Abstract
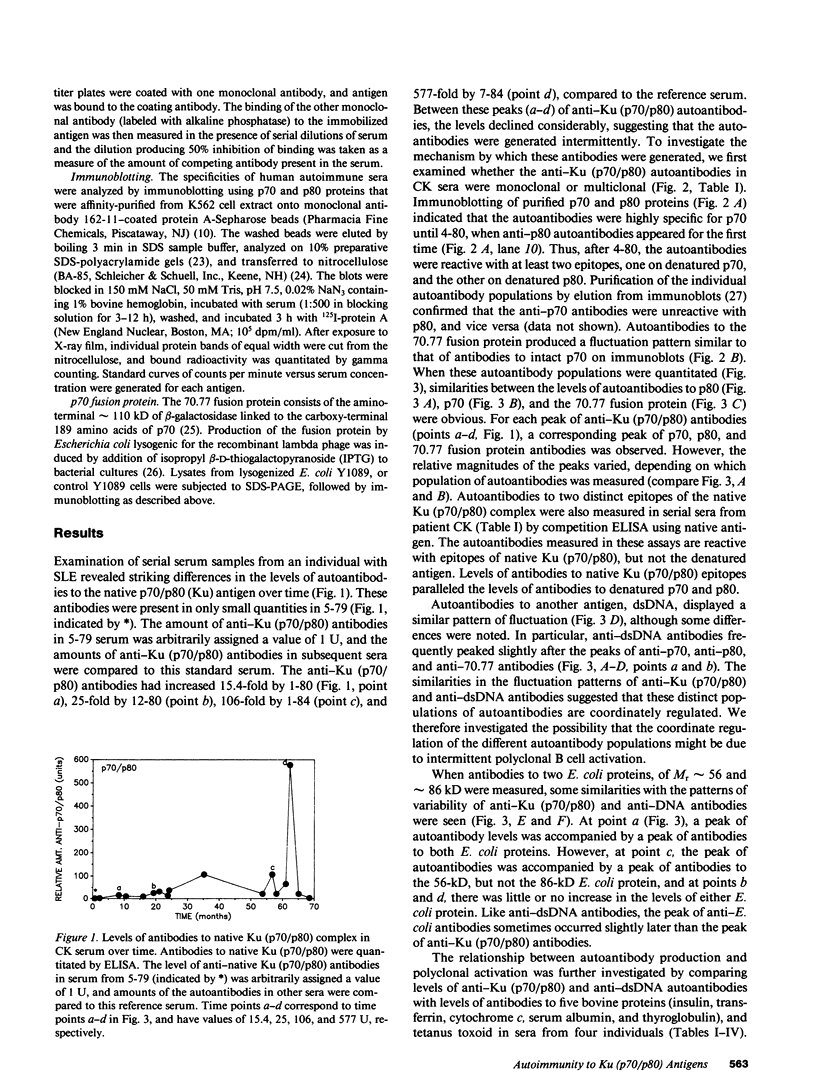
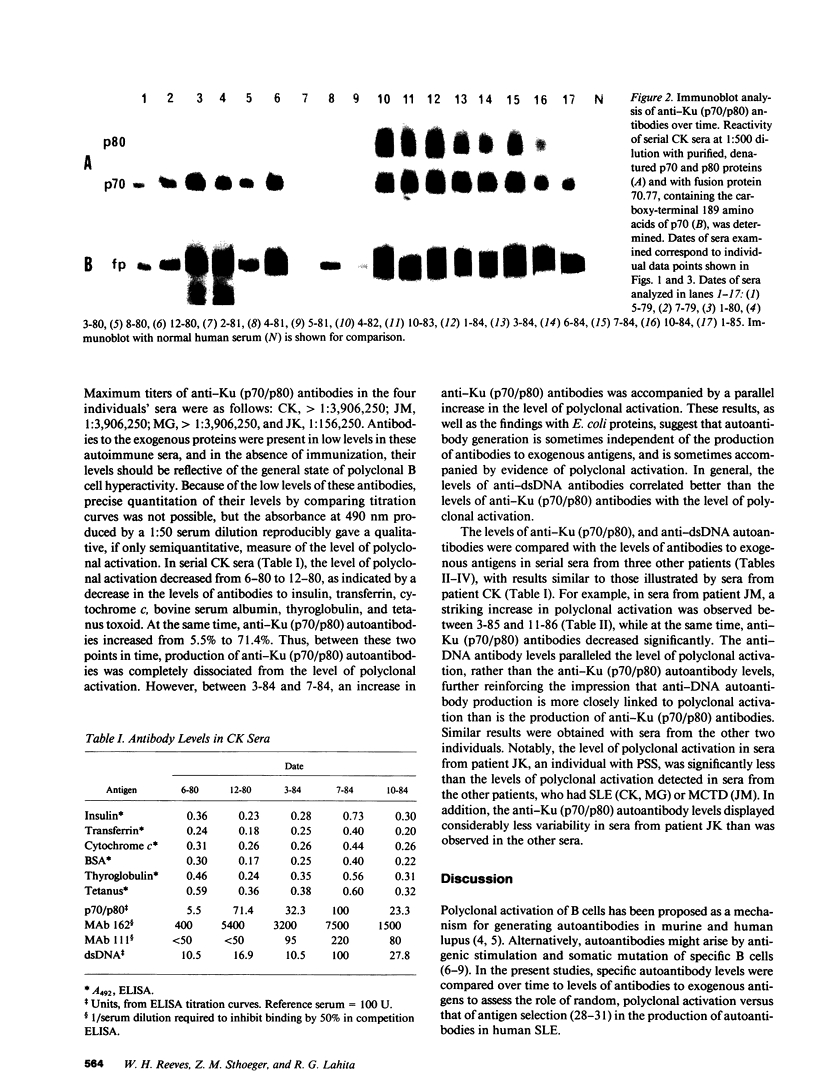
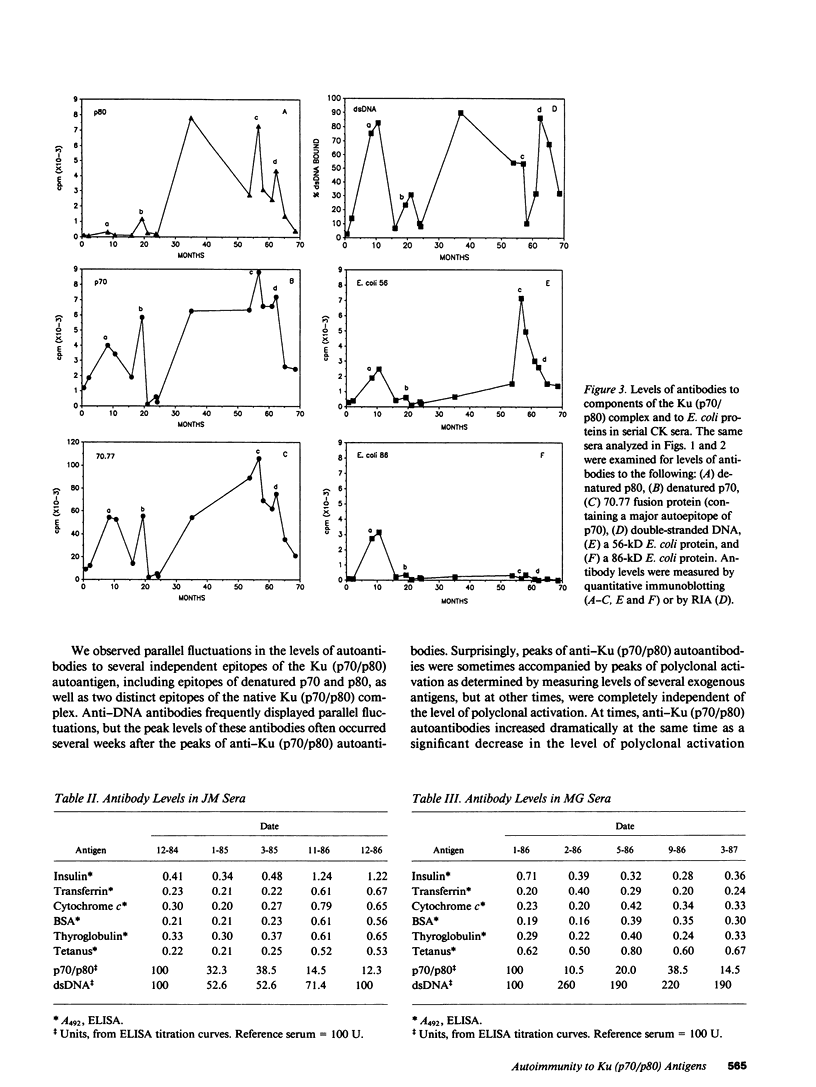
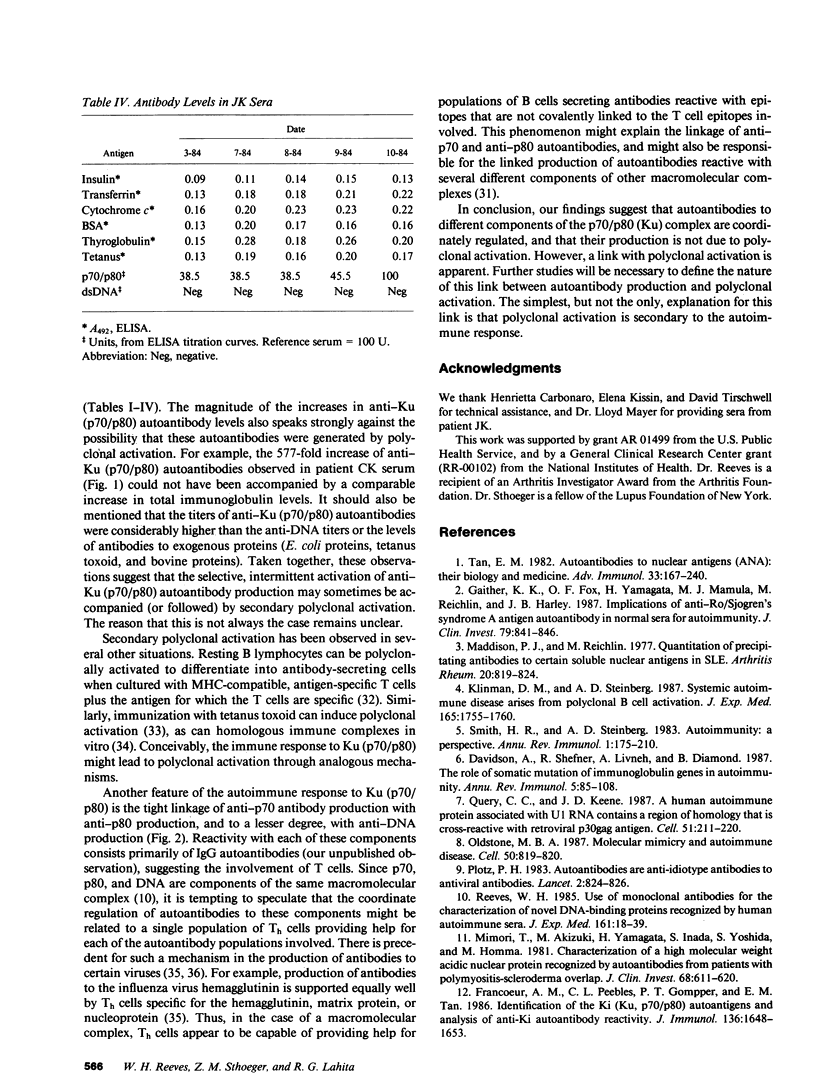
Levels of anti-Ku (p70/p80) antibodies were measured longitudinally in sera from four individuals with systemic lupus erythematosus or related disorders. Antibodies to the native Ku antigen (p70/p80 complex) varied over a range of up to 577-fold. Large fluctuations were also observed in the levels of autoantibodies to several distinct epitopes of the Ku (p70/p80) antigen. Levels of these individual autoantibody populations generally paralleled one another, suggesting that they are coordinately regulated. A similar pattern of anti-DNA antibody fluctuation was seen in some sera. To examine the possibility that these autoantibodies were generated by polyclonal B cell activation, the levels of anti-Ku (p70/p80) and anti-DNA antibodies were compared to the levels of antibodies to Escherichia coli proteins, tetanus toxoid, and bovine insulin, transferrin, cytochrome c, serum albumin, and thyroglobulin. In sera from the same individual, anti-Ku (p70/p80) antibodies were sometimes produced in the complete absence of polyclonal activation, and at other times were accompanied by increased polyclonal activation. Anti-DNA antibody levels more closely paralleled the level of polyclonal activation than did the anti-Ku (p70/p80) levels. These studies suggest that anti-Ku (p70/p80) antibodies are generated by an antigen-selective mechanism, but that polyclonal activation frequently, although not invariably, accompanies autoantibody production. This observation is consistent with the possibility that polyclonal activation might be secondary to autoantibody production.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonfa E., Golombek S. J., Kaufman L. D., Skelly S., Weissbach H., Brot N., Elkon K. B. Association between lupus psychosis and anti-ribosomal P protein antibodies. N Engl J Med. 1987 Jul 30;317(5):265–271. doi: 10.1056/NEJM198707303170503. [DOI] [PubMed] [Google Scholar]
- Chiorazzi N., Wasserman R. L., Kunkel H. G. Use of Epstein-Barr virus-transformed B cell lines for the generation of immunoglobulin-producing human B cell hybridomas. J Exp Med. 1982 Sep 1;156(3):930–935. doi: 10.1084/jem.156.3.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A., Shefner R., Livneh A., Diamond B. The role of somatic mutation of immunoglobulin genes in autoimmunity. Annu Rev Immunol. 1987;5:85–108. doi: 10.1146/annurev.iy.05.040187.000505. [DOI] [PubMed] [Google Scholar]
- DeFranco A. L., Ashwell J. D., Schwartz R. H., Paul W. E. Polyclonal stimulation of resting B lymphocytes by antigen-specific T lymphocytes. J Exp Med. 1984 Mar 1;159(3):861–880. doi: 10.1084/jem.159.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton R. B., Schnneider G., Schur P. H. Enzyme immunoassay for antibodies to native DNA. Specificity and quality of antibodies. Arthritis Rheum. 1983 Jan;26(1):52–62. doi: 10.1002/art.1780260109. [DOI] [PubMed] [Google Scholar]
- Fisher D. E., Reeves W. H., Wisniewolski R., Lahita R. G., Chiorazzi N. Temporal shifts from Sm to ribonucleoprotein reactivity in systemic lupus erythematosus. Arthritis Rheum. 1985 Dec;28(12):1348–1355. doi: 10.1002/art.1780281206. [DOI] [PubMed] [Google Scholar]
- Francoeur A. M., Peebles C. L., Gompper P. T., Tan E. M. Identification of Ki (Ku, p70/p80) autoantigens and analysis of anti-Ki autoantibody reactivity. J Immunol. 1986 Mar 1;136(5):1648–1653. [PubMed] [Google Scholar]
- Gaither K. K., Fox O. F., Yamagata H., Mamula M. J., Reichlin M., Harley J. B. Implications of anti-Ro/Sjögren's syndrome A antigen autoantibody in normal sera for autoimmunity. J Clin Invest. 1987 Mar;79(3):841–846. doi: 10.1172/JCI112892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giallongo A., Kochoumian L., King T. P. Enzyme and radioimmunoassays for specific murine IgE and IgG with different solid-phase immunosorbents. J Immunol Methods. 1982 Aug 13;52(3):379–393. doi: 10.1016/0022-1759(82)90009-6. [DOI] [PubMed] [Google Scholar]
- Hardin J. A. The lupus autoantigens and the pathogenesis of systemic lupus erythematosus. Arthritis Rheum. 1986 Apr;29(4):457–460. doi: 10.1002/art.1780290401. [DOI] [PubMed] [Google Scholar]
- Klinman D. M., Steinberg A. D. Systemic autoimmune disease arises from polyclonal B cell activation. J Exp Med. 1987 Jun 1;165(6):1755–1760. doi: 10.1084/jem.165.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison P. J., Reichlin M. Quantitation of precipitating antibodies to certain soluble nuclear antigens in SLE. Arthritis Rheum. 1977 Apr;20(3):819–824. doi: 10.1002/art.1780200310. [DOI] [PubMed] [Google Scholar]
- McCarty G. A., Rice J. R., Bembe M. L., Pisetsky D. S. Independent expression of autoantibodies in systemic lupus erythematosus. J Rheumatol. 1982 Sep-Oct;9(5):691–695. [PubMed] [Google Scholar]
- Milich D. R., McLachlan A., Thornton G. B., Hughes J. L. Antibody production to the nucleocapsid and envelope of the hepatitis B virus primed by a single synthetic T cell site. Nature. 1987 Oct 8;329(6139):547–549. doi: 10.1038/329547a0. [DOI] [PubMed] [Google Scholar]
- Mimori T., Akizuki M., Yamagata H., Inada S., Yoshida S., Homma M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest. 1981 Sep;68(3):611–620. doi: 10.1172/JCI110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Morgan E. L., Weigle W. O. Polyclonal activation of murine B lymphocytes by immune complexes. J Immunol. 1983 Mar;130(3):1066–1070. [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Plotz P. H. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet. 1983 Oct 8;2(8354):824–826. doi: 10.1016/s0140-6736(83)90740-7. [DOI] [PubMed] [Google Scholar]
- Query C. C., Keene J. D. A human autoimmune protein associated with U1 RNA contains a region of homology that is cross-reactive with retroviral p30gag antigen. Cell. 1987 Oct 23;51(2):211–220. doi: 10.1016/0092-8674(87)90148-6. [DOI] [PubMed] [Google Scholar]
- Reeves W. H. Antinuclear antibodies as probes to explore the structural organization of the genome. J Rheumatol Suppl. 1987 Jun;14 (Suppl 13):97–105. [PubMed] [Google Scholar]
- Reeves W. H., Sthoeger Z. M. Molecular cloning of cDNA encoding the p70 (Ku) lupus autoantigen. J Biol Chem. 1989 Mar 25;264(9):5047–5052. [PubMed] [Google Scholar]
- Reeves W. H. Use of monoclonal antibodies for the characterization of novel DNA-binding proteins recognized by human autoimmune sera. J Exp Med. 1985 Jan 1;161(1):18–39. doi: 10.1084/jem.161.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherle P. A., Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986 Oct 1;164(4):1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Smith D. E., Fisher P. A. Identification, developmental regulation, and response to heat shock of two antigenically related forms of a major nuclear envelope protein in Drosophila embryos: application of an improved method for affinity purification of antibodies using polypeptides immobilized on nitrocellulose blots. J Cell Biol. 1984 Jul;99(1 Pt 1):20–28. doi: 10.1083/jcb.99.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. R., Steinberg A. D. Autoimmunity--a perspective. Annu Rev Immunol. 1983;1:175–210. doi: 10.1146/annurev.iy.01.040183.001135. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Autoantibodies to nuclear antigens (ANA): their immunobiology and medicine. Adv Immunol. 1982;33:167–240. doi: 10.1016/s0065-2776(08)60836-6. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Cohen A. S., Fries J. F., Masi A. T., McShane D. J., Rothfield N. F., Schaller J. G., Talal N., Winchester R. J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982 Nov;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch M. J., Fong S., Vaughan J., Carson D. Increased frequency of rheumatoid factor precursor B lymphocytes after immunization of normal adults with tetanus toxoid. Clin Exp Immunol. 1983 Feb;51(2):299–304. [PMC free article] [PubMed] [Google Scholar]
- Wold R. T., Young F. E., Tan E. M., Farr R. S. Deoxyribonucleic acid antibody: a method to detect its primary interaction with deoxyribonucleic acid. Science. 1968 Aug 23;161(3843):806–807. doi: 10.1126/science.161.3843.806. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Ochs R., McRorie D. K., Zweig S., Busch H. Purification of an 86-70 kDa nuclear DNA-associated protein complex. Biochim Biophys Acta. 1985 Jul 26;841(1):22–29. doi: 10.1016/0304-4165(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]




