For decades researchers have investigated the efficacy of various methods for blunting the development of hyperthermia during exercise in the heat and the associated reduction in endurance performance. Somewhat recently, much of this research has examined the utility of cold fluid ingestion for this purpose. Although several studies have supported the notion that exercise performance can be improved with cold fluid ingestion, a fairly surprising finding has been that despite a greater internal heat loss, athletes are typically no cooler at the end of an exercise bout of fixed intensity and duration than with the ingestion of a thermoneutral fluid.1 We recently demonstrated that these similar core temperatures were likely due to fluid temperature-dependent alterations in sweating, whereby warm and cold fluid ingestion caused increases and decreases in sweating, respectively, which then resulted in parallel changes in evaporative heat loss that were approximately equal to the heat gained or lost to the ingested fluid.
Typically, one would expect this alteration in sweating to occur due to changes of core and/or skin temperature following cold fluid ingestion, thereby lowering the efferent hypothalamic signaling to sweat. Furthermore, the decrease in core temperature and sweating would not be expected to occur immediately following fluid ingestion, but would be delayed, as time would be required for adequate heat transfer to occur between the viscera, blood, and hypothalamus. Upon analysis of our continuous measurements of tympanic, rectal, and skin temperature, we observed no fluid temperature-dependent fluctuations in core or skin temperature (represented as mean body temperature in Fig. 1; lower left panel), whereas the local sweat rate changed sharply within a minute of ingestion (Fig. 1; upper left panel).2 Taken together, these observations indicated the existence of thermoreceptors along the gastrointestinal tract with the capacity to independently modulate sweating.
Figure 1.
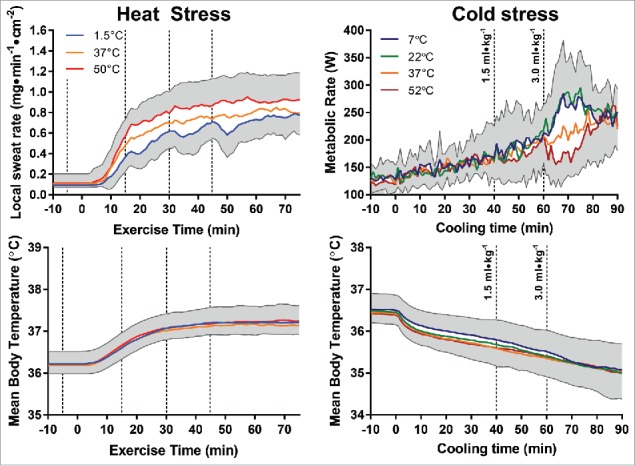
Redrawn thermometric (mean body temperature, bottom panels) and physiologic responses (sweat rate, top left panel; metabolic rate, top right panel) to warm and cold fluid ingestion during exercise in the heat from Morris et al. 20142 (left panels) and passive cold exposure from Morris et al. 20163 (right panels). Dashed lines denote time points at which fluid ingestion occurred.
To further examine the potential location of these thermoreceptors, we performed a follow-up experiment in which participants either swilled warm (50°C) or cold (1.5°C) water in their mouth only, or received a similar volume of fluid delivered directly into the stomach via a nasogastric tube.2 No fluid temperature-dependent alterations in sweating were observed with mouth swilling, but rapid alterations in sweating occurred in the nasogastric tube trial that were comparable to the sweating responses observed with regular ingestion. In parallel, similar core and skin temperatures were observed between all trials irrespective of ingested fluid temperature and method of administration, indicating that the thermoreceptors in question were located in the abdominal cavity.2
To further examine the nature of these thermoreceptors, we most recently assessed the effect of ingested fluid temperature on thermogenic shivering during cold stress (Fig. 1; right panels).3 In this study, participants ingested 1.5 or 3.0 mL/kg of 7°C, 22°C, 37°C or 52°C water, whereas 5°C water was circulated around a water perfused suit for 90 minutes to induce a cold stress. Similar to our earlier heat stress experiments, though there were no differences in core or skin temperatures (expressed as mean body temperature in Fig. 1; lower right panel) with the ingestion of different water temperatures, 52°C fluid ingestion dramatically and rapidly reduced the metabolic rate (Fig. 1; upper right panel) and 7°C and 22°C fluid ingestion similarly increased the metabolic rate. Electromyography measurements demonstrated that muscle activation was altered within 1 min of 7°C, 22°C, or 52°C fluid ingestion, which matched the temporal development of changes in sweating with different ingested fluid temperature during exercise in the heat. One notable difference in cold defense responses compared with the heat defense responses was that the 7°C and 22°C fluids altered shivering to a similar extent, whereas during exercise in the heat, graded alterations in sweating were observed depending on the heat sink introduced with 10°C, 1.5°C, and ice slushy ingestion.
In contrast to human thermoregulatory models that typically list the hypothalamus and skin as the sole locations of thermoreceptors that modify thermoregulatory responses, animal thermoregulatory models support our findings as many thermoreceptor locations including muscle, large veins, lower esophagus, stomach, and small intestine are accepted.4 Of particular note, visceral and cutaneous thermoreceptors follow similar central integration pathways and elicit similar responses to a given change in temperature.4 Additionally, both warm and cold thermoreceptors reside in these areas, which operate in concert to maintain thermal homeostasis by innervating areas of the brain that elicit both physiologic and behavioral thermoregulatory responses. Accordingly, it follows that the origin of visceral thermoreceptors likely served to defend against internal thermal stress during water ingestion in the wild, before the ability of humans to manipulate the temperature of the food and fluids they ingest.
With the consideration of this thermoregulatory circuit in mind, can the ingestion of cold fluids be beneficial for keeping cool under heat stress, and can warm fluids be beneficial for keeping warm under cold stress? Typically, the primary heat stressor in humans is the heat produced as a by-product of metabolism, inevitably leading to rises in core temperature. As any ingested internal thermal load is counter-balanced in advance of any alterations in core temperature, exercising athletes find it difficult to cool down with cold fluid ingestion. However, exceptions to this rule do exist. For example, the ingestion of cold fluids before the onset of exercise has been shown to reliably decrease core temperature,1 likely because sweating has not commenced and consequently cannot be reduced. Therefore, the internal heat sink is effective and core temperature decreases to the lower end of the interthreshold zone. However, it should be noted that this lowering of core temperature delays the onset of sweating once exercise has begun.2 Alternatively, cold fluid ingestion could also be beneficial under uncompensable conditions, wherein the ingested cold fluid provides an additional heat loss avenue and sweating efficiency is sufficiently compromised so that any reductions in sweating likely minimally affect evaporative heat loss. Finally, as feelings of thermal perception improve, cold fluid ingestion could be beneficial for inducing sensation of coolness and a subsequent increase in self-paced exercise intensity, which would be beneficial for performance.1 However, as exercise intensity increases, so would the metabolic rate, potentially leading to a paradoxical increase in core temperature.
Contrary to heat stress, cold stress is exclusively passive; meaning that cooling occurs from the outside-in. As such, core temperature is not greatly affected except in cases of prolonged moderate to severe cold exposure. However, under these conditions, core temperature is defended at the cost of prolonged shivering, both through prolonged increases in muscle tone as well as strong, asynchronous muscle contractions to produce sufficient metabolic heat to sustain a normothermic core temperature. These increases in muscle activity come at the cost of impaired gross motor performance, work efficiency, range of motion at the joints, conduction velocity along muscle fibers, and accuracy of proximal (but not distal) muscle groups as well as increased fatigue and mental confusion.5 Furthermore, while voluntarily suppressing shivering may help to reduce the associated decrements in gross motor performance, the consequent reduction in metabolic heat production may result in the development of hypothermia.5 Therefore, under these conditions, warm fluid ingestion may be an attractive and practical option to improve gross motor performance in the cold, both in the context of athletic endeavors as well as jobs involving manual labor, without threatening thermal homeostasis. However, it is worth noting that in our study3 shivering was reduced for only 10 min with ∼235 mL of 52°C fluid ingestion and therefore to prolong the benefits of warm fluid ingestion, larger fluid volumes may be required, which may result in an increased need to urinate.
In summary, we present strong evidence that thermoreceptors are located in the human abdomen, comparable to thermoreceptors described in other animals. Agreeing with animal models, the stimulation of these thermoreceptors modifies both physiologic and perceptual thermoregulatory responses to restore thermal homeostasis before core temperature being affected. As such, modifying the temperature of an ingested fluid under most circumstances is not beneficial for modifying core temperature but may be beneficial for improving the capacity to perform physical tasks by improving thermal comfort in the heat and gross motor performance in the cold.
References
- [1].Tan PMS, Lee JKW. The role of fluid temperature and form on endurance performance in the heat. Scand J Med Sci Sports. 2015; 25 Suppl 1:39-51. PMID:25943655; doi: 10.1111/sms.12366. [DOI] [PubMed] [Google Scholar]
- [2].Morris NB, Bain AR, Cramer MN, Jay O. Evidence that transient changes in sudomotor output with cold and warm fluid ingestion are independently modulated by abdominal, but not oral thermoreceptors. J Appl Physiol (1985). 2014;116:1088-1095. PMID:24577060; doi: 10.1152/japplphysiol.01059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Morris NB, Filingeri D, Halaki M, Jay O. Evidence of viscerally-mediated cold-defence thermoeffector responses in man. J Physiol. 2017;595(4):1201-1212. PMID:27929204; doi: 10.1113/JP273052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011; 301:R1207-R1228. PMID:21900642; doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- [5].Meigal A. Gross and fine neuromuscular performance at cold shivering. Int J Circumpolar Health. 2002;61:163-172. PMID:12078964; doi: 10.3402/ijch.v61i2.17449. [DOI] [PubMed] [Google Scholar]


