Significance
Type IV pili (T4P) are cell-surface appendages observed in prokaryotes that perform critical functions in cell motility, surface adhesion, virulence, and biofilm formation. Although the architecture of T4P has already been determined, the dynamics resulting from the response to various environmental signals remain unclear. Here we demonstrated the sequential process of T4P dynamics from stimulus to taxis at the single-cell level in a model cyanobacterium, which can recognize light direction. We directly visualized that T4P filaments dominantly appeared from the side of the cell opposite the illumination. This asymmetric activation is regulated on a timescale of minutes, and the process was transitioned between three sequential phases. These findings provide clues toward a general regulation mechanism of the T4P system.
Keywords: Synechocystis sp. PCC6803, twitching motility, phototaxis, signal transduction, fluorescence
Abstract
The type IV pili (T4P) system is a supermolecular machine observed in prokaryotes. Cells repeat the cycle of T4P extension, surface attachment, and retraction to drive twitching motility. Although the properties of T4P as a motor have been scrutinized with biophysics techniques, the mechanism of regulation remains unclear. Here we provided the framework of the T4P dynamics at the single-cell level in Synechocystis sp. PCC6803, which can recognize light direction. We demonstrated that the dynamics was detected by fluorescent beads under an optical microscope and controlled by blue light that induces negative phototaxis; extension and retraction of T4P was activated at the forward side of lateral illumination to move away from the light source. Additionally, we directly visualized each pilus by fluorescent labeling, allowing us to quantify their asymmetric distribution. Finally, quantitative analyses of cell tracking indicated that T4P was generated uniformly within 0.2 min after blue-light exposure, and within the next 1 min the activation became asymmetric along the light axis to achieve directional cell motility; this process was mediated by the photo-sensing protein, PixD. This sequential process provides clues toward a general regulation mechanism of T4P system, which might be essentially common between archaella and other secretion apparatuses.
Type IV pili (T4P) are fascinating supermolecular machines that drive twitching motility, protein secretion, and DNA uptake in prokaryotes (1). Twitching motility is now widely accepted as a form of bacterial translocation involving repetition of the cycle of extension and retraction of the pili (2, 3) (Fig. 1A), which is powered by assembly and disassembly ATPase at the base of helical pilus fibers (4). The mechanical response of a single pilus has been scrutinized in great detail using biophysical approaches such as optical tweezers methodology in Neisseria gonorrheae (3, 5–7), although the dynamic properties resulting from the response to various environmental signals remain less understood than those of bacterial flagella. T4P is also evolutionarily and structurally related to flagella in archaea, which have recently been designated archaella (8–11). Although newly developed techniques enable us to examine the dynamic properties of the machinery, little is known about the regulation mechanism of T4P because tens of components cooperate to orchestrate the dynamics of both the archaella and T4P system. Such complexity hampers the design of an experimental setup with quantitative and reproducible evaluations.
Fig. 1.
Negative phototaxis visualized under an optical microscope at the single-cell level. (A) Schematics of the T4P-based twitching motility. A cell moves via a cycle of extension, attachment, and retraction of T4P. These three processes are indicated by red arrows or circle, respectively, in A. (B) Diagram of the experimental setup. Lateral and vertical lights with a wavelength of 488 nm were separately irradiated onto the specimen on the sample stage. This setup enables quantification of the phototactic response of each cell movement. (C) Bright-field image of Synechocystis sp. PCC6803 and their moving trajectories for 240 s (color lines) on a glass substrate coated with 0.007% collodion. The blue arrow in the Inset represents the direction of light propagation. (Scale bar, 30 μm.) (D) Rose plots under lateral (Left), vertical (Middle), and no illumination (Right) of blue light against the sample stage (n = 200 cells). The number of cells moving more than 3 μm min−1 was counted. (E) Schematics of types of illumination in D. Thick arrows in the Insets and thin arrows represent the direction of light propagation and movement of cells, respectively. The fluence rate was 1,200 μmol m−2 s−1 from lateral or vertical illumination. BS, beam splitter; DM, dichroic mirror; Obj, objective lens.
To address how the T4P system is activated or repressed by environmental signals over a short timescale, we here used Synechocystis sp. PCC6803, a model cyanobacteria (12–14). This species exhibits T4P-dependent twitching motility on surfaces such as soft-agar plates at speeds of a few micrometers per minute, and the cell motility direction is regulated both positively and negatively along the light axis (15). The phototactic behavior is mediated by the photo-sensing proteins and its two component systems, and, particularly at the blue-light region of the spectrum, the cell exhibits negative phototaxis (16). A blue-light receptor, PixD, and response regulator, PixE, are related to the regulation of cyanobacterial phototaxis (17). However, it remains a mystery how the coccoid cell transmits the signals to the T4P machinery to achieve directional cell motility.
In this study, we directly visualized the dynamics of T4P under an optical microscope and showed that they were controlled by the blue-light exposure that induced the negative phototaxis. We provide the direct evidence that the local difference of light intensity in the cell induces the asymmetric activation of T4P to achieve directional cell motility. The blue-light receptor, PixD, mediates the suppression of the T4P dynamics in the opposite region to maintain the above activation. These findings highlight the light-signal processing system in cyanobacteria, which regulates T4P dynamics to navigate cells in a certain direction (18–20). This concept is in stark contrast to the mechanism of chemotaxis in bacteria, which changes the interval between random swimming and tumbling triggered by repellants (21).
Results
Observation of Phototaxis at the Single-Cell Level.
Phototaxis of bacteria has been visualized as colony migration on an agar plate (14, 22) or as the trajectory of cells (15, 18). To observe a detailed trajectory of negative phototaxis at the single-cell level, we constructed an optical setup that allowed us to simultaneously illuminate specimens on a glass substrate both laterally and vertically (Fig. 1B). Blue light with a wavelength of 488 nm was chosen to induce negative phototaxis, and the stimulus of light intensity was adjusted in the range of 10–10,000 μmol m−2 s−1 (photon flux density in the visible light wavelength) on the sample plane, which was calibrated using the intensity of fluorescent beads (Materials and Methods for more detail). The position of the cell was visualized by green light from a halogen lamp with a band-pass filter (532/40 nm) with a fluence rate of 1 μmol m−2 s−1, which was confirmed to have no effect on motility (Fig. 1 D and E, Right). When a blue light with 1,200 μmol m−2 s−1, which is half of direct sunlight (∼2,000 μmol m−2 s−1), was shone on cells from the side, most cells escaped from the direction of the light propagation on the glass coated with collodion (Fig. 1C and Movie S1). The effect of the collodion coating is shown in Fig. S1; a 0.007% solution of collodion was used because cells clearly showed negative phototaxis under this condition. The rose plot, a round histogram that intuitively and simultaneously presents the number of occurrences and direction, clearly indicates that the lateral light induced negative phototaxis (Fig. 1 D and E, Left), whereas cells move randomly under the vertical illumination (Fig. 1 D and E, Middle). These observations indicated that our experimental system successfully quantified the negative phototaxis of this species at the single-cell level. Note that in the absence of blue-light exposure (i.e., in the dark), cells did not show directional movement on the glass (Fig. 1 D and E, Right), suggesting that Synechocystis at least requires light exposure as a trigger to activate migration.
Fig. S1.
Effect of collodion coating on negative phototaxis. (A) Bright-field image of cells and their moving trajectories for 180 s (color lines) on a glass substrate coated with collodion. The concentration of collodion was presented at the upper right part of each image. The blue arrow in the Inset represents the direction of light propagation. (Scale bar, 30 μm.) (B) Rose plots under lateral illumination against the sample stage (n = 200 cells). Cell was subjected to the lateral light from the left side (270° in the image) at a fluence rate of 1,200 μmol m−2 s−1. The number of cells moving more than 3 μm min−1 was counted.
Direct Visualization of T4P Dynamics Through Fluorescent Beads.
To reveal the dynamics of T4P quantitatively, we fixed cells on the glass substrate. The motility of cells was hindered on the glass surface coating with high concentration of collodion, and when the concentration of collodion reached 0.2% the cells ceased to move entirely and remained immobilized on the surface (Fig. S1). Notably, in the presence of polystyrene beads in the aqueous solution, the immobilized cells were able to retract the beads from the solution to the cell surface (Fig. 2A and Movies S2 and S3). This observation suggests that the cells nonspecifically attached on the glass surface and thus were immobilized, but the ability of pili to extend, catch targets, and retract them remained intact. As a result, beads were accumulated on cells. However, cells treated by the chemical fixation with glutaraldehyde did not show such accumulation (Fig. S2). Beads stabilized by sulfate charges were effectively captured by pili, but neither carboxylated nor BSA-coated beads were accumulated (Fig. S2), suggesting the specificity of the tip of a single T4P filament. The beads accumulated on the cell surface were not released during our observation time, and thus the activity of T4P was directly visualized as the distribution of fluorescence intensity of beads accumulated on the surface (Fig. 2A).
Fig. 2.
Visualization of T4P dynamics through fluorescent beads. (A) (Top) schematics of the observation. The thick arrow in the Inset represents the direction of light propagation. Blue and red thin arrows represent the retraction and extension of pili, respectively. The light-illuminated region is shown in pale blue. (Bottom Left) merged image of the cell (red) and fluorescent beads (green). The Inset represents the direction of light propagation, which follows the axis perpendicular to the observation plane. (Bottom Right) averaged intensity profile of the beads in the micrograph (n = 20 cells). (B) Schematic, micrograph, and profile of the beads’ distribution when light was laterally illuminated. The micrograph is shown with the same color codes and visualization as at Bottom Left in A. The blue arrow in the Inset in both A and B represents the direction of light propagation. (C) Same sets as in B when lights were partially illuminated in the right half of the cell from the bottom as shown in the schematic. Partially covered marks in the Insets represent the localized illumination. (Scale bars, 1 μm in A and B, also applies to C.) (D) Tracking of beads: (Left, Middle, and Right) the results under vertical, lateral, and partial illumination, respectively, in D–F. Rounds at the center indicate the cell position. Blue and red colors code the movements toward the cell and the movements away from the cell, respectively, in D–F. Beads were visualized by vertical or lateral blue-light illumination. (E) Time courses of beads under different illuminations. (F) Histograms of the speed of displacement of beads. The average and SD were plotted (n = 100 in 15 cells). (G) (Left and Middle) histograms of the angle distribution of beads’ displacement, looking from the center of cells under vertical and lateral illumination, respectively. (Right) Schematic of the definition of the angle θ.
Fig. S2.
Preference of accumulation of beads on the cell surface. (A) Merged image of the cell (red) and fluorescent beads (green). The cell was immobilized onto the glass surface. (Top) Sulfate beads. Cells were chemically fixed with glutaraldehyde before adding beads. (Middle) Carboxylated beads. (Bottom) BSA-coated carboxylated beads. Beads were visualized by mercury lamp after chemical fixation of the cell with glutaraldehyde. (Scale bar, 1 μm.) (B) Averaged intensity profile of the beads in the micrograph (n = 20 cells).
In Fig. 2A, light was illuminated vertically onto the sample plane. In contrast, when light was laterally illuminated, the distribution of beads became not symmetric but asymmetric: beads were accumulated to the forward side of the cell along the lateral blue light (Fig. 2B and Movies S4 and S5). The asymmetric accumulation suggests that T4P filaments located around the right side of the cell can drive rightward motion, as in Fig. 1C, because lateral illumination induces negative phototaxis toward the right side in this setup (Fig. 1). However, when light was partially illuminated only at the right side of the cell, beads were accumulated only in the illuminated region (Fig. 2C). These observations could be explained by the microoptics effect of a cell, as previously reported (18) (Fig. S3) (Discussion).
Fig. S3.
Effect of microoptics. (A) Schimatics of microoptics effect. (B) (Top) Optical field distribution calculated by FDTD method. Cell was illuminated with lateral light from the left side (270° in the image). The color indicates relative light intensity. Dashed line indicates the cell outline. (Scale bar, 1 μm.) (Bottom) intensity profile along the cell outlines from 270° to 90° in Top. (C) Fluorescent beads with the size of 0.2 μm located at the surface of the cell. (Top) Bright-field image. (Middle) Fluorescent image visualized by vertical illumination. (Bottom) Fluorescent image visualized by lateral illumination. Cross-mark indicates the center of the cell. (Scale bar, 2 μm.) (Right) Schematic illustration of the effect of microoptics. The blue arrow in the Inset in A–C represents the direction of light propagation. (D) Intensity profile of the beads. Left and Right profiles came from the two different beads indicated by the red and blue arrows in C. Gaussian fitting of the intensity was presented by a solid line. (Top) vertical illumination. (Bottom) lateral illumination. The beads were marked by the arrows in C.
In the above three different illumination setups, the displacement of beads induced by T4P was accurately tracked at the single-pilus level (Fig. 2D). Most beads were retracted toward cells, but we also observed the movement of beads away from the cell body (red lines in Fig. 2 D and E). This particular movement was attributable to the elongation of a single pilus, and its speed was about one third compared with the retraction speed (Fig. 2F). The retraction and extension speeds were not substantially altered in the three different illumination setups, suggesting that the illumination condition of light in each setup was well regulated in our observation system, although the direction of light propagation was different, and so the asymmetric dynamics of T4P observed here may be a natural characteristic of this species. As predicted from the results shown in Fig. 2 A and B, the angle distribution of the beads’ movement was broadly distributed under the vertical illumination (Fig. 2G, Left), whereas it was asymmetric under the lateral illumination (Fig. 2G, Middle).
Visualization of T4P Filaments as a Fluorescent Image.
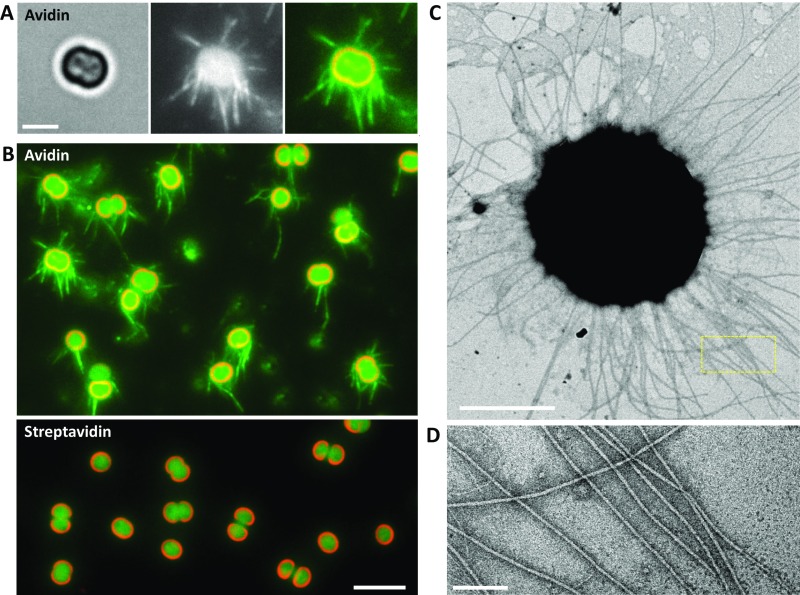
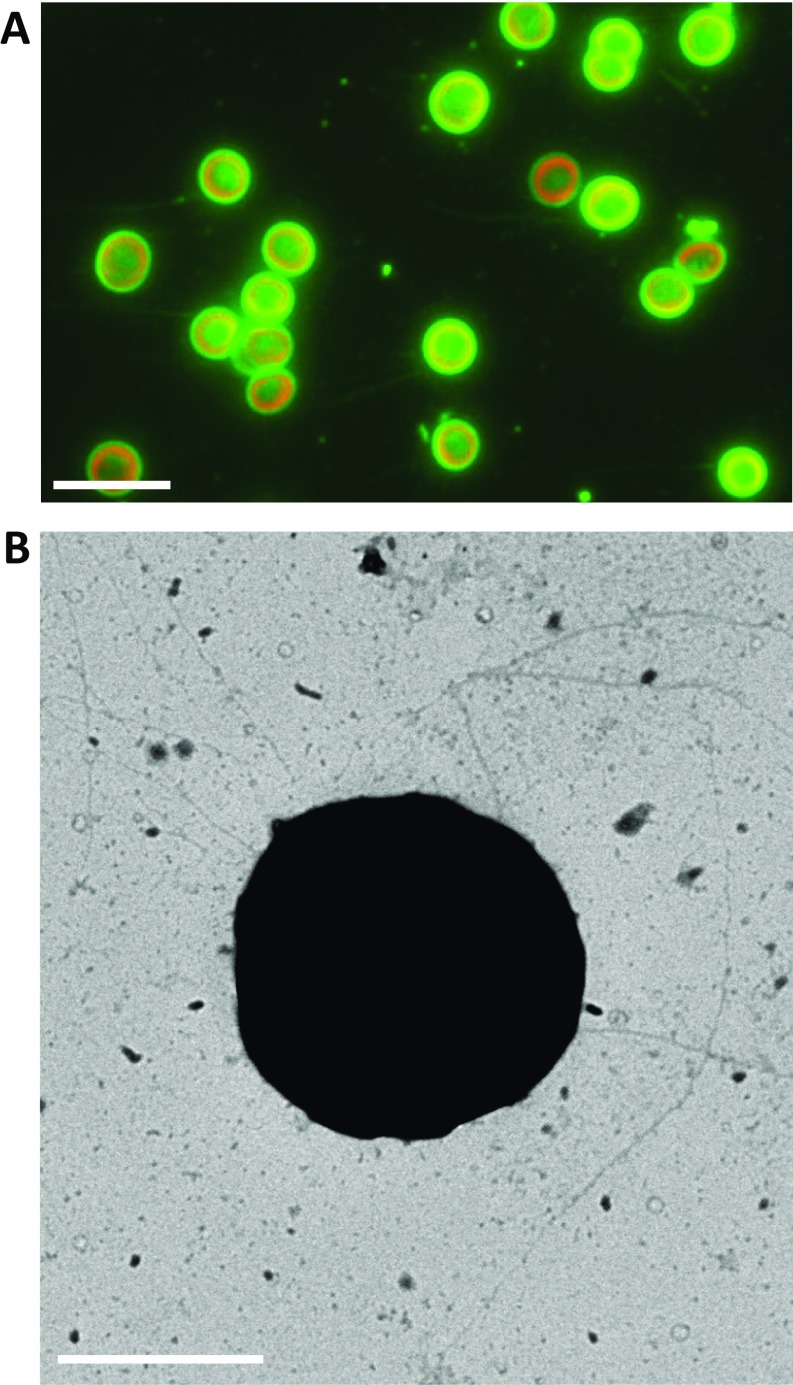
Although the accumulation of beads was quantified at the single-cell level (Fig. 2), the essence of the dynamics of T4P was still unresolved: i.e., we detected the extension and retraction only after the beads attached to T4P with the above experimental setup. To gain further insight into the regulation mechanism, we attempted to visualize pilus filaments with a fluorescent marker. We found that conventional labeling with fluorescent chemicals, such as FITC succinimidyl ester (2), did not work in Synechocystis. Instead, pilus filaments were sufficiently visualized with labeling by avidin conjugated with FITC (Fig. S4A). Several other types of biotin-binding proteins, such as streptavidin or neutravidin conjugated with FITC, were not applicable (Fig. S4B), indicating that the avidin-specific labeling of pili is caused by the glycosylated region of avidin (23). It is already known that the cell has two morphologically distinct pilus types, named thick and thin pili (13). To check which pilus filament is labeled with FITC–avidin, we applied this method to the GT strain, which is known to have nonmotile phenotype (24). We found that the cells have low fluorescent signal as a filament (Fig. S5A), and the intensity was much less than that in the PCC-P strain, a standard strain in this paper (Fig. S4 A and B). It is consistent with the EM observation: the PCC-P strain had thick pili as long filaments with a diameter of 8 nm and more than 2 μm in length (Fig. S4 C and D), corresponding with the morphotype of T4P, as previously described (13), but the number of thick pili in the GT strain was much less (Fig. S5B). Based on these observations, we conclude that we successfully established a method to visualize T4P of Synechocystis under an optical microscope.
Fig. S4.
Visualization of T4P filaments under an optical microscope. (A) (Left) magnified view of the bright-field image of the cell. (Scale bar, 2 μm.) (Middle) fluorescent micrograph labeled by FITC–avidin (see SI Materials and Methods for details). Cell was subjected to the vertical blue light at a fluence rate of 1,200 μmol m−2 s−1. Each T4P filament is clearly visualized. (Right) merged image of (Left) (red) and (Middle) (green). (B) Merged image of cells labeled by FITC–avidin (Top) and FITC–streptavidin (Bottom), respectively. (Scale bar, 5 μm.) (C) Image of a cell taken by the electron microscope with negative staining using ammonium molybdate. (Scale bar, 1 μm.) (D) Magnified image of the yellow boxed area in C. (Scale bar, 100 nm.)
Fig. S5.
Visualization of T4P filament in the GT strain. (A) Merged image of cells labeled by FITC–avidin. (Scale bar, 5 μm.) (B) Image of a cell taken by the electron microscope with negative staining using ammonium molybdate. (Scale bar, 1 μm.)
Here we applied the labeling procedure in Fig. S4A and directly quantified the effect of the lateral illumination on the extension of T4P (Fig. 3). As expected from the behavior of the accumulation of beads, T4P filaments dominantly appeared from the side of the cell opposite the illumination (Fig. 3A). This observation was consistent with the idea that the probability of the tip of a pilus binding to the target, in the case of a fluorescent bead in Fig. 2, is presumably stochastic, and negative phototaxis is mainly driven by the regulated asymmetric distribution of T4P filaments over the cell surface (Fig. 3B).
Fig. 3.
Distribution of T4P filaments in the wild type and ΔpixD mutant triggered by lateral illumination. (A) (Left) bright-field image of the cell, which was subjected to lateral light from the left side (270° in the image) at a fluence rate of 1,200 μmol m−2 s−1 (Materials and Methods for details). (Scale bar, 2 μm.) Middle: fluorescent micrograph. T4P were dominantly distributed around 90°. (Right) merged image of Left (red) and Middle (green). (B) Schematic showing the asymmetric tendency of the extension of T4P triggered by lateral light. The blue arrow in the Inset in both A and B represents the direction of light propagation. (C) Schematic of the definition of θ in rose plots (Left). Rose plot of the distribution of a T4P filament in the wild type (Middle), and the ΔpixD mutant (Right) (n = 20 cells). (D) Effects of lateral light intensity on the number of T4P per cell (Top), the pilus length (Middle), and the localization bias of pilus appearance (Bottom) as [(the number of pili in 0–180°)/(total number)] when light came from 270°, in the wild type (Left, circles) and ∆pixD mutant (Right, diamonds). The average and SD from 20 cells were plotted. Dashed lines represent the fitting of Michaelis–Menten kinetics.
The next question raised here is how the asymmetric distribution of T4P is regulated by the blue-light illumination. To evaluate the effect of the light, we applied a mutant that lacks PixD, a blue-light–sensing protein (15, 17). Unexpectedly, the T4P was generated in the ΔpixD mutant (Fig. 3C). The number of T4P increased with the increase in the power of light in both the wild type and ΔpixD mutant in a Michaelis–Menten manner (Fig. 3D, Top). The length of pili was similar in both samples (Fig. 3D, Middle), within a range of 2–4 μm on average, indicating that PixD is not required to induce the extension of T4P; i.e., there should be other photoreceptors. Notably, the distribution of T4P in the ΔpixD mutant was more uniform than that in the wild type (Figs. 3C and 3D, Bottom; see also Figs. S6 and S7). These observations suggest that PixD is responsible for the asymmetric distribution of T4P via an unknown mechanism, which suppresses the extension of T4P from the light-illuminated side.
Fig. S6.
Distribution of a single T4P triggered by lateral illumination in various light intensities. (A) Merged image of cell (red) and T4P (green). The cells of the wild type (Top) and ΔpixD mutant (Bottom) were subjected to the lateral light from the left side at various fluence rates, as presented in each image. The cells were subsequently chemically fixed by glutaraldehyde and labeled by FITC-avidin. The blue arrow in the Inset represents the direction of light propagation. (Scale bars, 5 μm.) (B) Rose plot of the distribution of a single T4P in the wild type (Top) and ΔpixD mutant (Bottom) (n = 20 cells). The definition of angle θ was the same in Fig. 3C. The fluence rate of the lateral illumination was presented in each graph.
Fig. S7.
Visualization of T4P dynamics through fluorescent beads in ΔpixD mutant. (A) Merged image of the cell (red) and fluorescent beads (green). Sulfate beads were visualized with a mercury lamp after chemical fixation of the cell with glutaraldehyde. (Inset) The direction of light propagation. (Top) Vertical. (Middle) lateral. (Bottom) partial. Partial blue light was applied to the right half of the cell, as shown in pale blue. The blue arrow in the Inset represents the direction of light propagation. (Scale bar, 1 μm.) (B) Averaged intensity profile of the beads in the micrograph (n = 20 cells). (C) Tracking of the beads under lateral illuminations. Rounds at the center indicate the cell and rough position of the cell surface: blue, movements toward the cell; red, movements away from the cell. Beads were visualized by vertical or lateral blue-light illumination. (D) Time courses of beads under lateral illuminations. The color codes of the lines are the same as in C. (E) Histogram of the speed of displacement of beads. The color codes of bars are the same as in C. Values in the graphs are the average± SD (n = 100 in 10 cells). (F) Histogram of the angle distribution of beads’ displacement looking from the center of cells under lateral illumination. The definition of angle θ was the same in Fig. 2G.
Finally, we reproduced the experiment in Fig. 2B under localized illumination without beads and visualized the dynamics of T4P at the single-cell level. In the wild type, T4P were extended only in the illuminated region (Fig. 4A). In contrast, T4P appeared uniformly when the cell body of the ΔpixD mutant was partially subjected to light (Fig. 4B). Quantification measurements clearly revealed the above tendency (Fig. 4C), indicating that a partial illumination was sufficient to trigger the extension of T4P from all regions (Fig. 4D). The role of PixD is presumably to suppress the extension in unilluminated areas, which is consistent with the scenario deduced from the results in Fig. 3. Such asymmetric distribution of T4P may be associated with the pilus assembly motor, PilB1, because the region of T4P production has a similar dimension to the “crescent” localization of PilB1 (25).
Fig. 4.
Distribution of T4P filaments in the wild type and ΔpixD mutant triggered by localized illumination. (A) (Top Left) magnified view of the bright-field image of a cell subjected to localized light from the bottom side (highlighted by pale blue) with a strength of 10,000 μmol m−2 s−1 (Materials and Methods for details). (Scale bar, 2 μm.) (Top Middle) fluorescent micrograph. T4P were dominantly distributed in the illuminated area, in this case the right half (0–180°) of the cell. (Top Right) merged image of Top Left (red) and Top Middle (green). (Bottom) multiple cells. (Scale bar, 5 μm.) (B) Three sets of images similar to the Top in A of the ΔpixD mutant. (Scale bar, 2 μm.) (C) Rose plots of the distribution of T4P in the wild type (Top) and ΔpixD mutant (Bottom) (n = 20 cells). (D) Effects of light intensity on the number of T4P per cell (Top), the pilus length (Middle), and the localization bias of pilus appearance (Bottom) as [(the number of pili in 0–180°)/(total number)] when the cells were illuminated only in the right-half region (0–180°), in the wild type (Left, circles), and ∆pixD mutant (Right, diamonds). The average and SD from 20 cells were plotted. Dashed lines represent the fitting of Michaelis–Menten kinetics. (E) Schematics of our observations in A and B. Red arrows represent the extension of T4P triggered by localized illumination. The thick blue arrow in the Inset represents the direction of light propagation. The thin pale blue arrows represent the region of the localized light illuminated in the right half of the cell.
Three Phases in the Realization of Phototaxis.
The results shown in Figs. 2, 3, and 4 suggest the possibility that the role of PixD is not to activate but rather to suppress T4P extension to achieve negative phototaxis. If this were the case, this suppression process could be quantitatively detected via the twitching motility of the cell body triggered by blue-light exposure. To test this hypothesis, we precisely analyzed both the directed motion of the wild type and the relatively random motion of the ΔpixD mutant under lateral illumination of blue light (Figs. 5 and S8 and Movies S1 and S6). The traces clearly showed the negative phototaxis in the wild type, whereas the ΔpixD mutant wiggled (Fig. 5A, Left and Middle). No substantial motility appeared without exposure (Fig. 5A, Right). We plotted displacements of 50 samples along the x axis, which is the direction of light propagation. In the wild type, the directed movement was barely observed for the first 1 min in most cells (Fig. 5B, Left). This tendency was clearly visible in the average of traces (Fig. 5C, Left) because the net displacement was almost zero for the first 1 min.
Fig. 5.
Analyses of twitching motions of the wild type and ΔpixD mutant triggered by lateral illumination. (Left) The wild type. (Middle) ΔpixD mutant. (Right) wild type without the illumination. (A) xy plots where light propagation of the lateral illumination was set parallel to the x axis (n = 50 cells). The blue arrow in the Inset represents the direction of light propagation. (B) Raw data of the time course of x (n = 50). (C) The average of x (thick blue line). The SD at each time point was plotted as a cyan perpendicular bar. (D) MSD plots (thick blue line). The cyan curve represents a hyperbolic fitting. The orange line represents a linear fitting. (E) A magnified view of D. In C–E, the pink region is from 0.2 to 0.9 min after the exposure; blue arrows in C–E indicate the transition from random movement to directed movement; and red arrows in E indicate the transition where the motion starts after the exposure. (F) Schematics of the cell response triggered by lateral blue-light illumination. Blue and red thin arrows represent the retraction and extension of pili, respectively. ON, the starting point of the light exposure.
Fig. S8.
Analyses of twitching motions triggered by lateral illumination in various light intensities. (A) Time course of the average displacement of cells along the light propagation of the lateral illumination (n = 50 cells). The color indicates the fluence rate of lateral illumination. (Left) The wild type. (Right) ΔpixD mutant. (B) Average of MSD plots (n = 50 cells). The color codes are the same as in A. (C) Effect of lateral light intensity on the cell velocity. The average and SD of velocity along the light propagation was plotted (n = 50 cells). ON, the starting point of the light exposure.
To address the question of how the cell movement transitions to the directed movement, we plotted the mean square displacement (MSD) of all cells (Fig. 5 D, Left, and E, Left). Remarkably, the average of MSD linearly increased with time from 0.2 to 1.0 min after the light exposure. This finding was more notable in the magnified view (Fig. 5E, Inset), proving that the twitching motility was activated randomly with an apparent diffusion coefficient of 1.2 μm2/s, which is good agreement with the scaling behavior of the motility in other bacteria (26, 27). The start point of the parabolic line indicates the phase transition to directed twitching motility as a result of the negative phototaxis.
In the ΔpixD mutant, the behavior of cells was different from that in the wild type with respect to directed or random motion. The raw traces were fluctuating, and the net displacement was zero (Fig. 5B, Middle). The linearity of MSD ensured that the twitching motility of the mutant was also activated, and the slope coincided with that of the wild type (Fig. 5 D, Right, and E, Right). However, no parabolic region appeared for the ΔpixD mutant, and MSD linearly increased for 10 min.
Our interpretation of the results in Fig. 5 is that the negative phototaxis accompanies transitions between three phases (Fig. 5F). First, cells are exposed to light, but a delay is needed before starting the wiggling motion within 0.2 min; this is the first phase. The delay is comparable to the time required for the extension of T4P filaments, a speed of 0.3 μm/s to be 3 μm in length (Figs. 2 and 3). In the second phase, pili uniformly appear from all directions of the cell surface, and so random twitching motility is activated both in the wild type and ΔpixD mutant. The random movement in the wild type is terminated by PixD within 1 min after the exposure, by suppression of the extension of T4P filaments only at the unilluminated region of the cell surface (Figs. 2, 3, and 4). Finally, in the third phase, Synechocystis drives the motion only by uninhibited pili, which directs the cell body away from the light illumination. These transitions between phases are the essence of negative phototaxis in this species, and other bacteria having photoreceptors might share a common mechanism.
Discussion
We demonstrated that the mechanism of taxis in Synechocystis is substantially different from the mechanism of motility in well-studied bacteria such as Salmonella (19, 20). Flagellated bacteria induce directional net displacement by a change in the frequency of the transition between tumbling and swimming (21). This strategy is reasonable, considering that the direction of the swimming in bacteria is fixed to the long axis of an ellipsoidal cell body; i.e., cells can only move on the axis along which flagella protrude. In contrast, T4P can extend from all directions of the body, and thus cells can move in any direction. Therefore, the taxis in Synechocystis is achieved by an asymmetric distribution of T4P in the cell body, although cells are fundamentally sphere-shaped, symmetric in rotation, and not polarized.
We visualized that the cell has the asymmetric distribution of T4P to move away from directional blue light (Fig. 3). Our data also suggest that the above polarization is triggered by the local difference of blue-light intensity, and the T4P extension was activated at the region where the intensity is higher (Fig. 4). These observations appear to have conflicting results because blue light would be expected to work as a “repellent.” However, this could be explained by the microoptics effect of the cell, as previously reported (18). When a single cell is exposed to collimated light, the cell condenses the light into the opposite side of the light source because the cell body works as an optics with a diffraction index higher than water. This model was proposed to explain positive phototaxis (18), and we extended the model to negative phototaxis based on the result of direct visualization of T4P and its dynamics. The fact that cell bodies function as an optics was directly confirmed in Chlamydomonas reinhardtii (28). The strategy seems reasonable because a microorganism with a size on the order of the visible wavelength cannot produce any structure to shield itself from light.
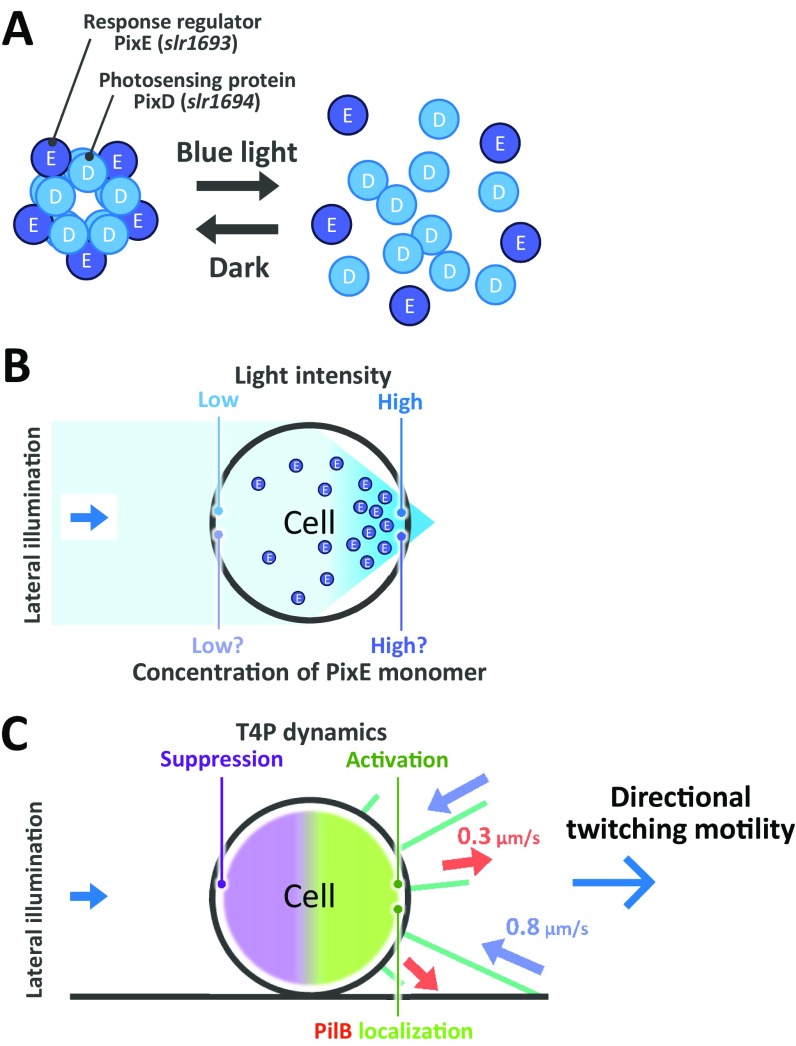
Here we elucidated the following sequence of events from stimulus to taxis in detail. First, blue-light stimulation induces the phototactic response as the uniform extension of T4P filaments. The localization of pilus assembly motor PilB1 may not be completed at the specific part of the cell in this stage (25). Second, after 1-min delay, T4P dynamics is regulated to be asymmetric along the light axis through the photoreceptor PixD, which mediates a suppression of the T4P dynamics in the region where the intensity is relatively low (Fig. 5F and Fig. S9). Because PixD is known to form a complex with the response regulator, PixE, and the complex is disassembled by the illumination of blue light in vivo (17, 29, 30), it is conceivable that the 1-min delay observed in Fig. 5 originates from the above reaction, whereas the role of PixE to the suppression is not yet clear. Finally, the cell produced directional cell motility as resultant negative phototaxis.
Fig. S9.
Schematic illustration of PixD-dependent negative phototaxis. (A) PixD forms complex with the response regulator PixE, and the complex is disassembled by the illumination of blue light. (B) PixD presumably releases PixE at the forward side of light illumination, where the intensity is relatively high, and this release mediates a suppression of the T4P dynamics in the region where the intensity is relatively low. (C) T4P dynamics is activated at the higher-intensity side, allowing navigation of directed twitching motility. The blue arrow in the Inset in both B and C represents the direction of light propagation. The pale red and blue arrows in C represent the extension and retraction of T4P, respectively.
Our experimental setup enables us to evaluate the regulation of T4P filament under an optical microscope on a timescale of minutes and thus could be an ideal tool to quantify the detail of phototactic responses in Synechocystis (14, 15, 18, 31). We also visualized the retraction of T4P by tracking a fluorescent bead that attached to the edge of a single pilus. This assay could contribute to quantification of the dynamic properties of T4P, which may be common in other bacteria such as Neisseria gonorrheae (6, 32) or Pseudomonas aeruginosa (33). The combination of our experimental system and mutants that lack other receptors or key proteins in various species will reveal more detailed mechanisms in the near future.
Materials and Methods
Strains and Culture Conditions.
The motile strains (PCC-P) of Synechocystis sp. PCC6803 and ΔpixD mutant (34, 35) were grown in BG-11 medium in moderate light (10 μmol m−2 s−1 photons) at 30 degrees with shaking to an optical density of around 1.0 at 750 nm.
Optical Microscopy and Data Analyses.
Cells were visualized under an inverted fluorescence microscope (IX83; Olympus) equipped with 20×, 60×, and 100× objective lenses (UPLAPO, 0.8 N.A., UPLAPO, 1.4 N.A., and UPLSAPO, 1.4 N.A.; Olympus), a filter set (FITC-5050A; Semrock), a CMOS camera (Zyla 4.2; Andor), and an optical table (RS-2000; Newport). The position of the cell was visualized by green light from a halogen lamp with a band-pass filter (FF01-531/40; Semrock) at a fluence rate of 1 μmol m−2 s−1, as needed. Projection of the image to the camera was made at 330 nm, 100 nm, and 67 nm per pixel. Sequential images of cells were captured as 16-bit images with a CMOS camera under 1-s or 0.1-s resolution and converted into a sequential TIF file without any compression. All data were analyzed by ImageJ 1.48v (rsb.info.nih.gov/ij/) and its plugins, particle tracker and multitracker.
Blue-Light Illumination.
Lateral and vertical lights with a wavelength of 488 nm from a blue laser (OBIS 488 LS; Coherent) were separately irradiated onto the specimen on the sample stage of a microscope (Fig. S10A) (SI Materials and Methods for details).
Fig. S10.
Experimental setup of light illumination on the microscope stage. (A) Diagram of lateral and vertical illuminations. Blue lasers with a wavelength of 488 nm were separately irradiated onto the specimen on the sample stage. (B) Diagram of partial illumination. The Ronchi-ruling, a constant-interval bar, was set at the conjugative position of sample plane to be a 2-μm interval on the sample stage. (C) Partial illumination of fluorescent beads. (Top) A 1-μm bead. (Bottom) A 2-μm bead. Partial blue light was applied to the right half of the cell, as shown in pale blue. (Scale bars, 2 μm.) (D) Calibration of blue-light intensity on the sample stage. Fluorescent intensity of beads with a size of 0.2 μm was linearly fitted by the vertical and lateral illumination. (E) Typical example of fluorescent intensity of beads in various blue-light intensities. (Left) vertical. (Right) lateral. (Scale bars, 0.5 μm.) (F) Diagram of mercury lamp. Accumulation of beads and FITC-labeled T4P was visualized by this setup. The blue arrow in the Inset in both D and E represents the direction of light propagation. BE, beam expander; BP, band-pass filter; BS, beam splitter; DM, dichroic mirror.
Phototaxis on Glass.
All procedures were done at room temperature (RT). The cell culture was poured into a tunnel assembled by taping a coverslip (36). The coverslip was coated with 0.007% (vol/vol) collodion in isoamyl acetate and air-dried before use. After incubation for 10 min in moderate light (10 μmol m−2 s−1 photons), the sample chamber was set on the microscope stage. The position of the cell was visualized by green light from a halogen lamp with a band-pass filter (FF01-531/40; Semrock) at a fluence rate of 1 μmol m−2 s−1, and the image was recorded at 1-s intervals. The cells were subjected to lateral or vertical illumination by the blue laser (SI Materials and Methods for details).
Beads’ Assay and Fluorescent Labeling of Pili.
Fluorescent polystyrene beads of size 0.2 μm (Sulfate: F8848; Thermo Fisher) were 100 times diluted to be 0.02% (wt/vol) in BG11 and used for beads’ assay. Avidin-FITC (Sigma) at the concentration of 0.035 mg/mL in BG-11 containing 2% BSA were used for labeling (SI Materials and Methods for details).
SI Materials and Methods
Beads’ Assay.
All procedures were done at RT. Fluorescent polystyrene beads of size 0.2 μm (Sulfate: F8848 and Carboxylate-modified: F8803; Thermo Fisher) were 100 times diluted to be 0.02% (wt/vol) in BG11, approximately, at a concentration of 5 × 1010 particles per milliliter. The cell culture was poured into a tunnel assembled by taping a coverslip. The coverslip was coated with 0.2% (vol/vol) collodion in isoamyl acetate and air-dried before use. After incubation for 10 min in moderate light (10 μmol m−2 s−1 photons), the floating cells were removed by replacing the BG-11 medium. The following procedure was conducted on the microscope stage. The cells were subjected to lateral, vertical, and partial illumination at fluence rates of 2,000, 2,000, and 10,000 μmol m−2 s−1, respectively, as described above. After illumination for 3 min, the fluorescent beads were added to the sample chamber, and their movement was visualized by the blue-light illumination at 0.1-s intervals (Fig. S10A). After adding beads for 2 min, the blue-light illumination from the laser was stopped, and the cells were chemically fixed by 1% (vol/vol) glutaraldehyde in BG-11 for 1 min. The fluorescent signal from the beads was visualized by a mercury lamp through a filter set (FITC-5050A; Semrock) (Fig. S10F).
Fluorescent Labeling of Pili.
All procedures were done at RT. The cell culture was poured into a tunnel assembled by taping a coverslip. The coverslip was coated with 0.2% (vol/vol) collodion in isoamyl acetate and air-dried before use. After incubation for 10 min in moderate light (10 μmol m−2 s−1 photons), the floating cells were removed by replacing the BG-11 medium. The following procedure was performed on the microscope stage. The cells were subjected to lateral, vertical, or partial illumination by the blue laser, as described above. After illumination for 3 min, the cells were chemically fixed by replacing 1% (vol/vol) glutaraldehyde in BG-11. After incubation for 1 min, the sample was washed with BG-11 three times and replaced with 2% (wt/vol) BSA in BG-11 for blocking. After incubation for 1 min, the sample was replaced with 0.035 mg/mL avidin–FITC (Sigma) in BG-11 containing 2% BSA for labeling. After incubation for 1 min, the sample was washed with BG-11 three times and then observed under a fluorescent microscope. Pili longer than 1 μm and the numbers of pili on the cells were manually measured by the projected image of cells.
Modeling of Microoptic Effects.
The optical field distribution was calculated by the software package FDTD Solutions (Lumerical), based on the finite-difference time-domain (FDTD) method. The refractive index of the cell relative to that of water used here was 1.05, as previously described (37).
Electron Microscopy.
Samples bound to the grids were stained with 2% (wt/vol) ammonium molybdate and observed by transmission electron microscopy, as previously described (36). Carbon-coated EM grids were glow-discharged by a PIB-10 hydrophilic treatment device (Vacuum Device), coated with 0.2% (vol/vol) collodion in isoamyl acetate, and air-dried before use. Cells were put on the EM grid and subjected with the illumination of blue light (2,000 μmol m−2 s−1 photons) for 3 min at RT. The cells were chemically fixed with 1% (vol/vol) glutaraldehyde in in BG-11 for 10 min at RT. After washing three times with BG-11, the cells were stained with 2% ammonium molybdate and air-dried. Samples were observed under a transmission electron microscope (JEM-1400, JEOL) at 100 kV. The EM images were captured by a charge-coupled device (CCD) camera, and analyzed by ImageJ 1.48v.
Blue-Light Illumination.
Lateral light illumination was applied from the left side of the microscope stage at an angle of 5 degrees. Vertical light illumination was applied from the objective lens with a dichroic mirror (Di02-R488; Semrock). For partial illumination, the Ronchi ruling (Edmond) was set at the conjugative position of the sample plane to be a 2-μm interval on the sample stage (Fig. S10 B and C). Calibration of the blue-light intensity was done as follows. Blue-light intensity from a halogen lamp with a band-pass filter (FF01-483/32; Semrock) was measured with a power meter (Q82017A; Advantest), and it was confirmed that the intensity of a fluorescent bead with a size of 0.2 μm (F8848; Thermo Fisher) through a filter set (FITC-5050A; Semrock) was increased with increasing light intensity (Fig. S10 D and E). The linearity was also confirmed by the lateral and vertical illumination with neutral-density filters in the range of 10–10,000 μmol m−2 s−1.
Supplementary Material
Acknowledgments
The authors thank S. Kojima for discussions that were critical in preparing the manuscript; and S. Masuda for supplying the wild type and ΔpixD mutant of Synechocystis sp. PCC6803. This study was supported by a Grant-in-Aid for Scientific Research on Innovative Areas [“Fluctuation & Structure” of JP16H00808 (to T.N.), “Cilia & Centrosomes” of JP87003306 (to T.N.), and “Motility Machinery” of JP15H01329 (to D.N.)]; Grant JP24117002 (to T.N.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan; and by Japan Society for the Promotion of Science KAKENHI [Grants JP16H06230 (to D.N.) and JP15H04364 (to T.N.)].
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702395114/-/DCSupplemental.
References
- 1.Korotkov KV, Sandkvist M, Hol WGJ. The type II secretion system: Biogenesis, molecular architecture and mechanism. Nat Rev Microbiol. 2012;10:336–351. doi: 10.1038/nrmicro2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skerker JM, Berg HC. Direct observation of extension and retraction of type IV pili. Proc Natl Acad Sci USA. 2001;98:6901–6904. doi: 10.1073/pnas.121171698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 4.Chang YW, et al. Architecture of the type IVa pilus machine. Science. 2016;351:aad2001. doi: 10.1126/science.aad2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biais N, Higashi DL, Brujic J, So M, Sheetz MP. Force-dependent polymorphism in type IV pili reveals hidden epitopes. Proc Natl Acad Sci USA. 2010;107:11358–11363. doi: 10.1073/pnas.0911328107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maier B, Koomey M, Sheetz MP. A force-dependent switch reverses type IV pilus retraction. Proc Natl Acad Sci USA. 2004;101:10961–10966. doi: 10.1073/pnas.0402305101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maier B, et al. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci USA. 2002;99:16012–16017. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinosita Y, Uchida N, Nakane D, Nishizaka T. Direct observation of rotation and steps of the archaellum in the swimming halophilic archaeon Halobacterium salinarum. Nat Microbiol. 2016;1:16148. doi: 10.1038/nmicrobiol.2016.148. [DOI] [PubMed] [Google Scholar]
- 9.Albers SV, Jarrell KF. The archaellum: How Archaea swim. Front Microbiol. 2015;6:23. doi: 10.3389/fmicb.2015.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahapure R, Driessen RP, Haurat MF, Albers SV, Dame RT. The archaellum: A rotating type IV pilus. Mol Microbiol. 2014;91:716–723. doi: 10.1111/mmi.12486. [DOI] [PubMed] [Google Scholar]
- 11.Jarrell KF, Albers SV. The archaellum: An old motility structure with a new name. Trends Microbiol. 2012;20:307–312. doi: 10.1016/j.tim.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Yoshihara S, Suzuki F, Fujita H, Geng XX, Ikeuchi M. Novel putative photoreceptor and regulatory genes required for the positive phototactic movement of the unicellular motile cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2000;41:1299–1304. doi: 10.1093/pcp/pce010. [DOI] [PubMed] [Google Scholar]
- 13.Bhaya D, Bianco NR, Bryant D, Grossman A. Type IV pilus biogenesis and motility in the cyanobacterium Synechocystis sp. PCC6803. Mol Microbiol. 2000;37:941–951. doi: 10.1046/j.1365-2958.2000.02068.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi J-S, et al. Photomovement of the gliding cyanobacterium Synechocystis sp. PCC 6803. Photochem Photobiol. 1999;70:95–102. doi: 10.1562/0031-8655(1999)070<0095:potgcs>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Ng WO, Grossman AR, Bhaya D. Multiple light inputs control phototaxis in Synechocystis sp. strain PCC6803. J Bacteriol. 2003;185:1599–1607. doi: 10.1128/JB.185.5.1599-1607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhaya D, Takahashi A, Grossman AR. Light regulation of type IV pilus-dependent motility by chemosensor-like elements in Synechocystis PCC6803. Proc Natl Acad Sci USA. 2001;98:7540–7545. doi: 10.1073/pnas.131201098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okajima K, et al. Biochemical and functional characterization of BLUF-type flavin-binding proteins of two species of cyanobacteria. J Biochem. 2005;137:741–750. doi: 10.1093/jb/mvi089. [DOI] [PubMed] [Google Scholar]
- 18.Schuergers N, et al. Cyanobacteria use micro-optics to sense light direction. eLife. 2016;5:e12620. doi: 10.7554/eLife.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Armitage JP, Hellingwerf KJ. Light-induced behavioral responses (;phototaxis’) in prokaryotes. Photosynth Res. 2003;76:145–155. doi: 10.1023/A:1024974111818. [DOI] [PubMed] [Google Scholar]
- 20.Häder DP. Photosensory behavior in procaryotes. Microbiol Rev. 1987;51:1–21. doi: 10.1128/mr.51.1.1-21.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berg HC. The rotary motor of bacterial flagella. Annu Rev Biochem. 2003;72:19–54. doi: 10.1146/annurev.biochem.72.121801.161737. [DOI] [PubMed] [Google Scholar]
- 22.Ragatz L, Jiang Z-Y, Bauer C, Gest H. Phototactic purple bacteria. Nature. 1994;370:104. [Google Scholar]
- 23.Livnah O, Bayer EA, Wilchek M, Sussman JL. Three-dimensional structures of avidin and the avidin-biotin complex. Proc Natl Acad Sci USA. 1993;90:5076–5080. doi: 10.1073/pnas.90.11.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuergers N, Wilde A. Appendages of the cyanobacterial cell. Life (Basel) 2015;5:700–715. doi: 10.3390/life5010700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuergers N, Nürnberg DJ, Wallner T, Mullineaux CW, Wilde A. PilB localization correlates with the direction of twitching motility in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology. 2015;161:960–966. doi: 10.1099/mic.0.000064. [DOI] [PubMed] [Google Scholar]
- 26.Marathe R, et al. Bacterial twitching motility is coordinated by a two-dimensional tug-of-war with directional memory. Nat Commun. 2014;5:3759. doi: 10.1038/ncomms4759. [DOI] [PubMed] [Google Scholar]
- 27.Holz C, et al. Multiple pilus motors cooperate for persistent bacterial movement in two dimensions. Phys Rev Lett. 2010;104:178104. doi: 10.1103/PhysRevLett.104.178104. [DOI] [PubMed] [Google Scholar]
- 28.Ueki N, et al. Eyespot-dependent determination of the phototactic sign in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA. 2016;113:5299–5304. doi: 10.1073/pnas.1525538113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanaka K, et al. Time-resolved tracking of interprotein signal transduction: Synechocystis PixD-PixE complex as a sensor of light intensity. J Am Chem Soc. 2012;134:8336–8339. doi: 10.1021/ja301540r. [DOI] [PubMed] [Google Scholar]
- 30.Yuan H, Bauer CE. PixE promotes dark oligomerization of the BLUF photoreceptor PixD. Proc Natl Acad Sci USA. 2008;105:11715–11719. doi: 10.1073/pnas.0802149105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chau RM, Ursell T, Wang S, Huang KC, Bhaya D. Maintenance of motility bias during cyanobacterial phototaxis. Biophys J. 2015;108:1623–1632. doi: 10.1016/j.bpj.2015.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biais N, Ladoux B, Higashi D, So M, Sheetz M. Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 2008;6:e87. doi: 10.1371/journal.pbio.0060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persat A, Inclan YF, Engel JN, Stone HA, Gitai Z. Type IV pili mechanochemically regulate virulence factors in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2015;112:7563–7568. doi: 10.1073/pnas.1502025112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugimoto Y, Nakamura H, Ren S, Hori K, Masuda S. Genetics of the blue light-dependent signal cascade that controls phototaxis in the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 2016;58:458–465. doi: 10.1093/pcp/pcw218. [DOI] [PubMed] [Google Scholar]
- 35.Kanesaki Y, et al. Identification of substrain-specific mutations by massively parallel whole-genome resequencing of Synechocystis sp. PCC 6803. DNA Res. 2012;19:67–79. doi: 10.1093/dnares/dsr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakane D, Sato K, Wada H, McBride MJ, Nakayama K. Helical flow of surface protein required for bacterial gliding motility. Proc Natl Acad Sci USA. 2013;110:11145–11150. doi: 10.1073/pnas.1219753110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aas E. Refractive index of phytoplankton derived from its metabolite composition. J Plankton Res. 1996;18:2223–2249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.