3.
- 5.Levy C, et al. Intronic miR-211 assumes the tumor suppressive function of its host gene in melanoma. Mol Cell. 2010;40:841–849. doi: 10.1016/j.molcel.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kretschmann A, et al. Different microRNA profiles in chronic epilepsy versus acute seizure mouse models. J Mol Neurosci. 2015;55:466–479. doi: 10.1007/s12031-014-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng J, et al. Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. J Mol Neurosci. 2013;50:291–297. doi: 10.1007/s12031-013-9953-3. [DOI] [PubMed] [Google Scholar]
- 8.Sano T, et al. MicroRNA-34a upregulation during seizure-induced neuronal death. Cell Death Dis. 2012;3:e287. doi: 10.1038/cddis.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omran A, et al. Interleukin-1β and microRNA-146a in an immature rat model and children with mesial temporal lobe epilepsy. Epilepsia. 2012;53:1215–1224. doi: 10.1111/j.1528-1167.2012.03540.x. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez-Mateos EM, et al. Silencing microRNA-134 produces neuroprotective and prolonged seizure-suppressive effects. Nat Med. 2012;18:1087–1094. doi: 10.1038/nm.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez-Mateos EM, et al. Antagomirs targeting microRNA-134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine-induced status epilepticus. Brain Struct Funct. 2015;220:2387–2399. doi: 10.1007/s00429-014-0798-5. [DOI] [PubMed] [Google Scholar]
- 12.Wang XM, Jia RH, Wei D, Cui WY, Jiang W. MiR-134 blockade prevents status epilepticus like-activity and is neuroprotective in cultured hippocampal neurons. Neurosci Lett. 2014;572:20–25. doi: 10.1016/j.neulet.2014.04.049. [DOI] [PubMed] [Google Scholar]
- 13.Spain E, et al. Direct, non-amplified detection of microRNA-134 in plasma from epilepsy patients. RSC Advances. 2015;5:90071–90078. [Google Scholar]
- 14.Ivens S, et al. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman G, et al. Acetylcholine-induced seizure-like activity and modified cholinergic gene expression in chronically epileptic rats. Eur J Neurosci. 2008;27:965–975. doi: 10.1111/j.1460-9568.2008.06070.x. [DOI] [PubMed] [Google Scholar]
- 16.Couey JJ, et al. Distributed network actions by nicotine increase the threshold for spike-timing-dependent plasticity in prefrontal cortex. Neuron. 2007;54(1):73–87. doi: 10.1016/j.neuron.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: Cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76:116–129. doi: 10.1016/j.neuron.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maslarova A, et al. Increased susceptibility to acetylcholine in the entorhinal cortex of pilocarpine-treated rats involves alterations in KCNQ channels. Neurobiol Dis. 2013;56:14–24. doi: 10.1016/j.nbd.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Shiba Y, et al. Spontaneous epileptic seizures in transgenic rats harboring a human ADNFLE missense mutation in the β2-subunit of the nicotinic acetylcholine receptor. Neurosci Res. 2015;100:46–54. doi: 10.1016/j.neures.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Motelow JE, et al. Decreased subcortical cholinergic arousal in focal seizures. Neuron. 2015;85:561–572. doi: 10.1016/j.neuron.2014.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho KO, et al. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cacheaux LP, et al. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bar-Klein G, et al. Losartan prevents acquired epilepsy via TGF-β signaling suppression. Ann Neurol. 2014;75:864–875. doi: 10.1002/ana.24147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gene Ontology C; Gene Ontology Consortium (2015) Gene Ontology Consortium: Going forward. Nucleic Acids Res 43:D1049–D1056. [DOI] [PMC free article] [PubMed]
- 25.He M, et al. Cell-type-based analysis of microRNA profiles in the mouse brain. Neuron. 2012;73:35–48. doi: 10.1016/j.neuron.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinjo ER, et al. Pilocarpine-induced seizures trigger differential regulation of microRNA-stability related genes in rat hippocampal neurons. Sci Rep. 2016;6:20969. doi: 10.1038/srep20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dibbens LM, et al. EPICURE Consortium Familial and sporadic 15q13.3 microdeletions in idiopathic generalized epilepsy: Precedent for disorders with complex inheritance. Hum Mol Genet. 2009;18:3626–3631. doi: 10.1093/hmg/ddp311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Forsingdal A, Fejgin K, Nielsen V, Werge T, Nielsen J. 15q13.3 homozygous knockout mouse model display epilepsy-, autism- and schizophrenia-related phenotypes. Transl Psychiatry. 2016;6:e860. doi: 10.1038/tp.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Endris V, et al. Homozygous loss of CHRNA7 on chromosome 15q13.3 causes severe encephalopathy with seizures and hypotonia. Am J Med Genet A. 2010;152A:2908–2911. doi: 10.1002/ajmg.a.33692. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, et al. Loss of MeCP2 in cholinergic neurons causes part of RTT-like phenotypes via α7 receptor in hippocampus. Cell Res. 2016;26:728–742. doi: 10.1038/cr.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broide RS, et al. Increased sensitivity to nicotine-induced seizures in mice expressing the L250T alpha 7 nicotinic acetylcholine receptor mutation. Mol Pharmacol. 2002;61:695–705. doi: 10.1124/mol.61.3.695. [DOI] [PubMed] [Google Scholar]
- 32.Paraskevopoulou MD, et al. DIANA-microT web server v5.0: Service integration into miRNA functional analysis workflows. Nucleic Acids Res. 2013;41:W169–173. doi: 10.1093/nar/gkt393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reczko M, Maragkakis M, Alexiou P, Grosse I, Hatzigeorgiou AG. Functional microRNA targets in protein coding sequences. Bioinformatics. 2012;28:771–776. doi: 10.1093/bioinformatics/bts043. [DOI] [PubMed] [Google Scholar]
- 34.Krol J, et al. Characterizing light-regulated retinal microRNAs reveals rapid turnover as a common property of neuronal microRNAs. Cell. 2010;141:618–631. doi: 10.1016/j.cell.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Odeh F, et al. Atlas of transgenic Tet-Off Ca2+/calmodulin-dependent protein kinase II and prion protein promoter activity in the mouse brain. Neuroimage. 2011;54:2603–2611. doi: 10.1016/j.neuroimage.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 36.Carvill GL, et al. GRIN2A mutations cause epilepsy-aphasia spectrum disorders. Nat Genet. 2013;45:1073–1076. doi: 10.1038/ng.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4:4. doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hansen KF, Sakamoto K, Pelz C, Impey S, Obrietan K. Profiling status epilepticus-induced changes in hippocampal RNA expression using high-throughput RNA sequencing. Sci Rep. 2014;4:6930. doi: 10.1038/srep06930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Darmanis S, et al. A survey of human brain transcriptome diversity at the single cell level. Proc Natl Acad Sci USA. 2015;112:7285–7290. doi: 10.1073/pnas.1507125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jackson KJ, et al. Role of alpha5 nicotinic acetylcholine receptors in pharmacological and behavioral effects of nicotine in mice. J Pharmacol Exp Ther. 2010;334:137–146. doi: 10.1124/jpet.110.165738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bailey CD, De Biasi M, Fletcher PJ, Lambe EK. The nicotinic acetylcholine receptor alpha5 subunit plays a key role in attention circuitry and accuracy. J Neurosci. 2010;30:9241–9252. doi: 10.1523/JNEUROSCI.2258-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johansson ME, et al. α7 Nicotinic acetylcholine receptor is expressed in human atherosclerosis and inhibits disease in mice–brief report. Arterioscler Thromb Vasc Biol. 2014;34:2632–2636. doi: 10.1161/ATVBAHA.114.303892. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Yamada M, Gomeza J, Basile AS, Wess J. Multiple muscarinic acetylcholine receptor subtypes modulate striatal dopamine release, as studied with M1-M5 muscarinic receptor knock-out mice. J Neurosci. 2002;22:6347–6352. doi: 10.1523/JNEUROSCI.22-15-06347.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darvesh S, Hopkins DA, Geula C. Neurobiology of butyrylcholinesterase. Nat Rev Neurosci. 2003;4:131–138. doi: 10.1038/nrn1035. [DOI] [PubMed] [Google Scholar]
- 45.Bomar JM, et al. Mutations in a novel gene encoding a CRAL-TRIO domain cause human Cayman ataxia and ataxia/dystonia in the jittery mouse. Nat Genet. 2003;35:264–269. doi: 10.1038/ng1255. [DOI] [PubMed] [Google Scholar]
- 46.Sun J, et al. BNIP-H recruits the cholinergic machinery to neurite terminals to promote acetylcholine signaling and neuritogenesis. Dev Cell. 2015;34:555–568. doi: 10.1016/j.devcel.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Wang C, Ji B, Cheng B, Chen J, Bai B. Neuroprotection of microRNA in neurological disorders (Review) Biomed Rep. 2014;2:611–619. doi: 10.3892/br.2014.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salas R, et al. Nicotine relieves anxiogenic-like behavior in mice that overexpress the read-through variant of acetylcholinesterase but not in wild-type mice. Mol Pharmacol. 2008;74:1641–1648. doi: 10.1124/mol.108.048454. [DOI] [PubMed] [Google Scholar]
- 49.Xia J, Gill EE, Hancock RE. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc. 2015;10:823–844. doi: 10.1038/nprot.2015.052. [DOI] [PubMed] [Google Scholar]
- 50.Mi H, Poudel S, Muruganujan A, Casagrande JT, Thomas PD. PANTHER version 10: Expanded protein families and functions, and analysis tools. Nucleic Acids Res. 2016;44:D336–D342. doi: 10.1093/nar/gkv1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fuchs EC, et al. Local and distant input controlling excitation in layer II of the medial entorhinal cortex. Neuron. 2016;89:194–208. doi: 10.1016/j.neuron.2015.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacLaren DA, Wilson DI, Winn P. Selective lesions of the cholinergic neurons within the posterior pedunculopontine do not alter operant learning or nicotine sensitization. Brain Struct Funct. 2016;221:1481–1497. doi: 10.1007/s00429-014-0985-4. [DOI] [PubMed] [Google Scholar]
- 53.Pabst M, et al. Astrocyte intermediaries of septal cholinergic modulation in the hippocampus. Neuron. 2016;90:853–865. doi: 10.1016/j.neuron.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 54.Noebels J. A perfect storm: Converging paths of epilepsy and Alzheimer’s dementia intersect in the hippocampal formation. Epilepsia. 2011;52:39–46. doi: 10.1111/j.1528-1167.2010.02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Friedman A, Kaufer D, Heinemann U. Blood-brain barrier breakdown-inducing astrocytic transformation: Novel targets for the prevention of epilepsy. Epilepsy Res. 2009;85:142–149. doi: 10.1016/j.eplepsyres.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olney JW, de Gubareff T, Labruyere J. Seizure-related brain damage induced by cholinergic agents. Nature. 1983;301:520–522. doi: 10.1038/301520a0. [DOI] [PubMed] [Google Scholar]
- 57.Soreq H. Checks and balances on cholinergic signaling in brain and body function. Trends Neurosci. 2015;38:448–458. doi: 10.1016/j.tins.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 58.Gnatek Y, et al. Acetylcholinesterase loosens the brain’s cholinergic anti-inflammatory response and promotes epileptogenesis. Front Mol Neurosci. 2012;5:66. doi: 10.3389/fnmol.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Engel T, Alves M, Sheedy C, Henshall DC. ATPergic signalling during seizures and epilepsy. Neuropharmacology. 2016;104:140–153. doi: 10.1016/j.neuropharm.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 60.Helbig I, et al. 15q13.3 microdeletions increase risk of idiopathic generalized epilepsy. Nat Genet. 2009;41:160–162. doi: 10.1038/ng.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spielmann M, et al. Homozygous deletion of chromosome 15q13.3 including CHRNA7 causes severe mental retardation, seizures, muscular hypotonia, and the loss of KLF13 and TRPM1 potentially cause macrocytosis and congenital retinal dysfunction in siblings. Eur J Med Genet. 2011;54:e441–e445. doi: 10.1016/j.ejmg.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, et al. A functional variant in the 3′-UTR of angiopoietin-1 might reduce stroke risk by interfering with the binding efficiency of microRNA 211. Hum Mol Genet. 2010;19:2524–2533. doi: 10.1093/hmg/ddq131. [DOI] [PubMed] [Google Scholar]
- 63.Rigau V, et al. Angiogenesis is associated with blood-brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- 64.Judson MC, et al. GABAergic neuron-specific loss of Ube3a causes Angelman syndrome-like EEG abnormalities and enhances seizure susceptibility. Neuron. 2016;90:56–69. doi: 10.1016/j.neuron.2016.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chitnis NS, et al. miR-211 is a prosurvival microRNA that regulates chop expression in a PERK-dependent manner. Mol Cell. 2012;48:353–364. doi: 10.1016/j.molcel.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pandis D, Scarmeas N. Seizures in Alzheimer disease: Clinical and epidemiological data. Epilepsy Curr. 2012;12:184–187. doi: 10.5698/1535-7511-12.5.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stevanin G, et al. Mutation in the catalytic domain of protein kinase C gamma and extension of the phenotype associated with spinocerebellar ataxia type 14. Arch Neurol. 2004;61:1242–1248. doi: 10.1001/archneur.61.8.1242. [DOI] [PubMed] [Google Scholar]
- 68.van de Leemput J, et al. Deletion at ITPR1 underlies ataxia in mice and spinocerebellar ataxia 15 in humans. PLoS Genet. 2007;3:e108. doi: 10.1371/journal.pgen.0030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stawicki TM, Zhou K, Yochem J, Chen L, Jin Y. TRPM channels modulate epileptic-like convulsions via systemic ion homeostasis. Curr Biol. 2011;21:883–888. doi: 10.1016/j.cub.2011.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiore R, et al. MiR-134-dependent regulation of Pumilio-2 is necessary for homeostatic synaptic depression. EMBO J. 2014;33:2231–2246. doi: 10.15252/embj.201487921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitsushima D, Sano A, Takahashi T. A cholinergic trigger drives learning-induced plasticity at hippocampal synapses. Nat Commun. 2013;4:2760. doi: 10.1038/ncomms3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berson A, et al. Cholinergic-associated loss of hnRNP-A/B in Alzheimer’s disease impairs cortical splicing and cognitive function in mice. EMBO Mol Med. 2012;4:730–742. doi: 10.1002/emmm.201100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vlachos IS, et al. DIANA miRPath v.2.0: Investigating the combinatorial effect of microRNAs in pathways. Nucleic Acids Res. 2012;40:W498–W504. doi: 10.1093/nar/gks494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaked I, et al. MicroRNA-132 potentiates cholinergic anti-inflammatory signaling by targeting acetylcholinesterase. Immunity. 2009;31:965–973. doi: 10.1016/j.immuni.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 77.Weissberg I, et al. Albumin induces excitatory synaptogenesis through astrocytic TGF-β/ALK5 signaling in a model of acquired epilepsy following blood-brain barrier dysfunction. Neurobiol Dis. 2015;78:115–125. doi: 10.1016/j.nbd.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Adeli H, Zhou Z, Dadmehr N. Analysis of EEG records in an epileptic patient using wavelet transform. J Neurosci Methods. 2003;123:69–87. doi: 10.1016/s0165-0270(02)00340-0. [DOI] [PubMed] [Google Scholar]
- 79.Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- 80.Hanin G, et al. Competing targets of microRNA-608 affect anxiety and hypertension. Hum Mol Genet. 2014;23:4569–4580. doi: 10.1093/hmg/ddu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim D, et al. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna: 2015. [Google Scholar]
- 83.Spink AJ, Tegelenbosch RAJ, Buma MOS, Noldus LPJJ. The EthoVision video tracking system—A tool for behavioral phenotyping of transgenic mice. Physiol Behav. 2001;73:731–744. doi: 10.1016/s0031-9384(01)00530-3. [DOI] [PubMed] [Google Scholar]
- 84.Du X, et al. FGFR3 stimulates stearoyl CoA desaturase 1 activity to promote bladder tumor growth. Cancer Res. 2012;72:5843–5855. doi: 10.1158/0008-5472.CAN-12-1329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.