Abstract
An intact orthographic processing system is critical for normal reading and spelling. Here we investigate the neural changes associated with impairment and subsequent recovery of the orthographic lexical processing system in an individual with an ischemic left posterior cerebral artery (PCA) stroke. This work describes a longitudinal case study of a patient, known as MMY, with impairments in orthographic lexical processing for reading and spelling at stroke onset, and who recovered these skills within 1 year post stroke. We tested the hypothesis that this acute impairment to reading and spelling would be associated with a selective loss of neural activation in the left fusiform gyrus, and that subsequent recovery would be associated with a gain of neural activation in this region. MMY’s case provided a unique opportunity to assess the selectivity of neural changes because she demonstrated a behavioral recovery of naming as well; i.e. if there is neural recovery for reading and spelling, but not naming, then these neural changes are selective to the recovery of orthographic processing. To test our hypothesis, we examined longitudinal behavioral and fMRI data of reading, spelling, and visual object naming acquired acutely, 3 weeks, 5 months, and one year post stroke. In confirmation of our hypothesis, the loss and subsequent gain of orthographic lexical processing was associated with up-regulation of neural activation in areas previously associated with orthographic lexical processing: i.e., the left mid-fusiform gyrus and inferior frontal junction. Furthermore, these neural changes were found to be selective to orthographic processing, as they were observed for reading and spelling, but not for visual object naming within the left mid-fusiform gyrus. This work shows that left PCA stroke can temporarily and selectively disrupt the orthographic lexical processing system, not only in the posterior region adjacent to the stroke, but also in relatively distant frontal orthographic processing regions.
Introduction
Functional neuroimaging techniques can provide a window into the neural basis of orthography and allow us to track changes in structure-function relationships during recovery after a brain lesion. Thus, functional neuroimaging can be used not only to localize functional activity associated with a particular area, but also through careful analysis to determine whether the absence/presence of a functional activity in a region is associated with specific type of orthographic deficit.
In neurotypical literate adults, the ability to read and write relies on a complex set of interacting cognitive functions. Central to these functions are processes specifically associated with retrieving, maintaining, and expressing orthographic information; these include orthographic lexical, sub-lexical, and working memory functions. In the brain, these central orthographic processes are typically associated with a left hemisphere set of regions including the left inferior frontal gyrus (IFG), intraparietal sulcus (IPS), and fusiform gyrus (FG) (Martin, Schurz, Kronbichler, & Richlan, 2015 for reading; Purcell, Turkeltaub, Eden, & Rapp, 2011 for spelling). Recently, a study of acquired dysgraphia indicates that damage to the left IFG and FG are associated with impairments in orthographic lexical processing in spelling, and damage to the left IPS is associated with impaired orthographic working memory (Rapp, Purcell, Hillis, Capasso, & Miceli, 2015). The left FG in particular has long been studied in the neuroimaging literature and is consistently associated with visual word processing. The left FG has been referred to as the Visual Word Form Area, due to: (1) its selective response to visual words in reading as compared to other non-word stimuli (e.g. Cohen et al., 2002; Dehaene & Cohen, 2011); (2) a clear association between activation in this area and development of literacy skills (Dehaene et al., 2010); and (3) an association between focal damage in this area and reading impairments (e.g., Gaillard et al., 2006). Specifically, the left FG has been associated with orthographic lexical processing in the neuroimaging literature in both reading (Glezer, Jiang, & Riesenhuber, 2009; Kronbichler et al., 2004; Szwed et al., 2011) and spelling (Rapp & Dufor, 2011; Rapp & Lipka, 2011).
That said, there is some debate regarding the selectivity of the left FG to orthographic lexical processing (for opposing views see Dehaene and Cohen, 2011; Price and Devlin, 2011). A unique perspective on this literature and debate involves an exploration of the neurotopographic changes across time associated with impairment and subsequent recovery of orthographic lexical processing after stroke. Such an investigation would allow for the testing of the hypothesis that transient impairment in orthographic processing in reading and spelling is associated with a transient decrease in activation specifically associated with orthographic tasks (i.e. reading and spelling) in the left FG and possibly other orthographic processing regions.
Although, to date, there is limited work examining written language longitudinally after stroke, there has been some work examining impairments in spoken language acutely. Generally, this previous work has indicated that recovery of language after a stroke involves multiple interacting mechanisms that are operative at different times over the course of recovery and vary across individuals. As such, the recovery of language after stroke is difficult to study, and therefore poorly understood. Some of these mechanisms are considered to include restoration of blood flow, recovery from diaschisis (i.e., impairment due to loss of input from a remote damaged brain area), and functional reorganization of a neural-cognitive system due to neuroplasticity (Jarso et al., 2013). Generally though, recovery of language function after stroke is considered to occur in three phases which occur within approximate time windows within the first year post stroke: acute phase is onset to 2–3 weeks post-stroke, subacute is 2–3 weeks to 4 months, and the chronic phase is greater than 4 months post stroke (Anglade, Thiel, & Ansaldo, 2014; Hillis et al., 2006). It is commonly understood that the bulk of cognitive, motor, or language recovery occurs within the acute and sub-acute phases post stroke (e.g., Kelly-Hayes et al., 1989). With respect to language function, this early post stroke recovery is thought to be primarily associated with a reperfusion of the indirectly damaged brain areas (e.g., Hillis et al., 2002, 2006; Jacquin et al., 2014). Late recovery of language function (i.e. in the chronic phase) is considered to be more marginal and is associated with a less well understood compensatory functional reorganization mechanisms (Anglade et al., 2014).
The functional neuroimaging literature on language recovery post-stroke is complex, and there has been difficulty identifying general principles of neural recovery. Some work suggests that recovery of language functions in aphasia is primarily accompanied by greater perilesional reorganization (e.g., Fridriksson, 2010; Perani et al., 2003; Postman-Caucheteux et al., 2010; Saur et al., 2006; Winhuisen et al., 2007), while other work suggests that good recovery is primarily associated with compensatory right hemisphere recruitment (Abo et al., 2004; Blank, Bird, Turkheimer, & Wise, 2003; Blasi et al., 2002; Turkeltaub et al., 2012). The diversity of these findings could be due to multiple factors such as the time post-stroke, severity of impairment, lesion location/size, and nature of the neural measure (Sebastian & Kiran, 2011). All of these factors may be differentially relevant across cases, and therefore influence recovery trajectories in vastly different manners across individuals.
At present, only a few studies have performed longitudinal functional neuroimaging in language recovery (Heiss, Kessler, Thiel, Ghaemi, & Karbe, 1999; Jarso et al., 2013; Saur et al., 2006; Sebastian et al., 2016). Heiss et al. (1999) examined patients with aphasia at 2 and 8 weeks post-stroke and reported that good recovery of language function may only be achieved if the language areas are preserved (but temporarily inactivated) and can subsequently be reintegrated into the original language network. Saur et al. (2006) used functional magnetic resonance imaging (fMRI) to examine the longitudinal reorganization in the language system after a stroke from the acute stage to the chronic stage. Saur et al. proposed that good recovery from aphasia is acutely associated with minimal activation, followed by an upregulation of the right homologue of language areas in the subacute phase, and finally a re-normalization shift back to the left hemisphere in the chronic phase. This previous work has focused primarily on the loss and recovery of spoken language. Only a nominal amount of work has examined the loss and recovery of orthographic function, (e.g., Cohen, Dehaene, McCormick, Durant, & Zanker, 2016); to our knowledge, none has examined the loss and recovery of orthographic lexical processing longitudinally from acute to the chronic phase of stroke.
The primary aim of this study is to examine the neural basis of recovery of orthographic lexical processing in an individual with left PCA stroke. Here we provide a novel fMRI study of a transient loss of orthographic lexical processing in an individual patient - MMY - who sustained a left ischemic PCA stroke, resulting in orthographic lexical impairments to both reading and spelling. These time points were acquired as part of a larger longitudinal language recovery study whose aim was to examine language recovery. Although, previous fMRI longitudinal post stroke studies have examined three time points post stroke, (e.g., acute, sub-acute, and chronic phases as reported in Saur et al., 2006), an additional chronic phase time point (i.e. a 1 year time point) was included in order to further explore the possibility of additional functional gains in the chronic post stroke phase. Therefore, MMY was tested on a language battery and fMRI experiments of reading, spelling and naming across the following time points within the first year post stroke: acutely (Time 1, i.e. T1), 3 weeks (T2), 5 months (T3), and one year (T4). We tested the hypothesis that a temporary orthographic behavioral deficit is associated with selective disruption of orthographic processing in the left fusiform gyrus, and that a recovery of orthographic processing is associated with re-normalization of orthographic specific neural activation in this area. MMY provided a unique opportunity to examine these orthographic selective changes because she also had a temporary visual object naming deficit, and this allowed for tracking neural changes associated with naming, as well as reading and spelling. Her recovery facilitated a method for assessing changes specific to orthography as opposed to more general language functions. In particular, if changes in neural activation are observed in both reading and spelling, but not naming, then they can be attributed to changes in orthographic processing.
Methods
Participants
MMY is a right-handed 54-year-old woman with 14 years of education who initially reported experiencing a sudden onset right-sided visual field loss. Further examination in the Emergency Department at Johns Hopkins Hospital revealed right-sided hemianopsia, and an initial language assessment revealed severely impaired oral reading, visual picture naming, and mild to moderate word retrieval deficits in conversation. MMY was recruited from the inpatient units for the ongoing longitudinal Stroke Cognitive Outcome and REcovery (SCORE) study. MMY received clinical MRI within 24 hours of symptom onset,; this revealed an acute ischemic left-hemisphere PCA stroke involving the occipital cortex, fusiform, lingual gyrus, and splenium. MMY did not have any have the following: contraindication for functional MRI (e.g., implanted ferrous metal, claustrophobia), a history of previous neurological disease (e.g., dementia), hearing impairment, or uncorrected vision.
Given the inherent variability in brain atrophy and performance due to such factors as age and education, and the inability to select numerous controls participants due unavoidable cost and time limitations, we selected a single control participant stringently well-matched to MMY. Therefore, we recruited a single control participant who had no knowledge of this study prior to participation. This control was matched to MMY on handedness, age, gender, and education (right-handed, 59 year-old female with 14 years education). This control had normal hearing and vision. The control had no history of neurological disorder (such as stroke, Parkinson’s disease, Alzheimer’s disease, epilepsy), psychological illness, or learning disability.
Language Testing
Both MMY and the Control underwent detailed language testing at the acute and three follow-up time points. Follow-up time points include 3 Weeks (subacute phase), 5 Months (chronic phase), and 1 year (chronic phase) post-stroke. Language tests included: Johns Hopkins University Dysgraphia Regularity sub-test and Parts of Speech sub-test (Beeson & Hillis, 2001; Goodman & Caramazza, 1985), Boston Diagnostic Aphasia Examination (BDAE-3; Goodglass, Kaplan, & Barresi, 2001), Boston Naming Test (BNT-2; Kaplan, Goodglass, & Weintraub, 2001), Hopkins Assessment of Naming Actions (HANA; Breining, Tippett, et al., 2015), and Pyramids and Palm Trees: Short Form (sPPT; Breining, Lala, et al., 2015).
Imaging
Both clinical and research scans were acquired from MMY at the acute time point. Whereas the clinical MRI scans were obtained within 24 h of admission to the hospital, Research Scans (discussed below) were acquired after the clinical scans, within 48h after admission. The clinical scans included T2, fluid attenuation inversion recovery (FLAIR), susceptibility weighted images (to evaluate for hemorrhage), Perfusion Weighted Imaging (PWI; to evaluate for hypoperfusion), Diffusion Weighted Imaging (DWI; to evaluate for acute ischemia), and MR angiography (to evaluate for stenosis, occlusion, aneurysm). Clinical scans were acquired on a Siemens 3.0 T Magnetom Trio scanner, equipped with an 8-channel head coil. High-resolution DWI images were acquired using a single-shot echo-planar imaging (EPI) pulse sequence with the following parameters: field of view (FOV) = 220×220 mm, matrix 160×160 mm, time to repetition (TR) = 5700 ms, echo time (TE) = 87 ms, slice thickness = 4 mm.
Research Scans
Research MR images were acquired at the acute time point (i.e. within 48h after admission to the hospital), and the follow-up time points including 3 Weeks (subacute phase), 5 Months (chronic phase), and 1 year (chronic phase) post-stroke. Research scans were acquired on a 3.0 T Philips Achieva MRI scanner, equipped with a 32-channel head coil. The MRI scanning session was 70 minutes. The data that is relevant to this work includes the following: a magnetization prepared rapid gradient echo (MPRAGE) anatomical scan and blood-oxygenation-level dependent (BOLD) echo planar imaging (EPI) acquisitions. MPRAGE scans were acquired as 170 sagittal slices, using a multishot, turbo field echo pulse sequence with an in-plane resolution of TR = 6800 ms, TE = 3.1 ms, inversion time (TI) = 850 ms, slice thickness = 1 mm. Functional MRI images were acquired using EPI sequences in the axial plane (FOV = 240×240 mm, TR = 2000 ms, TE = 30 ms, 35 axial slices, 3×3 mm in plane resolution, 3 mm slice thickness with 0.75 mm gap).
fMRI tasks
Data from reading, spelling, and naming fMRI experiments were acquired for MMY and Control. Approximately 30–45 min was spent training each participant outside the scanner in order to confirm that they understood and were able to perform the task. All of the experiments were developed in MATLAB Psychtoolbox. We employed an MRI compatible audio system to present audio files and an Optimo PK320 pico-projector system to present visual stimuli.
Stimuli
For the reading, spelling and naming experiments, all of the words were concrete nouns equated for number of syllables, frequency of occurrence (Kučera & Francis, 1983), length, and imageability (Gilhooly & Logie, 1980); see Table 1.
Table 1.
Stimuli parameters for the fMRI language experiments
| Reading | Spelling | Naming | |
|---|---|---|---|
| Frequency | 84.8 (107.6) | 71.8 (115.7) | 78.8 (108.1) |
| Length | 4.5 (0.5) | 4.2 (0.5) | 3.9 (0.8) |
| Imageability | 6.2 (0.4) | 6.1 (0.5) | 6.4 (0.6) |
Mean values with standard deviation are in parentheses
Reading Experiment
For this block design paradigm we presented pseudorandomly ordered 20 sec blocks of visual words, checkerboards, or a fixation cross resting condition. For the word and checkerboard conditions, 10 visual stimuli were presented for 500 ms each, with a 1500 ms fixation interval between items; this provided sufficient time for MMY to saccade to and view each stimulus. Each stimulus was presented to the foveal field of view and was approximately 2×4 degrees and was thus within MMY’s intact foveal field of view. For the word condition, subjects covertly read each word. For the checkerboard control condition, subjects were instructed to attend to all stimuli. We did not require a verbal response from the participants so as to avoid lengthening the inter-trial-interval to accommodate verbal response durations thus maintaining a block design; this is in keeping with the original experimental design from which ours was adapted (Cohen et al., 2002). Verbal confirmation was obtained immediately after the scan that the participant remained awake and viewed all of the stimuli. Data were acquired across two runs; each run was 4 min 46 sec.
Spelling Experiment
For this paradigm, the participants performed a handwriting task and a drawing task. At the start of each trial, the participants heard a cue of either “write” or “draw” (700 ms). If the cue was to “write” the participant heard a spoken word (1000 ms) that they then wrote on an MRI compatible tablet (this tablet was developed by Hybridmojo, LLC http://www.hybridmojo.com/). If the cue was “draw” the participant heard a spoken word (either “star” or “square”, 1000 ms) and drew an image of the appropriate shape on the tablet. There was 6700 ms allotted for recording responses. The total trial time was 8400 ms. There were a total of 40 spelling trials and 20 drawing trials; these were grouped into blocks of 5 trials each. Data were acquired across two runs; each run was 5 min 38 sec.
Naming Experiment
The experiment consisted of a cued picture naming task (Holland et al., 2011). Each picture was presented concurrently with one of three auditory cues: a whole word, an initial phoneme, or an unintelligible auditory noise. The picture-naming task used a set of 60 grayscale images taken from the International Picture Naming Project database (Bates et al., 2003). The order of pictures and accompanying cues was counterbalanced to ensure that the same picture and cue pairing was not presented during a single run. The order of presentation of each stimulus was randomized within each run. Each picture was preceded by a fixation cross for 500 ms and was then displayed for 3500 ms. A control condition consisted of passively viewing greyscale scrambled pictures. The scrambled pictures were derived by pixelating the images from the naming task using Adobe Photoshop 7.0. Trials were presented in blocks of six pictures, separated by the control condition of 7 sec. Noise cancelling microphone output from the scanner room was run through the penetration panel and connected to a Macintosh laptop computer in the scanner control room. Audacity software on the computer recorded verbal responses from each scanning run. These responses were scored for accuracy and reaction time off-line. There were 10 blocks of picture naming and 10 blocks of scrambled picture viewing in each run; data were acquired across two runs; each run was 5 min 24 sec.
Data analysis
Behavioral Analysis
Accuracy was analyzed for both the spelling and the naming experiments; verbal responses were not obtained for the reading experiment.
Spelling
Handwriting performance was obtained with an MRI compatible tablet. The x and y coordinates on the tablet were tracked in MATLAB Psychtoolbox during the experiment. Accuracy was determined for each trial by examining movies and snapshots that were generated in post-processing. Naming: Naming latencies were measured from recorded sound files as the duration between the onset of the stimulus and the onset of the participant’s response.
Structural and Lesion Analysis
The structural scan for each time point was aligned to the anterior commissure-posterior commissure (AC-PC) plane in BrainVoyager, and the T2, T3, and T4 time point structural cans were co-registered to the T1 structural scan using SPM12. A lesion was drawn at the T4 time point using MRIcron; the T1 (acute) structural scan was used as a reference in order to determine the amount of tissue loss around the ventricle.
In order to normalize the structural scans, we focused on the T1 time point. We did this because there was no discernable tissue loss at this time point, and therefore, no lesion mask was necessary for deriving the Montreal Neurological Institute (MNI) normalization parameters (i.e. it is convention to employ a lesion mask in order to accurately normalize brains with missing/damaged tissue (e.g., Andersen, Rapcsak, & Beeson, 2010)). The T1 structural scan was normalized via an SPM12 toolbox using an older adult MNI template developed by Rorden, Bonilha, Fridriksson, Bender, & Karnath (2012); these normalization parameters were then applied to the lesion in order to determine the standardized MNI volume and coordinate extent.
fMRI Statistical Analysis
All fMRI data were analyzed using BrainVoyager and SPM. Pre-statistical processing included slice-time correction, motion correction, high-pass temporal filtering, and spatial smoothing with full width at half maximum (FWHM) = 3 mm. Individual fMRI data from each time point were co-registered to the high-resolution anatomical image from the acute time point and normalized to Montreal Neurological Institute (MNI) standard space using SPM. Task related BOLD images were analyzed using a general linear model. In order to avoid employing slightly different normalization parameters for each of our time points due to the changes in structure across time points, we used the normalization parameters for T1 (acute) for all of the data analyses. This ensured that the all of the functional data were warped to the normal space in exactly the same way. For this reason, all of the functional data were co-registered to the T1 structural image, thereby allowing the employment of the same normalization parameters to each functional image.
Each condition was modeled separately by applying a hemodynamic response function (HRF) at the trial times for each condition. Only correct trials were included in each condition regressor; incorrect trials were assigned to a separate regressor of no-interest. The 6 direction motion parameters (x, y, z, roll, pitch, yaw) were entered as covariates to correct for head motion artifacts. In addition, physiological nuisance regressors were included by applying the CompCor approach, which involves performing a principle components analysis on the signal from voxels within cerebral spinal fluid and identifying those components most likely to be associated with physiological noise (Behzadi, Restom, Liau, & Liu, 2007). In all analyses, the contrasts between conditions were estimated via a fixed effects model in order to produce an average activation map for each individual.
For each of the language experiments, we identified a representative contrast that would optimize identifying regions associated with the language network. We applied the following representative contrasts from each paradigm for use in further analyses: Reading (Words > Checkerboards), Spelling (Handwriting > Fixation); and Naming (Phonemic & Word-cued naming > Scrambled images). We decided on these relatively low-level cognitive baselines because little is known regarding the functional activation at the acute time point, and low-level baselines provide a relatively inclusive neurotopographic map from which we can detect subtle changes across time. In order to control for multiple comparisons, we used Alphasim from the NeuroElf MATLAB toolbox, and a cluster-level correction of p < 0.05 in all analyses. For the single contrast analyses a cluster threshold of 154 was employed. For the conjunction analyses which examine joint voxel and extent thresholds, a cluster threshold of 36 was identified. For the initial analysis we examined the pattern of activation at each time point.
fMRI Longitudinal Analysis
For this analysis we determined whether or not the pattern of activation differed significantly between the acute scan and follow up time points for each participant. We contrasted the T4 (one year post-stroke) activation to that of T1 (acute time point).
Laterality Analysis
In order to track changes in laterality for each of the language experiments we performed a laterality analysis on the anatomically defined fusiform gyrus region associated with the ischemic PCA stroke. This ROI was obtained from the Harvard-Oxford Atlas (Desikan et al., 2006) anterior, middle, and posterior fusiform gyrus. The mean beta parameter estimates were obtained for each ROI. A laterality index (LI) was employed that captured both the extent and height of activation in each ROI. Briefly, this approach identifies a threshold of the mean of the 5% most activated voxels, and then sums the t-values above 50% of this mean (Fernandez et al., 2003). LI’s were obtained for each ROI for each time point in each language task. Laterality thresholds were set to LI > 0.2 for left lateralized, and < −0.2 for right lateralized (Seghier, 2008).
Results
Language Battery Behavioral Results
At the acute stage, MMY was impaired on many language and memory tasks. Her primary deficits were in reading, spelling, and naming visual objects. By T3 (5 months), MMY had generally normal language skills, but persistent short term memory problems. Her speech was fluent, grammatical, and well-articulated. She had mild word-finding difficulty in conversation, and had intact comprehension of spoken words and sentences.
Reading
MMY had a severe impairment in reading at the acute stroke phase. She was not able to read any of the words accurately. Her errors consisted of no-response errors; i.e., she attempted to read a given word, but could not, saying, “I don’t know.” At the T2 (sub-acute) phase, MMY had improved to 25.8% word accuracy, and expressed a trend towards a frequency effect, but not a length effect. Her errors primarily consisted of phonemic errors such as insertions (e.g. smush for mush), transpositions (e.g. star for tsar), and replacements (e.g. sare for dare); these comprised 78% of her errors. The remaining 22% were phonologically plausible errors (e.g. sord for cord; loff for laugh; pooch for pouch). MMY’s trend towards a frequency effect, lack of a length effect, and presence of phonologically plausible errors are all indicative of an orthographic lexical deficit in reading.
At the chronic phases T3 and T4, MMY had improved considerably to 94.4% and 97.8% word accuracy, respectively, and exhibited no frequency or length effects in reading. These results indicate that MMY had recovered from much of her reading impairment by the T3 time point and that this recovery persisted to the T4 time point.
Spelling
MMY had a moderate impairment in spelling at the acute stroke phase. Both word accuracy and letter accuracy were examined; whereas word accuracy provides a coarse measure, letter-level accuracy provides a sensitive measure of spelling performance. Therefore, letter-accuracy was used to determine frequency and length effects. Word accuracy indicated a moderate general deficit in spelling at the T1 and T2 time points with 62.5% and 68.3% accuracy. At the T3 and T4 time point she had recovered some of her spelling ability (79.6 and 79.8% accuracy respectively); her performance indicates that, although there was some recovery, there may still be a persistent spelling deficit at T4. We do not have samples of her pre-morbid spelling ability, and therefore cannot ascertain the severity of this potential persistent spelling deficit.
Throughout all four time points, her errors were predominantly phonologically plausible errors (e.g. ochen for ocean; loil for loyal; or grete for greet); MMY made 76%, 81%, 81%, and 84% phonologically plausible errors at each respective time point, in chronological order. Throughout, her remaining errors were predominantly letter errors such as replacements (e.g. clove for glove), insertions (e.g. bloud for loud), or deletions (e.g. frind for friend); a few remaining errors were no-responses or indeterminate word errors (e.g. hungry for hurry). The letter accuracy across the four time points indicates that for T1 and T2, MMY had a significant or trend towards significant frequency effect, but not a length effect. There was no frequency effect at the chronic T3 or T4 time points. Combined, these results suggest that MMY had an orthographic lexical deficit in spelling at T1 and T2, but that this deficit recovered by T3 and recovery persisted into T4.
Naming
These results are reported in a recent work (Patient P1 in Sebastian et al., 2016), and presented in Table 4. At T1 and T2, MMY was severely impaired at naming on the BNT-2 with 10% and 47% accuracy respectively. By T3, MMY had improved completely to 100% accuracy, which persisted to T4 with 97% accuracy. MMY’s errors were predominantly semantically related errors on naming such as explosion for volcano; something to ride for camel. MMY had relatively spared tactile naming, indicating that the impairment was associated with the visual modality. This object naming behavioral profile fits with an optic aphasia in that MMY does not have a general object naming deficit, but it is specific to the visual input modality (Beauvois, 1982).
Table 4.
Summary of Language Battery Scores
| MMY | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| T1 (Acute) | T2 (3W) | T3 (5M) | T4 (1YR) | T1 | T2 (4W) | T3 (5M) | T4 (1YR) | ||
| JHU Dysgraphia Battery* | 62.5 | 68.3 | 79.6 | 79.8 | 96.9 | 100 | |||
|
| |||||||||
| JHU Dyslexia Battery* | 0 | 26 | 94 | 98 | 100 | 100 | 100 | 100 | |
|
| |||||||||
| BNT-2 | 10 | 47 | 100 | 97 | 97 | 100 | 100 | 96.7 | |
|
| |||||||||
| sPPT | 60 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
|
| |||||||||
| BDAE Comprehension | Words | 62.5 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Commands | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
| Complex | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
|
| |||||||||
| BDAE Repetition | Words | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Sentences | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
Mean word accuracy for MMY summarized from Table 3
Other Language Processing
See Table 4 for a summary of performance on other language tasks. Generally, MMY had relatively intact comprehension and production of language as measured with the BDAE. At T1 she had a deficit in matching spoken words to pictures and impaired performance on the sPPT (a picture association test), but no impairment in comprehending spoken language (including commands and paragraphs) consistent with a deficit in accessing semantics from vision at the acute stage. Performance on the BDAE and sPPT was perfect for all follow up time points.
Structural and Lesion Results
As depicted in red outline in Figure 1, the lesion–defined by the tissue loss/damage observed at the T4 time point–included the left splenium and tissue medial to the left inferior/posterior horn of the lateral ventricle. The normalized lesion volume was 143 mm3; the superior-inferior extent was z=23 to −10, the lateral-medial extent was x = −34 to 0, and the posterior-anterior extent was y = −62 to −30. This lesion is primarily defined by enlargement of the left lateral ventricle, and therefore it is difficult to ascertain from the source of tissue loss, i.e. may be the case that tissue loss in the left fusiform or lingual or both contributed to ventricle enlargement. This subsequent loss of neural tissue can be observed clearly in Figure 1 which depicts T1 slices through the infarcted region.
Figure 1.
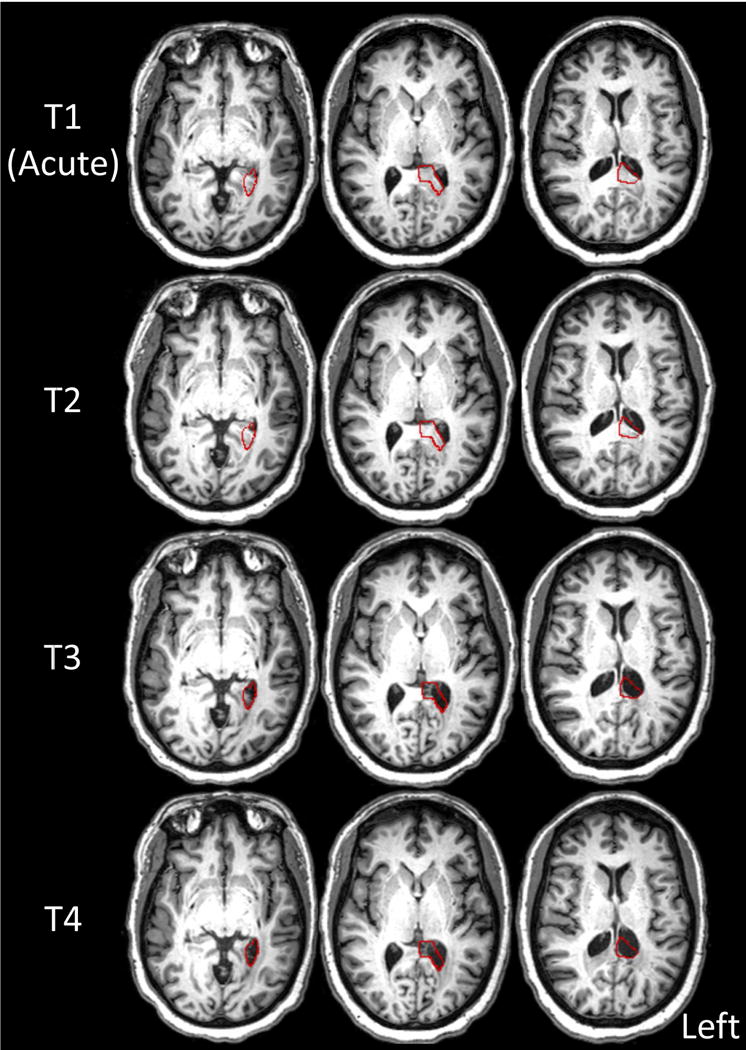
Structural axial slices in native space (aligned to the AC-PC plane) at each time point (T1: acute, T2: 3 weeks poststroke (PS), T3: 5 months PS, T4: 1 year PS). The red outline depicts the lesion volume defined by damaged or absent tissue at T4. The lesion includes the left splenium and tissue medial to the left inferior/posterior horn of the lateral ventricle.
fMRI Results
Behavioral Summary
MMY had an orthographic lexical deficit in Reading and Spelling in addition to an impairment in visual picture naming at both T1 and T2. Reading, spelling, and naming recovered considerably from these impairments by T3; this recovery persisted to T4.
As presented in Table 5, MMY’s performance in fMRI scanner tasks reflected her performance in the language battery, with impairment in both spelling and naming at T1 and T2. By T3 and T4, performance on both fMRI tasks was perfect. The Control performed perfectly on both tasks at all time points.
Table 5.
Summary of fMRI Behavior
| MMY | Control | |||||||
|---|---|---|---|---|---|---|---|---|
| T1 (Acute) | T2 (3W) | T3 (5M) | T4 (1YR) | T1 | T2 (4W) | T3 (5M) | T4 (1YR) | |
| Spelling | 40 | 70 | 100 | 100 | 100 | 100 | 100 | 100 |
| Naming | 55.5 | 79.9 | 100 | 100 | 100 | 100 | 100 | 100 |
All data are presented as % accuracy. Only the Phonemic and Word-cued accuracy was included here.
Longitudinal Imaging Results
Figure 2 depicts a summary of the activation maps in the Control and MMY across all four time points; for the list of coordinate peaks and associated brain regions see S1 for the Control and Table S2 for MMY. In order to provide a robust summary of the neural topography associated with all three tasks in the Control participant, the maps were combined across all four time points.
Figure 2.
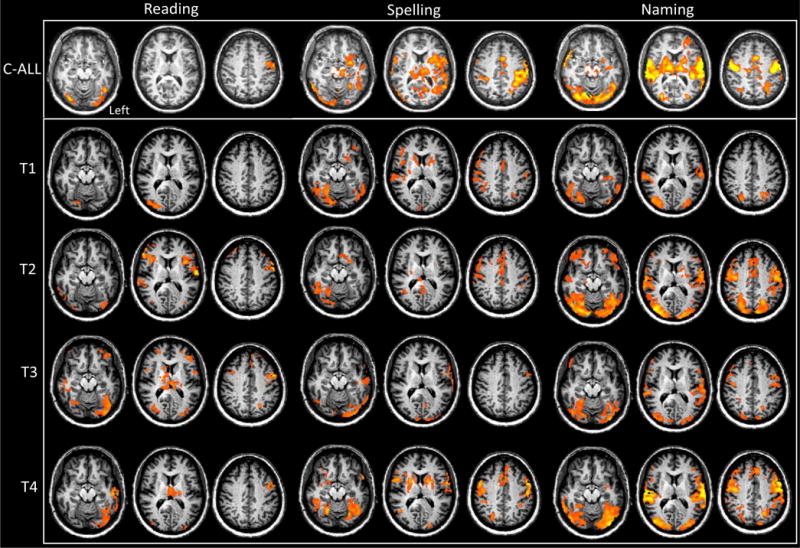
Reading, Spelling, and Naming fMRI results for the Control and for MMY. The rows depict axial slices for each task. Each row depicts axial slices (z slices = −20; 10; 35). The top row is the mean Control activity for each task (C-All). Rows 2 through 5 depict the activity for MMY at each time point (T1 = acute; T2 = 3week; T3 = 5month; T4 = 1yr). All data was cluster level corrected at a p < 0.05.)
For MMY at the acute time point, the most qualitatively abnormal activation map relative to the Control was in reading. This acute reading map indicates a very right lateralized and posterior activation pattern in the ventral occipitotemporal cortex. The Spelling and the Naming activation maps indicate numerous clusters that are in typical locations, but a relatively low activity. Although difficult to assess qualitatively across the four time points, MMY’s activation maps appear normal and robust at T4.
In order to identify the regions most associated with changes from the acute time point a comparison of T4–T1 activation was performed for Reading, Spelling, and Naming in the Control and MMY. The Control data are tabulated in table S4, and not discussed further here. MMY’s data are depicted in the top row of Figure 3 and Table S3. In MMY there was both considerable up- and down-regulation in BOLD response for the Reading and the Spelling tasks, and only up-regulation in the Naming task. For Reading, there was widespread up-regulation in the left occipital, temporal and middle/superior frontal cortices, as well as the thalamus; there was wide spread down-regulation in the left inferior frontal and left temporal lobe.
Figure 3.
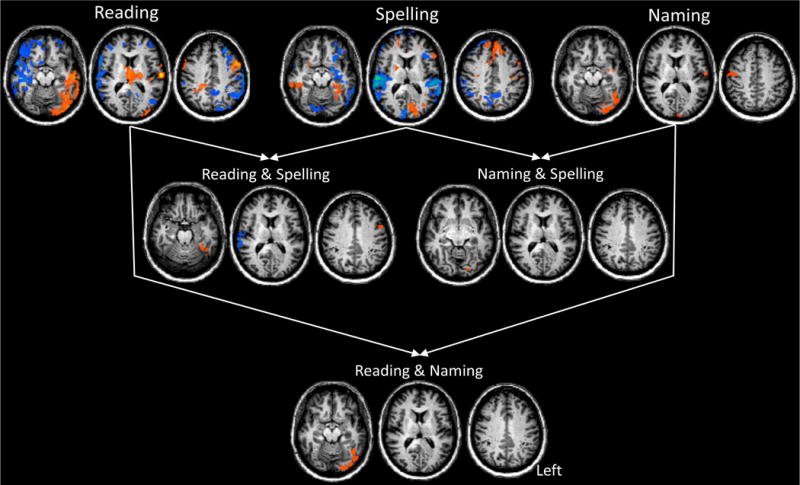
Longitudinal Analysis Results comparing the T4 (1 year) to the T1 (acute) time point for Reading, Spelling, and Naming. Each row depicts axial slices (z slices = −20; 10; 35). Blue clusters depict down regulation and orange depict up-regulation of activity from the T1 to T4 time point. The top row is the T4–T1 contrast for each of the Reading, Spelling, and Naming paradigms separately. The second row presents the conjunction of T4–T1 contrasts for Reading & Spelling (left) and Naming & Spelling (right). The bottom row shows the conjunction of T4–T1 contrasts for Reading & Naming. All data was cluster level corrected at a p < 0.05.
Next, we performed a series of conjunction analyses in the longitudinal data for both MMY and the Control in order to identify regions of converging changes in Reading, Spelling, and Naming tasks. Results for MMY are presented in Figure 3, Figure 4A and Table 6, and the results for the Control are presented in Figure S5.
Figure 4.
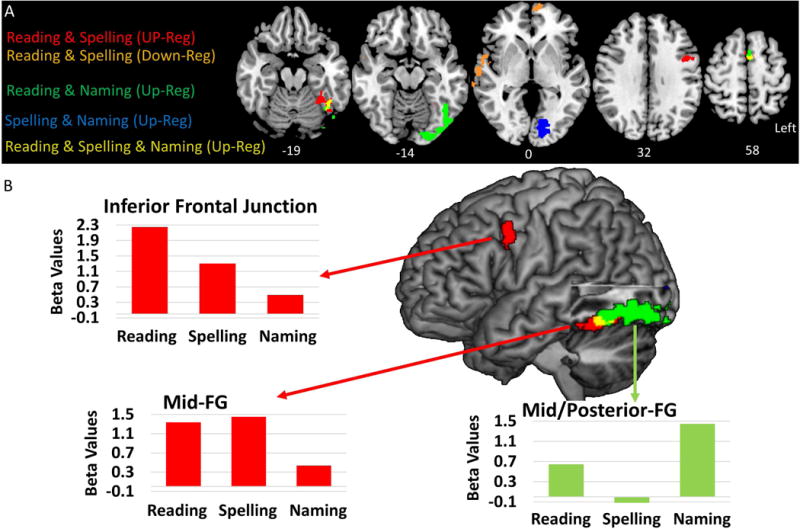
Summary of MMY Longitudinal Conjunction Analysis Results for the comparison of the T4 (1 year) to T1 (acute) time points. (A) provides a combined summary of the up and down-regulation conjunction analysis of T4–T1 clusters depicted in the bottom two rows of Figure 3. (B) A lateral rendering of the conjunction of T4–T1 contrasts. The bar plots are the mean beta-values derived from the clusters depicted in the rendered brain. Red bar plots show up-regulation of Reading & Writing in the left IFJ and mid-FG. The green bar plot shows the up-regulation of Reading & Naming in the left post-FG.
Table 6.
MMY Conjunction analysis clusters and peaks for T4 (1 year post-stroke) - T1 (Acute)
| Reading & Spelling | Reading & Naming | Spelling & Naming | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||||
| Region | k | X | Y | Z | t | Region | k | X | Y | Z | t | Region | k | X | Y | Z | t | |
| Up-Regulation | L IFJ | 42 | −51 | 9 | 42 | 3.3 | L FG | 198 | −18 | −87 | −9 | 4.8 | L LG | 90 | −18 | −84 | −3 | 3.4 |
| L FG | 50 | −30 | −45 | −18 | 3.2 | L FG | −42 | −69 | −12 | 3.8 | L Pre-CG | 33 | −48 | 0 | 21 | 2.9 | ||
| L FG | −42 | −60 | −18 | 2.3 | R SMA | 50 | 0 | 9 | 63 | 3.8 | ||||||||
| R SMA | 39 | 0 | 9 | 66 | 3.5 | |||||||||||||
|
| ||||||||||||||||||
| Down-Regulation | R STG | 186 | 66 | −12 | 0 | −6.1 | ||||||||||||
| R STG | 63 | −33 | 15 | −4.2 | ||||||||||||||
| R Temporal Pole | 57 | 6 | −15 | −2.5 | ||||||||||||||
Cluster peaks are right of the cluster size; local-maximum peaks are listed below these. k = cluster size. Anatomical Labels: Left - L; Right - R; Inferior Frontal Junction – IFJ; Superior Temporal Gyrus - STG; Supplementary Motor Area - SMA; Precentral Gyrus - Pre-CG; Fusiform Gyrus - FG; Lingual Gyrus - LG.
Reading & Spelling
In MMY there were three left hemisphere clusters associated with a conjoint up-regulation of activity associated with reading and spelling. First, there was a cluster with a peak in the left middle frontal gyrus (MFG) that was characterized as the inferior frontal junction (IFJ) due to its location in the inferior frontal sulcus at the junction of the pars opercularis, the premotor cortex, and the MFG (described in studies of cognitive control, e.g., Derrfuss, Brass, Neumann, & von Cramon, 2005; Brass, Derrfuss, Forstmann, & von Cramon, 2005). Second, there was a cluster in the midfusiform gyrus (FG). Third, there was also a cluster in the supplementary motor area (SMA); although the peak was at the mid-line (x = 0), the cluster was located in the left hemisphere. Finally, there was a conjunction of down-regulation observed in a large right superior temporal gyrus (STG) cluster which extended anteriorly into the temporal pole.
In the Control participant there was single cluster present which included the bilateral supplementary motor area, paracingulate, and anterior cingulate cortex (MNI peak = 8, 14, 47 with a peak t-value (990) = 3.01). This was the only significance cluster observed in the Control for the different combination of conjunction analyses in Reading, Spelling, and Naming data.
Reading & Naming
In MMY there was a left posterior (post-) FG cluster and right SMA cluster. The left post-FG cluster was large and extended posteriorly into the inferior lateral occipital gyrus. As for Reading & Spelling, the SMA cluster was also in the left hemisphere.
Spelling & Naming
In MMY there was a single cluster posterior in the left lingual gyrus (LG).
Reading & Spelling & Naming
In MMY there was a single significant up-regulation cluster that was shared across reading, spelling, and naming in the left SMA (MNI peak: −2, 9, 64; cluster size = 28 voxels). This cluster is presented in yellow in Figure 4A as the overlap of the up-regulation Reading & Spelling and Reading & Naming clusters. Note, that in Figure 4 there is also a yellow cluster overlap in the left FG, but this cluster did not pass a cluster level correction of p < 0.05 (cluster size = 18).
In Figure 4, the conjunction analyses results were combined to delineate selectivity of changes for each of the tasks. Of particular interest is the selectivity of changes in the left mid-FG and MFG associated with the recovery of orthographic processing (i.e. shared up-regulation across Reading & Spelling) as depicted by the red cluster in Figure 4A/B. In Figure 4B the mean beta values from these two were extracted for the Reading, Spelling, and Naming tasks in order to highlight the selectivity of changes for Reading & Spelling (i.e. orthographic processing) which are distinct from the relatively more posterior changes associated with Reading & Naming.
fMRI Laterality Results
This analysis was performed to explore similar or distinct patterns of lateralization across time for Reading, Spelling, and Naming in the regions associated with orthographic processing. Here, we focused on the FG and IFG; of primary interest was the FG, as this region in the left hemisphere was within the area affected by stroke (i.e. includes infarct and undamaged perilesional tissue).
Within the FG there were right lateralized activation patterns that were outside of the normal (per the Control) range of LIs for all three tasks - Reading, Spelling and Naming - at the acute time point, T1. For Reading, there was a gradual progression from right to left lateralization from T1 to T4. Spelling on the other hand revealed an abnormal right lateralization at T1 and T2, but by T3 and T4 activation had shifted to a left lateralized normal pattern. For Naming, there was an initial right lateralized response, which by T2 was bilateral, and left lateralized by T4.
Discussion
Here we report on an individual with an acute left PCA stroke who demonstrated an impairment in orthographic lexical processing at the acute and sub-acute phase of stroke, and who subsequently recovered this function by five months to one year post-stroke. By tracking recovery in the brain via fMRI language tasks, we observed four main results: First, there was a conjunction of neural recovery changes associated with both reading and spelling in areas the typical to orthographic processing network in normal adults, including the left mid-fusiform gyrus (mid-FG) and inferior frontal junction (IFJ). Second, neural changes associated with reading and spelling were distinct from changes associated with naming, and therefore were changes selective to orthographic processing. Third, in the acute phase, neural activation was associated with right lateralized activation in the FG for both reading and spelling (as well as naming). Finally, reading, spelling, and naming recovered to normal (either bilateral or left lateralized) activation by five months post-stroke and this recovery was still present at one year post-stroke.
Here we characterized the topography of neural recovery in MMY, who recovered from an orthographic lexical deficit that affected both reading and spelling. At the acute and sub-acute (three weeks post-stroke) phases, MMY had an impairment in orthographic lexical processing; by the chronic phase, including five months and one year post-stroke, MMY had recovered from this deficit. In order to track neural changes associated with this behavioral recovery, the neural activation associated with reading and spelling was evaluated at the acute phase of stroke, and compared to the one year poststroke time point, at which time MMY had both recovered and fully shifted to the chronic phase of stroke. In confirmation of our primary hypothesis, we observed a conjunction of neural changes for both reading and spelling in regions previously associated with orthographic processing, i.e. the left mid-FG, IFJ, and supplementary motor area (SMA). Although the left mid-FG was within the general brain region affected by the ischemic PCA stroke, the IFJ and SMA were not. Suppressed activation at the acute phase in these frontal regions that are cortically distant from the left PCA stroke is likely due to diaschisis in an orthographic lexical processing network, i.e. a selective loss of input to these frontal orthographic processing regions.
In this work, the left mid-FG and IFJ are associated with neural changes for reading and spelling from T1 to T4, but not naming, and therefore, these regions are associated with selective neural changes for orthographic processing. These are intriguing findings, given that the left mid-FG and IFJ have each been specifically implicated in orthographic processing for reading and spelling as measured with fMRI (Purcell, Napoliello, & Eden, 2011; Rapp & Lipka, 2011). Furthermore, damage to either the left FG or IFG has recently been associated specifically with impairments in orthographic lexical processing in spelling (Rapp et al., 2015), which supports the finding that these two regions are indeed associated with the loss and subsequent recovery of orthographic lexical processing.
The left mid-FG in particular has been the focus of numerous neuroimaging studies that have reported consistent activation selective to reading, and as such it has been labeled the Visual Word Form Area (VWFA) (e.g., Dehaene, Le Clec, Poline, Le Bihan, & Cohen, 2002; Dehaene & Cohen, 2011). This region has specifically been linked to orthographic lexical processing due to fMRI studies which have reported a sensitivity to the frequency of words as well as lexicality effects (i.e. words as compared to non-words) in reading (Glezer et al., 2009; Kronbichler et al., 2004; Szwed et al., 2011) as well as spelling (Ludersdorfer, Kronbichler, & Wimmer, 2015; Rapp & Dufor, 2011; Rapp & Lipka, 2011). Our findings support this role for the left mid-FG given MMY’s loss and subsequent re-gain of orthographic lexical processing is associated with up-regulation in this area.
The left IFJ has also been associated more recently with orthographic specific processing for both spelling and reading. Its role in orthographic specific processing has been reported in both the neuroimaging and lesion literature. In the neuroimaging literature, the left IFJ has been found to be functionally active in both reading and spelling, along with the left FG (Purcell, Jiang, & Eden, 2017; Purcell, Napoliello, et al., 2011; Rapp & Lipka, 2011). The lesion literature includes reports that damage to this region is associated with deficits in orthographic lexical processing in spelling (Hillis, Chang, Breese, & Heidler, 2004; Rapp et al., 2015). One proposed role of this region has been in coordinating the selection of the correct orthographic lexical representation among competing representations (Rapp & Lipka, 2011). This is in line with previous work associating it with cognitive control (Brass et al., 2005), and more recently it has been specifically associated with top-down control of linguistic functions (Muhle-Karbe et al., 2015).
Although not selective to orthographic processing, it was observed that the left SMA was associated with up-regulation in all three language tasks (reading, spelling, and naming), and is therefore non-selective to orthography. Previously, it has been associated with reading, with the suggestion that it is associated with goal directed attention while reading (e.g., Martin et al., 2015). Our work suggests that instead of an orthographic specific function, up-regulation associated with reading, spelling and naming may be associated with a domain general language function. Recent work on the SMA suggests that these general language functions could involve the control of inner speech or lexical selection (Alario, Chainay, Lehericy, & Cohen, 2006; Hertrich, Dietrich, & Ackermann, 2016). Furthermore, the only conjunction changes (reading & spelling) observed in the Control participant included the left SMA/Anterior cingulate (S5). This indicates that these SMA changes observed in MMY for reading and spelling may not be unique to recovery from stroke, and may instead be associated with unforeseen modulations in attention or familiarity associated with repeating the same language tasks over the course of a year.
The down-regulation observed in the right STG in MMY is a novel finding that may have relevance to compensatory neural mechanisms associated with recovery of orthographic lexical processing. Given that MMY made predominantly phonologically plausible spelling errors, her sublexical orthographic system was intact. Therefore, one interpretation is that this is a compensatory activation associated with increased reliance on or access to the sub-lexical orthographic representations. In line with this interpretation is previous neuroimaging work indicating that the left STG is associated with pseudoword spelling (Ludersdorfer et al., 2015). In MMY, the contra-lesional right STG may play a similar compensatory functional role; this compensatory role is reflected in hyperactivation here at the acute phase.
Overall, we propose that the recovery of language function in MMY was specific to the regain of neural function in both the original functional regions associated with orthographic lexical processing, and in separate regions associated with mapping visual objects to higher language regions (i.e. associated with phonology and semantics).
One ongoing debate in the study of the neural bases of orthography is the specificity of these neural substrates to orthography relative to other cognitive functions. The study of MMY provided a unique opportunity to address the question of specificity of neural substrates in recovery: she not only had an impairment in orthographic lexical processing at the acute and sub-acute phases, but also had a visual object naming deficit. We determined that there were regions associated with the recovery of reading and spelling within the left mid-FG, dorsal IFG, and SMA, but that were not associated with the recovery of visual object naming. Of particular interest was the left fusiform gyrus because it has long been at the center of a debate regarding whether it is specifically associated with processing to orthography or instead associated with more general language function (Dehaene & Cohen, 2011; Price & Devlin, 2011). Our findings, depicted in Figure 4, specifically indicate there was a heterogeneous pattern of functional recovery within the left fusiform: posteriorly, there was functional recovery associated with both reading and naming (green), and as one proceeds more anteriorly there was a relatively small recovery (i.e. a non-significant cluster) shared for reading, spelling, and naming (yellow), and anterior to this there was relatively selective recovery associated with reading and spelling (red). These findings indicate there is an a posterior-to-anterior progression from less-to-more specific recovery of orthographic representations. Stated differently, anteriorly there is a region that is specific to orthographic lexical processing that is distinct, anterior, but abutting those also utilized for object naming. Generally, the work of Dehaene and Cohen suggests that there are neural representations specific to orthographic processing within the left fusiform, whereas Price and Devlin suggest that this limited cortical region lacks neural representation specificity, instead claiming that representations are instantiated in a distributed manner across cortically distant language areas. Our findings support the position that although a portion of the left fusiform does lack specificity to the recovery of orthographic processing (i.e. left posterior FG yellow/green cluster in Figure 4), a relatively more anterior portion is in fact associated with the functional recovery that is specific to orthographic lexical processing (i.e. left mid-FG red cluster in Figure 4).
Another persistent issue in the field of post-stroke language recovery is the lateralization of activation associated with language recovery within the first year post-stroke. Some work suggests that that recovery of language function can be achieved if language areas are preserved (but temporarily inactivated), and later regain their original function (Heiss et al., 1999). Other work suggests that good recovery in aphasia is acutely associated with minimal activation, followed by an up-regulation the right homologous language areas in the subacute phase, and finally a re-normalization shift back to left hemispheric dominance in the chronic phase (Saur et al., 2006). The findings here support both of these proposed recovery trajectories. As reported in Table 4 there were considerable impairments at the acute phase (T1) for spelling, reading, and naming, and functionally this was associated with abnormal right lateralized activation in the fusiform gyrus for all three tasks (see Figure 5). By the sub-acute phase (T2), there was a considerable amount of recovery (i.e. greater than 20% recovery) for each task (although not as clear for overall word spelling scores, it is most evident by examining word accuracy in high frequency words in Table 3), and this recovery was associated with a shift from abnormal right lateralized fusiform gyrus activation, at least for reading and naming (spelling did not shift from normal abnormal right lateralized fusiform gyrus activation until T3 (chronic phase)). This shift from abnormal right lateralized activation was likely associated with moderate reperfusion of the left FG. This mechanism has been reported in previous studies of stroke recovery (e.g., Hillis et al., 2002, 2006; Jacquin et al., 2014), and was confirmed for MMY in a recent work which reported that there was reperfusion of the PCA territory by the sub-acute phase (T2) (i.e. MMY had normal perfusion by T2, see P1 Sebastian et al., 2016). One interpretation is that what can be observed as a “shift” may only reflect a depression of activation in the left hemisphere, and not a corresponding increase in activation levels in the right FG; this is confirmed by noting that whereas there was an increase in activation in reading, spelling, and naming in the left FG from T1 to T4, there was no change in activation in reading, spelling, or naming in the right FG from T1 to T4 (see S3). The further recovery of reading, spelling and naming from the sub-acute (T2) to the chronic phase (T3 and T4) was associated with relatively normal bilateral/left lateralized activation in the left FG, with normal left lateralization achieved for all three tasks at T4. This normal left lateralization in the FG by one year post stroke likely reflect regain of function to functionally selective cortical regions of the left FG (i.e. regions selective to orthographic lexical recovery and visual object naming recovery respectively, as discussed above), independent of changes in perfusion (because MMY normal perfusion by 3 weeks, see P1 in Sebastian et al., 2016).
Figure 5.
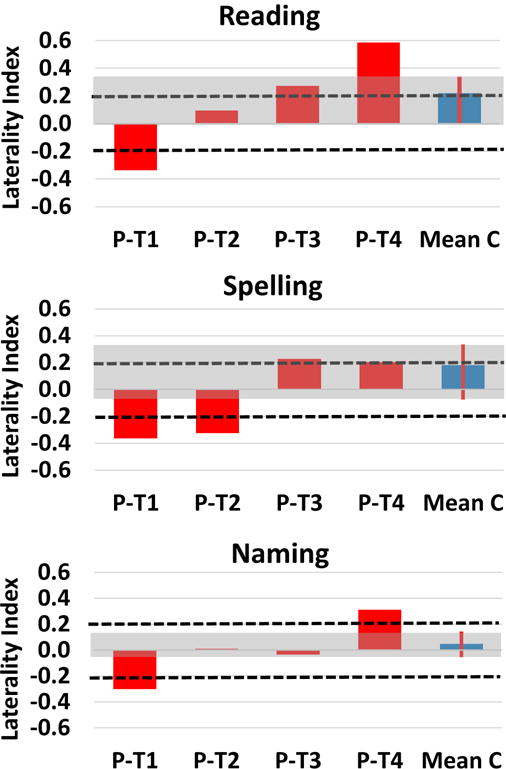
Longitudinal Laterality Analysis results in the fusiform gyrus (region defined by the Harvard Oxford Atlas). Each row depicts the laterality indices (LIs) for the Reading, Spelling, and Naming tasks. Dotted lines denote Laterality threshold of 0.2; >0.2 is left lateralized and <−0.2 is right lateralized. Red bar plots depict the LIs for MMY at each time point. The blue bar plot depicts the mean LI across all four time points for the Control participant. The red line and gray shading of the plot is the maximum and minimum range for the Control participant; this is used as a measure of normal laterality responses for each region and task.
Table 3.
Accuracy in spelling to dictation at all time points.
| T1 (acute) | T2 (3W) | T3 (5M) | T4 (1Yr) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| JHU Dysgraphia Battery-Part of Speech Sub-test | Word Accuracy | High Frequency | 64.3 | 83.3 | 85.7 | 83.3 |
| Low Frequency | 54.8 | 52.4 | 82.1 | 78.6 | ||
| Non-words | 64.7 | 8.8 | 70.6 | 79.4 | ||
|
| ||||||
| 4L | 77.8 | 66.7 | 77.8 | 77.8 | ||
| 5L | 68.8 | 62.5 | 79.2 | 79.2 | ||
| 6L | 55.8 | 74.4 | 76.7 | 79.1 | ||
| 7L | 50.0 | 25.0 | 75.0 | 100.0 | ||
|
| ||||||
| All Words | 62.5 | 68.3 | 79.6 | 81 | ||
|
| ||||||
| Letter Accuracy | High Frequency | 92.4 | 95.9 | 95.8 | 96.5 | |
| Low Frequency | 87.7 | 91.1 | 93.5 | 95.5 | ||
| Non-words | 91.3 | 20.4 | 92.9 | 94.6 | ||
|
| ||||||
| 4L | 97.2 | 91.7 | 94.4 | 94.4 | ||
| 5L | 87.5 | 90.9 | 95.6 | 95.6 | ||
| 6L | 90.9 | 95.3 | 93.4 | 95.2 | ||
| 7L | 89.3 | 85.7 | 92.9 | 100.0 | ||
|
| ||||||
| All Words | 89.8 | 92.7 | 95.4 | 95.6 | ||
|
| ||||||
| Letter Frequency effect (p-value) | 0.090 | 0.039 | 0.287 | 0.559 | ||
| Letter Length effect (p-value) | 0.442 | 0.123 | 0.276 | 0.766 | ||
Bolded are p-values that are significant (p < 0.05) or have a trend to significance (p < 0.1)
Limitations
The major limitation of this work is that it is in a single case study. This limitation is a consequence of the rarity of appropriate language profiles among patients enrolled in ongoing longitudinal studies of language recovery after acute stroke. Although this study does provide an indepth analysis of one single patient, it does not allow us to generalize our conclusions to other stroke patients.
Implications
This work has two major implications: First, it has implications generally for the study of language function recovery post-stroke in that it demonstrates how different language impairments (i.e. orthographic lexical deficit and optic aphasia) are reflected in a differentiable functional neurotopography of recovery within the same individual. This finding supports the idea that examining language recovery in the future should be approached with a diversity of language tasks (e.g. reading, spelling, and naming, in this work) in order to track the different aspects of language functions that are recovered. Second, it has implications for orthographic lexical processing network in that it confirms previous hypotheses proposing orthographic specific processing within the left FG; this is highlighted by the finding that unlike the left posterior FG which lacked neural changes specific to orthography, the neural changes in the left mid-FG were orthography specific. Essentially, this is a loss-of-function to gain-of-function study of orthographic lexical processing, which confirms the critical role of the left mid-FG and IFJ in intact orthographic processing.
Supplementary Material
Table 2.
MMY’s % accuracy in reading aloud at each time point
| T1 (acute) | T2 (3W) | T3 (5M) | T4 (1Yr) | ||
|---|---|---|---|---|---|
|
| |||||
| JHU Dyslexia Battery-Regularity Sub-test | High Frequency | 0.0 | 35.7 | 97.8 | 100.0 |
| Low Frequency | 0.0 | 13.6 | 91.1 | 95.6 | |
| Non-words | 0.0 | 20.0 | 92.0 | 100.0 | |
|
| |||||
| 3L | 0.0 | 50.0 | 100.0 | 100.0 | |
| 4L | 0.0 | 17.3 | 95.2 | 98.8 | |
| 5L | 0.0 | 22.2 | 88.9 | 97.2 | |
| 6L | 0.0 | 20.0 | 100.0 | 88.9 | |
| 7L | 0.0 | 0.0 | 100.0 | 75.0 | |
|
| |||||
| All Words | 0.0 | 25.8 | 94.4 | 97.8 | |
|
| |||||
| Frequency effect n (p-value) | n/a | 0.077 | 0.167 | 0.153 | |
| Length effect (p-value) | n/a | 0.456 | 0.933 | 0.462 | |
Bolded are p-values that are significant (p < 0.05) or have a trend to significance (p < 0.1)
Acknowledgments
The research reported in this paper was supported by the National Institutes of Health (National Institute of Deafness and Communication Disorders) through award R01 DC05375. The content is solely the responsibility of the authors and does not necessarily represent the views the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abo M, Senoo A, Watanabe S, Miyano S, Doseki K, Sasaki N, Yonemoto K. Language-related brain function during word repetition in post-stroke aphasics. Neuroreport. 2004;15(12):1891–4. doi: 10.1097/00001756-200408260-00011. https://doi.org/00001756-200408260-00011 [pii] [DOI] [PubMed] [Google Scholar]
- Alario FX, Chainay H, Lehericy S, Cohen L. The role of the supplementary motor area (SMA) in word production. Brain Research. 2006;1076(1):129–143. doi: 10.1016/j.brainres.2005.11.104. [DOI] [PubMed] [Google Scholar]
- Andersen SM, Rapcsak SZ, Beeson PM. Cost function masking during normalization of brains with focal lesions: still a necessity? NeuroImage. 2010;53(1):78–84. doi: 10.1016/j.neuroimage.2010.06.003. https://doi.org/10.1016/j.neuroimage.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade C, Thiel A, Ansaldo A. The complementary role of the cerebral hemispheres in recovery from aphasia after stroke: a critical review of literature. Brain Injury. 2014;28(2):138–145. doi: 10.3109/02699052.2013.859734. https://doi.org/10.3109/02699052.2013.859734. [DOI] [PubMed] [Google Scholar]
- Bates E, D’Amico S, Jacobsen T, Székely A, Andonova E, Devescovi A, Tzeng O. Timed picture naming in seven languages. Psychonomic Bulletin & Review. 2003;10(2):344–380. doi: 10.3758/bf03196494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvois MF. Optic aphasia: a process of interaction between vision and language. (Series B, Biological Sciences).Philosophical Transactions of the Royal Society of London. 1982;298(1089):35–47. doi: 10.1098/rstb.1982.0070. [DOI] [PubMed] [Google Scholar]
- Beeson PM, Hillis AE. Comprehension and production of written words. In: Chapey R, editor. Comprehension and production of written words. 4th. Baltimore: Williams and Wilkin; 2001. [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. https://doi.org/10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank SC, Bird H, Turkheimer F, Wise RJS. Speech production after stroke: The role of the right pars opercularis. Annals of Neurology. 2003;54(3):310–320. doi: 10.1002/ana.10656. https://doi.org/10.1002/ana.10656. [DOI] [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36(1):159–170. doi: 10.1016/s0896-6273(02)00936-4. https://doi.org/10.1016/S0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences. 2005;9(7):314–316. doi: 10.1016/j.tics.2005.05.001. https://doi.org/10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Breining B, Lala T, Martínez Cuitiño M, Manes F, Peristeri E, Tsapkini K, Hillis AE. A brief assessment of object semantics in primary progressive aphasia. Aphasiology. 2015;29(4):488–505. https://doi.org/10.1080/02687038.2014.973360. [Google Scholar]
- Breining B, Tippett D, Davis C, Posner J, Sebastian R, Oishi K, Hillis AE. Assessing dissociations of object and action naming in acute stroke. Monterey, CA: Clinical Aphasiology Conference; 2015. [Google Scholar]
- Cohen L, Dehaene S, McCormick S, Durant S, Zanker JM. Brain mechanisms of recovery from pure alexia:a single case study with multiple longitudinal scans. Neuropsychologia. 2016 doi: 10.1016/j.neuropsychologia.2016.07.009. https://doi.org/10.1016/j.neuropsychologia.2016.07.009. [DOI] [PubMed]
- Cohen L, Lehericy S, Chochon F, Lemer C, Rivaud S, Dehaene S. Language-specific tuning of visual cortex? Functional properties of the Visual Word Form Area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Le Clec HG, Poline JB, Le Bihan D, Cohen L. The visual word form area: a prelexical representation of visual words in the fusiform gyrus. Neuroreport. 2002;13(3):321–325. doi: 10.1097/00001756-200203040-00015. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Pegado F, Braga LW, Ventura P, Nunes Filho G, Jobert A, Cohen L. How learning to read changes the cortical networks for vision and language. Science. 2010;339(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- Dehaene Stanislas, Cohen L. The unique role of the visual word form area in reading. Trends in Cognitive Sciences. 2011;15(6):254–262. doi: 10.1016/j.tics.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: meta-analyses of switching and Stroop studies. Human Brain Mapping. 2005;25(1):22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. https://doi.org/10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Fernandez G, Specht K, Weis S, Tendolkar I, Reuber M, Fell J, Elger CE. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology. 2003;60(6):969–975. doi: 10.1212/01.wnl.0000049934.34209.2e. [DOI] [PubMed] [Google Scholar]
- Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2010;30(35):11558–11564. doi: 10.1523/JNEUROSCI.2227-10.2010. https://doi.org/10.1523/JNEUROSCI.2227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clemenceau S, Volle E, Hasboun D, Cohen L. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50(2):191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Gilhooly KJ, Logie RH. Age-of-acquisition, imagery, concreteness, familiarity, and ambiguity measures for 1,944 words. Behavior Research Methods & Instrumentation. 1980;12(4):395–427. https://doi.org/10.3758/BF03201693. [Google Scholar]
- Glezer LS, Jiang X, Riesenhuber M. Evidence for highly selective neuronal tuning to whole words in the “visual word form area. Neuron. 2009;62(2):199–204. doi: 10.1016/j.neuron.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H, Kaplan E, Barresi B. Boston Diagnostic Aphasia Examination (Third) Austin, TX: Linguisystems; 2001. [Google Scholar]
- Goodman RA, Caramazza A. The Johns Hopkins University Dysgraphia Battery. The Johns Hopkins University; Baltimore MD: 1985. [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Annals of Neurology. 1999;45(4):430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. http://doi.org/10.1002/1531-8249(199904)45:43.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Hertrich I, Dietrich S, Ackermann H. The role of the supplementary motor area for speech and language processing. Neuroscience and Biobehavioral Reviews. 2016;68:602–610. doi: 10.1016/j.neubiorev.2016.06.030. https://doi.org/10.1016/j.neubiorev.2016.06.030. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Chang S, Breese E, Heidler J. The crucial role of posterior frontal regions in modality specific components of the spelling process. Vol. 10. Department of Neurology Johns Hopkins University School of Medicine; Baltimore, MD 21287, USA: 2004. argye@JHMI.edu. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15788255. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kleinman JT, Newhart M, Heidler-Gary J, Gottesman RF, Barker PB, Chaudhry P. Restoring Cerebral Blood Flow Reveals Neural Regions Critical for Naming. The Journal of Neuroscience. 2006;26(31):8069–8073. doi: 10.1523/JNEUROSCI.2088-06.2006. https://doi.org/10.1523/JNEUROSCI.2088-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Metter EJ. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain: A Journal of Neurology. 2002;125(Pt 5):1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Holland R, Leff AP, Josephs O, Galea JM, Desikan M, Price CJ, Crinion JT. Speech Facilitation by Left Inferior Frontal Cortex Stimulation. Current Biology. 2011;21(16):1403–1407. doi: 10.1016/j.cub.2011.07.021. https://doi.org/10.1016/j.cub.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacquin A, Virat-Brassaud ME, Rouaud O, Osseby GV, Aboa-Eboulé C, Hervieu M, Béjot Y. Vascular aphasia outcome after intravenous recombinant tissue plasminogen activator thrombolysis for ischemic stroke. European Neurology. 2014;71(5–6):288–295. doi: 10.1159/000357428. https://doi.org/10.1159/000357428. [DOI] [PubMed] [Google Scholar]
- Jarso S, Li M, Faria A, Davis C, Leigh R, Sebastian R, Hillis AE. Distinct mechanisms and timing of language recovery after stroke. Cognitive Neuropsychology. 2013;30(7–8):454–75. doi: 10.1080/02643294.2013.875467. https://doi.org/10.1080/02643294.2013.875467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test (second) Philadelphia: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Kelly-Hayes M, Wolf PA, Kase CS, Gresham GE, Kannel WB, D’Agostino RB. Time Course of Functional Recovery After Stroke: The Framingham Study. Journal of Neurologic Rehabilitation. 1989;3(2):65–70. https://doi.org/10.1177/136140968900300202. [Google Scholar]
- Kronbichler M, Hutzler F, Wimmer H, Mair A, Staffen W, Ladurner G. The visual word form area and the frequency with which words are encountered: evidence from a parametric fMRI study. Neuroimage. 2004;21(3):946–953. doi: 10.1016/j.neuroimage.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Kučera H, Francis WN. Computational analysis of present-day American English. Providence, RI: Brown University Press; 1983. [Google Scholar]
- Ludersdorfer P, Kronbichler M, Wimmer H. Accessing orthographic representations from speech: The role of left ventral occipitotemporal cortex in spelling. Human Brain Mapping. 2015;36(4):1393–1406. doi: 10.1002/hbm.22709. https://doi.org/10.1002/hbm.22709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Schurz M, Kronbichler M, Richlan F. Reading in the brain of children and adults: A meta-analysis of 40 functional magnetic resonance imaging studies. Human Brain Mapping. 2015;36(5):1963–1981. doi: 10.1002/hbm.22749. https://doi.org/10.1002/hbm.22749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhle-Karbe PS, Derrfuss J, Lynn MT, Neubert FX, Fox PT, Brass M, Eickhoff SB. Co-Activation-Based Parcellation of the Lateral Prefrontal Cortex Delineates the Inferior Frontal Junction Area. Cerebral Cortex. 2015:bhv073. doi: 10.1093/cercor/bhv073. https://doi.org/10.1093/cercor/bhv073. [DOI] [PMC free article] [PubMed]
- Perani D, Cappa SF, Tettamanti M, Rosa M, Scifo P, Miozzo A, Fazio F. A fMRI study of word retrieval in aphasia. Brain and Language. 2003;85(3):357–368. doi: 10.1016/s0093-934x(02)00561-8. http://doi.org/10.1016/S0093-934X(02)00561-8. [DOI] [PubMed] [Google Scholar]
- Postman-Caucheteux WA, Birn RM, Pursley RH, Butman JA, Solomon JM, Picchioni D, Braun AR. Single-trial fMRI shows contralesional activity linked to overt naming errors in chronic aphasic patients. Journal of Cognitive Neuroscience. 2010;22(6):1299–1318. doi: 10.1162/jocn.2009.21261. http://doi.org/10.1162/jocn.2009.21261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The interactive account of ventral occipitotemporal contributions to reading. Trends in Cognitive Sciences. 2011;15(6):246–253. doi: 10.1016/j.tics.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Jiang X, Eden G. Shared orthographic neuronal representations for spelling and reading. NeuroImage. 2017;147:554–567. doi: 10.1016/j.neuroimage.2016.12.054. https://doi.org/10.1016/j.neuroimage.2016.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Napoliello EM, Eden GF. A combined fMRI study of typed spelling and reading. Neuroimage. 2011;55(2):750–762. doi: 10.1016/j.neuroimage.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell JJ, Turkeltaub PE, Eden GF, Rapp B. Examining the central and peripheral processes of written word production through meta-analysis. Frontiers in Psychology. 2011 Oct;2:239. doi: 10.3389/fpsyg.2011.00239. https://doi.org/10.3389/fpsyg.2011.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Dufor O. The neurotopography of written word production: an FMRI investigation of the distribution of sensitivity to length and frequency. Journal of Cognitive Neuroscience. 2011;23(12):4067–81. doi: 10.1162/jocn_a_00109. https://doi.org/10.1162/jocn_a_00109. [DOI] [PubMed] [Google Scholar]
- Rapp B, Lipka K. The literate brain: the relationship between spelling and reading. Journal of Cognitive Neuroscience. 2011;23(5):1180–1197. doi: 10.1162/jocn.2010.21507. https://doi.org/10.1162/jocn.2010.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp B, Purcell JJ, Hillis AE, Capasso R, Miceli M. Neural bases of orthographic long-term memory and working memory in dysgraphia. Brain. 2015:awv348. doi: 10.1093/brain/awv348. https://doi.org/10.1093/brain/awv348. [DOI] [PMC free article] [PubMed]
- Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath HO. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61(4):957–965. doi: 10.1016/j.neuroimage.2012.03.020. https://doi.org/10.1016/j.neuroimage.2012.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saur D, Lange R, Baumgaertner A, Schraknepper V, Willmes K, Rijntjes M, Weiller C. Dynamics of language reorganization after stroke. Brain. 2006;129(6):1371–1384. doi: 10.1093/brain/awl090. https://doi.org/10.1093/brain/awl090. [DOI] [PubMed] [Google Scholar]
- Sebastian R, Kiran S. Task-modulated neural activation patterns in chronic stroke patients with aphasia. Aphasiology. 2011;25(8):927–951. https://doi.org/10.1080/02687038.2011.557436. [Google Scholar]
- Sebastian R, Long C, Purcell JJ, Faria AV, Lindquist M, Jarso S, Hillis AE. Imaging network level language recovery after left PCA stroke. Imaging Network Level Language Recovery after Left PCA Stroke. 2016 doi: 10.3233/RNN-150621. https://doi.org/10.3233/RNN-150621. [DOI] [PMC free article] [PubMed]
- Seghier ML. Laterality index in functional MRI: methodological issues. Magnetic Resonance Imaging. 2008;26(5):594–601. doi: 10.1016/j.mri.2007.10.010. https://doi.org/10.1016/j.mri.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwed M, Dehaene S, Kleinschmidt A, Eger E, Valabrègue R, Amadon A, Cohen L. Specialization for written words over objects in the visual cortex. NeuroImage. 2011;56(1):330–344. doi: 10.1016/j.neuroimage.2011.01.073. https://doi.org/10.1016/j.neuroimage.2011.01.073. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Coslett HB, Thomas AL, Faseyitan O, Benson J, Norise C, Hamilton RH. The right hemisphere is not unitary in its role in aphasia recovery. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2012;48(9):1179–86. doi: 10.1016/j.cortex.2011.06.010. https://doi.org/10.1016/j.cortex.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winhuisen L, Thiel A, Schumacher B, Kessler J, Rudolf J, Haupt WF, Heiss WD. The Right Inferior Frontal Gyrus and Poststroke Aphasia: A Follow-Up Investigation. Stroke. 2007;38(4):1286–1292. doi: 10.1161/01.STR.0000259632.04324.6c. https://doi.org/10.1161/01.STR.0000259632.04324.6c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


