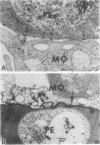
Abstract
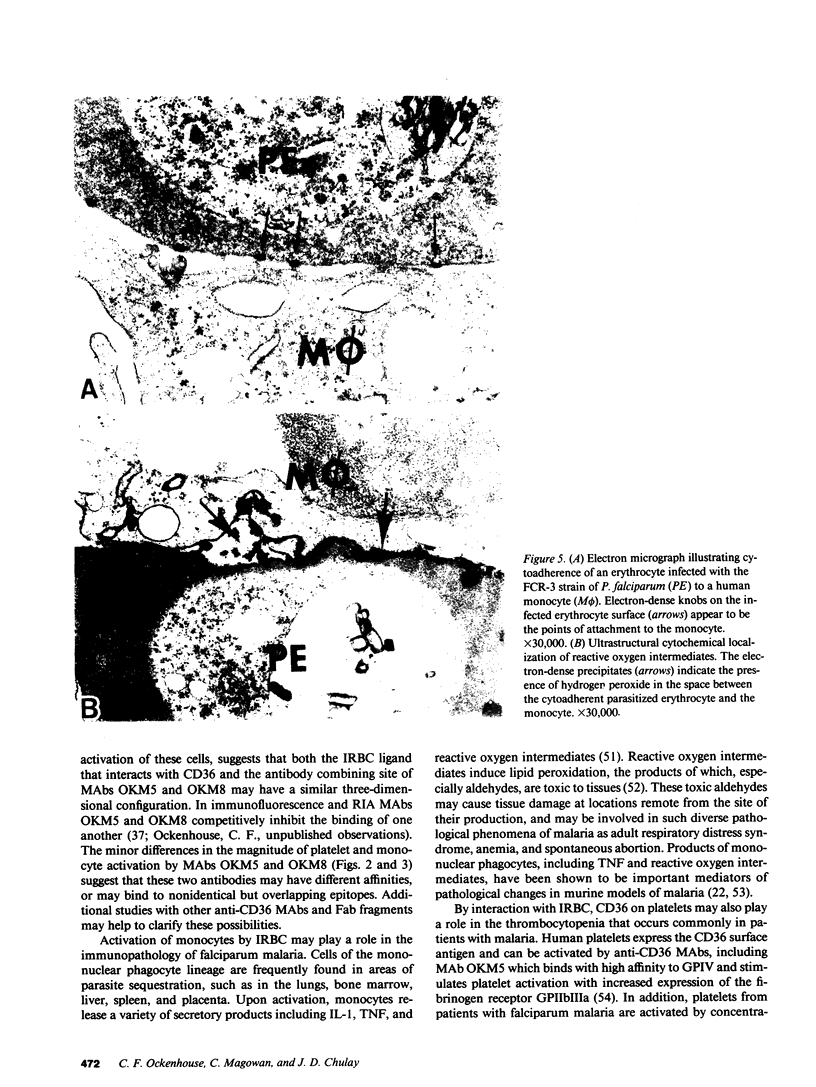
The CD36 leukocyte differentiation antigen, recognized by MAbs OKM5 and OKM8 and found on human monocytes and endothelial cells, has been implicated as a sequestration receptor for erythrocytes infected with the human malaria parasite Plasmodium falciparum (IRBC). CD36 is also expressed on platelets and appears to be identical to platelet glycoprotein IV. We investigated receptor activation of monocytes and platelets by anti-CD36 MAbs and by IRBC. Incubation of human monocytes with anti-CD36 MAbs or IRBC resulted in stimulation of the respiratory burst as measured by reduction of nitroblue tetrazolium and generation of chemiluminescence. Incubation of human platelets with anti-CD36 MAbs resulted in platelet activation as measured by aggregation or ATP secretion. Activation of monocytes and platelets required appropriate intracellular transmembrane signaling and was inhibited by calcium antagonists or by specific inhibitors of protein kinase C or guanine nucleotide binding proteins. Soluble CD36 inhibited binding of IRBC to both monocytes and platelets, suggesting that these interactions are mediated by the CD36 receptor. Using a cytochemical electron microscopic technique, the presence of reactive oxygen intermediates was identified at the interface between human monocytes and IRBC. These data provide support for the hypothesis that reactive oxygen intermediates produced by monocytes when IRBC ligands interact with cell surface receptors may play a role in the pathophysiology of falciparum malaria.
Full text
PDF

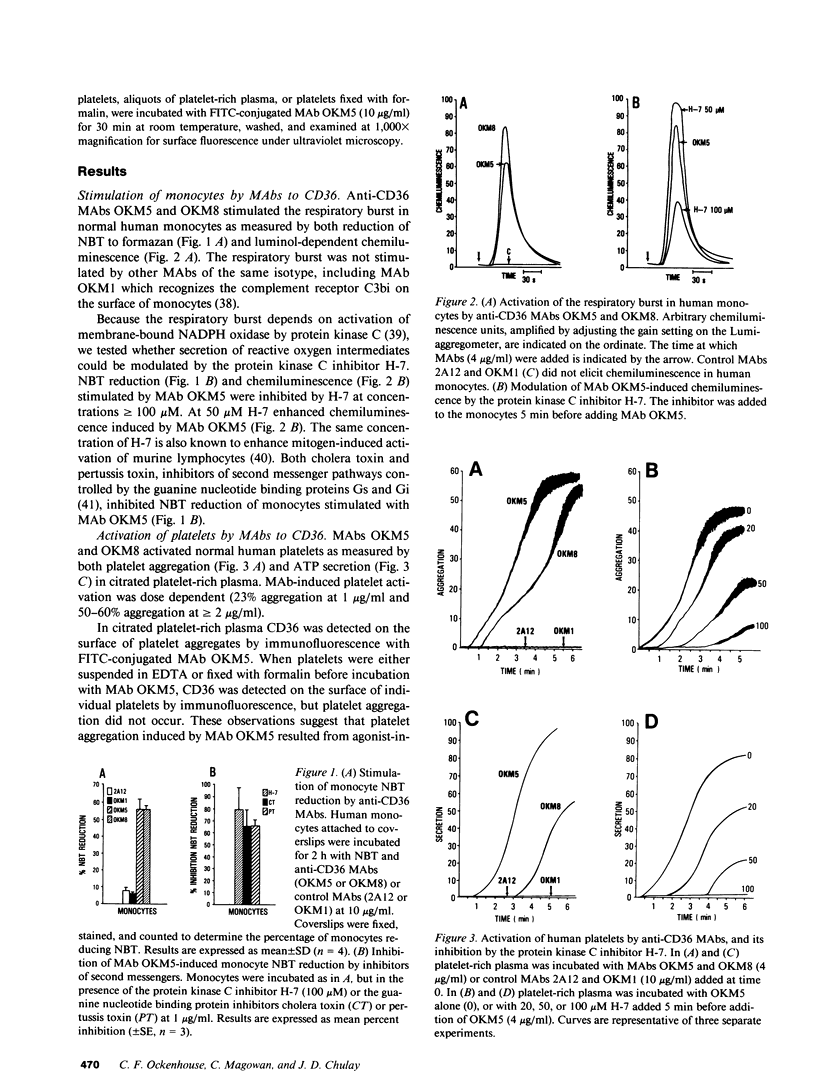
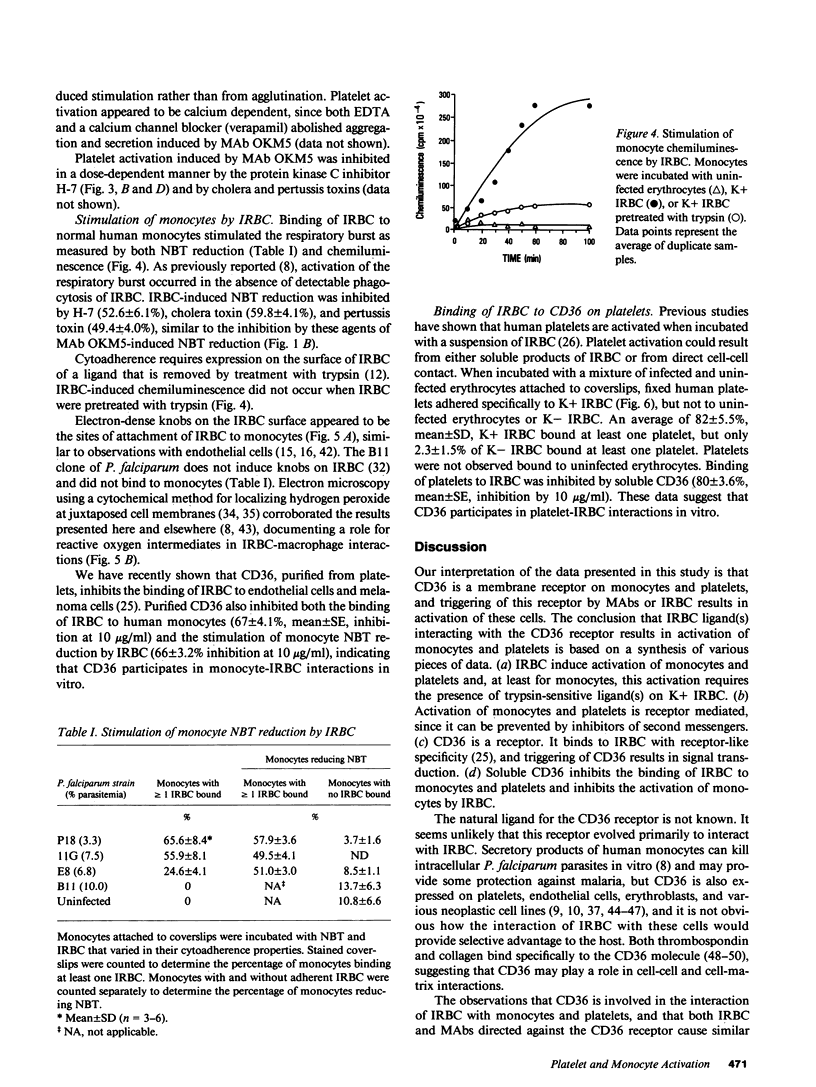



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Barnwell J., Silverstein R. L., Nachman R. L. Isolation of the thrombospondin membrane receptor. J Clin Invest. 1987 Apr;79(4):1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnwell J. W., Ockenhouse C. F., Knowles D. M., 2nd Monoclonal antibody OKM5 inhibits the in vitro binding of Plasmodium falciparum-infected erythrocytes to monocytes, endothelial, and C32 melanoma cells. J Immunol. 1985 Nov;135(5):3494–3497. [PubMed] [Google Scholar]
- Briggs R. T., Drath D. B., Karnovsky M. L., Karnovsky M. J. Localization of NADH oxidase on the surface of human polymorphonuclear leukocytes by a new cytochemical method. J Cell Biol. 1975 Dec;67(3):566–586. doi: 10.1083/jcb.67.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley P. J., Smith M. R., Braverman M. F., Dickson S. A. Human spleen contains phenotypic subsets of macrophages and dendritic cells that occupy discrete microanatomic locations. Am J Pathol. 1987 Sep;128(3):505–520. [PMC free article] [PubMed] [Google Scholar]
- Chulay J. D., Haynes J. D., Diggs C. L. Inhibition of in vitro growth of Plasmodium falciparum by immune serum from monkeys. J Infect Dis. 1981 Sep;144(3):270–278. doi: 10.1093/infdis/144.3.270. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Butcher G. A., Hunt N. H. Possible roles of tumor necrosis factor in the pathology of malaria. Am J Pathol. 1987 Oct;129(1):192–199. [PMC free article] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Cowden W. B. Oxygen-derived free radicals in the pathogenesis of parasitic disease. Adv Parasitol. 1986;25:1–44. doi: 10.1016/s0065-308x(08)60341-3. [DOI] [PubMed] [Google Scholar]
- Clark R. B., Love J. T., Jr, Sgroi D., Lingenheld E. G., Sha'afi R. I. The protein kinase C inhibitor H-7, inhibits antigen and IL-2-induced proliferation of murine T cell lines. Biochem Biophys Res Commun. 1987 Jun 15;145(2):666–672. doi: 10.1016/0006-291x(87)91016-3. [DOI] [PubMed] [Google Scholar]
- Cranston H. A., Boylan C. W., Carroll G. L., Sutera S. P., Williamson J. R., Gluzman I. Y., Krogstad D. J. Plasmodium falciparum maturation abolishes physiologic red cell deformability. Science. 1984 Jan 27;223(4634):400–403. doi: 10.1126/science.6362007. [DOI] [PubMed] [Google Scholar]
- David P. H., Hommel M., Miller L. H., Udeinya I. J., Oligino L. D. Parasite sequestration in Plasmodium falciparum malaria: spleen and antibody modulation of cytoadherence of infected erythrocytes. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5075–5079. doi: 10.1073/pnas.80.16.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte M. I., Corbett C. E., Boulos M., Amato Neto V. Ultrastructure of the lung in falciparum malaria. Am J Trop Med Hyg. 1985 Jan;34(1):31–35. doi: 10.4269/ajtmh.1985.34.31. [DOI] [PubMed] [Google Scholar]
- Essien E. M., Ebhota M. I. Platelet hypersensitivity in acute malaria (Plasmodium falciparum) infection in man. Thromb Haemost. 1981 Aug 28;46(2):547–549. [PubMed] [Google Scholar]
- Essien E. M., Ebhota M. I. Platelet secretory activities in acute malaria (Plasmodium falciparum) infection. Acta Haematol. 1983;70(3):183–188. doi: 10.1159/000206720. [DOI] [PubMed] [Google Scholar]
- Feinman R. D., Lubowsky J., Charo I., Zabinski M. P. The lumi-aggregometer: a new instrument for simultaneous measurement of secretion and aggregation by platelets. J Lab Clin Med. 1977 Jul;90(1):125–129. [PubMed] [Google Scholar]
- Fukuda Y., Nagura H., Imoto M., Koyama Y. Immunohistochemical studies on structural changes of the hepatic lobules in chronic liver diseases. Am J Gastroenterol. 1986 Dec;81(12):1149–1155. [PubMed] [Google Scholar]
- Grau G. E., Fajardo L. F., Piguet P. F., Allet B., Lambert P. H., Vassalli P. Tumor necrosis factor (cachectin) as an essential mediator in murine cerebral malaria. Science. 1987 Sep 4;237(4819):1210–1212. doi: 10.1126/science.3306918. [DOI] [PubMed] [Google Scholar]
- Graves P. M., Carter R., Keystone J. S., Seeley D. C., Jr Drug sensitivity and isoenzyme type in cloned lines of Plasmodium falciparum. Am J Trop Med Hyg. 1984 Mar;33(2):212–219. doi: 10.4269/ajtmh.1984.33.212. [DOI] [PubMed] [Google Scholar]
- Hommel M., David P. H., Oligino L. D. Surface alterations of erythrocytes in Plasmodium falciparum malaria. Antigenic variation, antigenic diversity, and the role of the spleen. J Exp Med. 1983 Apr 1;157(4):1137–1148. doi: 10.1084/jem.157.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inyang A. L., Sodeinde O., Okpako D. T., Essien E. M. Platelet reactions after interaction with cultured Plasmodium falciparum infected erythrocytes. Br J Haematol. 1987 Jul;66(3):375–378. doi: 10.1111/j.1365-2141.1987.tb06926.x. [DOI] [PubMed] [Google Scholar]
- Israeli A., Shapiro M., Ephros M. A. Plasmodium falciparum malaria in an asplenic man. Trans R Soc Trop Med Hyg. 1987;81(2):233–234. doi: 10.1016/0035-9203(87)90224-0. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Trager W. Plasmodium falciparum in culture: establishment of additional strains. Am J Trop Med Hyg. 1978 Jul;27(4):743–746. doi: 10.4269/ajtmh.1978.27.743. [DOI] [PubMed] [Google Scholar]
- Knowles D. M., 2nd, Tolidjian B., Marboe C., D'Agati V., Grimes M., Chess L. Monoclonal anti-human monocyte antibodies OKM1 and OKM5 possess distinctive tissue distributions including differential reactivity with vascular endothelium. J Immunol. 1984 May;132(5):2170–2173. [PubMed] [Google Scholar]
- Looareesuwan S., Ho M., Wattanagoon Y., White N. J., Warrell D. A., Bunnag D., Harinasuta T., Wyler D. J. Dynamic alteration in splenic function during acute falciparum malaria. N Engl J Med. 1987 Sep 10;317(11):675–679. doi: 10.1056/NEJM198709103171105. [DOI] [PubMed] [Google Scholar]
- Luse S. A., Miller L. H. Plasmodium falciparum malaria. Ultrastructure of parasitized erythrocytes in cardiac vessels. Am J Trop Med Hyg. 1971 Sep;20(5):655–660. [PubMed] [Google Scholar]
- MacPherson G. G., Warrell M. J., White N. J., Looareesuwan S., Warrell D. A. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985 Jun;119(3):385–401. [PMC free article] [PubMed] [Google Scholar]
- Magowan C., Wollish W., Anderson L., Leech J. Cytoadherence by Plasmodium falciparum-infected erythrocytes is correlated with the expression of a family of variable proteins on infected erythrocytes. J Exp Med. 1988 Oct 1;168(4):1307–1320. doi: 10.1084/jem.168.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makimura S., Brinkmann V., Mossmann H., Fischer H. Chemiluminescence response of peritoneal macrophages to parasitized erythrocytes and lysed erythrocytes from Plasmodium berghei-infected mice. Infect Immun. 1982 Aug;37(2):800–804. doi: 10.1128/iai.37.2.800-804.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ockenhouse C. F., Chulay J. D. Plasmodium falciparum sequestration: OKM5 antigen (CD36) mediates cytoadherence of parasitized erythrocytes to a myelomonocytic cell line. J Infect Dis. 1988 Mar;157(3):584–588. doi: 10.1093/infdis/157.3.584. [DOI] [PubMed] [Google Scholar]
- Ockenhouse C. F., Schulman S., Shear H. L. Induction of crisis forms in the human malaria parasite Plasmodium falciparum by gamma-interferon-activated, monocyte-derived macrophages. J Immunol. 1984 Sep;133(3):1601–1608. [PubMed] [Google Scholar]
- Ockenhouse C. F., Tandon N. N., Magowan C., Jamieson G. A., Chulay J. D. Identification of a platelet membrane glycoprotein as a falciparum malaria sequestration receptor. Science. 1989 Mar 17;243(4897):1469–1471. doi: 10.1126/science.2467377. [DOI] [PubMed] [Google Scholar]
- Oo M. M., Aikawa M., Than T., Aye T. M., Myint P. T., Igarashi I., Schoene W. C. Human cerebral malaria: a pathological study. J Neuropathol Exp Neurol. 1987 Mar;46(2):223–231. doi: 10.1097/00005072-198703000-00009. [DOI] [PubMed] [Google Scholar]
- Panton L. J., Leech J. H., Miller L. H., Howard R. J. Cytoadherence of Plasmodium falciparum-infected erythrocytes to human melanoma cell lines correlates with surface OKM5 antigen. Infect Immun. 1987 Nov;55(11):2754–2758. doi: 10.1128/iai.55.11.2754-2758.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Udeinya I. J., Leech J. H., Hay R. J., Aikawa M., Barnwell J., Green I., Miller L. H. Plasmodium falciparum malaria. An amelanotic melanoma cell line bears receptors for the knob ligand on infected erythrocytes. J Clin Invest. 1982 Aug;70(2):379–386. doi: 10.1172/JCI110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seim S., Espevik T. Toxic oxygen species in monocyte-mediated antibody-dependent cytotoxicity. J Reticuloendothel Soc. 1983 Jun;33(6):417–428. [PubMed] [Google Scholar]
- Snyderman R., Smith C. D., Verghese M. W. Model for leukocyte regulation by chemoattractant receptors: roles of a guanine nucleotide regulatory protein and polyphosphoinositide metabolism. J Leukoc Biol. 1986 Dec;40(6):785–800. doi: 10.1002/jlb.40.6.785. [DOI] [PubMed] [Google Scholar]
- Talle M. A., Rao P. E., Westberg E., Allegar N., Makowski M., Mittler R. S., Goldstein G. Patterns of antigenic expression on human monocytes as defined by monoclonal antibodies. Cell Immunol. 1983 May;78(1):83–99. doi: 10.1016/0008-8749(83)90262-9. [DOI] [PubMed] [Google Scholar]
- Tandon N. N., Kralisz U., Jamieson G. A. Identification of glycoprotein IV (CD36) as a primary receptor for platelet-collagen adhesion. J Biol Chem. 1989 May 5;264(13):7576–7583. [PubMed] [Google Scholar]
- Tandon N. N., Lipsky R. H., Burgess W. H., Jamieson G. A. Isolation and characterization of platelet glycoprotein IV (CD36). J Biol Chem. 1989 May 5;264(13):7570–7575. [PubMed] [Google Scholar]
- Tauber A. I. Protein kinase C and the activation of the human neutrophil NADPH-oxidase. Blood. 1987 Mar;69(3):711–720. [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Trager W., Rudzinska M. A., Bradbury P. C. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35(6):883–885. [PMC free article] [PubMed] [Google Scholar]
- Udeinya I. J., Schmidt J. A., Aikawa M., Miller L. H., Green I. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981 Jul 31;213(4507):555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- Walter P. R., Garin Y., Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol. 1982 Dec;109(3):330–342. [PMC free article] [PubMed] [Google Scholar]
- White N. J., Warrell D. A., Looareesuwan S., Chanthavanich P., Phillips R. E., Pongpaew P. Pathophysiological and prognostic significance of cerebrospinal-fluid lactate in cerebral malaria. Lancet. 1985 Apr 6;1(8432):776–778. doi: 10.1016/s0140-6736(85)91445-x. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]