INTRODUCTION
Varicella-zoster virus (VZV) is an exclusively human neurotropic α-herpesvirus. Primary infection causes varicella (chickenpox), after which virus becomes latent in neurons of cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis. With advancing age or immunosuppression as in, for example, organ transplant recipients and patients with cancer or acquired immunodeficiency syndrome (AIDS), cell-mediated immunity to VZV declines and virus reactivates from latency to cause zoster (shingles). Zoster is characterized by dermatomal distribution pain and rash on an erythematous base in one to three dermatomes. Figure 12.1 depicts primary infection (varicella) and reactivation (zoster) after latent infection in ganglia. Because VZV becomes latent in any and all ganglia, zoster can develop anywhere on the body. Skin lesions resolve within 1–2 weeks while complete cessation of pain usually takes 4–6 weeks. Unfortunately, zoster can be followed by chronic pain (postherpetic neuralgia: PHN), meningitis or meningoencephalitis, cerebellitis, isolated or multiple cranial nerve palsies (polyneuritis cranialis), vasculopathy, and myelopathy as well as various inflammatory disorders of the eye, the most common of which is progressive outer retinal necrosis (PORN) (Fig. 12.2). VZV reactivation can also produce chronic radicular pain without rash (zoster sine herpete). In fact, all the neurologic and ocular disorders listed above may also develop in the absence of rash. This review covers the clinical, laboratory, and pathologic features of the neurologic complications of VZV reactivation, including diagnostic testing needed to verify active VZV infection in the nervous system. The potential value of examining saliva for VZV DNA in patients with neurologic disease in the absence of rash is also discussed. Additional perspectives of VZV pathogenesis are provided by discussion of studies of the VZV genome, VZV latency, VZV immunity, and the value of immunization of elderly individuals with zoster vaccine to boost cell-mediated immunity to VZV and prevent VZV reactivation.
Fig. 12.1.

Varicella (chickenpox), latency, and zoster (shingles). Primary varicella-zoster virus (VZV) infection usually produces varicella in children, after which VZV becomes latent in ganglia. Decades later, VZV reactivation manifests as zoster, corresponding here to the right sixth thoracic dermatome.
Fig. 12.2.
Neurologic complications of varicella-zoster virus reactivation.
VZV GENOME
VZV and its simian counterpart simian varicella virus are members of the α-herpesvirus family. α-Herpesviruses are large double-stranded DNA viruses with similar genomic structure, replication, and assembly characteristics, and are able to become latent in ganglionic neurons. VZV was the first α-herpesvirus to be sequenced (Davison and Scott, 1986). The α-herpesvirus genome sizes range from 124 784 to 164 270 basepairs (bp) (Cohrs et al., 2009), with VZV and simian varicella virus at 125 000 bp having the smallest genomes among this virus family. Restriction endonuclease analysis of VZV DNA from virus isolated from an immunocompromised patient with varicella and a later episode of herpes zoster showed that the same strain of VZV caused both diseases, indicating the stability of the virus genome in its natural setting (Straus et al., 1983). From primary infection to latency, the virus undergoes about 20 cycles of replication, providing little opportunity for DNA mutations to accumulate (Grose et al., 2000) and resulting in little geographic diversity (Hawrami et al., 1996; Muir et al., 2002). Analysis of virus after propagation more than 1200 times in tissue culture revealed minimal mutations in the VZV genome (Hart et al., 2009). To date, sequence analysis of 23 VZV isolates has revealed the remarkable stability of this virus genome (Breuer, 2010). Thus, nucleotide sequencing, not virus gene expression, has been used to classify virus isolates and has identified five distinct phylogenetic clades (Breuer et al., 2010). The worldwide stability of the virus genome suggests that vaccines targeting essential but infrequently mutated virus surface glycoproteins will effectively reduce VZV-associated disease, a notion supported by protection against varicella after vaccination of children (Takahashi et al., 1975) and the considerable reduction in zoster after immunization of elderly adults (Oxman et al., 2005).
The VZV genome contains two unique segments covalently linked and bracketed by inverted repeat regions. Computer analysis initially predicted 71 open reading frames (ORFs), and transcripts for all of these protein-coding regions have been detected during productive virus infection (Cohrs et al., 2003; Kennedy et al., 2005; Nagel et al., 2009). Three VZV ORFs (ORFs 62, 63, and 64) are also located within repeated regions (ORFs 71, 70, and 69) of the genome. In addition to the predicted ORFs, three additional ORFs (ORFS 33.5, 94, and S/L) have been detected experimentally (Preston et al., 1997; Ross et al., 1997; Kemble et al., 2000). Overall, the VZV genome encodes a total of 71 genes, the fewest number of genes among the α-herpesviruses.
Characterization of VZV genes and their protein products is an essential step towards understanding the molecular biology of the virus. It is assumed that VZV gene expression, like that of the prototype human α-herpesvirus herpes simplex virus-1 (HSV-1), is regulated temporally into three classes (Roizman and Knipe, 2001). Immediate-early virus genes are expressed first and reprogram the cell transcriptional apparatus to recognize the virus genome. Early virus genes are expressed next and encode proteins required to replicate the virus genome. Virus structural proteins corresponding to late VZV genes are the last to be synthesized. Unlike HSV, VZV is highly cell-associated so that it is customarily propagated in tissue culture by cocultivation of infected cells with uninfected cells. The result is an unsynchronized and inefficient infection in which the ratio of infective-to-defective VZV particles approaches 1:40 000 (Carpenter et al., 2009).
VZV LATENCY
Since VZV becomes latent during primary varicella, as does the attenuated Oka strain of VZV used to vaccinate against varicella, virtually all humans are latently infected with VZV. Latency is characterized by the presence of virus DNA but limited expression of the virus genes, with resultant lack of production of infectious virus. The hallmark of VZV latency is its ability to reactivate and, in most instances, to produce disease more serious than during primary infection.
VZV replicates in multiple human cell types, but becomes latent in neurons. Virus gains access to the neuron during primary infection either by hematogenous spread or by retrograde transaxonal transport of virus from varicella vesicles in skin to ganglia. Importantly, ganglionic infection is established before the development of varicella in a simian model of varicella pathogenesis (Mahalingam et al., 2001). Unlike HSV latency, which is restricted to cranial nerve ganglia (HSV-1) or sacral ganglia (HSV-2), VZV becomes latent in cranial nerve ganglia, dorsal root ganglia, and autonomic ganglia along the entire neuraxis (Mahalingam et al., 1990; Terasaki et al., 1997; Kam et al., 1999; Gilden et al., 2001, 2011; Theil et al., 2004; Kushawaha et al., 2009). Once within the neuron, the virus DNA enters the nucleus, circularizes, and becomes associated with cellular histones, after which virus gene transcription is restricted (Cohrs et al., 1992; Clarke et al., 1995; Gary et al., 2006).
The study of VZV latency is hindered by the lack of a small-animal model that recapitulates this unique virus–host interaction. As a result, human ganglia removed at autopsy have been required in studying the physical state of VZV DNA and gene expression during latency. The VZV DNA copy number per 100 000 ganglionic cells during latency has been variously reported at 6–31 (Mahalingam et al., 1993), 258±38 (Pevenstein et al., 1999), and 9046±13 225 (Cohrs et al., 2000). While the full complement of 68 unique genes are transcribed during productive infection (Cohrs et al., 2003; Kennedy et al., 2005; Nagel et al., 2009), only 10 genes are transcribed during latency (Nagel et al., 2011a). VZV ORF 63 is the most prevalent and abundant gene transcribed in latency (Cohrs et al., 2008), with ORF 63 protein accumulating within the cytoplasm, where it may block VZV-induced neuronal apoptosis (Mahalingam et al., 1996; Hood et al., 2003, 2006). During productive infection, the protein encoded by ORF63 is highly phosphorylated and moderates the interferon response, possibly by modifying cellular histone complexes in infected cell nuclei (Ambagala and Cohen, 2007; Ambagala et al., 2009; Mueller et al., 2009).
Knowledge of VZV gene regulation within latently infected neurons is critical to understanding the mechanism by which the virus maintains latency and the early events of reactivation. VZV encodes six genes essential for virus propagation, five of which have been detected in latently infected ganglia (Cohrs et al., 2000, 2008). The sixth essential VZV gene whose transcription has not been detected during latency is ORF 9, the most abundant virus transcript found during productive infection (Cohrs et al., 2003; Nagel et al., 2011a).
VZV EPIDEMIOLOGY
Based on the prevalence of anti-VZV immunoglobulin G (IgG) antibodies in 21 288 individuals in the United States from 1988 to 1994, VZV infects approximately 99% of the US adult population (Kilgore et al., 2003). Virus becomes latent in all these individuals, one-third of whom will develop zoster during their lifetime (Yawn et al., 2007). In the United States, approximately one million new cases of zoster are diagnosed annually, of whom two-thirds are older than 50 years and nine in 10 are immunocompetent individuals; one in 10 experiences at least one zoster-related non-pain complication, while one in four experiences zoster-related pain that persists 30 days or more (Yawn et al., 2007). While a zoster vaccine is available, only 6.7% of individuals over age 60 years have received vaccine (Lu et al., 2011); thus, more than 900 000 individuals in the United States are still likely to develop zoster. The annual medical care cost of treating incident zoster cases in the United States is estimated at $1.1 billion, most of which is used to treat immunocompetent adults 50 years and older (Yawn et al., 2009). Since the risk of zoster increases with age, and the population age 65 and older is expected to double from 36 million in 2003 to 72 million in 2030, zoster and its attendant serious neurologic complications will continue to be a significant healthcare burden.
REACTIVATION OF VZV
Herpes zoster is characterized by pain and a vesicular eruption on an erythematous base in one or several dermatomes. Rash and pain usually develop within a few days of each other, although pain may precede rash by 7 to more than 100 days (Gilden et al., 1991). Any derma-tome may be involved. The trunk from T3 to L2 is the most heavily involved area (Table 12.1), followed by the face and, lastly, the extremities. Because the trunk is represented by 12 pairs of ganglia, while the face is supplied by afferent fibers only from the trigeminal ganglia, the latter are the most frequently latently infected ganglia, as is the case for HSV-1. The vesicles produced by HSV reactivation are patchy rather than dermatomal (Fig. 12.3), an important distinction for the clinician.
Table 12.1.
Segmental localization of 180 cases of zoster
| Right | Ganglion | Left |
|---|---|---|
| …………. | V | ………… |
| . | VII | . |
| C1 | ||
| . | 2 | …. |
| … | 3 | … |
| .. | 4 | .. |
| .. | 5 | . |
| .. | 6 | |
| . | 7 | … |
| . | 8 | |
| . | T1 | . |
| .. | 2 | . |
| …. | 3 | …. |
| …. | 4 | . |
| …….. | 5 | …….. |
| ….. | 6 | ……. |
| …. | 7 | ….. |
| .. | 8 | …….. |
| …….. | 9 | …. |
| ….. | 10 | …… |
| . | 11 | |
| … | 12 | …. |
| …. | L1 | …. |
| ….. | 2 | …. |
| .. | 3 | .. |
| 4 | ||
| . | 5 | .. |
| . | S1 | . |
| . | 2 | . |
| 3 | … | |
| 4 | ||
| 5 |
Each dot represents a single case of zoster.
Reproduced from Hope-Simpson (1965).
Fig. 12.3.

Vesicular eruptions produced by reactivation of herpes simplex virus (HSV) and varicella-zoster virus (VZV). Note patchy skin lesions produced by HSV (A) compared to more extensive dermatomal lesions characteristically produced by VZV (B).
Optic neuritis after zoster occurs in adults (Miller et al., 1986) and in children and may be bilateral (Selbst et al., 1983). Reports of visual loss in association with pain on eye movement and clinical findings of papillitis and central scotomata or optic atrophy weeks after VZV infection suggest an immunologic pathogenesis in some cases (Carroll and Mastaglia, 1979). The third nerve is affected more frequently than the sixth as a cause of zoster ophthalmoplegia. Least affected is the fourth, although isolated VZV-associated trochlear nerve palsies have been described (Grimson and Glaser, 1978). Combinations of third, fourth, and sixth nerve palsies are not unusual.
Zoster affects the head in approximately 19% of cases, with nearly all rashes (97%) in the trigeminal distribution (Burgoon et al., 1957; Hope-Simpson, 1965). In patients with trigeminal zoster, the ophthalmic division of the fifth nerve is most frequently affected. There have been 14 reported cases of trigeminal distribution zoster accompanied by alveolar bone necrosis and tooth loss (Manz et al., 1986). The next most commonly affected cranial nerve ganglion is the seventh. Weakness of facial muscles is usually associated with lesions in the ear (Ramsay Hunt syndrome; geniculate zoster). Vesicles may also be observed on the anterior two-thirds of the tongue (Payten and Dawes, 1972). Importantly, HSV-1 has been detected in endoneurial fluid in patients with Bell’s palsy (Murakami et al., 1996), which has implicated the virus in the pathogenesis of disease. Similarly, in patients with peripheral facial weakness and rash on the ear (zoster oticus), VZV is found in endoneurial fluid. The detection of either virus in endoneurial fluid in patients with facial weakness indicates reactivation from the geniculate ganglion. The facial paralysis is often more severe than in Bell’s palsy, and patients are less likely to recover completely (Robillard et al., 1986). Commonly associated with geniculate zoster are eighth-nerve deficit (Crabtree, 1968), fifth-nerve zoster or zoster of the occiput and neck (Klippel and Aynaud, 1899), ophthalmoplegia (Thomas and Howard, 1972), and multiple lower cranial nerve palsies (Crabtree, 1968; Steffen and Selby, 1972).
The mechanism by which the cranial nerves are involved in zoster is not clear. In one case of herpes zoster ophthalmicus with oculomotor nerve involvement, death occurred 4 days after cutaneous signs; pathologic examination revealed neuronal degeneration with microglial and lymphocytic infiltration in the oculomotor nuclear complex, and a leptomeningeal infiltrate most marked over the lower medulla and at the point of entry of the trigeminal and oculomotor nerve roots (Goodbody, 1953). Orbital changes consisted of perineural and intraneural lymphocytic infiltrates of the retrobulbar, episcleral, and intrascleral segments of the long posterior ciliary nerves, with minimal lymphocytic infiltrates and sarcolemmal proliferation in extraocular muscles. Thus,cranial neuropathymay be duetoa circumscribed orbital myositis or lymphocytic mononeuropathy. Like zoster-associated optic neuritis, ophthalmoplegia often appears weeks to months after cutaneous signs (Edgerton, 1945; Keane, 1975).
One possible explanation for VZV-induced late-onset cranial neuropathy is microinfarction. VZV particles could spread along trigeminal (and other ganglionic) afferent fibers to small vessels supplying cranial nerves in a manner thought to occur in VZV-related granulomatous arteritis. The blood supply of the cranial nerves comes from the carotid circulation (Lapresle and Lasjaunias, 1986) and may receive input from trigeminal afferent fibers, as has been demonstrated for larger extracranial and intracranial blood vessels (Mayberg et al., 1984). Careful serial sections of cranial nerves in patients who developed VZV-induced cranial mono- or polyneuropathies are needed.
Importantly, multiple forms of trigeminal (Easton, 1970; Hevner et al., 2003; Yamada et al., 2003) and facial (Morgan and Nathwani, 1992; Murakami et al., 1998; Terada et al., 1998) distribution zoster as well as polyneuritis cranialis due to VZV (Osaki et al., 1995; Murata et al., 2010) may occur in the absence of rash.
PATHOLOGY OF HERPES ZOSTER
The pathologic features of zoster are characterized by inflammation and hemorrhagic necrosis with associated neuritis, localized leptomeningitis, unilateral segmental poliomyelitis, and degeneration of related motor and sensory roots (Head and Campbell, 1900; Denny-Brown et al., 1944). Demyelination is seen in areas with mononuclear cell (MNC) infiltration and microglial proliferation. Intranuclear inclusions, viral antigen, and herpesvirus particles have been found in acutely infected ganglia (Cheatham et al., 1956; Esiri and Tomlinson, 1972; Ghatak and Zimmerman, 1973).
TREATMENT OF HERPES ZOSTER
Antiviral drugs (e.g., valacyclovir, 1 g three times daily for 7–10 days) speed healing of rash and shorten the duration of acute pain. In immunocompromised patients, intravenous acyclovir (5–10 mg/kg three times daily for 5–7 days) is recommended. Because zoster pain may be associated with the inflammatory response, many clinicians administer a short course of corticosteroids, e.g., oral prednisone, 1 mg/kg for 5–7 days, in addition to antiviral therapy.
NEUROLOGIC COMPLICATIONS OF ZOSTER
Postherpetic neuralgia
The most common neurologic complication of zoster is PHN, operationally defined as pain that persists for at least 3 months and sometimes years after resolution of zoster rash. Age is the most important factor in predicting the development of PHN (Table 12.2). Pain is usually constant, severe, stabbing, or burning and frequently associated with allodynia (increased sensitivity to light touch). The incidence of PHN appears to be slightly greater in women (Hope-Simpson, 1975) and after trigeminal distribution zoster (de Moragas and Kierland, 1957; Rogers and Tindall, 1971; Hope-Simpson, 1975).
Table 12.2.
Age predicts postherpetic neuralgia (PHN)
| Age (years) | Patients (no.) | PHN (%) | Reference |
|---|---|---|---|
| Adults | 590 | 9 | Ragozzino et al. (1982) |
| <70 | 756 | 4.2 | de Moragas and Kierland (1957) |
| >70 | 160 | 47.5 | de Moragas and Kierland (1957) |
| <60 | 333 | 15.9 | Rogers and Tindall (1971) |
| >60 | 243 | 46.9 | Rogers and Tindall (1971) |
The cause and pathogenesis of PHN are unknown. Two non-mutually exclusive theories are that: (1) excitability of ganglionic or even spinal cord neurons is altered; and (2) persistent or low-grade productive virus infection exists in ganglia. Smith’s (1978) analysis of ganglia from an early case of PHN of 2.5 months’ duration revealed diffuse and focal infiltration by chronic inflammatory cells (Fig. 12.4A), an observation confirmed by Watson et al. (1991), who found prominent collections of lymphocytes in ganglia from a patient with PHN of 2 years’ duration (Fig. 12.4B). The inflammatory response in ganglia of these subjects raised the possibility of prolonged viral infection. Further evidence that PHN may be produced by low-level ganglionitis has come from the detection of VZV DNA and proteins in blood MNCs of many patients with PHN (Vafai et al., 1988; Devlin et al., 1992; Mahalingam et al., 1995) and from the favorable response of some PHN patients to antiviral treatment (Terada et al., 1998; Gilden et al., 2003).
Fig. 12.4.

Hematoxylin and eosin-stained sections of dorsal root ganglia from patients with postherpetic neuralgia. Note the diffuse and focal infiltration by chronic inflammatory cells (A). Arrow in panel B points to a prominent collection of lymphocytes. (From Gilden et al. 2005 with permission from Wolters Kluwer Health).
Although not life-threatening, PHN is difficult to manage. Treatment is supportive, with use of neuroleptic drugs and various analgesics, including opiates to alleviate pain, but no universally accepted treatment exists. Pregabalin is given at 75–150 mg orally twice daily or 50–100 mg orally three times daily (150–300 mg/day). If minimal relief is obtained at 300 mg daily for 2 weeks, the dose can be increased to a maximum of 600 mg/day in two or three divided doses. In addition, oxycodone (controlled-release, 10–40 mg orally every 12 hours) or controlled-release morphine sulfate and tricyclic antidepressants are used (Dubinsky et al., 2004). Levorphanol produces morphine-like analgesia, at a dose of 2 mg orally every 6–8 hours as needed, with maximum doses of 6–12 mg daily (Rowbotham et al., 2003a, b). Combination treatment with morphine and gabapentin also decreases pain more than either drug alone or placebo (Gilron et al., 2005). Tricyclic antidepressants, including amitriptyline (10–25 mg orally at bedtime with a maximum dose of 150–200 mg/day), nortriptyline, mapotriline, and despramine, lessen the pain of PHN.
Zoster paresis
Zoster paresis (weakness) is manifest by arm weakness or diaphragmatic paralysis (Brostoff, 1966; Stowasser et al., 1990) after cervical distribution zoster, leg weakness after lumbar or sacral distribution zoster, and by urinary retention after sacral distribution zoster (Izumi and Edwards, 1973; Jellinek and Tulloch, 1976). Magnetic resonance imaging (MRI) of patients with zoster paresis reveals involvement not only of the posterior horn and posterior roots, but also of the anterior roots and anterior horn at the spinal level corresponding to the patient’s clinical deficit (Hanakawa et al., 1997; Umehara et al., 2011). Rarely, clinical deficit in cervical zoster paresis extends to the brachial plexus, confirmed by both electrodiagnostic testing and MRI (Choi et al., 2009). The prognosis varies. In one series of 45 patients with zoster paresis, 67% had near-complete recovery (Gupta et al., 1969), and in another 61 cases, 55% had complete functional recovery (Thomas and Howard, 1972).
VZV meningitis, meningoencephalitis, meningoradiculitis, and cerebellitis
Acute VZV infection may also present as meningitis or a meningoencephalitis. Many reported cases of VZV encephalitis may actually be VZV vasculopathy (Gilden, 2002). Recent reports of VZV meningitis (Habib et al., 2009; Klein et al., 2010), meningoradiculit is (Gunson et al., 2011), and cerebellitis (gait ataxia and tremor predominated) (Moses et al., 2006; Ratzka et al., 2006), all in the absence of rash and confirmed by the detection of VZV DNA and anti-VZV antibody in cerebrospinal fluid (CSF), revealed that VZV is not an uncommon cause of aseptic meningitis.
VZV vasculopathy
Another serious complication of VZV reactivation is VZV infection of human cerebral arteries (VZV vasculopathy) which causes both ischemic and hemorrhagic stroke. The exact incidence of VZV vasculopathy is unknown, although recent studies indicate that it is a significant stroke risk factor. In children, up to one-third of pediatric ischemic arteriopathies are associated with varicella (Amlie-Lefond et al., 2009; Braun et al., 2009; Ciccone et al., 2010). In adults with zoster, the risk of stroke is increased by 30% within the following year (Kang et al., 2009) and by 4.5-fold when zoster was in the ophthalmic distribution of the trigeminal nerve (Lin et al., 2010).
VZV vasculopathy affects both immunocompromised and immunocompetent individuals and can present as headache, mental status changes, and focal neurologic deficits. Both large and small vessels are involved (Nagel et al., 2008). Lesions at gray–white-matter junctions are frequently seen on brain imaging (Fig. 12.5). In more than two-thirds of patients, angiography reveals focal arterial stenosis and occlusion (Fig. 12.6), aneurysm, or hemorrhage. In 30 virologically verified cases of VZV vasculopathy, both large and small arteries were involved in 50%, small arteries in 37%, and large arteries alone in only 13%. Importantly, the diagnosis of VZV vasculopathy is often missed because: (1) up to one-third of patients do not have preceding zoster rash; (2) up to one-third do not have increased cells in CSF; (3) VZV DNA polymerase chain reaction analysis of CSF is only 30% sensitive; and (4) symptoms and signs often occur months after zoster (Nagel et al., 2008). The best test for diagnosis is detection of anti-VZV antibodies in CSF (Nagel et al., 2007). Once diagnosis is made, patients can be treated with intravenous acyclovir and prednisone.
Fig. 12.5.

Magnetic resonance imaging (MRI) scan of a patient with varicella-zoster virus multifocal vasculopathy. Proton density brain MRI scan shows multiple areas of infarction in both hemispheres, particularly involving white matter. Arrows point to gray–white-matter junction lesions.
Fig. 12.6.

Brain images of a patient with varicella-zoster virus vasculopathy and infarction. (A) Diffusion-weighted image (B value 1000) shows restricted diffusion in the right anterior cerebral artery territory, indicating acute infarction. (B) Three-dimensional time-of-flight magnetic resonance angiography at the time of infarction shows marked narrowing of anterior cerebral arteries, with a new flow gap at the junction of A1 and A2 segments of the right anterior cerebral artery, indicating occlusion on the right (long arrow) and marked stenosis on the left (short arrow). (C) Three-dimensional time-of-flight magnetic resonance angiography of the circle of Willis 5 months before infarction shows normal anterior cerebral arteries. (From Gilden et al. 2002. Copyright © Massachusetts Medical Society. Reprinted with permission).
VZV causes bland or hemorrhagic infarctions. Deep white-matter lesions often predominate and are ischemic or demyelinative, depending on the size of blood vessels involved (Fig. 12.7). Case reports have revealed a wide range of vascular pathology, ranging from neointimal proliferation to necrosis with and without inflammation (Kleinschmidt-DeMasters and Gilden, 2001). Infected cerebral arteries contain multinucleated giant cells, Cowdry A inclusion bodies, herpesvirus particles detected by electron microscopy, as well as VZV DNA, and VZV antigen (Fig. 12.8). VZV vasculopathy predominantly affects intracranial rather than systemic arteries, possibly due to inherent cellular and structural differences. For example, intracranial cerebral arteries do not contain the external elastic lamina that may affect transmural migration of virus and cells (Lee, 1995). Like horseradish peroxidase, which migrates from the external surface of cerebral arteries to trigeminal ganglia (Mayberg et al., 1981, 1984), reactivated VZV may also travel along ganglionic afferent fibers to the adventitia of cerebral arteries, a notion supported by the presence of viral antigen predominantly in the adventitia of cerebral arteries in early VZV vasculopathy (Salazar et al., 2011).
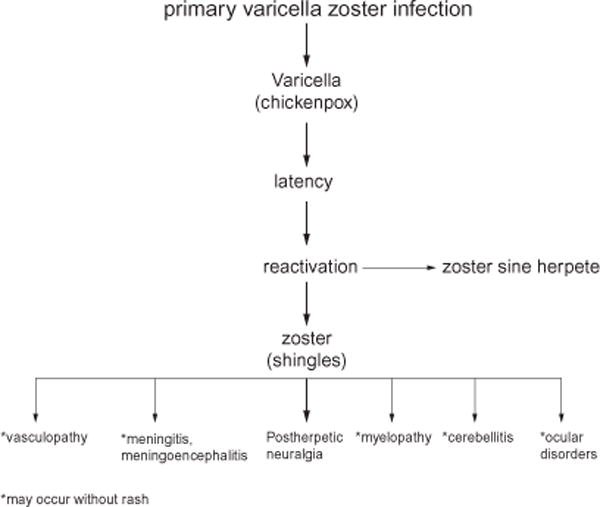
Fig. 12.7.
Kluver–Barrera-stained macroscopic section of brain from a patient who died of varicella-zoster-virus vasculopathy. Arrows denote multiple lesions of varying size at gray–white-matter junctions.
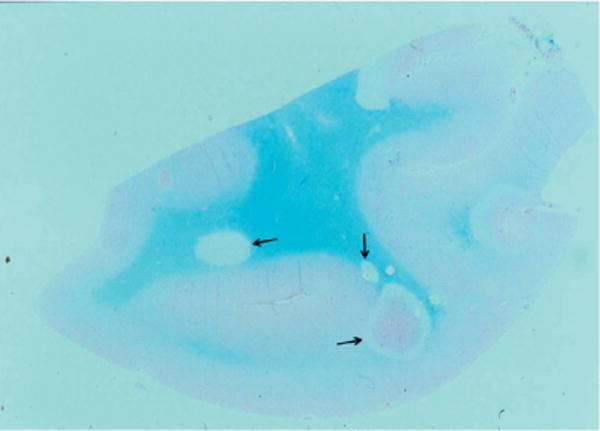
Fig. 12.8.
Pathologic and virologic findings in the arteries of patients who died from varicella-zoster virus (VZV) vasculopathy. (A) A cerebral artery with multinucleated giant cells (arrow). (B) Herpesvirions (arrow) within a cerebral artery. (C) VZV DNA in two (lanes 3 and 5) of five cerebral arteries. (D) VZV antigen (red) in the media of a cerebral artery. (From Gilden et al. 2009).
A recent study examining the histologic and immunohistochemical features of VZV-infected cerebral arteries from patients with VZV vasculopathy revealed several distinct features that may contribute to stroke: (1) a thickened arterial intima composed of myofibroblasts and cells of medial smooth-muscle origin; (2) a disrupted internal elastic lamina; and (3) decreased smooth-muscle cells (Nagel et al., 2011b). How these changes affect arterial contractility and lead to vessel occlusion remains to be determined.
VZV myelopathy
VZV myelopathy presents in various ways. One form is a self-limiting, monophasic spastic paraparesis, with or without sensory features and sphincter problems. This so-called postinfectious myelitis usually occurs in immunocompetent patients days to weeks after acute varicella or zoster. Its pathogenesis is unknown. The CSF usually contains a mild mononuclear pleocytosis, with a normal or slightly elevated protein. Steroids are used to treat these patients (Pina et al., 1997), although some improve spontaneously (Celik et al., 2001). Rarely, VZV myelitis recurs, even in immunocompetent patients (Gilden et al., 1994a). VZV myelopathy may also present as an insidious, progressive, and sometimes fatal myelitis, mostly in immunocompromised individuals. AIDS has commonly and increasingly become associated with VZV myelitis. MRI reveals longitudinal serpiginous enhancing lesions (Fig. 12.9). Diagnosis is confirmed by the presence of VZV DNA or anti-VZV IgG or both in CSF (Gilden et al., 1994a). Pathologic and virologic analyses of the spinal cord from fatal cases have shown frank invasion of VZV in the parenchyma (Kleinschmidt-DeMasters and Gilden, 2001) and, in some instances, spread of virus to adjacent nerve roots (Devinsky et al., 1991). Not surprisingly, some patients respond favorably to antiviral therapy (de Silva et al., 1996; Chua et al., 2001; Schvoerer et al., 2003). Importantly, VZV myelitis may develop without rash. Early diagnosis and aggressive treatment with intravenous acyclovir have been helpful, even in immunocompromised patients (de Silva et al., 1996). The benefit of steroids in addition to antiviral agents is unknown. VZV can also produce spinal cord infarction. identified by diffusion-weighted MRI and confirmed virologically (Orme et al., 2007). Thus, VZV vasculopathy can cause stroke in the spinal cord as well as in the brain.
Fig. 12.9.

Magnetic resonance imaging abnormality in a patient with varicella-zoster virus myelitis. Note cervical, longitudinal, serpiginous enhancing lesion (arrow). (From Gilden et al. 1994a with permission from Wolters Kluwer Health).
VZV-induced ocular disorders
VZV produces multiple ocular disorders, including both acute retinal necrosis and PORN. Acute retinal necrosis develops in both immunocompetent and immunocompromised hosts. Patients present with periorbital pain and floaters with hazy vision and loss of peripheral vision. Treatment is typically intravenous acyclovir, steroids, and aspirin followed by oral acyclovir (Bonfioli and Eller, 2005). Intravitreal injections of foscarnet and oral acyclovir have been used in early, milder cases. PORN presents with sudden painless loss of vision, floaters, and constricted visual fields with resultant retinal detachment. Multifocal, discrete opacified lesions begin in the outer retinal layers peripherally and/or posterior pole; only late in disease are inner retinal layers involved. Diffuse retinal hemorrhages and whitening with macular involvement bilaterally are characteristic findings (Fig. 12.10). Although PORN can be caused by HSV and cytomegalovirus, most cases are produced by VZV, primarily in AIDS patients with CD4+ counts typically less than 10 cells/mm3 of blood (Guex-Crosier et al., 1997), but also in other immunosuppressed individuals (Lewis et al., 1996). PORN may be preceded by retrobulbar optic neuritis and aseptic meningitis (Franco-Paredes et al., 2002), central retinal artery occlusion, or ophthalmic distribution zoster (Menerath et al., 1995), and may occur together with multifocal vasculopathy or myelitis. Treatment with intravenous acyclovir has given poor or inconsistent results (Johnston et al., 1993), and even when acyclovir helped, VZV retinopathy recurred when drug was tapered or stopped. PORN patients treated with ganciclovir alone or in combination with foscarnet had a better final visual acuity than those treated with acyclovir or foscarnet (Moorthy et al., 1997). The best treatment for PORN in AIDS patients may be prevention with highly active antiretroviral therapy, which appears to decrease its incidence (Austin, 2000).
Fig. 12.10.

Fundus photograph of a patient with varicella-zoster virus vasculopathy and progressive outer retinal necrosis. Note the diffuse retinal hemorrhages and whitening with macular involvement. (From Nakamoto et al. 2004 with permission from Wolters Kluwer Health).
As with VZV neurologic disease, ocular disorders caused by VZV can also occur in the absence of rash. Multiple cases of PORN (Friedman et al., 1993; Galindez et al., 1996), as well as a case of severe unremitting eye pain without rash, were proven to be caused by VZV based on the detection of VZV DNA in nasal and conjunctival samples (Goon et al., 2000). In addition, third cranial nerve palsies (Hon et al., 2005), retinal periphlebitis (Noda et al., 2001), uveitis (Akpel and Gottsch, 2000; Hon et al., 2005), iridocyclitis (Yamamoto et al., 1995), and disciform keratitis (Silverstein et al., 1997) occurred without rash and were confirmed virologically to be caused by VZV.
Zoster sine herpete (radicular pain in the absence of rash)
The notion of zoster sine herpete was initially provided by a report of multiple patients with dermatomal distribution radicular pain in areas distinct from pain with rash in zoster (Lewis, 1958). Currently, most clinicians regard zoster sine herpete exclusively as the occurrence of chronic radicular pain without rash. The first two virologically confirmed cases of zoster sine herpete were verified by detection of VZV DNA in CSF (Gilden et al., 1994b). A third case of thoracic distribution zoster sine herpete, in which electromyography of paraspinal muscles demonstrated frequent fibrillation potentials restricted to chronically painful thoracic root segments, was confirmed by detection of VZV DNA in blood MNCs and anti-VZV IgG antibody in CSF (Amlie-Lefond et al., 1996). In a recent report of a patient with zoster sine herpete, the CSF did not contain amplifiable VZV DNA, but did contain anti-VZV IgG with reduced serum/CSF ratios of anti-VZV IgG indicative of intrathecal synthesis (Blumenthal et al., 2011). Finally, the most compelling evidence that persistent radicular pain without rash can be caused by a chronic active VZV ganglionitis came from analysis of a trigeminal ganglionic mass removed from an immunocompetent adult who had experienced relentless trigeminal distribution pain for more than a year; pathologic and virologic analyses (Fig. 12.11) revealed that the patient’s zoster sine herpete was caused by an active VZV ganglionitis (Hevner et al., 2003).
Fig. 12.11.

Ganglionitis and intranuclear inclusions in zoster sine herpete. Hematoxylin and eosin staining of the trigeminal ganglion (top panel) shows widespread chronic inflammation with fibrosis and loss of neurons. Cells in some foci contain Cowdry type A intranuclear inclusions (arrow, inset) indicative of virus infection; the inflammatory cells are mainly lymphocytes with some plasma cells (arrowhead, inset). Immunohistochemical staining of the same ganglion (bottom panel) with mouse monoclonal antibody directed against varicella-zoster virus (VZV) gene 63 protein indicates VZV antigen (brown staining) in multiple cells throughout the ganglion. Adjacent sections stained with antibody directed against herpes simplex virus or with normal rabbit serum were negative (not shown). (From Hevner et al. 2003).
Other neurologic disease produced by VZV reactivation without rash
The detection of VZV DNA and anti-VZV IgG antibody with reduced serum/CSF ratios in the CSF of patients with meningoencephalitis, vasculopathy, myelitis, cerebellar ataxia, and polyneuritis cranialis, all without rash, has expanded the spectrum of neurologic disease produced by VZV in the absence of rash. An illustrative example is provided by the report of an immunocompetent 45-year-old woman who had multiple episodes of neurologic disease (multifocal vasculopathy, meningoencephalitis, recurrent myelitis, and inflammatory brainstem disease) over an 11-month period produced by VZV in which the CSF contained anti-VZV IgG antibody, but not VZV DNA, throughout her illness (Haug et al., 2010). Overall, if neurologic disease at any level of the neuraxis is thought to be due to VZV, the CSF and blood MNCs should be studied for VZV DNA, the CSF for the presence of anti-VZV IgG and IgM antibody, and the serum for anti-VZV IgM antibody (see below).
DIAGNOSTIC TESTS
The diagnosis of VZV-induced neurologic disease is straightforward when the characteristic dermatomal distribution rash of zoster is present. When zoster rash is not present in a patient with neurologic disease that can be caused by VZV (e.g., chronic radicular pain, meningoencephalitis with or without cerebellitis, vasculopathy, myelitis, or retinal necrosis), examination of CSF and blood MNCs for VZV is helpful. First, the presence of amplifiable VZV DNA in CSF in patients with any of the above disorders confirms the diagnosis of VZV-induced disease. VZV DNA is most likely to be found in patients with acute meningoencephalitis and cerebellitis and in some patients with VZV vasculopathy and myelitis. Importantly, many cases of VZV vasculopathy and myelitis are protracted, and the best virologic test to confirm VZV as the cause of neurologic disease is detection of anti-VZV IgG and/or anti-VZV IgM in CSF or anti-VZV IgM in serum. The detection of anti-VZV IgM in either serum or CSF is strong evidence of acute or chronic active infection produced by VZV. CSF most often contains only anti-VZV IgG and, rarely, both anti-VZV IgG and anti-VZV IgM. While the presence of anti-VZV IgG is strongly indicative of VZV-induced neurologic disease, clinching evidence comes from the demonstration of intrathecal synthesis of anti-VZV IgG antibodies to indicate chronic active infection. To determine intrathecal synthesis, the serum/CSF ratios for anti-VZV IgG (Qspec) and for total IgG (QIgG) are calculated in a non-traumatic lumbar puncture; an antibody index (Qspec/QIgG) equal to or greater than 1.5 indicates specific antibody synthesis in the CSF (Reiber and Lange, 1991). The detection of anti-VZV IgG antibody in CSF with intrathecal synthesis is superior to detection of VZV DNA in CSF to diagnose VZV vasculopathy (Nagel et al., 2007), as well as to establish the diagnosis of recurrent VZV myelopathy, VZV brainstem encephalitis, and VZV vasculopathy (Haug et al., 2010) and, most recently, to confirm the diagnosis of zoster sine herpete (Blumenthal et al., 2011). In 2 patients with zoster sine herpete, VZV DNA was found not only in CSF, but also in blood MNCs (Gilden et al., 1994b; Amlie-Lefond et al., 1996).
VZV DNA IN HUMAN SALIVA
In addition to the detection of VZV DNA in blood MNCs and CSF, the viral DNA may be present in saliva (Table 12.3). VZV DNA was present in the saliva of all of 54 patients with acute herpes zoster involving the face, trunk, and upper and lower extremities (Mehta et al., 2008), as well as in the saliva of most patients with the Ramsay Hunt syndrome (Furuta et al., 2001; Lackner et al., 2010). Interestingly, VZV DNA was found in the saliva of many patients with acute peripheral facial palsy without rash (Furuta et al., 2001, 2005), as well as in the saliva of 4 of 8 patients who experienced delayed facial palsy after orofacial surgery (Furuta et al., 2000). Importantly, VZV DNA persists for years in the saliva of individuals who developed zoster after 60 years of age (Nagel et al., 2011c). While it remains unclear why VZV DNA persists in the saliva of individuals with a history of zoster, the findings are consistent with earlier studies showing that the presence of VZV is not restricted to skin of the affected dermatome. For example, VZV DNA is also present in blood MNCs during acute zoster (Gilden et al., 1987) as well as in blood MNCs of some elderly individuals with no history of zoster (Devlin et al., 1992) and even in stressed healthy astronauts (Mehta et al., 2004; Cohrs et al., 2008). Overall, the detection of VZV DNA in saliva and blood indicates that, after reactivation from ganglia, virus does more than travel transaxonally retrograde to skin. The detection of VZV DNA in saliva of some elderly individuals for many years after zoster may reflect their inability to drive virus back to the latent state, just as a smoldering ganglionitis has been speculated to explain the development of PHN (Gilden et al., 2005). Both phenomena could readily be explained by individual differences in host cell-mediated immune responses to VZV.
Table 12.3.
Detection of varicella-zoster virus (VZV) DNA in saliva
| Clinical condition | Prevalence of VZV DNA in saliva |
Reference |
|---|---|---|
| Acute herpes zoster | 54/54 (100%) | Mehta et al. (2008) |
| Acute herpes zoster (Ramsay Hunt syndrome) | 13/25 (52%); 7/28 (25%) | Furuta et al. (2001); Aizawa et al. (2004) |
| 25/42 (60%); 9/10 (90%) | Yamakawa et al. (2007); Lackner et al. (2010) | |
| Acute peripheral facial paralysis without rash | 17/31 (55%); 3/10 (30%); 2/10 (20%) | Furuta et al. (2001, 2005); Lackner et al. (2010) |
| Delayed facial palsy after orofacial surgery | 4/8 (50%) | Furuta et al. (2000) |
| Elderly (>age 60) with history of zoster | 21/32 (66%) | Nagel et al. (2011c) |
| Elderly (>age 60) with no history of zoster | 2/17 (11%) | Nagel et al. (2011c) |
| Elderly (>age 60) after immunization | 18/36 (50%) | Pierson et al. (2011) |
| Healthy astronauts during and after space flight | 8/8 (100%); 2/3 (66%) | Mehta et al. (2004); Cohrs et al. (2008) |
Finally, the potential usefulness of saliva in diagnosis of patients with neurologic and ocular disease should be considered. Given that VZV may cause not only radicular pain in the absence of rash, but also cerebellitis, meningoencephalitis, vasculopathy, myelitis, and multiple serious ocular disorders without rash, future studies are needed to establish whether VZV DNA can be detected in the saliva of such patients.
VZV-SPECIFIC IMMUNITY
Primary VZV infection resulting in varicella is followed by the production of VZV-specific antibody and VZV-specific T-cell-mediated immunity (Kumagai et al., 1980; Arvin et al., 1986). Antibodies to VZV are detected throughout life, with the serum of individuals 60–94 years of age containing a variable presence of VZV-specific antibodies to VZV glycoproteins I–IV and to three non-glycosylated proteins; antibodies to VZV are also present in some elderly individuals with no history of varicella or zoster, indicative of subclinical infection (Vafai et al., 1988). Increased levels of antibody to VZV do not confer protection against zoster or PHN; in fact, increased levels of antibody to VZV after the onset of zoster are associated with more severe disease and a greater risk of PHN, perhaps because they reflect more extensive VZV replication (Weinberg et al., 2009).
T-cell immunity to VZV is more important than the antibody response, as evidenced in studies of natural VZV infection in agammaglobulinemic humans, who are unable to produce VZV-specific antibodies yet are protected against second episodes of varicella because of their ability to mount a VZV-specific T-cell-mediated immune response (Good and Zak, 1956). Individuals with T-cell-immune deficiency disorders have more severe disease than normal hosts (Gershon and Steinberg, 1979), and a significant increase in the incidence of zoster is associated with immunosuppression (Buchbinder et al., 1992). Even in human stem cell transplant recipients who received inactivated VZV vaccine, protection was correlated with VZV-specific T-cell immunity, not with anti-VZV antibody (Hata et al., 2002).
VZV-specific T-cell-mediated immunity maintains latent VZV in ganglia. The immune response can be subsequently boosted by subclinical reactivation of latent virus or environmental exposure to virus. Most importantly, the incidence of zoster increases with age as VZV-specific T-cell-mediated immunity declines. For example, the frequency of VZV-specific memory CD4 T cells is significantly influenced by age, as evidenced by their decrease during the first 3 years after varicella (Burke et al., 1982). After mid-adult life, the intensity and quality of antigenic stimulation provided by re-exposure and asymptomatic reactivation are not sufficient to maintain VZV-specific T-cell immunity. Furthermore, a comparison of the cell-mediated immune response to VZV antigen in vitro in young adults and individuals over age 60 years revealed fivefold fewer CD4 cells producing interferon-γor interleukin-4 and 5, as well as fewer CD4 early effectors and CD8 effector memory cells in the over-60 age group (Patterson-Bartlett et al., 2007).
While reduced cell-mediated immunity with age or after exposure to immunosuppressive regimens in cancer patients or bone marrow transplant recipients results in VZV reactivation (Dolin et al., 1978; Oxman et al., 2005), virus-specific T cells are rarely seen in human ganglia latently infected with VZV (Verjans et al., 2007). Analysis of human ganglia from donors who had zoster 1–5 months before death revealed VZV glycoprotein E in neurons and infiltration of non-cytolytic CD8+ T cells, but neurons that were positive for VZV glycoprotein E were neither positive for major histocompatibility complex (MHC) class I nor surrounded by T cells, suggesting that immune control of virus reactivation may not depend on direct contact with T cells (Gowrishankar et al., 2010). Since VZV has been shown to downregulate MHC I surface expression, virus latency is probably regulated by an innate immune response involving cytokines or chemokines (Cohen, 1998; Abendroth et al., 2001; Eisfeld et al., 2007). CXCL10 has been proposed as a potential driver of T-cell recruitment, based on its detection along with that of its receptor (CXCR3) in human ganglia from zoster patients (Steain et al., 2010).
Recognition of the essential role of cell-mediated immunity to VZV for protection against, and recovery from, varicella and zoster has led to studies designed to boost the cell-mediated immune response to VZV by immunization of elderly adults (see below).
PREVENTION OF ZOSTER
Attenuation of VZV by propagation in guinea pig cells has resulted in the development of the Oka VZV strain-based vaccine used to protect children from varicella and to prevent zoster in elderly adults. Propagation of this virus results in transcription of the complete complement of VZV genes seen in the wild-type virus (Hoover et al., 2006; Grinfeld et al., 2009). At least 63 mutations have been detected in the vaccine Oka strain as compared to the parental virus, but none of these mutations explains virus attenuation (Cohrs et al., 2005).
Zostavax is a licensed live, attenuated virus vaccine indicated for the prevention of herpes zoster in individuals 60 years and older. Zoster vaccine administered to people over 60 years of age leads to increased numbers of CD4 and CD8 cells, CD4 and CD8 effector memory T cells, and CD8 early-effector T cells; the half-life of the boost in T-cell immunity to VZV is at least 5 years (Levin et al., 1998). Zoster vaccine also boosts VZV-specific immunity in adults with a history of zoster before vaccination or with chronic illness. Demonstration of the ability of zoster vaccine to reverse VZV-specific T-cell deficiencies present before immunization was followed by the Shingles Prevention Study of Zostavax in a placebo-controlled, double-blind trial of more than 38 000 adults over age 60 years and randomized to receive a single dose of either zoster vaccine (n=19 270) or placebo (n=19 276). Racial distribution across both vaccination groups was similar (white (95%), black (2%), Hispanic (1%), and other (1%)), and gender distribution was 59% male and 41% female in both groups. All subjects were monitored for zoster. Endpoints included the burden of illness due to zoster and zoster-associated pain, as well as the incidence of clinically significant PHN. The most common side-effects reported by participants after zoster vaccination were redness, pain, itching, swelling, warmth or bruising at the injection site, and sometimes headache. Varicella-like rashes at the injection site were more common in zoster vaccine than in placebo recipients (0.1% versus 6.4%; p<0.05).
After a mean follow-up of 3 years, the Shingles Prevention Study found that Zostavax vaccine reduced the incidence of zoster by 51%. Subjects in the immunization group who developed zoster reported significantly less pain and discomfort than those in the placebo group, and PHN was less frequent (an overall 61% lower burden of disease). While the vaccine group had a significantly greater risk of a serious adverse event (1.9% versus 1.3%) and experienced more adverse events at the injection site (48.3% versus 16.6%) during the first 42 days after vaccination, no significant differences were seen between zoster vaccine and placebo groups in the incidence of vaccine-related serious adverse events (both <0.1%) at the end of the study.
Importantly, even immunized individuals who developed zoster displayed high VZV-specific cell-mediated immune responses associated with reduced zoster severity and a lower frequency of PHN than in participants with lower VZV-specific cell-mediated responses, whereas a high humoral response was associated with increased severity of zoster and a higher frequency of PHN than in participants with a lower humoral immune response (Weinberg et al., 2009).
In the United States, the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommend zoster vaccine for all persons over age 60 years with no prior contraindications and in persons regardless of a previous episode of zoster (http://www.cdc.gov/mmwr/PDF/rr/rr5705.pdf). Although zoster vaccine is not recommended for immunocompromised individuals, it appears that the current zoster vaccine could be safely administered to several groups of moderately immunocompromised adult patients, such as VZV-seropositive human immunodeficiency virus (HIV) patients with CD4 T-cell counts greater than 200 cells/mL, or even to patients with rheumatoid arthritis or psoriasis who are receiving moderate doses of methotrexate, steroids, or tumor necrosis factor inhibitors (Harpaz et al., 2008). In addition to the need to test the currently licensed zoster vaccine in moderately immunocompromised patients, higher-titer zoster vaccines should be also tested for safety and immunogenicity, especially in light of reports of the safe administration of heat-inactivated VZV vaccine to autologous bone marrow transplant recipients and their accelerated recovery of cell-mediated immunity to VZV and reduced incidence of zoster (Redman et al., 1997; Hata et al., 2002).
By 2008, 3 years after zoster vaccine was licensed and recommended by the Advisory Committee on Immunization Practices for persons age 60 years and older, less than 7% of this age group in the United States had been vaccinated (Lu et al., 2011). This disappointing rate reflects a combination of lack of patient awareness regarding the availability of a vaccine, physicians’ uncertainty about the duration of protection, and different cost-sharing plans for immunization. Zoster vaccine should be universally administered to all individuals over age 60 years and is currently under consideration for those age 50–59 years, since 15% of zoster episodes occur in this age group.
Acknowledgments
This work was supported in part by grants AG006127 (D.G.), AG032958 (D.G., R.J.C.), NS082228 (R.J.C.) and NS067070 (M.A.N.) from the National Institutes of Health.
We thank Marina Hoffman for editorial assistance and Cathy Allen for manuscript preparation.
References
- Abendroth A, Lin I, Slobedman B, et al. Varicella-zoster virus retains major histocompatibility complex class I proteins in the Golgi compartment of infected cells. J Virol. 2001;75:4878–4888. doi: 10.1128/JVI.75.10.4878-4888.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Ohtani F, Furuta Y, et al. Variable patterns of varicella-zoster virus reactivation in Ramsay Hunt syndrome. J Med Virol. 2004;74:355–360. doi: 10.1002/jmv.20181. [DOI] [PubMed] [Google Scholar]
- Akpel EK, Gottsch JD. Herpes zoster sine herpete presenting with hypema. Ocul Immunol Inflamm. 2000;8:115–118. [PubMed] [Google Scholar]
- Ambagala AP, Cohen JI. Varicella-zoster virus IE63, a major viral latency protein, is required to inhibit the alpha interferon-induced antiviral response. J Virol. 2007;81:7844–7851. doi: 10.1128/JVI.00325-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambagala AP, Bosma T, Ali MA, et al. Varicella-zoster virus immediate-early 63 protein interacts with human anti-silencing function 1 protein and alters its ability to bind his-tones h3.1 and h3.3. J Virol. 2009;83:200–209. doi: 10.1128/JVI.00645-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amlie-Lefond C, Mackin GA, Ferguson M, et al. Another case of virologically confirmed zoster sine herpete, with electrophysiologic correlation. J Neurovirol. 1996;2:136–138. doi: 10.3109/13550289609146547. [DOI] [PubMed] [Google Scholar]
- Amlie-Lefond C, Bernard TJ, Sébire G, et al. Predictors of cerebral arteriopathy in children with arterial ischemic stroke: results of the International Pediatric Stroke Study. Circulation. 2009;119:1417–1423. doi: 10.1161/CIRCULATIONAHA.108.806307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvin AM, Koropchak CM, Williams BR, et al. Early immune response in healthy and immunocompromised subjects with primary varicella-zoster virus infection. J Infect Dis. 1986;154:422–429. doi: 10.1093/infdis/154.3.422. [DOI] [PubMed] [Google Scholar]
- Austin RB. Progressive outer retinal necrosis syndrome: a comprehensive review of its clinical presentation, relationship to immune system status, and management. Clin Eye Vis Care. 2000;12:119–129. doi: 10.1016/s0953-4431(00)00052-7. [DOI] [PubMed] [Google Scholar]
- Blumenthal DT, Shacham-Shmueli E, Bokstein F, et al. Zoster sine herpete: virological verification by detection of anti-VZV IgG antibody in CSF. Neurology. 2011;76:484–485. doi: 10.1212/WNL.0b013e31820a0d28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfioli AA, Eller AW. Acute retinal necrosis. Semin Ophthalmol. 2005;20:155–160. doi: 10.1080/08820530500232027. [DOI] [PubMed] [Google Scholar]
- Braun KP, Bulder MM, Chabrier S, et al. The course and outcome of unilateral intracranial arteriopathy in 79 children with ischaemic stroke. Brain. 2009;132:544–557. doi: 10.1093/brain/awn313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuer J. VZV molecular epidemiology. Curr Top Microbiol Immunol. 2010;342:15–42. doi: 10.1007/82_2010_9. [DOI] [PubMed] [Google Scholar]
- Breuer J, Grose C, Norberg P, et al. A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24–25 July 2008. J Gen Virol. 2010;91:821–828. doi: 10.1099/vir.0.017814-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brostoff J. Diaphragmatic paralysis after herpes zoster. Br Med J. 1966;2:1571–1572. doi: 10.1136/bmj.2.5529.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchbinder SP, Katz MH, Hessol NA, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–1156. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- Burgoon CF, Burgoon JS, Baldridge GD. The natural history of herpes zoster. JAMA. 1957;164:265–269. doi: 10.1001/jama.1957.02980030041010. [DOI] [PubMed] [Google Scholar]
- Burke BL, Steele RW, Beard OW, et al. Immune responses to varicella-zoster in the aged. Arch Intern Med. 1982;142:291–293. [PubMed] [Google Scholar]
- Carpenter JE, Henderson EP, Grose C. Enumeration of an extremely high particle-to-PFU ratio for varicella-zoster virus. J Virol. 2009;83:6917–6921. doi: 10.1128/JVI.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll WM, Mastaglia FL. Optic neuropathy and ophthalmoplegia in herpes zoster oticus. Neurology. 1979;29:726–729. doi: 10.1212/wnl.29.5.726. [DOI] [PubMed] [Google Scholar]
- Celik Y, Tabak F, Mert A, et al. Transverse myelitis caused by varicella. Clin Neurol Neurosurg. 2001;103:260–261. doi: 10.1016/s0303-8467(01)00166-4. [DOI] [PubMed] [Google Scholar]
- Cheatham WJ, Dolan TF, Jr, Dower JC, et al. Varicella: report on two fatal cases with necropsy, virus isolation, and serologic studies. Am J Pathol. 1956;32:1015–1035. [PMC free article] [PubMed] [Google Scholar]
- Choi J-Y, Kang CH, Kim B-J, et al. Brachial plexopathy following herpes zoster infection: two cases with MRI findings. J Neurol Sci. 2009;285:224–226. doi: 10.1016/j.jns.2009.05.016. [DOI] [PubMed] [Google Scholar]
- Chua HC, Tjia H, Sitoh YY. Concurrent myelitis and Guillain–Barré syndrome after varicella infection. Int J Clin Pract. 2001;55:643–644. [PubMed] [Google Scholar]
- Ciccone S, Faggioli R, Calzolari F, et al. Stroke after varicella-zoster infection: report of a case and review of the literature. Pediatr Infect Dis J. 2010;29:864–867. doi: 10.1097/inf.0b013e3181ddefb6. [DOI] [PubMed] [Google Scholar]
- Clarke P, Beer T, Cohrs R, et al. Configuration of latent varicella-zoster virus DNA. J Virol. 1995;69:8151–8154. doi: 10.1128/jvi.69.12.8151-8154.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JI. Infection of cells with varicella-zoster virus down-regulates surface expression of class I major histocompatibility complex antigens. J Infect Dis. 1998;177:1390–1393. doi: 10.1086/517821. [DOI] [PubMed] [Google Scholar]
- Cohrs R, Mahalingam R, Dueland AN, et al. Restricted transcription of varicella-zoster virus in latently infected human trigeminal and thoracic ganglia. J Infect Dis. 1992;166(Suppl 1):S24–S29. doi: 10.1093/infdis/166.supplement_1.s24. [DOI] [PubMed] [Google Scholar]
- Cohrs RJ, Randall J, Smith J, et al. Analysis of individual human trigeminal ganglia for latent herpes simplex virus type 1 and varicella-zoster virus nucleic acids using real-time PCR. J Virol. 2000;74:11464–11471. doi: 10.1128/jvi.74.24.11464-11471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Hurley MP, Gilden DH. Array analysis of viral gene transcription during lytic infection of cells in tissue culture with varicella-zoster virus. J Virol. 2003;77:11718–11732. doi: 10.1128/JVI.77.21.11718-11732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, LaGuardia JJ, Gilden DH. Distribution of latent herpes simplex virus type-1 and varicella zoster virus DNA in human trigeminal ganglia. Virus Genes. 2005;31:223–227. doi: 10.1007/s11262-005-1799-5. [DOI] [PubMed] [Google Scholar]
- Cohrs RJ, Mehta SK, Schmid DS, et al. Asymptomatic reactivation and shed of infectious varicella zoster virus in astronauts. J Med Virol. 2008;80:1116–1122. doi: 10.1002/jmv.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohrs RJ, Koelle DM, Schuette MC, et al. Asymptomatic alphaherpesvirus reactivation. In: Gluckman TR, editor. Herpesviridae: Viral structure, life cycle and infections. Nova Science; Hauppauge, NY: 2009. pp. 113–166. [Google Scholar]
- Crabtree JA. Herpes zoster oticus. Laryngoscope. 1968;78:1853–1879. doi: 10.1288/00005537-196811000-00003. [DOI] [PubMed] [Google Scholar]
- Davison AJ, Scott JE. The complete DNA sequence of varicella-zoster virus. J Gen Virol. 1986;67:1759–1816. doi: 10.1099/0022-1317-67-9-1759. [DOI] [PubMed] [Google Scholar]
- de Moragas JM, Kierland RR. The outcome of patients with herpes zoster. Arch Dermatol. 1957;75:193–196. doi: 10.1001/archderm.1957.01550140037006. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D, Adams RD, Fitzgerald PJ. Pathologic features of herpes zoster: a note on “geniculate herpes”. Arch Neurol Psychiatry. 1944;51:216–231. [Google Scholar]
- de Silva SM, Mark AS, Gilden DH, et al. Zoster myelitis: improvement with antiviral therapy in two cases. Neurology. 1996;47:929–931. doi: 10.1212/wnl.47.4.929. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Cho ES, Petito CK, et al. Herpes zoster myelitis. Brain. 1991;114:1181–1196. doi: 10.1093/brain/114.3.1181. [DOI] [PubMed] [Google Scholar]
- Devlin ME, Gilden DH, Mahalingam R, et al. Peripheral blood mononuclear cells of the elderly contain varicella-zoster virus DNA. J Infect Dis. 1992;165:619–622. doi: 10.1093/infdis/165.4.619. [DOI] [PubMed] [Google Scholar]
- Dolin R, Reichman RC, Mazur MH, et al. NIH conference. Herpes zoster-varicella infections in immunosup-pressed patients. Ann Intern Med. 1978;89:375–388. doi: 10.7326/0003-4819-89-3-375. [DOI] [PubMed] [Google Scholar]
- Dubinsky RM, Kabbani H, El-Chami Z, et al. Practice parameter: treatment of postherpetic neuralgia: an evidence-based report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004;63:959–965. doi: 10.1212/01.wnl.0000140708.62856.72. [DOI] [PubMed] [Google Scholar]
- Easton HG. Zoster sine herpete causing acute trigeminal neuralgia. Lancet. 1970;2:1065–1066. doi: 10.1016/s0140-6736(70)90291-6. [DOI] [PubMed] [Google Scholar]
- Edgerton AE. Herpes zoster ophthalmicus: report of cases and review of literature. Arch Ophthalmol. 1945;34:40–62. 114–153. [PMC free article] [PubMed] [Google Scholar]
- Eisfeld AJ, Yee MB, Erazo A, et al. Downregulation of class I major histocompatibility complex surface expression by varicella-zoster virus involves open reading frame 66 protein kinase-dependent and -independent mechanisms. J Virol. 2007;81:9034–9049. doi: 10.1128/JVI.00711-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Tomlinson AH. Herpes zoster: demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J Neurol Sci. 1972;15:35–48. doi: 10.1016/0022-510x(72)90120-7. [DOI] [PubMed] [Google Scholar]
- Franco-Paredes C, Bellehemeur T, Merchant A, et al. Aseptic meningitis and optic neuritis preceding varicella- zoster progressive outer retinal necrosis in a patient with AIDS. AIDS. 2002;16:1045–1049. doi: 10.1097/00002030-200205030-00011. [DOI] [PubMed] [Google Scholar]
- Friedman SM, Mames RN, Sleasman JW, et al. Acute retinal necrosis after chickenpox in a patient with acquired immunodeficiency syndrome. Arch Ophthalmol. 1993;111:1607–1608. doi: 10.1001/archopht.1993.01090120029011. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Ohtani F, Fukuda S, et al. Reactivation of varicella-zoster virus in delayed facial palsy after dental treatment and oro-facial surgery. J Med Virol. 2000;62:42–45. [PubMed] [Google Scholar]
- Furuta Y, Ohtani F, Sawa H, et al. Quantitation of varicella-zoster virus DNA in patients with Ramsay Hunt syndome and zoster sine herpete. J Clin Microbiol. 2001;39:2856–2859. doi: 10.1128/JCM.39.8.2856-2859.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Ohtani F, Aizawa H, et al. Varicella-zoster virus reactivation is an important cause of acute peripheral facial paralysis in children. Pediatr Infect Dis J. 2005;24:97–101. doi: 10.1097/01.inf.0000151032.16639.9c. [DOI] [PubMed] [Google Scholar]
- Galindez OA, Sabates NR, Whitacre MM, et al. Rapidly progressive outer retinal necrosis caused by varicella zoster virus in a patient infected with human immunodeficiency virus. Clin Infect Dis. 1996;22:149–151. doi: 10.1093/clinids/22.1.149. [DOI] [PubMed] [Google Scholar]
- Gary L, Gilden DH, Cohrs RJ. Epigenetic regulation of varicella-zoster virus open reading frames 62 and 63 inlatently infected human trigeminal ganglia. J Virol. 2006;80:4921–4926. doi: 10.1128/JVI.80.10.4921-4926.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon AA, Steinberg SP. Cellular and humoral immune responses to varicella-zoster virus in immunocompromised patients during and after varicella-zoster infections. Infect Immun. 1979;26:170–174. doi: 10.1128/iai.25.1.170-174.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatak NR, Zimmerman HM. Spinal ganglion in herpes zoster. Arch Pathol. 1973;95:411–415. [PubMed] [Google Scholar]
- Gilden DH. Varicella zoster virus vasculopathy and disseminated encephalomyelitis. J Neurol Sci. 2002;195:99–101. doi: 10.1016/s0022-510x(02)00021-7. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Rozenman Y, Murray R, et al. Detection of varicella-zoster virus nucleic acid in neurons of normal human thoracic ganglia. Ann Neurol. 1987;22:377–380. doi: 10.1002/ana.410220315. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Dueland AN, Cohrs R, et al. Preherpetic neuralgia. Neurology. 1991;41:1215–1218. doi: 10.1212/wnl.41.8.1215. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Beinlich BR, Rubinstien EM, et al. Varicella-zoster virus myelitis: an expanding spectrum. Neurology. 1994a;44:1818–1823. doi: 10.1212/wnl.44.10.1818. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Wright RR, Schneck SA, et al. Zoster sine herpete, a clinical variant. Ann Neurol. 1994b;35:530–533. doi: 10.1002/ana.410350505. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Cohrs RJ, Mahalingam R, et al. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurology. 2009;8:731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilden DH, Lipton HL, Wolf JS, et al. Two patients with unusual forms of varicella-zoster virus vasculopathy. N Eng J Med. 2002;347 doi: 10.1056/NEJMoa020841. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Gesser R, Smith J, et al. Presence of VZV and HSV-1 DNA in human nodose and celiac ganglia. Virus Genes. 2001;23:145–147. doi: 10.1023/a:1011883919058. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Cohrs RJ, Hayward AR, et al. Chronic varicella zoster virus ganglionitis – a possible cause of postherpetic neuralgia. J Neurovirol. 2003;9:404–407. doi: 10.1080/13550280390201722. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Cohrs RJ, Mahalingam R. VZV vasculopathy and postherpetic neuralgia: progress and perspective on antiviral therapy. Neurology. 2005;64:21–25. doi: 10.1212/01.WNL.0000148484.19070.4D. [DOI] [PubMed] [Google Scholar]
- Gilden D, Mahalingam R, Nagel MA, et al. The neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441–446. doi: 10.1111/j.1365-2990.2011.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron I, Bailey JM, Tu D, et al. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:1324–1334. doi: 10.1056/NEJMoa042580. [DOI] [PubMed] [Google Scholar]
- Good RA, Zak SJ. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956;18:109–149. [PubMed] [Google Scholar]
- Goodbody RA. The pathology of acute herpes zoster ophthalmicus. J Pathol Bacteriol. 1953;65:221–227. doi: 10.1002/path.1700650123. [DOI] [PubMed] [Google Scholar]
- Goon P, Wright M, Fink C. Ophthalmic zoster sine herpete. J R Soc Med. 2000;93:191–192. doi: 10.1177/014107680009300409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowrishankar K, Steain M, Cunningham AL, et al. Characterization of the host immune response in human ganglia after herpes zoster. J Virol. 2010;84:8861–8870. doi: 10.1128/JVI.01020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson BS, Glaser JS. Isolatedtrochlear nerve palsiesin herpes zoster ophthalmicus. Arch Ophthalmol. 1978;96:1233–1235. doi: 10.1001/archopht.1978.03910060067013. [DOI] [PubMed] [Google Scholar]
- Grinfeld E, Ross A, Forster T, et al. Genome-wide reduction in transcriptional profiles of varicella-zoster virus vaccine strains compared with parental Oka strain using long oligonucleotide microarrays. Virus Genes. 2009;38:19–29. doi: 10.1007/s11262-008-0304-3. [DOI] [PubMed] [Google Scholar]
- Grose C, Ye M, Padilla J. Pathogenesis of primary infection. In: Arvin AM, Gershon AA, editors. Varicella-zoster virus: virology and clinical management. Cambridge University Press; Cambridge: 2000. pp. 105–122. [Google Scholar]
- Guex-Crosier Y, Rochat C, Herbort CP. Necrotizing herpetic retinopathies. A spectrum of herpes virus-induced diseases determined by the immune state of the host. Ocul Immunol Inflamm. 1997;5:259–265. doi: 10.3109/09273949709085066. [DOI] [PubMed] [Google Scholar]
- Gunson RN, Aitken C, Gilden D. A woman with acute headache and sacral dermatomal numbness. J Clin Virol. 2011;50:191–193. doi: 10.1016/j.jcv.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta SK, Helal BH, Kiely P. The prognosis in zoster paralysis. J Bone Joint Surg Br. 1969;51:593–603. [PubMed] [Google Scholar]
- Habib AA, Gilden D, Schmid DS, et al. Varicella zoster virus meningitis with hypoglycorrhachia in the absence of rash in an immunocompetent woman. J Neurovirol. 2009;15:206–208. doi: 10.1080/13550280902725550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanakawa T, Hashimoto S, Kawamura J, et al. Magnetic resonance imaging in a patient with segmental zoster paresis. Neurology. 1997;49:631–632. doi: 10.1212/wnl.49.2.631. [DOI] [PubMed] [Google Scholar]
- Harpaz R, Ortega-Sanchez IR, Seward JF, et al. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2008;57(RR-5):1–30. [PubMed] [Google Scholar]
- Hart J, Miller C, Tang X, et al. Stability of varicella-zoster virus and herpes simplex virus IgG monoclonal antibodies. J Immunoassay Immunochem. 2009;30:180–185. doi: 10.1080/15321810902782871. [DOI] [PubMed] [Google Scholar]
- Hata A, Asanuma H, Rinki M, et al. Use of an inactivated varicella vaccine in recipients of hematopoietic-cell transplants. N Engl J Med. 2002;347:26–34. doi: 10.1056/NEJMoa013441. [DOI] [PubMed] [Google Scholar]
- Haug A, Mahalingam R, Cohrs RJ, et al. Recurrent polymorphonuclear pleocytosis with increased red blood cells caused by varicella zoster virus infection of the central nervous system. J Neurol Sci. 2010;292:85–88. doi: 10.1016/j.jns.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawrami K, Harper D, Breuer J. Typing of varicella zoster virus by amplification of DNA polymorphisms. J Virol Methods. 1996;57:169–174. doi: 10.1016/0166-0934(95)01981-2. [DOI] [PubMed] [Google Scholar]
- Head H, Campbell AW. The pathology of herpes zoster and its bearing on sensory localization. Brain. 1900;23:353–523. doi: 10.1002/(sici)1099-1654(199709)7:3<131::aid-rmv198>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Hevner R, Vilela M, Rostomily R, et al. An unusual cause of trigeminal-distribution pain and tumour. Lancet Neurol. 2003;2:567–572. doi: 10.1016/s1474-4422(03)00506-4. [DOI] [PubMed] [Google Scholar]
- Hon C, Au WY, Cheng VC. Ophthalmic zoster sine herpete presenting as oculomotor palsy after marrow transplantation for acute myeloid leukemia. Haematologica. 2005;90(12 suppl):EIM04. [PubMed] [Google Scholar]
- Hood C, Cunningham AL, Slobedman B, et al. Varicella-zoster virus-infected human sensory neurons are resistant to apoptosis, yet human foreskin fibroblasts are susceptible: evidence for a cell-type-specific apoptotic response. J Virol. 2003;77:12852–12864. doi: 10.1128/JVI.77.23.12852-12864.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood C, Cunningham AL, Slobedman B, et al. Varicella-zoster virus ORF63 inhibits apoptosis of primary human neurons. J Virol. 2006;80:1025–1031. doi: 10.1128/JVI.80.2.1025-1031.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover SE, Cohrs RJ, Rangel ZG, et al. Downregulation of varicella-zoster virus (VZV) immediate-early ORF62 transcription by VZV ORF63 correlates with virus replication in vitro and with latency. J Virol. 2006;80:3459–3468. doi: 10.1128/JVI.80.7.3459-3468.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965;58:9–20. doi: 10.1177/003591576505800106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope-Simpson RE. Postherpetic neuralgia. J R Coll Gen Pract. 1975;25:571–575. [PMC free article] [PubMed] [Google Scholar]
- Izumi AK, Edwards J., Jr Herpes zoster and neurogenic bladder dysfunction. JAMA. 1973;224:1748–1749. [PubMed] [Google Scholar]
- Jellinek EH, Tulloch WS. Herpes zoster with dysfunction of bladder and anus. Lancet. 1976;2:1219–1222. doi: 10.1016/s0140-6736(76)91144-2. [DOI] [PubMed] [Google Scholar]
- Johnston WH, Holland GN, Engstrom RE, Jr, et al. Recurrence of presumed varicella-zoster virus retinopathy in patients with acquired immunodeficiency syndrome. Am J Ophthalmol. 1993;116:42–50. doi: 10.1016/s0002-9394(14)71742-8. [DOI] [PubMed] [Google Scholar]
- Kam AC, Dan NG, Maclean J, et al. Zoster related multiple cranial nerve palsies: an unusual complication following percutaneous balloon compression for trigeminal neuralgia. J Clin Neurosci. 1999;6:261–264. doi: 10.1016/s0967-5868(99)90519-2. [DOI] [PubMed] [Google Scholar]
- Kang JH, Ho JD, Chen YH, et al. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40:3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- Keane JR. Delayed trochlear nerve palsy in a case of zoster oticus. Arch Ophthalmol. 1975;93:382–383. doi: 10.1001/archopht.1975.01010020394016. [DOI] [PubMed] [Google Scholar]
- Kemble GW, Annuziato P, Lungu O, et al. Open reading frame S/L of varicella-zoster virus encodes a cytoplasmic protein expressed in infected cells. J Virol. 2000;74:11311–11321. doi: 10.1128/jvi.74.23.11311-11321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PG, Grinfeld E, Craigon M, et al. Transcriptomal analysis of varicella-zoster virus infection using long oligonucleotide-based microarrays. J Gen Virol. 2005;86:2673–2684. doi: 10.1099/vir.0.80946-0. [DOI] [PubMed] [Google Scholar]
- Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70:S111–S1118. doi: 10.1002/jmv.10364. [DOI] [PubMed] [Google Scholar]
- Klein NC, McDermott B, Cunha BA. Varicella-zoster virus meningoencephalitis in an immunocompetent patient without a rash. Scand J Infect Dis. 2010;42:631–633. doi: 10.3109/00365540903510716. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt-DeMasters BK, Gilden DH. Varicella zoster virus infections of the nervous systems – clinical and pathologic correlates. Arch Pathol Lab Med. 2001;125:770–780. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- Klippel M, Aynaud R. La paralysie faciale zostérienne. Rev Gen Clin Therapeut. 1899;13:225. [Google Scholar]
- Kumagai T, Chiba Y, Wataya Y, et al. Development and characteristics of the cellular immune response to infection with varicella-zoster virus. J Infect Dis. 1980;141:7–13. doi: 10.1093/infdis/141.1.7. [DOI] [PubMed] [Google Scholar]
- Kushawaha A, Mobarakai N, Tolia J. A 46-year-old female presenting with worsening headache, nuchal rigidity and a skin rash in varicella zoster virus meningitis: a case report. Cases J. 2009;2:6299. doi: 10.4076/1757-1626-2-6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner A, Kessler HH, Walch C, et al. Early and reliable detection of herpes simplex virus type 1 and varicella zoster virus DNAs in oral fluid of patients with idiopathic peripheral facial nerve palsy: decision support regarding antiviral treatment? J Med Virol. 2010;82:1582–1585. doi: 10.1002/jmv.21849. [DOI] [PubMed] [Google Scholar]
- Lapresle J, Lasjaunias P. Cranial nerve ischaemic arterial syndromes. Brain. 1986;109:207–215. doi: 10.1093/brain/109.1.207. [DOI] [PubMed] [Google Scholar]
- Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;66:149–173. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- Levin MJ, Barber D, Goldblatt E, et al. Use of a live attenuated varicella vaccine to boost varicella-specific immune responses in seropositive people 55 years of age and older: duration of booster effect. J Infect Dis. 1998;178(Suppl 1):S109–S112. doi: 10.1086/514264. [DOI] [PubMed] [Google Scholar]
- Lewis GW. Zoster sine herpete. Br Med J. 1958;2:418–421. doi: 10.1136/bmj.2.5093.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JM, Nagae Y, Tano Y. Progressive outer retinal necrosis after bone marrow transplantation. Am J Ophthalmol. 1996;122:892–895. doi: 10.1016/s0002-9394(14)70391-5. [DOI] [PubMed] [Google Scholar]
- Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–797. doi: 10.1212/WNL.0b013e3181d31e5c. [DOI] [PubMed] [Google Scholar]
- Lu PJ, Euler GL, Harpaz R. Herpes zoster vaccination among adults aged 60 years and older in the U.S., 2008. Am J Prev Med. 2011;40:e1–e6. doi: 10.1016/j.amepre.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Wolf W, et al. Latent varicella-zoster viral DNA in human trigeminal and thoracic ganglia. N Engl J Med. 1990;323:627–631. doi: 10.1056/NEJM199009063231002. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Lederer D, et al. Quantitation of latent varicella-zoster virus DNA in human trigeminal ganglia by polymerase chain reaction. J Virol. 1993;67:2381–2384. doi: 10.1128/jvi.67.4.2381-2384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Brucklier J, et al. Persistence of varicella-zoster virus DNA in elderly patients with postherpetic neuralgia. J Neurovirol. 1995;1:130–133. doi: 10.3109/13550289509111018. [DOI] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Cohrs R, et al. Expression of protein encoded by varicella-zoster virus open reading frame 63 in latently infected human ganglionic neurons. Proc Natl Acad Sci U S A. 1996;93:2122–2124. doi: 10.1073/pnas.93.5.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahalingam R, Wellish M, Soike K, et al. Simian varicella virus infects ganglia before rash in experimentally infected monkeys. Virology. 2001;279:339–342. doi: 10.1006/viro.2000.0700. [DOI] [PubMed] [Google Scholar]
- Manz HJ, Canter HG, Melton J. Trigeminal herpes zoster causing mandibular osteonecrosis and spontaneous tooth exfoliation. South Med J. 1986;79:1026–1028. doi: 10.1097/00007611-198608000-00028. [DOI] [PubMed] [Google Scholar]
- Mayberg MR, Langer RS, Zervas NT, et al. Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science. 1981;213:228–230. doi: 10.1126/science.6166046. [DOI] [PubMed] [Google Scholar]
- Mayberg MR, Zervas NT, Moskowitz MA. Trigeminal projections to supratentorial pial and dural blood vessels in cats demonstrated by horseradish peroxidase histochemistry. J Comp Neurol. 1984;223:46–56. doi: 10.1002/cne.902230105. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Cohrs RJ, Forghani B, et al. Stress-induced subclinical reactivation of varicella zoster virus in astronauts. J Med Virol. 2004;72:174–179. doi: 10.1002/jmv.10555. [DOI] [PubMed] [Google Scholar]
- Mehta SK, Tyring SK, Gilden DH, et al. Varicella-zoster virus in the saliva of patients with herpes zoster. J Infect Dis. 2008;197:654–657. doi: 10.1086/527420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menerath JM, Gerard M, Laurichesse H, et al. Bilateral acute retinal necrosis in a patient with acquired immunodeficiency syndrome. J Fr Ophtalmol. 1995;18:625–633. [PubMed] [Google Scholar]
- Miller DH, Kay R, Schon F, et al. Optic neuritis following chickenpox in adults. J Neurol. 1986;233:182–184. doi: 10.1007/BF00314431. [DOI] [PubMed] [Google Scholar]
- Moorthy RS, Weinberg DV, Teich SA, et al. Management of varicella zoster virus retinitis in AIDS. Br J Ophthalmol. 1997;81:189–194. doi: 10.1136/bjo.81.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Nathwani D. Facial palsy and infection: the unfolding story. Clin Infect Dis. 1992;14:263–271. doi: 10.1093/clinids/14.1.263. [DOI] [PubMed] [Google Scholar]
- Moses H, Nagel MA, Gilden DH. Acute cerebellar ataxia in a 41 year old woman. Lancet Neurol. 2006;5:984–988. doi: 10.1016/S1474-4422(06)70601-9. [DOI] [PubMed] [Google Scholar]
- Mueller NH, Graf LL, Orlicky D, et al. Phosphorylation of the nuclear form of varicella-zoster virus immediate-early protein 63 by casein kinase II at serine 186. J Virol. 2009;83:12094–12100. doi: 10.1128/JVI.01526-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir WB, Nichols R, Breuer J. Phylogenetic analysis of varicella-zoster virus: evidence of intercontinental spread of genotypes and recombination. J Virol. 2002;76:1971–1979. doi: 10.1128/JVI.76.4.1971-1979.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S, Mizobuchi M, Nakashiro Y, et al. Bell palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscle. Ann Intern Med. 1996;124:27–30. doi: 10.7326/0003-4819-124-1_part_1-199601010-00005. [DOI] [PubMed] [Google Scholar]
- Murakami S, Honda N, Mizobuchi M, et al. Rapid diagnosis of varicella zoster virus infection in acute facial palsy. Neurology. 1998;51:1202–1205. doi: 10.1212/wnl.51.4.1202. [DOI] [PubMed] [Google Scholar]
- Murata K, Miwa H, Kondo T. Polyneuritis cranialis caused by varicella zoster virus in the absence of rash. Neurology. 2010;74:85–86. doi: 10.1212/WNL.0b013e3181c7da35. [DOI] [PubMed] [Google Scholar]
- Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–1073. doi: 10.1212/01.wnl.0000258549.13334.16. [DOI] [PubMed] [Google Scholar]
- Nagel MA, Cohrs RJ, Mahalingam R, et al. The varicella zoster virus vasculopathies: clinical, CSF, imaging, and virologic features. Neurology. 2008;70:853–860. doi: 10.1212/01.wnl.0000304747.38502.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Gilden D, Shade T, et al. Rapid and sensitive detection of 68 unique varicella zoster virus gene transcripts in five multiplex reverse transcription-polymerase chain reactions. J Virol Methods. 2009;157:62–68. doi: 10.1016/j.jviromet.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Choe A, Traktinskiy I, et al. Varicella-zoster virus transcriptome in latently infected human ganglia. J Virol. 2011a;85:2276–2287. doi: 10.1128/JVI.01862-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Traktinskiy I, Azarkh Y, et al. Varicella zoster virus vasculopathy: analysis of virus-infected arteries. Neurology. 2011b;77:364–370. doi: 10.1212/WNL.0b013e3182267bfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel MA, Choe A, Cohrs RJ, et al. Persistence of VZV DNA in saliva after herpes zoster. J Infect Dis. 2011c;204:820–824. doi: 10.1093/infdis/jir425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto BK, Dorotheo EU, et al. Progressive outer retinal necrosis presenting with isolated optic neuropathy. Neurology. 2004;63:2423–2425. doi: 10.1212/01.wnl.0000147263.89255.b8. [DOI] [PubMed] [Google Scholar]
- Noda Y, Nakazawa M, Takahashi D, et al. Retinal periphlebitis as zoster sine herpete. Arch Ophthalmol. 2001;119:1550–1552. [PubMed] [Google Scholar]
- Orme HT, Smith G, Nagel MA, et al. VZV spinal cord infarction identified by diffusion-weighted magnetic resonance imaging (DWI) Neurology. 2007;69:398–400. doi: 10.1212/01.wnl.0000266390.27177.7b. [DOI] [PubMed] [Google Scholar]
- Osaki Y, Matsubayashi K, Okumiya K, et al. Polyneuritis cranialis due to varicella-zoster virus in the absence of rash. Neurology. 1995;45:2293–2294. doi: 10.1212/wnl.45.12.2293. [DOI] [PubMed] [Google Scholar]
- Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- Patterson-Bartlett J, Levin MJ, Lang N, et al. Phenotypic and functional characterization of ex vivo T cell responses to the live attenuated herpes zoster vaccine. Vaccine. 2007;25:7087–7093. doi: 10.1016/j.vaccine.2007.07.051. [DOI] [PubMed] [Google Scholar]
- Payten RJ, Dawes JDK. Herpes zoster of the head and neck. J Laryngol Otol. 1972;86:1031–1055. doi: 10.1017/s0022215100076179. [DOI] [PubMed] [Google Scholar]
- Pevenstein SR, Williams RK, McChesney D, et al. Quantitation of latent varicella-zoster virus and herpes simplex virus genomes in human trigeminal ganglia. J Virol. 1999;73:10514–10518. doi: 10.1128/jvi.73.12.10514-10518.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierson DL, Mehta SK, Gilden D, et al. Varicella zoster virus DNA at inoculation sites and in saliva after Zostavax immunization. J Infect Dis. 2011;203:1542–1545. doi: 10.1093/infdis/jir139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina MA, Ara JR, Capablo JL, et al. Myelitis and optic neuritis caused by varicella. Rev Neurol. 1997;25:1575–1576. [PubMed] [Google Scholar]
- Preston VG, Kennard J, Rixon FJ, et al. Efficient herpes simplex virus type 1 (HSV-1) capsid formation directed by the varicella-zoster virus scaffolding protein requires the carboxy-terminal sequences from the HSV-1 homologue. J Gen Virol. 1997;78:1633–1646. doi: 10.1099/0022-1317-78-7-1633. [DOI] [PubMed] [Google Scholar]
- Ragozzino MW, Melton LJ, III, Kurland LT, et al. Population-based study of herpes zoster and its sequelae. Medicine. 1982;61:310–316. doi: 10.1097/00005792-198209000-00003. [DOI] [PubMed] [Google Scholar]
- Ratzka P, Schlachetzki JC, Ba¨hr M, et al. Varicella zoster virus cerebellitis in a 66-year-old patient without herpes zoster. Lancet. 2006;367:182. doi: 10.1016/S0140-6736(06)67967-1. [DOI] [PubMed] [Google Scholar]
- Redman RL, Nader S, Zerboni L, et al. Early reconstitution of immunity and decreased severity of herpes zoster in bone marrow transplant recipients immunized with inactivated varicella vaccine. J Infect Dis. 1997;176:578–585. doi: 10.1086/514077. [DOI] [PubMed] [Google Scholar]
- Reiber H, Lange P. Quantitation of virus-specific antibodies in cerebrospinal fluid and serum: sensitive and specific detection of antibody synthesis in brain. Clin Chem. 1991;37:1153–1160. [PubMed] [Google Scholar]
- Robillard RB, Hilsinger RL, Jr, Adour KK. Ramsay Hunt facial paralysis: clinical analyses of 185 patients. Otolaryngol Head Neck Surg. 1986;95:292–297. doi: 10.1177/01945998860953P105. [DOI] [PubMed] [Google Scholar]
- Rogers RS, III, Tindall JP. Geriatric herpes zoster. J Am Geriatr Soc. 1971;19:495–503. doi: 10.1111/j.1532-5415.1971.tb01208.x. [DOI] [PubMed] [Google Scholar]
- Roizman B, Knipe DM. Herpes simplex viruses and their replication. In: Knipe DM, Howley PM, Griffin DE, et al., editors. Fields virology. 4th. Vol. 2. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. pp. 2399–2459. [Google Scholar]
- Ross J, Williams M, Cohen JI. Disruption of the varicella-zoster virus dUTPase and the adjacent ORF9A gene results in impaired growth and reduced syncytia formation in vitro. Virology. 1997;234:186–195. doi: 10.1006/viro.1997.8652. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Manville NS, Ren J. Pilot tolerability and effectiveness study of levetiracetam for postherpetic neuralgia. Neurology. 2003a;61:866–867. doi: 10.1212/01.wnl.0000079463.16377.07. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC, Twilling L, Davies PS, et al. Oral opioid therapy for chronic peripheral and central neuropathic pain. N Engl J Med. 2003b;348:1223–1232. doi: 10.1056/NEJMoa021420. [DOI] [PubMed] [Google Scholar]
- Salazar R, Russman AN, Nagel MA, et al. VZV ischemic optic neuropathy and subclinical temporal artery involvement. Arch Neurol. 2011;68:517–520. doi: 10.1001/archneurol.2011.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvoerer E, Frechin V, Warter A, et al. Persistent multiple pulmonary nodules in a nonimmunocompromised woman after varicella-related myelitis treated with acyclovir. J Clin Microbiol. 2003;41:4904–4905. doi: 10.1128/JCM.41.10.4904-4905.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbst RG, Selhorst JB, Harbison JW, et al. Parainfectious optic neuritis report and review following varicella. Arch Neurol. 1983;40:347–350. doi: 10.1001/archneur.1983.04050060047007. [DOI] [PubMed] [Google Scholar]
- Silverstein BE, Chandler D, Neger R, et al. Disciform keratitis: a case of herpes zoster sine herpete. Am J Ophthalmol. 1997;123:254–255. doi: 10.1016/s0002-9394(14)71044-x. [DOI] [PubMed] [Google Scholar]
- Smith FP. Pathological studies of spinal nerve ganglia in relation to intractable intercostal pain. Surg Neurol. 1978;10:50–53. [PubMed] [Google Scholar]
- Steain M, Gowrishankar K, Rodriguez M, et al. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella zoster virus infection. J Virol. 2010;85:626–631. doi: 10.1128/JVI.01816-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen R, Selby G. ‘Atypical’ Ramsay Hunt syndrome. Med J Aust. 1972;1:227–230. doi: 10.5694/j.1326-5377.1972.tb46771.x. [DOI] [PubMed] [Google Scholar]
- Stowasser M, Cameron J, Oliver WA. Diaphragmatic paralysis following cervical herpes zoster. Med J Aust. 1990;153:555–556. doi: 10.5694/j.1326-5377.1990.tb126199.x. [DOI] [PubMed] [Google Scholar]
- Straus SE, Hay J, Smith H, et al. Genome differences among varicella-zoster virus isolates. J Gen Virol. 1983;64:1031–1041. doi: 10.1099/0022-1317-64-5-1031. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Ikuno Y, Otsuka T, et al. Development of a live attenuated varicella vaccine. Biken J. 1975;18:25–33. [PubMed] [Google Scholar]
- Terada K, Niizuma T, Kawano S, et al. Detection of varicella-zoster virus DNA in peripheral mononuclear cells from patients with Ramsay Hunt syndrome or zoster sine herpete. J Med Virol. 1998;56:359–363. [PubMed] [Google Scholar]
- Terasaki S, Kameyama T, Yamamoto S. A case of zoster in the 2nd and 3rd branches of the trigeminal nerve associated with simultaneous herpes labialis infection – a case report. Kurume Med J. 1997;44:61–66. doi: 10.2739/kurumemedj.44.61. [DOI] [PubMed] [Google Scholar]
- Theil D, Horn AK, Derfuss T, et al. Prevalence and distribution of HSV-1, VZV, and HHV-6 in human cranial nerve nuclei III, IV, VI, VII, and XII. J Med Virol. 2004;74:102–106. doi: 10.1002/jmv.20152. [DOI] [PubMed] [Google Scholar]
- Thomas EJ, Howard FM., Jr Segmental zoster paresis – a disease profile. Neurology. 1972;22:459–466. doi: 10.1212/wnl.22.5.459. [DOI] [PubMed] [Google Scholar]
- Umehara T, Sengoku R, Mitsumura H, et al. Findings of segmental zoster paresis on MRI. J Neurol Neurosurg Psychiatry. 2011;82:694. doi: 10.1136/jnnp.2010.235127. [DOI] [PubMed] [Google Scholar]
- Vafai A, Wellish M, Gilden DH. Expression of varicella-zoster virus in blood mononuclear cells of patients with postherpetic neuralgia. Proc Natl Acad Sci U S A. 1988;85:2767–2770. doi: 10.1073/pnas.85.8.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verjans GM, Hintzen RQ, van Dun JM, et al. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A. 2007;104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CPN, Deck JH, Morshead C, et al. Postherpetic neuralgia: further post-mortem studies of cases with and without pain. Pain. 1991;44:105–117. doi: 10.1016/0304-3959(91)90124-G. [DOI] [PubMed] [Google Scholar]
- Weinberg A, Zhang JH, Oxman MN, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009;200:1068–1077. doi: 10.1086/605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Atsuta N, Tokunaga S, et al. Ipsilateral truncal sensory deficit in a patient with ophthalmic zoster sine herpete. Neurology. 2003;60:1049–1050. doi: 10.1212/01.wnl.0000052999.43687.7b. [DOI] [PubMed] [Google Scholar]
- Yamakawa K, Hamada M, Takeda T. Different real-time PCR assays could lead to a different result of detection of varicella-zoster virus in facial palsy. J Virol Methods. 2007;139:227–229. doi: 10.1016/j.jviromet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Tada R, Shimomura Y, et al. Detecting varicella-zoster virus DNA in iridocyclitis using polymerase chain reaction: a case of zoster sine herpete. Arch Ophthalmol. 1995;113:1358–1359. doi: 10.1001/archopht.1995.01100110018009. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Saddier P, Wollan PC, et al. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007;82:1341–1349. doi: 10.4065/82.11.1341. [DOI] [PubMed] [Google Scholar]
- Yawn BP, Itzler RF, Wollan PC, et al. Health care utilization and cost burden of herpes zoster in a community population. Mayo Clin Proc. 2009;84:787–794. doi: 10.4065/84.9.787. [DOI] [PMC free article] [PubMed] [Google Scholar]


