Abstract
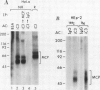
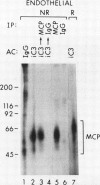
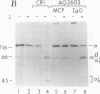
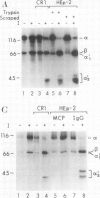
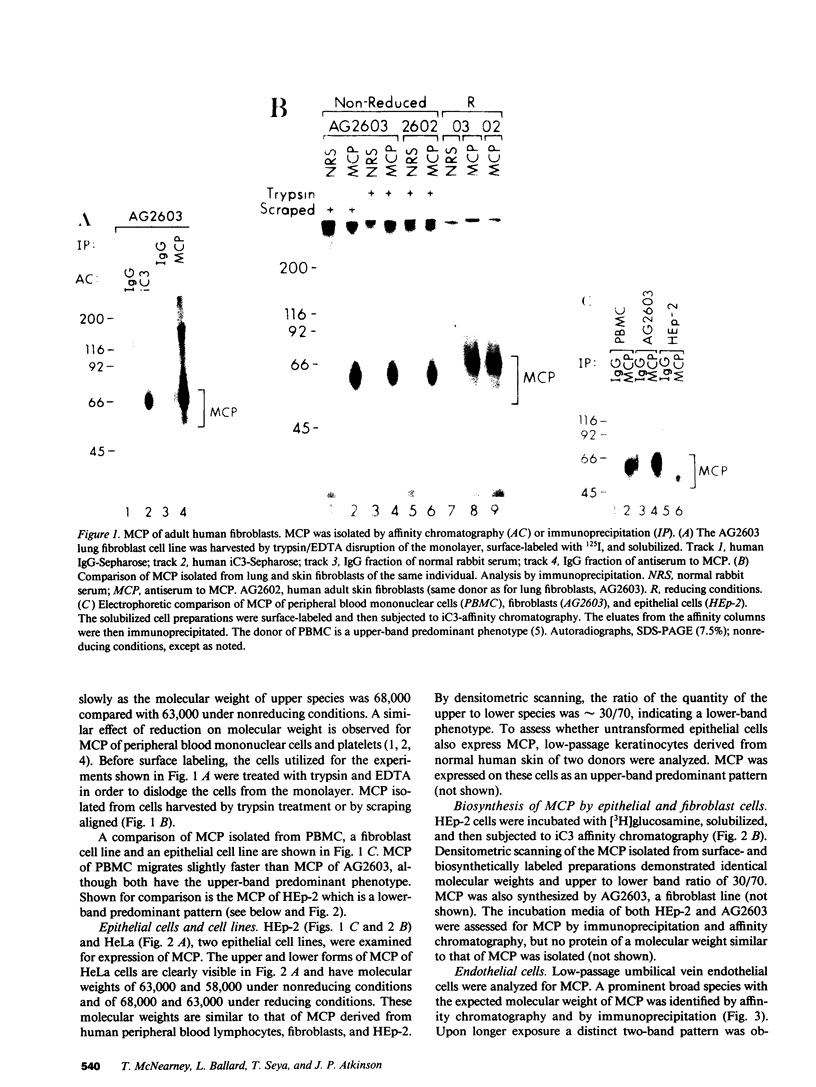
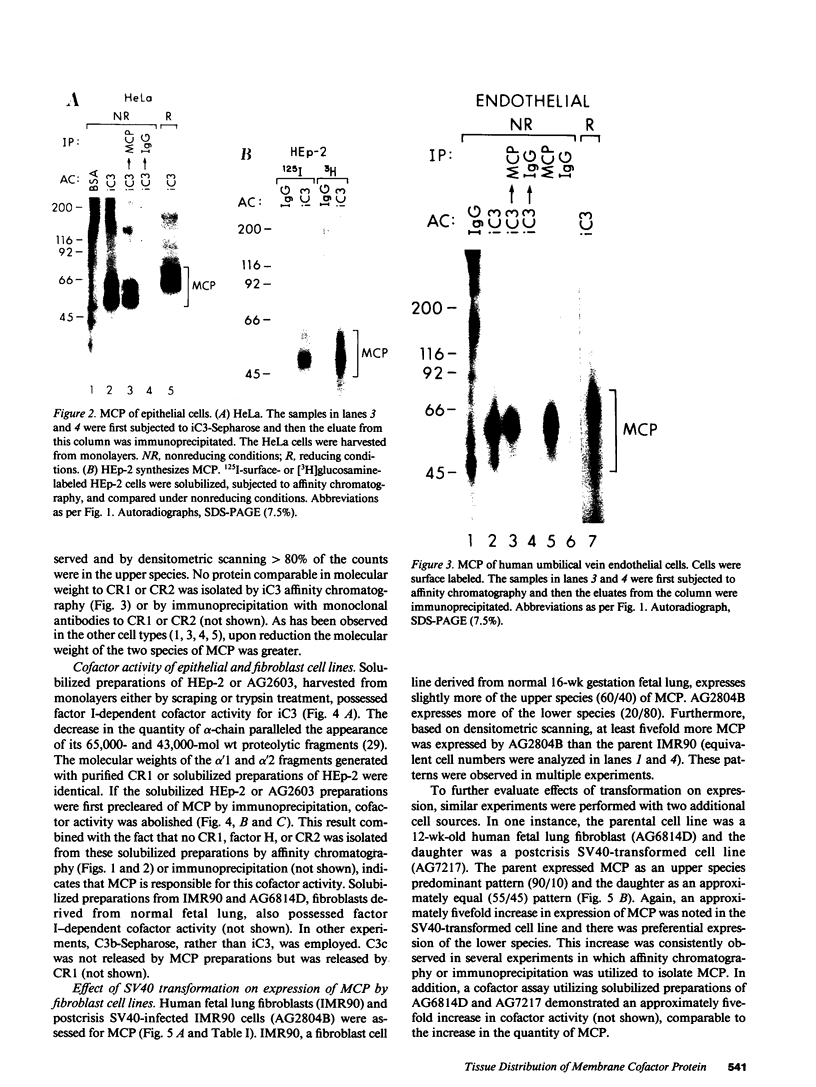
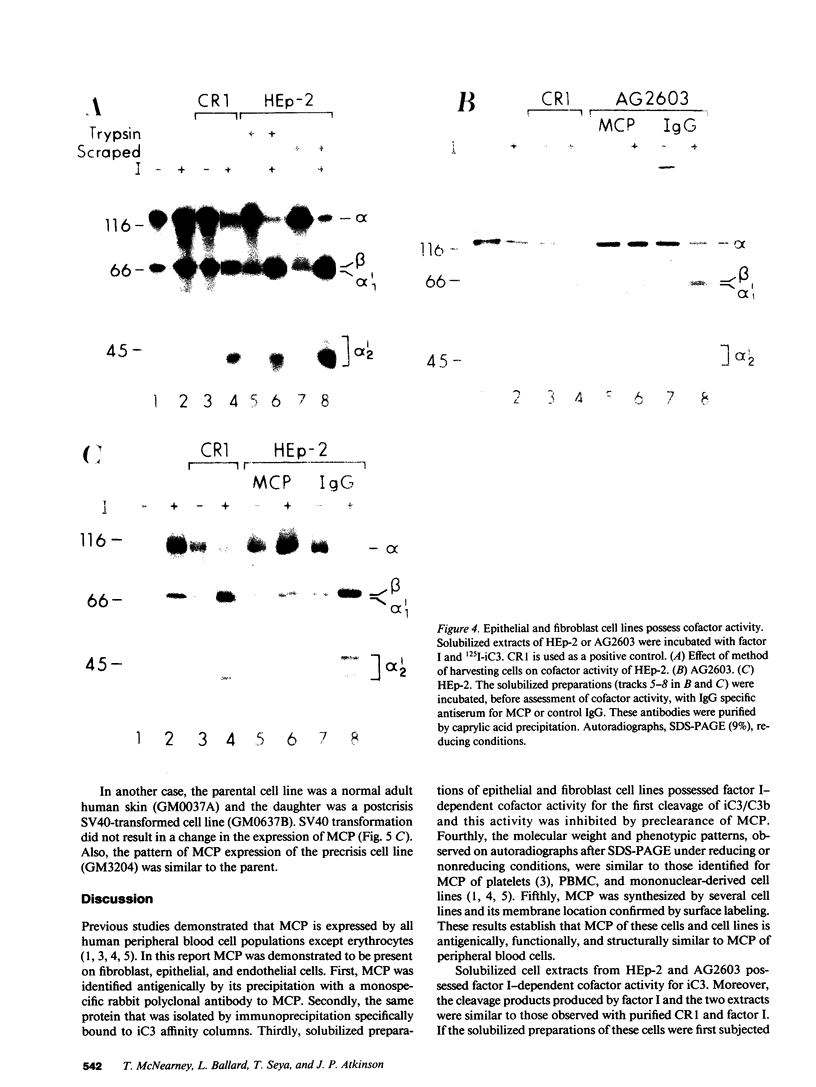
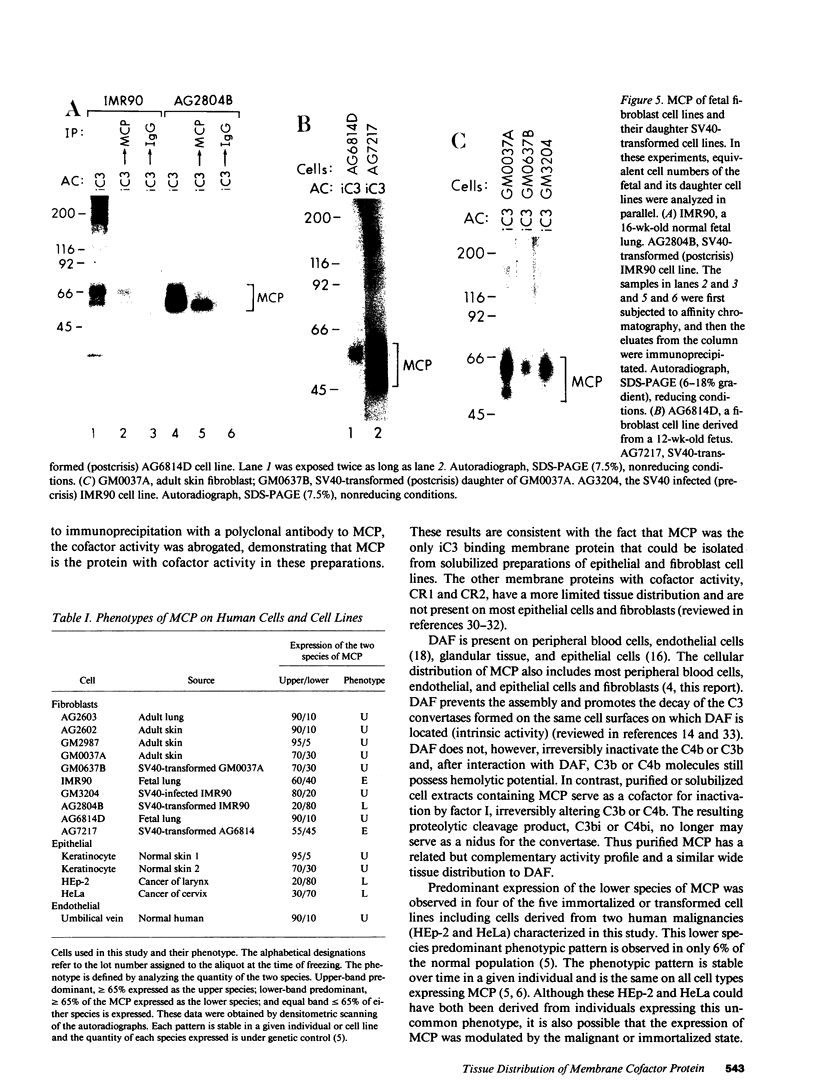
Membrane cofactor protein (MCP) of the complement system is a iC3/C3b binding molecule with cofactor activity that has been identified on all human peripheral blood cells except erythrocytes. Human mononuclear and platelet MCP is dimeric with molecular weights of 68,000 and 63,000 and is expressed in three phenotypic patterns. To further determine its tissue distribution, surface-labeled human fibroblast, epithelial, and endothelial cells and cell lines were assessed for the presence of MCP by iC3 affinity chromatography and by immunoprecipitation with a monospecific anti-MCP rabbit polyclonal antibody. All sources of adult and fetal fibroblast and epithelial cells and cell lines examined and umbilical vein endothelial cells expressed MCP. The molecular weight and phenotypic patterns of MCP were similar to those of peripheral blood cells. MCP was synthesized by fibroblast and epithelial cell lines. Solubilized extracts of these cell lines expressed factor I-dependent cofactor activity for the first cleavage of iC3/C3b which was abrogated by removal of MCP. Expression of MCP was modulated by SV40 transformation of two fetal fibroblast lines. There was a 5- to 10-fold increase in expression of MCP and a preferential expression of the lower species such that the phenotypic designation was changed. The wide tissue distribution and activity profile of MCP suggest that it is likely to play an important role in the regulation of the complement cascade.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Kinoshita T., Jaffe E. A., Nussenzweig V. Decay-accelerating factor is present on cultured human umbilical vein endothelial cells. J Exp Med. 1986 Jan 1;163(1):221–226. doi: 10.1084/jem.163.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard L. L., Bora N. S., Yu G. H., Atkinson J. P. Biochemical characterization of membrane cofactor protein of the complement system. J Immunol. 1988 Dec 1;141(11):3923–3929. [PubMed] [Google Scholar]
- Ballard L., Seya T., Teckman J., Lublin D. M., Atkinson J. P. A polymorphism of the complement regulatory protein MCP (membrane cofactor protein or gp45-70). J Immunol. 1987 Jun 1;138(11):3850–3855. [PubMed] [Google Scholar]
- Bora N. S., Lublin D. M., Kumar B. V., Hockett R. D., Holers V. M., Atkinson J. P. Structural gene for human membrane cofactor protein (MCP) of complement maps to within 100 kb of the 3' end of the C3b/C4b receptor gene. J Exp Med. 1989 Feb 1;169(2):597–602. doi: 10.1084/jem.169.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell R. D., Law S. K., Reid K. B., Sim R. B. Structure, organization, and regulation of the complement genes. Annu Rev Immunol. 1988;6:161–195. doi: 10.1146/annurev.iy.06.040188.001113. [DOI] [PubMed] [Google Scholar]
- Carroll M. C., Alicot E. M., Katzman P. J., Klickstein L. B., Smith J. A., Fearon D. T. Organization of the genes encoding complement receptors type 1 and 2, decay-accelerating factor, and C4-binding protein in the RCA locus on human chromosome 1. J Exp Med. 1988 Apr 1;167(4):1271–1280. doi: 10.1084/jem.167.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper N. R., Moore M. D., Nemerow G. R. Immunobiology of CR2, the B lymphocyte receptor for Epstein-Barr virus and the C3d complement fragment. Annu Rev Immunol. 1988;6:85–113. doi: 10.1146/annurev.iy.06.040188.000505. [DOI] [PubMed] [Google Scholar]
- Dykman T. R., Cole J. L., Iida K., Atkinson J. P. Polymorphism of human erythrocyte C3b/C4b receptor. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1698–1702. doi: 10.1073/pnas.80.6.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farries T. C., Finch J. T., Lachmann P. J., Harrison R. A. Resolution and analysis of 'native' and 'activated' properdin. Biochem J. 1987 Apr 15;243(2):507–517. doi: 10.1042/bj2430507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Human complement receptors for C3b (CR1) and C3d (CR2). J Invest Dermatol. 1985 Jul;85(1 Suppl):53s–57s. doi: 10.1111/1523-1747.ep12275473. [DOI] [PubMed] [Google Scholar]
- GIRARDI A. J., JENSEN F. C., KOPROWSKI H. SV40-INDUCED TRANFORMATION OF HUMAN DIPLOID CELLS: CRISIS AND RECOVERY. J Cell Physiol. 1965 Feb;65:69–83. doi: 10.1002/jcp.1030650110. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Rigby P. W., Lane D. P. Negative regulation of viral enhancers in undifferentiated embryonic stem cells. Cell. 1985 Sep;42(2):519–526. doi: 10.1016/0092-8674(85)90109-6. [DOI] [PubMed] [Google Scholar]
- Holers V. M., Seya T., Brown E., O'Shea J. J., Atkinson J. P. Structural and functional studies on the human C3b/C4b receptor (CR1) purified by affinity chromatography using a monoclonal antibody. Complement. 1986;3(2):63–78. doi: 10.1159/000467882. [DOI] [PubMed] [Google Scholar]
- Hourcade D., Holers V. M., Atkinson J. P. The regulators of complement activation (RCA) gene cluster. Adv Immunol. 1989;45:381–416. doi: 10.1016/s0065-2776(08)60697-5. [DOI] [PubMed] [Google Scholar]
- Hronis T. S., Steinberg M. L., Defendi V., Sun T. T. Simple epithelial nature of some simian virus-40-transformed human epidermal keratinocytes. Cancer Res. 1984 Dec;44(12 Pt 1):5797–5804. [PubMed] [Google Scholar]
- Hunter R. Standardization of the chloramine-T method of protein iodination. Proc Soc Exp Biol Med. 1970 Mar;133(3):989–992. doi: 10.3181/00379727-133-34611. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulczycki A., Jr, Krause V., Killion C. C., Atkinson J. P. Improved cell surface radioiodination of macrophages. J Immunol Methods. 1980;37(2):133–138. doi: 10.1016/0022-1759(80)90198-2. [DOI] [PubMed] [Google Scholar]
- Lublin D. M., Liszewski M. K., Post T. W., Arce M. A., Le Beau M. M., Rebentisch M. B., Lemons L. S., Seya T., Atkinson J. P. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J Exp Med. 1988 Jul 1;168(1):181–194. doi: 10.1084/jem.168.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda A. F., Babiss L. E., Fisher P. B. Transformation of human skeletal muscle cells by simian virus 40. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6581–6585. doi: 10.1073/pnas.80.21.6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitomo K., Fujita T., Iida K. Functional and antigenic properties of complement receptor type 2, CR2. J Exp Med. 1987 May 1;165(5):1424–1429. doi: 10.1084/jem.165.5.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosen C. E., Spicer D. B., Austen K. F. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985 May;65(5):1237–1244. [PubMed] [Google Scholar]
- Olander J. V., Bremer M. E., Marasa J. C., Feder J. Fibrin-enhanced endothelial cell organization. J Cell Physiol. 1985 Oct;125(1):1–9. doi: 10.1002/jcp.1041250102. [DOI] [PubMed] [Google Scholar]
- Pentland A. P., Needleman P. Modulation of keratinocyte proliferation in vitro by endogenous prostaglandin synthesis. J Clin Invest. 1986 Jan;77(1):246–251. doi: 10.1172/JCI112283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins S. J., Haris P. I., Sim R. B., Chapman D. A study of the structure of human complement component factor H by Fourier transform infrared spectroscopy and secondary structure averaging methods. Biochemistry. 1988 May 31;27(11):4004–4012. doi: 10.1021/bi00411a017. [DOI] [PubMed] [Google Scholar]
- Rey-Campos J., Rubinstein P., Rodriguez de Cordoba S. A physical map of the human regulator of complement activation gene cluster linking the complement genes CR1, CR2, DAF, and C4BP. J Exp Med. 1988 Feb 1;167(2):664–669. doi: 10.1084/jem.167.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. D., Medof M. E. Membrane complement receptors specific for bound fragments of C3. Adv Immunol. 1985;37:217–267. doi: 10.1016/s0065-2776(08)60341-7. [DOI] [PubMed] [Google Scholar]
- Seya T., Ballard L. L., Bora N. S., Kumar V., Cui W., Atkinson J. P. Distribution of membrane cofactor protein of complement on human peripheral blood cells. An altered form is found on granulocytes. Eur J Immunol. 1988 Aug;18(8):1289–1294. doi: 10.1002/eji.1830180821. [DOI] [PubMed] [Google Scholar]
- Seya T., Holers V. M., Atkinson J. P. Purification and functional analysis of the polymorphic variants of the C3b/C4b receptor (CR1) and comparison with H, C4b-binding protein (C4bp), and decay accelerating factor (DAF). J Immunol. 1985 Oct;135(4):2661–2667. [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg M. L., Defendi V. Transformation and immortalization of human keratinocytes by SV40. J Invest Dermatol. 1983 Jul;81(1 Suppl):131s–136s. doi: 10.1111/1523-1747.ep12540905. [DOI] [PubMed] [Google Scholar]
- Steinbuch M., Audran R. The isolation of IgG from mammalian sera with the aid of caprylic acid. Arch Biochem Biophys. 1969 Nov;134(2):279–284. doi: 10.1016/0003-9861(69)90285-9. [DOI] [PubMed] [Google Scholar]
- Tevethia M. J., Pipas J. M., Kierstead T., Cole C. Requirements for immortalization of primary mouse embryo fibroblasts probed with mutants bearing deletions in the 3' end of SV40 gene A. Virology. 1988 Jan;162(1):76–89. doi: 10.1016/0042-6822(88)90396-0. [DOI] [PubMed] [Google Scholar]
- Yu G. H., Holers V. M., Seya T., Ballard L., Atkinson J. P. Identification of a third component of complement-binding glycoprotein of human platelets. J Clin Invest. 1986 Aug;78(2):494–501. doi: 10.1172/JCI112601. [DOI] [PMC free article] [PubMed] [Google Scholar]