Significance
When the domestication of rice began in its homeland, China, is an enduring and important issue of debate for researchers from many different disciplines. Reliable chronological and robust identification criteria for rice domestication are keys to understanding the issue. Here, we first use phytolith dating to constrain the initial occupation of Shangshan, an important site with early rice remains located in the Lower Yangtze region of China. We then identify the rice phytoliths of Shangshan as partly domesticated based on their morphological characteristics. The results indicate that rice domestication may have begun at Shangshan in the Lower Yangtze during the beginning of the Holocene.
Keywords: rice domestication, radiocarbon dating, Shangshan, chronology, phytolith-occluded carbon
Abstract
Phytolith remains of rice (Oryza sativa L.) recovered from the Shangshan site in the Lower Yangtze of China have previously been recognized as the earliest examples of rice cultivation. However, because of the poor preservation of macroplant fossils, many radiocarbon dates were derived from undifferentiated organic materials in pottery sherds. These materials remain a source of debate because of potential contamination by old carbon. Direct dating of the rice remains might serve to clarify their age. Here, we first validate the reliability of phytolith dating in the study region through a comparison with dates obtained from other material from the same layer or context. Our phytolith data indicate that rice remains retrieved from early stages of the Shangshan and Hehuashan sites have ages of approximately 9,400 and 9,000 calibrated years before the present, respectively. The morphology of rice bulliform phytoliths indicates they are closer to modern domesticated species than to wild species, suggesting that rice domestication may have begun at Shangshan during the beginning of the Holocene.
Rice (Oryza sativa L.) is one of the world’s most important staple foods, sustaining more than half of the global population (1). Questions surrounding its origins and domestication have led to a considerable amount of attention in the last decade (e.g., refs. 2–5). The earliest phytolith remains identified as Oryza have been recovered at Diaotonghuan and Xianrendong, located in Jiangxi Province, China, and date from the end of the Pleistocene, about 12,000–9,000 calibrated years before the present (cal yr B.P.) (6, 7). However, few artifacts specialized for plant use, such as grinding stones, were recovered and most tools discovered—made of bone, antler, and shell—were likely used for hunting and gathering. These lines of evidence, together with their Pleistocene age, indicate that the rice was likely a wild species (8). Although other early rice remains in the form of phytoliths were found in a marine sediment core from the Okinawa Trough and dated to the Bølling-Allerød warm period (13,900–13,000 cal yr B.P.) (9), the nature of the rice remains as to their wild or domesticated status is unclear because of a limited application of available rice identification criteria. Rice remains (mainly from phytolith) found at the Shangshan site (N 29°27′21.636′′, E 119°58′15.42′′, 50 m above sea level) and dated to approximately 11,000–8,600 cal yr B.P. through the pottery sherds (10), may represent the first instance of rice cultivation (11). However, debate exists as to whether the rice is domesticated, wild, or transitional (12, 13). To date, the chronology of the Shangshan site has largely relied on 14C dating of organic materials extracted from pottery sherds (10), which might include a contribution from undetermined and older carbon sources, such as organics from the original clay source and aquatic food containing old carbon from freshwater or marine reservoirs (14). These substances may not directly reflect the age of the organic sources exploited by humans. Accordingly, further dating initiatives are needed to constrain the absolute-calendar time of the rice remains.
Radiocarbon dating of phytoliths, microscopic bodies of silica formed in plant cells, is an established technique that can be traced back to the 1960s (15). Phytoliths can occlude some organic carbon during their deposition, which is captured through photosynthesis from atmospheric CO2 during plant growth (16–20). Grasses, which are mainly short-lived annual plants, are high producers of phytoliths (21). 14C concentrations derived from the carbon inside the phytoliths should represent the atmospheric CO2 conditions under which the phytolith producer lived (16–19). A number of studies have recently shown that phytoliths can be confidently dated using rigorous phytolith isolation techniques that do not alter phytolith carbon or cause its leakage from silicified cell, and where suitable plant sources are used (i.e., from nonvolcanic environments) (16–18, 22, 23).
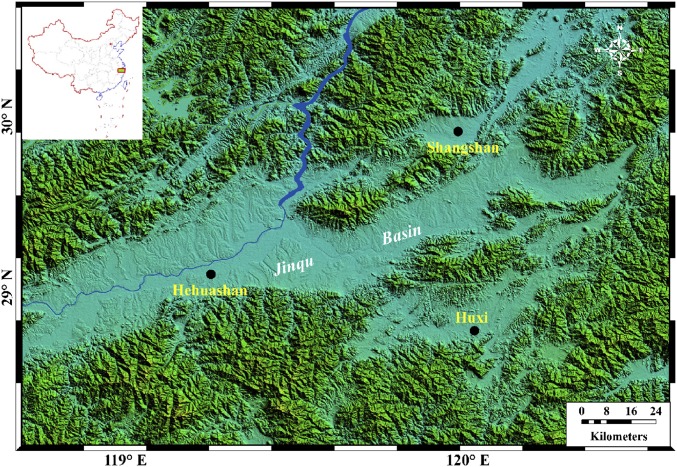
Previous studies demonstrated that abundant rice phytoliths occur in different cultural layers of Shangshan (24). Direct radiocarbon dating of the phytoliths may allow establishment of a firm rice chronology. Here we directly dated phytoliths using adjusted extracting protocols (22). We further validated the phytolith dates by comparing them with known radiocarbon ages of seeds and charcoal recovered from the same stratigraphic levels from a nearby site, Huxi (N 28°52′27.768′′, E 120°1′5.052′′, 92 m above sea level) (Fig. 1), an archaeological site dated from 9,000 to 8,300 cal yr B.P. that contains abundant remains of these types (Fig. S1B). With this evidence we used 14C dating of phytoliths retrieved from different cultural layers of Shangshan (Fig. S1A) and Hehuashan, located near Shangshan (N 29°2′19.60′′, E 119°14′57.48′′, 62 m above sea level) (Fig. S1C) to constrain the initial occupation and chronologies of the rice remains at these two sites. We also discuss the characteristics of these earliest rice remains with respect to their wild or domesticated status using established rice bulliform phytolith morphological differences (25) (Materials and Methods).
Fig. 1.
Locations of archaeological sites.
Fig. S1.
Soil profiles of Shangshan (A), Huxi (B), and Hehuashan (C) sites.
Results
Phytolith 14C Determinations and Comparison with Dates on Other Organic Materials.
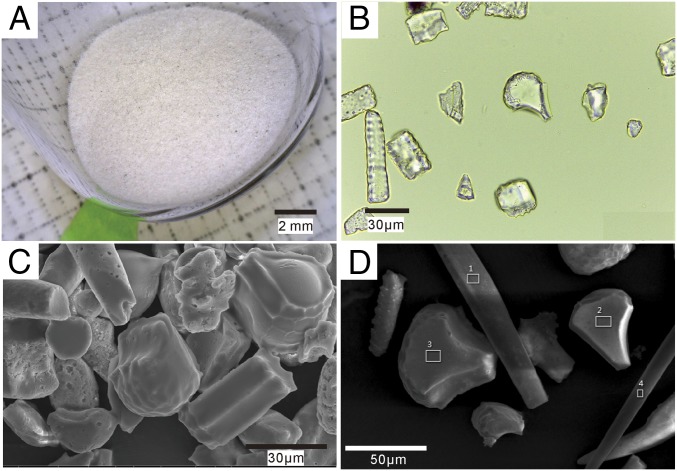
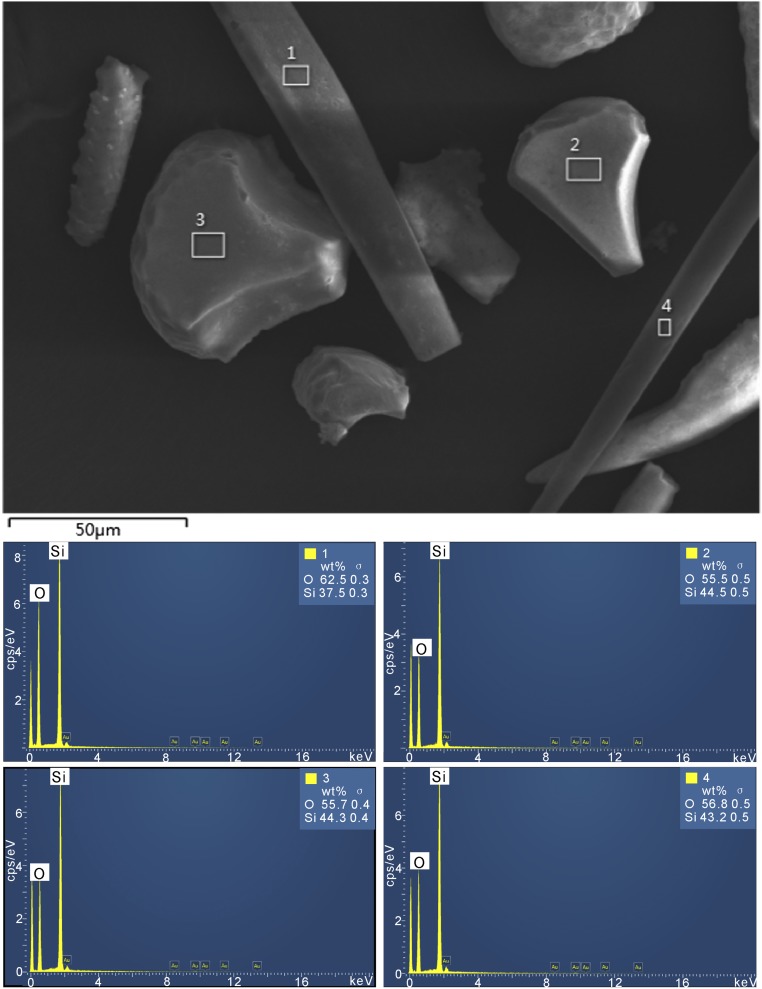
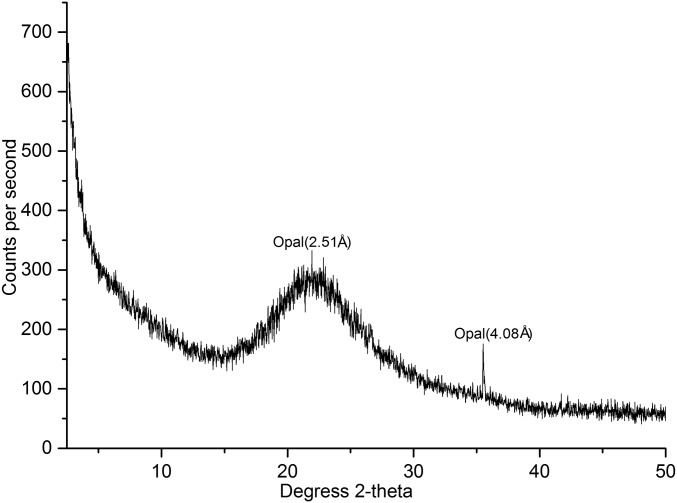
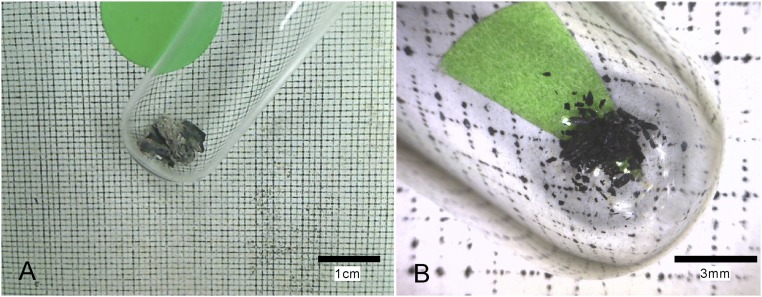
To assess the efficiency of phytolith-extracting protocols, we used multichecking methods, such as optical microscopy; scanning electron microscopy and energy-dispersive spectrometer (SEM-EDS); and X-ray diffraction (XRD). As Fig. 2 shows, the extracted phytoliths appear white. No extraneous organic materials were observed with an optical microscope and SEM-EDS analysis also showed the absence of exogenous contamination from organic materials on the phytolith surfaces (Fig. S2). XRD analysis shows that carbonate, clay, and other minerals were completely excluded (Fig. S3), indicating the efficiency of the phytolith extraction protocol. Thus, the measured 14C values of the phytoliths are shown with a high level of confidence to be from the phytolith samples themselves. We then obtained a total of 18 accelerator mass spectrometry (AMS) radiocarbon determinations, including nine on phytoliths. Other dates are from paired charcoal or seeds samples.
Fig. 2.
Images and EDS analysis of phytoliths extracted from cultural layers. (A) Images of phytoliths; (B) optical microscopy of phytoliths; (C) SEM images of phytoliths; and (D) EDS analysis of phytolith surface. (The white boxes are EDS spectra of phytoliths, which are shown in Fig. S2.)
Fig. S2.
EDS analysis of phytolith surfaces. 1 and 4 are EDS spectra of elongate phytoliths; 2 and 3 are EDS spectra of bulliform phytoliths. EDS analysis results show that the predominant elements of the phytolith surfaces are silicon and oxygen, and weight percent ratios of Si and O vary between 0.6 and 0.8. No carbon was detected by the EDS spectra of the phytolith.
Fig. S3.
X-ray powder diffractogram of phytoliths. XRD patterns of phytoliths show no typical crystalline phases in the curve, except for two small peaks at 2.51 and 4.08 Å, indicating that the extracted materials did not fully crystallize. The XRD results demonstrate that the extracted materials are opal (SiO2·n H2O, here called phytoliths).
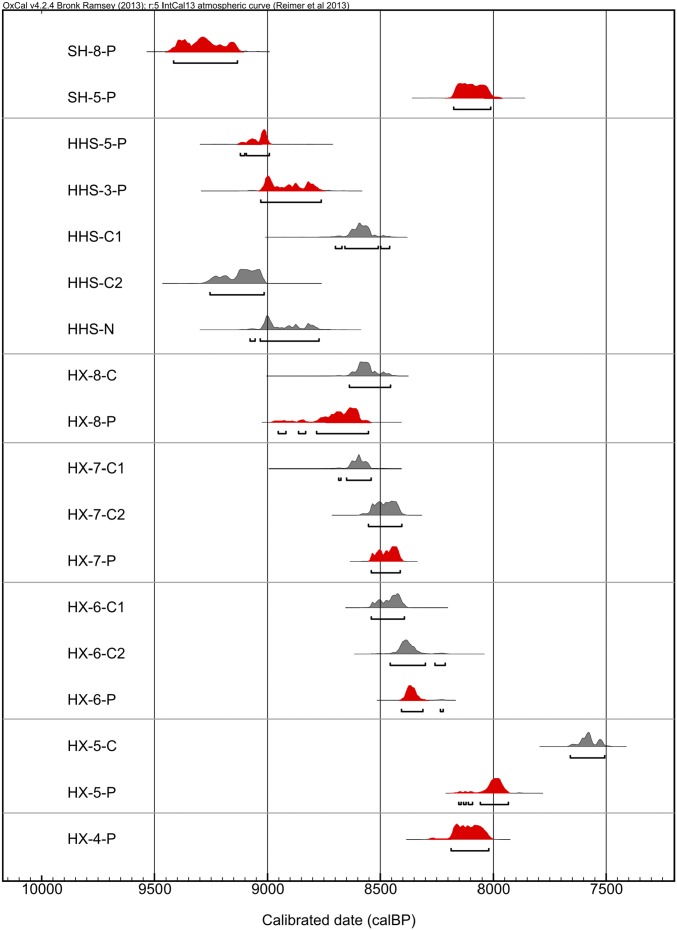
The Huxi site has been divided into seven subphases, including the Late Shangshan culture (26). Large amounts of charcoal, plant seeds, and phytoliths were preserved in the sediment because of the relatively anaerobic conditions of the site (27). From Huxi, we compared five phytolith dates with their paired charcoal samples (Fig. 3 and Table 1). Detailed carbon information, such as graphite, carbon yield rate, and fraction modern is shown in Table S1. The results show a high level of correspondence, with the exception of one outlier (HX-5-P). In four of the cases (HX-7-P, HX-7-C2, HX-6-P, and HX-6-C2), the 2σ probability distributions are virtually identical, and in one of the others there is a partial overlap. Two phytolith dates (HX-8-P and HX-7-P) from the eighth and seventh strata also show good consistency with published charcoal dates measured by the Peking University AMS Laboratory, which yielded ages of 8,790 ± 190 and 8,510 ± 80 B.P., respectively (27). The phytolith date (HX-5-P) that did not fall within the calibrated 2σ range of its paired charcoal sample (HX-5-C), may actually be the more reasonable determination. The charcoal sample dated was very small (<1 mg) (Fig. S4), having shown a substantial reaction on pretreatment facility with acid washes, indicating carbonate was present. Although alkaline treatment was done to remove humic acid, pretreatment could not be completed for fear that not enough would be left to date. Unremoved humics may have caused the discrepancy between it and its paired phytolith date. The consistency between the phytolith 14C ages and their paired charcoal samples indicate that phytolith dating is reliable and can be used either as single-factor dating when charcoal samples are limited or absent, or along with charcoal as additional sources of 14C determinations.
Fig. 3.
Calibrated 2σ probability distribution of radiocarbon assays on phytoliths (red) and other dating materials (gray). OxCal v4.2.4 (59); r;5 IntCal13 atmospheric curve (60).
Table 1.
AMS radiocarbon dating results
| Lab ID | Archaeological sites | Sample code | Dating materials | 13C/12C ratio | Conventional age (B.P.) | 2σ Calibration (cal B.P.) |
| 434204 | Shangshan | SH-8-P | Phytolith | NA* | 8280 ± 40 | 9,417–9,134 |
| 434203 | Shangshan | SH-5-P | Phytolith | NA* | 7280 ± 40 | 8,175–8,012 |
| 438410 | Hehuashan | HHS-5-P | Phytolith | −26.7 0/00 | 8130 ± 40 | 9,121–8,992 |
| 438409 | Hehuashan | HHS-3-P | Phytolith | −25.1 0/00 | 8040 ± 30 | 9,030–8,762 |
| 363178 | Hehuashan | HHS-C1 | Char | −26.3 0/00 | 7810 ± 40 | 8,699–8,459 |
| 363179 | Hehuashan | HHS-C2 | Char | −26.0 0/00 | 8170 ± 40 | 9,255–9,015 |
| 363180 | Hehuashan | HHS-N | Nutshell | −25.4 0/00 | 8050 ± 40 | 9,078–8,772 |
| 434202 | Huxi | HX-4-P | Phytolith | NA* | 7310 ± 40 | 8,186–8,021 |
| 438411 | Huxi | HX-5-P | Phytolith | −24.7 0/00 | 7180 ± 40 | 8,152–7,934 |
| 438412 | Huxi | HX-5-C | Char | −24.7 0/00 | 6710 ± 40 | 7,659–7,507 |
| 434199 | Huxi | HX-6-P | Phytolith | −25.9 0/00 | 7530 ± 30 | 8,406–8,221 |
| 434197 | Huxi | HX-6-C1 | Char | −24.6 0/00 | 7660 ± 40 | 8,540–8,393 |
| 358085 | Huxi | HX-6-C2 | Char | −27.5 0/00 | 7570 ± 50 | 8,456–8,212 |
| 406654 | Huxi | HX-7-P | Phytolith | −25.7 0/00 | 7680 ± 30 | 8,540–8,412 |
| 407469 | Huxi | HX-7-C1 | Char | −25.9 0/00 | 7820 ± 30 | 8,685–8,541 |
| 358084 | Huxi | HX-7-C2 | Char | −26.1 0/00 | 7690 ± 40 | 8,553–8,405 |
| 434200 | Huxi | HX-8-P | Phytolith | −25.4 0/00 | 7870 ± 40 | 8,953–8,553 |
| 434195 | Huxi | HX-8-C | Char | −23.5 0/00 | 7790 ± 40 | 8,637–8,455 |
Dating results include an uncertainty of ±2σ. All conventional ages were calibrated to calendar years using OXCal v4.2.4 and the IntCal13 calibration curve. NA, not applicable.
The original sample was too small to provide a δ13C on the original material. However, a ratio including both natural and laboratory effects was measured during 14C detection to calculate the true Conventional Radiocarbon Age.
Table S1.
Detailed carbon and other information for dated phytoliths
| Lab ID | Sample code | Dry soil weight (g) | Extracted (mg) | Analyzed (mg) | Graphite (mg C) | Carbon yield rate (%) | Fraction modern ± 1σ |
| 434204 | SH-8-P | 130.974 | 241 | 219.1 | 0.34 | 0.15 | 0.3567 ± 0.0018 |
| 434203 | SH-5-P | 131.880 | 232 | 207.5 | 0.17 | 0.08 | 0.4040 ± 0.0020 |
| 438410 | HHS-5-P | 150.000 | 1,000 | 463.7 | 0.80 | 0.20 | 0.3648 ± 0.0014 |
| 438409 | HHS-3-P | 150.000 | 1,313 | 455.1 | 0.80 | 0.20 | 0.3676 ± 0.0018 |
| 434202 | HX-4-P | 107.608 | 267 | 239.1 | 0.17 | 0.07 | 0.4025 ± 0.0020 |
| 438411 | HX-5-P | 150.358 | 1,144 | 415.3 | 0.50 | 0.10 | 0.4091 ± 0.0020 |
| 434199 | HX-6-P | 110.907 | 485 | 417.9 | 0.34 | 0.08 | 0.3916 ± 0.0015 |
| 406654 | HX-7-P | 201.773 | 726 | 688.0 | 1.02 | 0.15 | 0.3844 ± 0.0014 |
| 434200 | HX-8-P | 132.451 | 932 | 416.9 | 0.34 | 0.08 | 0.3754 ± 0.0019 |
Fig. S4.
Charcoal sample recovered from stratum 5 of Huxi (HX-5-C). (A) The whole sample before pretreatment. (B) The analyzed sample. There are some pieces that are lighter in color, although the sample received an alkali treatment (two times) to remove mobile humic acids in addition to the acid to remove carbonates. The sample is small (0.97 mg) and cannot receive any additional treatment for fear of it becoming too small. We cannot pick out the material or it will become too small.
The results of our phytolith dating at Shangshan are as follows. Two phytoliths dates from the early (upper layer of the eighth cultural stratum) and late stages (upper layer of the fifth cultural stratum) of the site range in age from 8280 ± 40 B.P. (±2σ: 9,417–9,134 cal yr B.P.) to 7280 ± 40 B.P. (±2σ: 8,175–8,012 cal yr B.P.). This finding suggests that the initial occupation of Shangshan may have occurred at about 9,400 cal yr B.P. or perhaps somewhat earlier, around 10,000 cal yr B.P., because phytolith sample SH-8 was retrieved between the seventh and eighth cultural strata of the site. The 8,175–8,012 cal yr B.P. phytolith date (SH-5-P) from a later stage of Shangshan is about 500 y younger than the pottery-based upper age limit of this site. This date probably reflects a transitional cultural period from the Shangshan to the Kuahuqiao culture (8,000–7,400 cal yr B.P.).
We then used phytolith dating to further construct the Hehuashan site chronology, previously sequenced as the Middle Shangshan culture (26). Three samples (two charcoal and one nutshell) recovered from pits at Hehuashan allowed us to further evaluate the reliability of phytolith dates. A phytolith date (HHS-5-P) completely overlaps with nutshell dates within ±2σ uncertainty (Fig. 3). Another phytolith date (HHS-3-P) is well within the dates obtained from charcoal samples. Thus, the phytolith dates provide a coherent chronology for Hehuashan, in good agreement with reliable, nonceramic-derived dating materials. The range of the phytolith dates from 8130 ± 40 B.P. (9,121–8,992 cal yr B.P.) to 8040 ± 30 B.P. (9,030–8,762 cal yr B.P.), suggest Hehuashan was initially occupied at ca. 9,000 cal yr B.P., later than the early habitation of Shangshan.
Identification of Oryza at Shangshan.
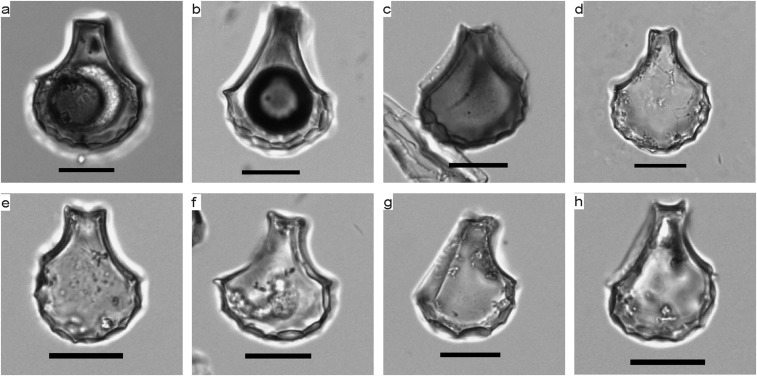
Whether the rice recovered from Shangshan is wild or domesticated has been a subject of debate in recent years (28). Although rice spikelet bases are considered key to documenting rice domestication (3), few of these were well-preserved at the site because of the acidic soil conditions. However, rice bulliform cell phytoliths, derived from the leaves, are highly resistant to decomposition, and their morphological characteristics can be used as a reliable proxy for domestication (24, 29, 30). Identification criteria include the number of fish-scale decorations on the lateral side of rice bulliform phytolith (Fig. S5). Previous studies have shown that domesticated rice phytoliths have more than nine decorations (9). Rice bulliform phytolith size also can be taken as an alternative proxy to determine the domestication rice from the wild rice (31). A few studies argued that phytolith size alone cannot be use to distinguish rice from related species (32, 33) because environmental conditions, such as CO2 concentrations (34) and evapotranspiration rates (35), may influence the phytolith size of different plants. However, in line with other evidence, this index at least can be used as semidomestication traits, in which rice bulliform phytoliths shifting toward larger sizes would imply that domesticated-type rice had evolved to dominate the rice populations being harvested (36, 37).
Fig. S5.
Rice bulliform phytoliths. (A–D) More than nine fish-scale decorations; (E–H) fewer than nine fish-scale decorations. (Scale bars, 20 μm.)
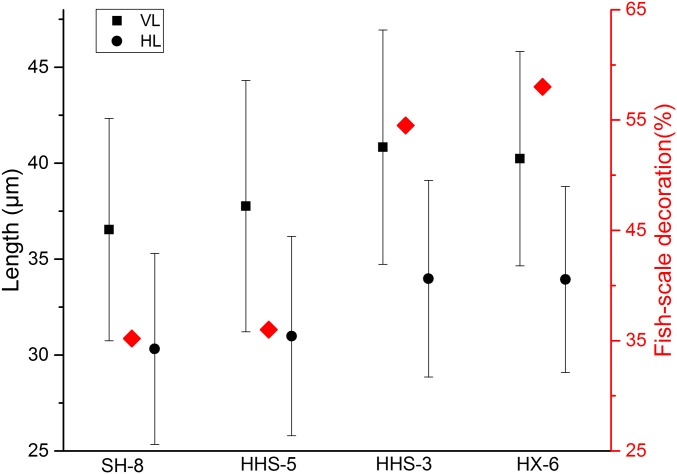
We counted the number of fish-scale decorations and measured the vertical and horizontal length of rice bulliform phytoliths identified from several selected cultural layers in Shangshan, Hehuashan, and Huxi. Approximately 36% had more than nine fish scales in the early occupation stages of Shangshan and Hehuashan (ca. 10,000–9,000 cal yr B.P.), in contrast to the ∼60% with more than nine counted from the late stages of Hehuashan and Huxi (ca. 9,000–8,200 cal yr B.P.) (Fig. 4). According to the study on the fish scales of modern Oryza phytoliths, the proportion of wild bulliform phytoliths with more than nine fish scales was 17.5 ± 8.3%, whereas for domesticated rice, the corresponding proportion was 63.7 ± 9.2% (25). There was a significantly larger amount of rice bulliforms with more than nine fish scales in the early stages of Shangshan and Hehuashan compared with modern wild rice, suggesting that the domestication process may have begun at these sites. The vertical and horizontal length measurements of rice bulliforms showed a shift toward larger sizes from the early (Shangshan site) to late stages (Huxi site) of Shangshan Culture, implying a long and improved cultivation or management processes of the rice during the Shangshan Culture period (Table S2).
Fig. 4.
Morphological characteristics of rice bulliform phytoliths from Shangshan, Hehuashan, and Huxi sites. HL, horizontal length (black circles); VL, vertical length of rice bulliform (black squares); black error bar represent ±1 SD; red diamonds represent the fish-scale decorations of rice bulliforms; SH-8 (9,417–9,134 cal yr B.P.), HHS-5 (9,121–8,992 cal yr B.P.), HHS-3 (9,030–8,762 cal yr B.P.), and HX-6 (8,406–8,221 cal yr B.P.).
Table S2.
Morphological characteristics of rice bulliform phytoliths identified from different cultural layers
| Archaeological sites | Cultural contexts | Sample code | Fish-scale decorations (%) | VL (SD, μm) | HL (SD, μm) |
| Shangshan | Stratum 8 | SH-8 | 35.2 | 36.5 (5.8) | 30.3 (5.0) |
| Hehuashan | Stratum 5 | HHS-5 | 36 | 37.8 (6.6) | 31.0 (5.2) |
| Hehuashan | Stratum 3 | HHS-3 | 54.5 | 40.8 (6.1) | 34.0 (5.1) |
| Huxi | Stratum 6 | HX-6 | 58 | 40.2 (5.6) | 34.0 (4.9) |
Discussion and Conclusions
We find again that phytolith dating is a reliable method for constraining the chronologies of archaeological sequences. 14C determinations presented here showed consistent results between the phytoliths and other dated materials from the same level or context, validating the reliability and accuracy of phytolith 14C dating. We find no evidence to support arguments from a few investigators (38–40) that old carbon absorbed from soils bias phytolith dates (see also SI Text). Our results here, combined with others from archaeological and paleoecological contexts and modern plants, indicate that phytolith ages are not significantly biased by extraneous carbon and can be used to establish reasonable chronological sequences in archaeological research (16–18, 22). Interestingly, our results in fact showed that phytolith dates were significantly younger than those derived from organic materials in pottery at Shangshan, which ranged between 11,348 and 8,608 cal yr B.P. (10), also refuting assertions that phytolith ages should be artificially old.
A number of sources, including contributions of older carbon from clays, older organics from freshwater or marine reservoirs, and postdepositional contamination may skew the accuracy of AMS dating of pottery sherds (41). The carbon source that is finally combusted to measure 14C content varies depending on the degree of the reservoir effect (if the sample obtains its carbon from a reservoir with a lower 14C level than the atmosphere, then the measured ages can be too old) at a particular site (42). Therefore, pottery-based dates from the Shangshan site may require evaluation by alternative dating methods.
Phytolith dates showed that Shangshan was occupied at about 9,400 cal yr B.P., earlier than the other two sites studied in this work. Huxi, where occupation occurred after 9,000 cal yr B.P., was the last site to be occupied. The dates of initial occupation of the three sites considered here constrained by the absolute chronologies of phytolith dates are consistent with the relative stratigraphies of the sites defined by archaeological typological sequences for the Shangshan Culture. The use of fiber-tempered ceramics declined gradually from the early stage of Shangshan to Huxi. Furthermore, the proportion of grinding stones in the lithic assemblage at Shangshan increased steadily, whereas the proportion of blades declined, also suggesting that Shangshan was occupied earlier than the other two sites.
Phytolith dates from this research provide direct chronologies of rice exploitation and domestication for three archaeological sites belonging to the Shangshan period that range from ∼10,000 to ∼8,000 cal yr B.P. Our results indicate that the process of rice domestication may have begun at least 9,400 cal yr B.P. in the Lower Yangtze basin.
Various sources of evidence, including rice husk phytoliths recovered from the residue of lithic tools (early Shangshan culture) (43, 44), use-wear traces of several flaked stone tools (44), pottery tempered with rice husks (Fig. S6), and the emergence of sedentary villages at Shangshan (10), indicate common rice presence and significant reliance on rice. This evidence, when combined with findings presented here on bulliform phytolith characteristics, make it likely that the process of rice domestication beginning with management or cultivation was taking place during the earliest occupation of Shangshan. Such an age for the beginnings of rice cultivation and domestication would agree with the parallel beginnings of agriculture in other regions of the world during a period of profound environmental change, when the Pleistocene was transitioning into the Holocene (45). For example, in the Old World, new archaeological evidence demonstrates millet (Setaria) exploitation and domestication beginning between approximately 11,000 and 9,000 cal yr B.P. in Northern China (46–48), although some dates are controversial (49). In the Near East, accumulated evidence from archaeobotanical remains demonstrates an initial management of wild plants at 11,500 cal yr B.P., with domestication evidenced about 10,000 cal yr B.P. (50). In the New World, the earliest crops, such as lerén (Calathea allouia), arrowroot (Maranta arundinacea), and Cucurbita moschata squash first appeared at approximately 10,200 cal yr B.P. in northern South America and maize was domesticated in Mexico by approximately 9,000 cal yr B.P. (51). The global climate experienced dramatic changes from cold glacial to warm interglacial during the transitional period between the Pleistocene and Holocene (52). In East Asia, a significant strengthening monsoon at about 9,500 y B.P. (53) was consistent with the initial occupation of Shangshan. It appears that during these periods, as hunter-gatherers in China were establishing the first sedentary villages (54), wild rice (Oryza rufipogon) was selected as the first plant to cultivate at the Shangshan site. Climatic amelioration during these transitional periods may thus serve as key factors in the process of rice domestication.
Fig. S6.
Several pottery sherds tempered with rice husks from Shangshan.
SI Text
A few studies have recently argued that old carbon absorbed by plant from soils may bias the accuracy of phytolith carbon-dating, making ages artificially old (38–40, 61). However, studies have shown how soil carbon is a very small organic constituent in plants compared with that derived from photosynthesis, in part because so much more carbon enters plant tissue via photosynthesis than through the xylem (vascular system) (16–20, 62). Furthermore, if present in any quantity, soil carbon would seemingly bias 14C study generally.
Materials and Methods
Sample Collection.
Soil samples (for extraction of phytoliths) and their paired charcoal or seeds samples were collected during the winter of 2015 from three archaeological sites (Shangshan, Hehuashan, and Huxi) in the Jinqu Basin of Zhejinang province (Fig. 1). These sites belong to the earliest Neolithic culture of the lower Yangtze River, the Shangshan Culture, which lasted from ∼11,000–8,600 cal yr B.P. (10). As Fig. S1B shows, the 2-m depth of the Huxi stratigrahphic profile consists of eight cultural layers. Sediment from the eighth to fourth strata developed during the middle and later phases of the Shangshan Culture (9,000–8,300 cal yr B.P.). Soil samples and charcoal were collected from the eighth to fifth strata. Only soil samples were collected in the fourth stratum because of the absence of charcoal and other dating materials. Two soil samples were collected from both the Shangshan and Hehuashan sites. Because of acidic soil conditions, few charcoal or seeds were recovered from Shangshan. Two charcoal samples and a nutshell were recovered after a large amount of flotation work was carried out on several pits in the Hehuashan site.
Phytolith Extraction.
A wet oxidation method that improves upon previous extraction processes was used for extracting phytoliths from the soil (21, 55–57). The detailed steps are as follows: (i) ∼130 g of dry soil was crushed and sieved to 500 µm; (ii) the sample was deflocculated with 5% sodium polyphosphates, then washed with distilled water three to four times; (iii) organic matter was first oxidized by 250 mL of H2O2 (30%) for 12 h at room temperature, and then heated in a water bath until the reaction stopped, then washed with distilled water three times; (iv) carbonates were eliminated using 200 mL of HCl (10%) by heating for 30 min, then washed with distilled water three times; (v) the >250-µm fraction was separated by wet sieving, and the remaining sample was disaggregated from the organic material and clay by ultrasonic treatment for 20 min; (vi) clays (<5 µm) were removed by gravity sedimentation until the sample was clear; (vii) the remaining higher-resistance materials were oxidized with 200 mL of HNO3 and a pinch of KClO3 by heating for 1 h, then washed with distilled water three to four times; (viii) phytoliths were extracted by washing three times with 200 mL of heavy liquid (ZnBr2) with a specific density of 2.35 g/cm3 and then three times with distilled water; (ix) extracted phytoliths were further sieved to 7 µm to remove clay, and then the recovered remains in the sieve were treated with 20 mL of H2O2 (30%) in a tube for 20 min, before being neutralized with distilled water; (x) finally, the recovered phytoliths were dried at 60 °C for 24 h for testing. Note that gravity sedimentation was used to wash samples before step (viii). However, a centrifuge was used to extract phytoliths and wash samples after step (viii).
Phytolith Purity Check.
The purity of phytolith concentration was first checked by optical microscopy, then further checked for extraneous organic materials by SEM-EDS analyses. EDS analysis was conducted to obtain quantitative information on the elemental compositions of the samples. It was used to verify phytolith purity and also to evaluate routine extraction processes (39, 58). In the EDS procedure, phytolith samples were first placed onto double-sided carbon tape and mounted on a stub. Then, a thin and highly conductive gold film was coated on the surface of samples using a vacuum sputter-coater to improve the quality of the SEM images. In this study, extracted phytoliths were analyzed using an SEM (Nova NanoSEM 450) linked to an EDS system (Aztec).
XRD is a rapid analytical technique mainly used for detection of crystalline structure. Here, we mainly used this technique to detect if carbonate, clays, and other minerals were still present in the extracted phytoliths. Air-dried phytoliths were first ground to <45 μm. Phytolith powders were placed into a sample holder. Then, the powders were smeared uniformly onto a glass slide, and a flat upper surface was ensured. Finally, the sample holder was placed into a sample container for detection. In this study, phytoliths were analyzed by XRD using a PANAlytical diffractometer at the Laboratory of Soil Structure and Materials, Institute of Geology and Geophysics, Chinese Academy of Sciences.
14C Measurement.
All phytolith and charcoal samples were sent to Beta Analytic Inc. Laboratory for radiocarbon dating. The submitted phytolith samples were suspended in deionized water and tested for neutral pH. All were found to have neutral pH. The samples were then centrifuged, vacuum desiccated, and weighed. In each case, 14C-free tin was added to approximately double the volume of the sample. The mixture was placed into a quartz container, which was inserted into a chemistry line and the line evacuated of all air. The chemistry line was evacuated to <0.2 mtorr and back-filled with 9.9999 Ar and 14C-free water six times to ensure the absence of any 14CO2 within the chemistry. The closed system was validated for vacuum integrity and then filled to 800 Torr with 9.9999 O2. Active O2 flow was established with collection traps encased in methanol slush to remove water and liquid nitrogen to collect combusted CO2. To combust the sample, a flame with a temperature higher than 1,000 °C was applied to the quartz container. In the process of combustion, the tin oxidized exothermically producing temperatures in excess of 1,400 °C. Upon completion of combustion, a small portion of gas was collected separately for δ13C analysis in an isotope ratio mass spectometer to obtain a value representative of the sample material. The remainder of the CO2 was collected in a quartz vessel containing a cobalt catalyst. Hydrogen (9.9999 purity) was added in a proportion of 3:1 H2 to CO2 and heated for 5 h at 595 °C. The evolved graphite was pressed directly from the graphitization vessel into a 14C-free cathode target and loaded into a 40 sample wheel designed for the National Electrostatics Corp (NEC) Source of Negative Ions by Cesium Sputtering source. Additionally, procedural blanks and standards run identically to the unknown were loaded into the wheel. The wheel was placed into an NEC 250Kv single-stage AMS system and subsequently calibrated and run over a 24-h period to derive the δ13C-corrected fraction of modern values relative to NIST-4990C (oxalic acid). The expected results for procedural standards were first validated, and the final radiocarbon age was then calculated and reported.
The standard acid-base-acid method was used to prepare the charcoal samples for radiocarbon analysis. Each sample was first visually inspected for size and durability. If necessary, it was reduced to 1- to 2-mm particles through dissecting and crushing followed by saturation with 0.1 N HCl heated to 70 °C for 1–2 h. After rinsing to neutral, 1–2% alkali was then repeatedly applied (50/50 wt NaOH) at 70 °C until no color change was observed or reasonable reduction in mass was observed. After rinsing to neutral, a final hot-acid wash (0.1 N HCl) was applied to ensure the alkali was neutralized, and once again rinsed to neutral. During this process, all roots and organic debris were eliminated. The sample was desiccated for 12–24 h, then microscopically examined for cleanliness, uniformity, and measured either as a whole or subsampled appropriately, where applicable. Combustion was then performed under 100% 9.9999 oxygen, as described for the phytoliths. Thereafter, they were subjected the same treatment process described above to produce graphite and the subsequent AMS results. No tin was needed because ready combustion was achieved at temperatures in excess of 1,000 °C.
All conventional 14C ages were calibrated using OXCal v4.2.2 (59) and the IntCal 13 atmospheric curve (60). A total of 18 samples were analyzed for their 14C contents. Of these, nine were phytolith samples, and the others were charcoal samples or nutshells.
Morphology of Rice Bulliform Phytoliths.
The fish-scale decorations on the edges of rice bulliforms can be used to differentiate wild rice from domesticated rice. It is well established that bulliform phytoliths of domesticated rice usually have more than nine fish-scale decorations, whereas wild rice varieties have fewer than nine (9, 25, 30) (Fig. S5). The vertical and horizontal lengths of rice bulliforms are an alternative, if possibly less secure, proxy for tracking the domestication process. Bulliforms of domesticated rice are usually, if not always, larger than wild ones (31). In this study, at least 50 rice bulliforms were counted and measured for each sample from Shangshan (stratum eight), Hehuashan (strata five and three), and Huxi (stratum six) sites.
Acknowledgments
We thank Prof. Dolores R. Piperno for her important suggestions, which subsequently improved this paper; Mr. Darden Hood and Ron Hatfield for their advice about phytolith dating; Prof. Chunxia Zhang and Ms. Bin Hu for their assistance with X-ray diffraction of the phytolith; Dr. Saihong Yang for her assistance with scanning electron microscopy and energy-dispersive spectrometer analysis; and Prof. Yunfei Zheng for providing several images of pottery sherds from Shangshan. This work was funded jointly by the 973 Program Grant 2015CB953801; the National Natural Science Foundation of China Grants 41230104, 41401230, 41430103, and 41472154; and the China Postdoctoral Science Foundation-funded project Grant 2014M561050.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704304114/-/DCSupplemental.
References
- 1.International Rice Research Institute . Bringing Hope, Improving Lives: Strategic Plan 2007–2015. IRRI; Manila, Philippines: 2006. pp. 1–61. [Google Scholar]
- 2.Nakamura S-i. The origin of rice cultivation in the Lower Yangtze Region, China. Archaeol Anthropol Sci. 2010;2:107–113. [Google Scholar]
- 3.Fuller DQ, et al. The domestication process and domestication rate in rice: Spikelet bases from the Lower Yangtze. Science. 2009;323:1607–1610. doi: 10.1126/science.1166605. [DOI] [PubMed] [Google Scholar]
- 4.Wei X, et al. Domestication and geographic origin of Oryza sativa in China: Insights from multilocus analysis of nucleotide variation of O. sativa and O. rufipogon. Mol Ecol. 2012;21:5073–5087. doi: 10.1111/j.1365-294X.2012.05748.x. [DOI] [PubMed] [Google Scholar]
- 5.Silva F, et al. Modelling the geographical origin of rice cultivation in Asia using the rice archaeological database. PLoS One. 2015;10:e0137024. doi: 10.1371/journal.pone.0137024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao Z, Piperno DR. Late Pleistocene/Holocene environments in the middle Yangtze River valley, China and rice (Oryza sativa L.) domestication: The phytolith evidence. Geoarchaeology. 2000;15:203–222. [Google Scholar]
- 7.Wu X, et al. Early pottery at 20,000 years ago in Xianrendong Cave, China. Science. 2012;336:1696–1700. doi: 10.1126/science.1218643. [DOI] [PubMed] [Google Scholar]
- 8.Fuller DQ. Pathways to Asian civilizations: Tracing the origins and spread of rice and rice cultures. Rice (N Y) 2011;4:78–92. [Google Scholar]
- 9.Lu HY, et al. Rice domestication and climatic change: Phytolith evidence from East China. Boreas. 2002;31:378–385. [Google Scholar]
- 10.Jiang L, Liu L. New evidence for the origins of sedentism and rice domestication in the Lower Yangzi River, China. Antiquity. 2006;80:355–361. [Google Scholar]
- 11.Gross BL, Zhao Z. Archaeological and genetic insights into the origins of domesticated rice. Proc Natl Acad Sci USA. 2014;111:6190–6197. doi: 10.1073/pnas.1308942110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Lee G-A, Jiang L, Zhang J. Evidence for the early beginning (c. 9000 cal. BP) of rice domestication in China: A response. Holocene. 2007;17:1059–1068. [Google Scholar]
- 13.Fuller DQ, Harvey E, Ling Q. Presumed domestication? Evidence for wild rice cultivation and domestication in the fifth millennium BC of the Lower Yangtze region. Antiquity. 2007;81:316–331. [Google Scholar]
- 14.Gilmore ZI. Direct radiocarbon dating of Spanish moss (Tillandsia usneoides) from early fiber-tempered pottery in the southeastern U.S. J Archaeol Sci. 2015;58:1–8. [Google Scholar]
- 15.Wilding LP, Brown RE, Holowaychuk N. Accessibility and properties of occluded carbon in biogenetic opal. Soil Sci. 1967;103:56–61. [Google Scholar]
- 16.Piperno DR. Phytolith radiocarbon dating in archaeological and paleoecological research: A case study of phytoliths from modern Neotropical plants and a review of the previous dating evidence. J Archaeol Sci. 2016;68:54–61. [Google Scholar]
- 17.Piperno DR. Standard evaluations of bomb curves and age calibrations along with consideration of environmental and biological variability show the rigor of phytolith dates on modern neotropical plants: Review of comment by Santos, Alexandre, and Prior. J Archaeol Sci. 2016;71:59–67. [Google Scholar]
- 18.Asscher Y, Weiner S, Boaretto E. A new method for extracting the insoluble occluded carbon in archaeological and modern phytoliths: Detection of 14C depleted carbon fraction and implications for radiocarbon dating. J Archaeol Sci. 2017;78:57–65. [Google Scholar]
- 19.Hodson MJ. The development of phytoliths in plants and its influence on their chemistry and isotopic composition. Implications for palaeoecology and archaeology. J Archaeol Sci. 2016;68:62–69. [Google Scholar]
- 20.Soter S. Radiocarbon anomalies from old CO2 in the soil and canopy air. Radiocarbon. 2011;53:55–69. [Google Scholar]
- 21.Piperno D. Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists. Altamira Press; Lanham, MD: 2006. pp. 1–21. [Google Scholar]
- 22.Zuo X, et al. Radiocarbon dating of prehistoric phytoliths: A preliminary study of archaeological sites in China. Sci Rep. 2016;6:26769. doi: 10.1038/srep26769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin H, et al. 2014. A primary study on AMS 14C dating of phytolith at Tianluoshan site, Zhejiang Province. Quat Sci 34:1–7. Chinese.
- 24.Ma Y, et al. Rice bulliform phytoliths reveal the process of rice domestication in the Neolithic Lower Yangtze River region. Quat Int. 2016;426:126–132. [Google Scholar]
- 25.Huan X, et al. Bulliform phytolith research in wild and domesticated rice paddy soil in South China. PLoS One. 2015;10:e0141255. doi: 10.1371/journal.pone.0141255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang L. 2013. The early Neolithic age of the Qiantangjiang Basin and its cultural lineage. Southeast Culture, 44–53. Chinese.
- 27.Zheng Y, Crawford GW, Jiang L, Chen X. Rice domestication revealed by reduced shattering of archaeological rice from the Lower Yangtze valley. Sci Rep. 2016;6:28136. doi: 10.1038/srep28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuller DQ, et al. Consilience of genetics and archaeobotany in the entangled history of rice. Archaeol Anthropol Sci. 2010;2:115–131. [Google Scholar]
- 29.Ball T, et al. Phytoliths as a tool for investigations of agricultural origins and dispersals around the world. J Archaeol Sci. 2016;68:32–45. [Google Scholar]
- 30.Wu Y, Jiang L, Zheng Y, Wang C, Zhao Z. Morphological trend analysis of rice phytolith during the early Neolithic in the Lower Yangtze. J Archaeol Sci. 2014;49:326–331. [Google Scholar]
- 31.Zheng Y, Matsui A, Fujiwara H. Phytoliths of rice detected in the Neolithic sites in the Valley of the Taihu Lake in China. Environ Archaeo. 2003;8:177–183. [Google Scholar]
- 32.Pearsall D, et al. Distinguishing rice (Oryza sativa Poaceae) from wild Oryza species through Phytolith analysis: Results of preliminary research. Econ Bot. 1995;49:183–196. [Google Scholar]
- 33.Gu YS, Zhao ZJ, Pearsall DM. Phytolith morphology research on wild and domesticated rice species in East Asia. Quat Int. 2013;287:141–148. [Google Scholar]
- 34.Ge Y, Jie DM, Guo JX, Liu HM, Shi LX. Response of phytoliths in Leymus chinensis to the simulation of elevated global CO2 concentrations in Songnen Grassland, China. Chin Sci Bull. 2010;55:3703–3708. [Google Scholar]
- 35.Issaharou-Matchi I, et al. Intraspecific biogenic silica variations in the grass species Pennisetum pedicellatum along an evapotranspiration gradient in South Niger. Flora. 2016;220:84–93. [Google Scholar]
- 36.Fuller DQ. Contrasting patterns in crop domestication and domestication rates: Recent archaeobotanical insights from the Old World. Ann Bot (Lond) 2007;100:903–924. doi: 10.1093/aob/mcm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fuller DQ, Qin L. 2009. Archaeobotany in the origin of the rice agriculture. Southern Cultural Relics, 38–45. Chinese.
- 38.Reyerson PE, et al. Unambiguous evidence of old soil carbon in grass biosilica particles. Biogeosciences. 2016;13:1269–1286. [Google Scholar]
- 39.Santos GM, et al. Possible source of ancient carbon in phytolith concentrates from harvested grasses. Biogeosciences. 2012;9:1873–1884. [Google Scholar]
- 40.Yin J, Yang X, Zheng Y. Influence of increasing combustion temperature on the AMS 14C dating of modern crop phytoliths. Sci Rep. 2014;4:6511. doi: 10.1038/srep06511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janz L, Feathers JK, Burr GS. Dating surface assemblages using pottery and eggshell: Assessing radiocarbon and luminescence techniques in Northeast Asia. J Archaeol Sci. 2015;57:119–129. [Google Scholar]
- 42.Taylor RE, Bar-Yosef O. Radiocarbon Dating: An Archaeological Perspective. 2nd Ed Left Coast Press; Walnut Creek, CA: 2014. [Google Scholar]
- 43.Yang X, et al. Barnyard grasses were processed with rice around 10000 years ago. Sci Rep. 2015;5:16251. doi: 10.1038/srep16251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Jiang L. 2016. A primary analysis on use-wear and residues of flaked stone tools from the Shangshan site, Zhejiang Province. Southern Cultural Relics, 117–121. Chinese.
- 45.Larson G, et al. Current perspectives and the future of domestication studies. Proc Natl Acad Sci USA. 2014;111:6139–6146. doi: 10.1073/pnas.1323964111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, et al. Early millet use in northern China. Proc Natl Acad Sci USA. 2012;109:3726–3730. doi: 10.1073/pnas.1115430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu H, et al. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc Natl Acad Sci USA. 2009;106:7367–7372. doi: 10.1073/pnas.0900158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhao Z. The process of origin of agriculture in China: Archaeological evidence from flotation results. Quat Sci. 2014;34:73–84. [Google Scholar]
- 49.Zhao Z. New archaeobotanic data for the study of the origins of agriculture in China. Curr Anthropol. 2011;52:S295–S306. [Google Scholar]
- 50.Zeder MA. The origins of agriculture in the Near East. Curr Anthropol. 2011;52:S221–S235. [Google Scholar]
- 51.Piperno DR. The origins of plant cultivation and domestication in the New World Tropics: Patterns, process, and new developments. Curr Anthropol. 2011;52:S453–S470. [Google Scholar]
- 52.Shakun JD, et al. Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation. Nature. 2012;484:49–54. doi: 10.1038/nature10915. [DOI] [PubMed] [Google Scholar]
- 53.Dong J, et al. A high-resolution stalagmite record of the Holocene East Asian monsoon from Mt Shennongjia, central China. Holocene. 2010;20:257–264. [Google Scholar]
- 54.Cohen DJ. The beginnings of agriculture in China. Curr Anthropol. 2011;52:S273–S293. [Google Scholar]
- 55.Zuo X, Lu H, Gu Z. Distribution of soil phytolith-occluded carbon in the Chinese Loess Plateau and its implications for silica–carbon cycles. Plant Soil. 2014;374:223–232. [Google Scholar]
- 56.Santos GM, et al. The phytolith 14C puzzle: A tale of background determinations and accuracy tests. Radiocarbon. 2010;52:113–128. [Google Scholar]
- 57.Carter JA. Atmospheric carbon isotope signatures in phytolith-occluded carbon. Quat Int. 2009;193:20–29. [Google Scholar]
- 58.Corbineau R, Reyerson PE, Alexandre A, Santos GM. Towards producing pure phytolith concentrates from plants that are suitable for carbon isotopic analysis. Rev Palaeobot Palynol. 2013;197:179–185. [Google Scholar]
- 59.Bronk Ramsey C, Lee S. Recent and planned developments of the program OxCal. Radiocarbon. 2013;55:720–730. [Google Scholar]
- 60.Reimer PJ, et al. IntCal13 and marine13 radiocarbon age calibration curves 0–50,000 years cal BP. Radiocarbon. 2013;55:1869–1887. [Google Scholar]
- 61.Alexandre A, et al. Direct uptake of organic carbon by grass roots and allocation in leaves and phytoliths: 13C labeling evidence. Biogeosciences. 2016;15:1693–1703. [Google Scholar]
- 62.Casey WH, Kinrade SD, Knight CTG, Rains DW, Epstein E. Aqueous silicate complexes in wheat, Triticum aestivum L. Plant Cell Environ. 2004;27:51–54. [Google Scholar]