Abstract
Objective
The relationship between cannabis use and the onset of psychosis is well-established. Aberrant salience processing is widely thought to underpin many of these symptoms. Literature explicitly investigating the relationship between aberrant salience processing and cannabis use is scarce; with those few studies finding that acute THC administration (the main psychoactive component of cannabis) can result in abnormal salience processing in healthy cohorts, mirroring that observed in psychosis. Nevertheless, the extent of and mechanisms through which cannabis has a modulatory effect on aberrant salience, following both acute and chronic use, remain unclear.
Methods
Here, we systematically review recent findings on the effects of cannabis use – either through acute THC administration or in chronic users – on brain regions associated with salience processing (through functional MRI data); and performance in cognitive tasks that could be used as either direct or indirect measures of salience processing. We identified 13 studies either directly or indirectly exploring salience processing. Three types of salience were identified and discussed – incentive/motivational, emotional/affective, and attentional salience.
Results
The results demonstrated an impairment of immediate salience processing, following acute THC administration. Amongst the long-term cannabis users, normal salience performance appeared to be underpinned by abnormal neural processes.
Conclusions
Overall, the lack of research specifically exploring the effects of cannabis use on salience processing, weaken any conclusions drawn. Additional research explicitly focused on salience processing and cannabis use is required to advance our understanding of the neurocognitive mechanisms underlying the association between cannabis use and development of psychosis.
Keywords: Marijuana smoking, Cannabis, Psychotic disorders, Cognition disorders, Functional neuroimaging
Introduction
The endocannabinoid system and cognitive dysfunction
Though cannabis is one of the most widely used illicit drugs in the western world (Burns, 2013), its use is also recognised as one of the most preventable risk factors for onset, and relapse, of psychotic disorders (Di Forti et al., 2015, Moore et al., 2007, Schoeler et al., 2016a, Patel et al., 2016, Schoeler et al., 2016b, Schoeler et al., 2016c). The relationship between cannabis use and schizophrenia symptomology – for example, disturbances in behaviours, thoughts, and perceptions – is widely accepted. However, the neural mechanisms underlying this relationship in schizophrenia, and the relationship between cannabis use and psychotic like symptoms and cognitive dysfunction in healthy populations, remain unclear.
Studies show that the effects of delta-9-tetrahydrocannabinol (THC), the main psychoactive ingredient in cannabis, are mediated through the central cannabinoid (CB1) receptors in the brain (Pamplona and Takahashi, 2012), which are components of what is known as the "endocannabinoid system" (Mechoulam and Parker, 2013, Pamplona and Takahashi, 2012) . The endocannabinoid system is comprised of endogenous cannabinoid receptors, their ligands (known as endocannabinoids), and enzymes, involved in the degradation of these ligands. Its primary functions include maintaining homeostasis in the central nervous system, cognition and memory processes, and control of motor function and signs of analgesia, through reactions on the CB1 receptors, and modulation of immune responses, through the activation of CB2 receptors, expressed largely throughout the peripheral nervous system, and to a lesser extent in the central nervous system (CNS) and other tissues (Mechoulam and Parker, 2013, Pamplona and Takahashi, 2012). Mounting evidence, reviewed in Appiah-Kusi et al., shows this system to be dysregulated in schizophrenia – for example, abnormal levels of cannabinoid receptors and endocannabinoids both in vivo and post-mortem (Leweke et al., 1999, Appiah-Kusi et al., 2016); thus highlighting the importance of the endocannabinoid system as a potential target for schizophrenia treatment – and, more immediately, the importance of further elucidating the role of the eCB system in the symptomology of schizophrenia (Bossong et al., 2014b).
Cannabis and symptoms of schizophrenia
Both positive and negative symptoms of schizophrenia can be induced by cannabis intake (Stefanis et al., 2004, D'Souza et al., 2004, Bhattacharyya et al., 2012, Bhattacharyya et al., 2009, Bhattacharyya et al., 2015a), with these induced psychotic effects being linked to modulation of prefrontal, medial temporal, and striatal functioning (Bhattacharyya et al., 2012). There is also evidence that a range of cognitive deficits observed in schizophrenia can be induced in healthy populations by acute THC administration – aberrant salience processing being of greatest interest here (Bhattacharyya et al., 2012, Bhattacharyya et al., 2015b). Specifically, abnormal salience processing and attribution is widely thought to underlie much of the psychotic symptoms observed in schizophrenia (Kapur, 2003), and is thus of particular interest in relation to research exploring the psychosis like effects of cannabis.
Salience attribution and different types of saliencies
In salience attribution literature, a stimulus that is more prominent or noticeable than others around it is said to be salient; with the appropriate detection of this saliency playing a key role in the allocation of cognitive resources, such as attention. For this review, we focused on the most prominent categories of saliency, described as follows. Incentive/motivational salience (Charboneau et al., 2013, Filbey et al., 2009, Wolfling et al., 2008) refers to the 'wanting' individuals feel when confronted with reward-related stimuli. Incentive or motivational salience is influenced by inputs like learning and memory, but also by neurobiological factors, such as dopamine expression, and states such as drug and appetite states (as demonstrated by the cannabis cue tasks) (Berridge, 2012). Emotional/affective salience (Metrik et al., 2015, Ballard et al., 2012, Phan et al., 2008, Somaini et al., 2012) is closely related to incentive salience, and assigns importance to a stimulus based on the appraisal of potential pleasant or unpleasant outcomes (as demonstrated in the emotional evaluation tasks) (Niu et al., 2012). Attentional salience (Bhattacharyya et al., 2012, Hooker and Jones, 1987, Grant et al., 2012, Gruber and Yurgelun-Todd, 2005, Kober et al., 2014, Eldreth et al., 2004) assigns attentional resources to a stimulus or an environmental event, in some cases interrupting ongoing processes, or selectively focuses on an event while filtering out other stimuli (as seen in the Stroop test), resulting in attention and the associated cognitive resources being directed to the salient event (Shinn-Cunningham, 2008, Bowman et al., 2011). All three aspects of salience are closely related, and through a combined effort they enable the processing and assignment of appropriate cognitive resources to salient and non-salient stimuli in everyday life.
The aberrant salience hypothesis of schizophrenia
The aberrant salience hypothesis of schizophrenia posits that dysregulated dopamine levels in the dopamine receptor rich salience network result in the inappropriate processing of external and internal stimuli, which in turn induces psychotic symptoms through the aberrant attribution of salience to everyday experiences and stimuli (Kapur, 2003). Regions of the brain involved in salience attribution are implicated in a range of social cognition processes, including empathy for pain (Singer et al., 2004), emotional and attentional dimensions of pain (Peyron et al., 2000), hunger, metabolic status, pleasurable touch (Craig, 2002), identifying faces of loved ones or allies (Bartels and Zeki, 2004), and social rejection (Eisenberger et al., 2003). Given the range of cognitive processes that also involve brain regions implicated in salience processing, it follows that the extent of aberrant attribution of salience to everyday experiences and stimuli in those with psychosis is also thought to be closely correlated with severity of psychotic symptoms (Jensen and Kapur, 2009).
Brain regions involved in salience processing
Prior studies describe a salience network consisting primarily of several regions in the brain. Core nodes of the salience network include the dorsal anterior cingulate cortex (dACC), and the anterior insula (AI) – thought to be involved in the integration of external sensory input, and interoceptive autonomic processing; though its broader functions also rely on the amygdala, ventral striatum, and the substantia nigra/ventral tegmental area – central components of the reward pathway neuro-circuitry (Wylie and Tregellas, 2010, Menon, 2015). These regions are often co-activated alongside the lateral prefrontal cortex and parietal regions, and are thus likely also involved in attention, working memory, and response selection (Seeley et al., 2007). These regions also express high levels of both dopamine receptors – as reviewed by Winton-Brown et al. (2014) in the context of the aberrant salience hypothesis of schizophrenia (Winton-Brown et al., 2014) – and endogenous CB1 receptors, to which THC binds – with a simultaneous high expression of both being found in the dorsal and ventral striatum, and the substantia nigra (Pertwee, 2008, Beaulieu and Gainetdinov, 2011).
Functional abnormalities of the salience network in psychosis
Reduced functional connectivity of brain regions including the striatum, cingulate cortex, and insula are said to relate to aberrant salience processing in psychosis (White et al., 2010). It is thought that this functional dysconnectivity may disrupt appropriate switching between contextually relevant functional brain states (Menon, 2011, Manoliu et al., 2014), leading to further cognitive impairment. At the neurochemical level, abnormalities within the dopaminergic system, which are characteristic of schizophrenia, are thought to contribute to the aberrant salience processing within the disorder (Kapur, 2003). Increased striatal dopamine levels during aberrant salience processing have been recorded in people with psychotic illnesses, and in healthy individuals following THC challenge, during aberrant salience processing (Winton-Brown et al., 2014, Bossong et al., 2009). Experimental analyses by Boehme et al. (2015) also found aberrant salience processing to be positively correlated with striatal dopamine levels in healthy individuals, with no intervention (Boehme et al., 2015). However, direct experimental evidence of any relationships between the three is still needed.
Cannabis and salience
The extent and mechanisms underlying the modulatory effect of cannabis on aberrant salience in those with psychotic illness, and following acute and chronic use in healthy individuals, remain unclear. As mentioned previously, acute THC administration in healthy samples can induce transient psychosis like symptoms, including impaired salience processing (Bhattacharyya et al., 2012). Other studies show a reduced stress response to environmental stimuli resulting from cannabinoid ingestion – an effect also potentially related to impaired reward processing, and cannabis abuse (Gardner and Vorel, 1998, Ballard et al., 2012). Indeed, THC has been shown to increase dopamine release in the nucleus accumbens and ventral tegmental area in both rats and humans, which may also contribute to abnormalities in salience processing (Chen et al., 1990, Bossong et al., 2009), though human evidence regarding the effects of THC/cannabis use on the dopaminergic system is equivocal (Sami et al., 2015).
Aims of the Study
The aim of this article was to systematically review current findings on the effects of acute and chronic cannabis use/THC on salience processing. The studies reviewed included both behavioural and imaging research in humans, and also included both observational and interventional methodologies. By consolidating current literature on the topic, we hope to bring clarity and insight to the role of the endocannabinoid system in the pathophysiology of aberrant salience processing, and schizophrenia.
Methods
In accordance with the evidence-based principles of the PICO structure (Boudin, 2010), the review question employed was: “Is there a relationship between cannabis or its main psychoactive ingredient, Δ9-THC, and salience processing?”
Search Strategy and Selection Criteria
Following recommended guidelines for systematic reviews (Higgins and Green, 2008), a systematic search strategy was used to identify all relevant studies. Searches were conducted in the Medline, EMBASE, and PsychInfo databases; employing the OvidSP platform; and using the following combination of Boolean and MeSH search terms (where available) describing cannabis use or cannabinoids, and salience processing: (cannab* OR hashish OR marij* OR marih* OR tetrahydrocannabinol OR THC OR cannabidiol OR CBD) AND (salience OR salient) were used. As there are currently no specific MeSH terms referring to salience processing, the standard PubMed author keyword of “salience” and its variant “salient” were used. Further searches using the same search terms were undertaken on the Cochrane Clinical Trials Database. All human studies published in English up to and including the search date of November 20th 2015, and indexed in the above databases, were assessed. A further search of PubMed on July 7th 2016, using the same search terms as before, identified no additional studies. All identified relevant studies, reviews, and conference abstracts were hand-searched for any additional relevant publications. Inclusion criteria for the studies required that they were: (1) studies of healthy humans; (2) investigating the acute or long-term effects of cannabis use, or effects of acute administration of THC; (3) measuring salience processing; (4) MR imaging studies. Exclusion criteria were: (1) studies where cannabis/THC exposure was not investigated; (2) studies where effect on salience of stimuli/salience processing was not reported; (3) studies including any clinical participant groups (i.e. any patient groups); (4) animal studies.
Study Selection
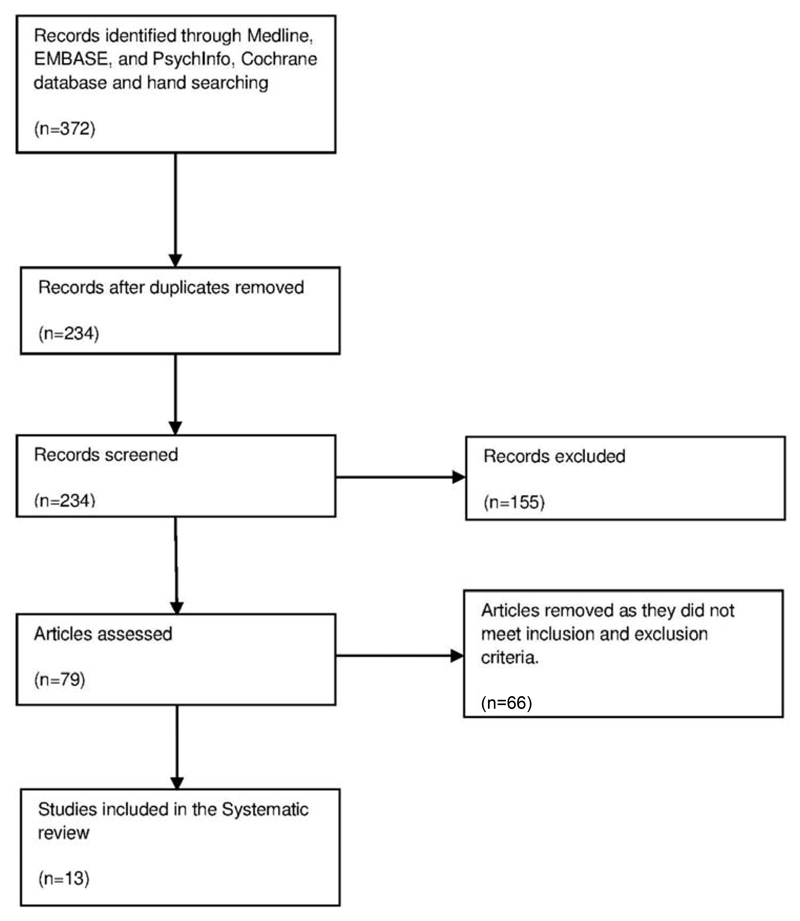
Initially, 372 studies were identified for review. These were primarily screened by one reviewer, assisted by a second reviewer. Any disagreements between the reviewers were resolved through discussion, or with a third reviewer (Dr Sagnik Bhattacharyya). After excluding irrelevant or duplicate studies and those not satisfying our inclusion criteria, a total of 13 studies were included in the final review (Figure 1).
Figure 1.
Flow-chart describing the number of studies excluded at each stage of the study selection process.
Of the 13 studies included, five reported only behavioural effects, and the remaining eight reported neuroimaging (functional MRI; fMRI) results, in addition to the results of the behavioural tasks. Five (three behavioural, and two functional and behavioural) of the 13 studies reported the effects of acute administration of THC in individuals who were occasional cannabis users or minimally exposed to the drug; whereas the remaining eight studies investigated the long-term effects of cannabis use in individuals with varying duration of exposure to cannabis (two behavioural, and six functional and behavioural) (see Table 1).
Table 1.
Summary of the studies and findings included in the review.
| Author | Participants | Study design | Task | Main measures | Main findings of target group vs. control | |
|---|---|---|---|---|---|---|
| Acute THC Challenge | Metrik et al. (2015) 1 | Regular cannabis users 15hrs cannabis abstinence. N=89 (mostly male) |
Within subject, repeat measures, placebo controlled. Dose: 2.7-3% THC cigarette. Tasks: 17min, and 30min post THC. |
Pleasantness rating of affective and cannabis related stimuli, and emotional Stroop task | Behavioural (affective salience) |
- Increased response latency specifically to negative and cannabis related stimuli - Generally, no change in processing of emotional stimuli - Except in dependent subgroup who showed attentional bias to positive word stimuli |
| Ballard et al. (2012) 1 | Healthy occasional cannabis users. N=25 (male/female) |
Within subject, repeat measures, placebo controlled. Dose: 7.5mg, and 15mg THC capsules. Tasks: 90mins, and 120mins post THC. |
Evaluation of emotional images | Behavioural (affective salience) |
- Impaired recognition of facial fear and anger - Recognition of happy/sad faces was unchanged - Neutral images were rated as slightly more negative in the lower THC dose condition |
|
| Bhattacharyya et al. (2012) 2 | Healthy, minimal previous cannabis use. N=15 (male) |
Within subject, repeat measures, placebo controlled. Dose: 10mg THC capsule. Tasks: 60-120mins post THC. |
Visual oddball detection paradigm | Behavioural + Functional activation (attentional salience) |
- Reduced response latency to standard relative to oddball stimuli - Increased PFC activity - Decreased activity in right caudate - Effects in right caudate neg. correlated with severity of induced symptoms |
|
| Phan et al. (2008) 1 | Recreational users 24hrs cannabis abstinence. N=16 (male) |
Within subject, repeat measures, placebo controlled. Dose: 7.5mg THC capsule. Tasks: approx. 120mins post THC. |
Emotional face processing task | Behavioural + Functional activation (affective salience) |
- Responses were more accurate and faster for the non-threat vs. threat stimuli, after THC - THC significantly reduced amygdala activation in response to threatening faces - No subjective effect on anxiety |
|
| Hooker et al. (1987) 2 | Healthy, occasional cannabis users. N=12 (male) |
Within subject, repeat measures, placebo controlled. Dose: 10.7mg THC. Tasks: 15mins post THC. |
Stroop Colour and Word Test, and Randt Memory Battery and the Controlled Oral Word Association Test | Behavioural (attentional salience) |
- Significantly increased Stroop interference effect - Increased memory recall intrusions - Immediate and sustained attention and controlled retrieval from semantic memory unaffected |
|
| Effects of long term cannabis use | Charboneau et al. (2013) 3 | Non treatment seeking, cannabis dependent users. 8hr cannabis abstinence. N=16 (mostly female) |
Within subject, repeat measures. No THC administered. |
Cannabis cue exposure | Behavioural + Functional activation (motivational salience) |
- Increased activity in inferior OFC/PCC/parahippocampal gyrus/hippocampus/amygdala/superior temporal pole/occipital cortex - Craving scores correlated with activation only in the 1st fMRI run - Exposure to cannabis cues increased craving |
| Somaini et al. (2012) 1 | Cannabis dependent active users (N=14) vs. abstinent cannabis users (N=14); and non-users (N=14) (mostly male) |
Between groups. No THC administered. |
Evaluation of emotional responses to neutral and unpleasant images | Behavioural + Functional activation (affective salience) |
- Active users displayed increased pleasantness and lower arousal ratings in response to the emotional task - Hyperactivity of the HPA axis, especially amongst active users, and impaired hormonal reaction to negative emotions |
|
| Filbey et al. (2009) 3 | Regular cannabis users. 72hrs cannabis abstinence. N=38 (mostly male) |
Within subject – no control sample. No THC administered. 72hrs between testing. |
Tactile cannabis cue exposure | Behavioural + Functional activation (motivational salience) |
- Increased activity in reward pathway - OFC and NAc activation pos. correlated with volume of problems related to cannabis use |
|
| Grant et al. (2012) 1 | Non treatment seeking users (N=16) vs. non-users (N=214) (male/female) |
Between groups. No THC administered. |
Rapid Visual Information Processing task (as part of CANTAB battery) | Behavioural (attentional salience) |
- No significant group differences in target detection or false alarms | |
| Kober et al. (2014) 2 | Treatment seeking cannabis dependent users (N=20) vs. non-users controls (N=20). (male) | Between groups, cannabis users received intervention; Follow up assessments with cannabis users only. No THC administered. |
Cognitive control (Stroop) task | Behavioural + Functional activation (attentional salience) |
- No differences in Stroop response times - Reduced cannabis related activity in prefrontal regions, and ventral and dorsal striatum (caudate), amygdala/parahippocampal gyrus, thalamus, and midbrain regions - Greater dACC activity was associated with less cannabis use during treatment - Greater activity in ventral striatum associated with less cannabis use at 1 yr follow up |
|
| Wölfling et al. (2008) 3 | Chronic heavy cannabis dependent users (N=15) 12hrs cannabis abstinence vs. healthy non-users (N=15) (male/female) |
Between groups. No THC administered. |
Exposure to cannabis related images | Behavioural (motivational salience) |
- Cannabis stimuli perceived as more arousing and pleasant - Increased cannabis craving in relation to cannabis stimulus exposure - Increased physiological arousal in relation to cannabis stimulus exposure |
|
| Gruber et al. (2005) 2 | Heavy cannabis users (N=10) vs. non-users (N=10). (mostly male) |
Between groups. No THC administered. |
Cognitive control (Stroop) task | Behavioural + Functional activation (attentional salience) |
- No significant between groups differences in Stroop performance - Lower ACC activity in cannabis users - Increased mid cingulate activity in cannabis users - Abnormal bilateral activation of the DLPFC in cannabis users |
|
| Eldreth et al. (2004) 2 | Heavy cannabis users (N=11) 25d cannabis abstinence vs. non-users. (male) | Between groups. No THC administered. |
Cognitive control (Stroop) task | Behavioural + Functional activation (attentional salience) |
- No cannabis related deficits in Stroop performance - Hypo-activity in the left perigenual ACC and left LPFC of cannabis users - Hyperactivity in bilateral hippocampus of cannabis users |
Summarizing the findings of the studies included in the review. PFC = prefrontal cortex, OFC = orbitofrontal cortex, PCC = posterior cingulate cortex, HPA axis = hypothalamic-pituitary-adrenal axis, NAc = nucleus accumbens, dACC = dorsal anterior cingulate cortex. 1= affective salience, 2 = attentional salience, 3 = motivational salience.
As the results of our search for relevant research shows, few studies have directly investigated the effects of acute/chronic cannabis use on salience processing. Consequently, not all the studies included in the review assessed these effects specifically. As the salience network is not task specific, it can be assessed indirectly by measuring a range of cognitive processes – including pain, attention, working memory, uncertainty, and other homeostatic changes. Therefore, the outcomes of specific tasks used in these studies, though not specific to salience, were used to infer various aspects of salience, via related cognitive processes. The findings, and also the limitations of using such an approach, will be discussed in detail here.
Data Extraction
For each selected study, demographic and methodological variables and outcome data were extracted, and conflicts resolved, in the same manner as the study screening. As the review included a small number of studies, data was not coded, and instead was summarized and included in the attached tables. Given the potential breadth, and yet, lack of definition in current salience processing research, the data extraction outcome was not strictly decided in advance. The primary outcomes of interest that were established in advance were the effects of cannabis on different measures that could be related to attentional, affective, or motivational salience. In the acute experimental studies, the effects of cannabis or THC were compared with those of a placebo, using a repeated, within-subject design. Those studies examining the effects of long-term cannabis use involved either a non-cannabis using control group for comparison, or employed a within-subject design. The studies were first separated according to their assessment of either acute administration in non-users, or assessment of regular cannabis users; then further separated into studies investigating behavioural effects of cannabis use, and studies investigating both behavioural effects and functional neuroimaging findings related to cannabis use. None of the relevant studies identified involved structural MRI techniques.
Risk of bias
As the methodologies of the included studies were largely heterogeneous, a suitably flexible and inclusive approach to the risk of bias and quality assessment was needed. To ensure this, criteria for interventional and observational trials were adapted as appropriate from the Agency for Healthcare Research and Quality (AHRQ) guidelines for rating the strength of scientific evidence were used (West et al., 2002) (see Tables 3 and 4).
Table 3.
Methodological quality of studies of effect of acute THC challenge on salience processing
| Study | Defined Study Population | Random-isation | Blinding | Inter-vention | Adequate control | Outcome measurement | Statistical analysis | Drop-out rate specified | Excluded/adjust-ment for tobacco con-founding | Funding or Sponsor-ship |
|---|---|---|---|---|---|---|---|---|---|---|
| Metrik et al. (2015) | ✓ Healthy regular cannabis users |
✕ Not specified |
✓ Double |
✓ Marijuana cigarettes |
✓ Placebo cigarette |
✓ Emotional processing task |
✓ GEE analyses |
✓ 26/115 (22.6%) |
✕ Tobacco in cigarettes, tobacco smokers allowed smoke after CO test |
✓ Declared |
| Ballard et al. (2012) | ✓ Healthy occasional cannabis users |
✕ Not specified |
✓ Double |
✓ THC capsule |
✓ Placebo |
✓ Emotional processing task |
✓ Two-way repeated measures ANOVA |
✕ Not specified |
✕ Normal amounts of caffeine and nicotine consumed |
✓ Declared |
| Bhattacharyya et al. (2012) | ✓ Healthy volunteers, minimal cannabis use |
✕ Not specified |
✓ Double |
✓ THC capsule |
✓ Placebo |
✓ -Visual oddball task performance - Regional brain activity |
✓ Pairwise comp-arisons; repeated measures ANOVA |
✕ Not specified |
✕ Not specified |
✓ Declared |
| Phan et al. (2008) | ✓ Healthy occasional cannabis users |
✓ | ✓ Double |
✓ THC capsule |
✓ Placebo |
✓ - Emotional processing task - Regional brain activity |
✓ ANOVA |
✓ 2/16 (12.5) |
✕ Not specified, asked to abstain from all drugs 24h before, and 23h after session |
✓ Declared |
| Hooker et al. (1987) | ✓ Healthy volunteers, occasional cannabis use |
✕ Not specified |
✓ Double |
✓ THC cigarette |
✓ Placebo |
✓ Stroop, memory and attention task performance |
✓ Repeated measures ANOVA |
✕ Not specified |
✕ Not specified |
✕ Not specified |
Occasional use refers to exposure more the 10 x in their lives, but not using daily. Regular use refers to use at least 2 days a week in the past month, at least weekly the previous 6 months. Minimal use <15 times/lifetime. THC – tetrahydrocannabinol; ANOVA – Analysis of variance
Table 4.
Methodological quality of studies of effect of chronic cannabis use on salience processing
| Study | Defined study population | Comparability of subjects | Adequate exposure | Control | Outcome Measurement | Statistical analysis | Excluded/adjustment for tobacco confounding? | Funding or sponsorship |
|---|---|---|---|---|---|---|---|---|
| Charboneau et al. (2013) | ✓ Non-treatment seeking cannabis dependent users (8hrs abstinent) |
✓ Within subject |
✓ Met MINI criteria for cannabis dependence; positive urine cannabis screen |
✓ Within Subject |
✓ - Cannabis craving score - Regional brain activation |
✓ - Friedman test - Spearman correlation |
✕ Not specified |
✓ Declared |
| Somaini et al. (2012) | ✓ Cannabis dependent active users |
✓ | ✓ Met DSM IV criteria for cannabis dependence; urine screening at baseline, and twice weekly thereafter. |
✓ Abstinent cannabis users; non-users |
✓ - Evaluation of affective images - Regional brain activation |
✓ - One-way ANOVA |
✓ Participants abstained from tobacco for 12hrs before study |
✕ Not specified |
| Filbey et al. (2009) | ✓ Regular cannabis users (72hrs abstinent) |
✓ Within subject |
✓ Self-reported cannabis use of at least four times/week for previous 6 months |
✓ Within subject |
✓ - Cannabis craving - Regional brain activation |
✓ - Multiple linear regression analysis |
✕ Not specified |
✓ Declared |
| Grant et al. (2012) | ✓ Non-treatment seeking users |
✓ | ✓ Self-reported cannabis use of ±3.1 times/week for previous 12 months |
✓ Non-users |
✓ - Performance on attentional processing task |
✓ - ANOVA |
✕ No |
✓ Declared |
| Kober et al. (2014) | ✓ Treatment seeking cannabis dependent users |
✓/✕ Age/sex matched. No info on psychiatric illness, additional substance use, or psychotropic medication for users. |
✓ Met DSM IV SCID criteria for cannabis dependence; weekly self-report and urine drug screening |
✓ Non-users |
✓ - Performance on attentional processing task |
✓ - Random effects analysis |
✕ No |
✓ Declared |
| Wölfling et al. (2008) | ✓ Chronic heavy cannabis dependent users (12hrs abstinent) |
✓/✕ Demographic characteristics matched. More tobacco smokers in the dependent users group. |
✓ Met ICD 10 criteria for cannabis dependence |
✓ Non-users |
✓ - Cannabis craving scores - Arousal |
✓ - One-way ANOVA |
✓ Participants abstained from tobacco for 2hrs before study |
✕ Not specified |
| Gruber et al. (2005) | ✓ Heavy cannabis users |
✓ | ✓ Self-report; positive urine cannabis screen |
✓ Non-users |
✓ - Performance on attentional processing task - Regional brain activation |
✓ - Random effects t test analysis |
✕ Not specified |
✓ Declared |
| Eldreth et al. (2004) | ✓ Heavy cannabis users (25d abstinent) |
✓ | ✓ Screened with DUSQ, ASI, DIS, DSM IV, self-report, and positive urine drug screen for cannabis |
✓ Non-users |
✓ - Performance on attentional processing task - Regional brain activation |
✓ - Random effects two-sample t test |
✓ Participants abstained from tobacco for 3hrs before study |
✓ Declared |
DSM IV – Diagnostic and Statistical Manual of Mental Disorders; SCID – Structured Clinical Interview for DSM Disorders; ICD 10 – International Statistical Classification of Diseases and Related Health Problems; DUSQ – Drug Use Survey Questionnaire, ASI – Addiction Severity Index, DIS – Diagnostic Interview Schedule; ANOVA – Analysis of variance
Results
Behavioural findings
Regarding the effects of acute THC administration on attentional salience related behaviours, Bhattacharyya et al. found that, using a visual oddball paradigm, response times to all stimuli were reduced in the THC group, compared to placebo (Bhattacharyya et al., 2012). In particular, reaction times to the standard stimuli were significantly reduced compared to the oddball stimuli, in the THC condition. The authors also reported that THC caused a significant increase in the severity of psychotic symptoms (Bhattacharyya et al., 2012). There was no relationship between the effect of THC on response latency to the standard stimuli, and its effect on the severity of psychotic symptoms (Bhattacharyya et al., 2012). In an early study by Hooker et al., acute marijuana ingestion resulted in an increased Stroop interference effect and increased memory intrusions during a free recall task (Hooker and Jones, 1987). Immediate sustained attention and controlled retrieval from semantic memory were unaffected.
Studies investigating the influence of acute THC administration on affective salience suggest an effect dependent on stimulus type. Both Ballard et al. and Phan et al. reported a pronounced impairment in recognition of threat-related facial emotions (e.g. fear and anger) (Ballard et al., 2012, Phan et al., 2008). For the Ballard et al. study, recognition of happiness and sadness remained relatively unaffected, and there was no consistent effect on the “valence or arousal” of images of emotional scenes, with neutral pictures being rated as slightly more negative and arousing under the lower dose of THC (Ballard et al., 2012). The participants of the Phan et al. study did not report a decrease in subjective anxiety (Phan et al., 2008). A separate study reported increased response latency in relation to negative stimuli and cannabis stimuli (versus neutral stimuli) after acute THC administration, and an attentional bias towards positive word stimuli specifically amongst a subgroup who were cannabis dependent (Metrik et al., 2015).
The studies exploring the effects of long-term cannabis use on motivational and attentional salience processing behaviours were also reasonably consistent. Of the studies included, three investigated effects on motivational salience processing in relation to cannabis cues in long term users. All three reported increases in drug craving, following exposure to cannabis cues versus neutral stimuli (Filbey et al., 2009, Charboneau et al., 2013, Wolfling et al., 2008). Wolfling et al. also reported increases in physiological arousal (via skin conductance and EEG readings) in the cannabis group, in response to the cannabis cues (Wolfling et al., 2008). The same group also rated the cannabis-associated stimuli as significantly more pleasant and more arousing than other stimuli (Wolfling et al., 2008).
Both Kober et al. and Gruber et al. observed a strong Stroop effect across all groups. However, there was no significant difference between the cannabis dependent individuals and the controls in terms of response times, magnitude of Stroop effect, or number of errors made (Kober et al., 2014, Gruber and Yurgelun-Todd, 2005). Eldreth et al. reported the same finding for users after 25 days of cannabis abstinence (Eldreth et al., 2004). Similarly, Grant et al. reported no differences in error detection or false alarm scores in the Rapid Visual Information Processing task, between healthy long-term cannabis users and non-users (Grant et al., 2012).
Regarding affective salience processing in long term users, Somaini et al. found higher pleasantness ratings and lower arousal ratings during induced negative emotional states in current cannabis users, compared to participants who were 6 months abstinent, and healthy controls (Somaini et al., 2012).
Neuroimaging findings
Eight of the included studies incorporated analysis of functional MRI data, and reported on brain regions involved in salience processing that were affected by cannabis or THC intake. Two of these investigations were in the acute THC setting, whilst the others examined the long-term effects of cannabis use on dependant, regular, and recreational users. Table 2 shows a summary of the regions that displayed abnormal activity during salience processing, under either the acute THC or long-term cannabis use conditions.
Acute effects of cannabis on functional brain activity in non-using healthy volunteers, during salience processing
Table 2.
Summary of the regions displaying abnormal activity during salience processing, under either the acute THC or long-term cannabis use conditions.
| Author and Year of Publication | Comparison: Cannabis vs Control | Striatum | Insula | ACC | Hippocampus | Thalamus | Frontal Lobe | Amgydala | Substantia Nigra | VTA | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acute THC challenge | Phan et al. (2008) | THC capsule vs Placebo. Recreational cannabis users; 24hrs cannabis abstinent processing of facial emotions | - | - | - | - | - | - | Significant ↓ in right lateral amyg. Non significant ↓ in left amyg. | - | - | - |
| Bhattacharyya et al. (2012) | THC capsule vs Placebo Healthy participants, minimal previous cannabis use; oddball paradigm | ↓ In: caudate head, putamen | ↓ In: insula | - | - | ↓ In: Right thalamus | ↑ In: Right inferior, medial, and superior frontal gyrus. Right orbitofrontal cortex. frontal pole | - | - | - | - | |
| Effects of long term cannabis use | Charboneau et al. (2013) | Non treatment seeking, cannabis dependent users; 8hrs cannabis abstinent; Cannabis cue exposure | - | ↓ In: Left - insula, superior temporal gyrus | - | ↑ In: Right - parahippocampal gyrus, hippocampus; Left - fusiform gyrus, parahippocampal gyrus, hippocampus, |
↑ In: thalamus | ↑ In: Left - superior frontal gyrus, medial frontal gyrus, inferior orbitofrontal; Right - superior frontal gyrus | ↑ In: amygdala. | - | - | ↑ In: Right occipital cortex, Left and right middle occipital gyrus. ↓ In: Right and left superior temporal; ↑ In: left superior temporal pole, left middle temporal pole. ↑ In: Right posterior cingulate, Left posterior cingulate and precuneus. ↓ In: Inferior parietal; supramarginal gyrus; angular gyrus |
| Somaini et al. (2012) | Cannabis dependent active users vs. abstinent cannabis users; and non-users; Emotional responses to neutral and unpleasant images | - | - | - | - | - | - | - | - | - | persistent ↑ of the HPA axis; impaired hormonal reaction to negative emotional stimuli | |
| Filbey et al. (2009) | Regular cannabis users; 72hrs cannabis abstinent; Cannabis cue exposure | - | ↑ in insula | ↑ In: dorsal anterior cingulate cortex | - | ↑ In: thalamus | ↑ In: inferior frontal gyrus | ↑ In: amygdala. | - | ↑ In: VTA | ↑ In: cerebellum, ↑ In: pre and post central gyri, superior temporal gyrus. ↓ In: inferior parietal lobe | |
| Kober et al. (2014) | Treatment seeking, cannabis dependent users vs healthy controls; Stroop task | ↓ In: ventral striatum, dorsal striatum | ↓ In: right insula/superior temporal gyrus | - | ↓ In: amygdala/para-hippocampal gyrus | ↓ In: thalamus, sub-thalamic nucleus | ↓ In: bilateral dorsolateral and dorsal anterior prefrontal cortex | ↓ In: amygdala/para-hippocampal gyrus | ↓ In: substantia nigra | ↓ In: VTA | ↓ In: midbrain. Right posterior superior/middle temporal gyrus | |
| Gruber et al. (2005) | Heavy cannabis users vs. non-users; Stroop task | - | - | ↓ In: ACC | - | - | abnormal bilateral of DLPFC | - | - | - | ↑ In: mid cingulate cortex | |
| Eldreth et al. (2004) | Heavy cannabis users 25d cannabis abstinent vs. non-users; Stroop task | - | - | ↓ In: Left perigenual ACC | ↑ In: bilateral hippocampus | - | ↓ In: left LPFC | - | - | - | - |
Summarizing the regions displaying abnormal activity either under the acute THC or long term cannabis use conditions, during salience processing. ↑ = increase in activation; ↓ = decrease in activation; ACC = anterior cingulate cortex; DLPFC/LPFC = dorsolateral prefrontal cortex/lateral; VTA = ventral tegmental area
Healthy volunteers who were administered THC acutely showed a decreased BOLD signal in the caudate head, putamen, insula, and thalamus during oddball relative to standard stimuli conditions (attentional salience processing) (Bhattacharyya et al., 2012). The authors also reported an augmented BOLD response in the frontal brain region, including the inferior frontal gyrus, medial frontal gyrus, superior frontal gyrus, orbitofrontal gyrus and the frontal pole – when compared to placebo. Reduced activity in the caudate while viewing oddball relative to standard stimuli was negatively correlated with the severity of psychotic symptoms, and with the significantly greater reduction in response latency for standard stimuli under the THC condition (Bhattacharyya et al., 2012). In response to processing of threat-related facial emotions (affective salience processing), THC significantly attenuated activity in the right lateral amygdala of recreational cannabis users, whilst a non-significant reduction was seen in the right amygdala of the same group. (Phan et al., 2008)
Non-acute effects of recreational, regular, and dependent cannabis use on functional brain activity, during salience processing
Charboneau et al. and Filbey et al. investigated the effects of visual and tactile cannabis cues, respectively, on drug craving – a behaviour related to motivational salience. Both studies reported similar findings in the midbrain, temporal, frontal, and occipital regions; however, differing effects were seen in the insula, and some parietal regions (Charboneau et al., 2013, Filbey et al., 2009). Charboneau et al. reported increased activity in the frontal, limbic (amygdala and hippocampus), occipital and temporal regions, and the posterior cingulate cortex. They also found decreased activity in the parietal and insula regions (Charboneau et al., 2013). Filbey et al. reported a greater BOLD activation in the ventral tegmental area, dorsal anterior cingulate cortex, cerebellum, thalamus, pre- and post-central gyri, thalamus, amygdala, fusiform gyrus, pre- post central gyri, and superior temporal gyrus (Filbey et al., 2009). But unlike Charboneau et al., activation was also recorded in the inferior frontal gyrus/insula, and inferior parietal lobe (Filbey et al., 2009). In the Charboneau et al. study, craving scores were significantly correlated with activation in the limbic, paralimbic, and visual regions for the first fMRI task run, but not for the later runs (Charboneau et al., 2013).
Using the Stroop colour word interference task to explore cognitive control – related to attentional salience – in cannabis users before, and at two time-points after addiction intervention, Kober et al. found diminished activation in several prefrontal regions, including the bilateral dorsolateral and dorsal anterior prefrontal cortex (Kober et al., 2014) – findings echoed by the Gruber et al. and Eldreth et al. studies (Gruber and Yurgelun-Todd, 2005, Eldreth et al., 2004). Decreased activation was also reported for the ventral and dorsal striatum, amygdala/parahippocampal gyrus, thalamus, and midbrain regions, including the ventral tegmental area and substantia nigra, right insula/superior temporal gyrus, and right posterior superior/middle temporal gyrus in cannabis dependent users of the Kober et al. study (Kober et al., 2014). The authors found greater activation in the dACC, and ventral striatum in participants who used less cannabis during treatment, and who used less cannabis in the year following the intervention (respectively) (Kober et al., 2014). This is in keeping with the findings of decreased ACC activity in current heavy users, which persists in heavy users after 25 days of abstinence, in the Gruber et al. and Eldreth et al. studies, respectively (Gruber and Yurgelun-Todd, 2005, Eldreth et al., 2004).
Finally, abnormal persistent hyperactivity of the hypothalamic–pituitary–adrenal axis (HPA axis) was observed in previous (6 months abstinent) cannabis users, with a stronger effect observed in current cannabis users (Somaini et al., 2012). Additionally, an impaired hormonal reaction to unpleasant images was observed in the group of active users (Somaini et al., 2012).
Discussion
The aim of this paper was to systematically review the recent cannabis and cognition research, with a view to summarizing the existing findings about the effects of cannabis on salience processing – based on both behavioural observations, and functional neuroimaging findings. To the best of our knowledge, this is the first systematic review to specifically explore this topic.
As is its nature, salience can be inferred and measured through many different direct and indirect means. As a result, cautious comparisons must be made between the different study designs, given the different methods used, and the different aspects of salience being elicited. For the purpose of this review, studies were included if they measured – directly or indirectly – at least one aspect of the three categories of salience processing described earlier (e.g. attentional, affective, or motivational salience). The designs investigated both acute administration, and long-term use; and either behavioural changes, or changes in both behaviours and functional activation.
Regarding the behavioural findings, all the acute THC administration studies demonstrated significant effects on salience processing. For the oddball task, acute THC administration decreased response times, especially for the standard stimuli. The authors suggest that this is a result of inappropriate attribution of attentional salience to the standard stimuli, and that this may give insight into the development of psychotic symptoms, through a similar inappropriate salience response to non-salient elements (Bhattacharyya et al., 2012). It is important to note that no direct relationship was observed between the effect of THC on response times, and its effect on severity of psychotic symptoms. The acute THC Stroop study also reported an increased Stroop interference effect after marijuana ingestion; however, no difference was observed in immediate and sustained attention, controlled retrieval from semantic memory, and speed of reading and naming colours (Hooker and Jones, 1987). These findings suggest an initial impairment in immediate attribution of attentional salience that is less evident in memory recall and other cognitive processes. In the behavioural studies of the long-term effects of cannabis use, a similar effect on attentional salience was not observed. Stroop task performance of long-term cannabis users was within the normal range, compared to healthy controls, for all the relevant studies (Kober et al., 2014, Eldreth et al., 2004, Gruber and Yurgelun-Todd, 2005). The same was found in the Rapid Visual Information Processing task (Grant et al., 2012). Investigating the consequences of acute THC administration in long-term cannabis users, focusing on whether effects on attentional salience are attenuated with prolonged exposure to cannabis, may benefit future research, as it is well-accepted that tolerance to the effects of THC can develop in regular users (D'Souza et al., 2008). Though no significant performance effects were observed for the long-term users in the Rapid Visual Information Processing task or the Stroop test, reduced activity in regions strongly related to cognitive control and salience/reward processing were noted in all the Stroop task studies (Kober et al., 2014, Eldreth et al., 2004, Gruber and Yurgelun-Todd, 2005). This could indicate greater demand on cognitive control mechanisms during cognitive control processes and salience detection in cannabis users (Kober et al., 2014). It may also reflect a change in neural strategy to meet the cognitive demands of the task, or some other form of neural inefficiency, as an earlier review of the effect of cannabis use on different cognitive functions suggested (Bossong et al., 2014a). Alternatively, this could simply be the result of the studies in chronic users being sufficiently powered to detect differences in neurophysiology, but not powered to detect changes at the behavioural level. Nevertheless, the finding of increased ACC activity associated with cannabis abstinence may also hold strong value in terms of future intervention plans – as treatments focusing on cognitive control and attentional salience attribution, as well as abstinence, may improve cessation outcomes (Kober et al., 2014).
The findings of the acute THC studies focusing on emotion processing introduce another aspect to this discussion. When tasked with more emotive stimuli, the acute THC challenge resulted in impaired recognition of facial threat stimuli (e.g. fear and anger) (Phan et al., 2008, Ballard et al., 2012). This impairment was not reflected in the processing of sad or happy faces, nor was there a consistent effect on the processing of emotional scenes (Ballard et al., 2012, Metrik et al., 2015). This suggests that though general affective salience may remain reasonably intact, acute THC administration results in abnormalities in the perception of threat cues, possibly due to a dysregulated allocation of affective salience in relation to particular emotive stimuli.
Regarding the neuroimaging findings relating to acute THC administration, Bhattacharyya et al. reported an attenuation of striatal activity, specifically in the caudate, but found an augmentation of activity in the prefrontal cortex, in participants performing the oddball task (Bhattacharyya et al., 2012). These regions are strongly functionally connected, and their roles in salience processing are thought to be highly interconnected (Jung et al., 2014). In a secondary analysis of their data, Bhattacharyya et al. found attenuated fronto-striatal connectivity under THC, which was related to its effect on task performance, while this connectivity was augmented under the influence of cannabidiol, a cannabinoid with opposing effects to that of THC (Bhattacharyya et al., 2015b). Thus, an alteration of activity in these regions could result in the abnormal attentional salience processing observed during the oddball task. Phan et al. reported a THC related decrease in amygdala activation during the processing of threat-related facial stimuli specifically, which is consistent with the pivotal role the amygdala is thought to play in the processing of affective facial stimuli, and stress responses (Phan et al., 2008, Liberzon et al., 2003) – though a debate still exists as to whether the processing role of the amygdala is less specific and applies to facial stimuli in general, regardless of the stimulus being affective/non-affective (Santos et al., 2011). However, no associated reduction in anxiety was reported (Phan et al., 2008). The study by Somaini et al. also reported abnormal functioning of the innate hormonal stress system – the HPA axis – particularly in reaction to negative emotions and threat (Somaini et al., 2012). Given the importance of the amygdala in regulating activation of HPA axis responses (Smith and Vale, 2006, Appiah-Kusi et al., 2016), it is possible that the abnormal functioning of the amygdala and HPA axis may have a combined effect, resulting in the specific aberrant processing of threat stimuli observed after acute THC challenge.
The effects of cannabis use on motivational and incentive salience are harder to decipher. It is clear that long-term cannabis users experience increases in craving after exposure to cannabis cues. However, this effect is also reported in studies of other drug dependencies (Epstein et al., 2009), suggesting that it is not a direct effect of chronic cannabis use, but an effect of chronic drug dependency on attribution of incentive salience related to drug cue exposure. Indeed, both the Filbey et al. and the Charboneau et al. studies reported increased activity in regions forming part of the neural reward circuitry in relation to cannabis cues (Filbey et al., 2009, Charboneau et al., 2013), which may also mediate visually cued aspects of drug craving, or alternatively, other aspects of cues including affective memory. The findings of the two studies did differ in relation to activation in the insula and some parietal regions, though this could be related to differences in the study sample, i.e. non-treatment seeking cannabis dependent individuals, versus regular cannabis users. Charboneau et al. found that the correlation between craving scores and neural activation was not repeated in the limbic, paralimbic, and visual regions after the first task time-point (Charboneau et al., 2013). It was suggested that this was the result of initial involvement of the visual salience regions in cue associated craving being inhibited by higher brain regions, after prolonged exposure (Charboneau et al., 2013). If a direct effect exists for chronic cannabis use on visual salience and cue-induced craving, it is likely that this is a conditioned response, related to the effect of overall drug dependency on the reward pathway.
Indeed, it is important to note that, as salience processing was not explicitly measured in the majority of the studies included, the direct neural effects of cannabis use on salience processing should be interpreted cautiously, as the influence of other variables, such as craving, may be misleading.
That being said, it is possible that those effects on salience processing that are attributable to THC may be a consequence of its actions in the endocannabinoid system, and the resultant modulation of dopamine expression. Though direct human experimental evidence demonstrating dopamine release with THC administration is still equivocal (Sami et al., 2015), there is considerable evidence from animal studies in support of this (Cheer et al., 2004, Chen et al., 1990, Malone and Taylor, 1999). THC is an agonist of the endocannabinoid system, and activation of the endocannabinoid system can modulate the expression of various neurotransmitters, including dopamine, via direct and indirect methods (Appiah-Kusi et al., 2016, Pertwee, 2008). Given the high expression of both dopamine and endocannabinoid receptors in the striatum and substantia nigra – critical regions implicated in salience processing – and some studies suggesting altered dopamine release following acute THC administration in those genetically predisposed or at risk of psychosis (Sami et al., 2015), a role for both the dopaminergic and endocannabinoid systems in the context of aberrant salience processing, at least in psychosis, may be hypothesized. In chronic users, however, evidence exists suggesting a blunting of dopamine synthesis as well as release capacity over time, as identified in a review by Sami et al. (2015) (Sami et al., 2015, Bloomfield et al., 2014, D'Souza et al., 2008, Mizrahi et al., 2014). Hence, the precise nature of the relationship between acute and chronic exposure to cannabis and THC and dopaminergic alterations are unclear. Additional studies exploring this are still required – particularly in relation to acute vs chronic cannabis use.
Several limitations of this review impede the development of decisive conclusions based on the findings; the overarching limitation being the lack of research investigating the effects of cannabis use on salience processing, specifically. Thus, the papers included here involved a variety of tasks and measures, from which varieties of salience were inferred. Additional limitations include the analysis of different types of cannabis use – specifically, the administration of acute THC, and the effects of long term cannabis use on dependent and regular users. Given the shallow pool of research on the topic, it was necessary to gather as much information as possible. Lack of adequate control elements was a methodological limitation of some of the included studies. The study by Grant et al. did not include an equally matched control group, whilst neither the Filbey et al. nor the Charboneau et al. studies included a control group. As discussed earlier, salience processing was not directly measured in the majority of studies, and thus it is possible that the results have been influenced by other cognitive processes, particularly in the cannabis cues/craving studies.
While there are many studies linking cannabis use to psychosis, and aberrant salience has been strongly linked to the generation of psychotic symptoms, few studies have investigated what cannabis does to salience processing acutely, or following regular use. Acutely, THC appears to have a significant effect on the perception of salience; whilst following long-term use, normal salience performance appears to be underpinned by abnormal neural processes. Overall, more studies need to be carried out specifically looking at salience processing as the main outcome measure related to cannabis intake, to allow a better understanding of the neurocognitive mechanisms underlying the increased risk of development of psychosis associated with cannabis use.
Summation
Few studies have explicitly explored the relationship between cannabis use and abnormal salience processing. However, the outcomes of some cognitive studies may be used to infer aspects of salience, as reported here.
Impaired salience processing as a result of acute cannabis intake, particularly in relation to threatening facial emotions, e.g. fear and anger, was most consistently reported.
Long term cannabis use did not result in impaired salience processing. However, these performances were associated with reduced activity in regions strongly related to cognitive control and salience/reward processing – a reduction which also appeared to be mediated by cannabis abstinence.
Considerations
Due to the paucity of research on the topic, it is difficult to bring absolute clarity to the relationship between abnormal salience processing and cannabis use.
Adding to this is the heterogeneity of methods used, and outcomes from which salience was inferred for this review. Direct exploration of this relationship is not impossible, however, and may be achieved in future research.
Acknowledgments
Financial Support: Dr Sagnik Bhattacharyya has received support from the NIHR (NIHR Clinician Scientist Award; NIHR CS-11-001), and the UK MRC (MR/J012149/1), and from the NIHR Mental Health Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King's College London. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Authors' Contributions: Surapi Bhairavi Wijayendran and Aisling O'Neill contributed equally to the manuscript. Surapi Bhairavi Wijayendran contributed to the conception and design of the study, undertook the original systematic review, and produced an early draft of the manuscript; Aisling O'Neill updated the systematic review, analysed and interpreted the data, and revised the manuscript; Sagnik Bhattacharyya contributed to the conception and design of the study, and gave final approval of the version to be published.
Conflict of Interest: None.
References
- Appiah-Kusi E, Leyden E, Parmar S, Mondelli V, Mcguire P, Bhattacharyya S. Abnormalities in neuroendocrine stress response in psychosis: the role of endocannabinoids. Psychol Med. 2016;46:27–45. doi: 10.1017/S0033291715001786. [DOI] [PubMed] [Google Scholar]
- Ballard ME, Bedi G, de Wit H. Effects of delta-9-tetrahydrocannabinol on evaluation of emotional images. J Psychopharmacol. 2012;26:1289–98. doi: 10.1177/0269881112446530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Berridge KC. From prediction error to incentive salience: mesolimbic computation of reward motivation. Eur J Neurosci. 2012;35:1124–43. doi: 10.1111/j.1460-9568.2012.07990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Atakan Z, Martin-Santos R, Crippa JA, Kambeitz J, Malhi S, Giampietro V, Williams S, Brammer M, Rubia K, Collier DA, et al. Impairment of inhibitory control processing related to acute psychotomimetic effects of cannabis. Eur Neuropsychopharmacol. 2015a;25:26–37. doi: 10.1016/j.euroneuro.2014.11.018. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Crippa JA, Allen P, Martin-Santos R, Borgwardt S, Fusar-Poli P, Rubia K, Kambeitz J, O'carroll C, Seal ML, Giampietro V, et al. Induction of psychosis by Delta9-tetrahydrocannabinol reflects modulation of prefrontal and striatal function during attentional salience processing. Arch Gen Psychiatry. 2012;69:27–36. doi: 10.1001/archgenpsychiatry.2011.161. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Falkenberg I, Martin-Santos R, Atakan Z, Crippa JA, Giampietro V, Brammer M, Mcguire P. Cannabinoid modulation of functional connectivity within regions processing attentional salience. Neuropsychopharmacology. 2015b;40:1343–52. doi: 10.1038/npp.2014.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Fusar-Poli P, Borgwardt S, Martin-Santos R, Nosarti C, O'carroll C, Allen P, Seal ML, Fletcher PC, Crippa JA, Giampietro V, et al. Modulation of mediotemporal and ventrostriatal function in humans by Delta9-tetrahydrocannabinol: a neural basis for the effects of Cannabis sativa on learning and psychosis. Arch Gen Psychiatry. 2009;66:442–51. doi: 10.1001/archgenpsychiatry.2009.17. [DOI] [PubMed] [Google Scholar]
- Bloomfield MA, Morgan CJ, Egerton A, Kapur S, Curran HV, Howes OD. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol Psychiatry. 2014;75:470–8. doi: 10.1016/j.biopsych.2013.05.027. [DOI] [PubMed] [Google Scholar]
- Boehme R, Deserno L, Gleich T, Katthagen T, Pankow A, Behr J, Buchert R, Roiser JP, Heinz A, Schlagenhauf F. Aberrant Salience Is Related to Reduced Reinforcement Learning Signals and Elevated Dopamine Synthesis Capacity in Healthy Adults. J Neurosci. 2015;35:10103–11. doi: 10.1523/JNEUROSCI.0805-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, Jager G, Bhattacharyya S, Allen P. Acute and non-acute effects of cannabis on human memory function: a critical review of neuroimaging studies. Curr Pharm Des. 2014a;20:2114–25. doi: 10.2174/13816128113199990436. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Jansma JM, Bhattacharyya S, Ramsey NF. Role of the endocannabinoid system in brain functions relevant for schizophrenia: an overview of human challenge studies with cannabis or 9-tetrahydrocannabinol (THC) Prog Neuropsychopharmacol Biol Psychiatry. 2014b;52:53–69. doi: 10.1016/j.pnpbp.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Bossong MG, Van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD, van Gerven JM, Ramsey NF, Lammertsma AA, Kahn RS. Delta 9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology. 2009;34:759–66. doi: 10.1038/npp.2008.138. [DOI] [PubMed] [Google Scholar]
- Boudin F, J-Y N, Dawes M. Clinical Information Retrieval using Document and PICO Structure; Human Language Technologies: The 2010 Annual Conference of the North American Chapter of the ACL; Los Angeles, California. Association for Computational Linguistics; 2010. pp. 822–830. [Google Scholar]
- Bowman H, Su L, Wyble B, Barnard PJ. Salience Sensitive Control, Temporal Attention and Stimulus-Rich Reactive Interfaces. In: Roda C, editor. Human Attention in Digital Environments. Cambridge University Press; 2011. [Google Scholar]
- Burns L. World Drug Report 2013 By United Nations Office on Drugs and Crime. Drug and Alcohol Review. 2013;33:216. [Google Scholar]
- Charboneau EJ, Dietrich MS, Park S, Cao A, Watkins TJ, Blackford JU, Benningfield MM, Martin PR, Buchowski MS, Cowan RL. Cannabis cue-induced brain activation correlates with drug craving in limbic and visual salience regions: preliminary results. Psychiatry Res. 2013;214:122–31. doi: 10.1016/j.pscychresns.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JP, Paredes W, Li J, Smith D, Lowinson J, Gardner EL. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl) 1990;102:156–62. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- D'souza DC, Perry E, Macdougall L, Ammerman Y, Cooper T, Wu YT, Braley G, Gueorguieva R, Krystal JH. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology. 2004;29:1558–72. doi: 10.1038/sj.npp.1300496. [DOI] [PubMed] [Google Scholar]
- D'souza DC, Ranganathan M, Braley G, Gueorguieva R, Zimolo Z, Cooper T, Perry E, Krystal J. Blunted psychotomimetic and amnestic effects of delta-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology. 2008;33:2505–16. doi: 10.1038/sj.npp.1301643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Forti M, Marconi A, Carra E, Fraietta S, Trotta A, Bonomo M, Bianconi F, Gardner-Sood P, O'Connor J, Russo M, Stilo SA, et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry. 2015;2:233–8. doi: 10.1016/S2215-0366(14)00117-5. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–20. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci U S A. 2009;106:13016–21. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner EL, Vorel SR. Cannabinoid transmission and reward-related events. Neurobiol Dis. 1998;5:502–33. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- Grant JE, Chamberlain SR, Schreiber L, Odlaug BL. Neuropsychological deficits associated with cannabis use in young adults. Drug Alcohol Depend. 2012;121:159–62. doi: 10.1016/j.drugalcdep.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Yurgelun-Todd DA. Neuroimaging of marijuana smokers during inhibitory processing: a pilot investigation. Brain Res Cogn Brain Res. 2005;23:107–18. doi: 10.1016/j.cogbrainres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series. Wiley Online Library; 2008. [Google Scholar]
- Hooker WD, Jones RT. Increased susceptibility to memory intrusions and the Stroop interference effect during acute marijuana intoxication. Psychopharmacology (Berl) 1987;91:20–4. doi: 10.1007/BF00690920. [DOI] [PubMed] [Google Scholar]
- Jensen J, Kapur S. Salience and psychosis: moving from theory to practise. Psychol Med. 2009;39:197–8. doi: 10.1017/S0033291708003899. [DOI] [PubMed] [Google Scholar]
- Jung WH, Jang JH, Park JW, Kim E, Goo EH, Im OS, Kwon JS. Unravelling the intrinsic functional organization of the human striatum: a parcellation and connectivity study based on resting-state FMRI. PLoS One. 2014;9:e106768. doi: 10.1371/journal.pone.0106768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kober H, Devito EE, Deleone CM, Carroll KM, Potenza MN. Cannabis abstinence during treatment and one-year follow-up: relationship to neural activity in men. Neuropsychopharmacology. 2014;39:2288–98. doi: 10.1038/npp.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leweke FM, Giuffrida A, Wurster U, Emrich HM, Piomelli D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport. 1999;10:1665–9. doi: 10.1097/00001756-199906030-00008. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–33. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Malone DT, Taylor DA. Modulation by fluoxetine of striatal dopamine release following Delta9-tetrahydrocannabinol: a microdialysis study in conscious rats. Br J Pharmacol. 1999;128:21–6. doi: 10.1038/sj.bjp.0702753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoliu A, Riedl V, Zherdin A, Muhlau M, Schwerthoffer D, Scherr M, Peters H, Zimmer C, Forstl H, Bauml J, Wohlschlager AM, et al. Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40:428–37. doi: 10.1093/schbul/sbt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Parker LA. The endocannabinoid system and the brain. Annu Rev Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Menon V. Salience Network. In: Toga AW, editor. Brain Mapping: An Encyclopedic Reference. Academic Press: Elsevier; 2015. [Google Scholar]
- Metrik J, Aston ER, Kahler CW, Rohsenow DJ, McGeary JE, Knopik VS. Marijuana's acute effects on cognitive bias for affective and marijuana cues. Exp Clin Psychopharmacol. 2015;23:339–50. doi: 10.1037/pha0000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizrahi R, Kenk M, Suridjan I, Boileau I, George TP, McKenzie K, Wilson AA, Houle S, Rusjan P. Stress-induced dopamine response in subjects at clinical high risk for schizophrenia with and without concurrent cannabis use. Neuropsychopharmacology. 2014;39:1479–89. doi: 10.1038/npp.2013.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, Lewis G. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–28. doi: 10.1016/S0140-6736(07)61162-3. [DOI] [PubMed] [Google Scholar]
- Niu Y, Todd RM, Anderson AK. Affective salience can reverse the effects of stimulus-driven salience on eye movements in complex scenes. Front Psychol. 2012;3:336. doi: 10.3389/fpsyg.2012.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Takahashi RN. Psychopharmacology of the endocannabinoids: far beyond anandamide. J Psychopharmacol. 2012;26:7–22. doi: 10.1177/0269881111405357. [DOI] [PubMed] [Google Scholar]
- Patel R, Wilson R, Jackson R, Ball M, Shetty H, Broadbent M, Stewart R, McGuire P, Bhattacharyya S. Association of cannabis use with hospital admission and antipsychotic treatment failure in first episode psychosis: an observational study. BMJ Open. 2016;6:e009888. doi: 10.1136/bmjopen-2015-009888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. Ligands that target cannabinoid receptors in the brain: from THC to anandamide and beyond. Addict Biol. 2008;13:147–59. doi: 10.1111/j.1369-1600.2008.00108.x. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000) Neurophysiol Clin. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, Onyewuenyi I, Popovska A, de Wit H. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–9. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sami MB, Rabiner EA, Bhattacharyya S. Does cannabis affect dopaminergic signaling in the human brain? A systematic review of evidence to date. Eur Neuropsychopharmacol. 2015;25:1201–24. doi: 10.1016/j.euroneuro.2015.03.011. [DOI] [PubMed] [Google Scholar]
- Santos A, Mier D, Kirsch P, Meyer-Lindenberg A. Evidence for a general face salience signal in human amygdala. Neuroimage. 2011;54:3111–6. doi: 10.1016/j.neuroimage.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Schoeler T, Monk A, Sami MB, Klamerus E, Foglia E, Brown R, Camuri G, Altamura AC, Murray R, Bhattacharyya S. Continued versus discontinued cannabis use in patients with psychosis: a systematic review and meta-analysis. Lancet Psychiatry. 2016a;3:215–25. doi: 10.1016/S2215-0366(15)00363-6. [DOI] [PubMed] [Google Scholar]
- Schoeler T, Petros N, Di Forti M, Klamerus E, Foglia E, Ajnakina O, Gayer-Anderson C, Colizzi M, Quattrone D, Behlke I, Shetty S, et al. Effects of continuation, frequency, and type of cannabis use on relapse in the first 2 years after onset of psychosis: an observational study. The Lancet Psychiatry. 2016b doi: 10.1016/S2215-0366(16)30188-2. [DOI] [PubMed] [Google Scholar]
- Schoeler T, Petros N, Di Forti M, Pingault J, Klamerus E, Foglia E, Small A, Murray R, Bhattacharyya S. Examining the association between continued cannabis use and risk of relapse in first episode psychosis: a quasi-experimental investigation within an observational study. JAMA Psychiatry. 2016c doi: 10.1001/jamapsychiatry.2016.2427. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinn-Cunningham BG. Object-based auditory and visual attention. Trends Cogn Sci. 2008;12:182–6. doi: 10.1016/j.tics.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Smith SM, Vale WW. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin Neurosci. 2006;8:383–95. doi: 10.31887/DCNS.2006.8.4/ssmith. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somaini L, Manfredini M, Amore M, Zaimovic A, Raggi MA, Leonardi C, Gerra ML, Donnini C, Gerra G. Psychobiological responses to unpleasant emotions in cannabis users. Eur Arch Psychiatry Clin Neurosci. 2012;262:47–57. doi: 10.1007/s00406-011-0223-5. [DOI] [PubMed] [Google Scholar]
- Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99:1333–41. doi: 10.1111/j.1360-0443.2004.00806.x. [DOI] [PubMed] [Google Scholar]
- West S, King V, Carey TS, Lohr KN, McKoy N, Sutton SF, Lux L. Systems to rate the strength of scientific evidence. Evid Rep Technol Assess (Summ) 2002:1–11. [PMC free article] [PubMed] [Google Scholar]
- White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123:105–15. doi: 10.1016/j.schres.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Winton-Brown TT, Fusar-Poli P, Ungless MA, Howes OD. Dopaminergic basis of salience dysregulation in psychosis. Trends Neurosci. 2014;37:85–94. doi: 10.1016/j.tins.2013.11.003. [DOI] [PubMed] [Google Scholar]
- Wolfling K, Flor H, Grusser SM. Psychophysiological responses to drug-associated stimuli in chronic heavy cannabis use. Eur J Neurosci. 2008;27:976–83. doi: 10.1111/j.1460-9568.2008.06051.x. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]