Significance
As an E3 ubiquitin ligase, CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) directly ubiquitinates and triggers the degradation of various downstream targets and acts as a central repressor of light signaling. In this study, our data reveal that a Ser/Thr kinase, PINOID (PID), promotes photomorphogenic development. PID directly interacts with COP1 and phosphorylates it at the site of Ser20, thereby repressing the activity of COP1 in plants. Thus, our findings provide insight into the precise regulatory mechanism in maintaining appropriate COP1 activity in response to dynamically changing light conditions in plants.
Keywords: Arabidopsis, photomorphogenesis, phosphorylation, PID, COP1
Abstract
CONSTITUTIVELY PHOTOMORPHOGENIC 1 (COP1) plays crucial roles in various cellular processes via its E3 ubiquitin ligase activity in organisms, ranging from fungi to humans. As a key component in regulating various biological events, COP1 itself is precisely controlled at multiple layers. Here, we report a negative regulator of COP1, PINOID (PID), which positively mediates photomorphogenic development. Specifically, PID genetically and physically interacts with COP1 and directly phosphorylates COP1 at Ser20. As a result, this posttranslational modification serves to repress COP1 activity and promote photomorphogenesis. Our findings signify a key regulatory mechanism for precisely maintaining COP1 activity, thereby ensuring appropriate development in plants.
Plants have evolved a complicated but delicate regulation system for adapting to internal and external cues throughout their life cycle. Light, one of the key environmental factors in regulating various developmental processes in plants, is perceived mainly by multiple known photoreceptors, including phytochromes, cryptochromes, phototropins, and UVR8 (1–5). In response to light, Arabidopsis seedlings undergo photomorphogenic development with short hypocotyls and opened cotyledons, whereas skotomorphogenic processes occur with elongated hypocotyls, closed cotyledons, and apical hooks in the absence of light (6).
During the dark to light transition, CONSTITUTIVELY PHOTOMORPHOGENIC/DE-ETIOLATED/FUSCA (COP1/DET/FUS) play critical roles and act as key repressors of photomorphogenesis (7, 8). Of these, COP1 functions as an E3 ubiquitin ligase and targets ELONGATED HYPOCOTYL 5 (HY5), HYH, LAF1, HFR1, BBX21/STH2, BBX22/STH3, and PIL1 (9–17), as well as phytocromes (phyA–phyE) (18–20) for ubiqutination and degradation, thereby repressing photomorphogenesis in darkness. Accumulating evidence shows that COP1 is also involved in flowering, the circadian clock, and root development by ubiquitinating CO, ELF3, GI, and SCAR1 (21-23).
Mammalian COP1 is a key regulator of the tumor development; it targets tumor suppressor p53 as well as the oncogene c-jun, ETS for ubiquitination and degradation (24–27). Mammalian COP1 itself is tightly controlled through multiple regulatory mechanisms. In response to DNA damage, the ataxia telangiectasia mutated (ATM) protein kinase directly targets Ser387 of COP1 for phosphorylation, subsequently promoting COP1 self-ubiquitination and degradation (28). In the meanwhile, 14-3-3σ preferentially interacts with phosphorylated COP1 and facilitates its export from the nucleus to the cytoplasm (29). Thus, it appears that phosphorylation of COP1 is a key step in the inactivation of COP1 and regulation of COP1-mediated cellular processes in mammalian cells. However, the responsible kinase and a possible role of COP1 phosphorylation in Arabidopsis has remained unclear.
In this study, we show that Ser/Thr kinase PINOID (PID) phosphorylates COP1 and promotes photomorphogenesis. A point mutation in PID that leads to a premature stop code at Arg383 completely suppresses the drastically short hypocotyls of cop1-6 in darkness. Overexpression of PID transgenic seedlings exhibits a constitutively photomorphogenic phenotype with expanded cotyledons in the dark. In the light, pid mutant seedlings display elongated hypocotyls, whereas PID overexpressors show shorter hypocotyls compared with wild-type (WT) seedlings. The YFP-COP1 S20D transgenic plants show a larger hook unfolding angle in darkness and shorter hypocotyls in red light compared with WT, YFP-COP1, and YFP-COP1 S20A transgenic lines. Collectively, PID directly phosphorylates COP1 at Ser20 and represses its activity, which in turn serves to promote photomorphogenesis.
Results
Mutation in CSU3 Suppresses Constitutively Photomorphogenic Phenotype of cop1-6 in Darkness.
To explore factors involved in regulating COP1 and COP1-mediated processes, in previous work we performed a forward genetic screen for suppression of cop1-6 mutant phenotype in darkness (30). One recessive allele, cop1 suppressor 3 (csu3), was recovered from this screen. The hypocotyl length of the csu3 cop1-6 double mutant was similar to that of WT in the dark (Fig. 1 A and B). On phenotypic analysis, approximately 50% of csu3 cop1-6 plants exhibited three cotyledons at the seedling stage, and all of the csu3 cop1-6 adult plants displayed a pin-like phenotype, whereas none of the cop1-6 plants exhibited these abnormal phenotypes (Fig. S1 A–C). Although the cotyledon angle of csu3 cop1-6 was a little larger than that of WT, it was significantly smaller than that of the cop1-6 single mutant (Fig. 1 C and D). Moreover, the greening rate of csu3 cop1-6 on transfer from dark to white light was similar to that of WT, significantly higher than that of the cop1-6 single mutant (Fig. 1 E and F). Intriguingly, csu3 partially rescued the short hypocotyl length of the cop1-6 mutant in the continuous white (W), blue (B), red (R), and far-red (FR) light conditions tested (Fig. S1 D–K). Furthermore, the csu3 cop1-6 double mutant exhibited an intermediate adult phenotype between WT and the cop1-6 dwarf phenotype under the long-day condition (16-h light/8-h dark) at 8 wk (Fig. S1C). Collectively, these genetic results suggest that csu3 suppresses the phenotype of cop1-6 almost completely in the dark but only partially in the light.
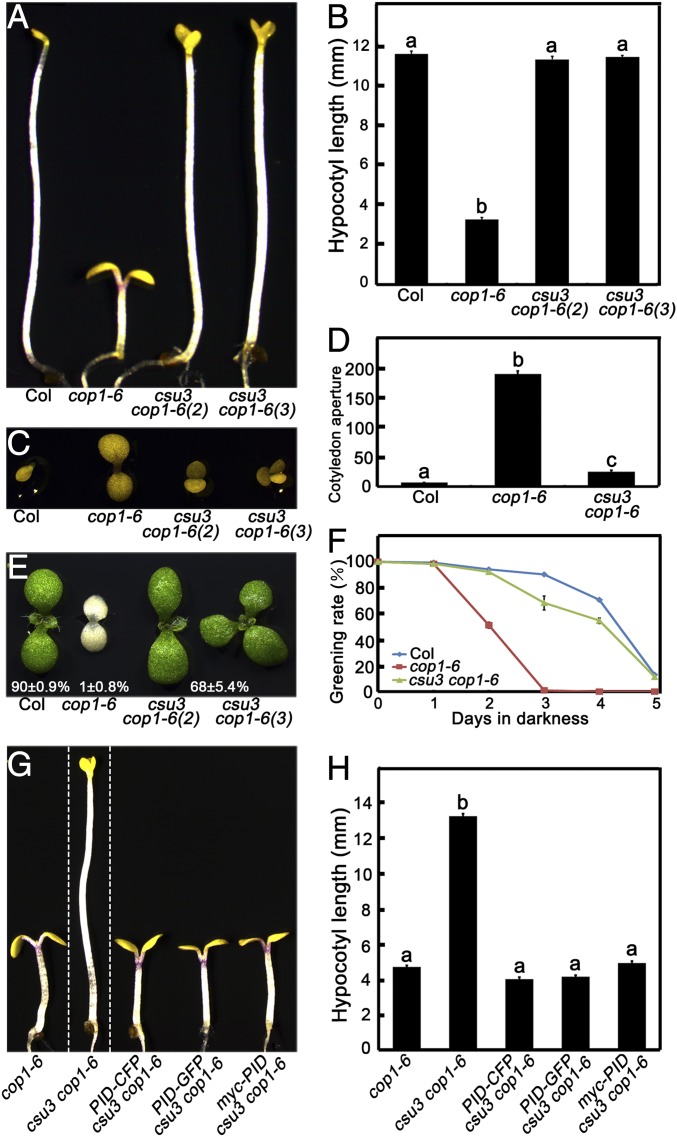
Fig. 1.
csu3 suppresses the cop1-6 mutant in darkness. (A and B) Hypocotyl phenotypes and lengths of Col, cop1-6, and csu3 cop1-6 mutant etiolated seedlings were grown in the dark for 5 d. csu3 cop1-6 (2) and csu3 cop1-6 (3) indicate csu3 cop1-6 seedlings with two and three cotyledons, respectively. Error bars represent SE (n ≥ 20). (C and D) Cotyledon phenotypes and angles (°) of Col, cop1-6, and csu3 cop1-6 mutant etiolated seedlings grown in darkness for 5 d. Error bars represent SE (n ≥ 25). (E and F) Cotyledon greening phenotypes and ratios of Col, cop1-6, and csu3 cop1-6 mutant etiolated seedlings grown in darkness for the indicated days and then transferred to W light for an additional 2 d. Error bars represent SE (n ≥ 100). (G and H) Complementation test, showing hypocotyl phenotypes and lengths of cop1-6, csu3 cop1-6, PID-CFP csu3 cop1-6, PID-GFP csu3 cop1-6, and myc-PID csu3 cop1-6 seedlings grown in the dark for 5 d. cop1-6, csu3 cop1-6, and various PID transgenic seedlings are separated by dotted lines. Error bars represent SE (n ≥ 20). In B, D, and H, letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post hoc analysis.
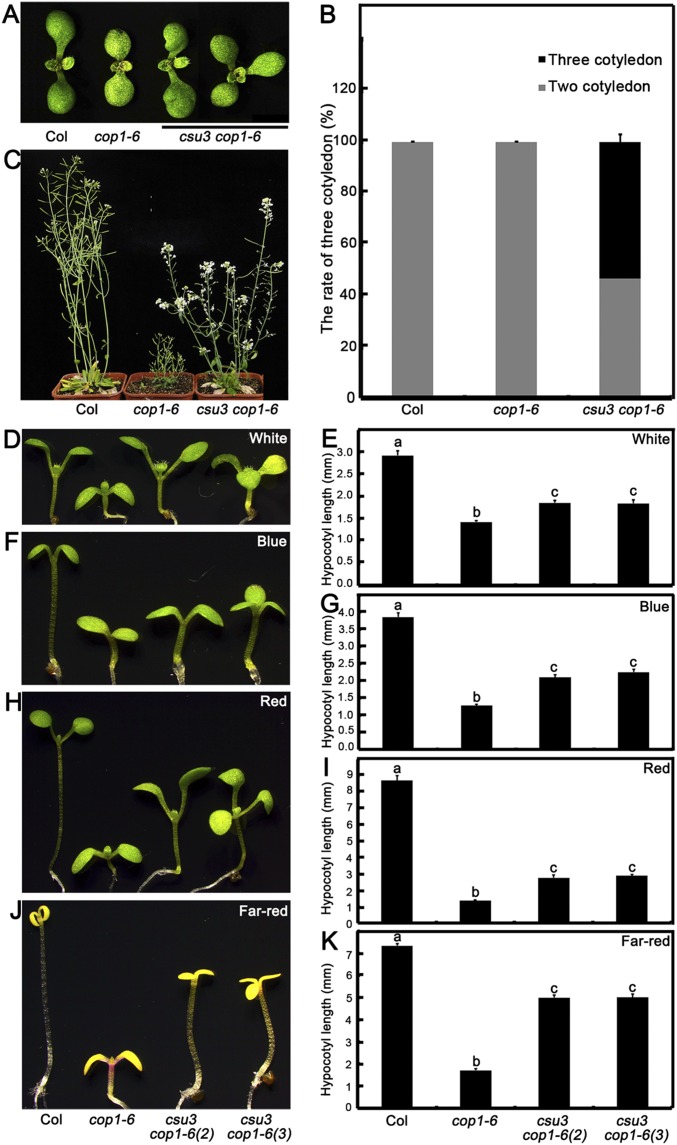
Fig. S1.
The csu3 cop1-6 mutant shows a three-cotyledon phenotype, and csu3 partially suppresses cop1-6 in the light. (A and B) The cotyledon phenotypes (A) and ratios of three cotyledons (B) of Col, cop1-6, and csu3 cop1-6 mutant seedlings grown under white light conditions for 5 d. (C) The adult phenotypes of Col, cop1-6, and csu3 cop1-6 mutant seedlings grown in long-day (16-h light/8-h dark) conditions for 52 d. (D–K) Hypocotyl phenotypes and lengths of 5-d-old Col, cop1-6, and pid-15 cop1-6 mutant seedlings grown under white (D and E), blue (F and G), red (H and I), and far-red (J and K) light conditions. Error bars represent SE (n ≥ 20). csu3 cop1-6 (2) and csu3 cop1-6 (3) indicate csu3 cop1-6 seedlings with two cotyledons and three cotyledons, respectively. In E, G, I, and K, letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post hoc analysis.
CSU3 Encodes Protein Kinase PINOID.
Considering that abnormal features of csu3 cop1-6 appear at both the seedling and adult stages, and that only pin-formed 1 (pin1) (31, 32), pinoid (pid) (33, 34), and monopteros (mp) (35, 36) have been reported to show these similar properties, we sequenced genomic sequences of PIN1, PID, and MP individually in csu3 cop1-6 and identified a C-to-T substitution at position 1453 from the start codon in the PID gene, causing a premature stop codon at the site of Arg383 (Fig. S2 A and B). Fourteen independent pid alleles (pid-1 to pid-14) have been identified and characterized previously (33, 34, 37), so csu3, which is a previously unidentified pid allele, has been designated pid-15.
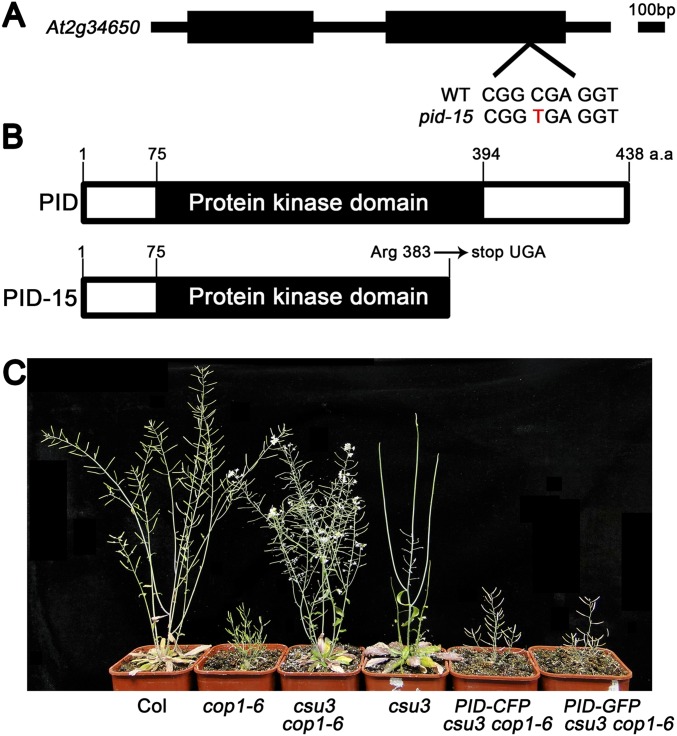
Fig. S2.
Identification of CSU3. (A and B) CSU3 encodes PINOID protein kinase. The gene structure of CSU3 is shown. Black blocks indicate exons, and the intron is represented by a black line. The point mutation in csu3/pid-15 is shown in red. (B) The protein structure of PID and PID-15. a.a., amino acids. (C) Complementation test. The adult phenotypes of Col, cop1-6, csu3 cop1-6, csu3, PID-CFP csu3 cop1-6, and PID-GFP csu3 cop1-6 were grown under long-day (16-h light/8-h dark) conditions for 8 wk.
To further confirm that the suppression of cop1-6 was indeed caused by the disruption of PID, we individually transformed PID-CFP, PID-GFP, and myc-PID into csu3 cop1-6 double mutant. PID-CFP csu3 cop1-6, PID-GFP csu3 cop1-6, and myc-PID csu3 cop1-6 displayed phenotypes resembling cop1-6 in the dark (Fig. 1 G and H). Moreover, the adult phenotypes of PID-CFP csu3 cop1-6 and PID-GFP csu3 cop1-6 plants were indistinguishable from those of cop1-6 (Fig. S2C). These results suggest that a functional PID gene indeed complements the phenotype conferred by the pid-15 mutation in cop1-6.
PID Is a Positive Regulator of Light Signaling.
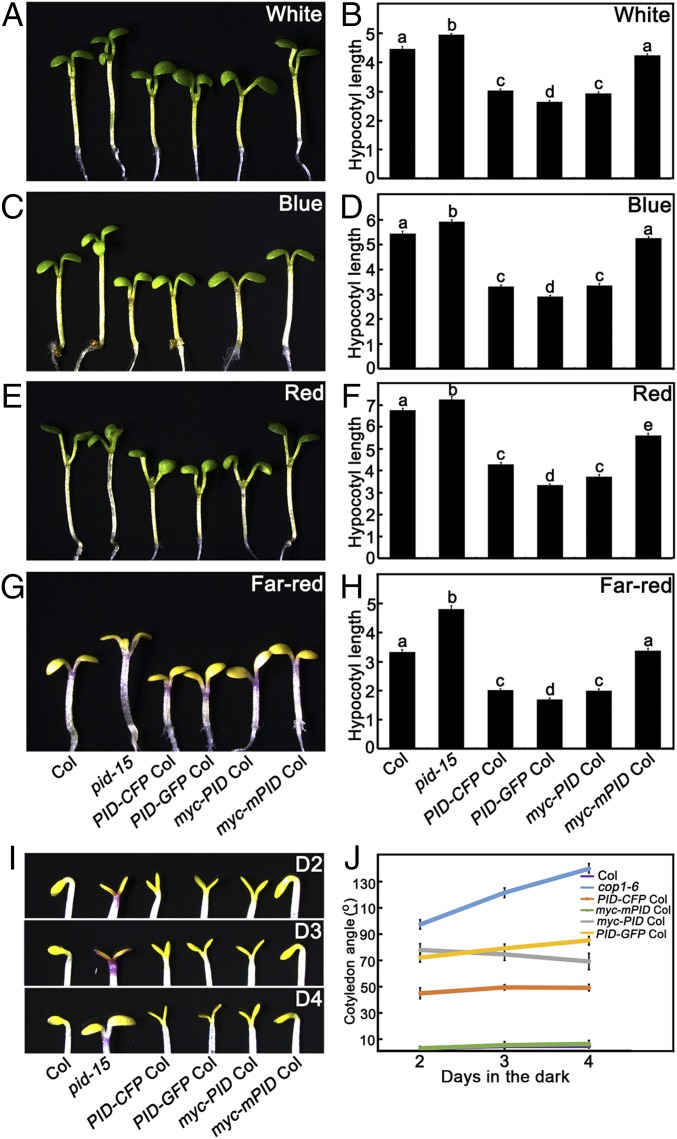
To illustrate the role of PID in light signaling, we isolated pid-15 single mutant from F2 generation of pid-15 cop1-6 crossed with WT (Col). However, the homozygous pid-15 mutant was nearly sterile, barely producing seeds (Fig. S2C), indicating that pid-15 was a strong allele. We then maintained the pid-15 in a heterozygous state. We generated transgenic lines PID-CFP, PID-GFP, myc-PID, and myc-mPID in the Col background (Fig. S3 A–E). Like other pid alleles (33, 34), a majority of homozygous pid-15 showed three cotyledons at the seedling stage. We measured the hypocotyl length of pid-15 with three cotyledons and found that they developed longer hypocotyls in the W, B, R, and FR light conditions tested (Fig. 2 A–H). In contrast, the hypocotyls of PID-CFP, PID-GFP, and myc-PID transgenic lines were shorter than WT under those light conditions (Fig. 2 A–H). In the dark, PID-CFP, PID-GFP, and myc-PID transgenic seedlings did not show any significant difference in hypocotyl length compared with WT (Fig. S3 F and G); however, they displayed obviously opened cotyledons mimicking the cop1 mutant phenotype in darkness (Fig. 2 I and J). Overexpression of myc-mPID (Fig. S3 C and E), which shows defects in kinase activity (38), exhibited phenotypes resembling WT (Fig. 2). Taken together, these findings suggest that PID functions as a positive regulator of photomorphogenesis in Arabidopsis.
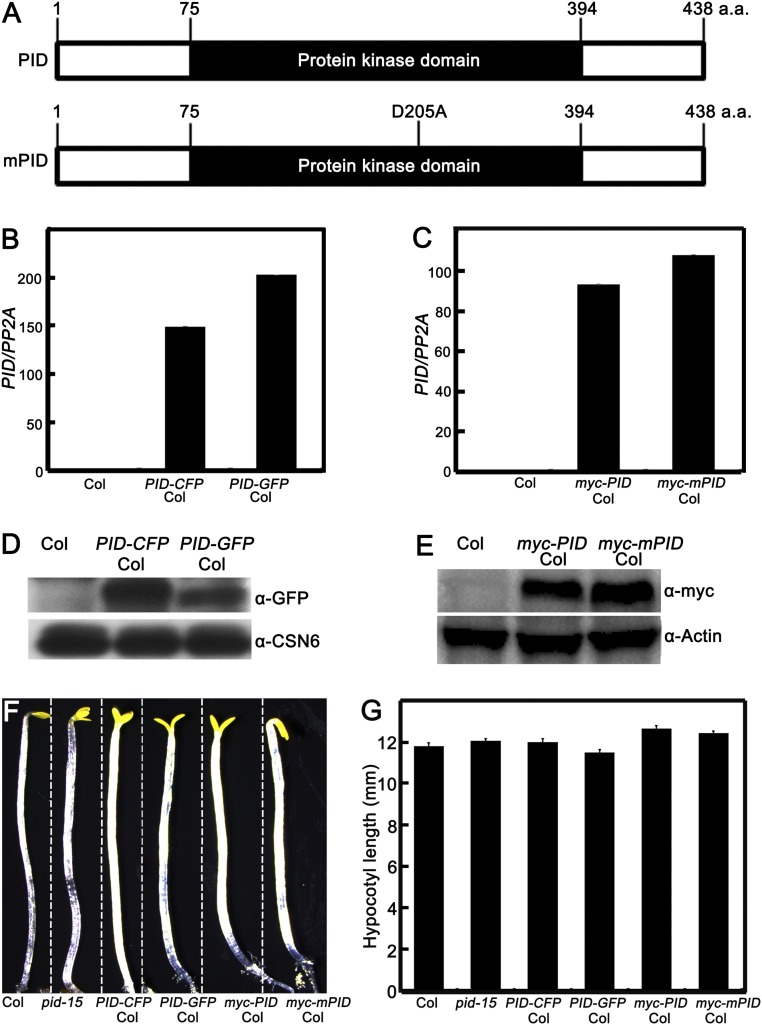
Fig. S3.
PID or mutated PID gene and protein expression levels in transgenic lines. (A) Protein structure of PID and mutated PID (mPID). The site of D205 was substituted by A, which locates at the ATP binding center in PID kinase domain. (B and C) PID mRNA expression levels in transgenic lines. Quantitative real-time PCR analysis shows the expression of PID in Col, PID-CFP Col, PID-GFP Col, myc-mPID Col, and myc-PID Col seedlings. (D and E) Immunoblot analysis showing the protein levels of PID-CFP, PID-GFP, myc-mPID, and myc-PID in the Col, PID-CFP Col, PID-GFP Col, myc-mPID Col, and myc-PID Col seedlings, respectively. α-GFP monoclonal antibodies were used to detect the PID-CFP and PID-GFP, and α-myc monoclonal antibodies were used to detect the myc-mPID and myc-PID. CSN6 and tubulin served as loading controls. (F and G) Hypocotyl lengths are comparable among Col, pid mutant, and PID transgenic lines in darkness. Shown are hypocotyl phenotypes (F) and lengths (G) of 5-d-old Col, pid-15, PID-CFP Col, PID-GFP Col, myc-PID Col, and myc-mPID Col seedlings grown in darkness. Error bars represent SE (n ≥ 20). In F, Col, pid-15, and various transgenic lines are separated by dotted lines.
Fig. 2.
Overexpression of PID is hypersensitive to light and results in expanded cotyledons in the dark. (A–H) Hypocotyl phenotypes and lengths of Col, cop1-6, pid-15, PID-CFP Col, PID-GFP Col and pid-15 cop1-6 seedlings grown under white (A and B), blue (C and D), red (E and F), and far-red (G and H) light conditions for 5 d. Error bars represent SE (n ≥ 20). (I and J) The cotyledon apertures of Col, cop1-6, PID-CFP Col, PID-GFP Col, myc-PID Col, and myc-mPID Col transgenic seedlings grown in darkness for 2 d (D2), 3 d (D3), or 4 d (D4). Error bars represent SE (n ≥ 20). In B, D, F, and H, letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post hoc analysis.
PID Physically Interacts with COP1.
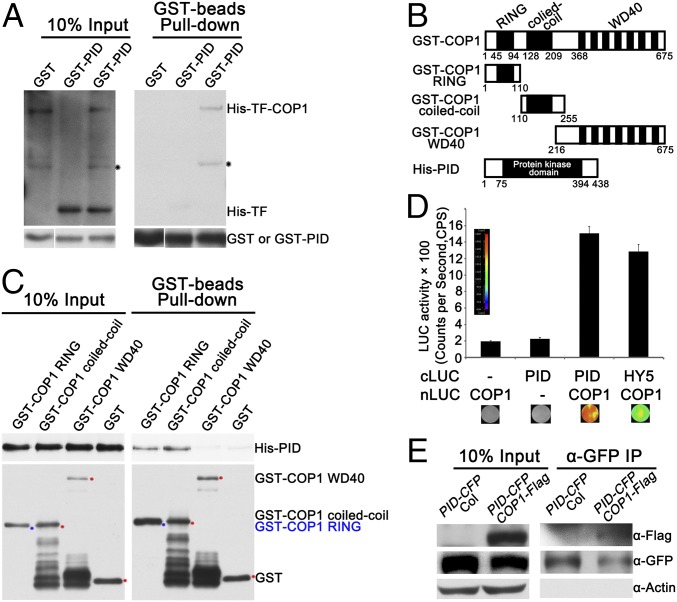
Given that the strong genetic interaction between PID and COP1, we tested the protein–protein interaction between PID and COP1. We performed pull-down assays using recombinant His-Trigger Factor (TF)-COP1 and GST-PID expressed in Escherichia coli strain BL21 (DE3) host strains. Neither His-TF-COP1 nor His-TF could be pulled down by GST; however, His-TF-COP1, but not His-TF alone, was pulled down by GST-PID (Fig. 3A). To map the domain of COP1 required for interacting with PID, we continued the pull-down assays using recombinant GST-fused three truncation fragments of COP1 and His-PID (Fig. 3B). Either GST-COP1 RING or GST-COP1 coiled-coil alone could pull down His-PID, whereas GST-COP1 WD40 and GST could not (Fig. 3C).
Fig. 3.
PID physically interacts with COP1. (A) Pull-down assay showing that GST-PID pulled down His-TF-COP1, but not His-TF. Pull-down assays were performed using GST, GST-PID, His-TF, and His-TF-COP1. His-TF and His-TF-COP1 proteins were detected by immunoblotting using anti-His antibodies. GST and GST-PID proteins were detected using anti-GST antibodies. Black stars indicate nonspecific bands. (B) Schematics of various GST-COP1 truncated fragments and His-PID. (C) The RING-finger and coiled-coil domains of COP1 pulled down His-PID. The in vitro pull-down assay was performed using GST or various truncated COP1 fragments fused with GST to pull down His-PID. Bound and 10% of input His-PID fractions were detected by immunoblotting using anti-His antibodies. Immobilized GST and GST-COP1 fusion proteins were detected with anti-GST antibodies. The GST and GST-COP1 truncated protein bands are indicated by red and blue stars, respectively. (D) Firefly LCI assay showing COP1 interacting with PID in tobacco leaf cells. The PID-cLUC and COP1-nLUC constructs were transiently coinfiltrated in tobacco leaves, and luminescence intensity was detected using LB985 NightSHADE. HY5-cLUC and COP1-nLUC served as positive controls. (E) Co-IP assay showing COP1 interacting with PID in vivo. Here 4-d-old white light-grown PID-CFP Col and COP1-Flag PID-CFP transgenic seedlings were transferred to darkness for 24 h and then subjected to co-IP using anti-GFP agarose, with the immunoprecipitates detected using anti-Flag and anti-GFP antibodies, respectively. Actin served as a negative control.
Next, we further verified the PID–COP1 interaction using firefly luciferase complementation imaging (LCI) assays in tobacco leaves. Agrobacterium-mediated transient expression with cLUC-PID and COP1-nLUC were coexpressed in Nicotiana benthamiana leaves, and high luciferase activity could be readily detected after the addition of luciferin (Fig. 3D); however, no luciferase activity was detected when coexpressed with cLUC and COP1-nLUC or cLUC-PID and nLUC (Fig. 3D). Moreover, in coimmunoprecipitation (co-IP) assays using transgenic seedlings carrying both PID-CFP and COP1-Flag, immunoprecipitation of PID-CFP pulled down COP1-Flag in PID-CFP and COP1-Flag transgenic lines (Fig. 3E), demonstrating that PID associates with COP1 in vivo. Taken together, these results suggest that PID physically interacts with COP1, and that both COP1 RING-finger and coiled-coil domains are responsible for the COP1–PID interaction.
PID Phosphorylates COP1 Directly in Vitro.
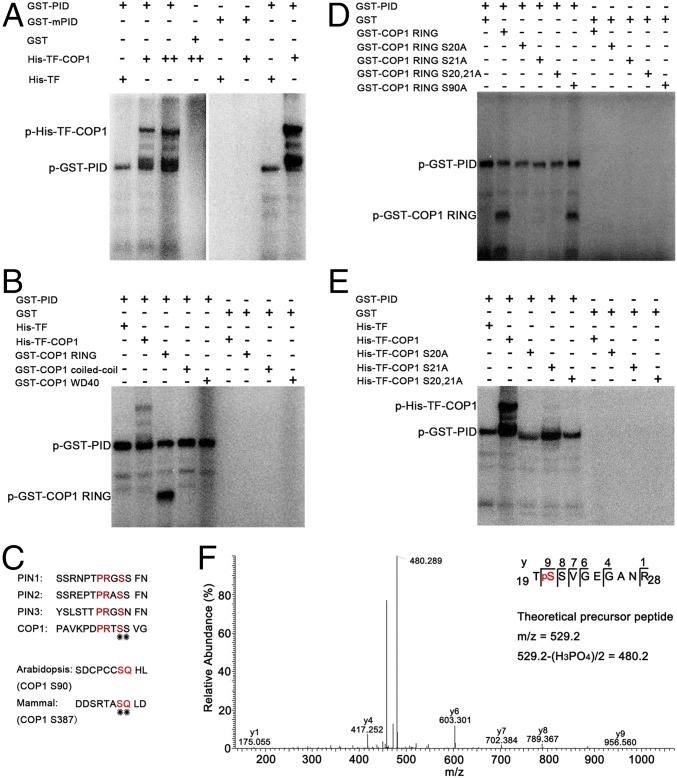
As a Ser/Thr kinase, PID directly targets PIN1 on Ser231, Ser252, and Ser290 for phosphorylation and modulates its polarization activity (37). Considering that PID has kinase activity and physically interacts with COP1, we suspected that PID might target COP1 for phosphorylation. To explore this possibility, we performed in vitro phosphorylation assays using recombinant GST-PID and His-TF-COP1. Consistent with previous studies (34, 39), PID exhibited autophosphorylation activity in vitro. Phosphorylated His-TF-COP1 was detected when GST-PID was added in the reactions. As the amount of His-TF-COP1 increased, more phosphorylated His-TF-COP1 was observed. The negative control His-TF was not phosphorylated by GST-PID (Fig. 4A). GST-mPID, which carries an amino acid substitution (D205A) in its ATP-binding center lacking its autophosphorylation activity (38), could not phosphorylate His-TF-COP1 (Fig. 4A). These findings suggest that PID is able to phosphorylate COP1 in vitro.
Fig. 4.
PID targets Ser20 on COP1 for phosphorylation in vitro. (A) In vitro protein phosphorylation assay showing GST-PID efficiently phosphorylated His-TF-COP1. (B) PID phosphorylated the RING-finger domain of COP1. The numbers indicate the amino acid residues of COP1. (C) Predicted phosphorylation sites on Arabidopsis COP1. (D and E) PID phosphorylated the Ser20 and/or Ser21 of COP1 in an in vitro kinase assay. (F) Collision-induced dissociation mass spectrum clearly showing that the Ser20 on COP1 was phosphorylated by PID. The proteins were separated by SDS/PAGE and purified by PHOS-Select Iron Affinity Gel, then subjected to MS/MS spectrometric analyses.
To map the phosphorylation sites on COP1, we used COP1 truncation fragments fused with GST (Fig. 3B) to conduct in vitro phosphorylation assays. As shown in Fig. 4B, GST-PID could phosphorylate COP1 RING, but not COP1 coiled-coil and WD40, implying that the phosphorylation sites for PID reside within the COP1 RING-finger domain. To further identify the exact phosphorylation site(s) of COP1, we analyzed the COP1 RING-finger amino acid sequence using the NetPhos program (NetPhos software prediction score >0.5) (40) and identified two potential putative phosphosites with high prediction scores, Ser20 and Ser21. It has been shown that PID phosphorylates PINs by a central serine residue within the highly conserved TPRXS (N/S) motif (37). By analyzing the COP1 RING-finger domain amino acids, we identified one conserved phosphorylation motif (DPRTSS) for PID in this region (Fig. 4C). In addition, the phosphorylation site on mammalian COP1 is Ser387-locating in the serine-glutamine (SQ) motif (28).
The Ser90 in Arabidopsis COP1 RING-finger domain is the sole potential SQ motif within this region (Fig. 4C); therefore, we substituted Ser-to-Ala at these three sites (S20A, S21A, and S90A) alone or together and conducted in vitro phosphorylation assays. GST-PID was not able to phosphorylate COP1 RING S20A, COP1 RING S21A, and COP1 RING S20, 21A, but could phosphorylate COP1 RING S90A in vitro (Fig. 4D). Consistently, GST-PID could not phosphorylate full-length COP1 carrying a substitution at either S20A or S21A (Fig. 4E). Either S20A or S21A substitution might affect COP1 conformation and phosphorylation by PID in vitro; thus, we performed LC-MS/MS analysis on the recombinant COP1 by adding purified GST-PID in the phosphorylation reaction to identify the exact phosphosite. A tryptic peptide (TSSVGEGANR) derived from the predicted phosphorylation motif was found in a phosphorylated state. On analysis of six other tryptic peptides, only Ser20, but not Ser21, was unambiguously identified to be phosphorylated (Fig. 4F). Taken together, these findings support the idea that PID targets COP1 for phosphorylation, and that the phosphorylation site on COP1 is Ser20.
PID Promotes the Degradation of PIF3.
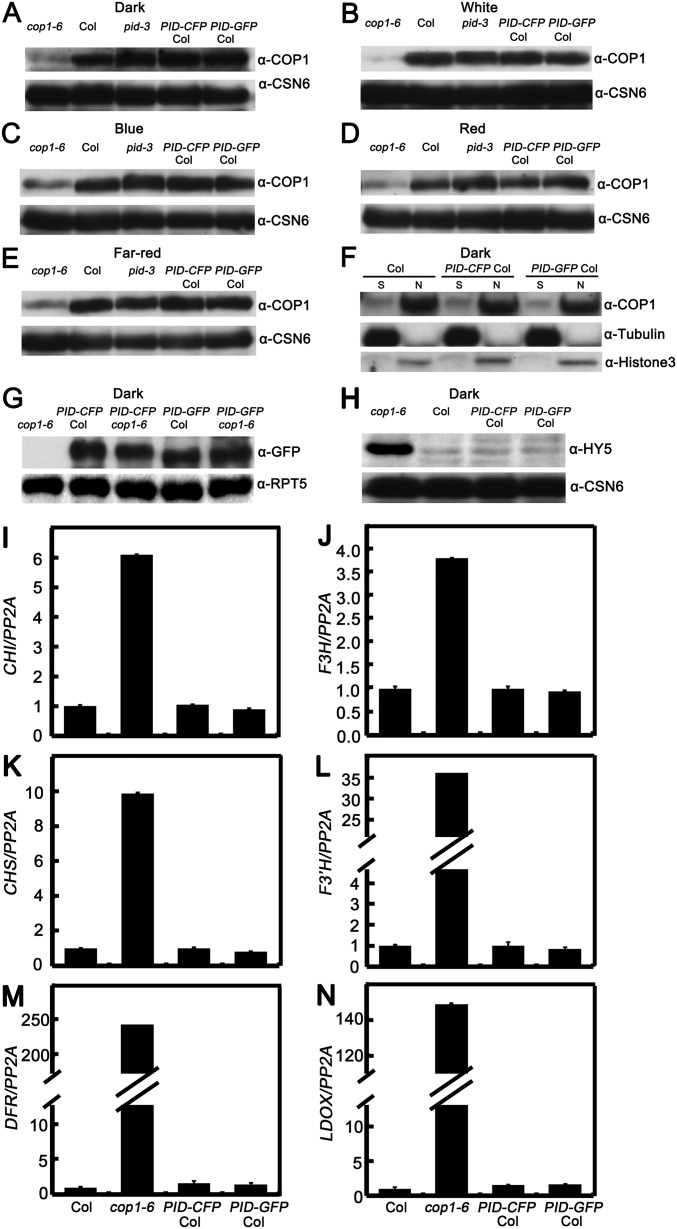
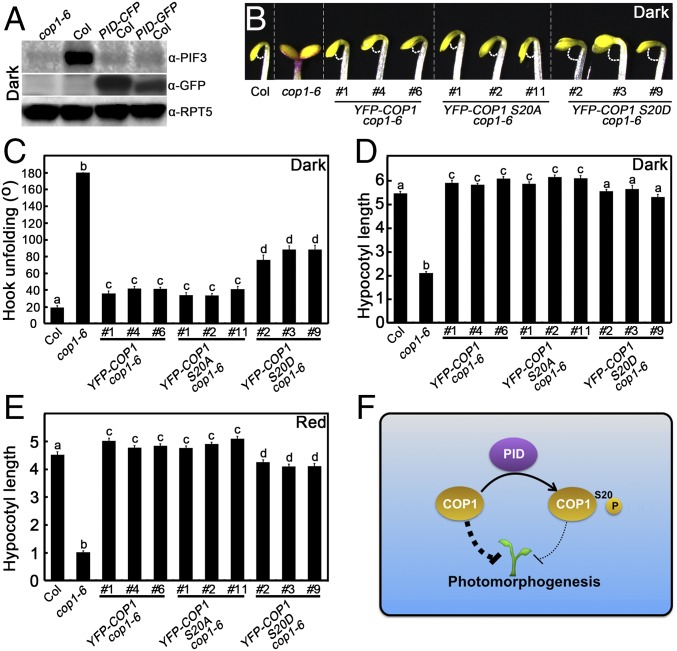
To further explore the function of PID in phosphorylating COP1, we detected the abundance of COP1 in Arabidopsis seedlings. The amount of COP1 was comparable in WT and PID-overexpressing transgenic lines grown in various light conditions (dark and W, B, FR, and R) (Fig. S4 A–E). In addition, COP1 was found to localize in the nucleus in both WT and PID transgenic lines grown in the dark (Fig. S4F). These results indicate that PID might have no effect on the COP1 protein level and its partitioning between the nucleus and the cytoplasm. To explore whether COP1 regulates PID, we introgressed the PID-CFP and PID-GFP into the cop1-6 background by genetic crossing. As shown in Fig. S4G, the PID protein levels in the cop1-6 background were not markedly different from those in the Col background, indicating that COP1 might not affect the PID abundance.
Fig. S4.
Protein levels of COP1 in Col, pid-3, and PID transgenic lines. (A–E) Immunoblots analysis showing the COP1 protein levels in Col, pid-3, PID-CFP Col, and PID-GFP Col transgenic seedlings grown in the dark (A) and in white (B), blue (C), red (D), and far-red (E) light. CSN6 served as a loading control. (F) Biochemical detection of COP1 localization in the cytoplasm and nucleus. The nuclear proteins were extracted from seedlings grown in darkness for 5 d. N, nuclear protein; S, soluble fraction, cytoplasmic protein. Immunoblotting was performed using anti-COP1, anti-histone 3, and anti-tubulin antibodies. (G) Immunoblot analysis showing the PID protein levels in various transgenic lines. PID protein levels in cop1-6, PID-CFP Col, PID-GFP Col, PID-CFP cop1-6, and PID-GFP cop1-6 seedlings grown in darkness as detected by GFP antibodies. Plant total proteins were extracted from 5-d-old seedlings grown in the dark. cop1-6 served as a negative control. RPT5 protein served as a loading control. (H) HY5 abundance in Col, cop1-6, PID-CFP Col, and PID-GFP Col seedlings as detected by HY5 antibodies. Plant total proteins were extracted from 5-d-old seedlings grown in the dark. CSN6 protein levels served as loading controls. (I–N) Quantitative real-time PCR analysis showing HY5-regulated gene expression in Col, cop1-6, PID-CFP Col, and PID-GFP Col seedlings. Plant total RNA was extracted from 2.5-d-old seedlings grown in the dark. Gene expression levels were normalized to the PP2A gene.
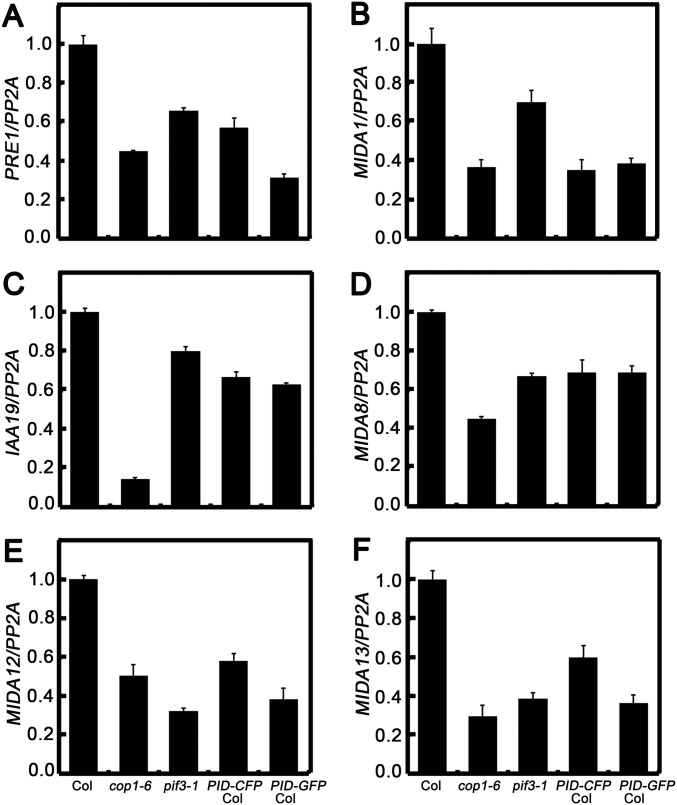
We next investigated whether PID affects the biochemical activity of COP1. In darkness, COP1 destabilizes HY5 (10) but stabilizes PIF3 (41). Likely owing to the already extremely low activity of HY5 and the requirement of light triggered event, HY5 protein abundance and HY5-regulated gene expression were not altered in PID transgenic seedlings grown in darkness (Fig. S4 H–N); however, PIF3 protein level was dramatically reduced in the dark-grown PID transgenic lines (Fig. 5A). In addition, PIF3-controlled gene expression was changed accordingly in the dark-grown PID-overexpressing transgenic lines (Fig. S5). These results suggest that PID negatively regulates the abundance of PIF3.
Fig. 5.
Phenotypic analysis of YFP-COP1 S20A cop1-6 and YFP-COP1 S20D cop1-6 transgenic seedlings. (A) PIF3 and PID protein levels in cop1-6, Col, PID-CFP Col, and PID-GFP Col seedlings grown in darkness as detected by PIF3 and GFP antibodies. Plant total proteins were extracted from 5-d-old seedlings grown in the dark. cop1-6 served as a negative control. RPT5 protein levels were used as loading controls. (B–D) Hook phenotypes (B), unfolding angles (C), and hypocotyl lengths (D) of Col, cop1-6, YFP-COP1 cop1-6, YFP-COP1 S20A cop1-6, and YFP-COP1 S20D cop1-6 seedlings grown in darkness for 2 d. Error bars represent SE (n ≥ 20). (E) Hypocotyl lengths of Col, cop1-6, YFP-COP1 cop1-6, YFP-COP1 S20A cop1-6, and YFP-COP1 S20D cop1-6 plants grown in red light (58.7 μmol·m−2·s−1) for 4 d. Error bars represent SE (n ≥ 20). (F) A working model showing how PID mediates in maintaining appropriate COP1 activity. PID directly phosphorylates COP1 on Ser20 to repress its activity, which in turn serves to precisely control COP1 activity to ensure normal plant development. In B, C, and D, letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post hoc analysis.
Fig. S5.
PIF3-controlled gene expression in Col, cop1-6, and PID transgenic lines. Quantitative real-time PCR analysis shows PIF3-regulated gene expression in Col, cop1-6, pif3-1, PID-CFP Col, and PID-GFP Col seedlings. Plant total RNA was extracted from 2.5-d-old seedlings grown in the dark. Both cop1-6 and pif3-1 served as negative controls. Gene expression levels were normalized to the PP2A gene.
Phosphomimic COP1 Exhibits Reduced Biological Activity in Arabidopsis.
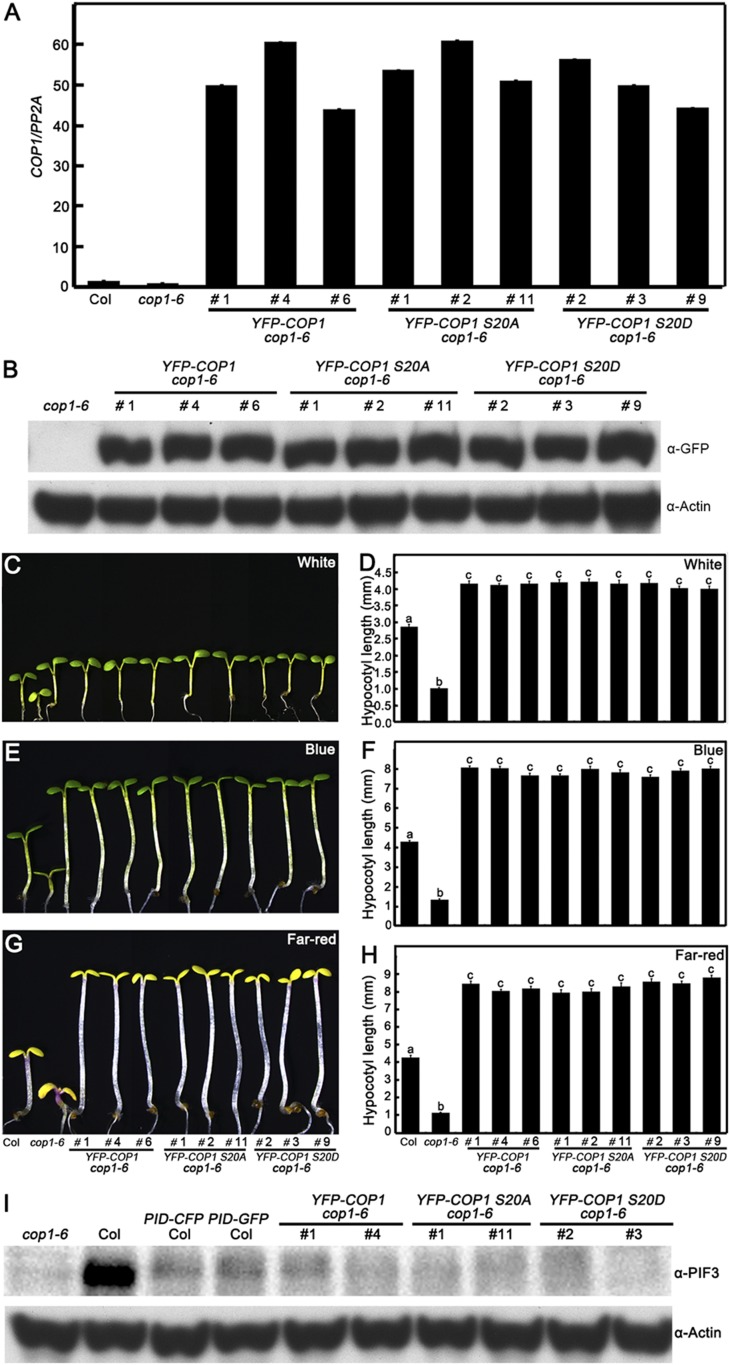
To investigate the biological significance of the Ser20 of COP1 in regulating plant development, we transformed the 35S promoter-drived WT (YFP-COP1), nonphosphorylatable (YFP-COP1 S20A), and phosphomimic (YFP-COP1 S20D) forms of COP1 into a cop1-6 mutant background. The various independent YFP-COP1 cop1-6, YFP-COP1 S20A cop1-6, and YFP-COP1 S20D cop1-6 transgenic lines, in which WT or mutated COP1 were overexpressed (Fig. S6 A and B), displayed an etiolated phenotype in darkness (Fig. 5), indicating that YFP-COP1, YFP-COP1 S20A, and YFP-COP1 S20D transgenes are largely functional and can rescue the cop1-6 short hypocotyls in darkness. However, the hook unfolding angles of YFP-COP1 cop1-6 and YFP-COP1 S20A cop1-6 were significantly smaller compared with those in YFP-COP1 S20D transgenic plants in darkness (Fig. 5 B and C). YFP-COP1 S20D transgenic seedlings developed shorter hypocotyls compared with YFP-COP1 cop1-6 and YFP-COP1 S20A cop1-6 seedlings in the dark and R light conditions (Fig. 5 D and E), but not in the W, B, and FR light conditions (Fig. S6 C–H). These results indicate that phosphomimic or phosphorylated COP1 has reduced activity in regulating photomorphogenesis in planta.
Fig. S6.
COP1 or mutated COP1 gene and protein levels in various independent transgenic lines. (A) COP1 mRNA expression level. Quantitative real-time PCR analysis shows the expression of COP1 transcript levels in Col, cop1-6, YFP-COP1 cop1-6, YFP-COP1 S20A cop1-6, and YFP-COP1 S20D cop1-6 transgenic lines. (B) Immunoblot analysis showing the protein levels of YFP-COP1, YFP-COP1 S20A, and YFP-COP1 S20D in cop1-6, YFP-COP1 cop1-6, YFP-COP1 S20A cop1-6, and YFP-COP1 S20D cop1-6 transgenic lines. Anti-GFP monoclonal antibodies were used to detect the YFP-COP1, YFP-COP1 S20A, and YFP-COP1 S20D. Actin served as a loading control. (C–H) YFP-COP1 cop1-6, YFP-COP1 S20A cop1-6, and YFP-COP1 S20D cop1-6 transgenic seedlings display elongated hypocotyls in the light. The hypocotyl phenotypes and lengths of Col, cop1-6, YFP-COP1 cop1-6, YFP-COP1 S20A cop1-6, and YFP-COP1 S20D cop1-6 seedlings grown under white (C and D), blue (E and F), and far-red (G and H) light for 5 d. Error bars represent SE (n ≥ 20). In D, F, and H, letters above the bars indicate significant differences (P < 0.05) as determined by one-way ANOVA with Tukey’s post hoc analysis. (I) Immunoblot analysis showing the PIF3 protein levels in cop1-6, Col, PID-CFP Col, PID-GFP Col, YFP-COP1 cop1-6, YFP-COP1 S20A cop1-6, and YFP-COP1 S20D cop1-6 seedlings grown in darkness as detected by PIF3 antibodies. Plant total proteins were extracted from 5-d-old seedlings grown in the dark. cop1-6 served as a negative control. Actin protein level served as a loading control.
Discussion
Extensive studies have demonstrated that COP1 acts as a central regulator in multiple developmental processes in plants via its E3 ubiquitin ligase activity (7, 8). Using a forward genetic screen, we have identified PID as a negative regulator of COP1. Biochemical studies showed that PID directly targets COP1 Ser20 for phosphorylation. Overexpression of PID in Arabidopsis led to shortened hypocotyls in the light and expended cotyledons in darkness. Phosphomimic COP1 exhibited lower activity in the regulation of photomorphogenesis in planta. These results suggest a critical role for PID in modulating COP1 activity and promoting photomorphogenic development.
cop1-6 is a weak mutant, not a null mutant, that produces a functional COP1-6 mutant protein at a lower level (30). The pid-15 mutant might work by enhancing the activity of a functional COP1-6 to promote effective suppression. This might explain the finding that PID regulates COP1 activity through phosphorylation, but pid-15 is epistatic to cop1-6 (Fig. 1). The pid-15 single mutant develops elongated hypocotyls (Fig. 2), whereas pid-15 cop1-6 shows an intermediate phenotype in hypocotyls in various light conditions (Fig. S1), indicating that COP1 and PID may work synergistically in regulating photomorphogenesis. PID directly targets COP1 at the site of Ser20 for phosphorylation (Fig. 4), and COP1 does not regulate PID abundance (Fig. S4G), implying that PID-COP1 might not provide feedback regulation for PID stability.
COP1 stabilizes PIF3 in the dark, as demonstrated by the significantly lower PIF3 protein levels in the absence of COP1 (41) (Fig. 5A). YFP-COP1, YFP-COP1 S20A, and YFP-COP1 S20D could not rescue the PIF3 protein levels in the cop1-6 background (Fig. S6I), whereas they completely complemented the constitutively photomorphogenic phenotype of the cop1-6 mutant (Fig. 5 A–C), suggesting that the etiolated phenotype conferred by these transgenes in cop1-6 is largely independent of PIF3. There are several possible explanations for these observations: (i) degradation of PIF3 by PID might occur in a COP1-independent manner; (ii) phosphorylation of COP1 by PID might affect the abundance, activity, or action of an unidentified target rather than PIF3; and (iii) it is possible that YFP-COP1, YFP-COP1 S20A, and YFP-COP1 S20D transgenes lack the ability to rescue PIF3 abundance in the cop1-6 background.
Either loss or gain of COP1 function leads to abnormal phenotypes from the seedling stage to the adult stage (42–45), indicating that appropriate COP1 abundance and activity are essential for normal plant development. Recent studies have revealed that the precise abundance and activity of COP1 is under the tight control of various factors through multiple mechanisms (30, 46–48). COP1 is evolutionally conserved from plants to human. Mammalian COP1 has E3 ubiquitin ligase activity and targets various downstream substrates for ubiquitination as well. In response to DNA damage, activated ATM directly phosphorylates mammalian COP1 at the site of Ser387 (28), and subsequently triggers its self-ubiquitination and degradation as well as 14-3-3σ–mediated nucleus exportation (29). In Arabidopsis, PID directly targets COP1 Ser20 for phosphorylation (Fig. 4); however, unlike phosphorylated mammalian COP1, phosphorylated Arabidopsis COP1 likely is not required for self-ubiquitination, self-degradation, and nucleocytoplasmic repartitioning, given that overexpression of PID did not affect COP1 abundance or nuclear localization pattern (Fig. S4 A–F). PID-mediated phosphorylation of COP1 promotes photomorphogenesis, at least in part by repressing the COP1 activity. It appears that COP1 activity is precisely modulated via multiple regulatory mechanisms. PIF1, CSU2, SIZ1, and PID are all involved in the regulation of COP1 activity through distinct mechanisms (46–48). Consistently, PID transgenic plants mimicked the cop1 phenotype with respect to cotyledons in darkness and exhibited shorter hypocotyls in the light (Fig. 2). YFP-COP1 S20D, which is a phosphomimic form, showed reduced activity in Arabidopsis (Fig. 5 B–E). However, it appears that PID-mediated phosphorylation is only partially responsible for the repression of COP1 activity, considering that neither PID nor YFP-COP1 S20D cop1-6 transgenic seedlings fully resembled the cop phenotype in either the dark or the light (Figs. 2 and 5 B–E).
Phosphorylation of phytochromes results in the attenuation of phytochrome signaling in plants. Phosphorylation of phyA Ser598 disrupts the interaction with its downstream partners and leads to attenuating phyA signal (49). Light triggers phosphorylation of phyB Tyr104 and represses its activity (50). Similarly, PID directly phosphorylates COP1 on Ser20 and negatively regulate the ubiquitin ligase activity of COP1 to tightly modulate seedling photomorphogenesis. Phosphorylation of COP1 on Ser20 is likely a key mechanism in the precise control of COP1 E3 ubiquitin ligase activity (Fig. 5F). PIFs are phosphorylated at multiple sites in a light-dependent manner before their ubiquitination and degradation (51–53). Thus, it is likely that phosphorylation modification is a critical strategy in the control of appropriate photomorphogenic development in plants through distinct mechanisms.
Materials and Methods
Plant Materials and Growth Conditions.
The cop1-6 (44), hy5-215 (54), pid-15, and pid-15 cop1-6 (this study) seedlings are of the Columbia (Col) ecotype. Seeds were surface-sterilized for 10 min with 30% commercial Chlorox bleach and washed three times with sterile water, then sown on solid Murashige and Skoog medium supplemented with 0.4% Bacto-agar (Difco) and 1% sucrose for phenotypic analysis and biochemical assays. The plates were stratified at 4 °C for 3 d in darkness and then transferred to continuous W light (91 μmol·m−2·s−1) and maintained at 22 °C for 8 h. Then the seeds were incubated in dark and in R (14.7 μmol·m−2·s−1), FR (1.205 μmol·m−2·s−1), B (1.57 μmol·m−2·s−1) and W (15.75 μmol·m−2·s−1) light for phenotypic analysis. The light intensity was measured with a LI-COR LI-250 light meter.
In Vitro Kinase Assays.
Approximately 2 μg of purified protein were added into the kinase reaction mix (30 μL total volume), containing 1× kinase buffer (25 mM Tris⋅HCl pH 7.5, 1 mM DTT, and 5 mM MgCl2) and 1× ATP solution (100 μM MgCl2/ATP and 2 μCi γ-[32P]ATP). The reaction mixtures were incubated at 30 °C for 30 min, the reaction was terminated by the addition of 6 μL of 5× SDS loading buffer, and the mixtures were then boiled at 100 °C for 5 min and separated over 10% SDS/PAGE gels. The gels were dried with a gel dryer (model 583; Bio-Rad) and subsequently exposed to a phosphor screen, and signals were detected with an Amersham Typhoon FLA 7000 phosphor imager (GE Healthcare). Coomassie Brilliant Blue R 250 stain was used for SDS/PAGE.
Details of the experimental procedures for plasmid construction, genomic complementation testing, mass spectrometry, immunoblot analysis, firefly LCI analysis, in vitro pull-down analysis, measurement of hypocotyl length and cotyledon aperture, and quantitative real-time PCR assays are provided in SI Materials and Methods.
SI Materials and Methods
Plasmid Construction.
To generate pEarleyGateway-PID-CFP, pEarleyGateway-PID-GFP, pEarley Gateway-myc-PID, and pEarleyGateway-myc-mPID constructs, the full-length PID ORF with attB sites was amplified from Col cDNA. The mutated full-length PID D205A (mPID) coding sequence was amplified using a site-directed mutagenesis method. The target fragments were cloned into the gateway vector pDONR-221 using BP Clonase (Invitrogen). The destination fragments then were introduced into the plant pEarleyGateway 102, pEarleyGateway 103, and pEarleyGateway 203 binary vectors (55) using LR Clonase (Invitrogen).
COP1 RING-finger, COP1 coiled-coil, and COP1 WD40 domains were amplified from full-length COP1 and cloned into pGEX-4T-1 with EcoRI and XhoI. GST-COP1 RING S20A, GST-COP1 RING S21A, GST-COP1RING S20 and 21A, and GST-COP1 RING S90A were constructed with the template of GST-COP1 RING by site-directed mutagenesis method. pCold-His-TF-COP1 and pCambia1307-COP1-Flag constructs have been described previously (47). COP1S20A, COP1S21A, and COP1S20, 21A were introduced into the pCold-His-TF vector with KpnI and PstI. COP1 S20A, COP1 S21A, and COP1S20 and 21A were cloned into pDONR-221 by BP reaction and then introduced into pEarleyGateway 104 using LR Clonase.
Measurement of Hypocotyl Length and Cotyledon Aperture.
Seeds were surface-sterilized for 10 min and kept at 4 °C for 3 d for stratification, and then incubated in continuous white light for 8 h to induce uniform germination. Then, the seeds were transferred to darkness and various light conditions (W, B, R, and FR) (17). ImageJ software was used to measure hypocotyl length, cotyledon aperture, and hook angle of seedlings.
Genomic Complementation Test.
The pEarleyGateway-PID-CFP, pEarleyGateway-PID-GFP, and pEarley Gateway-myc-PID plasmids were transformed into Agrobacterium tumefaciens GV3101, then transformed into the flowering csu3 cop1-6 double-mutant plants through the floral dip method (56).
Mass Spectrometry.
For MS analysis, GST-PID and His-TF-COP1 were used to perform a kinase assay, after which the proteins were separated by SDS/PAGE. After trypsin digestion, phosphopeptides were enriched using PHOS-Select Iron Affinity Gel (Sigma-Aldrich) following the manufacturer’s instructions. For LC-MS/MS analysis, the phosphopeptides were reconstituted in 0.2% formic acid, loaded onto a 100 μm × 1.5 cm precolumn, and separated on a 75 μm × 15 cm capillary column with a laser-pulled sprayer. Both columns were packed in-house with 4 μm of C18 bulk material (InnosepBio). An Easy nLC 1000 system (Thermo Fisher Scientific) was used to deliver the following HPLC gradient: 5–30% B for 75 min, 30–75% B for 6 min, then held at 75% B for 18 min (A, 0.1% formic acid in water; B, 0.1% formic acid in acetonitrile). The eluted peptides were sprayed into an Orbitrap Velos Pro mass spectrometer (Thermo Fisher Scientific) equipped with a nano-ESI source. The mass spectrometer was operated in data-dependent mode with a full MS scan in FT mode at a resolution of 120,000, followed by collision-induced dissociation and ETD. MS/MS scans were done on the 10 most abundant ions in the initial MS scan. The target value was 1e6 for Fourier transform MS full scans and 2e5 for ion trap MS scans, the anion target value was 1e6, and ETD activation time was 150 ms.
MSConvert was used to convert the raw data files to mascot generic format (“.mgf”) before submission for the database search. Mascot version 2.3.02 was used to carry out a database search with the following parameters: carbamidomethyl (Cys) as fixed modification, oxidation (Met), phosphorylation (Ser, Thr, and Tyr) as variable modification; ± 5 ppm for peptide pass tolerance and ± 0.6 Da for fragment mass tolerance; maximum missed cleavages, 2. The experiments were performed with three biological replicates, which yielded comparable results.
Immunoblot Analysis and Immunoprecipitation.
To perform immunoblot assays, Arabidopsis seedlings were homogenized in liquid nitrogen and resuspended in a protein extraction buffer [10 mM Tris pH 8.0, 100 mM NaH2PO4, 8 M urea, 1 mM PMSF and 1× Complete Protease Inhibitor Mixture (Roche)]. Total extracts were centrifuged at 4 °C at 15,000 × g for 10 min, and the supernatants were then subjected to protein SDS/PAGE gels. Immunoblotting were performed using anti-COP1 (57), anti-PIF3 (58), anti-HY5 (10), anti-GFP (Abmart), anti-CSN6 (59), anti-myc (Sigma-Aldrich), anti-actin (Sigma-Aldrich), and anti-tubulin (Sigma-Aldrich).
In Vitro Pull-Down Assays.
In vitro pull-down assays were conducted as described previously with minor modifications (58). Here 10 μL of GST beads and 1 μg of purified GST or GST fusion proteins were mixed in GST binding buffer (20 mM Tris⋅HCl pH 7.5, 250 mM NaCl, 10% glycerol, and 1 mM PMSF) and rotated for 2 h at 4 °C. Then 1 μg of His-TF, His-TF-COP1, or His-PID was added to the mixtures with an additional 1 h of rotation. Then GST beads were washed with binding buffer four times and boiled with 5× SDS loading buffer. The protein samples were subjected to 10% SDS/PAGE. Input and pull-down prey proteins were detected by immunoblotting using anti-His and anti-GST monoclonal antibodies (Sigma-Aldrich).
Firefly LCI Assay.
The firefly LCI transient expression assay was performed as described previously (60). N. benthamiana leaves were coinfiltrated with A. tumefaciens strains carrying the binary plasmids of pCAMBIA1300:COP1-nLUC and pCAMBIA1300-cLUC-PID. The corresponding empty vector pCAMBIA1300-nLUC or pCAMBIA1300-cLUC was infiltrated as a negative control. After a 2-d incubation in darkness at 22 °C and an additional 1-d incubation under a 16-h light/8-h dark photoperiod, the leaves were harvested, and 1 mM D-luciferin (BD Monolight; BD Biosciences) solution was sprayed onto the tobacco leaves. Luciferase activity was measured with the LB 985 NightSHADE Spectrum imaging system (Berthold).
Quantitative Real-Time RT-PCR.
The RNeasy Plant Mini Kit (Qiagen) was used to extract total RNA of Arabidopsis seedlings. Here 2 μg of total RNA was applied to synthesize cDNA using the 5× All-In-One RT MasterMix system (Applied Biological Materials) according to the manufacturer’s instructions. Quantitative real-time PCR analysis was performed on a 7500 Fast Real-Time PCR Amplifier (Applied Biosystems) with a SYBR Premix Ex Taq Kit (TaKaRa). Each experiment was repeated with three biological replicates, and each sample was analyzed in triplicate. The expression levels of target genes were normalized to the PP2A gene. The primers used in these assays are listed in Table S1.
Table S1.
Primers used in this study
| Primer name | Primer sequences | ||
| Plasmid constructs | |||
| PIDattB1 | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGTTACGAGAATCAGACGG | pDONR221-PID | |
| PIDattB2 | GGGGACCACTTTGTACAAGAAAGCTGGGTCAAAGTAATCGAACGCCGCTGG | ||
| mPID-F1 | TCATCTACAGAGCTCTGAAGCCTG | pDONR221-mPID | |
| mPID-R1 | CAGGCTTCAGAGCTCTGTAGATGA | ||
| PID-KpnI-F | CGGGGTACCATGTTACGAGAATCAGACGGTG | pCambia1300-n/cLuc-PID | |
| PID-SalI-R | ACGCGTCGACAAAGTAATCGAACGCCGCT | ||
| COP1-SpeI-F | GGACTAGTATGGAAGAGATTTCGACGGATCCG | pCambia1307-COP1-Flag | |
| COP1-HindIII R | CCCAAGCTTCGCAGCGAGTACCAGAACTTTG | ||
| COP1-S90A-F1 | ATGGAAGAGATTTCGACGGATCCG | pGEX-4T-1-COP1S90A | |
| COP1-S90A-R1 | TGAGGTGTTGGGCACAACAGG | ||
| COP1-S90A-F2 | CCTGTTGTGCCCAACACCTCA | ||
| COP1-S90A-R2 | CGCAGCGAGTACCAGAACTTTG | ||
| COP1-KpnI-F | CGGGGTACCATGGAAGAGATTTCGACGGATCCG | pCold-His-TF-COP1 | |
| COP1-PstI-R | AAAACTGCAGTCACGCAGCGAGTACCAGAACTTTG | ||
| COP1-attB1 | GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGAAGAGATTTCGACGGATC | pEarlygateway-104-YFP-COP1 | |
| COP1-attB2 | GGGGACCACTTTGTACAAGAAAGCTGG GTCTCACGCAGCGAGTACCAGAAC | ||
| COP1-RING-EcoRI-F | CCGGAATTC ATGGAAGAGATTTCGACGGATC | pGEX-4T-1-COP1 1–110 aa | |
| COP1-RING-XhoI-R | CCGCTCGAGCACATGCCGAGCTGAAGTTTT | ||
| COP1-coil-EcoRI-F | CCGGAATTCAAAACTTCAGCTCGGCATGTG | pGEX-4T-1-COP1 110–255 aa | |
| COP1-coil-XhoI-R | CCGCTCGAGTCAATAATTGCCTACAAAATTTCCTCC | ||
| COP1WD40-EcoRI-F | CCGGAATTCAAGTTGCGGATGCTCGGAG | pGEX-4T-1 -COP1 216–675 aa | |
| COP1WD40-XhoI-R | CCGCTCGAGTCACGCAGCGAGTACCAGAAC | ||
| COP1 RING S20A-F | CGAGAACAGCTTCAGTTGGTGA | pGEX-4T-1 -COP1 RING S20A | |
| COP1 RING S20A-R | TTCACCAACTGAAGCTGTTCTCG | ||
| COP1 RING S21A-F | CGAGAACATCTGCAGTTGGTGA | pGEX-4T-1 -COP1 RING S20A | |
| COP1 RING S21A-R | TTCACCAACTGCAGATGTTCTCG | ||
| COP1 S20D-F | CGAGAACAGATTCAGTTGGTGA | pGEX-4T-1 -COP1 S20A | |
| COP1 S20D-R | TTCACCAACTGAATCTGTTCTCG | ||
| Real-time qPCR | |||
| PID-RT-F | TAGACAACCTCACCGGCGATTC | ||
| PID-RT-R | AGAGCATAATGTGACCGTCGGA | ||
| COP1-RT-F | AAGAGTGTAGTACGGAGGGAAGG | ||
| COP1-RT-R | TAGACGACTGTAGCGAGTGAAGG | ||
| PP2A-RT-F | TATCGGATGACGATTCTTCGTGCAG | ||
| PP2A-RT-R | GCTTGGTCGACTATCGGAATGAGAG | ||
| CHS-RT-F | AGCTGATGGACCTGCAGGCATCTTGGC | ||
| CHS-RT-R | TGCATGTGACGTTTCCGAATTGTCGAC | ||
| CHI-RT-F | ATGTCTTCATCCAACGCCTGCGCC | ||
| CHI-RT-R | GACGGTGAAGATCACGAATTTACC | ||
| DFR-RT-F | ATGGTTAGTCAGAAAGAGACCGTGTGTG | ||
| DFR-RT-R | CGTTTCGCAAATCAAGAAGATGTTGTAC | ||
| LDOX-RT-F | TGGGTCACTGCAAAATGTGT | ||
| LDOX-RT-R | CGGAGACTCAACACTCACCA | ||
| F3H-RT-F | ATGGCTCCAGGAACTTTGACTGAGCTA | ||
| F3H-RT-R | GATCTGACGGCAGATCTCTCCTCTTTT | ||
| F3′H-RT-F | GTTGAGAAAGATTAGTTCTGTTCATCT | ||
| F3′H-RT-R | GTTGACTACACACATGTTCACCAACTG | ||
| MIDA1-RT-F | GATTGAGTGGGGTTGTCGG | ||
| MIDA1-RT-R | TACAGAGTACTACTACGTACACC | ||
| MIDA8-RT-F | CCACCTCGAGTTCCTGCAAG | ||
| MIDA8-RT-R | GCTTGCAGGATACCGTGGTG | ||
| MIDA12-RT-F | ATGGAGAAGGCAGTTCATTTATCC | ||
| MIDA12-RT-R | CGCAGAGATGTTGGTGGATT | ||
| MIDA13-RT-F | CACGTGACGGATCAGAGAACTT | ||
| MIDA13-RT-R | GGATCGTGACCATAACAAGCG | ||
| IAA19-RT-F | TTTCATCTGGTGGTGACGCT | ||
| IAA19-RT-R | CATACCCTAACCCCACTTTCG | ||
| PRE1-RT-F | GTTCTGATAAGGCATCAGCCTCG | ||
| PRE1-RT-R | CATGAGTAGGCTTCTAATAACGGCG | ||
The underscored nucleotides indicate the (5′→3′) restriction sites for cloning.
Acknowledgments
This work was supported by National Natural Science Foundation of China Grants 31330048 and 31621001, Peking-Tsinghua Center for Life Sciences, US National Institutes of Health (GM-47850), and Southern University of Science and Technology.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702984114/-/DCSupplemental.
References
- 1.Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: Structure, evolution, and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad M, Cashmore AR. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- 3.Christie JM. Phototropin blue-light receptors. Annu Rev Plant Biol. 2007;58:21–45. doi: 10.1146/annurev.arplant.58.032806.103951. [DOI] [PubMed] [Google Scholar]
- 4.Guo H, Yang H, Mockler TC, Lin C. Regulation of flowering time by Arabidopsis photoreceptors. Science. 1998;279:1360–1363. doi: 10.1126/science.279.5355.1360. [DOI] [PubMed] [Google Scholar]
- 5.Rizzini L, et al. Perception of UV-B by the Arabidopsis UVR8 protein. Science. 2011;332:103–106. doi: 10.1126/science.1200660. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan JA, Deng XW. From seed to seed: The role of photoreceptors in Arabidopsis development. Dev Biol. 2003;260:289–297. doi: 10.1016/s0012-1606(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 7.Huang X, Ouyang X, Deng XW. Beyond repression of photomorphogenesis: Role switching of COP/DET/FUS in light signaling. Curr Opin Plant Biol. 2014;21:96–103. doi: 10.1016/j.pbi.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Lau OS, Deng XW. The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci. 2012;17:584–593. doi: 10.1016/j.tplants.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Ang LH, et al. Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol Cell. 1998;1:213–222. doi: 10.1016/s1097-2765(00)80022-2. [DOI] [PubMed] [Google Scholar]
- 10.Osterlund MT, Hardtke CS, Wei N, Deng XW. Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature. 2000;405:462–466. doi: 10.1038/35013076. [DOI] [PubMed] [Google Scholar]
- 11.Holm M, Ma LG, Qu LJ, Deng XW. Two interacting bZIP proteins are direct targets of COP1-mediated control of light-dependent gene expression in Arabidopsis. Genes Dev. 2002;16:1247–1259. doi: 10.1101/gad.969702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seo HS, et al. LAF1 ubiquitination by COP1 controls photomorphogenesis and is stimulated by SPA1. Nature. 2003;423:995–999. doi: 10.1038/nature01696. [DOI] [PubMed] [Google Scholar]
- 13.Jang IC, Yang JY, Seo HS, Chua NH. HFR1 is targeted by COP1 E3 ligase for post-translational proteolysis during phytochrome A signaling. Genes Dev. 2005;19:593–602. doi: 10.1101/gad.1247205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu D, et al. BBX21, an Arabidopsis B-box protein, directly activates HY5 and is targeted by COP1 for 26S proteasome-mediated degradation. Proc Natl Acad Sci USA. 2016;113:7655–7660. doi: 10.1073/pnas.1607687113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo Q, et al. COP1 and phyB physically interact with PIL1 to regulate its stability and photomorphogenic development in Arabidopsis. Plant Cell. 2014;26:2441–2456. doi: 10.1105/tpc.113.121657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang CS, Maloof JN, Wu SH. COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis. Plant Physiol. 2011;156:228–239. doi: 10.1104/pp.111.175042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Datta S, et al. LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell. 2008;20:2324–2338. doi: 10.1105/tpc.108.061747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seo HS, Watanabe E, Tokutomi S, Nagatani A, Chua NH. Photoreceptor ubiquitination by COP1 E3 ligase desensitizes phytochrome A signaling. Genes Dev. 2004;18:617–622. doi: 10.1101/gad.1187804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang IC, Henriques R, Seo HS, Nagatani A, Chua NH. Arabidopsis PHYTOCHROME INTERACTING FACTOR proteins promote phytochrome B polyubiquitination by COP1 E3 ligase in the nucleus. Plant Cell. 2010;22:2370–2383. doi: 10.1105/tpc.109.072520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Debrieux D, Trevisan M, Fankhauser C. Conditional involvement of constitutive photomorphogenic1 in the degradation of phytochrome A. Plant Physiol. 2013;161:2136–2145. doi: 10.1104/pp.112.213280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang S, et al. Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J. 2008;27:1277–1288. doi: 10.1038/emboj.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu JW, et al. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol Cell. 2008;32:617–630. doi: 10.1016/j.molcel.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyachok J, et al. SCAR mediates light-induced root elongation in Arabidopsis through photoreceptors and proteasomes. Plant Cell. 2011;23:3610–3626. doi: 10.1105/tpc.111.088823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dornan D, et al. The ubiquitin ligase COP1 is a critical negative regulator of p53. Nature. 2004;429:86–92. doi: 10.1038/nature02514. [DOI] [PubMed] [Google Scholar]
- 25.Migliorini D, et al. Cop1 constitutively regulates c-Jun protein stability and functions as a tumor suppressor in mice. J Clin Invest. 2011;121:1329–1343. doi: 10.1172/JCI45784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vitari AC, et al. COP1 is a tumour suppressor that causes degradation of ETS transcription factors. Nature. 2011;474:403–406. doi: 10.1038/nature10005. [DOI] [PubMed] [Google Scholar]
- 27.Lu G, et al. Phosphorylation of ETS1 by Src family kinases prevents its recognition by the COP1 tumor suppressor. Cancer Cell. 2014;26:222–234. doi: 10.1016/j.ccr.2014.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dornan D, et al. ATM engages autodegradation of the E3 ubiquitin ligase COP1 after DNA damage. Science. 2006;313:1122–1126. doi: 10.1126/science.1127335. [DOI] [PubMed] [Google Scholar]
- 29.Su CH, et al. Nuclear export regulation of COP1 by 14-3-3σ in response to DNA damage. Mol Cancer. 2010;9:243. doi: 10.1186/1476-4598-9-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu D, et al. The RING-Finger E3 ubiquitin ligase COP1 SUPPRESSOR1 negatively regulates COP1 abundance in maintaining COP1 homeostasis in dark-grown Arabidopsis seedlings. Plant Cell. 2014;26:1981–1991. doi: 10.1105/tpc.114.124024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell. 1991;3:677–684. doi: 10.1105/tpc.3.7.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gälweiler L, et al. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science. 1998;282:2226–2230. doi: 10.1126/science.282.5397.2226. [DOI] [PubMed] [Google Scholar]
- 33.Bennett SRM, Alvarez J, Bossinger G, Smyth DR. Morphogenesis in Pinoid mutants of Arabidopsis-thaliana. Plant J. 1995;8:505–520. [Google Scholar]
- 34.Christensen SK, Dagenais N, Chory J, Weigel D. Regulation of auxin response by the protein kinase PINOID. Cell. 2000;100:469–478. doi: 10.1016/s0092-8674(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 35.Berleth T, Jurgens G. The role of the monopteros gene in organizing the basal body region of the Arabidopsis embryo. Development. 1993;118:575–587. [Google Scholar]
- 36.Hardtke CS, Berleth T. The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J. 1998;17:1405–1411. doi: 10.1093/emboj/17.5.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang F, et al. Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell. 2010;22:1129–1142. doi: 10.1105/tpc.109.072678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zegzouti H, Anthony RG, Jahchan N, Bögre L, Christensen SK. Phosphorylation and activation of PINOID by the phospholipid signaling kinase 3-phosphoinositide-dependent protein kinase 1 (PDK1) in Arabidopsis. Proc Natl Acad Sci USA. 2006;103:6404–6409. doi: 10.1073/pnas.0510283103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benjamins R, Ampudia CS, Hooykaas PJ, Offringa R. PINOID-mediated signaling involves calcium-binding proteins. Plant Physiol. 2003;132:1623–1630. doi: 10.1104/pp.103.019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol. 1999;294:1351–1362. doi: 10.1006/jmbi.1999.3310. [DOI] [PubMed] [Google Scholar]
- 41.Bauer D, et al. Constitutive photomorphogenesis 1 and multiple photoreceptors control degradation of phytochrome interacting factor 3, a transcription factor required for light signaling in Arabidopsis. Plant Cell. 2004;16:1433–1445. doi: 10.1105/tpc.021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng XW, Caspar T, Quail PH. cop1: A regulatory locus involved in light-controlled development and gene expression in Arabidopsis. Genes Dev. 1991;5:1172–1182. doi: 10.1101/gad.5.7.1172. [DOI] [PubMed] [Google Scholar]
- 43.Deng XW, et al. COP1, an Arabidopsis regulatory gene, encodes a protein with both a zinc-binding motif and a G beta homologous domain. Cell. 1992;71:791–801. doi: 10.1016/0092-8674(92)90555-q. [DOI] [PubMed] [Google Scholar]
- 44.McNellis TW, et al. Genetic and molecular analysis of an allelic series of cop1 mutants suggests functional roles for the multiple protein domains. Plant Cell. 1994;6:487–500. doi: 10.1105/tpc.6.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McNellis TW, von Arnim AG, Deng XW. Overexpression of Arabidopsis COP1 results in partial suppression of light-mediated development: Evidence for a light-inactivable repressor of photomorphogenesis. Plant Cell. 1994;6:1391–1400. doi: 10.1105/tpc.6.10.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin XL, et al. An Arabidopsis SUMO E3 ligase, SIZ1, negatively regulates photomorphogenesis by promoting COP1 activity. PLoS Genet. 2016;12:e1006016. doi: 10.1371/journal.pgen.1006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu D, et al. Arabidopsis COP1 SUPPRESSOR 2 represses COP1 E3 ubiquitin ligase activity through their coiled-coil domains association. PLoS Genet. 2015;11:e1005747. doi: 10.1371/journal.pgen.1005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu X, et al. PHYTOCHROME INTERACTING FACTOR1 enhances the E3 ligase activity of CONSTITUTIVE PHOTOMORPHOGENIC1 to synergistically repress photomorphogenesis in Arabidopsis. Plant Cell. 2014;26:1992–2006. doi: 10.1105/tpc.114.125591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim JI, et al. Phytochrome phosphorylation modulates light signaling by influencing the protein-protein interaction. Plant Cell. 2004;16:2629–2640. doi: 10.1105/tpc.104.023879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nito K, Wong CC, Yates JR, 3rd, Chory J. Tyrosine phosphorylation regulates the activity of phytochrome photoreceptors. Cell Reports. 2013;3:1970–1979. doi: 10.1016/j.celrep.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Sady B, Ni W, Kircher S, Schäfer E, Quail PH. Photoactivated phytochrome induces rapid PIF3 phosphorylation prior to proteasome-mediated degradation. Mol Cell. 2006;23:439–446. doi: 10.1016/j.molcel.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Ni W, et al. A mutually assured destruction mechanism attenuates light signaling in Arabidopsis. Science. 2014;344:1160–1164. doi: 10.1126/science.1250778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ni W, et al. Multisite light-induced phosphorylation of the transcription factor PIF3 is necessary for both its rapid degradation and concomitant negative feedback modulation of photoreceptor phyB levels in Arabidopsis. Plant Cell. 2013;25:2679–2698. doi: 10.1105/tpc.113.112342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ang LH, Deng XW. Regulatory hierarchy of photomorphogenic loci: Allele-specific and light-dependent interaction between the HY5 and COP1 loci. Plant Cell. 1994;6:613–628. doi: 10.1105/tpc.6.5.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Earley KW, et al. Gateway-compatible vectors for plant functional genomics and proteomics. Plant J. 2006;45:616–629. doi: 10.1111/j.1365-313X.2005.02617.x. [DOI] [PubMed] [Google Scholar]
- 56.Clough SJ, Bent AF. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 57.Saijo Y, et al. The COP1-SPA1 interaction defines a critical step in phytochrome A-mediated regulation of HY5 activity. Genes Dev. 2003;17:2642–2647. doi: 10.1101/gad.1122903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong J, et al. Arabidopsis DE-ETIOLATED1 represses photomorphogenesis by positively regulating phytochrome-interacting factors in the dark. Plant Cell. 2014;26:3630–3645. doi: 10.1105/tpc.114.130666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Peng Z, Serino G, Deng XW. Molecular characterization of subunit 6 of the COP9 signalosome and its role in multifaceted developmental processes in Arabidopsis. Plant Cell. 2001;13:2393–2407. doi: 10.1105/tpc.010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen H, et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 2008;146:368–376. doi: 10.1104/pp.107.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]