SUMMARY
Objective
The goals of this study were (1) to quantify proteoglycan 4 (PRG4) gene expression; (2) to assess lubricin immunostaining; and (3) to measure synovial fluid lubricin concentrations in clinical and experimental models of equine carpal osteoarthritis (OA).
Design
Lubricin synovial fluid concentrations and cartilage and synovial membrane PRG4 expression were analyzed in research horses undergoing experimental OA induction (n = 8) and in equine clinical patients with carpal OA (n = 58). Lubricin concentrations were measured using a custom sandwich enzyme-linked immunosorbent assay, and PRG4 expression was quantified using qRT-PCR. Lubricin immunostaining was assessed in synovial membrane and osteochondral sections in the experimental model.
Results
Lubricin concentrations increased in synovial fluid following induction of OA, peaking at 21 days post-operatively in OA joints vs sham-operated controls (331 ± 69 μg/mL vs 110 ± 19 μg/mL, P = 0.001). Lubricin concentrations also increased in horses with naturally occurring OA as compared to control joints (152 ± 32 μg/mL vs 68 ± 4 μg/mL, P = 0.003). Synovial membrane PRG4 expression increased nearly 2-fold in naturally occurring OA (P = 0.003), whereas cartilage PRG4 expression decreased 2.5-fold (P = 0.025). Lubricin immunostaining was more pronounced in synovial membrane from OA joints as compared to controls, with intense lubricin localization to sites of cartilage damage.
Conclusions
Although PRG4 gene expression decreases in OA cartilage, synovial membrane PRG4 expression, synovial fluid lubricin concentrations and lubricin immunostaining all increase in an equine OA model. Lubricin may be elevated to protect joints from post-traumatic OA.
Keywords: Proteoglycan 4, Lubricin, Superficial zone protein, Osteoarthritis, Cartilage, Synovium
Introduction
Lubricin, a mucinous glycoprotein encoded by the proteoglycan 4 (PRG4) gene, functions as both a boundary lubricant and chondroprotective agent in synovial joints. Patients with camptodactyly-arthropathy-coxa vara-pericarditis (CACP) fail to express PRG4 and subsequently develop early-onset polyarthropathy1,2. Studies have revealed that lubricin-deficient synovial fluid in a subset of patients with osteoarthritis (OA) fails to lubricate cartilage and that boundary lubrication can be restored by the addition of exogenous recombinant lubricin3. In human patients with anterior cruciate ligament (ACL) injury4 and in rodent models of ACL injury4–6, synovial fluid lubricin concentrations are decreased in injured joints as compared to controls. Synovial fluid lubricin concentrations were also decreased in a population of human patients with late-stage OA and RA7. Mounting evidence suggests that lubricin supplementation, either through genetic overexpression8 or recombinant lubricin supplementation9–12 delays the progression of OA in rodent models. Accordingly, there is considerable interest in the use of lubricin supplementation as a potential therapeutic for OA, but information about lubricin expression and tissue localization in large animal naturally occurring OA and translational large animal experimental models of OA is limited.
Large animal models such as the horse more closely recapitulate OA in humans than small animal models because cartilage thickness and joint volume more closely approximate human cartilage13–15, and cartilage is subject to loading forces of similar or greater magnitude than human cartilage15. Moreover, the equine carpal fragment model has the added benefits of allowing repeated synovial fluid sampling and controlled, athletic exercise15,16. However, there is substantial controversy about how lubricin is altered in large animal models of arthritis. In one report, PRG4 mRNA levels were significantly decreased in a sheep meniscectomy model 3 months post-operatively, and gene expression correlated with decreased lubricin immunostaining in degenerative articular cartilage17. Conversely, lubricin synovial fluid concentrations and boundary lubrication properties were similar between operated and contralateral control limbs, leading investigators to conclude that lubricin should be evaluated at earlier time points in the development of OA18. In dogs undergoing unilateral cranial cruciate ligament transection, lubricin immunohistochemical staining did not differ between OA and control limbs 13 weeks post-operatively19, and quantitation of lubricin using Western blot analysis revealed an 83% increase in lubricin in acutely injured (≤3 weeks) equine joints20. Thus, although several studies in rodent models suggest that lubricin decreases in experimental arthritis, it remains unclear whether lubricin levels are increased, decreased or unchanged in large animal models of OA and over what time course these changes occur.
Furthermore, some investigations report increased synovial fluid lubricin concentrations in human patients with intra-articular fracture21 or late-stage OA22, bringing into question whether it is appropriate to extrapolate from rodent models to humans. A primary limitation of studies to date evaluating lubricin synovial fluid concentrations in human patients3,5 and in most experimental animal models of OA is that synovial fluid lubricin concentrations are only evaluated at a single time point6,18,20. A recent longitudinal analysis of synovial fluid lubricin concentrations in an ovine anterior cruciate ligament transection model revealed increased lubricin concentrations at 2 and 4 weeks post-injury as compared to 20 weeks; however, synovial fluid samples were not evaluated prior to injury23. Because serial lubricin quantitation has been limited, it is difficult to make inferences as to how lubricin concentrations change over the course of OA, whether altered lubricin concentrations precede the development of OA, and whether or not intraarticular lubricin supplementation may be indicated in clinically relevant large animal models or humans. To our knowledge, no studies have evaluated PRG4 expression, serial synovial fluid lubricin concentrations and immunostaining in the same model.
Our objective, therefore, was to assess changes in synovial fluid lubricin concentrations at serial intervals both before and after osteochondral fragmentation in an equine OA model16, and to assess PRG4 expression and immunostaining from articular cartilage and synovium at study termination 70 days post-OA induction. In addition, we sought to quantify PRG4 and lubricin glycoprotein expression in cartilage, synovial membrane and synovial fluid samples from horses with naturally occurring carpal OA injuries similar to the carpal osteochondral fragment experimental model. We hypothesized that PRG4 expression, lubricin synovial fluid concentrations and lubricin synovial membrane and cartilage immunostaining would decrease in both experimental and naturally occurring OA in horses.
Methods
Samples
PRG4 expression and lubricin synovial fluid concentrations were analyzed in samples from two equine cohorts: equine clinical patients or research animals with carpal OA (n = 36) or healthy carpal joints (n = 22), and research horses undergoing carpal osteochondral fragmentation for OA induction (n = 8). All experimental protocols were approved by the Cornell University Institutional Animal Care and Use Committee, and all synovial fluid and tissue samples were collected with informed owner consent.
Naturally occurring OA
Healthy and OA synovial fluid, synovial membrane and cartilage tissues were harvested from the antebrachiocarpal (ACJ) and middle carpal (MCJ) joints of horses presenting to the Cornell University Equine Hospital for arthroscopy or from horses donated to the hospital for research purposes. Cytokine and catabolic enzyme expression from this cohort of primarily Thoroughbred horses ranging in age from 2 to 11 years has been previously described24. Radiographic evaluation of all OA joints was performed prior to surgery or euthanasia, and joints were assigned a score of normal, mild, moderate or severe OA according to the radiographic presence of osteophytes, enthesiophytes, osteoproliferation, joint space narrowing, or chronic fracture lines, with additional arthroscopic/gross scoring used to corroborate the radiographic score. Thirty-six horses with carpal OA underwent radiography prior to surgery or euthanasia, and 22 horses with normal joints were included in the study.
Experimental OA
The arthroscopically created equine carpal osteochondral fragment-exercise model of OA has been previously described16,25. Briefly, an 8 mm osteochondral fragment was created at the distal dorsal aspect of the radial carpal bone of one randomly assigned forelimb. The contralateral forelimb was sham-operated without creation of a fragment. Synovial fluid samples were obtained from both limbs at the time of initial arthroscopy and at weekly intervals post-operatively. Horses were maintained in 3.65 m × 3.65 m box stalls and were exercised on a high-speed treadmill 5 times weekly beginning 2 weeks post-operatively and continuing for the study duration to simulate race training. Horses were walked (5 km/h) for 4 min, trotted (16–19 km/h) for 2 min, galloped (28–32 km/h) for 2 min, and trotted (16–19 km/h) for 2 min, for a total exercise time of 10 min. On day 70 post-induction, horses were euthanized with an overdose of barbiturate, and synovial membrane biopsies obtained for RNA expression. Synovial fluid was collected, and synovial membrane and osteochondral blocks, including the radial carpal bone fragment and parent bone and the opposite third carpal bone, were harvested for histological processing and immunohistochemistry. Eight Thoroughbred horses (n = 5 females and n = 3 castrated males), aged 2–6 years old, were used for the study.
Isolation of RNA and real-time quantitative PCR lubricin assay
Synovial membrane tissue and cartilage was snap-frozen in liquid nitrogen, pulverized and stored at −80°C prior to isolation of RNA using the PerfectPure RNA Tissue Kit (5Prime, Gaithersburg, MD), and RNA purity and concentration were assessed with UV microspectrophotometry (NanoDrop 2000 Spectrophotometer, Thermo Scientific, Waltham, MA). PRG4 gene expression was quantified by real-time PCR using the Taqman One-Step RT-PCR technique (Absolute Quantitative PCR; ABI PRISM 7900 HT Sequence Detection System, Applied Biosystems, Foster City, CA). Primer Express Software Version 2.0 (Applied Biosystems, Foster City, CA) was used to design primers and dual-labeled fluorescent probes (6-carboxyfluorescein (FAM) as the 5′ reporter dye and Iowa Black® FQ as the 3′ quenching dye) for quantification of equine PRG4. Primers were designed as follows: Fwd: 5′ – TGCGGTGCTTCCCCATAC – 3′; Rev: 5′ – AAACAGGAACCCATCAGAAAGTG – 3′; Probe: 5′ –/56FAM/ATAGCAGGCCGCCTTCCCGG/3IABkFQ/– 3′. The total copy number of mRNA was determined using a validated standard curve of equine PRG4 C-terminal cDNA, and these values were normalized to the housekeeping gene 18S.
Purification of equine synovial fluid lubricin
Synovial fluid was collected from two healthy 4 to 6-year-old horses, clarified by high-speed centrifugation at 10,000 × g for 1 h and stored at −80°C. Synovial fluid was digested with 1 U/mL Streptomyces hyaluronidase (Calbiochem®, EMD Chemicals, San Diego, CA) in 50 mM NaAc buffer, pH 5.5 at 4°C for 16 h on an end-over-end rocker and purified using a HiTrap™ diethylaminoethyl (DEAE) fast flow sepharose FPLC column (GE Healthcare Life Sciences, Little Chalfont, UK), similar to previously described methodology26. Lubricin was confirmed by Coomassie staining (Teknova, Hollister, CA) and immunoblotting with a C-terminal lubricin polyclonal antibody (ab28484, Lot: GR116636-3, Abcam®, Cambridge, UK), anti-mucin lubricin monoclonal antibody 9G3 (MABT401; EMD Millipore, Darmstadt, Germany) and anti-mucin lubricin monoclonal antibody 6a8 (courtesy of G Jay) (Supplementary Fig. 1). The final protein concentration of the highest purity lubricin fraction (400–600 mM) was quantitated using a bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL).
Synovial fluid lubricin (sandwich ELISA)
A sandwich ELISA using peanut agglutinin (PNA) (Sigma–Aldrich, St. Louis, MO) and anti-lubricin monoclonal antibody 9G3 (MABT401; EMD Millipore, Darmstadt, Germany) has been previously described4 and was used for quantification of equine synovial fluid lubricin. Twelve hours after coating high binding 96-well plates (Corning Inc., Corning, NY) with 10μg/mL of PNA in 50 mM sodium bicarbonate buffer, pH 9.5, plates were blocked with phosphate buffered saline (PBS) + 3% EIA-grade BSA (Sigma–Aldrich, St. Louis, MO) for 1 h. Serial dilutions of purified equine lubricin and diluted equine SF samples were incubated for 1 h at room temperature, followed by washing in PBS+ 0.1% Tween20. Monoclonal Ab 9G3 was added to the plate at a dilution of 1:10,000 for 1 h at room temperature and washed with PBS + 0.1% Tween20. Chicken anti-mouse IgG-horseradish peroxidase (EMD Millipore, Darmstadt, Germany) was added at 1:2000 dilution for 1 h, followed by washing three times in PBS + 0.1% Tween20 with a final rinse in PBS alone. TMB reagent was added (Pierce, Rockford, IL), the reaction was stopped with 2N H2SO4, and absorbance was measured at 450 nm with 540 nm background subtraction.
Histological processing
Sagittal osteochondral sections were obtained from the weight-bearing regions of the fragmented radial carpal bone and parent bone, in addition to the articulating third carpal bone opposite the fragment in the experimental animals described above. Sections were fixed in 4% paraformaldehyde, decalcified in 10% EDTA, dehydrated in alcohol, cleared, paraffin embedded and sectioned at 6 mm, as previously described27. Both osteochondral and synovial membrane sections were incubated with 10% hyaluronidase solution for 30 min at 37°C prior to rinsing and quenching endogenous peroxidase activity for immunohistochemistry. Histological staining using lubricin-specific monoclonal antibody 6a8 (courtesy of G Jay) at a 1:1000 dilution was performed using a similar protocol to that described for anti-lubricin mAb-7h12, beginning with the quenching of endogenous peroxidase activity28. Safranin O/Fast Green staining was performed to assess cartilage sGAG content. Synovial membrane sections from the MCJ tissue adjacent to the fracture site were obtained for mAb 6a8 lubricin staining and hematoxylin and eosin staining. Representative synovial membrane and osteochondral sections were imaged using an Aperio Scan-Scope slide scanner (Leica, Wetzlar, Germany) at 20 × magnification, exported as. tif files with ImageScope software (Leica, Wetzlar, Germany) and compiled using Adobe Illustrator CS5.1 software (Adobe Systems Inc., San Jose, CA).
Statistical analysis
Naturally occurring OA
ELISA and qRT-PCR data were analyzed using a 1-way ANOVA with Tukey’s HSD post hoc tests. Comparisons between horses with normal joints and horses with OA joints (1, 2, and 3 grouped together) were made using an independent sample t-test for comparison of means. Log values were used to normalize lubricin ELISA and cartilage gene expression data in order to satisfy model assumptions, and summary statistics were performed on the untransformed data. The association between results of the lubricin ELISA and PRG4 expression data and previously published TNFα ELISA data and catabolic cytokine expression data24 was investigated. Since transformation of the previously reported data did not result in satisfactory results for the assumption of normality in all cases, Spearman correlation was performed. P-values < 0.05 were considered significant.
Experimental OA
Lubricin ELISA data were analyzed using with a mixed model because each horse was repeatedly measured on each limb and each limb repeatedly over days. The fixed effects in the model included treatment (control vs OA), day and a treatment*day interaction term, and random effects included horse and individual limb nested within horse to account for the non-independence of the observations. This analysis was followed with post hoc comparisons of specific contrasts for each time point to assess the difference between control vs OA joints. A Bonferroni correction for multiple comparisons was applied. Synovial membrane qRT-PCR data were analyzed using a paired t-test. P-values < 0.05 were considered significant. Statistical analysis was performed using JMP Pro 11.0 software (SAS Institute Inc., Cary, NC).
Results
Naturally occurring OA
Synovial membrane PRG4 gene expression increased in naturally occurring equine carpal OA [Fig. 1(A)]; whereas cartilage PRG4 transcription levels decreased [Fig. 1(B)]. Differences in PRG4 gene expression were statistically significant between control and moderate OA cases for both synovial membrane (40.3 × 104 ± 0.4 × 104 copies/ng vs 77.9 × 104 ± 1.0 × 104copies/ng, P = 0.003) cartilage (45.5 × 104 ± 1.4 × 104 copies/ng vs 18.8 × 104 ± 0.8 ×104 copies/ng, P = 0.025). Synovial fluid lubricin concentrations in horses with naturally occurring OA (n = 47) demonstrated a trend for increased lubricin levels in all individual OA grades as compared to controls. Because there were no significant differences in lubricin concentrations between mild, moderate and severe (1, 2, and 3) OA groups and because the OA grading criteria did not account for variables such as duration of injury, all OA grades were combined for analysis. Lubricin concentrations were significantly elevated in OA vs control joints (152 ± 32 μg/mL vs 68 ± 4 μg/mL, P = 0.003) when all OA grades were combined [Fig. 1(C)]. Association analysis revealed moderate correlation between synovial membrane PRG4 expression and ADAMTS5 [Fig. 1(D), ρ = 0.488, P < 0.001] and TNFα [Fig. 1(E), ρ = 0.508, P < 0.001] expression. Cartilage PRG4 expression was moderately correlated with cartilage ADAMTS5 expression (ρ = 0.596, P = 0.009), and synovial fluid lubricin concentrations were moderately correlated with TNFα concentrations [Fig. 1(F), ρ = 0.520, P = 0.016].
Fig. 1.
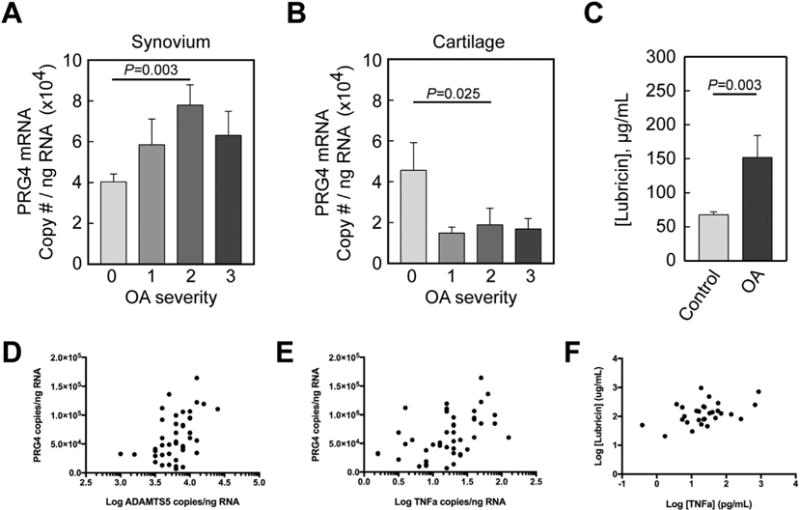
Proteoglycan 4 (PRG4) gene expression in synovial membrane (A) and cartilage (B) from normal equine carpal joints (n = 22) and equine carpal joints with naturally occurring OA (n = 36). Categories were numbered as normal (0), mild OA (1), moderate OA (2) and severe OA (3). Synovial membrane PRG4 expression was significantly increased (A) and cartilage PRG4 expression was significantly decreased (B) in the moderate OA group as compared to the control group. (C) Synovial fluid lubricin concentrations were significantly elevated in OA joints as compared to controls. Data are presented as mean ± S.E.M. Scatterplots showing the correlation between (D) synovial membrane PRG4 expression and synovial membrane ADAMTS5 (log transformed) (ρ = 0.488, P < 0.001) and (E) TNFα (log transformed) (ρ = 0.508, P < 0.001) expression. (F) Scatterplots showing the correlation between synovial fluid lubricin concentrations (log transformed) vs TNFα concentrations (log transformed) (ρ = 0.520, P < 0.001).
Experimental OA
All experimental horses were assessed to be free of lameness by one boarded surgeon (AEW) and to have no radiographic evidence of OA by two boarded surgeons (AJN, AEW) prior to the beginning of the study. At day 70, the severity of OA in the carpal osteochondral fragment model corresponded to grade 1 OA (mild OA) in the naturally occurring cohort. Lubricin concentrations increased in synovial fluid following induction of OA. Lubricin concentrations were significantly elevated in OA joints vs controls on days 21, 28, and 42 following induction of OA (P < 0.01) [Fig. 2(B)]. Twenty-one days post-operatively, synovial fluid lubricin concentrations were increased 3-fold in joints with carpal fragmentation as compared to sham-operated controls (331 ± 69 μg/mL vs 110 μg/mL ± 19 μg/mL, P = 0.001). In the mixed effects model, the main effect of treatment (control vs OA) was F(statistic = 1, df =16) = 16.56 (P = 0.001), the main effect of day was F(9,115) = 2.92, (P = 0.004), and the main effect of the treatment*day interaction term was F(9,115) = 1.19, (P = 0.310). Despite the non-significant interaction term, we followed the analysis with post hoc comparisons because we performed the analysis with specific contrasts in mind, namely, comparing the effect of treatment at each specific day. The elevation in lubricin concentrations in synovial fluid did not correspond to an increase in synovial membrane PRG4 gene expression in horses with OA vs controls (1.9 × 105 ± 0.2 × 105 copies/ng vs 1.8 × 105 ± 0.2 ×105 copies/ng) at day 70 post-carpal fragment induction [Fig. 2(A)].
Fig. 2.
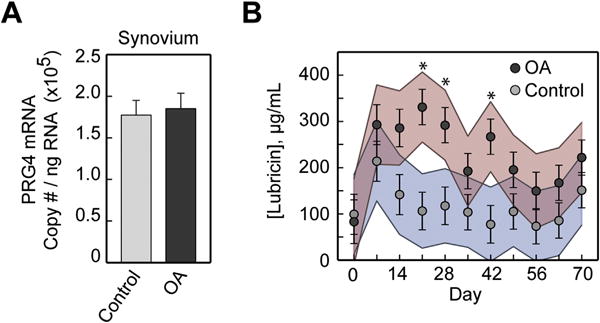
(A) Proteoglycan 4 (PRG4) gene expression in synovial membrane from sham-operated control and osteochondral fragment-induced OA joints in experimental horses 70 days post-fragmentation (n = 8). (B) Serial lubricin synovial fluid concentrations from day 0 (immediately prior to fragment induction) to day 70 post-fragmentation in OA and control joints (n = 8). Lubricin synovial fluid concentrations were increased in joints with carpal fragmentation vs sham-operated controls on days 21 (P < 0.001), 28 (P = 0.003) and 42 (P = 0.001) following induction of OA. Data are presented as predicted (least squares) means ± S.E.M. with colored lines representing 95% confidence intervals. * = P < 0.01.
Lubricin immunoreaction was increased in intensity and distribution within the synovial membrane of all OA limbs vs controls (n = 8), most prominently within the superficial intimal layers and surrounding vasculature (Fig. 3). Lubricin immunostaining of the articular surface of the third carpal bone, which articulates with the osteochondral fragment, was variably affected in the OA limb as compared to the control, depending upon the degree of damage to the articular surface. The most dramatic third carpal bone lubricin staining was observed in damaged articular surfaces, including surface fissuring, fibrillation, and proteoglycan loss; whereas lubricin localization to the lamina splendens and superficial zone chondrocytes was similar to controls at sites distant to the osteochondral fragment (Fig. 4). Within the radial carpal bone fragment, lubricin immunostaining was increased, particularly within the superficial 100–200 mm of fibrocartilaginous repair tissue covering the articular margin of the fragment at the transition to normal, hyaline cartilage of the parent bone (Fig. 5, ROI 1–2). Lubricin immunoreaction was increased within the vascular lining cells, most notably within the fracture fragment and at the site of reintegration (Fig. 5, ROI 3).
Fig. 3.
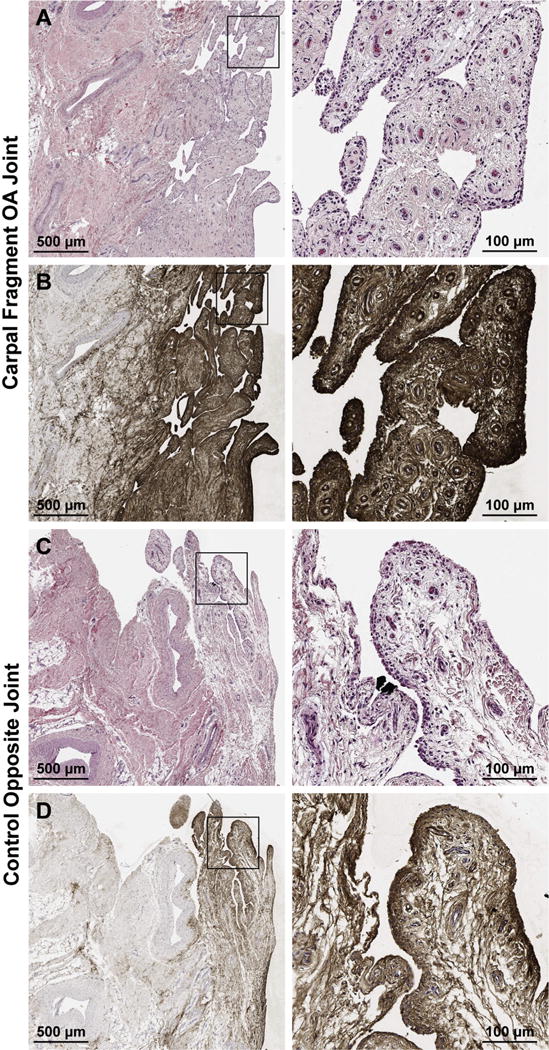
Hematoxylin and eosin (A, C) and lubricin immunostaining with monoclonal antibody 6a8 (B, D) of synovial tissues from the equine carpal osteochondral fragment model 70 days after fragment induction. Lubricin immunostaining is increased throughout the entire synovial villus of the OA limb (B) as compared to the control (D).
Fig. 4.
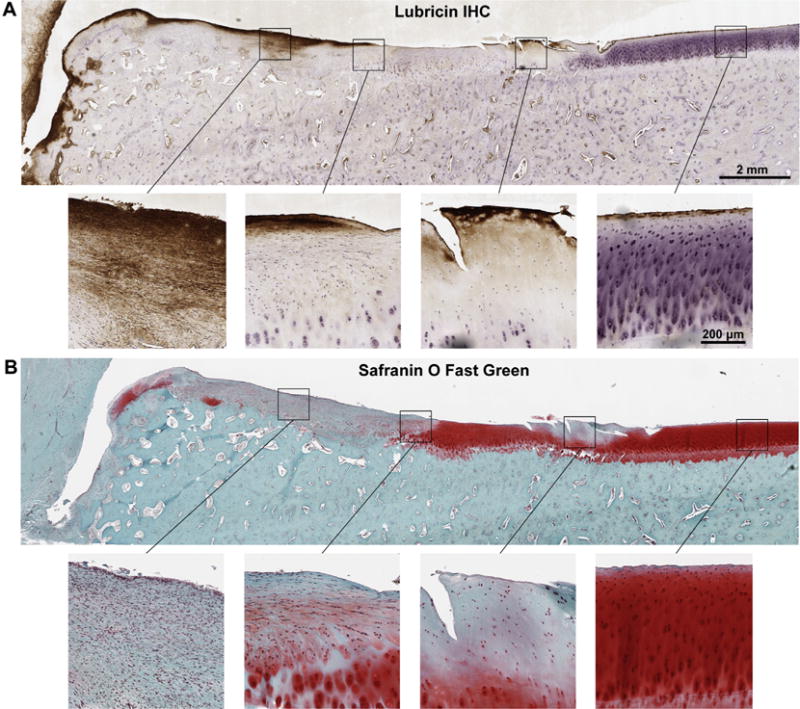
Sagittal osteochondral section obtained from the third carpal bone of the equine carpal osteochondral fragment model 70 days after fragment induction, stained with monoclonal antibody 6a8 for lubricin (A) and with Safranin O Fast Green for sulfated proteoglycans (B). (A) Lubricin immunoreaction is significantly increased within the fibrocartilaginous repair tissue articulating with the radial carpal bone (RCB) fragment (A, left two images). Lubricin localization is primarily confined to areas devoid of Safranin O staining (A, B second image from the left). Lubricin intensely stains a region of fissured and fibrillated cartilage (A, second image from right) demonstrating significant cell loss and loss of proteoglycan (B, second image from right) as compared to staining of the superficial 2–3 cell layers in adjacent healthy cartilage distant from the RCB fragment (A, B rightmost images).
Fig. 5.
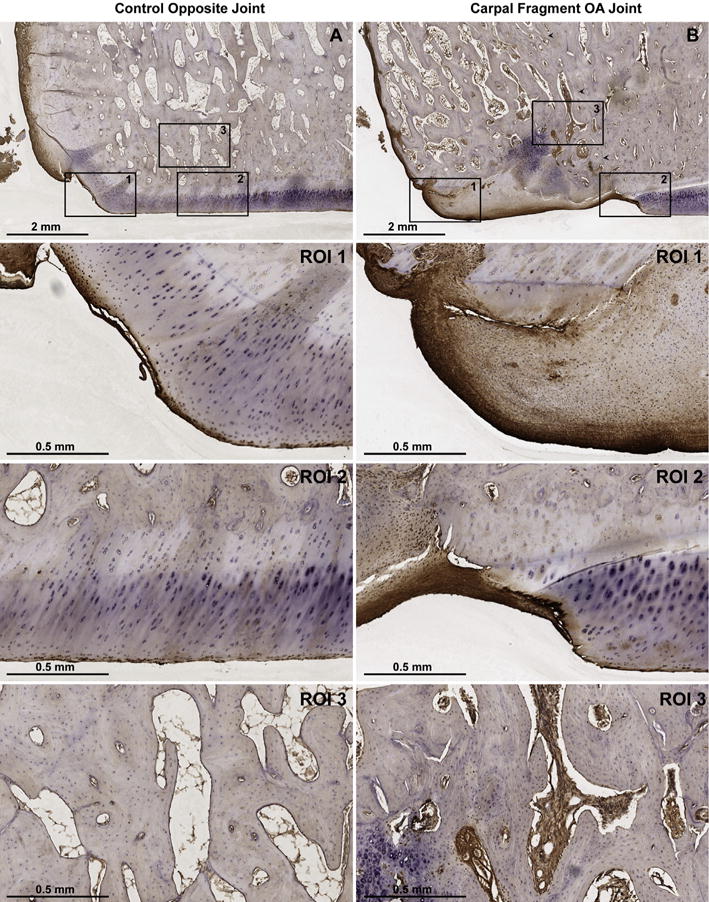
Lubricin immunostaining of the equine carpal osteochondral fragment-exercise model 70 days after fragment induction using monoclonal antibody 6a8. (A) Control and (B) fragmented articular surfaces of the distal radial carpal bone (RCB) at 1 × magnification. Arrowheads in B delineate the site of reattachment of the osteochondral fragment with the parent bone. Regions of interest (ROI) 1, 2, and 3 represent 5 × magnifications of the peripheral articular margin, the weight-bearing articular surface, and the bone within the fracture fragment (B), respectively. Lubricin immunoreaction is localized to both the surface and deeper layers of fibrocartilage (B, 1–2) as compared to the superficial layers of control hyaline cartilage (A, 1–2). Lubricin staining is also increased within the vascular channels of the RCB chip fracture fragment (B, 3) as compared to the healthy RCB (A, 3).
Discussion
In the present study, we evaluated the time course of synovial fluid lubricin concentrations before and at weekly intervals after osteochondral fragment induction of OA in an equine treadmill exercise model. Contrary to prior studies in anterior cruciate ligament deficient small animal models4,6, our results indicate that synovial fluid lubricin concentrations increase in response to osteochondral fragmentation in horses [Fig. 2(B)]. These findings in the experimental model were corroborated by increased synovial fluid lubricin concentrations in horses with naturally occurring carpal OA [Fig. 1(C)], in which abundant expression of TNFα in synovial membrane and cartilage and IL-1β in cartilage have been previously reported24. Interestingly, synovial fluid lubricin concentrations were moderately correlated with synovial fluid TNFα concentrations [Fig. 1(F)]. Furthermore, lubricin immunostaining was most pronounced in the synovial tissues of OA as compared to control limbs of experimental horses (Fig. 3), with the most intense lubricin localization observed at sites of cartilage fibrillation (Fig. 4) and at sites of osteochondral re-integration and repair (Fig. 5). Taken together, these results suggest that lubricin may be upregulated as part of the endogenous repair response in post-traumatic carpal OA in horses.
Mild traumatic changes were observed in the sham-operated joint in some animals. One explanation for these findings is that horses undergo compensatory overloading of the sham-operated limb during exercise due to lameness associated with the osteochondral fracture. Although there may be significant advantages to pairing limbs within the same horse in terms of reducing the number of experimental animals and reducing statistical variability, sham-operated joints may not be truly representative of healthy joints due to supraphysiologic loading associated with the rigorous high-speed treadmill exercise29. Recent investigations have revealed that controlled, low intensity treadmill exercise correlates with increased lubricin cartilage immunolocalization in exercised rats30. However, after anterior cruciate ligament transection, forced joint exercise decreases cartilage Prg4 expression31, suggesting that exercise may promote lubricin production in healthy joints but not in the presence of joint instability.
Other authors have hypothesized that lubricin may be upregulated in OA as a repair mechanism, but that the endogenous response may still be insufficient to prevent disease progression8. Our data revealing correlations between synovial fluid lubricin and TNFα concentrations supports a possible link between joint inflammation and lubricin production. To our knowledge, this is the first study to evaluate serial synovial fluid lubricin concentrations before and after osteochondral injury, revealing that synovial fluid lubricin concentrations increase shortly after injury, followed by a gradual return to baseline [Fig. 2(B)]. An approximately two-fold increase in lubricin synovial fluid concentrations have been detected following tibial fractures in humans21.
As has been previously reported in sheep models of OA17, our studies demonstrated decreased PRG4 gene expression in naturally occurring equine OA cartilage. Interestingly, we observed significant elevations in PRG4 gene expression from synovial tissues of horses with naturally occurring OA as compared to controls [Fig. 1(A)], and synovial PRG4 expression was moderately correlated with synovial ADAMTS5 and TNFα expression [Fig. 1(D)e(E)]. Although no significant differences were observed in PRG4 gene expression from synovial tissues in the experimental model [Fig. 2(A)], it is likely that 70 days after injury is too late to detect any change in gene expression. The equine osteochondral fragment model of OA induces synovitis and progressive OA changes; however, it is a self-limiting model of OA. Based upon peak synovial fluid lubricin concentrations at 21 days post-injury, we would predict synovial membrane PRG4 gene expression to peak much earlier than day 70.
Limitations of the naturally occurring OA cohort are the qualitative nature of the OA grading criteria and limited information on duration of injury. In order to address this constraint and because there were no statistically significant differences in the lubricin ELISA data between the three severity grades, all OA grades (1, 2, and 3) were combined together and compared to normal joints. OA grading criteria and duration of injury were not limitations in the experimental OA study, and lubricin ELISA results revealed significant increases in lubricin concentrations in arthritic joints as compared to healthy joints. Monoclonal antibody 9G3 detects a combined peptide and O-linked glycosylated epitope within lubricin’s KEPAPTTTT mucin-rich domain which is relatively insensitive to the presence of sialic acids but sensitive to the presence of O-linked glycans28. The PNA lectin used in the ELISA analysis also binds to lubricin’s mucin-rich domain, specifically to non-sialylated O-linked glycans. Recent glycosylation analysis of equine synovial fluid lubricin suggests that equine joints with OA or osteochondral fragmentation have elevated levels of non-sialylated O-linked glycans as compared to normal joints32. Although it is possible that differences in sialylation may contribute in part to differences detected using the mAb 9G3 PNA ELISA, these 2% differences are modest compared to the 3-fold increases observed in synovial fluid lubricin concentrations in our study. Another limitation of this study is the lack of information about the molecular degradation of lubricin within the joint. Although it is clear that an O-linked glycosylated epitope of lubricin is increased in both experimental and naturally occurring OA in horses, it is not obvious whether this comparatively more populous epitope is still attached to functional N- and C-terminal domains. We cannot rule out the possibility that the elevated lubricin detected in synovial fluid has been sheared off of the articular cartilage and does not reflect increased lubricin synthesis in response to injury. Nonetheless, the ELISA data suggesting that lubricin is increased in arthritis, coupled with the increase in PRG4 synovial membrane gene expression and the increased lubricin immunostaining in OA joints provides supporting evidence that lubricin expression may be upregulated in post-traumatic arthritis.
Here, we demonstrate pronounced lubricin immunostaining in fibrocartilaginous repair tissue and in the healing osteochondral fragments, suggesting that lubricin may be an important mediator in intra-articular fracture repair and healing of damaged hyaline cartilage. Lubricin has been localized to multiple fibrocartilaginous structures, including meniscus33,34, intervertebral disc35,36 and the fibrocartilaginous disc of the temporomandibular joint37–39 and a variety of other tissues. Lubricin’s biological effects extend beyond its role as a mechanical lubricant, with known roles in chondroprotection through inhibition of chondrocyte apoptosis40, inhibition of protein deposition and pannus formation41 and normalization of subchondral bone remodeling9. Furthermore, Prg4 expression has been shown to regulate transcriptional networks involved in chondrocyte hypertrophy and catabolism8. These findings suggest that lubricin, in addition to enhancing lubrication, may possess anti-inflammatory functions distinct from its lubricating properties in joint trauma. Recently, lubricin has been shown to inhibit synovial inflammation via binding to both the CD44 receptor42 and Toll-like receptors 2 and 443,44.
Lubricin is an important boundary lubricant with diverse biological roles within the synovial environment. Lubricin supplementation has been proposed as a novel OA therapy in humans, with mounting evidence for its clinical application. Prior to translating lubricin supplementation therapy into humans, further investigation is warranted in clinically relevant large animal models such as the horse and in distinct types of joint injury, including both instability and intra-articular fracture models. The majority of evidence indicating that lubricin is depleted in joint injury has been derived from rodent models of knee instability4–6 or in clinical cases of cruciate injury and late-stage knee OA in humans5,7, whereas data in human patients with intra-articular tibial plateau fracture21 and in our equine osteochondral fragmentation model, lubricin is increased. Further mechanistic work is required to understand what role lubricin has following intra-articular fracture.
Acknowledgments
The authors thank Ryan Peterson for his assistance with scanning immunohistochemistry images, Sabine Mann for manuscript review and Francoise Vermeylen of the Cornell Statistical Consulting Unit for assistance with statistical analysis and data modeling.
Role of the funding source
Supported by the Cornell University Harry M. Zweig Memorial Fund for Equine Research, NIH grant number T32ODO11000 from the National Center for Research Resources, and the Grayson-Jockey Club Storm Cat Career Development Award. The study sponsors had no involvement in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Footnotes
Author contributions
HLR contributed to the conception and design of the project; acquired, analyzed and interpreted data; drafted the article; and approved the final submitted version of the article. AEW contributed to the design of the project and collection of data; critically revised of the article for important intellectual content; and approved the final submitted version of the article. GDJ contributed to the analysis and interpretation of data; critically revised the article for important intellectual content; and approved the final submitted version of the article. HOM provided statistical expertise for the analysis and interpretation of data; critically revised the article for important intellectual content; and approved the final submitted version of the article. AJN contributed to the conception and design of the project; obtained funding for the project; contributed to the analysis and interpretation of data; critically revised the article for important intellectual content; and approved the final submitted version of the article. AJN (ajn1@cornell.edu) and HLR (hlr42@cornell.edu) take full responsibility for the integrity of the work as a whole, from inception to finished article.
Conflict of interest
HLR, AEW, HOM and AJN have no competing interests to declare. GDJ has authored and was granted a United States patent “Tribonectin polypeptides and uses thereof,” no. 6743774 for the therapeutic use of lubricin in joints. Dr. Jay receives support from a Phase II STTR for clinical translation efforts related to his patent. The present paper neither materially nor financially affects Dr. Jay’s patent relating to rhPRG4. Case Western Reserve University has licensed mAb-9g3 (for GDJ) to EMD Millipore (Billerica, MA).
Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.joca.2016.07.021.
References
- 1.Bahabri S, Suwairi W, Laxer R, Polinkovsky A, Dalaan A, Warman M. The camptodactyly-arthropathy-coxa vara-pericarditis syndrome: clinical features and genetic mapping to human chromosome 1. Arthritis Rheum. 1998;41(4):730–5. doi: 10.1002/1529-0131(199804)41:4<730::AID-ART22>3.0.CO;2-Y. http://dx.doi.org/10.1002/1529-0131(199804)41:4<730::AIDART22>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 2.Marcelino J, Carpten JD, Suwairi WM, Gutierrez OM, Schwartz S, Robbins C, et al. CACP, encoding a secreted proteoglycan, is mutated in camptodactyly-arthropathy-coxa vara-pericarditis syndrome. Nat Genet. 1999;23(3):319–22. doi: 10.1038/15496. http://dx.doi.org/10.1038/15496. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig TE, McAllister JR, Lun V, Wiley JP, Schmidt TA. Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthritis Rheum. 2012;64(12):3963–71. doi: 10.1002/art.34674. http://dx.doi.org/10.1002/art.34674. [DOI] [PubMed] [Google Scholar]
- 4.Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009;60(10):2997–3006. doi: 10.1002/art.24800. http://dx.doi.org/10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elsaid K, Fleming B, Oksendahl H, Machan J, Fadale P, Hulstyn M, et al. Decreased lubricin concentrations and markers of joint inflammation in synovial fluids from patients with anterior cruciate ligament injury. Arthritis Rheum. 2008;58(6):1707–15. doi: 10.1002/art.23495. http://dx.doi.org/10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teeple E, Elsaid K, Fleming BC, Jay GD, Aslani K, Crisco JJ, et al. Coefficients of friction, lubricin, and cartilage damage in the anterior cruciate ligament-deficient guinea pig knee. J Orthop Res. 2008;26(2):231–7. doi: 10.1002/jor.20492. http://dx.doi.org/10.1002/jor.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kosinska MK, Ludwig TE, Liebisch G, Zhang R, Siebert H-C, Wilhelm J, et al. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS One. 2015;10(5):e0125192. doi: 10.1371/journal.pone.0125192. http://dx.doi.org/10.1371/journal.pone.0125192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruan MZC, Erez A, Guse K, Dawson B, Bertin T, Chen Y, et al. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5(176):176ra34. doi: 10.1126/scitranslmed.3005409. http://dx.doi.org/10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Z, Xu C, Li X, Song J, Yu B. Treatment with recombinant lubricin attenuates osteoarthritis by positive feedback loop between articular cartilage and subchondral bone in ovariectomized rats. Bone. 2015;74:37–47. doi: 10.1016/j.bone.2014.12.065. http://dx.doi.org/10.1016/j.bone.2014.12.065. [DOI] [PubMed] [Google Scholar]
- 10.Teeple E, Elsaid KA, Jay GD, Zhang L, Badger GJ, Akelman M, et al. The effects of supplemental intra-articular lubricin and hyaluronic acid on the progression of post-traumatic arthritis in the anterior cruciate ligament deficient rat knee. Am J Sports Med. 2011;39(1):164–72. doi: 10.1177/0363546510378088. http://dx.doi.org/10.1177/0363546510378088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jay GD, Fleming BC, Watkins BA, McHugh KA, Anderson SC, Zhang LX, et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following ACL transection. Arthritis Rheum. 2010;62(8):2382–91. doi: 10.1002/art.27550. http://dx.doi.org/10.1002/art.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flannery CR, Zollner R, Corcoran C, Jones AR, Root A, Rivera-Bermúdez MA, et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 2009;60(3):840–7. doi: 10.1002/art.24304. http://dx.doi.org/10.1002/art.24304. [DOI] [PubMed] [Google Scholar]
- 13.Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19(3):142–6. [PubMed] [Google Scholar]
- 14.McIlwraith CW, Fortier LA, Frisbie DD, Nixon AJ. Equine models of articular cartilage repair. Cartilage. 2011;2(4):317–26. doi: 10.1177/1947603511406531. http://dx.doi.org/10.1177/1947603511406531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16(1):105–15. doi: 10.1089/ten.teb.2009.0452. http://dx.doi.org/10.1089/ten.TEB.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McIlwraith CW, Frisbie DD, Kawcak CE. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. 2012;1(11):297–309. doi: 10.1302/2046-3758.111.2000132. http://dx.doi.org/10.1302/2046-3758.111.2000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young AA, McLennan S, Smith MM, Smith SM, Cake MA, Read RA, et al. Proteoglycan 4 downregulation in a sheep meniscectomy model of early osteoarthritis. Arthritis Res Ther. 2006;8(2):R41. doi: 10.1186/ar1898. http://dx.doi.org/10.1186/ar1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barton KI, Ludwig TE, Achari Y, Shrive NG, Frank CB, Schmidt TA. Characterization of proteoglycan 4 and hyaluronan composition and lubrication function of ovine synovial fluid following knee surgery. J Orthop Res. 2013;31(10):1549–54. doi: 10.1002/jor.22399. http://dx.doi.org/10.1002/jor.22399. [DOI] [PubMed] [Google Scholar]
- 19.Desrochers J, Amrein MW, Matyas JR. Microscale surface friction of articular cartilage in early osteoarthritis. J Mech Behav Biomed Mater. 2013;25:11–22. doi: 10.1016/j.jmbbm.2013.03.019. http://dx.doi.org/10.1016/j.jmbbm.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 20.Antonacci JM, Schmidt TA, Serventi LA, Cai MZ, Shu YL, Schumacher BL, et al. Effects of equine joint injury on boundary lubrication of articular cartilage by synovial fluid: role of hyaluronan. Arthritis Rheum. 2012;64(9):2917–26. doi: 10.1002/art.34520. http://dx.doi.org/10.1002/art.34520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballard BL, Antonacci JM, Temple-Wong MM, Hui AY, Schumacher BL, Bugbee WD, et al. Effect of tibial plateau fracture on lubrication function and composition of synovial fluid. J Bone Joint Surg. 2012;94(10):e64. doi: 10.2106/JBJS.K.00046. http://dx.doi.org/10.2106/JBJS.K.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neu CP, Reddi AH, Komvopoulos K, Schmid TM, Di Cesare PE. Increased friction coefficient and superficial zone protein expression in patients with advanced arthritis. Arthritis Rheum. 2010;62(9):2680–7. doi: 10.1002/art.27577. http://dx.doi.org/10.1002/art.27577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atarod M, Ludwig TE, Frank CB, Schmidt TA, Shrive NG. Cartilage boundary lubrication of ovine synovial fluid following anterior cruciate ligament transection: a longitudinal study. Osteoarthritis Cartilage. 2015;23(4):640–7. doi: 10.1016/j.joca.2014.12.017. http://dx.doi.org/10.1016/j.joca.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Kamm JL, Nixon AJ, Witte TH. Cytokine and catabolic enzyme expression in synovium, synovial fluid and articular cartilage of naturally osteoarthritic equine carpi. Equine Vet J. 2010;42(8):693–9. doi: 10.1111/j.2042-3306.2010.00140.x. http://dx.doi.org/10.1111/j.2042-3306.2010.00140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frisbie DD, Ghivizzani SC, Robbins PD, Evans CH, McIlwraith CW. Treatment of experimental equine osteoarthritis by in vivo delivery of the equine interleukin-1 receptor antagonist gene. Gene Ther. 2002;9(1):12–20. doi: 10.1038/sj.gt.3301608. http://dx.doi.org/10.1038/sj.gt.3301608. [DOI] [PubMed] [Google Scholar]
- 26.Reesink HL, Bonnevie ED, Liu S, Shurer CR, Hollander MJ, Bonassar LJ, et al. Galectin-3 binds to lubricin and reinforces the lubricating boundary layer of articular cartilage. Sci Rep. 2016 May;6:25463. doi: 10.1038/srep25463. http://dx.doi.org/10.1038/srep25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nixon AJ, Begum L, Mohammed HO, Huibregtse B, O’Callaghan MM, Matthews GL. Autologous chondrocyte implantation drives early chondrogenesis and organized repair in extensive full- and partial-thickness cartilage defects in an equine model. J Orthop Res. 2011;29(7):1121–30. doi: 10.1002/jor.21366. http://dx.doi.org/10.1002/jor.21366. [DOI] [PubMed] [Google Scholar]
- 28.Ai M, Cui Y, Sy M-S, Lee DM, Zhang LX, Larson KM, et al. Antilubricin monoclonal antibodies created using lubricinknockout mice immunodetect lubricin in several species and in patients with healthy and diseased joints. PLoS One. 2015;10(2):e0116237. doi: 10.1371/journal.pone.0116237. http://dx.doi.org/10.1371/journal.pone.0116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teeple E, Jay GD, Elsaid KA, Fleming BC. Animal models of osteoarthritis: challenges of model selection and analysis. AAPS J. 2013;15(2):438–46. doi: 10.1208/s12248-013-9454-x. http://dx.doi.org/10.1208/s12248-013-9454-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Musumeci G, Castrogiovanni P, Trovato FM, Imbesi R, Giunta S, Szychlinska MA, et al. Physical activity ameliorates cartilage degeneration in a rat model of aging: a study on lubricin expression. Scand J Med Sci Sport. 2014:222–30. doi: 10.1111/sms.12290. http://dx.doi.org/10.1111/sms.12290. [DOI] [PubMed]
- 31.Elsaid KA, Zhang L, Waller K, Tofte J, Teeple E, Fleming BC, et al. The impact of forced joint exercise on lubricin biosynthesis from articular cartilage following ACL transection and intra-articular lubricin’s effect in exercised joints following ACL transection. Osteoarthritis Cartilage. 2012;20(8):940–8. doi: 10.1016/j.joca.2012.04.021. http://dx.doi.org/10.1016/j.joca.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 32.Svala E, Jin C, Ruetschi U, Ekman S, Lindahl A, Karlsson NG, et al. Characterisation of lubricin in synovial fluid from horses with osteoarthritis. Equine Vet J. 2015 doi: 10.1111/evj.12521. http://dx.doi.org/10.1111/evj.12521. [DOI] [PubMed]
- 33.Musumeci G, Trovato FM, Loreto C, Leonardi R, Szychlinska MA, Castorina S, et al. Lubricin expression in human osteoarthritic knee meniscus and synovial fluid: a morphological, immunohistochemical and biochemical study. Acta Histochem. 2014;116(5):965–72. doi: 10.1016/j.acthis.2014.03.011. http://dx.doi.org/10.1016/j.acthis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Cheriyan T, Martin SD, Gomoll AH, Schmid TM, Spector M. Lubricin distribution in the torn human anterior cruciate ligament and meniscus. J Orthop Res. 2011;29(12):1916–22. doi: 10.1002/jor.21473. http://dx.doi.org/10.1002/jor.21473. [DOI] [PubMed] [Google Scholar]
- 35.Shine KM, Spector M. The presence and distribution of lubricin in the caprine intervertebral disc. J Orthop Res. 2008;26(10):1398–406. doi: 10.1002/jor.20614. http://dx.doi.org/10.1002/jor.20614. [DOI] [PubMed] [Google Scholar]
- 36.Shine KM, Simson JA, Spector M. Lubricin distribution in the human intervertebral disc. J Bone Joint Surg Am. 2009;91(9):2205–12. doi: 10.2106/JBJS.H.01344. http://dx.doi.org/10.2106/JBJS.H.01344. [DOI] [PubMed] [Google Scholar]
- 37.Leonardi R, Almeida LE, Loreto C. Lubricin immunohistochemical expression in human temporomandibular joint disc with internal derangement. J Oral Pathol Med. 2011;40(7):587–92. doi: 10.1111/j.1600-0714.2011.01012.x. http://dx.doi.org/10.1111/j.1600-0714.2011.01012.x. [DOI] [PubMed] [Google Scholar]
- 38.Leonardi R, Rusu MC, Loreto F, Loreto C, Musumeci G. Immunolocalization and expression of lubricin in the bilaminar zone of the human temporomandibular joint disc. Acta Histochem. 2012;114(1):1–5. doi: 10.1016/j.acthis.2010.11.011. http://dx.doi.org/10.1016/j.acthis.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 39.Wei L, Xiong H, Li B, Cheng Y, Long X. Boundary-lubricating ability and lubricin in synovial fluid of patients with temporomandibular joint disorders. J Oral Maxillofac Surg. 2010;68(10):2478–83. doi: 10.1016/j.joms.2010.01.018. http://dx.doi.org/10.1016/j.joms.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 40.Waller KA, Zhang LX, Elsaid KA, Fleming BC, Warman ML, Jay GD. Role of lubricin and boundary lubrication in the prevention of chondrocyte apoptosis. Proc Natl Acad Sci USA. 2013;110(15):5852–7. doi: 10.1073/pnas.1219289110. http://dx.doi.org/10.1073/pnas.1219289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhee DK, Marcelino J, Baker M, Gong Y, Smits P, Lefebvre V, et al. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest. 2005;115(3):622–31. doi: 10.1172/JCI200522263. http://dx.doi.org/10.1172/JCI22263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Sharif A, Jamal M, Zhang LX, Larson K, Schmidt TA, Jay GD, et al. Lubricin/proteoglycan 4 binding to CD44 receptor: a mechanism of the suppression of proinflammatory cytokineinduced synoviocyte proliferation by lubricin. Arthritis Rheumatol. 2015;67(6):1503–13. doi: 10.1002/art.39087. http://dx.doi.org/10.1002/art.39087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iqbal SM, Leonard C, Regmi SC, De Rantere D, Tailor P, Ren G, et al. Lubricin/proteoglycan 4 binds to and regulates the activity of toll-like receptors in vitro. Sci Rep. 2016;6:18910. doi: 10.1038/srep18910. http://dx.doi.org/10.1038/srep18910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alquraini A, Garguilo S, D’Souza G, Zhang LX, Schmidt TA, Jay GD, et al. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther. 2015;17:353. doi: 10.1186/s13075-015-0877-x. http://dx.doi.org/10.1186/s13075-015-0877-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


