Significance
The nitrogen cycle and the fixation of atmospheric N2 into ammonium are crucial to global food production. The industrial Haber–Bosch process facilitates half the global nitrogen fixation in the form of ammonia but it is energy- and resource-intensive, using natural gas as the source of energy and hydrogen at elevated temperature and pressure. Our alternative approach synthesizes ammonium from N2 and H2O at ambient conditions powered by water splitting, which may be driven renewably. The inorganic–biological hybrid system fixes atmospheric nitrogen into NH3 or soluble biomass with high fluxes and energy efficiency. Simultaneously, this system cultivates a living soil bacterium that acts as a potent biofertilizer amenable to boosting crop yields.
Keywords: nitrogen fixation, Xanthobacter, ammonia synthesis, fertilizer, solar
Abstract
We demonstrate the synthesis of NH3 from N2 and H2O at ambient conditions in a single reactor by coupling hydrogen generation from catalytic water splitting to a H2-oxidizing bacterium Xanthobacter autotrophicus, which performs N2 and CO2 reduction to solid biomass. Living cells of X. autotrophicus may be directly applied as a biofertilizer to improve growth of radishes, a model crop plant, by up to ∼1,440% in terms of storage root mass. The NH3 generated from nitrogenase (N2ase) in X. autotrophicus can be diverted from biomass formation to an extracellular ammonia production with the addition of a glutamate synthetase inhibitor. The N2 reduction reaction proceeds at a low driving force with a turnover number of 9 × 109 cell–1 and turnover frequency of 1.9 × 104 s–1⋅cell–1 without the use of sacrificial chemical reagents or carbon feedstocks other than CO2. This approach can be powered by renewable electricity, enabling the sustainable and selective production of ammonia and biofertilizers in a distributed manner.
The reduction of N2 into NH3 is essential for maintaining the global biogeochemical nitrogen (N) cycle (1). Fixed, organic N in food, biomass, and waste is eventually returned to the atmosphere as N2 through biological denitrification. As a ubiquitous, synthetic nitrogenous fertilizer, NH3 synthesized from atmospheric N2 via the Haber–Bosch process has been added to agricultural soils to drive global increases in crop yields (2). Despite its high efficiency and scalability, the Haber–Bosch process unsustainably employs natural gas as a H2 feedstock, operates at high temperatures and pressures, and relies on a significant infrastructure for NH3 distribution (1). A distributed approach toward NH3 synthesis from renewable energy sources at ambient conditions would enable on-site deployment and reduce CO2 emissions. To this end, significant effort has been devoted to promoting the reduction of nitrogen to NH3 with the use of transition metal catalysts (3–5), electrocatalysts (6), photocatalysts (7–11), purified nitrogenases (N2ases) (11, 12), and heterotrophic diazotrophs (13, 14), potentially powered by renewable energy and operating at ambient conditions. Such approaches, however, typically use sacrificial reductants to drive conversion at low turnover or suffer poor selectivity.
More broadly, the limitations of synthetic NH3 as a fertilizer have become apparent in recent years as decreasing efficiency of fertilizer use, coupled to environmental damage, has provided an imperative for the development of sustainable biofertilizers (15, 16). Soil microorganisms facilitate efficient nutrient uptake and recycling (17), pathogen resistance (18), environmental adaptation (19), and long-term soil productivity (15). However, the diminished yields of organic/sustainable agriculture have demonstrated that nutrient cycling alone, accentuated by natural variabilities in the soil microbiome, is insufficient to meet an increasing worldwide food demand (20). Attempts to establish robust, productive soil communities through microbial inocula have shown promise (21), but the limited natural flow of organic carbon into these soils results in a bottleneck in the biological activity of these largely heterotrophic biomes (22). An alternative solution would leverage the increasing abundance of renewable energy to cultivate and feed such soil microbiomes, effectively supplementing the natural process of microbial N2 fixation and plant beneficial interactions.
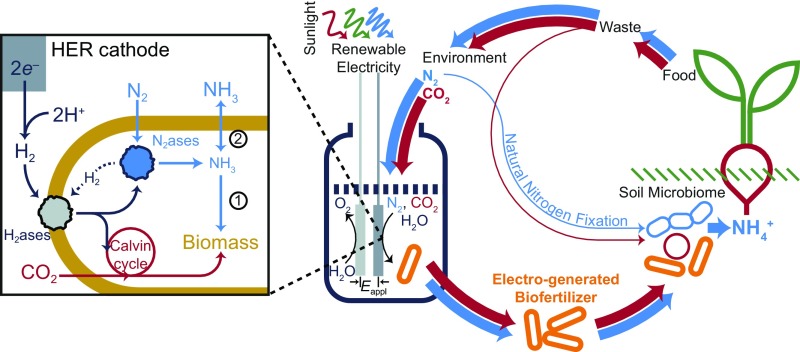
To further the development of distributed fertilization and natural N cycling, we demonstrate the reduction of N2 coupled to H2O oxidation by interfacing biocompatible water-splitting catalysts with the growth of N2-reducing, autotrophic, biofertilizing microorganisms in a single reactor (Fig. 1). This nitrogen cycle builds on our previous efforts that use H2 from water splitting to power a bioengineered microorganism to fix CO2 to biomass and liquid fuels at energy efficiencies far exceeding natural photosynthesis (23, 24). The biocompatible catalysts, a cobalt–phosphorus (Co–P) alloy for the hydrogen evolution reaction (HER) and an oxidic cobalt phosphate (CoPi) for the oxygen evolution reaction (OER), permit low driving voltages (Eappl) under mild conditions (pH 7, 30 °C). The combination of these electrocatalysts with H2-oxidizing microbes yields CO2 reduction efficiencies (ηelec,CO2) up to ∼50% (24). The modular design of this renewable synthesis platform may be leveraged beyond fuel production, toward more complex reactions such as the nitrogen reduction reaction (NRR), as well as cultivation of living whole-cell biofertilizers depending on the specific synthetic capabilities of the microorganism. We exploit this design flexibility to perform the efficient synthesis of NH3 from N2 and H2O by driving the NRR within the H2-oxidizing, autotrophic microorganism Xanthobacter autotrophicus. This Gram-negative diazotrophic bacterium can use H2 under microaerobic conditions (<5% O2) as its sole energy source to fix CO2 and N2 into biomass (25). We further demonstrate that X. autotrophicus functions as a potent electrogenerated biofertilizer, increasing yields of radishes (Raphanus sativus L. var. “Cherry Belle”), a fast-growing model food crop.
Fig. 1.
Schematic of the electroaugmented nitrogen cycle. A constant voltage (Eappl) is applied between CoPi OER and Co–P HER electrodes for water splitting. The H2ases of X. autotrophicus oxidize the generated H2, fueling CO2 reduction in the Calvin cycle and N2 fixation by N2ases. The generated NH3 is typically incorporated into biomass (pathway 1), but can also diffuse extracellularly by inhibiting biomass formation (pathway 2). This process is powered by renewable, solar-derived electricity taking N2 and CO2 from the environment. Cells of X. autotrophicus form an electrogenerated biofertilizer which may be added to soils to improve plant growth. The pathway of natural N cycling/N2 fixation is indicated, with line width denoting relative flux of these pathways. Red pathways indicate carbon cycling; blue pathways indicate N cycling.
Results and Discussion
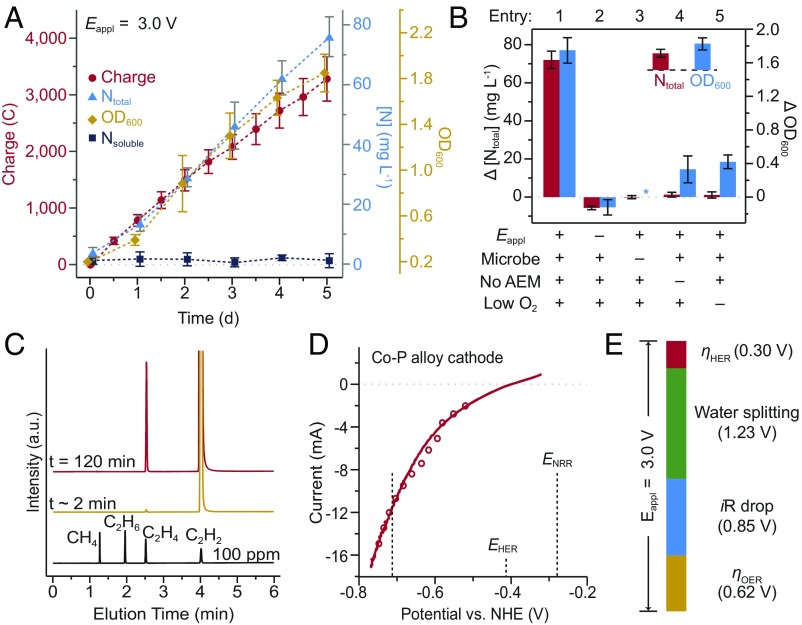
When interfaced with CoPi | Co–P water-splitting catalysts (Fig. 1), X. autotrophicus accumulates fixed N derived from the NRR. An O2/CO2/N2 gas mixture (2/20/78) was maintained in the single-chamber reactor housing the Co–P HER cathode and the CoPi OER anode (SI Appendix, Fig. S1). At the beginning of each experiment, X. autotrophicus was inoculated into the organic-free, N-free minimal medium. A constant driving voltage (Eappl = 3.0 V) was applied to the CoPi | Co–P catalyst system, and aliquots from the reactor were periodically sampled for the quantification of biomass (optical density at 600 nm, OD600) as well as fixed N (detected by two colorimetric assays, SI Appendix, Fig. S2A). The H2 generated from water splitting provides the biological energy supply for X. autotrophicus to perform the NRR, as well as CO2 reduction, into biomass without the need for sacrificial reagents (Fig. 2). The amount of faradaic charge passed into water splitting was proportional to biomass accumulation (OD600) as well as the total N content in the medium (Ntotal) during 5-d experiments (Fig. 2A). No biofilm formation was observed on either electrode. The fixed N was assimilated into biomass as evidenced by no change in the extracellular soluble N content (Nsoluble). Over the course of the experiment, 72 ± 5 mg L–1 of Ntotal, as well as 553 ± 51 mg L–1 of dry cell weight accumulated (n = 3, entry 1 in Fig. 2B and SI Appendix, Table S1). In contrast, no accumulation of Ntotal is observed in controls that omit one of the following elements in our experiment: H2 from water splitting, X. autotrophicus, a single-chamber reactor, or a microaerobic environment (entry 2–5 in Fig. 2B and SI Appendix, Table S1). The small increases in OD600 for entries 4 and 5 in Fig. 2B are likely due to light scattering from the accumulation of poly(3-hydroxybutyrate) (PHB). In the dual-chamber experiment where cathode and anode are separated by an anion-exchange membrane (AEM) (entry 4 in Fig. 2B), the absence of Ntotal accumulation is concurrent with an increase of soluble Co2+ (as determined by inductively coupled plasma mass spectroscopy, ICP-MS) in the medium from 0.9 ± 0.2 μM to 40 ± 6 μM over the course of 24 h, which approaches the ∼50-μM half-maximum inhibitory concentration (IC50) of X. autotrophicus (SI Appendix, Fig. S2B). As the AEM is not permeable to cations including Co2+, its use prevents the redeposition of adventitious Co2+ onto the CoPi anode via self-healing, as we have previously described (24, 26), emphasizing the importance of biocompatibility in our system.
Fig. 2.
N2 reduction on the CoPi | Co–P | X. autotrophicus hybrid system. (A) OD600, the amount of charge passed through, the concentration of total N content (Ntotal), and soluble N content (Nsoluble) are plotted versus the duration of experiments. n ≥ 3; error bars denote SEM. (B) Change of Ntotal and OD600 under different experimental conditions in 5-d experiments (SI Appendix, Table S1). “No AEM” indicates a single-chamber reaction without an anion-exchange membrane. *, not applicable because no bacteria were introduced. n ≥3; error bars denote SEM. (C) A qualitative gas chromatography comparison of the whole-cell acetylene reduction with 100-ppm standard sample. t, incubation time after C2H2 injection. (D) Linear scan voltammetry (line, 10 mV s–1) and chronoamperometry (circle, 30-min average) of Co–P HER cathode in X. autotrophicus medium, iR corrected. The thermodynamic values of HER and NRR (EHER, ENRR) are displayed. (E) Contributions of voltage drops within the applied Eappl = 3.0 V, as calculated SI Appendix. ηHER and ηOER, overpotentials of HER and OER. a.u., arbitrary units.
Whole-cell acetylene (C2H2) reduction (Fig. 2C) demonstrated high N2ase activity in X. autotrophicus grown in our hybrid system. This assay provides a measure of the NRR turnover number (TON) by using C2H2 as an isoelectronic surrogate of N2 (25, 27). Aliquots of X. autotrophicus sampled directly from our hybrid system reduced C2H2 exclusively to C2H4 at a rate of 127 ± 33 µM h–1 OD600–1 (n = 3), when the whole-cell aliquots were incubated under a C2H2/O2/H2/CO2/Ar gas environment (10/2/9/9/70). The measured C2H2-reduction activity corresponds to a rate of N2 fixation at ∼12 mg Ntotal L–1⋅d–1 for cultures of OD600 = 1.0, consistent with the measured Ntotal accumulation during the 5-d experiments (entry 1 in Fig. 2B and SI Appendix, Table S1). Combining a measured cell density of 3.8 ± 0.7 × 108 cells mL–1, this C2H2-reduction activity translates to an NRR turnover frequency (TOF) of 1.9 × 104 s–1 per bacterial cell, or roughly ∼4 s–1⋅N2ase–1, assuming a N2ase copy number of about 5,000 (28). The similarity between the measured NRR TOF per N2ase and the values reported in previous studies (12, 27, 29) indicated that the N2ases are fully functional in our experiments, highlighting the biocompatibility of the water-splitting catalysts in the CoPi | Co–P | X. autotrophicus hybrid system. The equivalent TON is ∼9 × 109 bacterial cell–1 and 2 × 106 N2ase–1, at least two orders of magnitude higher than that previously reported for synthetic (3–5, 7) and biological (11, 29) catalysts (SI Appendix, Table S2).
This hybrid system displays high faradaic efficiency for the NRR. A current of 10–12 mA for 100 mL of X. autotrophicus culture was needed to maintain sufficient microbial growth. This current was achieved with a cell potential of Eappl = 3.0 V (SI Appendix, Fig. S2C); this high driving voltage is due to the dilute medium salinity as X. autotrophicus is sensitive to high salt concentrations (25). The contributions of the HER and the OER overpotentials were determined by examining these half-reactions in the low salinity culture medium. Fig. 2D shows the I–V characteristics of the Co–P cathode for the HER vs. the normal hydrogen electrode (NHE). A 12-mA current is achieved for a driving voltage of –0.72 V vs. NHE or at a 0.3-V HER overpotential (ηHER) (EHER = –0.41 V at pH = 7). Following a similar procedure for the OER half-reaction, we determined that the OER overpotential (ηOER) is 0.62 V. As summarized in Fig. 2E, with the measured overpotentials, the contribution of ohmic resistance due to low ionic conductivity is ∼28% of Eappl (∼0.85 V). Given the standard potential difference between the HER and the NRR (EHER = −0.28 V), the driving force for the NRR by X. autotrophicus is ∼0.43 V, which is lower than the previously reported values that are summarized in SI Appendix, Table S2 (3–5, 29, 30). Based on the overall cell potential of 3.0 V, the energy efficiency of NRR (ηelec,NRR) is 1.8 ± 0.3% (n = 3) for a 5-d experiment (SI Appendix, Table S1). Parallel to NRR, X. autotrophicus fixes CO2 into cellular biomass (Fig. 2A) at a measured ηelec,CO2 = 11.6 ± 1.9% (n = 3). The theoretical value of ηelec,NRR at Eappl = 3.0 V is 7.5 ∼11.7% based on the reaction stoichiometry of N2ase and upstream biochemical pathways. Thus, our observation of ηelec,NRR = 1.8 ± 0.3% is 15 ∼23% of the theoretical ηelec,NRR. The calculated NRR faradaic efficiency is 4.5%, higher than the faradaic efficiencies or quantum yields of other reported NRR systems (6–11) at ambient conditions (SI Appendix, Table S2).
Modeling shows that linear microbial growth may be achieved by controlling the H2 concentration relative to the Michaelis constant of H2ase (24). Fig. 2A shows that linear growth conditions may be achieved for X. autotrophicus by balancing the H2 produced from water splitting and microbial H2 oxidation. Importantly, the H2 inhibition constant of N2ase is Ki(D2) ∼11 kPa (31); the low H2 partial pressure generated by water splitting (roughly 0.3% or 0.3 kPa H2, depending on gas flow rate) does not impede N2 fixation and/or reduce the NRR energy efficiency. When the reactor is pressurized with an external H2 source, microbial growth is attenuated (SI Appendix, Fig. S3A). This contention is illustrated by numerical simulations (SI Appendix, Fig. S3B), which show slower biomass accumulation in the case of microbes under high H2 concentration but linear growth in CoPi | Co–P water splitting. In this regard, the direct hybrid device provides potential benefit, as the generated H2 from water splitting not only provides cellular energy but also controls metabolic pathways, thus allowing growth conditions to be controlled with fidelity.
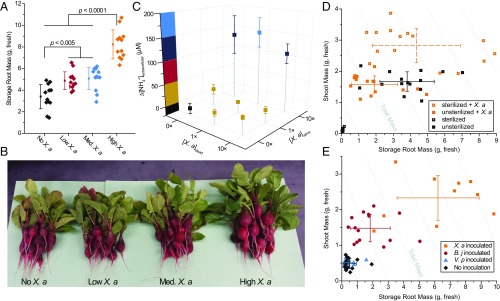
X. autotrophicus cells can be applied directly to promote plant growth and in this regard is, in effect, a living biofertilizer. Cultures of X. autotrophicus were collected, washed, resuspended in 50 mM NaCl saline, and applied to greenhouse radish growth experiments to assess their ability to improve harvest yields (Fig. 3). Increasing amounts of X. autotrophicus, applied weekly, increased edible radish storage root mass by up to ∼130% compared with unfertilized controls from 3.4 ± 1.2 g plant–1 to 8.2 ± 1.4 g plant–1 (n = 12 radishes for both) (Fig. 3 A and B and SI Appendix, Fig. S4A), and total mass by up to ∼100% (SI Appendix, Fig. S4 B and C). To evaluate a potential mechanism of improving plant growth, in vitro fertilization assays were conducted by suspending X. autotrophicus cells in a 50 mM NaCl solution and measuring extracellular secretion of N and P. Only living cells of X. autotrophicus released NH4+ and PO43– over the course of the 7-d experiment, with ethanol-killed controls displaying no or negative nutrient release behavior (Fig. 3C and SI Appendix, Fig. S5 A and B). Under these saline “starvation” conditions, these bacteria maintained ∼50% cell viability, likely metabolizing stored PHB carbon reserves (SI Appendix, Fig. S5A) (32, 33), demonstrating their capacity to be self-sustaining even in the absence of external energy inputs. Whereas actively growing X. autotrophicus cells do not secrete Nsoluble (Fig. 2A), under these starvation conditions, leaky reutilization pathways potentially slowly release N and P from polyglutaminyl-rich cell membranes (34) and polyphosphate granules (32). From this, we contend that living X. autotrophicus cells improve plant growth as a slow-release source of bioavailable N and P, demonstrating the capability of this hybrid inorganic–biological NRR cycle to effectively bridge the gap between atmospheric N2 and plant biomass.
Fig. 3.
X. autotrophicus biofertilization of radishes. (A) Yields of radish storage roots from biofertilization with different amounts of X. autotrophicus (X. a) (n = 12 radishes per treatment) in as-supplied potting media. No X. a: OD600 = 0, Low X. a: OD600 = 0.03, Med. X. a: OD600 = 0.3, High X. a: OD600 = 3.0, applied at t = 7, 14 d. Corresponding dry masses and shoot masses are given in SI Appendix, Fig. S4 A–C. Significance (P value) calculated by a two-tailed, heteroscedastic Student’s t test. (B) Photographs of radishes from A. (C) Extracellular NH4+ release from live and dead X. autotrophicus after 7 d in 50 mM NaCl starvation conditions. Dead cells were prepared by 70% EtOH sterilization. 0×: OD600 = 0, 1×: OD600 = 0.5, 10×: OD600 = 5 (n = 3 biological replicates). (D) Growth yields of radish seeds with and without seed sterilization by hypochlorite treatment, and preinoculation with and without X. autotrophicus (n = 15). Experiments conducted in sterilized potting medium. (E) Growth yields of radish seeds sterilized and inoculated with X. autotrophicus, B. japonicum, V. paradoxus, or no inoculation, fertilized at t = 7, 14 d with X. autotrophicus biofertilizer in sterilized potting medium. All error bars indicate the SD centered on the arithmetic mean.
X. autotrophicus engages in specific plant–microbe and soil microbe–microbe interactions. To study this, we evaluated the effect of soil microbiome composition on X. autotrophicus biofertilizer performance. As-supplied potting media (Promix HP MYCORRHIZAE) contains a plant-growth-promoting fungal inoculant. Reuse of the potting media or sterilization by autoclaving effectively removes these microbial species (SI Appendix, Fig. S5 C and D), allowing more direct assessment of radish–X. autotrophicus interactions. Interestingly, reused potting media previously fertilized with X. autotrophicus exhibit a carryover effect, demonstrating that this biofertilizer improves plant yields over multiple planting cycles (SI Appendix, Fig. S5E), although comparatively lower yields from cycle 1 to cycle 2 suggest continued biofertilization produces the best radish growth. Radish seeds themselves also carry along native microbial inoculants on their seed coat and can be sterilized by quick treatment with a dilute hypochlorite solution (35). Radish seeds sterilized by this method showed lower growth yields (Fig. 3D) compared with unsterilized controls, attesting to the importance of plant–microbe commensal interactions. Radish seeds preincubated with X. autotrophicus before sowing, in the method of biopriming (35), were able to compensate for the loss of the native radish microbiome, and in fact improve total plant mass by ∼40% compared with unsterilized controls (Fig. 3D). This interaction was found to be somewhat specific to X. autotrophicus as inoculation of sterilized and unsterilized radish seeds with another diazotroph, Bradyrhizobium japonicum [American Type Culture Collection (ATCC) 10324], and a plant-growth-promoting bacterium, Variovorax paradoxus (ATCC 17713) (36), showed similar yields as unsterilized radish seeds (SI Appendix, Fig. S4D). To discern the soil microbe–microbe interactions, radish seeds were inoculated with different plant-growth-promoting bacteria, and fertilized weekly with X. autotrophicus cells (Fig. 3E). Uninoculated radish seeds in sterile soil showed poor growth even with X. autotrophicus fertilization, suggesting that biofertilization is most effective in cooperation with preestablished plant–microbe relationships (from biopriming or soil fungal inoculants) to facilitate nutrient uptake. Growth improved upon inoculation with B. japonicum and no significant change was observed with V. paradoxus, although the greatest growth increase was for X. autotrophicus inoculated and fertilized radishes, at an average storage root mass of 6.2 ± 2.7 g plant–1 (n = 10) compared with 0.4 ± 0.2 g plant–1 (n = 14) for uninoculated controls, an improvement of ∼1,440%. These results suggest that X. autotrophicus not only works as a biofertilizer in conjunction with other members of the soil microbiome (as represented by B. japonicum), but that X. autotrophicus itself engages in a unique plant-growth-promoting mechanism with radishes to realize increased yields. Its function as a soil and seed inoculant in addition to acting as a N and P source positions it as a potential strategy to revive biologically degraded and nonarable soils (21). Further studies will be required to unambiguously define the mechanism of X. autotrophicus–plant interactions.
X. autotrophicus interacts favorably with sustainable abiotic fertilizers. One such natural fertilizer is human urine, which is an attractive alternative soluble N and P fertilizer (37), as well as a potential growth substrate for soil bacteria. X. autotrophicus was found to grow autotrophically in a synthetic defined urine medium (38) diluted to an appropriate ionic concentration (SI Appendix, Fig. S5F). This bacterium was also tolerant to the variable loading of N and P associated with their concentration in natural urine sources (SI Appendix, Fig. S5G) (39, 40). These results suggest that this biofertilizer is compatible with existing sustainable fertilizers as a potential medium for X. autotrophicus cultivation and in-soil propagation. As observed for other soil bacteria (15), the ability of X. autotrophicus to transform the labile N and P in urine into stable, slow-release forms is an intriguing prospect for future longitudinal field studies (20).
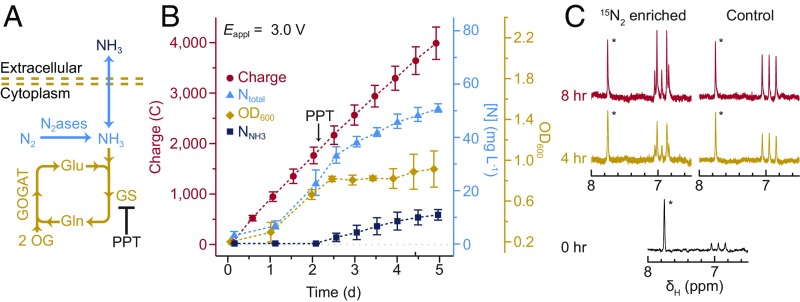
In addition to fixing N2 in the form of soluble biomass, the hybrid device can be induced to excrete synthesized NH3 directly into the extracellular medium. Genome sequencing of the strain of X. autotrophicus used here (SI Appendix, Table S3) indicates that the NH3 generated from N2ase is incorporated into biomass via a two-step process mediated by glutamine synthetase (GS) and glutamate synthase (GOGAT) (Fig. 4A), as is consistent with previous biochemical assays (41). If the functionality of this NH3 assimilation pathway is disrupted, direct production of extracellular NH3 should occur. Noting that GS inhibitors can induce NH3 secretion in sugar-fermenting diazotrophs (14), we turned to the specific GS inhibitor phosphinothricin (PPT) (42) to block the NH3 assimilation pathway and allow NH3 to passively diffuse out into the extracellular medium (pathway 2 in Figs. 1 and 4A). After adding a sufficiently high concentration of phosphinothricin (50 μM) to inhibit GS (42), the biomass accumulation of X. autotrophicus slows, while Ntotal and the concentration of free NH3 in the solution (NNH3) continues to increase (Fig. 4B), indicating that N2 fixation after phosphinothricin addition is in the form of extracellular NH3. 15N isotope labeling experiments confirm that the generated NH3 is from N2 (Fig. 4C). After exposing the microbes to 15N-enriched N2, the intensity of the 15NH4+ doublet peaks (6.91 ppm, J1NH = 72.7 Hz) in the 1H NMR spectrum of the supernatant monotonically increases. This 15NH4+ signal is not detectable in control samples under N2 of natural abundance; the 1H NMR exhibits only the triplet signal of 14NH4+ (6.95 ppm, J1NH = 50.0 Hz). The concentration of N in NH3 (NNH3) after terminating the experiment was 11 ± 2 mg L–1 (∼0.8 mM) and the accumulated Ntotal reached 47 ± 3 mg L–1 (n = 3, SI Appendix, Table S1). The rate of N2 fixation decreases in the latter phase of experiment due to down-regulation at transcriptional and posttranscriptional levels (43).
Fig. 4.
NH3 production in the extracellular media. (A) Biochemical diagram of NH3 synthesis from N2. OG, 2-oxoglutarate; PPT, phosphinothricin. (B) The OD600, the amount of charge passed through, the concentration of Ntotal and NNH3 are plotted versus the duration of experiments. The addition of PPT as GS inhibitor was displayed. n = 3; error bars denote SEM. (C) The 1H NMR spectrum evolution of generated NH4+ under 15N-enriched and naturally abundant N2. Time counted as the duration after providing 15N2. * denotes resonances for the internal standard of H–CON(CH3)2. Gln, glutamine; Glu, glutamic acid.
Conclusion
We demonstrate herein the synthesis of solid N biomass and NH3 from N2, driven by water splitting at appreciable TON and TOF, under ambient conditions. By driving water splitting with solar energy and renewable electricity, the approach can potentially provide a renewable synthesis platform for the NRR. We demonstrate that the production of NH3 and X. autotrophicus biofertilizer allows this hybrid inorganic–biological NRR system to effectively connect atmospheric N2 to plant biomass. The hybrid inorganic–biological approach illustrated for the NRR may be generalized to a renewable biological and chemical synthesis platform, depending on the biomachinery to which water splitting is coupled.
Supplementary Material
Acknowledgments
We thank N. Li for ICP-MS measurement; M. Lewandowski for the acetylene reduction assay; B. Greene for numerical simulation; H. Chung for assistance on genome variant calling; S. Zhang for 1H NMR; and D. Gygi, S. Hays, C. Lemon, X. Ling, C. Myrvhold, and Y. Song from Harvard University, and X. Ling from Nanyang Technological University for helpful discussions. K.K.S. thanks K. J. Woodruff and the Harvard University Arnold Arboretum for assistance with greenhouse studies. C.L. was supported by a Lee Kuan Yew Postdoctoral Fellowship, K.K.S was supported by a Harvard University Center for the Environment Fellowship, and B.C.C. was supported by a predoctoral fellowship from the National Science Foundation Graduate Research Fellowships Program. P.A.S. acknowledges grant support from the Office of Naval Research Multidisciplinary University Research Initiative Award N00014-11-1-0725 and the Wyss Institute for Biologically Inspired Engineering. P.A.S. and D.G.N. thank the Harvard University Climate Change Solutions Fund and the Campus Sustainability Innovation Fund for support. This work was performed at Harvard University under the support of the First 100 W Project, provided by the TomKat Foundation.
Footnotes
Conflict of interest statement: A provisional patent application has been filed related to the technology described in this paper.
Data deposition: The sequence reported in this paper has been deposited in the National Center for Biotechnology Information Sequence Read Archive (NCBI SRA) database (accession no. SRP073266).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706371114/-/DCSupplemental.
References
- 1.Smil V. Detonator of the population explosion. Nature. 1999;400:415. [Google Scholar]
- 2.Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 3.Yandulov DV, Schrock RR. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science. 2003;301:76–78. doi: 10.1126/science.1085326. [DOI] [PubMed] [Google Scholar]
- 4.Arashiba K, Miyake Y, Nishibayashi Y. A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nat Chem. 2011;3:120–125. doi: 10.1038/nchem.906. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JS, Rittle J, Peters JC. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature. 2013;501:84–87. doi: 10.1038/nature12435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lan R, Irvine JTS, Tao S. Synthesis of ammonia directly from air and water at ambient temperature and pressure. Sci Rep. 2013;3:1145. doi: 10.1038/srep01145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, et al. Nitrogenase-mimic iron-containing chalcogels for photochemical reduction of dinitrogen to ammonia. Proc Natl Acad Sci USA. 2016;113:5530–5535. doi: 10.1073/pnas.1605512113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu D, Zhang L, Ruther RE, Hamers RJ. Photo-illuminated diamond as a solid-state source of solvated electrons in water for nitrogen reduction. Nat Mater. 2013;12:836–841. doi: 10.1038/nmat3696. [DOI] [PubMed] [Google Scholar]
- 9.Oshikiri T, Ueno K, Misawa H. Selective dinitrogen conversion to ammonia using water and visible light through plasmon-induced charge separation. Angew Chem Int Ed Engl. 2016;55:3942–3946. doi: 10.1002/anie.201511189. [DOI] [PubMed] [Google Scholar]
- 10.Ali M, et al. Nanostructured photoelectrochemical solar cell for nitrogen reduction using plasmon-enhanced black silicon. Nat Commun. 2016;7:11335. doi: 10.1038/ncomms11335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown KA, et al. Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid. Science. 2016;352:448–450. doi: 10.1126/science.aaf2091. [DOI] [PubMed] [Google Scholar]
- 12.Milton RD, et al. Nitrogenase bioelectrocatalysis: Heterogeneous ammonia and hydrogen production by MoFe protein. Energy Environ Sci. 2016;9:2550–2554. [Google Scholar]
- 13.Shanmugam KT, Valentine RC. Microbial production of ammonium ion from nitrogen. Proc Natl Acad Sci USA. 1975;72:136–139. doi: 10.1073/pnas.72.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz-Marquez JCF, Do Nascimento M, Curatti L. Metabolic engineering of ammonium release for nitrogen-fixing multispecies microbial cell-factories. Metab Eng. 2014;23:154–164. doi: 10.1016/j.ymben.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Chaparro JM, Sheflin AM, Manter DK, Vivanco JM. Manipulating the soil microbiome to increase soil health and plant fertility. Biol Fertil Soils. 2012;48:489–499. [Google Scholar]
- 16.Tilman D, Balzer C, Hill J, Befort BL. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA. 2011;108:20260–20264. doi: 10.1073/pnas.1116437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carvalho TLG, Balsemão-Pires E, Saraiva RM, Ferreira PCG, Hemerly AS. Nitrogen signalling in plant interactions with associative and endophytic diazotrophic bacteria. J Exp Bot. 2014;65:5631–5642. doi: 10.1093/jxb/eru319. [DOI] [PubMed] [Google Scholar]
- 18.Rudrappa T, Czymmek KJ, Paré PW, Bais HP. Root-secreted malic acid recruits beneficial soil bacteria. Plant Physiol. 2008;148:1547–1556. doi: 10.1104/pp.108.127613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodriguez RJ, et al. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008;2:404–416. doi: 10.1038/ismej.2007.106. [DOI] [PubMed] [Google Scholar]
- 20.Mäder P, et al. Soil fertility and biodiversity in organic farming. Science. 2002;296:1694–1697. doi: 10.1126/science.1071148. [DOI] [PubMed] [Google Scholar]
- 21.Wubs ERJ, van der Putten WH, Bosch M, Bezemer TM. Soil inoculation steers restoration of terrestrial ecosystems. Nat Plants. 2016;2:16107. doi: 10.1038/nplants.2016.107. [DOI] [PubMed] [Google Scholar]
- 22.Kramer C, Gleixner G. Variable use of plant- and soil-derived carbon by microorganisms in agricultural soils. Soil Biol Biochem. 2006;38:3267–3278. [Google Scholar]
- 23.Torella JP, et al. Efficient solar-to-fuels production from a hybrid microbial-water-splitting catalyst system. Proc Natl Acad Sci USA. 2015;112:2337–2342. doi: 10.1073/pnas.1424872112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Colón BC, Ziesack M, Silver PA, Nocera DG. Water splitting-biosynthetic system with CO2 reduction efficiencies exceeding photosynthesis. Science. 2016;352:1210–1213. doi: 10.1126/science.aaf5039. [DOI] [PubMed] [Google Scholar]
- 25.Malik KA, Schlegel HG. Enrichment and isolation of new nitrogen-fixing hydrogen bacteria. FEMS Microbiol Lett. 1980;8:101–104. doi: 10.1007/BF00446833. [DOI] [PubMed] [Google Scholar]
- 26.Bediako DK, Ullman AM, Nocera DG. Catalytic oxygen evolution by cobalt oxido thin films. Top Curr Chem. 2016;371:173–213. doi: 10.1007/128_2015_649. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman BM, Lukoyanov D, Yang Z-Y, Dean DR, Seefeldt LC. Mechanism of nitrogen fixation by nitrogenase: The next stage. Chem Rev. 2014;114:4041–4062. doi: 10.1021/cr400641x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider K, et al. The molybdenum nitrogenase from wild-type Xanthobacter autotrophicus exhibits properties reminiscent of alternative nitrogenases. Eur J Biochem. 1995;230:666–675. doi: 10.1111/j.1432-1033.1995.0666h.x. [DOI] [PubMed] [Google Scholar]
- 29.Danyal K, et al. Uncoupling nitrogenase: Catalytic reduction of hydrazine to ammonia by a MoFe protein in the absence of Fe protein-ATP. J Am Chem Soc. 2010;132:13197–13199. doi: 10.1021/ja1067178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuriyama S, et al. Catalytic formation of ammonia from molecular dinitrogen by use of dinitrogen-bridged dimolybdenum-dinitrogen complexes bearing PNP-pincer ligands: Remarkable effect of substituent at PNP-pincer ligand. J Am Chem Soc. 2014;136:9719–9731. doi: 10.1021/ja5044243. [DOI] [PubMed] [Google Scholar]
- 31.Guth JH, Burris RH. Inhibition of nitrogenase-catalyzed NH3 formation by H2. Biochemistry. 1983;22:5111–5122. doi: 10.1021/bi00291a010. [DOI] [PubMed] [Google Scholar]
- 32.Wiegel J, Wilke D, Baumgarten J, Opitz R, Sschlegel HG. Transfer of the nitrogen-fixing hydrogen bacterium Corynebacterium autotrophicum Baumgarten et al. to Xanthobacter gen. nov. Int J Syst Bacteriol. 1978;28:573–581. [Google Scholar]
- 33.Trainer MA, Charles TC. The role of PHB metabolism in the symbiosis of rhizobia with legumes. Appl Microbiol Biotechnol. 2006;71:377–386. doi: 10.1007/s00253-006-0354-1. [DOI] [PubMed] [Google Scholar]
- 34.Kandler O, König H, Wiegel J, Claus D. Occurrence of poly-γ-D-glutamic acid and poly-α-L-Glutamine in the genera Xanthobacter, Flexithrix, Sporosarcina and Planococcus. Syst Appl Microbiol. 1983;4:34–41. doi: 10.1016/S0723-2020(83)80032-0. [DOI] [PubMed] [Google Scholar]
- 35.Mahmood A, Turgay OC, Farooq M, Hayat R. 2016. Seed biopriming with plant growth promoting rhizobacteria: A review FEMS Microbiol Ecol 92: fiw112 10.1093/femsec/fiw112. [DOI] [PubMed]
- 36.Satola B, Wübbeler JH, Steinbüchel A. Metabolic characteristics of the species Variovorax paradoxus. Appl Microbiol Biotechnol. 2013;97:541–560. doi: 10.1007/s00253-012-4585-z. [DOI] [PubMed] [Google Scholar]
- 37.Heinonen-Tanski H, van Wijk-Sijbesma C. Human excreta for plant production. Bioresour Technol. 2005;96:403–411. doi: 10.1016/j.biortech.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 38.Anderson MS, Ewert MK, Keener JF, Wagner SA, editors. Life Support Baseline Values and Assumptions Document. NASA; Houston: 2015. Tech Rep NASA/TP-2015-218570, Document ID 20150002905. [Google Scholar]
- 39.Wydeven T, Golub MA. Generation Rates and Chemical Compositions of Waste Streams in a Typical Crewed Space Habitat. NASA, Moffett Field; CA: 1990. Tech Rep NASA/TM-102799, Document ID 19900019017. [Google Scholar]
- 40.Rose C, Parker A, Jefferson B, Cartmell E. The characterization of feces and urine: A review of the literature to inform advanced treatment technology. Crit Rev Environ Sci Technol. 2015;45:1827–1879. doi: 10.1080/10643389.2014.1000761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murrell JC, Lidstrom ME. Nitrogen metabolism in Xanthobacter H4-14. Arch Microbiol. 1983;136:219–221. [Google Scholar]
- 42.Gill HS, Eisenberg D. The crystal structure of phosphinothricin in the active site of glutamine synthetase illuminates the mechanism of enzymatic inhibition. Biochemistry. 2001;40:1903–1912. doi: 10.1021/bi002438h. [DOI] [PubMed] [Google Scholar]
- 43.Dixon R, Kahn D. Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol. 2004;2:621–631. doi: 10.1038/nrmicro954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.