Abstract
Historically, intra-arterial (IA) drug administration for malignant brain tumors including glioblastoma multiforme (GBM) was performed as an attempt to improve drug delivery. With the advent of percutaneous neuorovascular techniques and modern microcatheters, intracranial drug delivery is readily feasible; however, the question remains whether IA administration is safe and more effective compared to other delivery modalities such as intravenous (IV) or oral administrations. Preclinical large animal models allow for comparisons between treatment routes and to test novel agents, but can be expensive and difficult to generate large numbers and rapid results. Accordingly, we developed a murine model of IA drug delivery for GBM that is reproducible with clear readouts of tumor response and neurotoxicities. Herein, we describe a novel mouse model of IA drug delivery accessing the internal carotid artery (ICA) to treat ipsilateral implanted GBM tumors that is consistent and reproducible with minimal experience. The intent of establishing this unique platform is to efficiently interrogate targeted anti-tumor agents that may be designed to take advantage of a directed, regional therapy approach for brain tumors.
Keywords: glioblastoma multiforme, GBM, intra-arterial, infusion, regional cancer therapy, mouse
Introduction
Glioblastoma muliforme (GBM) is the most common and lethal type of primary brain tumor with a median overall survival of 15 month and a 2-year survival rate of only 26% [1, 2]. Despite recent advances in surgery, radiotherapy and chemotherapy, GBM remains incurable and challenging to treat [3–7]. One method to increase the efficacy of chemotherapy is to selectively increase the dose delivered to the tumor by using intra-arterial (IA) delivery. IA therapy has long been considered a potential method to improve chemotherapy delivery to GBM with the advantages of higher tumor drug exposure compared to non-targeted tissue and a more localized delivery than intravenous (IV) administration [8]. Clinical comparisons between IA and IV administration exist; however, these studies are limited and lack robust preclinical rationale [9]. Currently, IA administration is controversial as clinical applications have suggested, but have not proven, an improved efficacy [10–12].
Preclinical infusion of the internal carotid artery (ICA) has been accomplished in large animals including the rat, rabbit, cat, and monkey [13–15]. However, large animal models are expensive to house and maintain and evaluation of therapeutic effect and neurotoxicity can be difficult. Murine models for the regional therapy of cancers by directed catheter infusions have been previously described [16–19]. Murine models, particularly mouse models, are relatively inexpensive, are easily handled and assessed, can generate large numbers quickly, and take advantage of numerous existing mouse-specific reagents. Furthermore, non-invasive imaging techniques are readily available and allow for longitudinal studies of response and potential toxicity.
Extending techniques developed for the regional therapies of other cancers, including the use of the venerable drug melphalan, we developed a mouse intracranial infusion model for the selective treatment of GBM tumors. Melphalan is a nitrogen mustard alkylating chemotherapy that is the most commonly used agent for the regional therapy of a variety of cancers and sites including the limb [20], pelvis [21], and liver [22]. Based upon its drug kinetics, tumor uptake, and synergy with hyperthermia, the regional administration of melphalan may be effective for any tumor type and was therefore tested for ICA infusion in our proposed system. The model described here was developed independently based upon our experience with regional therapies at other tumor sites [16] and has potential advantages compared to the only other similar mouse models published to date [18, 19]. Firstly, our model of ICA infusion was designed to be delivered antegrade to ensure complete delivery of infusate unlike the retrograde approach via the external carotid artery [18]. Theoretically, the retrograde approach may spill into the systemic circulation depending upon the mouse’s mean arterial pressure and the pressure applied to the infusate. Secondly, our catheter diameters are well defined and constant unlike models that require manual creation of small diameter catheters from larger ones [19]. Hand-made catheters have the potential to create variability in inner diameters and subsequently influence drug delivery rates and pressures. Also, by using pre-designed catheters, comparisons between experiments and investigators are standardized. Thus, the described platform may have advantages compared to existing models, was readily reproducible, and exhibited minimal procedure-related morbidity.
Materials and Methods
Mice
Female athymic mice aged 8 weeks were purchased from the National Cancer Institute (Frederick, MD) for use in this study. Mice were fed a standard laboratory diet and housed under standard light and accommodation conditions. All experimental protocols were approved by the Roswell Park Cancer Institutional Animal Care and Use Committee.
Cell lines
Human primary glioblastoma cells, U87MG cells (ATCC, Manassas, VA), were cultured in DMEM high glucose (Gibco #11995) supplemented with 10% fetal bovine serum, 100 units/ml penicillin, 100 μg/ml streptomycin, and 10mM HEPES buffer (Life Technologies). Cells were harvested before confluence and used for tumor inoculation.
Orthotopic tumor inoculation
Mice were anesthetized with isoflurane gas (induction with 4% isoflurane, maintenance with ~2% isoflurane, Abbott Laboratories, Chicago, IL). Animals were then secured in a stereotaxic frame and isoflurane anesthesia was administered through a nose cone adapted for the stereotaxic frame to maintain an appropriate level of anesthesia. The appropriate depth of anesthesia was verified by toe pinch and observation of respiration. A midline incision was made to the cranium and the pericranium was stripped to expose the skull. A dental drill was used to create a 1mm burr hole in the skull at 1.0 mm anterior to the bregma suture and 2.0 mm lateral to the midline suture. A Hamilton syringe (10 μl with a 26 gauge needle, Hamilton Co., Reno, NV, USA) was passed into the brain 3.0 mm from dura and 2.5 μl of U87MG cell suspension was injected over a period of 3 minutes, the needle was left in place for 5 minutes and then withdrawn over 4 minutes. The skin was re-approximated with 5-0 silk and Vetbond adhesive. The animals were monitored for recovery from anesthesia, returned to the animal care facilities, and resumed their normal activity in cages.
Drugs for ICA infusion
1 mg Melphalan (Sigma-Aldrich, St. Louis, MO) was pre-dissolved with 20 μL of 100% ethanol and 0.4 μL of 6N hydrochloric acid, and then infused at a concentration of 30 or 60 μg/mL in normal saline. 2% of Evans Blue dye (Sigma-Aldrich, St. Louis, MO) was infused with normal saline for studies examining infusate distribution.
ICA infusion
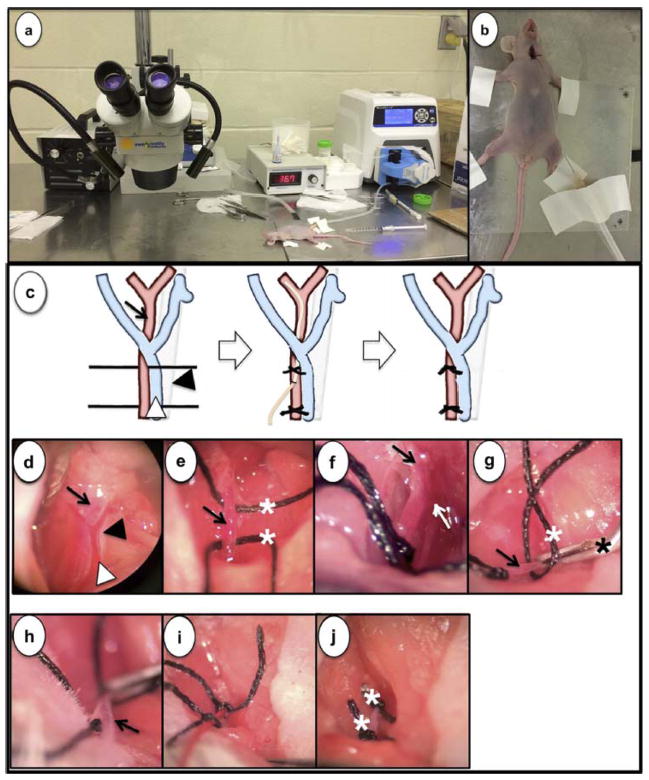
After allowing 7 days for tumor establishment, ICA infusion was performed by a single investigator (MK). Tumor bearing mice received inhaled anesthesia with isoflurane to an appropriate level. Mice were placed on a warming platform to maintain normothermia throughout the procedure and a dissecting microscope (magnification ×6.5 ~ ×35) (VWR, West Chester, PA) facilitated visualization of the anatomy (Fig. 1a). The set up allowed for a prolonged infusion with the catheter stabilized to the surgical platform at the completion of the procedure (Fig. 1b). The neck of the mouse was prepped with alcohol and betadine, and a 1.5 cm longitudinal midline neck incision was made using scissors. The left sternomastoid and paratracheal muscles were retracted laterally and medially, respectively. Microscopically, a view of the common carotid artery (CCA), internal jugular vein (IJV) and left vagus nerve were obtained (Fig. 1c, d) via a midline neck incision and individually dissected. A 5-0 silk tie was placed around the proximal and distal parts of the CCA respectively (Fig. 1c, e). The proximal 5-0 silk tie was then tied (Fig. 1f) and a vascular clip was placed beyond the distal silk tie (Fig. 1g). An arteriotomy was created using microscissors (Roboz, Gaitherburg, MD) in the left CCA between the proximal silk tie and the vascular clip which allowed for cannulation with a sterile microcatheter (Fig. 1h). The cannula consisted of low-density polyethylene with a fixed inner diameter of 0.2 mm and outer diameter of 0.36 mm (Scientific Commodities, Lake Havasu City, AZ). Under direct visualization, the microcatheter was advanced into the left ICA and secured with the distal 5-0 silk ties (Fig. 1c, i). The microcatheter was then attached to a peristaltic infusion pump (Masterflex L/S; Cole-Parmer Instrument Co., Vernon Hills, IL). Using a reservoir of chemotherapy, infusion was commenced at 0.15 ml/min for 10 minutes. At the completion of the infusion, the cannula was removed and discarded as chemotherapeutic waste. The silk ties were used to fully occlude the left CCA cannulation site in order to prevent bleeding (Fig. 1c, j). The skin was re-approximated with 5-0 silk suture and Vetbond adhesive. All mice were recovered on a warming blanket and injected subcutaneously with buprenorphine (0.2 mg/kg body weight) for pain control.
Fig. 1.
The necessary instruments needed to perform ICA infusions are commonly available in most labs including a light source, dissecting microscope, a hotplate, and a peristaltic pump (a). Following either inhaled or injected anesthesia, neck hyperextension is required for exposure of the carotid triangle (b). Schematic representation of each step of the ICA infusion in the carotid triangle containing CCA (black arrow), IJV (white arrowhead), and vagus nerve (black arrowhead) including catheter placement and eventual CCA ligation (c). Photomicrograph via a dissecting microscope (magnification, 6.5X to 35X) shows CCA (black arrow), IJV (white arrowhead) and vagus nerve (black arrowhead) (d). CCA was anchored with 5-0 black silk (white asterisk) (e). CCA was visualized as ICA (black arrow) and ECA (white arrow) diverged (f). A small arteriotomy (arrow) was made in the CCA with microscissors after applying 5-0 black silk (white asterisk) and a vascular clip (black asterisk) (g). A microcatheter (arrow) was cannulated through the arteriotomy (h) and secured with black silk ties (i). Two black silk ties (white asterisk) were permanently applied after removing the catheter (j).
Neurological evaluation
Mice were evaluated by one investigator (MK) for neurological deficits using the Garcia test (Table 1a). Mice were given a score of 3 to 18 (normal) [23] as the scoring system consists of 6 tests (spontaneous activity, axial sensation, vibrissae proprioception, symmetry of limb movement, forelimb outstretching, climbing) with a possible score range of 0 to maximum 3.
Table 1.
Garcia neurological evaluation criteria and scoring (a). Procedure efficiency and morbidity (b).
| (a) | ||||
|---|---|---|---|---|
|
| ||||
| Neurological exam | Score | |||
|
| ||||
| 3 | 2 | 1 | 9 | |
| Spontaneous activity | Moved freely, explored environment and reached the upper rim ≥3 sides of cage | Moves hesitantly, explored, and reached the upper rim <3 sides of cage | Limited movement, did not rise on hindlimbs | No purposeful movement |
| Symmetry of limb movement | All limbs extended symmetrically | Limbs on one side outstretched less(or more slowly) than contralateral side | limbs on one side showed minimal movement | No movement on one side |
| Forelimb movement | Both forelimbs outstretched, walked symmetrically | Impaired forelimb walked with asymmetric outstretch | limbs on one side showed minimal movement | No forelimb movement on one side |
| Climbing | Climbed to cage top easily with a frim grip | Asymmetric or impaired climbing, weak grip | Failed to climb or circled instead | |
| Axial sensation | Equally startled to the stimulus on both sides of trunk | Slower reaction on one side compared to contralateral side | No response to stimulus on one side | |
| Vibrissae proprioception | Turned head equally to cotton wisp on both sides | Reacted more slowly on one side compared to contralateral side | No response to stimulus on one side | |
|
| ||||
| (b) | ||||
|
| ||||
| Mouse # | Cannulation time (min) | Procedure complications | Garcia score mean | |
|
| ||||
| 1–10 | 23.0 ±2.8 | 1 fatal hemorrhage | 18 | |
| 11–25 | 17.5 ± 1.0* | None | 18 | |
p <0.05
Distribution of Evans Blue dye
A 2% solution of Evans Blue dye in normal saline was infused into the left ICA to examine the qualitative perfusate distribution. Immediately following Evans Blue dye administration into two mice, the brains were fixed by transthoracic cardiac infusion with a 4% paraformaldehyde solution (Electron Microscopy Sciences, Hatfield, PA). The brains were harvested by making a T-shaped incision that was centered on the vertical midline scalp extending from the level of the cervical-cranial junction to the nose. A horizontal incision was made at the level of the cervical-cranial with fine scissors (Roboz, Gaitherburg, MD). The calvarium was opened by scissors blades, and then the skull edges were extracted. Within 10 minutes after the end of ICA injection, the brains were completely removed and then fixed in 4% paraformaldehyde solution for an additional 4 hours ex vivo.
Gross findings and staining of brain tissue
Mice were randomized 7 days after tumor implantation and divided into three groups [vehicle (normal saline) vs. melphalan (30 or 60 μg/mL in normal saline)] given ICA. In these preliminary studies designed to test the ICA infusion model, a total of 9 mice received vehicle, 2 mice melphalan 30 μg/mL, and 2 mice 60 μg/mL. 7 days after treatments, the mice were anesthetized with Ketamine (100mg/kg) and Xylazine (10mg/kg) for the brain fixation, and then brains were cryoprotected by incubation with 15%–30% sucrose in PBS (overnight in each sucrose solution) and embedded in the cryo-medium Neg-50 (Fisher Scientific, Waltham, MA). Coronal cryosections 20 μm thick were prepared on Leica CM1900 cryostat. Gross findings were acquired with Stereomicroscope Discovery V12 using AxioCam HRc (Carl Zeiss, Jena, Germany). H&E staining (StatLab Medical Products, McKinney, TX) was performed for overall tumor pathology. Giemsa (Sigma-Aldrich, St. Louis, MO) and DAPI (Life Technologies, Grand Island, NY) were used for nuclei identification, and then images were acquired with Zeiss Axio Imager Z1 fluorescence microscope using AxioCam MRc & MRm-Fl (Carl Zeiss, Jena, Germany). After fixation the specimens were washed in PBS, embedded in OCT compound, and serially sectioned at 12 μm with a Leica CM1850 cryotome. Apoptotic cells were stained by the indirect terminal deoxynucleotidyl transferase- mediated deoxyuridine triphosphate nick end labeling (TUNEL) method with a Fluorescein conjugated reporter (Millipore Corporation, Billerica, MA). Before immunohistochemistry staining, sections were dried at room temperature, washed with PBS, incubated for 30 min with blocking solution (10% bovine serum/0.4% Triton X-100 in PBS), and treated for 2 hours at room temperature with a mouse antibody against Ki67 (abcam, Cambridge, MA).
Statistics
The duration of ICA cannulation was compared between the first 10 and subsequent 15 mice using Student’s t-test with significance defined as p<0.05.
Results
Cannulation proficiency and morbidity
Mouse ICA cannulation became more proficient and successful over time and had limited morbidity (Table 1b). A single investigator (MK) performed a total of 25 ICA cannulations with an average time of 19.7 ± 1.3 min (range, 13 to 40 min). The average procedure time for the first 10 cases was 23 ± 2.8 min, with an improved mean 17.5 ± 1.0 min to complete the cannulation after 10 cases. Increasing experience was directly related to a decreased ICA cannulation time (p = 0.04). A single procedural complication and eventual mortality was noted in one mouse during the initial 10 cannulations which consisted of bleeding from attempted cannulation. The ICA infusion procedure was well tolerated in all animals with no neurologic deficits detected in animals administered vehicle (normal saline). Furthermore, in animals treated with melphalan at either 30 or 60 μg/mL, no neurological deficits were noted at 24 hours post procedure (Garcia score = 18).
Distribution of ICA infusate
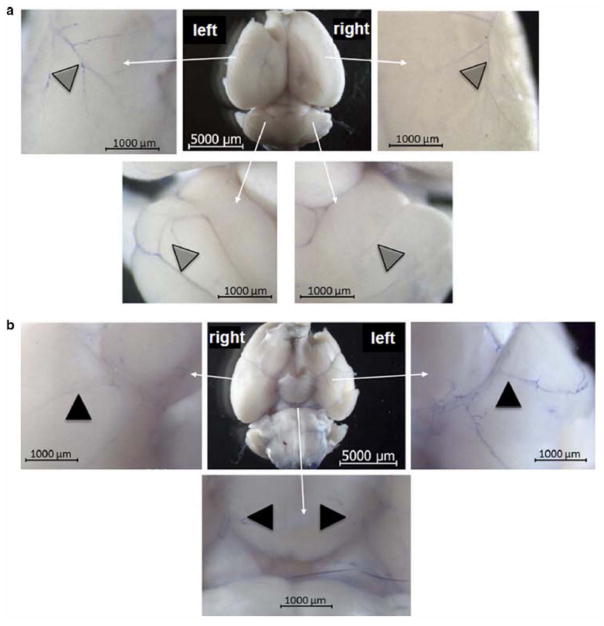
Following 2% Evans Blue dye administration to the left ICA, harvested brains of two mice demonstrated consistent macroscopically visible distribution within the neurovasculature. Viewing the dorsal aspect of the brain, the left hemispheric vessels were more prominently blue compared to the right hemisphere (Fig. 2a). Similar observations were made when examining the ventral aspect of the brain with the left hemisphere demonstrating a more intense blue-stained vasculature (Fig. 2b). In both the dorsal and ventral views, the vessels appeared intact and supported blood flow as noted by the presence of blue dye. As the brains were immediately harvested after Evans Blue delivery, perfusion into the brain parenchyma was not evaluable. Overall, in this qualitative assessment, it appeared that Evans Blue dye presence favored the ipsilateral side of infusion suggestive of targeted delivery.
Fig. 2.
Representative photomicrograph of gross examination of brain after infusion with 2% Evans Blue dye demonstrates selective delivery with ICA infusion. The infused left hemispheric vessels were intact and stained predominantly in the ventral (a) and dorsal views (b) compared to the right hemisphere. (n=2 mice)
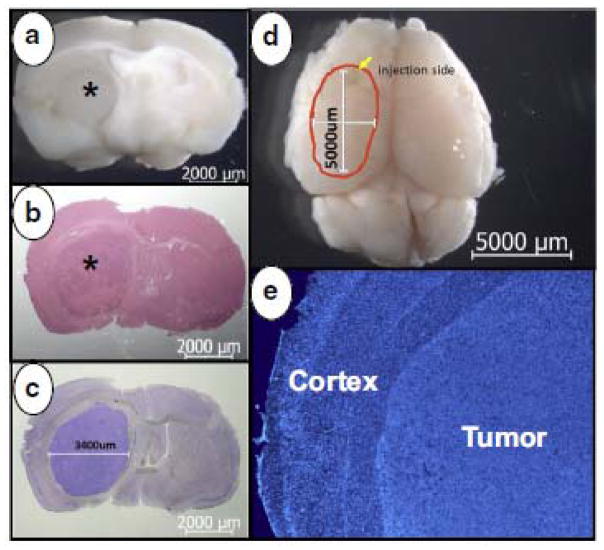
Measurement of tumor response
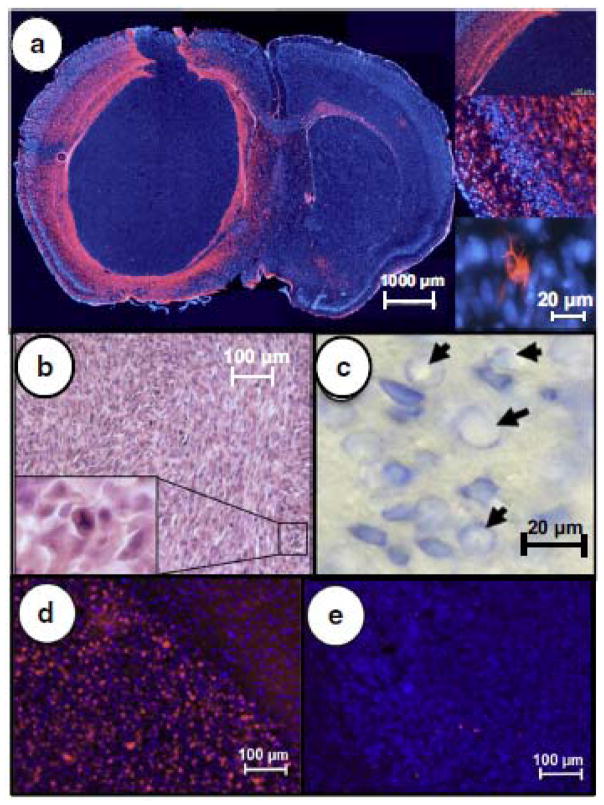
Tumors were clearly delineated from normal brain parenchyma by IHC and formed well defined, visible tumors (Fig. 3a). Tumor tissue was contrasted by simple H+E staining (Fig. 3b), but was further contrasted with Giemsa staining (Fig. 3c). Tumors were consistent and typical measurements (5 x 3.4 mm) at the time of treatment (day 7 after implantation) are shown in perpendicular views (Fig. 3c, 3d). The identification of cell nuclei at a higher magnification by DAPI staining (Fig. 3e), also demonstrated a clear delineation between the tumor and the overlying brain cortex. The U87MG cells injected recapitulated human GBM tumors with a similar distribution of glial fibrillary acidic protein (GFAP) around the tumor periphery and diffusely throughout the tumor (Fig 4a). While pseudopalisading necrosis which is commonly noted in GBM was not clearly evident, multiple bizarre mitotic figures were observed similar to human disease (Fig. 4b). The enhanced view of the brain parenchyma seen with Giemsa staining could detect toxicity in the form of vacuolated nuclei (Fig. 4c). Further evaluation of tumor response was possible by measuring Ki67 levels in the treated tumors (Fig. 4d) and the degree of apoptosis present by TUNEL staining (Fig. 4e). In these experiments designed to examine the feasibility of IA drug delivery, there were no differences in tumor responses at the doses of melphalan tested compared to vehicle controls. Typical measurements showed >70% of tumor cells being Ki67 positive and ~1% of tumor cells showing apoptosis in vehicle and melphalan treated mice. The IHC staining for Ki67 and apoptosis confirm the aggressive nature of these tumors and their inherent resistance to treatment. However, while responses were not detected to treatment, no significant toxicity was noted with either vehicle or melphalan treatment, suggesting that the technique was safe and well tolerated.
Fig. 3.
Orthotopic U87MG xenografts were assessed by gross (a), H&E (b), and Giemsa staining (c). Tumors (asterisk) were large and well established in the left hemisphere and measured 3.4 x 5 mm in maximum diameters in perpendicular axes (c, d). Striatum destruction and cortex compression were detected microscopically with DAPI staining (e).
Fig. 4.
Similar to human GBM, GFAP (red) with DAPI (blue) counterstaining demonstrated the diffuse distribution of astrocytes with a well-defined border and scattered GFAP positive cells throughout the tumor (a) and typical branched cell morphology at higher magnification (inset). Bizarre mitotic figures are visualized throughout the tumor including a tripolar mitotic figure (b) magnified in inset. Reflectinig toxicity, vacuolated nuclei were noted with Giemsa staining after melphalan ICA infusion (c). Background DAPI staining of tumor nuclei (blue), Ki67 staining of tumor cells (red) shows a high level of mitotic activity and no response to melphalan (d), which corresponded to minimal apoptotic tumor cells (red) detected by TUNEL staining (e).
Discussion
GBMs are highly challenging to treat due to their locally aggressive behavior. Early invasion of tumor cells into normal brain tissue usually results in incomplete resections [24, 25]. The standard treatment includes maximal surgical resection and radiation therapy. There is limited benefit for adjuvant chemotherapy which is usually reserved for tumor recurrence [26, 27]. The resistance of primary CNS malignancies to therapy may be related to an inability to achieve tumoricidal concentrations of therapeutic agents in tumors with acceptable neurologic and systemic toxicities [28]. Myelosuppression and systemic toxicity have been limiting factors in achieving effective tumor concentrations with systemic chemotherapy. Directed intra-arterial administration of drugs can theoretically increase the tumor concentrations of therapeutic agents and limit systemic toxicity. The benefit of intra-arterial administration may be further amplified by the use of drugs with a significant first-pass uptake in the infused region that are rapidly cleared in the peripheral circulation. The optimal preclinical model to serve as a platform for novel agent testing has yet to be defined. The aim of the present study was to establish a reliable, safe, and readily reproducible intra-arterial administration model for brain tumors in the mouse. Rapid readouts of tumor response were also examined by the current study to facilitate efficient agent testing.
Our murine model of ICA infusion was feasible and had only limited procedure related morbidity. There were no infarctions, intracranial hemorrhages or detectable neurologic deficits in 90% of the first 10 animals treated, and 100% were without complications following our initial experience. One mouse died from bleeding during the ICA cannulation, but this was strictly technical in nature and occurred early in the series. Also, the average time of ICA cannulations improved following the initial 10 cases. The morbidity of the procedure was minimal as all animals achieved a normal Garcia score within the first 24 hours of infusion. Even though our model has a risk of ischemia with ligation of the CCA, tissue histology did not appear to have any significant degree of ischemia and there were no clinical signs or symptoms of ischemia by standardized neurologic testing. As this was done as an open procedure, anesthesia was necessary for the cannulation and subsequent infusion; however, future clinical applications using percutaneously placed catheters may have the added benefit of minimal sedation and real time neurologic evaluation throughout the infusion procedure.
The directed infusion appeared to selectively favor the ipsilateral side of the brain as noted by Evans Blue dye presence. Since the circulation was not isolated, a visible, but relatively weak appearance of Evans Blue was noted in the contralateral vasculature. As clinical infusions are likely to be performed in a similar manner, it is important to note the effect of the tested agent on the contralateral side of the brain which can be determined in our model system. Furthermore, the relative concentration of drug can be measured in various parts of the tumor and surrounding brain tissue using this model. Ideally, novel agents that have a high first-pass uptake into tumor tissue may take full advantage of an intra-arterial delivery and these agents could be readily tested on this platform.
Melphalan is a standard drug for the regional therapy of cancers, so it was selected for evaluation in our model system. Doses of 30 or 60 μg/mL melphalan were relatively non-toxic, but did not have any significant anti-tumor effects compared to vehicle infused controls. As the nature of the GBM tumor chosen was highly aggressive and resistant to therapy, a lack of measurable response was not unanticipated. The use of U87MG cells and their consistent reproduction of an aggressive GBM tumor in mice, sets a high bar to evaluate potential treatment regimens, but does have the benefit of being well-characterized as its entire genome sequence has been published [29]. Indeed, this model proved to be quite aggressive and our initial attempts at ICA infusion therapy had minimal to no anti-tumor effect. Additionally, the numbers of mice treated were insufficient to detect any potential minor differences in treatments; however, the treatments were performed primarily to determine the feasibility and reproducibility of the procedure. Thus, the model did demonstrate feasibility with a low level of morbidity which may allow for the further investigation of a wide range of reagents.
Responses were measured at the tissue level using IHC to examine cell architecture, mitotic figures, Ki67 levels, and apoptosis of tumor cells. Similar measures were possible to determine the degree of normal brain parenchyma damage. These measures were simple to perform and allowed for the comparison of various treatments. Not described in our current report, but readily available, is the sequential measurement of tumor growth or response with non-invasive imaging such as small animal MRI or CT. Also, tumor cell lines transduced to express luciferase may also be useful for following anti-tumor responses over time as previously reported [18]. It must be emphasized that the current studies only describe the technique of ICA drug delivery, methods of monitoring toxicity and morbidity, and anti-tumor responses; drug efficacy was not measured in this series. In summary, the described murine model provides an exciting opportunity to evaluate therapeutic efficacy of a variety of novel agents and appears to have advantages over existing models.
Acknowledgments
This manuscript was supported in part by Roswell Park Cancer Institute and National Cancer Institute (P30 CA016056).
Footnotes
Compliance with ethical standards
Conflicts of interests
The authors have no financial interests to disclose regarding this manuscript.
References
- 1.Andratschke N, Grosu AL, Molls M, Nieder C. Perspectives in the treatment of malignant gliomas in adults. Anticancer research. 2001;21:3541–3550. [PubMed] [Google Scholar]
- 2.Lefranc F, Rynkowski M, DeWitte O, Kiss R. Present and potential future adjuvant issues in high-grade astrocytic glioma treatment. Advances and technical standards in neurosurgery. 2009;34:3–35. doi: 10.1007/978-3-211-78741-0_1. [DOI] [PubMed] [Google Scholar]
- 3.Nieder C, Adam M, Molls M, Grosu AL. Therapeutic options for recurrent high-grade glioma in adult patients: recent advances. Critical reviews in oncology/hematology. 2006;60:181–193. doi: 10.1016/j.critrevonc.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L, Williams PM, Modrusan Z, Feuerstein BG, Aldape K. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer cell. 2006;9:157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Zustovich F, Cartei G, Ceravolo R, Zovato S, Della Puppa A, Pastorelli D, Mattiazzi M, Bertorelle R, Gardiman MP. A phase I study of cisplatin, temozolomide and thalidomide in patients with malignant brain tumors. Anticancer research. 2007;27:1019–1024. [PubMed] [Google Scholar]
- 6.Zustovich F, Lombardi G, Della Puppa A, Rotilio A, Scienza R, Pastorelli D. A phase II study of cisplatin and temozolomide in heavily pre-treated patients with temozolomide-refractory high-grade malignant glioma. Anticancer research. 2009;29:4275–4279. [PubMed] [Google Scholar]
- 7.Frenay M, Lebrun C, Lonjon M, Bondiau PY, Chatel M. Up-front chemotherapy with fotemustine (F) / cisplatin (CDDP) / etoposide (VP16) regimen in the treatment of 33 non-removable glioblastomas. Eur J Cancer. 2000;36:1026–1031. doi: 10.1016/s0959-8049(00)00048-4. [DOI] [PubMed] [Google Scholar]
- 8.Eckman WW, Patlak CS, Fenstermacher JD. A critical evaluation of the principles governing the advantages of intra-arterial infusions. Journal of pharmacokinetics and biopharmaceutics. 1974;2:257–285. doi: 10.1007/BF01059765. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt JK, Riina HA, Shin BJ, Moliterno JA, Hofstetter CP, Boockvar JA. Intra-arterial chemotherapy for malignant gliomas: a critical analysis. Interventional neuroradiology : journal of peritherapeutic neuroradiology, surgical procedures and related neurosciences. 2011;17:286–295. doi: 10.1177/159101991101700302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newton HB, Slivka MA, Stevens CL, Bourekas EC, Christoforidis GA, Baujan MA, Chakeres DW. Intra-arterial carboplatin and intravenous etoposide for the treatment of recurrent and progressive non-GBM gliomas. Journal of neuro-oncology. 2002;56:79–86. doi: 10.1023/a:1014498225405. [DOI] [PubMed] [Google Scholar]
- 11.Fortin D, Desjardins A, Benko A, Niyonsega T, Boudrias M. Enhanced chemotherapy delivery by intraarterial infusion and blood-brain barrier disruption in malignant brain tumors: the Sherbrooke experience. Cancer. 2005;103:2606–2615. doi: 10.1002/cncr.21112. [DOI] [PubMed] [Google Scholar]
- 12.Guillaume DJ, Doolittle ND, Gahramanov S, Hedrick NA, Delashaw JB, Neuwelt EA. Intra-arterial chemotherapy with osmotic blood-brain barrier disruption for aggressive oligodendroglial tumors: results of a phase I study. Neurosurgery. 2010;66:48–58. doi: 10.1227/01.NEU.0000363152.37594.F7. discussion 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French JD, West PM, Von Amerongen FK, Magoun HW. Effects of intracarotid administration of nitrogen mustard on normal brain and brain tumors. Journal of neurosurgery. 1952;9:378–389. doi: 10.3171/jns.1952.9.4.0378. [DOI] [PubMed] [Google Scholar]
- 14.Zink WE, Foley CP, Dyke JP, Synan MJ, Chakrapani AL, Ballon DJ, Olbricht WL, Gobin YP. Novel microcatheters for selective intra-arterial injection of fluid in the rat brain. AJNR American journal of neuroradiology. 2009;30:1190–1196. doi: 10.3174/ajnr.A1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassenbusch SJ, Anderson JH, Colvin OM. Predicted and actual BCNU concentrations in normal rabbit brain during intraarterial and intravenous infusions. Journal of neuro-oncology. 1996;30:7–18. doi: 10.1007/BF00177438. [DOI] [PubMed] [Google Scholar]
- 16.Kim M, Camoriano M, Muhitch JB, Kane JM, 3rd, Skitzki JJ. A novel mouse model of isolated limb perfusion for extremity melanoma. The Journal of surgical research. 2012;178:294–298. doi: 10.1016/j.jss.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 17.Kim M, Blum AB, Haslinger ML, Donahue MJ, Fisher DT, Skitzki JJ, Park IY. Quinacrine for extremity melanoma in a mouse model of isolated limb perfusion (ILP) Surgery today. 2015;45:355–362. doi: 10.1007/s00595-014-0952-y. [DOI] [PubMed] [Google Scholar]
- 18.Burkhardt JK, Hofstetter CP, Santillan A, Shin BJ, Foley CP, Ballon DJ, Pierre Gobin Y, Boockvar JA. Orthotopic glioblastoma stem-like cell xenograft model in mice to evaluate intra-arterial delivery of bevacizumab: from bedside to bench. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2012;19:1568–1572. doi: 10.1016/j.jocn.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 19.Jacobs JD, Hopper-Borge EA. Carotid artery infusions for pharmacokinetic and pharmacodynamic analysis of taxanes in mice. Journal of visualized experiments : JoVE. 2014:e51917. doi: 10.3791/51917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nieweg OE, Kroon BB. Isolated limb perfusion with melphalan for melanoma. Journal of surgical oncology. 2014;109:332–337. doi: 10.1002/jso.23558. [DOI] [PubMed] [Google Scholar]
- 21.Uzan C, Goere D, Dumont F, Gouy S, Muret J, Hakime A, De Baere T, Bonvalot S. Isolated pelvic perfusion in irradiated unresectable recurrence of pelvic tumor: preliminary outcome and ongoing study. Journal of visceral surgery. 2014;151(Suppl 1):S11–15. doi: 10.1016/j.jviscsurg.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Rashid OM, Sloot S, Zager JS. Regional therapy in metastatic melanoma: an update on minimally invasive intraarterial isolated limb infusion and percutaneous hepatic perfusion. Expert opinion on drug metabolism & toxicology. 2014;10:1355–1364. doi: 10.1517/17425255.2014.951330. [DOI] [PubMed] [Google Scholar]
- 23.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke; a journal of cerebral circulation. 1995;26:627–634. doi: 10.1161/01.str.26.4.627. discussion 635. [DOI] [PubMed] [Google Scholar]
- 24.Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, Frank A, Bayazitov IT, Zakharenko SS, Gajjar A, Davidoff A, Gilbertson RJ. A perivascular niche for brain tumor stem cells. Cancer cell. 2007;11:69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Charles N, Holland EC. The perivascular niche microenvironment in brain tumor progression. Cell Cycle. 2010;9:3012–3021. doi: 10.4161/cc.9.15.12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fine HA, Dear KB, Loeffler JS, Black PM, Canellos GP. Meta-analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults. Cancer. 1993;71:2585–2597. doi: 10.1002/1097-0142(19930415)71:8<2585::aid-cncr2820710825>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 27.Petersdorf SH, Livingston RB. High dose chemotherapy for the treatment of malignant brain tumors. Journal of neuro-oncology. 1994;20:155–163. doi: 10.1007/BF01052725. [DOI] [PubMed] [Google Scholar]
- 28.Gobin YP, Cloughesy TF, Chow KL, Duckwiler GR, Sayre JW, Milanese K, Vinuela F. Intraarterial chemotherapy for brain tumors by using a spatial dose fractionation algorithm and pulsatile delivery. Radiology. 2001;218:724–732. doi: 10.1148/radiology.218.3.r01mr41724. [DOI] [PubMed] [Google Scholar]
- 29.Clark MJ, Homer N, O’Connor BD, Chen Z, Eskin A, Lee H, Merriman B, Nelson SF. U87MG decoded: the genomic sequence of a cytogenetically aberrant human cancer cell line. PLoS genetics. 2010;6:e1000832. doi: 10.1371/journal.pgen.1000832. [DOI] [PMC free article] [PubMed] [Google Scholar]