Abstract
Rarity has been a central topic for conservation and evolutionary biologists with the ultimate goal of determining the species characteristics that cause extinction risk. More recently, beyond the rarity of species, the rarity of functions or functional traits, called functional rarity, has gained momentum in helping to understand the impact of biodiversity decline on ecosystem functioning. However a conceptual framework for defining and quantifying functional rarity is still lacking. Here, we introduce twelve different forms of functional rarity along gradients of species scarcity and trait distinctiveness. We then highlight the potential key role of functional rarity in the long-term and large-scale maintenance of ecosystem processes, as well as the necessary linkage between functional and evolutionary rarity.
Keywords: Biodiversity-ecosystem functioning, conservation target, functional distinctiveness, species scarcity, functional redundancy
The multiple facets of rarity
Rarity has fascinated ecologists and evolutionary biologists [1], and has become the cornerstone of research fields especially conservation biology [2–4]. Why do species become rare? Why are there so many rare species on Earth? Many studies have examined the biological characteristics of species to explain the reasons for their rarity [e.g., 5,6–9] and the potential consequences of their extirpation [3,4]. Rare species perform different functions in ecosystems, some being redundant with those of many other rare and common species while some are unique [10–13]. Surprisingly, few studies have investigated the rarity of functions (functional rarity hereafter) within communities and its importance for the functioning of ecosystems [12,14,15].
While human societies have often placed higher value on rare species relative to common ones, rarity and commonness remain generic and vague concepts. Indeed some species can be commonly found at a large geographic scale while being locally rare within communities, such as apex predators. Others can be commonly found within communities but possess unique traits or genes. These examples point out that rarity and commonness have multiple facets [16,17]. Therefore, just as definitions and estimates of biodiversity have been recently expanded to include spatial, phylogenetic and functional dimensions [18–20], our definitions of rarity and commonness need to be revised with a broader quantitative framework that captures additional dimensions of biodiversity. The seminal paper of Rabinowitz [21] provided the foundation for such a framework with seven forms of rarity based on three species characteristics: geographic range, habitat specificity and local abundance. This typology of rarity is able to take into account the main aspects related to the spatial distribution of species, but it remains silent on species’ functions. Given the increasingly important role of functional diversity in community ecology, biogeography and conservation biology [22–26], there is thus an urgent need to develop a framework of functional rarity and associated metrics that directly combine functional trait information and species abundances across scales.
Better characterizing functional rarity goes beyond the issue of the mere understanding of why species are rare or common, it can also be key to better understanding the relationship between biodiversity and ecosystem functioning (BEF). Growing consensus suggests that BEF relationships are driven by the diversity of functions carried out by species and their individuals within an ecosystem [27–29]. In parallel, the disproportionate effect of some rare species (for example, keystone species) on ecosystem processes is increasingly reported [12,15,30]. This calls for a deeper integration of functional rarity in BEF studies particularly to meet the challenge of maintaining multiple processes under global changes [31].
In this paper, we propose a conceptual framework that builds on the Rabinowitz’ classification to define and quantify functional rarity. For this we identify four crossed species scarcity - trait distinctiveness dichotomies and two geographic rarity categories (restricted vs. widespread species), leading to twelve different forms of functional rarity. Next, we discuss the potential effect of each form of functional rarity on the functioning of ecosystems. As a perspective, we propose future directions, including the necessary linkage between functional and evolutionary rarity, an important avenue for both BEF research and conservation biology.
On the importance of functional rarity
The maintenance of scarce and unique phenotypes in communities is a well-known phenomenon, as lower frequency and greater distinctiveness limit both intra- and inter-specific competition (negative frequency dependence) [32]. It has also been described as a “strategy” for a species to expand its niche width via a release of intraspecific competition or the exploitation of alternative resources [33]. In addition, both microbial experiments and theoretical studies emphasized the positive role of rare phenotypes in the rescue of ecological communities in face of severe environmental stresses [34,35]. However this principle has not been tested over large scales where functional rarity needs to be well defined and assessed.
There is contrasting evidence about the importance of rare species on ecosystem functioning [13,36]. An intuitive line of reasoning assumes that rare species have very little impact on ecosystems according to the ‘mass ratio hypothesis’ [37]. This common belief lies in the long tradition of using total biomass or productivity as a proxy for ecosystem functioning, where dominant species have strong effects while rare species have marginal influence. However, the need to deal with ecosystem multi-functionality, resilience or resistance across time and disturbances or dependence upon some keystone species challenges this simplistic view [13,30]. For instance, even at low abundance, predators can have disproportionate impacts on ecosystem functioning through top-down control along the trophic chain and the associated energy fluxes. Since predators are often among the most endangered [38,39], their loss will likely have strong effects on ecosystems. A good example is given by the giant moray eel (Gymnothorax javanicus) that hunts at night within the labyrinth of coral reefs. This species possesses distinct functional characteristics (elongated shape and strong olfactory capacities) and has no equivalent in its ability to prey on hard-to-access dead or weak animals, thus accelerating nutrient cycling in oligotrophic ecosystems [40]. The influence that the giant moray eel has on ecosystem functioning appears irreplaceable as suggested by its very unique combination of traits. Despite the potential importance of functional rarity on ecosystem functioning, there are only a handful of studies in the literature addressing this issue [10,12,40]. This is certainly partly due to a lack of framework for estimating functional rarity across scales and species pools.
Here we propose an ecology of outliers dedicated to better understand (i) how to define and identify those outliers given their local or regional abundances and trait distinctiveness, (ii) the consequences of the persistence of those outliers for the structure and dynamics of communities and ecosystems, (iii) the distribution of these outliers across the Tree of Life.
Functional rarity: a conceptual framework
The definition of functional rarity is the most critical conceptual point before making significant progress in this new ecology of outliers.
For decades ecological rarity has been estimated at the species level using three main characteristics ultimately related to extinction risk [41]: geographical range, habitat specificity and local abundance. The combination of these three characteristics define seven forms of species rarity [21], with the rarest species having small range, a high level of habitat specificity and locally low abundance. Our proposed facets of functional rarity are partly based on these basic forms (for instance local abundance in Figure I of Box 1). Complementing this, quantifying functional rarity must include the extent to which species traits, used as proxies to represent functions, trophic links and niche axes [42–47], are more or less distinct or redundant within local communities or larger-scale species assemblages [40,48,49] (Box 1).
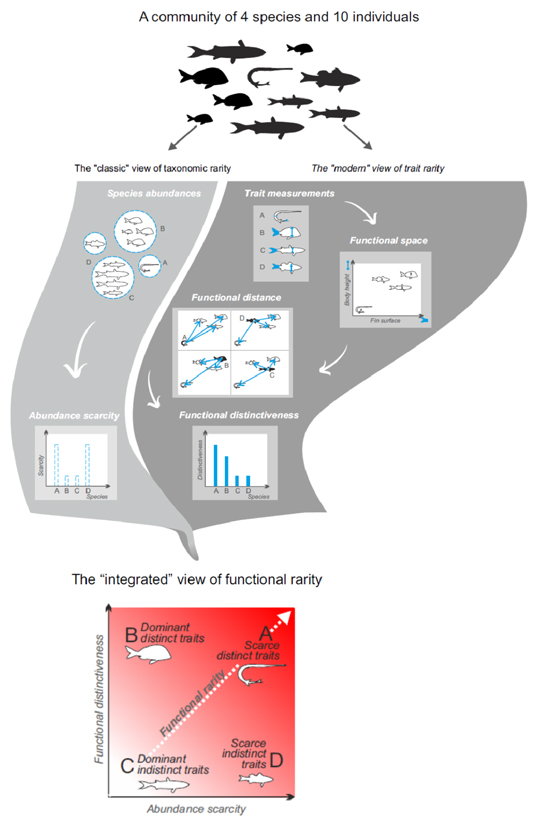
Figure I (Box 1).
Functional rarity types in local communities are assessed in both abundance and trait space by combining the classical view of taxonomic rarity and the modern view of trait rarity. Using a 10-individual community of 4 species, we highlight different facets of functional rarity integrated into a single framework. The 4 species correspond to archetypal situations at the extremes of the abundance scarcity and functional distinctiveness gradients, species A being the ecological outlier (highest functional rarity value) in the community, while species C is the ecological norm (lowest functional rarity value).
Box 1. From the rarity of species to the rarity of functions.
As the use of functional traits rapidly expands, the question of which traits, or combinations of traits, can be the most informative is critical since certain traits may reveal different information about the functional distinctiveness of a species. Moreover, if traits that are selected are highly correlated with each other, then the ‘true’ functional distinctiveness, which may become evident if other traits or combinations of traits were considered, can be obscured. It is also important to note that, although trait databases have emerged in many different kingdoms [83–86], they are often biased towards traits measured on common species [87–89], which can impede an accurate assessment of functional distinctiveness.
When selecting and analyzing functional trait information for the identification of functionally distinct species, researchers would do best to identify traits which can have implications for multiple ecological functions [29]. Given this complexity, there seems to be three main approaches that have emerged. A first is to use a few traits where the functional consequences are well understood. If the ecological consequences of traits are ambiguous, a second approach is to use a multitude of traits as a way to capture overall ecological distinctiveness. The third approach is a hybrid option, where well-understood traits are either analyzed separately or combined with ambiguous traits to assess how trait inclusion alters the interpretation of functional distinctiveness.
Once traits have been selected for the whole set of species, the functional distances between all pairs of species need to be quantified (Figure I). Several metrics are classically used depending on trait categories and potential missing values [58,59]. Then the functional distinctiveness of a given species can be assessed using its functional distance to the rest of the community (see Box 2).
The last step is to combine species rarity, for instance based on local abundance (Figure I), and trait distinctiveness into an index of functional rarity; the functionally rarest species having low abundance and the most distinct traits (species A in Figure I), while the functionally commonest species are those with highest abundances and the least distinct traits (species C in Figure I).
Using a set of dichotomies for species characteristics related to their frequencies and their traits, we propose to introduce the different facets of functional rarity. For the distribution of species we follow the steps of Rabinowitz [21] with two levels of rarity across scales. At local scale (e.g., at the community scale), we discriminate scarce vs. abundant species, while at the regional level we define restricted vs. widespread species (Table 1). In the same vein, we propose to differentiate the rarity vs. commonness of species traits compared to a given pool at the local and the regional levels. At local scale we choose to define functionally distinct species as those having traits dissimilar from those of other species and functionally redundant species as those having the traits most abundant at local scale. At the regional scale a dichotomy can be made between species possessing unique traits, i.e. not shared by any other species in the pool, and species possessing shared traits. Based on these four crossed dichotomies we can define 16 potential forms of functional rarity. Of these 16, four are never met since species cannot be functionally redundant at the local scale while being unique at the regional scale (Table 1). We therefore end up with 12 potential forms of functional rarity among which we identify two extremes cases: rare traits, exhibited by a few scarce, range-restricted species, and common traits, supported by many widespread and locally abundant species.
Table 1.
The 12 forms of functional rarity.
| Species frequency | ||||||
|---|---|---|---|---|---|---|
| Geographically Restricted | Geographically Widespread | |||||
| Locally Scarce | Locally Abundant | Locally Scarce | Locally Abundant | |||
| Species Traits | Geographically Unique | Locally Distinct |
Rare traits whatever the scale and the species pool |
Specialized traits supported by few species | Widespread traits supported by few scarce species | Traits supported by few common species |
| Locally Redundant | Impossible | Impossible | Impossible | Impossible | ||
| Geographically Shared | Locally Distinct | Traits supported by many rare species that do not co-occur | Specialized traits supported by many species | Traits supported by many widespread but locally sparse species that do not co-occur | Traits supported by many common species that do not co-occur | |
| Locally Redundant | Traits supported by many rare species | Specialized traits supported by many species | Traits supported by many widespread but locally sparse species |
Common traits whatever the scale and the species pool |
||
At each spatial scale, we can also visualize functional rarity vs. commonness with a biplot based on relative species frequencies and traits (illustration at local scale on Fig. I in Box 1). Category A corresponds to rare traits while category C is for common traits in a community. Since scarce species and redundant traits tend to be the most frequent within communities [40], we expect to find a majority of species belonging to category D while species from category B, i.e. those dominating communities and possessing distinct traits, may be the least frequent [17,50]. Given the heterogeneous distribution of species richness among these categories we suggest defining the bounds of each category with quantile values. To better discriminate rare vs. common traits, an alternative is to use the 5% most extreme values as a cut-off.
Although this framework is focused on defining functional rarity at the species level, it can be easily applied to a variety of taxonomic and population-level scales. For example, the recent awareness that intra-specific functional variability can have important impacts not only on local adaptation but also on community assembly and ecosystem functioning [51–53] has led to increased measurement of traits of individuals within species at different locations [54], as well as the development of new diversity metrics [55,56]. Our framework can be easily extended to include intra-specific variability, as functional rarity can be calculated at the individual level [48]. This can be further extended to lower levels of integration such as genotypes, genes or transcriptomes as well.
Measuring functional rarity
For over three decades a myriad of metrics have been developed to quantify many facets of biodiversity [57–63]. However this prolific field has poorly integrated the measurement of functional rarity vs. commonness.
To combine the different facets of rarity (Table 1, Box 1) into a single index, we propose an “integrated” view of functional rarity that accounts for both species functional distinctiveness/uniqueness (based on traits, Box 2) and its scarcity/restrictedness based on local and regional frequencies, Box 3). Functional rarity of species i can be expressed at the local scale as:
With Di being species functional distinctiveness and Si being species scarcity within a given community. At the regional scale, functional rarity of species i is expressed as:
With Ui being species functional uniqueness at the regional scale and Ri its geographic restrictedness.
Box 2. Measuring functional distinctiveness and uniqueness.
The main difference between functional distinctiveness and uniqueness is the scale at which the rarity of species traits is assessed. At the local scale, functional distinctiveness takes into account all species within the community to measure whether species i is more or less functionally close to the rest of the community [40]. At the regional scale, functional uniqueness relies on the functionally nearest species to measure the extent to which species i has no functional equivalent (or redundancy) in the pool [90]. These two indices simply correspond to the Mean Pairwise Distance (MPD) and the Mean Nearest Taxon Distance (MNTD) measuring the isolation (based on phylogenetic relationships) of each species from all the others and to its closest relative, respectively [91].
Functional distinctiveness of species i is thus defined as the mean functional distance to the N other species:
Where N is the number of species within the community and dij is the functional distance between species i and j. dij is scaled between 0 and 1 by diving all functional distances between species by the maximum value among pairs within the pool.
Functional distinctiveness can also be weighted by species relative abundance since a species is even more distinct if not sharing traits with the most abundant species within the community:
To avoid considering the abundance of focal species i in the calculation of functional distinctiveness, since it is already acknowledged to assess its local scarcity (Box 3), Abj is the relative abundance of species j among the N-1 remaining species.
Di is low when species i is functionally close to many others and/or to the most dominant within the community (high Abj values). As an extreme case Di tends to 0 when a species is hyper-dominant (Abi tends to 1, and the others to 0) and/or when all species are redundant with species i (dij tend to 0). At the opposite Di tends to 1 when the most distant species j (dij=1) is hyper-dominant (Abj tends to 1), or when all species have the maximum distance to species i within the community. Di ranges between 0 and 1.
Functional uniqueness (Ui) is measured by the functional distance to the nearest neighbor (or to the k nearest neighbors) within the regional species pool as:
Ui is high when species i has a unique combination of traits compared to other species and more particularly has a high functional distance even with its closest species. At the opposite Ui is 0 when species i shares exactly the same traits as another species in the pool, i.e. is perfectly redundant. Ui scales between 0 and 1 since dij scales between 0 and 1.
Box 3. Measuring species scarcity and restrictedness.
To measure species scarcity within communities we can simply use the inverse of relative abundance with two constraints: the index should range between 0 and 1 to have the same weight as distinctiveness in functional rarity measures (Box 2) and should have a pivotal value of 0.5 for a species with a relative abundance corresponding to 1/N, N being the number of species in the community. When the relative abundance of species i (Abi) is higher than 1/N (expectation under the perfect even distribution of abundance among species) the species tend to be dominant while the species tend to be scarcer than expected when Abi < 1/N. We can thus express scarcity as:
A species with a very low abundance will have a value close to 1 while dominant species (Abi close to 1) in species rich communities (N high) will tend to have low values. If Abi = 1/N then Si=0.5.
At the regional scale we can measure species restrictedness using the extent of occurrence or the area of occupancy, the most geographically restricted species receiving a value of 1 while widespread species will tend to values close to 0. In this case there is no need to use the pivotal value of 1/N since species geographical extents are independent. Instead we can use the geographic extent of the most widespread species to standardize restrictedness, which ranges from 0 to 1 [92].
Other rarity indices with multiple cut-off points can also be used [93] to assess species restrictedness but they are sensitive to species geographic range distributions.
The integration of both facets of rarity can be implemented in many ways. The simplest way is to build upon the additive framework measuring the Evolutionarily Distinct and Globally Endangered (EDGE) score [63,64]. By analogy, the functional rarity of species i, at a given scale, can be estimated as the addition of Di and Si at local scale or Ui and Ri at regional scale. This simple integration may be useful in a conservation perspective to provide a comprehensive picture of functional rarity. However, more complex frameworks can be proposed to combine both facets of rarity to weight them differently or to give a low value if one of the two is low (multiplicative).
A critical step is the choice of the traits to be included in the estimation of functional rarity (Box 1). Obviously it depends on the question being investigated. Trait-based theory has identified two types of species’ traits with respect to their potential functions [44]. ‘Effect traits’ determine the effect species have on ecosystem functioning, and they are distinguished from ‘response traits’, which determine the response of species to the environment [44]. This distinction has irrigated many fields of ecology [25] and helps identify relevant response and effect traits related to species’ impacts on ecosystem functioning on the one hand and species persistence and coexistence on the other. From a conservation perspective, it is however unclear which traits should be accounted for.
Once traits have been chosen for a specific research objective, pairwise species functional distances can be calculated using the Euclidean distance if traits are quantitative (after trait standardization to give the same weight) or the Gower distance if at least one trait is qualitative or if some values are missing [65]. Many ecological distinctiveness measures have been developed, most of them being designed within a phylogenetic perspective and based on tree branches linking species [66]. By analogy, we propose to measure functional distinctiveness (Di) and uniqueness (Ui) using a functional space where species are placed according to their traits (Fig. I in Box 1). The main difference between these two measures is that Di takes into account all species within the community and their abundances while Ui is based on the distance to the functionally nearest species in the regional pool. In other words distinctiveness assesses whether a species is more or less functionally close to the rest of the community while uniqueness estimates the extent to which a species has no functional equivalent in the regional pool (Box 2). Box 3 develops how to measure species scarcity and restrictedness.
Functional rarity and ecosystem functioning
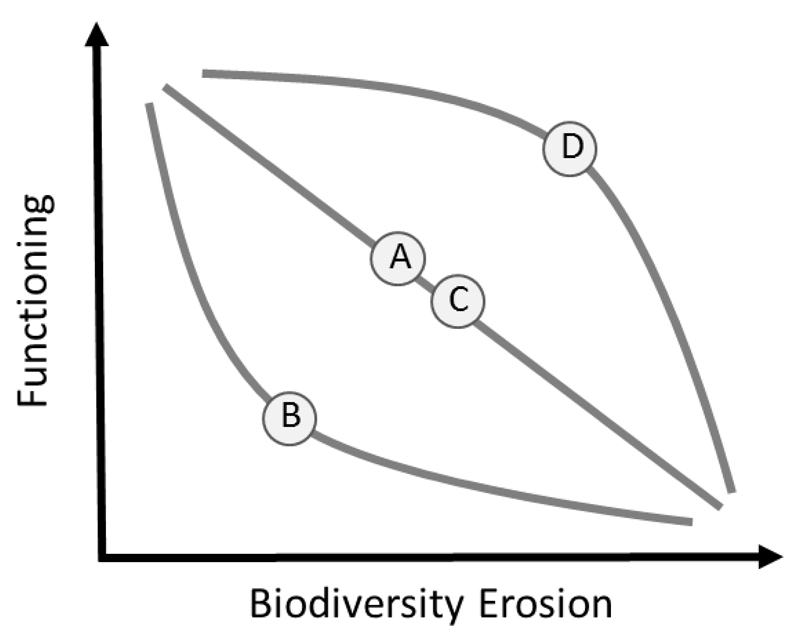
Assessing the importance of functional rarity in BEF will require appropriate design to disentangle the effect of species functional distinctiveness and of species scarcity. To this end, we propose hypothetical scenarios where the influence of biodiversity loss on the shape of BEF relationships (Fig. 1) depends on species functional rarity according to the four categories identified at local scale in Fig. I. Indeed if ecosystem functions like productivity are expected to decrease with biodiversity loss [27], we hypothesize that the shape of this decline will depend on the traits of the first species getting extirpated from the community (Fig. 1). When extirpated species support dominant but distinct traits (category B), the functioning will be strongly impacted in the first stage of biodiversity decline since irreplaceable traits will be lost. Conversely, this initial impact will be limited when the first extirpated species bear scarce indistinct traits (category D) because remaining species can perform the same functions. Intermediate relationships are expected when the extirpated species bear either scarce distinct or dominant indistinct traits (categories A and C). The long-term stability of ecosystem functioning [67] should also depend on the traits of species extirpated first, and on the type of traits. The response-effect trait framework has been especially useful for conceptualizing the maintenance (or resilience) of ecosystem functions. When extirpated species support dominant but indistinct (effect) traits (category C), the stability of ecosystem functioning will be strongly impacted (loss of functional redundancy). Moreover distinct (effect) traits (categories A and B) can become the common traits thus contributing to the long term insurance of ecosystem functioning [68]. When focusing on the long-term stability, the dynamics of communities is also at play [34] and accounting for response traits is thus of tremendous importance. The loss of species supporting scarce distinct (response) traits (category A) is expected to strongly impact the long-term stability of ecosystem functioning. To summarize, it is less straightforward to make qualitative predictions for the stability of ecosystem functioning because response and effect traits are both involved. We encourage ecologists to explore these scenarios theoretically and experimentally. This can come for example from experiments with microorganisms using dilution protocol where the rare species are lost first [69,70].
Figure 1.
Hypothetical consequences of biodiversity loss on local ecosystem functioning, for the four scenarios of functional rarity (i.e. when species of each group are extirpated first when biodiversity declines). The letters correspond to the categories on the distinctiveness-scarcity biplot at local scale, as described in the Fig. I of Box 1.
Functional rarity across the Tree of Life
An evolutionary perspective on functional rarity can shed light on the processes that are at the origin of functional rarity across the tree of life and allow its maintenance. Although no work has been done so far following our suggested framework, there is a long tradition in evolutionary biology to investigate how ecological specialisation evolve (e.g., refs. [71,72]). Pioneering work by Futuyma & Moreno [73] has focused on specialization for resource in terms of diet and feeding behaviour. However the general hypotheses around a framework to investigate the evolution of functional rarity still need to be developed [74]. Proposing a theoretical evolutionary approach to the integrated view of functional rarity (Figure I) is a long-term perspective. Indeed both species abundance and trait rarity (functional distinctiveness or uniqueness) are at play. Complex eco-evolutionary models will thus be required to answer this question. Examining the phylogenetic signal of trait rarity is a first key step. For instance, the question of whether specialist species or functionally distinct species are also phylogenetically distinct is poorly known (but see ref. [75]). In other words, is there any correlation between functional and phylogenetic distinctiveness or uniqueness? When examining the global evolutionary and functionally uniqueness of mammals, we did not find species that were both evolutionary and functionally unique (Fig. 2). The species pool under study is obviously critical in such relationships as is the set of traits used to estimate species functional uniqueness (Fig. 2, global vs. Europe). Interestingly, the shape of the relationship remains stable whether we restricted the analysis to the scale of Europe or to body mass as the sole trait (Fig. 2). This can have tremendous consequences for conservation biology [76] in case a geographical mismatch between taxonomic, phylogenetic and functional rarity hotspots is found (Box 4). If such pattern is confirmed at the community scale, this will also prevent using phylogenetic distinctiveness as a proxy for functional rarity in BEF research [77] or this will urge functional ecologists to better understand why phylogenetic diversity or dissimilarity matters for ecosystem functioning [78] (Box 4).
Figure 2.
Relationship between evolutionary and functional uniqueness of mammals at both global and European scales calculated with two different sets of traits. All mammals of the words that contained both traits and phylogenetic information were included (4616 species). Functional uniqueness was calculated without accounting for abundance. The global mammal functional distance matrices (Gower distance for multiple traits and Euclidean distance for log transformed body-mass) together with the phylogenetic distances were extracted from [81]. The list of mammal species for Europe was extracted from [82]. Colours represent the 10 and the 5 most frequent orders at global and European scales, respectively. The remaining orders (e.g. monotrema) are grouped into the Others category.
Box 4. Outstanding questions.
What are the ecological drivers of the maintenance of functional rarity in communities?
Elucidating the drivers of functional rarity requires considering the effects of both niche-based and neutral community assembly processes [94–97] on both functional distinctiveness and taxonomic scarcity. Indeed niche-based processes affect functional diversity [22,98] – and thus functional distinctiveness -, while neutral processes influence taxonomic diversity patterns by affecting species demography [99] – and thus species’ relative abundances.
Which evolutionary forces generate functional rarity?
Future work should not only focus on the relationship between phylogenetic distinctiveness and functional distinctiveness across different clades and regions (Fig. 2 for an example), but also investigate what are the mechanisms generating such patterns. Focusing on the extremes, it will be fundamental to understand through the lens of evolutionary processes why some old clades could emerge as functionally distinct/unique and others not.
Is there a geographic congruence (or mismatch) of hotspots of taxonomic, functional and phylogenetic rarity?
Mapping functional rarity at a global scale should be a primary objective of functional biogeography [24]. Potential mismatches between the geographic distributions of the different facets of rarity can imply to refine priority conservation areas (e.g., ref. [100]).
Concluding remarks
Our framework for measuring functional rarity paves the way of an ecology of outliers, which allows for a deeper understanding of the role of individuals, genotypes or species bearing distinct trait values within populations, ecosystems or biomes. A conservation strategy for ecological outliers can also emerge beyond the identification of areas where functional and evolutionary distinctiveness tend to aggregate [79]. For instance the effectiveness of protected areas for ecological outliers is still untested while the conditions (environment, human pressure) under which populations of ecological outliers can persist are unknown. This framework can also contribute to bridge the gap between evolutionary biology and ecology (Box 4). A combination of theoretical, observational and experimental work across the Tree of Life will help to explore this framework and identify the level at which functional rarity should be considered. This work is urgently needed, as rare taxa will be the first victims of what is now called the 6th extinction crisis [80].
Glossary
- Ecology of outliers
research area that studies how and why species (or organisms) are outliers given their local or regional abundances and trait distinctiveness, and the consequences of the persistence of those outliers for the structure and dynamics of communities and ecosystems.
- Functional distinctiveness (or trait distinctiveness)
local-scale characteristics of a species (or an organism) having traits dissimilar from those of other species (organisms) in the community. A metrics of functional distinctiveness assesses whether a species (or an organism) is more or less functionally close to the rest of the community.
- Functional uniqueness (or trait uniqueness)
regional-scale characteristics of a species (or an organism) possessing unique traits, i.e. not shared by any other species in the regional pool. A metrics of functional uniqueness assesses the extent to which a species (or an organism) has no functional equivalent in the regional pool.
- Functional rarity (or trait rarity)
characteristics of a species (or an organism) that integrates both functional distinctiveness and taxonomic scarcity at local scale, or both functional uniqueness and taxonomic restrictedness at regional scale. Functionally-rare species are ecological outliers. They possess the highest functional rarity value in the community (local scale) or in the regional pool (regional scale).
- Functional trait
any fitness-related morphological, physiological, phenological or behavioral feature measurable at the individual level.
- Taxonomic restrictedness (or species restrictedness)
regional-scale characteristics of a species being geographically restricted (e.g., small extent of occurrence or small area of occupancy).
- Taxonomic scarcity (or species scarcity)
local-scale characteristics of a species with low relative abundance (in terms of number of individuals or biomass) in the community.
References
- 1.Kunin W, Gaston K. The biology of rarity: patterns, causes, and consequences. Trends Ecol Evol. 1993;8:298–301. doi: 10.1016/0169-5347(93)90259-R. [DOI] [PubMed] [Google Scholar]
- 2.Soulé M. Conservation Biology: the Science of Scarcity and Diversity. Sinauer Associates; 1986. [Google Scholar]
- 3.Gaston K. Rarity. Chapman & Hall; 1994. [Google Scholar]
- 4.Kunin W, Gaston K. The Biology of Rarity: Causes and Consequences of Rare-Common Differences. Chapman & Hall; 1997. [Google Scholar]
- 5.Soulé M. What do we really know about extinction? In: Schonewald-Cox C, et al., editors. Genetics and Conservation: a Reference for Managing Wild Animal and Plant Populations. Benjamin/Cummings; 1983. [Google Scholar]
- 6.Murray B, et al. How plant life-history and ecological traits relate to species rarity and commonness at varying spatial scales. Ecology. 2002;27:291–310. [Google Scholar]
- 7.Lavergne S, et al. Do rock endemic and widespread plant species differ under the Leaf-Height-Seed plant ecology strategy scheme? Ecol Lett. 2003;6:398–404. [Google Scholar]
- 8.Gaston K, Blackburn T. Rarity and body size: generality is important. Conserv Biol. 1996;10:1295–1298. [Google Scholar]
- 9.Espeland E, Emam T. The value of structuring rarity: the seven types and links to reproductive ecology. Biol Conserv. 2011;20:963–985. [Google Scholar]
- 10.Jain M, et al. The importance of rare species: a trait-based assessment of rare species contributions to functional diversity and possible ecosystem function in tall-grass prairies. Ecol Evol. 2014;4:104–112. doi: 10.1002/ece3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker B, et al. Plant attribute diversity, resilience and ecosystem function: the nature and significance of dominant and minor species. Ecosystems. 1999;2:95–113. [Google Scholar]
- 12.Bracken M, Low N. Realistic losses of rare species disproportionately impact higher trophic levels. Ecol Lett. 2012;15:461–467. doi: 10.1111/j.1461-0248.2012.01758.x. [DOI] [PubMed] [Google Scholar]
- 13.Lyons K, et al. Rare species and ecosystem functioning. Conserv Biol. 2005;19:1019–1024. [Google Scholar]
- 14.Chase J. An inordinate foundness of rarity. PloS Biol. 2013;11:e1001573. doi: 10.1371/journal.pbio.1001573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pendleton RM, et al. Loss of rare fish species from tropical floodplain food webs affects community structure and ecosystem multifunctionality in a mesocosm experiment. PloS One. 2004;9:e8456. doi: 10.1371/journal.pone.0084568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Godet L, et al. Dissociating several forms of commonness in birds sheds new light on biotic homogenization. Global Ecol Biogeogr. 2015;24:416–426. [Google Scholar]
- 17.Calba S, et al. Measuring and explaining large-scale distribution of functional and phylogenetic diversity in birds: separating ecological drivers from methodological choices. Global Ecol Biogeogr. 2014;23:669–678. [Google Scholar]
- 18.Naeem S, et al. The functions of biological diversity in an age of extinction. Science. 2012;336:1401–1406. doi: 10.1126/science.1215855. [DOI] [PubMed] [Google Scholar]
- 19.McGill BJ, et al. Fifteens forms of biodiversity trend in the Anthropocene. Trends Ecol Evol. 2015;30:104–113. doi: 10.1016/j.tree.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 20.Pereira HM, et al. Essential biodiversity variables. Science. 2013;339:277–278. doi: 10.1126/science.1229931. [DOI] [PubMed] [Google Scholar]
- 21.Rabinowitz D. Seven forms of rarity. In: Synge H, editor. The Biological Aspects of Rare Plant Conservation. Wiley; 1981. pp. 205–217. [Google Scholar]
- 22.McGill BJ, et al. Rebuilding community ecology from functional traits. Trends Ecol Evol. 2006;21:178–185. doi: 10.1016/j.tree.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 23.Shipley B. From Plant Traits to Vegetation Structure Chance and Selection in the Assembly of Ecological Communities. Cambridge University Press; 2010. [Google Scholar]
- 24.Violle C, et al. The emergence and promise of functional biogeography. Proc Natl Acad Sci USA. 2014;111:13690–13696. doi: 10.1073/pnas.1415442111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laughlin DC. Applying trait-based models to achieve functional targets for theory-driven ecological restoration. Ecol Lett. 2014;17:771–784. doi: 10.1111/ele.12288. [DOI] [PubMed] [Google Scholar]
- 26.Cadotte MW, et al. Predicting communities from functional traits. Trends Ecol Evol. 2015;30:500–511. doi: 10.1016/j.tree.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Hooper DU, et al. Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 28.Diaz S, et al. Functional Diversity - at the Crossroads between Ecosystem Functioning and Environmental Filters. In: Canadell J, et al., editors. Terrestrial Ecosystems in a Changing World. The IGBP Series, Springer-Verlag; 2007. [Google Scholar]
- 29.Cadotte MW, et al. Beyond species: functional diversity and the maintenance of ecological processes and services. J Appl Ecol. 2011;48:1079–1087. [Google Scholar]
- 30.Power ME, et al. Challenges in the quest for keystones. BioScience. 1996;46:609–620. [Google Scholar]
- 31.Isbell F, et al. The biodiversity-dependent ecosystem service debt. Ecol Lett. 2015;18:119–134. doi: 10.1111/ele.12393. [DOI] [PubMed] [Google Scholar]
- 32.Levine J, HilleRisLambers J. The importance of niches for the maintenance of species diversity. Nature. 2009;461:254–257. doi: 10.1038/nature08251. [DOI] [PubMed] [Google Scholar]
- 33.Roughgarden J. Evolution of niche width. Am Nat. 1972;106:683–718. [Google Scholar]
- 34.Loreau M, et al. Biodiversity as spatial insurance in heterogeneous landscapes. Proc Natl Acad Sci USA. 2003;100:12765–12770. doi: 10.1073/pnas.2235465100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low-Décarie E, et al. Community rescue in experimental metacommunities. Proc. Natl Acad Sci USA. 2015;112:14307–14312. doi: 10.1073/pnas.1513125112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solan M, et al. Extinction and ecosystem function in the marine benthos. Science. 2004;306:1177–1180. doi: 10.1126/science.1103960. [DOI] [PubMed] [Google Scholar]
- 37.Grime JP. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol. 1998;86:902–910. [Google Scholar]
- 38.Ricciardi A, Ramussen JB. Extinction rates of North American freshwater fauna. Conserv Biol. 1999;13:1220–1222. [Google Scholar]
- 39.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423:280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 40.Mouillot D, et al. Rare species support vulnerable functions in high-diversity ecosystems. PloS Biol. 2013;11:e1001569. doi: 10.1371/journal.pbio.1001569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harnik PG, et al. Long-term differences in extinction risk among the seven forms of rarity. Proc R Soc B. 2002;279:4969–4976. doi: 10.1098/rspb.2012.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Westoby M, et al. Plant ecological strategies: some leading dimensions of variation between species. Annu Rev Ecol Evol Syst. 2002;33:125–159. [Google Scholar]
- 43.Lavorel S, et al. A novel framework for linking functional diversity of plants with other trophic levels for the quantification of ecosystem services. J Veg Sci. 2013;24:942–948. [Google Scholar]
- 44.Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol. 2002;16:545–556. [Google Scholar]
- 45.Violle C, et al. Let the concept of trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- 46.Violle C, Jiang L. Towards a trait-based quantification of species niche. J Plant Ecol-UK. 2009;2:87–93. [Google Scholar]
- 47.Gravel D, et al. The meaning of functional trait composition of food webs for ecosystem functioning. Phil Tran R Soc B. 2016;371:20150268. doi: 10.1098/rstb.2015.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Umana MN, et al. Commonness, rarity, and intraspecific variation in traits and performance in tropical tree seedlings. Ecol Lett. 2015;8:1329–1337. doi: 10.1111/ele.12527. [DOI] [PubMed] [Google Scholar]
- 49.Leitão RP, et al. Rare species contribute disproportionately to the functional structure of species assemblages. Proc R Soc London Ser B. 2016;283:20160084. doi: 10.1098/rspb.2016.0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thompson K, et al. Little evidence for limiting similarity in a long-term study of a roadside plant community. J Ecol. 2010;98:480–487. [Google Scholar]
- 51.Albert C, et al. On the importance of intraspecific variability for the quantification of functional diversity. Oikos. 2012;121:116–126. [Google Scholar]
- 52.Jung V, et al. Intraspecific variability and trait-based community assembly. J Ecol. 2010;98:1134–1140. [Google Scholar]
- 53.Zuppinger-Dingley D, et al. Selection for niche differentiation in plant communities increases biodiversity effects. Nature. 2014;515:108–111. doi: 10.1038/nature13869. [DOI] [PubMed] [Google Scholar]
- 54.Siefert A, et al. A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecol Lett. 2015;18:1406–1419. doi: 10.1111/ele.12508. [DOI] [PubMed] [Google Scholar]
- 55.Fontana S, et al. Individual-level trait diversity concepts and indices to comprehensively describe community change in multidimensional trait space. Funct Ecol. 2015;30:808–818. [Google Scholar]
- 56.Violle C, et al. The return of the variance: intraspecific variability in community ecology. Trends Ecol Evol. 2012;27:244–252. doi: 10.1016/j.tree.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 57.Mason NWH, et al. Functional richness, functional evenness and functional divergence: the primary components of functional diversity. Oikos. 2005;111:112–118. [Google Scholar]
- 58.De Bello F, et al. Which trait dissimilarity for functional diversity: trait means or trait overlap? J Veg Sci. 2013;24:807–819. [Google Scholar]
- 59.Pavoine S, et al. On the challenge of treating various types of variables: application for improving the measurement of functional diversity. Oikos. 2009;118:391–402. [Google Scholar]
- 60.Chao A, et al. Phylogenetic diversity measures based on Hill numbers. Phil Trans Roy Soc B. 2010;365:3599–3609. doi: 10.1098/rstb.2010.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mouchet M, et al. Functional diversity measures: an overview of their redundancy and their ability to discriminate community assembly rules. Funct Ecol. 2010;24:867–876. [Google Scholar]
- 62.Cadotte MW, et al. The ecology of differences: integrating evolutionary and functional distances. Ecol Lett. 2013;16:1234–1244. doi: 10.1111/ele.12161. [DOI] [PubMed] [Google Scholar]
- 63.Cadotte M, Davies T. Rarest of the rare: advances in combining evolutionary distinctiveness and scarcity to inform conservation at biogeographical scales. Divers Distrib. 2010;16:376–385. [Google Scholar]
- 64.Isaac NJB, et al. Mammals on the EDGE: conservation priorities based on threat and phylogeny. PloS One. 2007;2:e296. doi: 10.1371/journal.pone.0000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Legendre P, Legendre L. Numerical Ecology. Elsevier; 1998. [Google Scholar]
- 66.Redding DW, et al. Measuring evolutionary isolation for conservation. PloS One. 2014;9:e113490. doi: 10.1371/journal.pone.0113490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: the insurance hypothesis. Proc Natl Acad Sci USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mariotte P, et al. Subordinate plant species enhance community resistance against drought in semi-natural grasslands. J Ecol. 2013;101:763–773. [Google Scholar]
- 69.Peter H, et al. Function-specific response to depletion of microbial diversity. ISME J. 2011;5:351–361. doi: 10.1038/ismej.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bouvier T, et al. Contrasted effect of temporal and spatial insurance in marine bacterioplankton communities. PloS One. 2012;7:e37620. doi: 10.1371/journal.pone.0037620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bonetti MF, Wiens JJ. Evolution of climatic niche specialization: a phylogenetic analysis in amphibians. Proc R Soc B-Biol Sci. 2014;281 doi: 10.1098/rspb.2013.3229. 20133229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cornwell WK, et al. Functional distinctiveness of major plant lineages. Funct Ecol. 2014;102:345–356. [Google Scholar]
- 73.Futuyma DJ, Moreno G. The evolution of ecological specialization. Annu Rev Ecol Evol Syst. 1988;19:207–233. [Google Scholar]
- 74.Fernandez MH, Vrba ES. Macroevolutionary processes and biomic specialization: testing the resource-use hypothesis. Evol Ecol. 2005;19:199–219. [Google Scholar]
- 75.Thuiller W, et al. Conserving the functional and phylogenetic trees of life of European tetrapods. Phil Tran R Soc B. 2015 doi: 10.1098/rstb.2014.0005. 20140005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winter M, et al. Phylogenetic diversity and nature conservation: where are we? Trends Ecol Evol. 2013;28:199–204. doi: 10.1016/j.tree.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 77.Srivastava D, et al. Phylogenetic diversity and the functioning of ecosystems. Ecol Lett. 2012;15:637–648. doi: 10.1111/j.1461-0248.2012.01795.x. [DOI] [PubMed] [Google Scholar]
- 78.Cadotte MW. Experimental evidence that evolutionarily diverse assemblages result in higher productivity. Proc Natl Acad Sci USA. 2013;110:8996–9000. doi: 10.1073/pnas.1301685110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jetz W, et al. Distribution and conservation of global evolutionary distinctness in birds. Curr Biol. 2014:919–930. doi: 10.1016/j.cub.2014.03.011. 2014. [DOI] [PubMed] [Google Scholar]
- 80.Ceballos G, et al. Accelerated modern human - induced species losses: Entering the sixth mass extinction. Science Adv. 2015;1:1–5. doi: 10.1126/sciadv.1400253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mazel F, et al. Multifaceted diversity-area relationships reveal global hotspots of mammalian species, trait and lineage diversity. Global Ecol Biogeogr. 2014;23:836–847. doi: 10.1111/geb.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zupan L, et al. Spatial mismatch of phylogenetic diversity across three vertebrate groups and protected areas in Europe. Divers Distrib. 2014;20:674–685. doi: 10.1111/ddi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kattge J, et al. TRY: a global database of plant traits. Global Change Biol. 2011;17:2905–2935. [Google Scholar]
- 84.Dell AI, et al. Trait database for size and temperature dependence of species ineractions. Ecology. 2013;94:1205–1206. [Google Scholar]
- 85.Wilman H, et al. EltonTraits 1.0: Species-level foraging attributes of the world's birds and mammals. Ecology. 2014;95:2027–2027. [Google Scholar]
- 86.Homburg K, et al. Carabids.org - A dynamic online database of ground beetle species traits (Coleoptera, Carabidae) Insect Conserv Divers. 2014;7:195–205. [Google Scholar]
- 87.Sandel B, et al. Estimating the missing species bias in plant trait measurements. J Veg Sci. 2015;26:828–838. [Google Scholar]
- 88.Violle C, et al. Trait databases: misuses and precautions. J Veg Sci. 2015;26:826–827. [Google Scholar]
- 89.Violle C, et al. Vegetation Ecology meets Ecosystem Science: permanent grasslands as a functional biogeography case study. Sci Tot Env. 2015;534:43–51. doi: 10.1016/j.scitotenv.2015.03.141. [DOI] [PubMed] [Google Scholar]
- 90.Buisson L, et al. Toward a loss of functional diversity in stream fish assemblages under climate change. Global Change Biol. 2013;19:387–400. doi: 10.1111/gcb.12056. [DOI] [PubMed] [Google Scholar]
- 91.Webb CO, et al. Phylogenies and community ecology. Annu Rev Ecol Evol Syst. 2002;33:475–505. [Google Scholar]
- 92.Dapporto L, Dennis RLH. Species' richness, rarity and endemicity of Italian offshore islands: complementary signals from island-focused and species-focused analyses. J Biogeogr. 2008;35:664–674. [Google Scholar]
- 93.Leroy B, et al. Improving occurrence based rarity metrics in conservation studies by including multiple rarity cut-off points. Insect Conserv Divers. 2012;5:159–168. [Google Scholar]
- 94.Adler P, et al. A niche for neutrality. Ecol Lett. 2007;10:95–104. doi: 10.1111/j.1461-0248.2006.00996.x. [DOI] [PubMed] [Google Scholar]
- 95.Vergnon R, et al. Niches versus neutrality: uncovering the drivers of diversity in a species-rich community. Ecol Lett. 2009;12:1079–1090. doi: 10.1111/j.1461-0248.2009.01364.x. [DOI] [PubMed] [Google Scholar]
- 96.Holt RD. Emergent neutrality. Trends Ecol Evol. 2006;21:531–533. doi: 10.1016/j.tree.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 97.Gravel D, et al. Reconciling niche and neutrality: the continuum hypothesis. Ecol Lett. 2006;9:399–409. doi: 10.1111/j.1461-0248.2006.00884.x. [DOI] [PubMed] [Google Scholar]
- 98.Enquist BJ, et al. Scaling from traits to ecosystems: developing a general Trait Driver Theory via integrating trait-based and metabolic scaling theories. Adv Ecol Res. 2015;52 [Google Scholar]
- 99.Hubbell SP. The Unified Neutral Theory of Biodiversity and Biogeography. Princeton University Press; 2001. [DOI] [PubMed] [Google Scholar]
- 100.Devictor V, et al. Spatial mismatch and congruence between taxonomic, phylogenetic and functional diversity: the need for integrative conservation strategies in a changing world. Ecol Lett. 2010;13:1030–1040. doi: 10.1111/j.1461-0248.2010.01493.x. [DOI] [PubMed] [Google Scholar]