Abstract
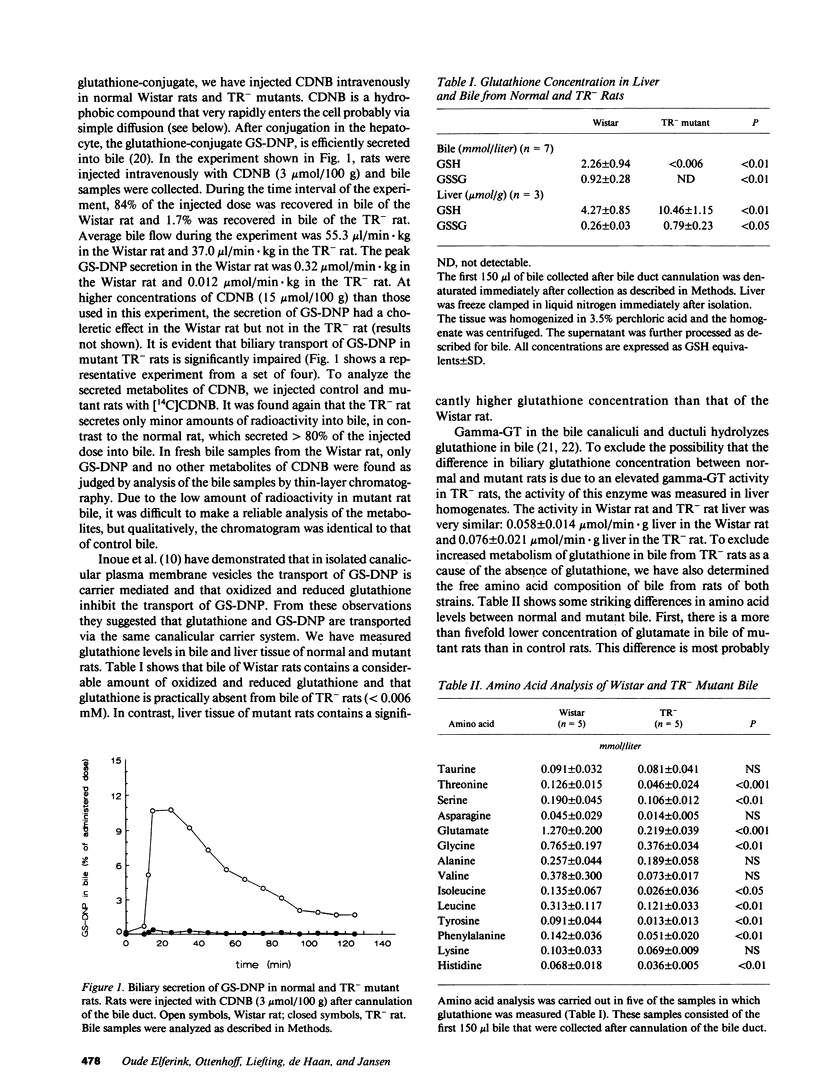
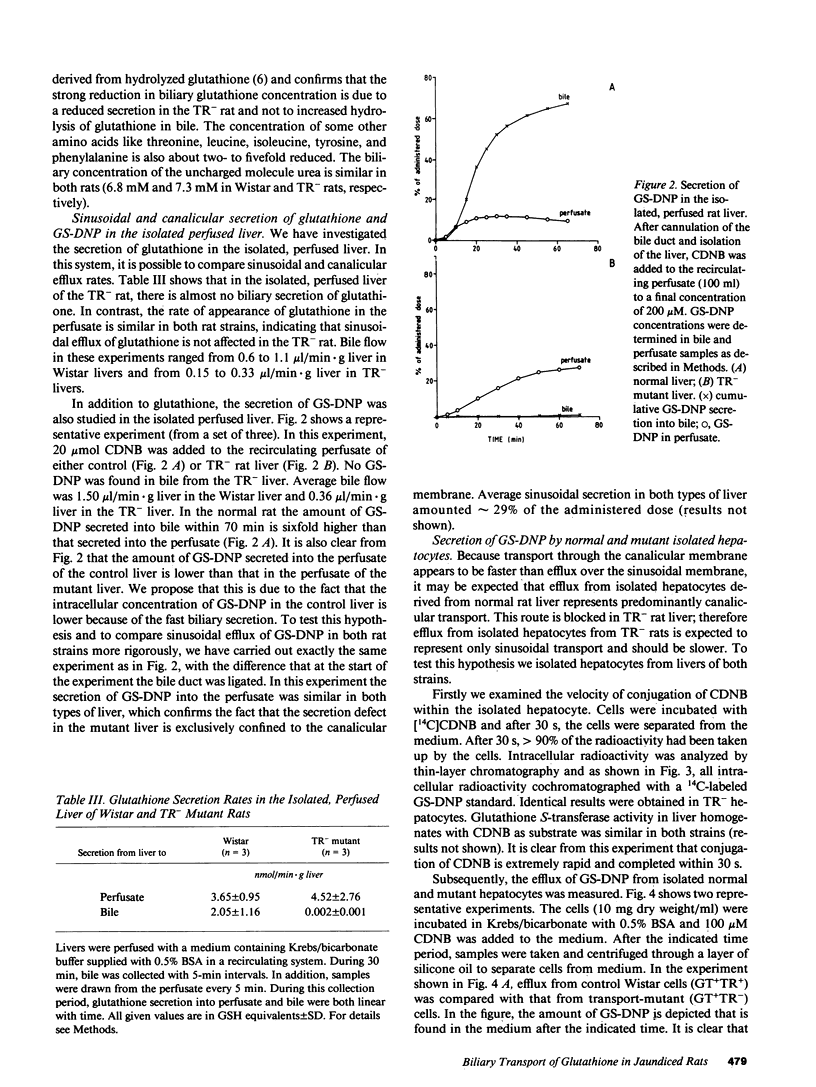
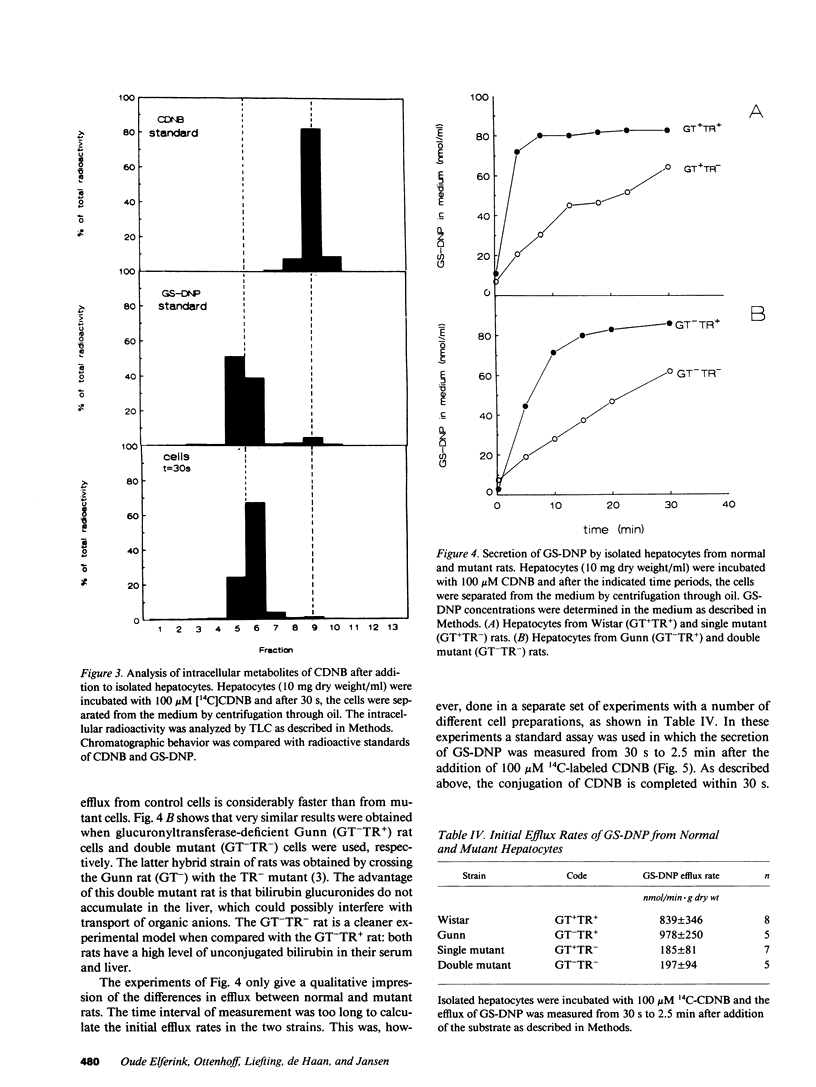
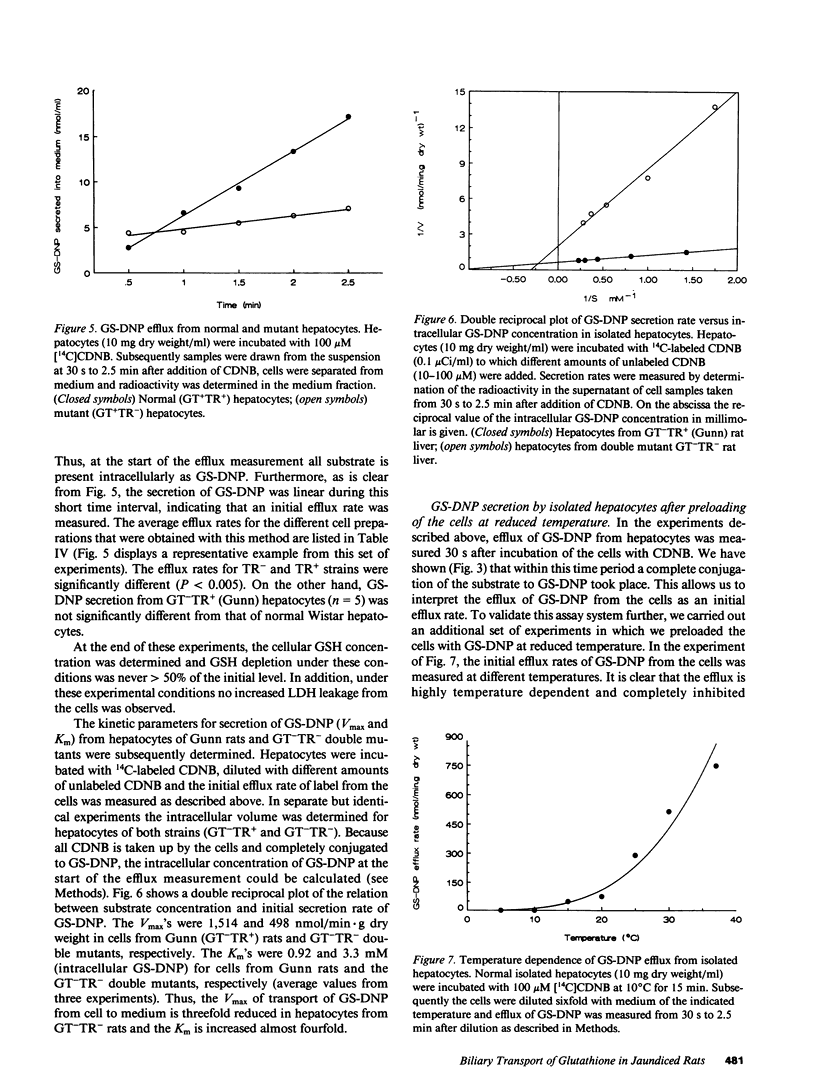
TR- mutant rats have an autosomal recessive mutation that is expressed as a severely impaired hepatobiliary secretion of organic anions like bilirubin-(di)glucuronide and dibromosulphthalein (DBSP). In this paper, the hepatobiliary transport of glutathione and a glutathione conjugate was studied in normal Wistar rats and TR- rats. It was shown that glutathione is virtually absent from the bile of TR- rats. In the isolated, perfused liver the secretion of glutathione and the glutathione conjugate, dinitrophenyl-glutathione (GS-DNP), from hepatocyte to bile is severely impaired, whereas the sinusoidal secretion from liver to blood is not affected. The secretion of GS-DNP was also studied in isolated hepatocytes. The secretion of GS-DNP from cells isolated from TR- rat liver was significantly slower than from normal hepatocytes. Efflux of GS-DNP was a saturable process with respect to intracellular GS-DNP concentration: Vmax and Km for efflux from TR- cells was 498 nmol/min.g dry wt and 3.3 mM, respectively, as compared with 1514 nmol/min.g dry wt and 0.92 mM in normal hepatocytes. These results suggest that the canalicular transport system for glutathione and glutathione conjugates is severely impaired in TR- rats, whereas sinusoidal efflux is unaffected. Because the defect also comes to expression in isolated hepatocytes, efflux of GS-DNP from normal hepatocytes must predominantly be mediated by the canalicular transport mechanism, which is deficient in TR- rats.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott W. A., Meister A. Intrahepatic transport and utilization of biliary glutathione and its metabolites. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1246–1250. doi: 10.1073/pnas.83.5.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerboom T. P., Bilzer M., Sies H. Competition between transport of glutathione disulfide (GSSG) and glutathione S-conjugates from perfused rat liver into bile. FEBS Lett. 1982 Apr 5;140(1):73–76. doi: 10.1016/0014-5793(82)80523-1. [DOI] [PubMed] [Google Scholar]
- Awasthi Y. C., Garg H. S., Dao D. D., Partridge C. A., Srivastava S. K. Enzymatic conjugation of erythrocyte glutathione with 1-chloro-2,4-dinitrobenzene: the fate of glutathione conjugate in erythrocytes and the effect of glutathione depletion on hemoglobin. Blood. 1981 Oct;58(4):733–738. [PubMed] [Google Scholar]
- Ballatori N., Clarkson T. W. Sulfobromophthalein inhibition of glutathione and methylmercury secretion into bile. Am J Physiol. 1985 Feb;248(2 Pt 1):G238–G245. doi: 10.1152/ajpgi.1985.248.2.G238. [DOI] [PubMed] [Google Scholar]
- Ballatori N., Jacob R., Boyer J. L. Intrabiliary glutathione hydrolysis. A source of glutamate in bile. J Biol Chem. 1986 Jun 15;261(17):7860–7865. [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J. L., Smith G. D., Peters T. J. Purification and properties of gamma-glutamyl transferase from normal rat liver. Biochim Biophys Acta. 1981 Feb 13;657(2):334–343. doi: 10.1016/0005-2744(81)90319-3. [DOI] [PubMed] [Google Scholar]
- Durand-Schneider A. M., Maurice M., Dumont M., Feldmann G. Effect of colchicine and phalloidin on the distribution of three plasma membrane antigens in rat hepatocytes: comparison with bile duct ligation. Hepatology. 1987 Nov-Dec;7(6):1239–1248. doi: 10.1002/hep.1840070611. [DOI] [PubMed] [Google Scholar]
- Eberle D., Clarke R., Kaplowitz N. Rapid oxidation in vitro of endogenous and exogenous glutathione in bile of rats. J Biol Chem. 1981 Mar 10;256(5):2115–2117. [PubMed] [Google Scholar]
- Gregus Z., Stein A. F., Klaassen C. D. Age-dependent biliary excretion of glutathione-related thiols in rats: role of gamma-glutamyltransferase. Am J Physiol. 1987 Jul;253(1 Pt 1):G86–G92. doi: 10.1152/ajpgi.1987.253.1.G86. [DOI] [PubMed] [Google Scholar]
- Gregus Z., Stein A. F., Klaassen C. D. Effect of inhibition of gamma-glutamyltranspeptidase on biliary and urinary excretion of glutathione-derived thiols and methylmercury. J Pharmacol Exp Ther. 1987 Jul;242(1):27–32. [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Huber M., Guhlmann A., Jansen P. L., Keppler D. Hereditary defect of hepatobiliary cysteinyl leukotriene elimination in mutant rats with defective hepatic anion excretion. Hepatology. 1987 Mar-Apr;7(2):224–228. doi: 10.1002/hep.1840070204. [DOI] [PubMed] [Google Scholar]
- Inoue M., Akerboom T. P., Sies H., Kinne R., Thao T., Arias I. M. Biliary transport of glutathione S-conjugate by rat liver canalicular membrane vesicles. J Biol Chem. 1984 Apr 25;259(8):4998–5002. [PubMed] [Google Scholar]
- Inoue M., Kinne R., Tran T., Biempica L., Arias I. M. Rat liver canalicular membrane vesicles. Isolation and topological characterization. J Biol Chem. 1983 Apr 25;258(8):5183–5188. [PubMed] [Google Scholar]
- Jansen P. L., Groothuis G. M., Peters W. H., Meijer D. F. Selective hepatobiliary transport defect for organic anions and neutral steroids in mutant rats with hereditary-conjugated hyperbilirubinemia. Hepatology. 1987 Jan-Feb;7(1):71–76. doi: 10.1002/hep.1840070116. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Oude Elferink R. P. Hereditary hyperbilirubinemias: a molecular and mechanistic approach. Semin Liver Dis. 1988 May;8(2):168–178. doi: 10.1055/s-2008-1040537. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Peters W. H., Lamers W. H. Hereditary chronic conjugated hyperbilirubinemia in mutant rats caused by defective hepatic anion transport. Hepatology. 1985 Jul-Aug;5(4):573–579. doi: 10.1002/hep.1840050408. [DOI] [PubMed] [Google Scholar]
- Jansen P. L., Peters W. H., Meijer D. K. Hepatobiliary excretion of organic anions in double-mutant rats with a combination of defective canalicular transport and uridine 5'-diphosphate-glucuronyltransferase deficiency. Gastroenterology. 1987 Nov;93(5):1094–1103. doi: 10.1016/0016-5085(87)90574-9. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Adams J. D., Mitchell J. R. Hepatic glutathione homeostasis in the rat: efflux accounts for glutathione turnover. Hepatology. 1984 Jul-Aug;4(4):586–590. doi: 10.1002/hep.1840040402. [DOI] [PubMed] [Google Scholar]
- Lauterburg B. H., Smith C. V., Hughes H., Mitchell J. R. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. J Clin Invest. 1984 Jan;73(1):124–133. doi: 10.1172/JCI111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwall G., Boyer T. D. Excretion of glutathione conjugates by primary cultured rat hepatocytes. J Biol Chem. 1987 Apr 15;262(11):5151–5158. [PubMed] [Google Scholar]
- Meijer D. K., Keulemans K., Mulder G. J. Isolated perfused rat liver technique. Methods Enzymol. 1981;77:81–94. doi: 10.1016/s0076-6879(81)77013-7. [DOI] [PubMed] [Google Scholar]
- Ookhtens M., Hobdy K., Corvasce M. C., Aw T. Y., Kaplowitz N. Sinusoidal efflux of glutathione in the perfused rat liver. Evidence for a carrier-mediated process. J Clin Invest. 1985 Jan;75(1):258–265. doi: 10.1172/JCI111682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetta P., Di Cola D., Federici G. Alkaline hydrolysis of N-ethylmaleimide allows a rapid assay of glutathione disulfide in biological samples. Anal Biochem. 1986 Apr;154(1):205–208. doi: 10.1016/0003-2697(86)90516-6. [DOI] [PubMed] [Google Scholar]
- Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969 Mar;27(3):502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- Wahlländer A., Sies H. Glutathione S-conjugate formation from 1-chloro-2,4-dinitrobenzene and biliary S-conjugate excretion in the perfused rat liver. Eur J Biochem. 1979 Jun 1;96(3):441–446. doi: 10.1111/j.1432-1033.1979.tb13056.x. [DOI] [PubMed] [Google Scholar]



