Abstract
Pterygium internum (external eye layer) shows great recurrence tendency after surgical removal. Its etiology is still unclear and represents a significant problem. The main goal of our study was to explore the interrelationships of pathohistological characteristics of pterygium, namely presence of inflammation, vascularisation degree and fibrinoid changes and on the basis of their analysis to test the possibility of predicting its evolution and recurrence. The analysis was performed on the material taken from 55 patients surgically treated by the technique of Arlt. The specimens were stained using the classical histochemical methods: hematoxylin-eosin (HE), Masson’s trichrome, Gomori’s reticulin stain and PAS technique. Pterygium is mostly covered by conjunctival epithelium, while in the cap region shows morphology of modified stratified squamous epithelium of the cornea. Structural basis of the epithelium is composed of continuous basal lamina and continuous connective fibers underneath. This connective basis shows fibrinoid changes in the form of oval islets of different size, parallel to convexity of pterygium, or is in the form of unified focus. The number, caliber and the type of blood vessels showed excessive variability.
Pathohistological analysis of morphological characteristics of pterygium is adequate basis for prediction of recurrences; as they present the biggest concern in treatment of this widely spread disease.
Keywords: pterygium, recurrence, pathohistological characteristics
INTRODUCTION
MPterygium is one of the most common ophthalmic disorders whose pathogenesis is still unclear. The literature reports the following etiological factors which may be the cause of occurrence of pterygium: ultraviolet radiation (UV), chronic eye inflammation, toxic effects of chemical substances. Recently, some viruses have also been mentioned as possible etiological factors. Pterygium may be regarded as a neoplastic change as well [1-5]. Destructive effect of UV rays leads to decrease of limbal stem cells of the cornea, i.e. causes limbal insufficiency. It activates the tissue growth factors which induce angiogenesis and cell proliferation [6-8]. It has been observed through microscopy that the pterygium is composed of fibro-vascular tissue whose collagen fibers often present elastosis. The pterygium is covered by conjunctival epithelium, except on its top. On the top of the pterygium, a wedge-like extensions of fibrous tissue can microscopically be seen, and the head of the pterygium enroaches to cornea, so Browman’s layer is invaded and fragmented.
The cavities are filled with fibrous connective tissue [9]. Pterygia posses many histopathological hallmarks of chronic inflammation. They are: the lymphocytic infiltration consisting predominately of T lymphocytes, then plasma cells and mastocytes. Mastocytes (mast cells) play an important role in chronic inflammation accompanied with angiogenesis and fibroses. The increase of newly-formed blood vessels, number of fibroblasts, grouping of degeneratively changed collagen fibers, and the presence of abnormal elastic fibers also confirm the presence of chronic inflammation [10-12]. The aim of the paper is to explore the interrelationships of pathohistological characteristics of pterygium and on the basis of their anaysis test the possibility of predicting its evolution and the recurrence. The aim of the research was to investigate the clinical characteristics of pterygium as well, such as: the presence of Fuchs’ spots and occurrence of recidive postoperatively.
MATERIALS AND METHODS
A total of fifty-five patients with pterygium, surgically treated by the technique of Arlth were examined. In order to observe the recurrence episodes, all the patients were postoperatively followed-up for one year. Excised pterygium tissue was fixed in 10% buffered formalin and processed routinely for paraffin-embedded sections.
In order to observe general composition and topohistological characteristics of the pterygia, 3-5 micron thick samples were stained by classic histochemical methods: hematoxylin-eosin (HE); Mason’s Trichrome staining; Gomori’s reticulin staining and PAS technique. Pathohistologic changes were semi-quantitatively classified into 5 groups. Inflammation intensity: group o - no inflammatory infiltrate; group 1- sporadic, perivascular presence of lymphocytes; group 2 - multifocal chronic inflammatory infiltrate (mostly lymphocytes); group 3 - multifocal chronic inflammatory infiltrate (domination of plasmocytes); group 4 - diffusive chronic inflammatory infiltrate. Degree of vascularisation: group o - vascularisation of pterygium resembles normal conjunctiva; group 1 - arteriole blood vessels presence is dominant in the region of the vascular stalk; group 2 - presence of arterial blood vessels in the center of pterygium; group 3 - a stronger presence of capillary blood vessels subepithelially and in the advanced region; group 4 - more expressed presence of arteriole blood vessels subepithelially and in advanced region. Fibrinoid change: group owithout presence; group 1 - rare focal, perivascular fibrinoid changes; group 2 - a greater number of focal perivascular changes; group 3 - a greater number of focal changes and sporadically subepithelial; group 4 - a greater number of focal and diffusively subepithelial and/or massive change in the progressive part. The results of examination of histopathological and clinical characteristics of pterygium were comparatively analyzed. Statistical analysis of the obtained results was carried out by a statistical programme package - Statistical Package for Social Science (SPSS) software, version 11. Depending on the tested markers, the following methods of descriptive statistics were used: mean value (X); parametric: Student’s t-test, and non-parametric test: Mann-Whitney test (M-W).
RESULTS
Pathohistological changes ofthe pterygia are shown in Chart 1. As seen in the Chart 1, in most of the analyzed pterygia the inflammation intensity was expressed in the form of sporadic, perivascular presence of lymphocytes (group 1). Chart also shows that in most of the analyzed pterygium the degree of vascularisation was expressed in terms of presence arterial blood vessels in the center of pterygium (group 2), and the fibrinoid change was present in the form of a greater number of focal perivascular changes and sporadically subepithelial changes (group 3). Histopathological features 10-11 of the 55 analyzed pterygia resembles the structure of normal conjunctiva (Chart 1).
CHART 1.

Intensity of pathohistological changes of the pterygia
Histomorphologically, all the analyzed pterygia can be characterized by excessive fibrovascular proliferations covered by conjunctival epithelium. The analysis involved pterygia of different size, 1-6 mm, whose development ranges from 1 to 20 years.
The pterygium epithelium covers the stroma (Figure 1). The vascular stem showed multiple branching in the neck toward the anterior segment (Figure 2) and loose connective tissue (edematous fibrous tissue) with always present fibrinoid change (hyaline degeneration), especially in the part progressing to the cornea center (Figure 3).
FIGURE 1.

Abundant pterygium stroma mostly covered by conjunctival epithelium (HE, 10x).
FIGURE 2.

Vascular stalk of the pterygium neck and its branching towards the progressing part of pterygium (HE, 10x).
FIGURE 3.

Appearance of an edematous tissue with fibrinoid change elements in the progressive part of pterygium (HE, 20x).
Epithelial structural basis shows continuous basal lamina and in most cases underlying continuous connective tissue. This binding basis shows fibrinous changes in the form of oval islets of different size, parallel to convexivity (Figure 4) or is a fibrinoid change in the form of a unique focus (Figure 5). Fibrinoid change is manifested by granular protein mass with fragmented collagen fibers of different thickness at its perifery part. De-arrangement of collagen fibers is more notable in the pterygium of longer evolution. Fibrinoid change in the superior segment of lamina propria was observed at the corneal - conjunctival junction (Figure 6).
FIGURE 4.

Subepithelial connective tissue with focal fibrinoid change (HE, 40x).
FIGURE 5.

Fibrinoid change of subepitheli-al connective tissue with confluent patches (HE, 40x).
FIGURE 6.

Collagen connective tissues disorder, as well as their fibrinoid transformation of granular protein mass appearance (HE, 40x).
The number, caliber, and the type of blood vessels showed extreme variability (Figure 7 and Figure 8). Abundant vascular network and widely branched capillaries in superior propria, accompanied by focal active epithelium with wide fibrinoid change of propria elasticity, are the characteristics of slowly progressive larger pterygia. Developed lesions with expressed fibrinoid degeneration in the advanced portion are actively progressive lesions accompanied by visible Fuchs’ patches. There is scarce epithelium in two or three epithelial layers above it.
FIGURE 7.

Edo-matous stroma of the pterygium with delicate capillary network and poorly expressed fibrinoid change in the pterygium of shorter evolution (HE, 20x).
FIGURE 8.

Richly branched capillaries with wide lumen in the pterygia of longer evolution (HE, 20x).
Comparative analysis of recurrence of pterigya and the presence of Fuchs’ spots are shown in Chart 2. The presence of Fuchs’ spots was observed in 75% of patients with recurrence (6 of 8), and in those without recurrence Fuchs’ spots were present in only 27.7% cases (13 of 47). It was confirmed by Mann-Whitney test (M-W = 99.0; p < 0.05) that the difference is highly statistically significant. It could be said that the presence of Fuchs’ patches shows statistically significant relatedness to the rate of recurrence. The results of comparative analysis between the studied histopathological and clinical characteristics of pterygia are shown in Chart 3 and Chart 4.
CHART 2.

Comparative analysis: Recurrence of the Fuchs’ spot
CHART 3.

Comparative analysis: Pathohistological changes - Fuchs’ spots
CHART 4.
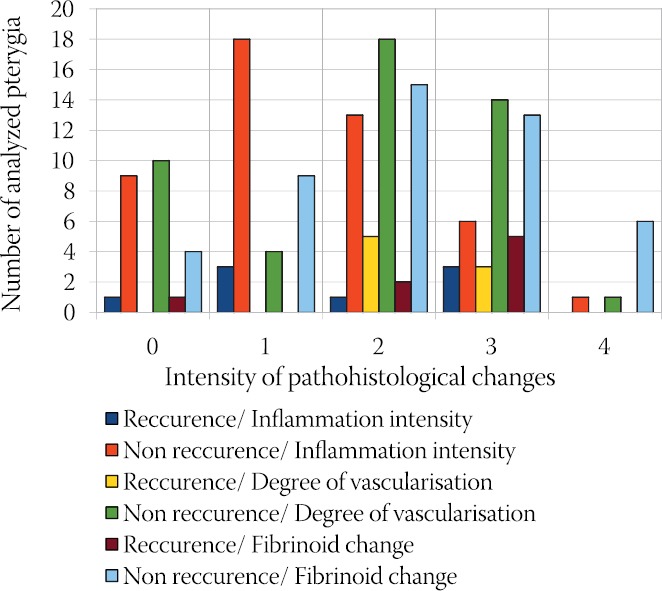
Comparative analysis: Recurrence of the Pathohistological changes
In patients with Fuchs’ spots the inflammation was more intense, but this difference is not statistically significant, as confirmed by Mann-Whitney test (M-W = 302.5; p > 0.05).
Vascularisation was also more intense in these patients in comparison to the patients without Fuchs’ spots, and MannWhitney test (M-W = 234.0; p < 0.05) confirmed the difference as statistically significant. The intensity of fibrinoid change was higher than in patients without Fuchs’ spots, but this difference is not statistically significant, as confirmed by Mann-Whitney test (M-W = 325.0; p > 0.05) (Chart 3). Mean value of inflammation intensity in patients with recurrence was 1,06 and in patients without recurrence 0.78. The observed difference is not statistically significant, as confirmed by t-test (t=1.026; DF=53; p>0.05). Vascularisation was more intense in patients with recurrence (=1.37) than in patients without recurrence (=1.08). The observed difference is not statistically significant, as confirmed by t-test (t=0.99; DF =53; p>0.05). In patients with recurrence the presence of fibrinoid change was more intense (=1.50) than in patients without recurrence (=1.35). Yet, the observed difference is not statistically significant, as confirmed by t-test (t =0.440; DF = 53; p >0.05) (Chart 4).
DISCUSSION
According to their general characteristics (shape, structure components, topography), examined pterygia correspond with the expected, standardized morphological findings obtained by Fuchs [13], Cilova [14] and Seifert [15]. Histomorphologically, all the analyzed pterygia could be defined as fibrovascular proliferations, mostly covered by conjunctival epithelium. However, as reported by other authors, it was impossible to see topographic division (toward the longer axis) to the cap, head, neck and body in the examined pterygia. Seifert et al. [15] called the cap of pterygium the top, and Cilova [14] avascular part of the head. Besides this, she mentioned the other parts as well: vascular part of the head and scleral part [16].
The epithelium of pterygium: according to our results, pterygium is mostly covered by conjunctival epithelium, while in the head of pterygium it exhibits the morphology of modified stratified squamous epithelium of the cornea, what is in accordance with literature data of Cilova [14] and Seifert [15]. However, it should be pointed out that the changes in the epithelium in our material are of somewhat more important variability in comparison to data of the aforementioned authors. In the epithelium of this region, cellular and nuclear polymorphism was found, especially expressed in the surface layer of the epithelium, where young cells with large nuclei were found. It was just the lamina propria of these segments that showed dominant fibrinoid change. Such associated pathomorphology could be the basis of macroscopic feature of pterygium progression, Fuchs’ patches. In the rest of the pterygium epithelium, conjunctival epithelium was dominant, with weaker histo-differentiation, as reflected in smaller number of mucous-secreting cells. While studying histo-morphology of the body of pterygium, not many authors dealt with epithelium. Cilova showed that scleral epithelium of progressive pterygium consisted of more layers in comparison to stationary pterygium. It makes up numerous wrinkles, invaginations, as well as partial and isolated epithelial canals. Cilova [14] found stronger mucous dystrophy and progressive pterygium. The epithelium of progressive pterygium shows significantly higher hyperplasia and basal germinative layer activity than stationary pterygium. Particularly clearly defined hyperplasia and activity of the basal layer of the epithelium were found in the progressive pterygium base, where numerous invaginations and epithelial canals can be seen. However, Seifert et al. [15] proved that these larger invaginations, as seen in longitudinal cross-sections, are in fact a gap between lateral lobe of pterygium and its base. Seifert et al. [15] also claim that the epithelium is extremely attenuated in certain parts (especially in the lateral parts of the lobe), due to epithelium stretching caused by enlargement of connective tissue mass of pterygium. Measurement (quantification) of the degree of division of pterygium epithelial cells showed no increase [17]. It is certainly possible that during pterygium development over the years, proliferative activity varies. The recent studies indicate increased telomerase activity, a biomarker of cell proliferation [18], in 2/3 of the examined pterygiums [19]. Weinstein et al. [20] found abnormal expression of p53 in pterygium epithelium, suggesting that pterygium can be the result of uncontrolled cell proliferation, but not degenerative lesion. There is no connection regarding abnormal expression of p53 among the groups with or without recurrences.
Connective tissue in pterygium: the vascular stalk with multiple branching in the neck towards anterior segment and edomatous fibrous connective tissue with always present fibrinous change, especially in the part that progresses to the center of the cornea, is dominant in the stroma [21].
Cilova [14] found the signs of young, newly-formed connective tissue in avascular part of the progressive pterygium head. This tissue strongly resembles the corneal connective tissue, except that the lamellas are thinner and irregularly intertwined. It has significantly more cell elements of connective tissue, roundly shaped, in comparison to normal cornea. Immature character of this newly formed avascular connective tissue is also confirmed by histochemical analyses (van Gieson, Azan, PAS, dialysed iron and toluidin blue). Besides these, she found thickening and de-layering of the basement membrane on the specimens stained with PAS reaction and the method of Gomora. The membrane sends abundance of filaments towards the underlying connective tissue, so it appears to be a part of its origin (takes part in its formation).
In the distal parts of stationary pterygium head, she, as well as Fusch [13], found more compact connective tissue than in progressive pterygium, composed of thicker corneal-like lamellas, but not so regularly arranged. This tissue contains significantly lower number of cell elements (thin and hardly seen) in comparison to newly-formed connective tissue in progressive pterygium. This study shows that aforementioned tissue is stained with Van Gieson stain, Azan stain, PAS, toluidine blue by Hale’s method, alcian blue, resembling normal corneal tissue. These data indicate that connective tissue breakdown occurs. The basal epithelial membrane in avascular part of the stationary pterygium is less thickened and less de-layered than in progressive pterygium. Cilova [14] showed that there were variations in histomorphologic structure of vascularised connective tissue of progressive and stationary pterygium head.
The main part (mass) of the pterygium is composed of connective tissue that, in the course of pterygium evolution, undergoes pathological changes that are accompanied by the increase of the mass of the pterygium itself. The newlyformed structure in the pinguecular part of pterygium is located subepithelially and progresses towards the cornea. In the corneal part of pterygium, this newly formed and well vascularised conjunctiva is positioned between the epithelium and the rough original layers of stroma (including Bowman’s layer). In such a way changes in extracellular matrix responsible for physical properties of tissue occur [22]. As a result, mechanical stability of tissue is lost. Mechanical stress cannot be absorbed anymore, so the tissue is bulged above the eyeball surface. According to statistic data, this tissue protuberation then forms lobuses laterally to pterygium basis, with simultaneous modification and expansion of epithelial surface. For these reasons, during the surgical procedures not only this surface loose tissue in the region of cornea and sclera should be removed, but the tissue underneath and around lateral margins as well, to prevent recurrences of residual pathologically changed cells.
In reference to characterise elastotic material within pterygium, the term “elastotic degeneration” was introduced to describe tissue susceptibility to Weigert’s and Verfhoff s elastic tissue stains, but at the same time the lack of tissue degradation by pancreatic elastase. Since this specific staining property is not universal for pterygia, it is generally accepted that the elastic fibers within pterygium are abnormal. Historically, Hogan and Alvarado [22] found that elastotic material within pterygium is formed in four ways: 1) by degenerated collagen, 2) by degeneration of pre-existing elastic fibers, 3) by abnormal fibroblastic activity and 4) by ground substance disorder. Recently, ultrastructural analysis by Austin et al. [23] attributed the elastotic degeneration only to abnormal fibroblastic activity with the production of abnormal maturational forms of elastic fibers. Cameron [24], according to EM study of pterygia, reported the presence of active fibroblasts in the nature tissue planes surrounding Bowman’s layer.
Cell composition and the number of cells: Karukonda et al. [17] found no difference in the cellular proliferation between pterygial and normal conjunctival tissue, and suggested that pterygium was not a disorder of overexpressed cellular proliferation. By transmission electron microscopy they proved that extracellular matrix is a dominant component of pterygium, and that cellular proliferation of primary and recurrent pterygium does not significantly differ. In the study of Tai et al. [25] it is confirmed that fibroblast of pterygium, thanks to its adherent ability and higher growth kinetics, is superior to fibroblasts of normal conjunctiva. This might explain the high grow ability in ocular pterygium. Cilova [12] found significantly higher number of mastocytes in progressive pterygium in comparison to stationary one, with dominating de-granulated forms.
Characteristics and topography of extracellular matrix: electron-microscopic studies have revealed elastogenesis in substantia propria in normal conjunctiva, then in deeper episcleral connective tissue, as well as at the level of Tenon’s capsule. Ultrastructural examinations also disposed evidence of elastogenesis in the pinguecular part of pterygia, but with distorted morphogenetic sequence of fiber formation. In substantia propria, elastodysplasia (immature formation of elastic fibers) and elastodystrophy (degenerative changes of elastic fibers and formation of electron-dense inclusions) were verified. The amorphous materials in the pinguecular part of pterygium are composed of large number of hollow-centered microfibrils, precursors of elastic fibers that show the tendency to agglomerate centrally in the form of large aggregated envelopes, and to acquire electron-dense inclusion as well [23]. In previous studies, these changes were considered a reflection of an increased accumulation of elastic fibers in normal bulbar conjunctiva, and it was thought that they resulted from degeneration of collagen fibers as the result of sun exposure and subsequent intensitivity of elastose digestion [26, 27]. But, Austin et al. [23] reported that these irregular elastic fibers exist in the substantia propria of the pinguecular part of pterigia, and that the pinguecular fibroblasts might have a role in their formation. Wang et al. [28] immunohistochemically confirmed in their study that these abnormal tortuous fibers are made of elastin. These fibers are elastic and are not formed by elastotic degeneration of collagen fibers. Contrary to this, there was no expression of elastin in the substantia propria of normal conjunctiva. The accumulation (deposition) of abnormal elastic fibers was the same as in previously studied solar elastosis of sun damaged skin (actinic keratosis) [29, 30]. Subepithelial connective tissue in progressive pterygia is composed of young connective tissue rich with blood vessels. The vascular part of the head of the pterygia is characterised by the lack or extremely low presence of hyaline and “elastoid” dystrophy of the connective tissue. In the vascular part of the head of pterygium, in significant number of cases there are cross sections with hyaline and “elas- toid” dystrophy. Elastoid or hyaline dystrophic changes are rare or not present at all in the connective tissue of the body of progressive pterygium. Subepithelial connective tissue of the body of stationary pterygium is composed of compact connective tissue. There are more mature histiocytic elements and vessels present. In great number of cases hyaline and elastoid dystrophy are present [14].
Pterygium vascularity: the number, calibre and type of blood vessels showed extreme variability. Fragile capillary network of multiple blood vessels in edematous stroma of pterygia with discretely exposed fibrinoid change can be seen in evolutive shorter forms, while the complexes with abundant branching of well-developed capillaries with greater lumen are characteristic for other evolutive forms. In the study of Markowich et al. [31], pterygia specimens had a greater number of blood vessels positive to vWF (fon Wilebrand growth factor) and VEGF (vascular endothelial growth factor) (p=0.0012), then stronger staining intensity to VEGF in epithelial, stromal and endothelial cells (p<0.0001) and greater number of VEGF positive cells (p<0.0001) than in normal conjunctiva. Over-expression of VEGF in pterygium tissue, along with abundance of vWF stained blood vessels, can support previous hypotheses that angiogenesis plays an important role in formation of pterygium. When the blood vessels are in question, Cilova [14] found more abundant vascularisation in the body of the progressive pterygium in comparison to the body of stationary one. The patients with Fuchs’ patches present, representing the zones of pterygium progression, had higher inflammation in pterygium tissue in comparison to the group of patients without progression zones. This was expected, since Fuchs’patches are a significant, primarily clinical indicator of progressivness or activity of the pterygium tissue. Since the presence of Fuchs’ patches represents a potential risk of recurrence, the patients with recurrence are expected to have higher inflammation in comparison to those without recurrence. This was confirmed in our study, yet without statistical significance. It would have probably been more significant if the number of examined patients had been greater [21]. Fuchs’ patches have been a reliable marker of pterygium progression for a long time and showed statistically significant positive correlation with the intensity of vascularisation of excised pterygia in our results. This correlation shows a moment - period of rapid growth rate in pterygium progression. For its further progression and genesis of the new connective tissue, a greater number of blood vessels, as well as their greater calibre are necessary. A greater degree of pterygium vascularisation in patients with recurrence following the surgical intervention in comparison to the other group of patients, in spite of absence of statistical significance, was expected, since the genesis of the new recurrent connective tissue requires a greater number of blood vessels as well [21]. In patients with observed presence of Fuchs’ patches (progression zones), as well as in patients with recurrence of the disease, the intensity of fibrinoid change was higher. This could be explained by enormous evolution of pterygium and by accumulation of degenerative changes in pterygium tissue [21].
CONCLUSION
Pathohistological analysis of morphological characteristics of pterygium is a basis for assessment of its evolution, and along with clinical parameters it can be used in case when repeated surgery might be required, for adjuvant therapy application and for prediction of recurrences. That is why the analysis of pathohistological characteristics of the pterygium becomes more significant in everyday ophthalmologic practice.
DECLARATION OF INTEREST
Authors declare they have no conflict of interest in regard to this study.
REFERENCES
- 1.Coroneo MT. Pterygim as an early indicator ofultraviolet insolation: a hypothesis. Br J Ophthalmol. 1993;77:734–739. doi: 10.1136/bjo.77.11.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Detorakis ET, Sourvinos G, Spandidos DA. Detection of herpes simplex virus and human papilloma virus in ophthalmic pterygium. Cornea. 2001;20(2):164–167. doi: 10.1097/00003226-200103000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher MJ, Giannoudis A, Herrington CS, Hiscott P. Human papillomavirus in pterygium. Br J Ophthalmol. 2001;85(7):782–784. doi: 10.1136/bjo.85.7.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saw SM, Tan D. Pterygium: prevalence, demography and risk factors. Ophthalmic Epidemiol. 1999;6:219–228. doi: 10.1076/opep.6.3.219.1504. [DOI] [PubMed] [Google Scholar]
- 5.Džunić B, Jovanović P, Petrovič A. Uporedna analiza kliničkih karakteristika pterigijuma. Acta Fac Med Naiss. 2009;26(2):77–83. [Google Scholar]
- 6.Kruse FE, Chen JJY, Tsai RJF, Tseng SCG. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci. 1990;31:1903–1913. [PubMed] [Google Scholar]
- 7.Huang AJW, Tseng SCG. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:96–105. [PubMed] [Google Scholar]
- 8.Chen JJY, Tseng SCG. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1991;31:1301–1314. [PubMed] [Google Scholar]
- 9.Lukas RD. Greer’s ocular pathology. 4th ed. Oxford: Blackwell Scientific Publications; 1989. [Google Scholar]
- 10.Nakagami T, Watanabe I, Murakami A, Okisaka S, Ebihara N. Expression of stem cell factor in pterygium. Jpn J Ophthalmol. 2000;44:193–197. doi: 10.1016/s0021-5155(99)00214-2. [DOI] [PubMed] [Google Scholar]
- 11.Anđelković Z, Somer LJ, Matavulj M, Lačković V, Lalošević D, Nikolić I, et al. Ćelija i tkiva. Niš: Bonafides; 2002. [Google Scholar]
- 12.Cilova-Atanasova B. The mastocyte raeaction in pterygium. Folia Med. 1971;13:21–25. [PubMed] [Google Scholar]
- 13.Fuchs E. Ueber das Pterygium. Graefe’s Arch Ophthalmol. 1892;38(2):1–90. [Google Scholar]
- 14.Cilova-Atanasova B. Differences in the histomophologic and histochemical structure of the so called “progresive” and “stationary” pterygium. Folia Med. 1974;16:77–81. [PubMed] [Google Scholar]
- 15.Seifert P, Eckert J, Spitznas M. Topological-histological investigation of the pterygium. Graefe’s Arch Clin Exp Ophthalmol. 2001;239:288–293. doi: 10.1007/s004170100262. [DOI] [PubMed] [Google Scholar]
- 16.ЧиловаАтанасоваБ. НаучныетрудыаспирантовиординаторовПервогомедицинскогоМасковскогоисститутаимениИ. М. Сеченова. Москва1967.r;стр.108, (Bulgarian) [Google Scholar]
- 17.Karukonda SR, Thompson HW, Beuermann RW, Lam DS, Wilson R, Chew SJ, Steinemann TL. Cell cycle kinetics in pterygium at three latitudes. Br J Ophthalmol. 1995;79:313–317. doi: 10.1136/bjo.79.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadota Y. Morphological study on the pathogenesis of pterygium. Acta Soc Ophthalmol Jpn. 1987;91:324–334. [PubMed] [Google Scholar]
- 19.Shimmura S, Ishioka M, Hanada K, Shimazaki J, Tsubota K. Telomerase activity and p53 exspression in pterygia. Invest Ophthalmol Vis Sci. 2000;41:1364–1369. [PubMed] [Google Scholar]
- 20.Weinstein O, Rosenthal G, Zirkin H, Monos T, Lifshitz T, Argov S. Overexpression of p53 tumor suppressor gene in pterygia. Eye. 2002;16(5):619–621. doi: 10.1038/sj.eye.6700150. [DOI] [PubMed] [Google Scholar]
- 21.Džunić B, Jovanović P, Zlatanović G, Veselinović D, Petrović A, Stefanović I. Uporedna analiza patohistoloških i kliničkih karakteristika pterigijuma. Vojnosanit Pregl. 2010;67(2):159–165. doi: 10.2298/vsp1002159d. Serbian. [DOI] [PubMed] [Google Scholar]
- 22.Hogan MJ, Alvorado J. Pterygium and pinguecula: electron microscopic study. Arch Ophthalmol. 1967;78:174–186. doi: 10.1001/archopht.1967.00980030176010. [DOI] [PubMed] [Google Scholar]
- 23.Austin P, Jakobiec FA, Iwamoto T. Elastodysplasia and elastodystrophy as the pathologic basis of ocular pterygia and pinguecula. Ophthalmol. 1983;90:96–106. doi: 10.1016/s0161-6420(83)34594-2. [DOI] [PubMed] [Google Scholar]
- 24.Cameron ME. Histology of pterygium: an electron microscopic study. Br J Ophthalmol. 1983;67:604–860. doi: 10.1136/bjo.67.9.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tai MC, Chen CH, Yieh FS, Chang CJ. Morphology and growth kinetics of human pterygium fibroblast in primary culture. J Med Sci. 2003;1:23–28. [Google Scholar]
- 26.Ansen MW, Rahi AHS, Shukla BR. Pseudoelastic nature of pterygium. Br J Ophthalmol. 1970;54:473–476. doi: 10.1136/bjo.54.7.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vass P, Tapaszto I. The histochemical examination of fibres of pterygium by elastase. Acta Ophthalmol. 1964;42:849–854. doi: 10.1111/j.1755-3768.1964.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang IJ, Hu FR, Chen PJ, Lin CT. Mechanism of abnormal elasti gene expression in the pinguecular part of pterygia. Am J Pathol. 2000;157:1269–1276. doi: 10.1016/S0002-9440(10)64642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montagna W, Kirchner S, Carlisle K. Histology of sun-damaged skin. J Am Acad Dermatol. 1989;21:907–918. doi: 10.1016/s0190-9622(89)70276-0. [DOI] [PubMed] [Google Scholar]
- 30.Uitto J, Christiano AM. Elastic fiber. Dermatol Gen Med. 1993;1:339–349. [Google Scholar]
- 31.Marcovich AL, Morad Y, Sandbank J, Huszar M, Rosner M, Pollack A, et al. Angiogenesis in pterygium: morphometric and immunohistochemical study. Curr Eye Res. 2002;25(1):17–22. doi: 10.1076/ceyr.25.1.17.9959. [DOI] [PubMed] [Google Scholar]


