Abstract
The objective of this study was to choose a suitable anesthetic combination for use in experimental surgical models by comparing the anesthetic and cardio-respiratory changes. Fourteen healthy male sheep were randomly assigned to two different drug regimens. In Group 1 the sheep were anesthetized with ketamine + xylazine (22 mg/kg im. + 0.2 mg/kg i.m., respectively). Anesthetic combination of ketamine + diazepam (22 mg/kg im. + 0.4 mg/kg i.m., respectively) was used in Group 2. Heart rate, respiratory rate and mean arterial pressures were evaluated before anesthesia, after induction of anesthesia up to 30 minutes in 5 minute intervals and during recovery. In all sheep, duration of anesthesia induction, duration of anesthesia and duration of recovery were recorded. Quality of induction, anesthesia, analgesia and recovery were evaluated. Cardio-respiratory parameters decreased below baseline values after anesthesia induction in both groups. However, no profound effects on cardio-respiratory functions were observed during study. In Group 2, it was observed that; anesthesia induction time was longer, the depth of anesthesia was inadequate during the osteotomy stage of the surgical procedure and recovery time was longer in comparison to Group 1. Otherwise the quality of anesthesia induction, anesthesia, analgesia and recovery was better in Group 1 than Group 2. These findings indicate that both drug combinations can provide short time anesthesia for minor surgical procedures. Ketamine+xylazine combination can be used as a more suitable anesthetic combination in experimental surgical procedures such as maxillofacial surgery than ketamine+diazepam combination, in sheep.
Keywords: anesthesia, general, oral surgical procedures, xylazine
INTRODUCTION
In the practice of anesthesia, drug combinations are frequently used when optimal conditions for anesthesia are generated. Since drugs manifest different effects when used separately or in combination, changes that might occur during combined drug usage should be understood and recognized. In humans, the addition of a sedative-hypnotic drug to ketamine, an anesthetic drug which is also used commonly in animal studies, is a frequent anesthetic application to augment ketamine’s anesthetic effects, decrease its side effects and also to provide necessary depth of anesthesia and surgical comfort [1]. In contrast to the majority of anesthetics, ketamine increases heart rate and mean arterial pressure, stimulates cardiovascular functions and when used as a single agent it can induce undesired effects such as muscular hypertonicity, myoclonus, and convulsions [2]. To minimize these unwanted and restricting effects, ketamine is administered in combination with drug groups such as benzodiazepines, and alpha-2 agonists. Diazepam is a potent hypnoticsedative and produces muscle relaxation; it is a long-acting drug due to its slow metabolism and it has relatively weaker cardiovascular effects when compared with other sedative drugs [3]. In combination with ketamine, diazepam alleviates unwanted cardiovascular effects of ketamine and demonstrates anticonvulsive, amnestic and muscle relaxant effects via central mechanisms [3]. Xylazine, an alpha-2 agonist used in animal experiments, stimulates alpha-2 adrenergic receptor in cerebral presynaptic nerve ends, inhibits release of cathecolamines and dopamine resulting in analgesic and sedative effects, and hinders nerve conduction in the central nervous system leading to relaxation of striated muscles [4]. Xylazine is usually used in combination with ketamine during anesthetic applications [3].
In veterinary medicine as well as in scientific investigations sheep are preferred as experimental animals because of their unique characteristic features. Among these characteristics their easy and perfect adaptations to laboratory conditions with their body weights and sizes similar to those of human beings can be enumerated [5]. Although the effects of most of anesthetics in humans have been investigated, and elucidated, studies in literature investigating anesthetic effects of drug combinations in sheep undergoing major surgery for research or treatment are scarce in number. In this study, comparison of anesthetic drug combinations as diazepam-ketamine and as xylazine-ketamine in spontaneously breathing sheep exposed to experimental maxillofacial surgery was targeted.
MATERIALS AND METHODS
After obtaining approval from the local ethics committee, 7-8 month old 14 male sheep, weighing between 21-28 kg, bred and raised under similar conditions, were randomized into 2 groups. In xylazine group (Group 1, n=7) after 16 hours of fasting and 12 hours of thirst, ketamine (22 mg/ kg, i.m.) was administered 10 minutes after atropine (0.05 mg/kg, i.m.) + xylazine (0.2 mg/kg, i.m.) premedication. For diazepam group (Group2, n=7), 10 minutes after premedication with atropine (0.05 mg/kg, i.m.) + diazepam (0.4 mg/ kg, i.m.), ketamine (22 mg/kg, i.m.) was injected. Induction of anesthesia was performed after observation of findings of sedation in all sheep which were head drop and palpebral ptosis. The animals were maintained in spontaneous respiration and as an analgesic flunixine meglumine (9 mg/ kg, i.m.) was introduced just before surgical intervention. During surgery, when animals started to move, additional 4 mg/kg intravenous ketamine was given to Groups 1 and 2 through external jugular vein to achieve rapid onset of effect. Heart rates, respiratory rates and blood pressures of animals were assessed. Induction of anesthesia, anesthesia and analgesia, and recovery from anesthesia were qualitatively evaluated as good, moderate and worse in accordance with Aydilek and co-workers study [6] (Table 1). Besides, duration of induction, anesthesia, surgery and postoperative recovery period were recorded. The induction period was assessed as the time for the sheep to lie sideways, and the presence and absence of response to painful stimuli after administration of ketamine. The duration of anesthesia was determined as the period between the first injection and the moment of the first spontaneous elevation of the sheep’s head. The recovery period was the time passed from the last injection up to the time the animal stands up on its legs, and maintains its erect posture. The heart rates per minute were determined by auscultation with a stethoscope, and respiratory rates per minute were assessed by observation of chest and abdomen movements, and auscultation with a stethoscope. Systolic and diastolic blood pressures were measured by placing a cuff around the tail root in a noninvasive oscillometric method and mean arterial pressures were calculated with the diastolic blood pressure + 1/3 (systolic -diastolic blood pressure) formula. Hemodynamic parameters were measured before premedication, at 5-minute intervals for 30 minutes starting from the onset of the induction of anesthesia, and also during the recovery period.
TABLE 1.
Scoring criteria used for assessing quality of induction, anesthesia and analgesia, and recovery.
Surgical methods
Distraction Group; In all 7 sheep, submandibular incision was made to expose the right mandibular body. After the mucoperiosteal flap was reflected, vertical corticotomies were performed from mesial to the decidious first premolar tooth mesial to the first molar under saline irrigation. The 25 mm mandibular bone segment was removed. To create a transport segment, vertical corticotomy was performed approximately 2 cm posterior to the distal edge of the defect. The distraction device (LOGIC distractor system, straight, right, TX, USA), was oriented perpendicular to the corticotomy line and was fixed in place. Graft group; The recipient site, the right mandible was prepared by making 25 mm segmental resection between the canine and first molar. Block grafts measuring 25 mm long, 20 mm high, 8 mm wide were harvested from the right iliac crest. The block graft was then placed into the defect and fixed in place.
Statistical analysis
Because of the distribution of continuous variables were normal, according to Klomogorov Smirnov normality test; two independent sample t tests were used to compare vital findings, and durations of surgeries, inductions, recovery periods, heart rates, respiratory rates and blood pressures between two groups. One way repeated measures ANOVA was used to compare the heart rates, respiratory rates and blood pressures among 10 follow-up periods separately, for each group (for multiple comparison Bonferroni test was used). Two way repeated measures ANOVA was used to compare the alteration of heart rates, respiratory rates and blood pressures between two groups. Variables were presented as mean ± standard deviation and minimum and maximum values. A p values <0.05 were considered as statistically significant. Analyses were performed using commercial statistical software (SPSS, ver. 16.0 demo, Chicago, IL).
RESULTS
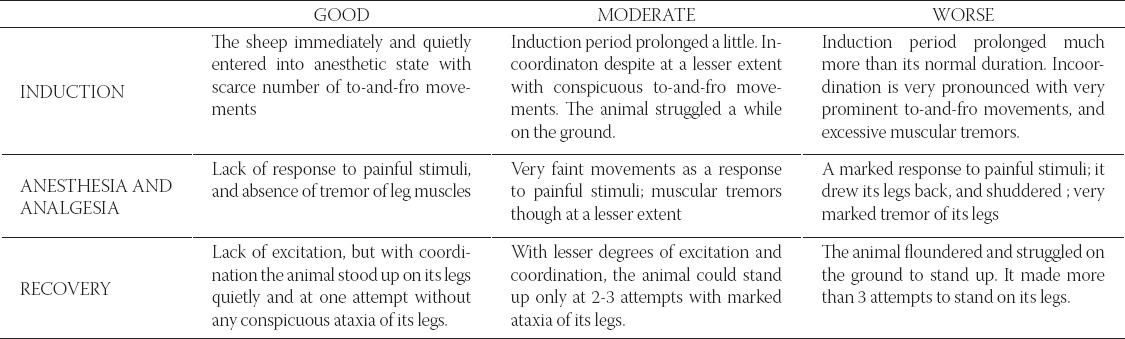
During the study period, animals remained hemodynamically stable and any problem requiring medical support was not seen. In both groups heart rates, respiratory rates and mean arterial pressures during the surgical procedure were rated within physiological limits. There was statistically significant difference between the groups (p<0.05) in heart rates, which were measured soon after premedication, and at minute 10, 20, 25 and 30. In with-in group assessments, there was statistically significant difference among 10 measures of heart rate separately for two groups (for Groups 1 and 2, p=0.001, p<0.001 respectively) (Table 2). There was no statistically significant difference between Group 1 and Group 2 in alteration of heart rates during the procedure (p=0.070). In both groups heart rates increased with the start of surgical procedure after the induction period (p<0.05). The mean arterial pressures measured at minute 20 and 30 were significantly higher in Group 2. In within group assessments, there was statistically significant difference among 10 measures of mean arterial pressure separately for two groups (for Groups 1 and 2, p=0.004, p<0.001 respectively) (Table 2). There was no statistically significant difference between Group 1 and Group 2 in alteration of mean arterial pressures during the procedure (p=0.683). In both groups the mean arterial pressures increased with the start of surgical procedure after the induction period. The respiratory rates at minute 20, 25 and 30 and recovery were significantly higher in Group 2 (p<0.05). With-in group assessments, there was statistically significant difference among 10 measures of respiratory rate in two groups (for Groups 1 and 2, p=0.013, p=0.002 respectively) (Table 2). There was statistically significant difference between Group 1 and Group 2 when alteration of respiratory rates was concerned during the procedure (p<0.001). In both groups, respiratory rates increased with the start of surgical procedure after the induction period. Especially, in Group 2 the respiratory rate was in a trend to increase after the 15th minute, meanwhile decrease in respiratory rates was observed in Group 1 after the 15th minute.
TABLE 2.
Mean values of heart rate, respiratory rate and mean blood pressures in two groups
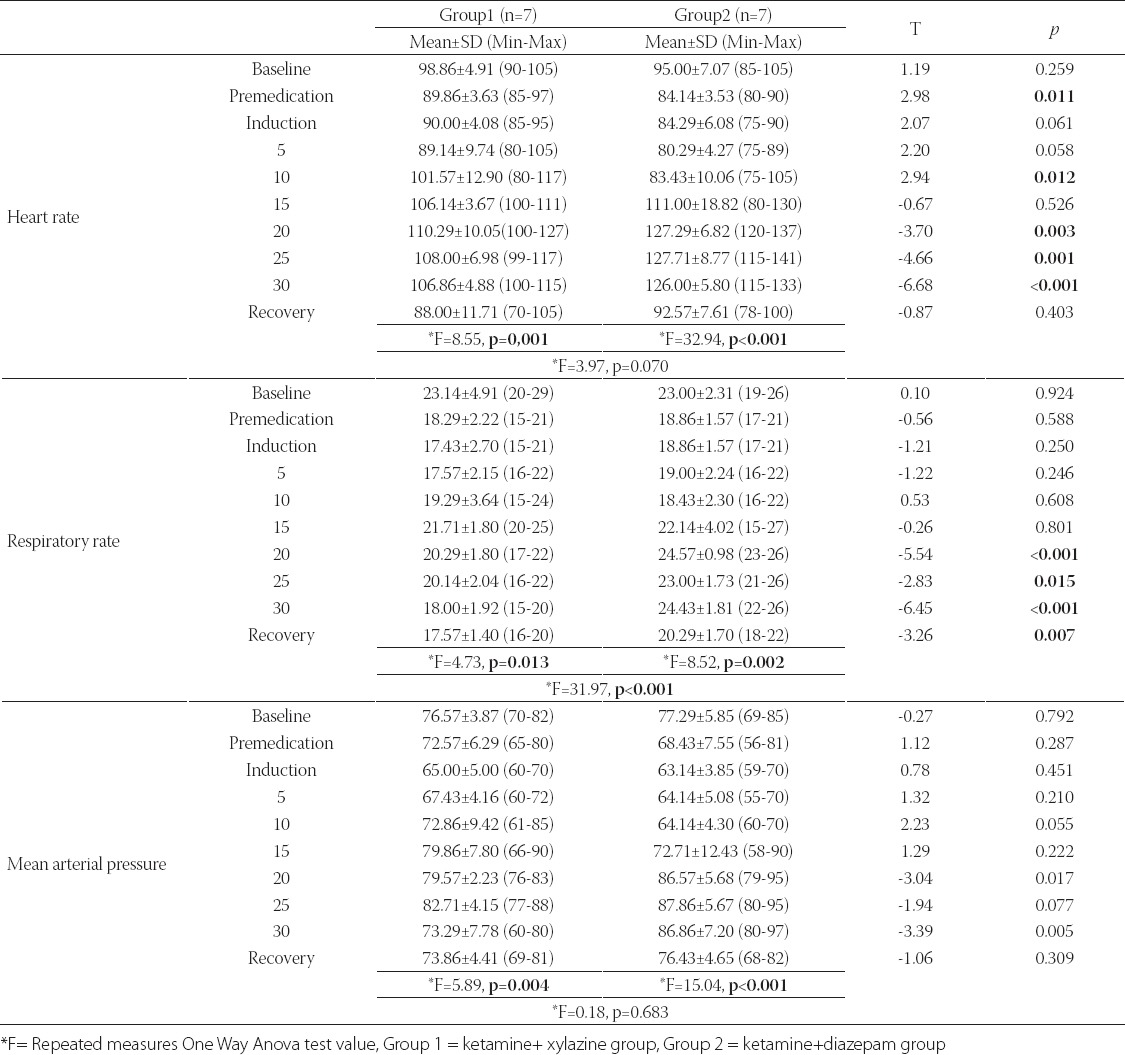
The duration of anesthetic and surgical procedures was shown in Table 3. Sufficient depth in anesthesia was achieved after 8.43 minutes in Group 1 and 13 minutes in Group 2 after ketamine administration. Time to the induction of anesthesia was found to be significantly shorter in Group 1 (p= 0.006). The quality of induction was deemed to be good in both groups. In Group 1, the duration of anesthesia ranged between 26 and 45 minutes (mean 40.1417.31 minutes) after administration of ketamine. In Group 2, upon the initiation of the osteotomy phase of the operation, involuntary movements in animals were observed, and additional doses were instituted. Anesthesia and analgesia in all phases of surgery in Group 1 were of good quality. In this group, 1-shot additional dose was administered to three animals dependent on the operative times, and degree of surgical comfort and satisfaction were assessed to be good after additional doses. In Group 2, the quality of anesthesia and analgesia up to the osteotomy phase of the surgery was of moderate degree, while during the osteotomy phase it was evaluated as “worse” in quality. In this Group, during the osteotomy phase all animals received additional doses. Two animals were administered second, and one animal was administered third additional doses. After administration of dosages surgical satisfaction and comfort were evaluated as of moderate degree. In Group 1 total duration of surgery, and postoperative recovery time ranged between 40-75, and 75-105 minutes, respectively. In Group 2, operative time, and postoperative recovery times varied between 45-115, and 120-180 minutes, respectively. In Group 1 postoperative recovery time was detected to be significantly shorter than Group 2 (p<0.001). The quality of recovery was evaluated as “good” in Group 1 and “moderate” in Group 2. Although any statistically significant difference between operative times was not found, relatively longer durations were observed in Group 2.
TABLE 3.
Mean values of duration of anesthetic and surgical procedures in two groups
DISCUSSION
Ketamine can be used singly or in combination for preand intraoperative sedation, induction, and maintenance of anesthesia, balanced anesthetic applications, regional and spinal anesthesia, and postoperative analgesia [7-9]. The pharmacodynamic and clinical effects of ketamine which has been used since the 1960s on bodily systems, have been determined in experimental and clinical studies. Ketamine had come into the foreground especially during the last decade due to prevention of its side effects by using drug combinations and thus expansion of its area of usage [7-9]. Sedative preanesthetic drugs like diazepam and xylazine can possess hypotensive and hypoxic effects by depressing cardiovascular and respiratory activities. In contrast to most of the anesthetic drugs, ketamine has been shown to possess incremental effects on the heart rate, blood pressure and respiratory rate due to increase in sympathetic activation [9-14]. Ketamine has desired effects such as maintenance and stimulation of respiration, bronchodilation, maintenance of functional residual capacity and achievement of equivalent minute ventilation rates both in spontaneously breathing individuals and in those wide awake [15]. It had also unwanted respiratory effects such as increase in respiratory secretions [9, 16]. It has been demonstrated that anti-muscarinic drugs like atropine have decreasing effect on hypersecretion induced with ketamine [17]. In our study, atropine incorporated in premedication decreased hypersecretion which was induced during spontaneous breathing. Benzodiazepines decrease cardiovascular effects of ketamine [18-20]. Similarly, xylazine decreases the effects of ketamine by lowering blood pressure and depressing the cardiovascular system. In these studies, dependent on the stage of anesthesia, it was demonstrated that the drugs used in combinations can cause fluctuations in heart rate, blood pressure, respiratory rate and catecholamine levels varying with the degree of stress [4, 6, 7, 9]. Similar to the results of other studies, our study demonstrated that hemodynamic findings such as heart rates, respiratory rates and mean blood pressures fell under baseline values after premedication phase and climbed over baseline values after induction and during surgical intervention. We suggest that in addition to sympathetic effects of ketamine, higher values of the three hemodynamic parameters in the ketamine-diazepam Group can be attributed to relatively lesser contribution of diazepam to the induction of anesthesia compared to xylazine. Mouallem et al. [21] in a study conducted in sheep emphasized that ketamine-diazepam combination significantly reduced heart rates and respiratory rates, however Coulson et al. [22] investigated cardiovascular effects of ketamine-di- azepam and ketamine-xylazine combinations in sheep and stressed lack of any meaningful effects on heart rates and respiratory hemodynamics. In our study incremental or decremental alterations in hemodynamic variables caused by both drug combinations remained within physiologic limits. Ketamine anesthesia which demonstrates its main impact via central nervous system, has anesthesic, analgesic, and amnesic effects as well as unwanted effects such as delirium, hallucination and nightmares during the recovery period [9]. In combination with benzodiazepines and an alpha-2 agonist xylazine, an increase in the quality of analgesia, sedation, anesthesia and recovery, alleviation of anxiety and a decrease in the unwanted ketamine have been demonstrated [9, 10, 19, 20]. In our study prolongation of anesthetic induction and recovery period, inadequate depth of intraoperative anesthesia were observed in premedication with diazepam in ketamine-diazepam group. Xylazine premedication in ketamine-xylazine Group was found to be adequate. Compared with ketamine-diazepam combination, faster anesthetic induction, an anticipated duration of anesthesia, improved surgical comfort and satisfaction at every stage of anesthesia have been observed by using ketaminexylazine combination. On the other hand postoperative recovery period was shorter and requirement for additional dosages was less compared to -ketamine-diazepam combination. In a study investigating the effects of both combinations in sheep for minor surgeries of shorter duration both combinations were emphasized to be appropriate [22]. In our study both drug combinations have provided adequate anesthesia up to the osteotomy stage, however anesthesia achieved in ketamine-diazepam Group during osteotomy stage was not sufficient. Sumitra et al. [20] emphasized that ketamine-diazepam combination affected respiratory and cardiac functions relatively at a lesser extent than ketaminexylazine combination, and ketamine-diazepam combination can be a suitable anesthetic regimen for surgical models in rats. Lin et al. [23] investigated the effects of two different anesthetic regimens, namely ketamine-diazepam and ketamine-diazepam-xylazine combinations in sheep and found that the latter combination resulted in a prolonged duration of anesthesia. They finally emphasized that both combinations can be used to anaesthetize sheep. In the same study Lin et al. [23] stated that intravenous ketamine and xylazine infusions provide an improved anesthesia with subsequent satisfactory recovery period lasting for 96 minutes after cessation of infusion. In an investigation performed in children with a benzodiazepine-ketamine combination unconscious state was achieved within 13 minutes and stressed that anesthesia and analgesia was maintained with intravenous ketamine boluses without any side effects excluding delay in recovery [24]. Also in our study, anesthetic states was reached within 13 minutes and with repeated doses were observed delay in awakening and prolonged recovery times in ketamine-diazepam group. On the other hand in ketaminexylazine group anesthesia was achieved within approximately 8 minutes. With repeated doses delayed awakening and longer recovery times were seen in this group, too.
CONCLUSION
Though ketamine-xylazine and ketamine-diazepam combinations are frequently used anesthetic methods in clinical and experimental studies, both methods can be preferred in painful minor surgeries. However in major surgical interventions involving bone tissue as maxillofacial surgery, ketamine-xylazine anesthesia can be preferred over ketaminediazepam anesthesia in that it achieves more rapid anesthetic induction, better surgical comfort, maintenance of physiological parameters within optimal limits and faster recovery.
DECLARATION OF INTEREST
No authors have affiliations or financial involvement with any organization or entity with a direct financial interest in the subject matter or materials discussed in the manuscript. The authors declare that they have no competing interests.
REFERENCES
- 1.Muir WW, 3rd, Lerche P, Robertson JT, et al. Comparison of four drug combinations for total intravenous anesthesia of horses undergoing surgical removal of an abdominal testis. J Am Vet Med Assoc. 2000;15:869–873. doi: 10.2460/javma.2000.217.869. [DOI] [PubMed] [Google Scholar]
- 2.Haskins SC, Farver TB, Patz JD. Ketamine in dogs. Am J Vet Res. 1985;46:1855–1860. [PubMed] [Google Scholar]
- 3.Koshy TA, Mahabala TH, Srikantu J, Sanmathi S. Thiopentonemidazolam mixture as an induction agent for general anesthesia on ‘in-patients’. Ind J Anesth. 2003;47:129–133. [Google Scholar]
- 4.Takeshita H, Okuda Y, Sari A. The effects of ketamine on cerebral circulation and metabolism in man. Anesthesiology. 1972;36:69–75. doi: 10.1097/00000542-197201000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Bosanquet A, Goss AN. The sheep as a model for temporomandibular joint surgery. Int J Oral Maxillofac Surg. 1987;16:600–603. doi: 10.1016/s0901-5027(87)80113-3. [DOI] [PubMed] [Google Scholar]
- 6.Aydilek N, Ceylan C, Ipek H, Gundogdu U. Effects of xylazine-diazepam-ketamine and xylazine-tiletamine-zolazepam anesthesia on some coagulation parameters in horses. YYU Vet Fak Derg. 2007;18:55–58. [Google Scholar]
- 7.Akeson J, Björkman S, Messeter K, Rosén I, Helfer M. Cerebral pharmacodynamics of anesthetic and subanesthetic doses of ketamine in the normoventilated pig. Acta Anaesthesiol Scand. 1993;37:211–218. doi: 10.1111/j.1399-6576.1993.tb03703.x. [DOI] [PubMed] [Google Scholar]
- 8.Annetta MG, Iemma D, Garisto C, Tafani C, Proietti R. Ketamine: new indications for an old drug. Curr Drug Targets. 2005;6:789–794. doi: 10.2174/138945005774574533. [DOI] [PubMed] [Google Scholar]
- 9.Wyatt JD, Scott RA, Richardson ME. The effects of prolonged ketamine-xylazine intravenous infusion on arterial blood pH, blood gases, mean arterial blood pressure, heart and respiratory rates, rectal temperature and reflexes in the rabbit. Lab Anim Sci. 1989;39:411–416. [PubMed] [Google Scholar]
- 10.Muir WW, Sharda RT, Milne DW. Evaluation of xylazine and ketamine hydrochloride for anesthesia in horses. J Am Vet Med Assoc. 1977;38:195–201. [PubMed] [Google Scholar]
- 11.Clarke KW, Taylor PM, Watkins SB. Detomidine/ketamine anesthesia in the horse. Acta Vet Scand. 1986;82:167–179. [PubMed] [Google Scholar]
- 12.Lin HC, Branson KR, Thurmon JC, et al. Ketamine, telazol, xylazine and detomidine. A comparative anesthetic drug combinations study in ponies. Acta Vet Scand. 1992;33:109–115. doi: 10.1186/BF03547317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzikiti TB, Chanaiwa S, Mponda P, Sigauke C, Dzikiti LN. Comparison of quality of induction of anesthesia between intramuscularly administered ketamine, intravenously administered ketamine and intravenously administered propofol in xylazine premedicated cats. J S Afr Vet Assoc. 2007;78:201–204. doi: 10.4102/jsava.v78i4.323. [DOI] [PubMed] [Google Scholar]
- 14.Lin HC, Tyler JW, Welles EG, Spano JS, Thurmon JC, Wolfe DF. Effects of anesthesia induced and maintained by continuous intravenous administration of guaifenesin, ketamine, and xylazine in spontaneously breathing sheep. Am J Vet Res. 1993;54:1913–1916. [PubMed] [Google Scholar]
- 15.Tokics L, Strandberg A, Brismar B, Lundquist H, Hedenstierna G. Computerized tomography of the chest and gas exchange measurements during ketamine anesthesia. Acta Anaesthesiol Scand. 1987;31:684–692. doi: 10.1111/j.1399-6576.1987.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 16.von Ungern-Sternberg BS, Regli A, Frei FJ, et al. A deeper level of ketamine anesthesia does not affect functional residual capacity and ventilation distribution in healthy preschool children. Paediatr Anaesth. 2007;17:1150–1155. doi: 10.1111/j.1460-9592.2007.02335.x. [DOI] [PubMed] [Google Scholar]
- 17.Mogensen F, Mueller D, Valentin N. Glycopyrrolate during ketamine/diazepam anesthesia. Acta Anaesthesiol Scand. 1986;30:332–336. doi: 10.1111/j.1399-6576.1986.tb02425.x. [DOI] [PubMed] [Google Scholar]
- 18.Jackson APF, Dhadphale PR, Callaghan ML. Haemodynamic studies during induction of anesthesia for open-heart surgery using diazepam and ketamine. Br J Anaesth. 1978;50:375–377. doi: 10.1093/bja/50.4.375. [DOI] [PubMed] [Google Scholar]
- 19.Morse Z, Sano K, Kanri T. Effects of a midazolam-ketamine admixture in human volunteers. Anesth Prog. 2004;51:76–79. [PMC free article] [PubMed] [Google Scholar]
- 20.Sumitra M, Manikandan P, Rao KV, Nayeem M, Manohar BM, Puvanakrishnan R. Cardiorespiratory effects of diazepam-ketamine, xylazine-ketamine and thiopentone anesthesia in male Wistar ratsa comparative analysis. Life Sci. 2004;27:1887–1896. doi: 10.1016/j.lfs.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Mouallem H. Comparative studies of general anesthesia of sheep with ketamine and Etomidate. Pol Arch Weter. 1988;28:113–127. [PubMed] [Google Scholar]
- 22.Coulson NM, Januszkiewicz AJ, Dodd KT, Ripple GR. The cardiorespiratory effects of diazepam-ketamine and xylazine-ketamine anesthetic combinations in sheep. Lab Anim Sci. 1989;39:591–597. [PubMed] [Google Scholar]
- 23.Lin HC, Wallace SS, Tyler JW, Robbins RL, Thurmon JC, Wolfe DF. Comparison of tiletamine-zolazepam-ketamine and tiletamine-zolazepam-ketamine-xylazine anesthesia in sheep. Aust Vet J. 1994;71:239–242. doi: 10.1111/j.1751-0813.1994.tb03419.x. [DOI] [PubMed] [Google Scholar]
- 24.Holm-Knudsen R, Sjorgren P, Laub M. Midazolam and ketamin for rectal premedication and induction of anesthesia in children. Anaesthesist. 1990;39:255–257. [PubMed] [Google Scholar]


