Abstract
Bridging cellular reproduction and survival is essential for all life forms. Aspergillus fungi primarily reproduce by forming asexual spores called conidia, whose formation and maturation is governed by the central genetic regulatory circuit BrlA→AbaA→WetA. Here, we report that WetA is a multi-functional regulator that couples spore differentiation and survival, and governs proper chemical development in Aspergillus flavus. The deletion of wetA results in the formation of conidia with defective cell walls and no intra-cellular trehalose, leading to reduced stress tolerance, a rapid loss of viability, and disintegration of spores. WetA is also required for normal vegetative growth, hyphal branching, and production of aflatoxins. Targeted and genome-wide expression analyses reveal that WetA exerts feedback control of brlA and that 5,700 genes show altered mRNA levels in the mutant conidia. Functional category analyses of differentially expressed genes in ΔwetA RNA-seq data indicate that WetA contributes to spore integrity and maturity by properly regulating the metabolic pathways of trehalose, chitin, α-(1,3)-glucan, β-(1,3)-glucan, melanin, hydrophobins, and secondary metabolism more generally. Moreover, 160 genes predicted to encode transcription factors are differentially expressed by the absence of wetA, suggesting that WetA may play a global regulatory role in conidial development. Collectively, we present a comprehensive model for developmental control that bridges spore differentiation and survival in A. flavus.
Introduction
Coordination of cellular reproduction and survival is fundamental to the existence and propagation of all living organisms. From the simplest single cell organisms to complex multicellular plants and animals, regulatory and signalling systems have evolved to ensure that future viability of the reproductive cells. Fungi primarily reproduce through spore propagation; fungal spores are adapted for dispersal and are resistant to desiccation, heat, oxidative and UV stresses, properties which also render them very capable of establishing infections [1]. Fungal sporulation involves coordinated control of morphological, physiological, and metabolic (chemical) developmental processes.
The genus Aspergillus includes several organisms that are commonly found in the human environment. For example, the widely distributed Aspergillus flavus is an opportunistic pathogen of plants and humans [2], and can produce the mycotoxin aflatoxin B1 (AFB1), the most potent carcinogen found in nature. The main means of dissemination of this fungus is producing a massive number of asexual spores (conidia), which are dispersed in the soil and air. In agricultural fields, these spores are carried to corn ears by insects or the wind where they grow in maize kernels and produce AFB1 [3]. AFB1 can be present in oil-seed crops, such as corn, cereals, sorghum, and peanuts, and when AFB1 is present in the feed consumed by a cow, it can be metabolised to AFM1 (M for milk), which is also highly toxic and carcinogenic [4]. Consumption of high doses of AFB1 in humans can lead to acute aflatoxicosis, liver necrosis, and even death. Due to their carcinogenicity and toxicity, levels of aflatoxins in foods and feeds are strictly regulated worldwide [5]. As conidiation and AF production are tightly correlated in A. flavus, understanding the mechanisms bridging cellular and chemical development may provide novel insights into controlling the dissemination of the fungus and subsequent contamination of crops by AFB1 [6–8].
The asexual reproductive cycle of Aspergillus fungi can be divided into two distinct phases: growth and development. The growth phase involves germination of the conidium and formation of an undifferentiated network of hyphal cells that form the mycelium. Once nutritional resources begin to be limiting, some of the hyphal cells stop mycelial growth and begin asexual development (conidiation) by forming complex structures called conidiophores that bear multiple chains of conidia (Fig 1A), completing the asexual reproductive cycle [9]. Conidiation in Aspergillus involves distinct morphological and chemical processes [9]. For example, a key morphological process is the formation of a large number of conidia with specialized cell walls. Similarly, key primary metabolic processes include the acquisition of pigments and massive biogenesis of trehalose within the spore (up to 15% of dry weight), providing protection and long-term viability [10].
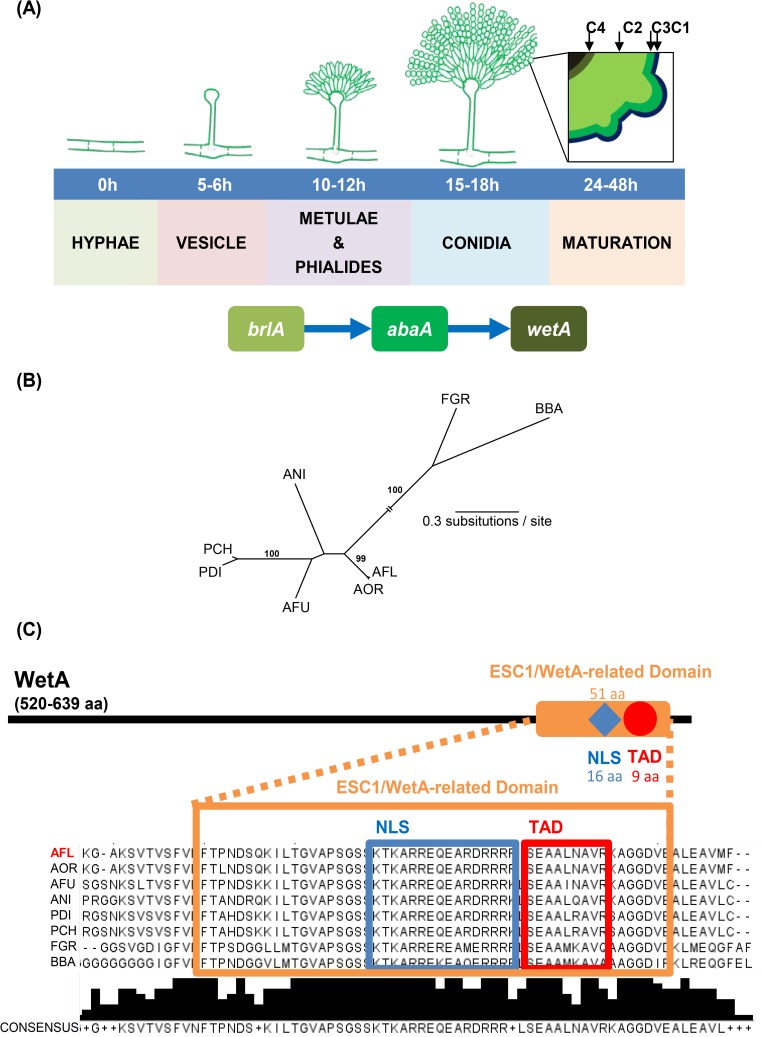
Fig 1. WetA is required for proper conidial maturation and contains both a transcription activation domain and a nuclear localization signal in a variety of fungi.
(A) A model for the roles of the central regulators in Aspergillus conidiogenesis. WetA is activated by AbaA and is responsible for conidia wall maturation. The black square illustrates the graphic view of the wall structure of the mature conidium, including the crenulated electron-dense outer layer C1, the carbohydrate-condensed layer C3, the electron-thin layer C2, and the innermost layer C4. Note: some Aspergillus species lack metulae (ex. A. parasiticus), and some species can have both metulae-phialides or phialides-only conidiophores (ex. some A. flavus variants) [11]. (B) Unrooted phylogeny of WetA amino acid sequences of A. flavus NRRL3357 XP_002383329.1 (AFL), A. fumigatus Af293 XP_751508.1 (AFU), A. nidulans FGSC4 XP_659541.1 (ANI), A. oryzae RIP40 XP_001816745.1 (AOR), Penicillium chrysogenum Wisconsin 54–1255 XP_002564365.1 (PCH), P. digitatum Pd1 XP_014534725.1 (PDI), Fusarium graminearum PH-1 I1S0E2.2 (FGR), and Beauveria bassiana ARSEF 2860 XP_008599445.1 (BBA) [12–18]. The sequences were aligned using MAFFT, version 7.1.5 [19]. The WetA protein phylogeny was calculated using the maximum likelihood optimality criterion, as implemented in PAUP [20], version 4.0a152; we used the WAG model of amino acid evolution [21], with empirical amino acid frequencies and allowing for rate heterogeneity among sites. Values near internal branches correspond to bootstrap support values (only values above 70% are shown). Branch lengths correspond to the estimated number of amino acid substitutions per site–the internal branch leading to the FGR and BBA sequences has been truncated for optimal visualization. (C) The predicted WetA protein architecture. The red circle and the red box represent the transcription activation domain (TAD) which was predicted by 9aaTAD using the “Less stringent Pattern” setting [22]. The blue diamond and the blue box represent the nuclear localization signal (NLS) predicted by NLStradamus using the 4 state HMM static model [23]. The orange rectangle and the orange box represent the ESC1/WetA-related domain (PTHR22934) predicted by the PANTHER classification system [24]. The consensus sequence and the consensus histogram are shown under the amino acid sequence multiple sequence alignment.
A key and essential step for conidiophore development in Aspergillus is activation of brlA, which encodes a C2H2 zinc finger transcription factor (TF) (Fig 1A) [9,25]. Further genetic and biochemical studies identified abaA and wetA as genes that are also important for conidiation. The abaA gene, activated by BrlA during the middle stages of conidiation, has been reported to function in the differentiation and functionality of the cells that produce conidia, which are known as phialides [26,27]. The wetA gene is activated by AbaA at the late phase of conidiation and functions in the synthesis of crucial conidial wall components, such as the inner C4 layer, which makes conidia impermeable and mature [28,29]. These three genes define a central regulatory pathway that acts in concert with other genes to control conidiation-specific gene expression and determine the order of gene activation during conidiophore development and spore maturation [30].
In this report, we have characterised the functions of WetA (wet-white A) in an aflatoxigenic A. flavus strain (NRRL3357) employing genetic, analytical, and genomic approaches as a way to better understand the developmental and chemical biology of this important plant pathogen that dramatically impacts human health. Similar to what has been described in A. nidulans [29], the deletion (Δ) of wetA in A. flavus resulted in various defects, including the formation of wet-white conidia that take up water and autolyze rather than undergoing the final stages of maturation. A. flavus ΔwetA conidia are defective in the formation of a complex cell wall and lack pigments. TEM analysis indicates that many of the ΔwetA mutant conidia are misshaped and lack cytoplasm. Moreover, the ΔwetA mutant conidia lack trehalose and are highly sensitive to heat and oxidative stress. Importantly, WetA is also necessary for proper vegetative growth and AFB production. RNA-seq analyses of conidia indicate over 5,700 genes are differentially expressed between wild-type (WT) and the mutant conidia including 160 genes predicted to encode (putative) TFs, indicating a global regulatory role of WetA in conidiogenesis. Collectively, we propose that the evolutionarily conserved WetA protein plays a global regulatory role in governing growth, development, and bridging spore differentiation and survival in A. flavus.
Materials and methods
Strains, media, and culture conditions
Aspergillus strains used in this study are listed in S1 Table. The fungal strains were grown on minimal medium (MM) with appropriate supplements as described previously [31,32] and incubated at 30°C. To determine the number of conidia, WT and mutant strains were point-inoculated and grown on solid MM at 30°C for 2 days. The conidia were collected in ddH2O from the entire colony and counted using a hemocytometer for further experiments. For liquid submerged cultures, conidia of WT and mutant strains were inoculated in liquid MM and incubated at 30°C, 220 rpm. When comparing the conidiation levels, the conidia were collected from mycelial mats of the same size and were counted 2 days after conidiation induction. Conidiation induction was performed as previously described [33]. Escherichia coli strains, DH5α and BL21 (DE3), were grown in Luria-Bertani medium with ampicillin (50 mg/ml) for plasmid amplification.
Generation of wetA deletion and complemented strains
The oligonucleotides used in this study are listed in S1 Table. Double-joint PCR was used to generate the deletion constructs of wetA [34]. Briefly, the deletion constructs containing A. fumigatus pyrG marker with 5’ and 3’ flanking regions of wetA were introduced into the recipient strain NRRL3357.5 [35]. To generate complemented strains, a WT wetA gene region including its upstream 2 kb region and downstream 1 kb region was amplified and introduced into the recipient strain. Multiple wetA deletion mutants (ΔwetA) in A. flavus were generated, which all behaved the same in every assay tested. We also generated three independent complemented strains (C’wetA), and they all behaved identically to one another as well. We chose TMY1 (ΔwetA) and TMY2 (C’wetA) as the testing strains for further experiments.
Nucleic acid manipulation
To isolate genomic DNA, about 106 conidia of relevant strains were inoculated in 2 ml liquid MM and stationary cultures at 30°C for 2 days. The mycelial mat was collected, squeeze-dried, and genomic DNA was isolated as described [34]. Total RNA isolation for Northern blot analyses was performed as described [33,34,36]. For RNA-seq, 2-day-old conidia of WT and ΔwetA strains were harvested from solid MM. Total RNA was extracted and submitted to ProteinCT Biotechnologies (Madison, WI) and the University of Wisconsin Biotechnology Center (Madison, WI) for library preparation and sequencing.
Conidia viability, autolysis, and stress response test
To check conidial viability, 2-day-old conidia of WT, ΔwetA, and C’wetA strains were collected and spread onto solid MM and cultured at 30°C. At 2-, 5-, 7-, 14-, and 20-days post-inoculation, conidia were collected from MM plates, and approximately 200 conidia were inoculated onto solid MM and cultured until colonies appeared. Survival rate was calculated as the ratio of the actual colony number to expected colony number. To test for conidial autolysis, approximately 100 conidia of WT, ΔwetA, and C’wetA strains were inoculated onto solid MM and incubated at 30°C for 4, 7, and 18 days. Conidia from the entire plate were collected and counted. Relative conidial number was compared to the number of conidia derived from a 4-day-old plate of each strain.
Two-day-old conidia of WT, ΔwetA, and C’wetA strains were collected to examine stress tolerance. For thermal stress tolerance tests, conidia were incubated at 50°C for 0, 10, and 60 minutes, and then spread onto solid MM. For UV stress tolerance tests, conidia were spread on solid MM and exposed to varying UV intensities (0, 100, and 200 J/cm2). For osmotic stress tolerance tests, conidia were spread onto solid MM with different concentrations (0.0, 0.6, and 2.4 M) of KCl. Finally, for oxidative stress tolerance tests, conidia were spread onto solid MM with different concentrations (0, 2, and 4 mM) of H2O2. All plates were incubated at 30°C until colonies appeared. Colony numbers were counted and calculated as a percentage of the untreated control.
Conidia content quantification
Two-day-old conidia of WT, ΔwetA, and C’wetA strains were collected. Trehalose and β-glucan quantification was performed as previously described [13,37].
Transmission electron microscopy (TEM)
Two-day-old conidia of WT and ΔwetA were collected from MM plates. Sample preparation was performed as previous described [31]. TEM analyses were done by the UW Electron Microscope Facility.
Vegetative growth rate and hyphal branching rate tests
Conidia of WT, ΔwetA, and C’wetA strains were point inoculated onto solid MM and cultured at 30°C. Colony diameter was measured daily until day 7 post inoculation. To measure the hyphal branching rate, conidia of WT and ΔwetA strains were inoculated and cultured for 18 hours at 30°C, 220 rpm in liquid MM (V18). The mycelium aggregates were transferred to solid MM and cultured for 8 hours (A8). The average peripheral growth unit (PGU) is defined as the distance between the first two branching points from the hyphal tips. At least 15 PGUs were measured for each strain.
AFB1 quantification
Two-day-old conidia of WT, ΔwetA, and C’wetA strains were inoculated into 100 ml MM (103/ml) and cultured for 5 days at 30°C, 220 rpm. Individual liquid cultures were filtered by a single layer of Miracloth. The mycelium aggregates were squeezed with a paper towel to remove as much of the medium as possible. The mycelium mat was placed in a 65°C oven for 2 hours and then its dry weight was quantified. Two milliliters of the culture (with or without filtration to remove the mycelium) were mixed with an equal volume of chloroform, vigorously vortexed, and centrifuged. The chloroform (bottom) layer (750 μl) of each sample was transferred and evaporated in a glass tube overnight. Then each dried sample was dissolved in 500 μl methanol anhydrous and filtrated by a 0.45 μm filter syringe into HPLC vials. Each sample was injected into the HPLC (Agilent 1200 series) at a flow rate of 0.8ml/min with water:acetonitrile anhydrous:methanol anhydrous (20:40:40, v/v), and AFB was detected by a diode array detector at a wavelength of 365 nm [38]. The injected volume was 10 μl and the separation was performed via Agilent HPLC column Zorbax Eclipse XDB-C18, 5um, 4.6 x 250mm cart.
RNA sequencing
A strand-specific library was prepared from total RNA using the Illumina TruSeq Strand-specific RNA sample preparation system. Briefly, mRNA was extracted from total RNA using poly-A selection, followed by RNA fragmentation. The strand-specific library was constructed by first-strand cDNA synthesis using random primers, sample cleanup, and second-strand synthesis using DNA Polymerase I and RNase H. A single 'A' base was added to the cDNA fragments followed by ligation of the adapters. Final cDNA library was achieved by further purification and enrichment with PCR, then quality checked using a Bioanalyzer 2100. The library was sequenced (SE100bp) using the Illumina HiSeq2500, and over 19 million high-quality reads per sample were achieved. All RNA-seq data files are available from the NCBI Gene Expression Omnibus database (Accession number: GSE95711).
Gene expression analysis
The quality of the raw sequence reads was verified using version 0.11.5 of FastQC [39]. The A. flavus genome and gene annotations were downloaded from NCBI (GCF_000006275.2_JCVI-afl1-v2.0_genomic.gff) and used for mapping. Mapping of the raw sequence reads to the genome was carried out with version 2.1.1 of Tophat2 [40], and the default options were used except for the maximum intron length was set to 4,000 bases (—max-intron-length 4000). Most (77–93%) of the reads from each of the samples mapped to the A. flavus genome. The alignment BAM files were compared against the gene annotation GFF file, and raw counts for the number of reads mapping to each gene were generated using version 0.6.1 of HTSeq-count [41]. Approximately 70–80% of mappable reads from each of the samples could be assigned to genes. Differential expression analysis of the raw counts was carried out using version 1.14.1 of DESeq2 [42]. Genes were considered differentially expressed between the WT and ΔwetA conidia if their adjusted p-value was less than 0.05 and their log2 fold change was less than -1 or greater than 1.
Functional enrichment analysis
GO annotations for A. flavus genes were downloaded from the AmiGO 2 website (version 2.4) on February 8, 2017 [43], and terms enriched in either the WetA-activated or -repressed gene lists were detected using version 3.0.3 of the BiNGO application [44] for Cytoscape (version 3.4.0) [45]. Version 1-26-17 of the Gene Ontology (go.obo) [46] was used to establish GO term relationships. GO terms were considered enriched if their p-value, following the Benjamini-Hochberg correction as implemented in BiNGO, was less than 0.05.
Results
Protein sequence features of WetA in A. flavus
The A. flavus wetA (XM_002383288.1) ORF comprises 1,692 bp with no introns and is predicted to encode a 563 amino-acid (aa) long protein. BlastP analysis against eight previously characterized WetA amino acid sequences reveals that A. flavus WetA has 99%, 61%, 57%, 53%, 53%, 68%, and 35% aa identity with WetA of A. oryzae, A. fumigatus, A. nidulans, Penicillium chrysogenum, P. digitatum, Beauveria bassiana, and Fusarium graminearum, respectively. Phylogenetic analysis revealed that A. flavus WetA is nested within a clade that also contains the A. oryzae, A. nidulans, P. digitatum, and P. chrysogenum WetA sequences, while B. bassiana WetA clusters with the F. graminearum sequence. Moreover, A. flavus WetA is almost identical to A. oryzae WetA and is relatively more similar to A. fumigatus WetA compared to A. nidulans WetA (Fig 1B). A. flavus WetA, along with all other WetA proteins included in our analyses, has a conserved 51-aa-length ESC1/WetA-related domain (PTHR22934: SF29) with the putative DNA-binding ability originating near the C terminus (Fig 1C) [14,47]. This highly conserved domain is further predicted by 9aaTAD and NLStradamus [22,23] to contain a 9-aa-length transcription activation domain (TAD) and a 16-aa-length nuclear localization signal (NLS), suggesting that WetA is a potential TF (Fig 1C).
The role of WetA in conidia
To understand the biological functions of WetA, we generated multiple wetA deletion mutants and complement strains in A. flavus. The wetA null mutant forms colourless (white) conidia which start to autolyze (wet) and collapse at 2 to 3 days after conidiation, forming an aggregated sphere structure (Fig 2A).
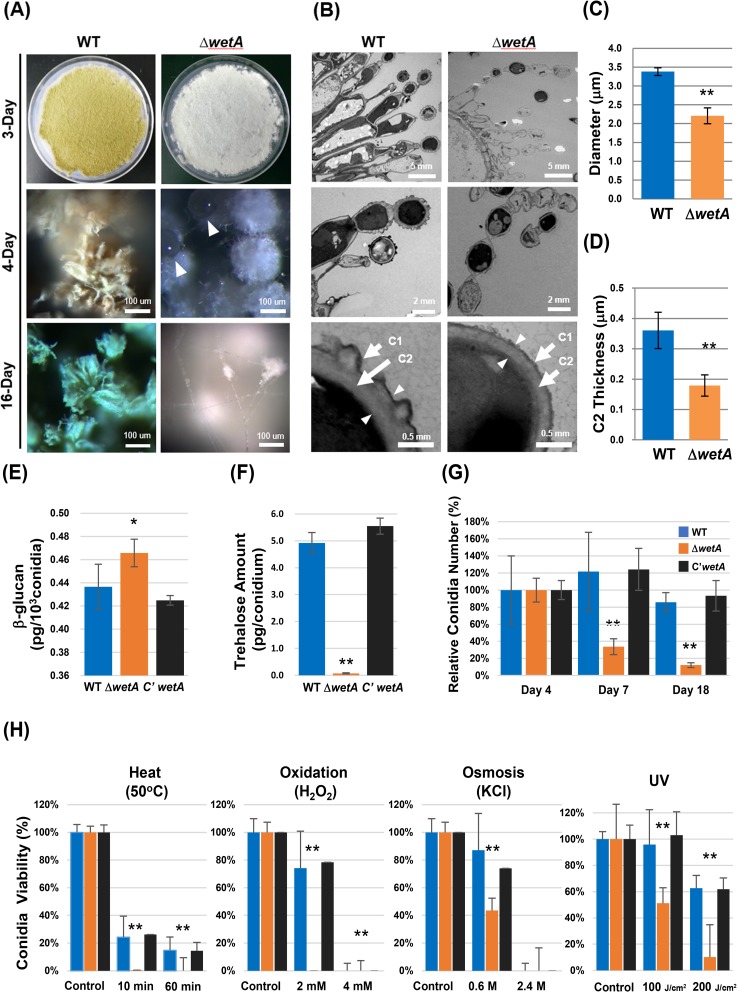
Fig 2. WetA is necessary for the proper formation of conidia in Aspergillus flavus.
(A) Phenotypes of WT (NRRL3357), ΔwetA, and C’wetA grown on solid MM at 30°C for 3, 4, 16 days after asexual induction. The white triangles indicate the liquid droplets formed on the autolyzing conidiophores of ΔwetA strain. (B) TEM images of 2-day-old conidiophores/conidia of WT and ΔwetA strains. Note: the remnant of lysed conidia formed a wet-vesicle-like structure on the top of the conidia chain, and most of the conidiophore/conidia contents were lost in the ΔwetA strain. The bottom panels show the conidia wall structures of WT and ΔwetA strains. Arrows indicate the locations of the C1 and C2 layers while the arrowheads indicate the C2 layer thickness. (C, D) The average diameter of conidia and thickness of the C2 layer of WT and ΔwetA conidia. At least seven WT and ΔwetA intact conidia from different sample slices were measured. (E, F) Quantification of conidia content (β-(1,3)-glucan (E) and trehalose (F)) of WT, ΔwetA, and C’wetA 2-day-old conidia The error bars indicate one standard deviation from the mean and the asterisks the level of significance (*, p < 0.05; **, p < 0.01). (G) The relative viability of WT, ΔwetA, and C’wetA conidia grown on solid MM at 30°C for 4, 7, 18 days after inoculation. The conidial viability at day 4 of each strain was set as 100%. ** (p < 0.01). The error bars indicate one standard deviation from the mean viability of triplicates. (H) Tolerance of WT, ΔwetA, and C’wetA 2-day-old conidia to heat (50°C), oxidative (H2O2), osmotic (KCl), and UV stresses. The control indicates untreated conidia. The viability of the untreated conidia of each strain was set as 100%. ** (p < 0.01). The error bars indicate one standard deviation from the mean viability of triplicates.
To check the detailed structural defects of the ΔwetA conidia, we carried out transmission electron microscopy (TEM) of conidiophores of WT and ΔwetA strains. As shown in Fig 2B, while WT formed intact conidial chains, the ΔwetA mutant showed fewer intact conidia and a high number of lysed conidial remnants. The WT conidial diameter is about 153% longer than that observed with ΔwetA (Fig 2C). Moreover, the WT conidium shows a crenulated electron-opaque outer layer (C1) and an electron-translucent inner layer (C2), as reported in A. nidulans and A. fumigatus [13,29]. Although ΔwetA conidium forms the C1 and C2 layers, the C1 outer layer is smooth and the C2 layer is more condensed and thinner than that of WT (Fig 2D).
Although the TEM results show that the intact ΔwetA conidium is as electron-opaque as the WT conidium, the intact ΔwetA conidium contains more β-(1,3)-glucan but less trehalose when compared to the WT conidium (Fig 2E and 2F).
Somewhat surprisingly, while the loss of wetA leads to systemic defects in conidia, about 10% of the total ΔwetA conidia appears to be intact even at day 16 (Fig 2A). Besides, the survivors exert consistent phenotypes with the original ΔwetA mutants. However, as the majority of the ΔwetA conidia autolyze and disintegrate, the total number of the ΔwetA conidia is dramatically decreased at day 7 and 18 post inoculation, whereas no significant changes in the viability of WT and C’wetA conidia were observable even at day 18 (Fig 2A and 2G). Finally, we examined whether the ΔwetA conidia show altered responses to various stresses. As shown in Fig 2H, the wetA null conidia are sensitive to osmotic (KCl) and oxidative (H2O2) stresses, and highly sensitive to heat (50°C) and UV stress (Fig 2H). Taken together, these results suggest that WetA plays an essential role in the proper maturation and stress tolerance of conidia in A. flavus.
The roles of WetA in growth, hyphal branching, AFB production, and developmental control
We further tested the roles of WetA in governing other biological processes. We found that, in addition to conidiation, WetA is associated with proper hyphal growth. The WT and C’wetA strains showed higher colony growth rate than the ΔwetA mutant on solid minimal medium (MM), regardless of the presence or absence of the light (Fig 3A and 3C). However, WetA appears to affect conidiation and hyphal development in response to light. Under dark condition, the ΔwetA colony exhibits highly reduced conidiation levels (Fig 3A). The WT colony edge can be divided into three regions, the single layer vegetative hyphae region (Vs), the multi-layer vegetative hyphae region (Vm), and the dense aerial hyphae region (Ad). In comparison to WT, the ΔwetA colony’s edge does not contain the Vs region and instead has a sparse aerial hyphae region (As) between the Vm and Ad regions (Fig 3B). In addition, the As region of the ΔwetA colony is expanded when grown in the dark environment (Fig 3A and 3B and S1 Fig). Furthermore, the absence of wetA results in about 2.5-fold lower hyphal branching rate in both the submerged culture and the solid culture (Fig 3D and 3E), which is consistent with our previous observation of the same phenotype in ΔwetA in A. fumigatus [13]. We noticed that both the apical branching and lateral branching rates were reduced in ΔwetA mutants, suggesting that WetA is involved in both branching systems. To further elucidate the role of WetA, we examined AFB production in WT, ΔwetA, and C’wetA strains shake-cultured for 5 days by HPLC. The results show that the ΔwetA strain was unable to produce AFB1 and AFB2 in the submerged culture (Fig 3F and 3G).
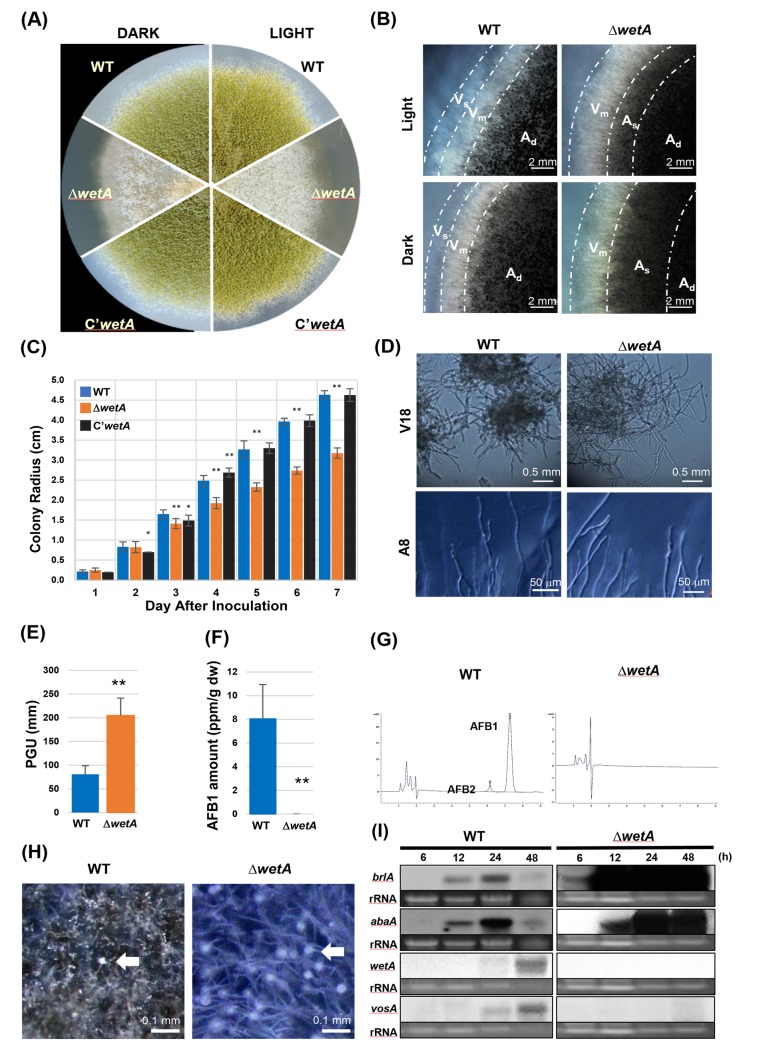
Fig 3. Multiple roles of WetA.
(A-C) WetA affects vegetative growth. (A) The colony image of WT, ΔwetA, and C’wetA strains on solid MM at 5 days after point inoculation under light and dark conditions. (B) Colony edge image of WT and ΔwetA strains under light and dark conditions. Vs: single-layer vegetative hyphae region. Vm: multi-layer vegetative region. As: sparse aerial hyphae region. Ad: dense aerial hyphae region. (C) Colony growth rates of WT, ΔwetA, and C’wetA strains after point inoculation on solid MM. The error bars indicate one standard deviation. * (p < 0.05) and ** (p < 0.01). (D, E) Hyphal branching rates of WT and ΔwetA strains. (D) Microscopy images show WetA regulates hyphal branching. Loss of wetA leads to reduced hyphal branching rate in both solid and submerged cultures. (E) Average PGU values of A8. ** (p < 0.01). The error bars indicate one standard deviation. (F, G) Aflatoxin quantification by HPLC of WT and ΔwetA submerged culture after 5-days cultivation. (F) AFB1 amount (per g dry weight) in WT and ΔwetA vegetative cells. ** (p < 0.01). The error bars indicate one standard deviation. (G) The HPLC chromatograms of AFB1 and AFB2 in the culture medium of WT and ΔwetA strains. (H) WT and ΔwetA strains were induced for asexual development and observed after 8 h incubation at 30°C on solid MM plate. The white arrows indicate conidiophores. Note: the abundant conidiophore formation in ΔwetA culture. (I) Northern blot analysis of brlA, abaA, wetA, and vosA mRNA levels in WT and ΔwetA strains at 6, 12, 24, 48 h after conidiation induction.
We found that the absence of wetA resulted in precocious conidiophore development. The ΔwetA mutant started to generate abundant conidiophores at 6 h post asexual developmental induction, an hour earlier than WT and complement strains, and by 8 hours the difference in conidiophore production was striking (Fig 3H). This observation was corroborated by Northern blot analysis. As shown in Fig 3I, loss of wetA leads to early accumulation of mRNA from the asexual reproduction-inducing gene brlA at 6 h after asexual developmental induction, whereas brlA transcript only started to accumulate at 12 h after induction in WT. In WT, mRNA levels of brlA and abaA, another conidia-inducing regulator, reach their highest at 24 h and drop at 48 h after induction. In contrast, transcript levels of brlA and abaA dramatically increase at 12 h and stay at high levels at 24 and 48 h after asexual induction in the ΔwetA mutant. In WT, the mRNA for the regulator vosA started to accumulate at 24 h post-induction, while vosA mRNA accumulation was hardly detected in ΔwetA strain (Fig 3I). These results indicate that in A. flavus, WetA is a key feedback negative controller of brlA expression and conidiation. Overall, our data suggest that WetA plays multiple roles in cellular and chemical development in A. flavus.
Genome-wide expression analyses in conidia
To shed more light on the multiple regulatory roles WetA appeared to play in A. flavus biology, we carried out genome-wide expression analyses in WT and mutant conidia using RNA-seq. Poly-A mRNA from three technical replicates of 2-day-old conidia of WT and ΔwetA strains were purified and sequenced as described in the methods section; one technical replicate of the ΔwetA was discarded following failure in multiple quality control analyses. Examination of global gene expression differences between the WT and mutant wetA indicate that WetA plays a broad regulatory role in conidia. Out of the 13,485 mapped A. flavus genes, 5,755 (42.68% of the total) showed differential accumulation of mRNAs in the ΔwetA conidia in comparison to WT conidia. Among 5,755 differentially expressed genes (DEGs), mRNA levels of 2,856 (21.18%) genes were lower (Down) in the ΔwetA conidia compared to WT conidia, and those of 2,899 (21.50%) genes were higher (Up) in the ΔwetA conidia compared to WT conidia (Fig 4A).
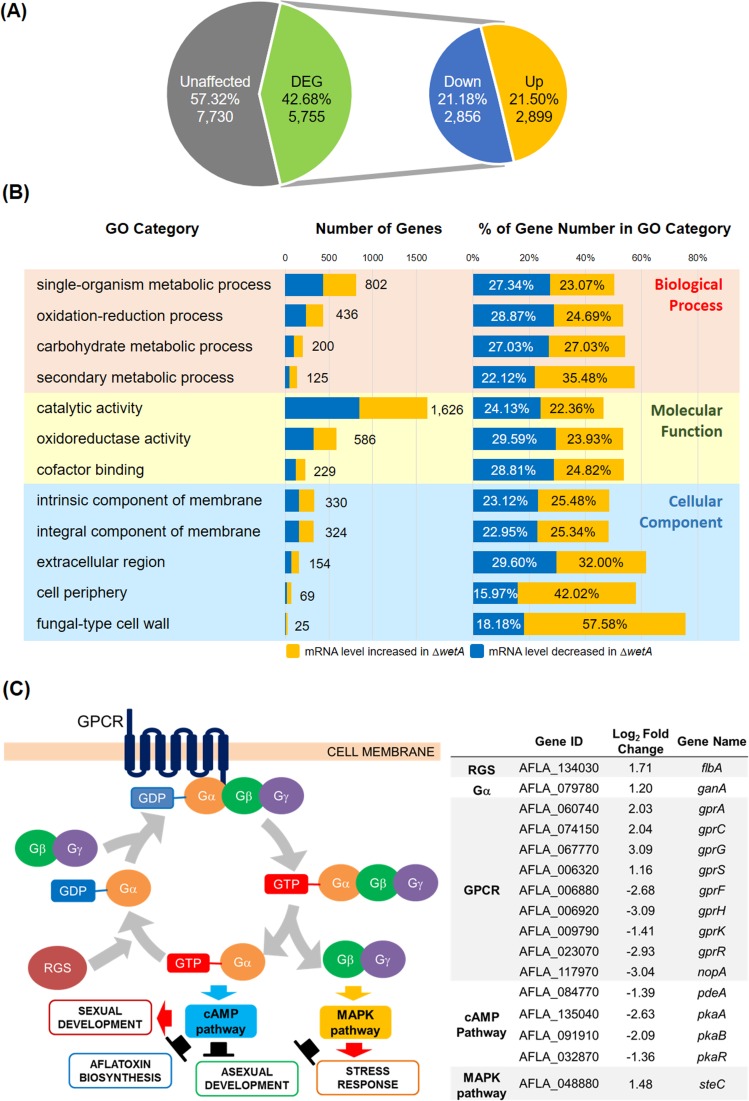
Fig 4. RNA-seq analyses of conidia.
(A) The numbers of genes whose mRNA levels were similar (Unaffected, grey), or different between WT and ΔwetA conidia (DEG, green), with down (blue) and up (yellow) in the ΔwetA conidia compared to WT. A DEG is defined by having a > 2-fold change of mRNA levels between WT and ΔwetA conidia and an adjusted p-value of less than 0.05. (B) Functional categories of DEGs in conidia. The yellow bars represent genes whose mRNA levels increased in the ΔwetA conidia, whereas the blue bars represent those genes whose mRNA levels decreased in the ΔwetA conidia. The pink shaded box represents the biological process GO categories; the yellow shaded box represents the molecular function GO categories; the blue shaded box represents the cellular component GO categories. “Number of Genes”: the total number of DEGs assigned to the specific GO category. “% of Gene Number in GO”: the number of DEGs divided by the total number of genes assigned to the specific GO category in the genome as a whole. (C) The schematic diagram and mRNA expression profile of the G-protein regulatory pathways controlling development, stress response, and aflatoxin biosynthesis.
Functional category analysis was carried out by determining Gene Ontology (GO) terms that were enriched in DEGs. The top enriched biological process GO categories are “single-organism metabolic process”, “oxidation-reduction process”, “carbohydrate metabolic process”, and “secondary metabolic process”. The top enriched molecular function GO categories are “catalytic activity”, “oxidoreductase activity”, and “cofactor binding”. The top enriched cellular component GO categories are “intrinsic component of membrane”, “integral component of membrane”, “extracellular region”, “cell periphery”, and “fungal-type cell wall” (Fig 4B). Of note, over 50% of all genes in the A. flavus genome annotated with the GO terms “carbohydrate metabolic process” (54.05%), “secondary metabolic process” (57.60%), and “fungal-type cell wall” (75.76%), were regulated by WetA in our RNA-seq data, consistent with our phenotypic data (Fig 4B). The top enriched GO categories for genes whose mRNA levels decreased or increased in the ΔwetA conidia are listed in S2 and S3 Tables, and the top 100 DEGs with decreased/increased mRNA accumulation levels in the ΔwetA conidia are listed in S4 and S5 Tables, respectively.
To explore the molecular roles of WetA in conidiation, we checked mRNA levels of those genes assigned to the GO term “Asexual Development” (GO:0019954) and other known genes related to asexual development [16]. In total, 77 genes related to asexual development are differentially expressed by the absence of WetA: 29 genes (37.66%) and 48 genes (62.34%) showed decreased and increased mRNA levels in the ΔwetA conidia, respectively (see Table 1 and S6 Table). These data corroborate our working hypothesis that WetA is an important feedback regulator of conidiation, likely by activating several conidiation repressors and repressing key conidiation activators.
Table 1. DEGs of interest.
| mRNA level decreased in ΔwetA | mRNA level increased in ΔwetA | |
|---|---|---|
| Asexual Development | argB, bem1, dewA, fluG, fphA, kex1, nce102, nudG, osaA, pbcR, pkaA, pkaB, pkaR, ppoA, ppoB, rft1, rhbA, ricA, rodB, sfgA, swoM, tcpA, tmpA, tpsA ortholog, tpsC, veA, vosA, wA/pksP, wetA | abaA, ams1, atg1, atgH, brlA, cch1, chsA, chsB, chsE, chsF, chsG, crzA, esdC, fbx15, figA, flbA, flbC, ime2, llmB, llmF, medA, midA, mob1, msdS, mtfA, nsdC, nsdD, nudA, odeA, pac2/osaB, pcl1, phnA, ppoC, ppoD, prpA, rgdA, rho1, rodA, sidB, sltA, ssc1, steC, stuA, ugtA, vapA, wsc1, wsc3, zipA |
| Transcription Factor* | aflR, aflYd, amdA, amdR, aro80, atf21, clrA, ctf1B, fcr1, fkh1, galX, metR, nosA, nscR, pbcR, pcaG, prnA, rdr1, regA, scfA, sdrA, sfgA, sfp1, silA | abaA, amdX, aoiH, brlA, cnjB, cpcA, crzA, devR/hpa3, egd1, egr2, flbC, glcD, hacA, mtfA, ndtA, nsdC, nsdD, pacC, rap1, rfeB, rfeG, rgdA, rpn4, seb1, sltA, srbA, steA, stuA, zipA |
| Aflatoxin Cluster | aflA, aflR, aflS, aflYd | aflB, aflC, aflT, aflU, aflW, alfYa |
*only those genes with annotation are listed
As the GO terms “intrinsic component of membrane” and “integral component of membrane” were enriched in functional analysis, we further examined mRNA levels of the membrane-receptor-encoded-genes like G-protein coupled receptors (GPCRs). A heterotrimeric G-protein pathway can be involved in the activation of cAMP pathways and MAPK pathways, which then lead to the repression of asexual development [48]. The A. flavus genome contains 15 GPCRs [49], of which 9 showed altered mRNA levels (4 up, 5 down) in the ΔwetA conidia compared to WT (Fig 4C). The ganA gene predicted to encode a G protein alpha subunit (Gα), and the flbA gene predicted to encode a regulator of G protein signaling (RGS) protein showed increased mRNA levels in the ΔwetA conidia (Fig 4C). Genes involved in the cAMP pathway like pdeA, pkaA, pkaB, and pkaR, showed decreased mRNA levels in the ΔwetA conidia (Fig 4C). Gene involved in the MAPK pathway, steC, show increased mRNA levels in the ΔwetA conidia (Fig 4C). Our results suggest that the WetA-mediated regulation may be associated with signal transduction pathways.
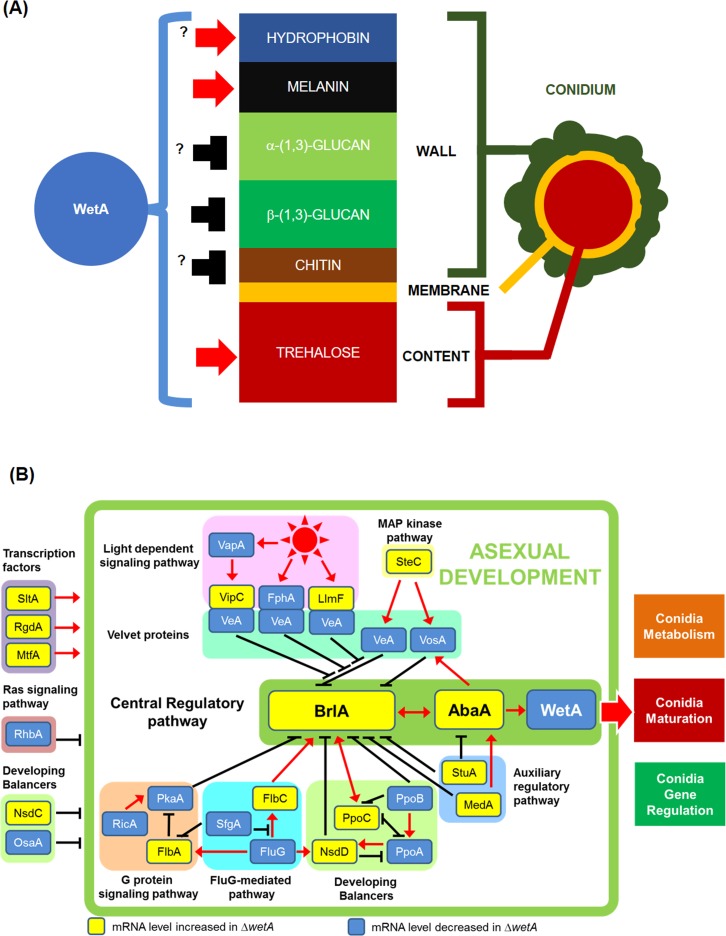
Next, we checked the expression levels of genes involved in conidia content and conidial wall integrity. As shown in Table 2, genes associated with the biosynthesis of trehalose, melanin, and hydrophobins, as well as the degradation of β-glucan, showed decreased mRNA levels in the ΔwetA conidia. Conversely, mRNA levels of genes associated with biosynthesis of chitin and β-(1,3)-glucan were increased in the ΔwetA conidia. These transcriptomic data and our direct measurement of β-glucan and trehalose indicate that WetA governs the integrity of conidia by coordinating intracellular contents and conidial wall biogenesis (Fig 5A, Table 2 and S7 Table).
Table 2. DEGs involved in spore maturation.
| mRNA level decreased in ΔwetA | mRNA level increased in ΔwetA | |
|---|---|---|
| Trehalose Biosynthesis | tppB, tppC, rfaB, ccg-9, tpsC, AFLA_087630 | |
| Trehalose Degradation | treA | |
| Chitin Biosynthesis | chsA, chsC, chsE, chsF, chsG, chsZ | |
| Chitin Degradation | chiA, chiB, cts2, ctcB, nagA, AFLA_031380, AFLA_107830, AFLA_057680 | |
| α-(1,3)-glucan Biosynthesis | ags1 | ags2 |
| α-(1,3)-glucan Degradation | agnD, agnE | AFLA_091790 |
| β-(1,3)-glucan Biosynthesis | fksP, gel1, gel2, gel4, gel5, gel6, gel7, AFLA_107790, AFLA_064920 | |
| β-(1,3)-glucan Degradation | bgt1, exg1, AFLA_023650 | engl1, eng3, eng4, eng8, exg0, exg2 |
| Melanin Biosynthesis | wA (pksP), ayg1 | |
| Hydrophobin | dewA, rodB, AFLA_063080, AFLA_098980 | rodA, AFLA_094600 |
Fig 5. Summary of WetA functions and a model for WetA-mediated developmental regulation in A. flavus.
(A) Schematic diagram of the WetA-mediated regulatory model of conidia architecture. The question mark indicates that the WetA-mediated activation/repression function needs to be verified by further experiments. (B) A comprehensive model for WetA-mediated regulation of asexual development based on transcriptomic, genetic, and biochemical data. In this model, those genes with increased and decreased mRNA levels in the ΔwetA conidia are labeled in yellow (WetA-inhibited) and blue (WetA-activated), respectively. The functions and references of each gene in this model are listed in S9 Table.
The GO term “secondary metabolic process” is enriched in the WetA-influenced transcriptome. We examined the differentially expressed genes belonging to secondary metabolite gene clusters (SMG clusters). We predicted the A. flavus SMG clusters with antiSMASH and used the cluster boundaries identified by Inglis et al. for A. oryzae clusters if they were conserved in A. flavus [50,51]. If the clusters were not identified by Inglis et al., we used the default boundaries provided by antiSMASH. There are 660 SMGs distributed in the 74 SMG clusters in A. flavus (S8 Table). We found that 306 genes (46.37%) distributed in 68 SMG clusters (92%) showed altered mRNA levels in the ΔwetA conidia (136 down and 170 up, see S8 Table). All of the genes located in Clusters 23, 35, 41, 46, 48, 52, 54, and 71 showed altered mRNA levels in the ΔwetA conidia. Interestingly, all genes in Clusters 23 and 52 showed decreased mRNA levels in the ΔwetA conidia, whereas all genes in Cluster 71 showed increased mRNA levels the ΔwetA conidia. These data indicate an important role of WetA governing secondary metabolic chemical development in conidia.
Finally, we focused on putative TFs showing altered mRNA levels in the ΔwetA conidia as this functional category was enriched among WetA-induced DEGs (S2 Table. We found that 160 genes predicted to encode TFs exhibited altered mRNA accumulation in the presence and absence of WetA in conidia: 100 (62.50%) showed decreased mRNA levels and 60 (37.50%) showed increased mRNA levels in the ΔwetA conidia. Approximately 80% of these putative TFs have a zinc binding domain, including 18 TFs with a C2H2 domain and 64 TFs with a Zn(II)2Cys6 (or C6) domain (Table 1 and S10 Table); important classes for the regulation of fungal development and metabolism. Taken together, WetA governs proper expression of various signaling, regulatory, structural, and metabolic elements that coordinate cellular and chemical development of conidia.
Discussion
Asexual development has been studied in Aspergilli and other fungi for many years [13–18,28–30,52–59]. In addition to Aspergillus fungi, the function of WetA is highly conserved in other Ascomycetes. In P. digitatum, the lack of wetA also results in abnormal conidia, delayed germination, and reduced stress tolerance [15]. Moreover, P. chrysogenum wetA can fully complement the A. nidulans wetA deletion mutation, suggesting that the WetA-mediated sporulation regulatory mechanisms are conserved in A. nidulans and P. chrysogenum [18]. In F. graminearum, loss of wetA causes deficient conidia, reduced oxidative and heat stress tolerance, and reduced chronological spore viability. F. graminearum WetA suppresses microcycle conidiation and then further maintains conidial dormancy [14]. In B. bassiana, wetA null mutants produce deficient conidia which are sensitive to environment stresses [17].
Previous studies suggest that WetA is responsible for activating a set of genes whose products comprise or direct the assembly of the conidial wall layers and ensure proper cytoplasmic status [28]. In A. nidulans, WetA together with AbaA activate genes which are expected to encode spore-specific functions (Class B Genes). WetA together with both BrlA and AbaA activate Class C and Class D genes, which are expected to encode phialide-specific functions [28,30,60]. However, WetA alone is sufficient to activate Class B and some Class D genes [28]. At least one gene (wA), whose mRNA accumulates in phialide cells instead of in conidia, is activated by WetA, indicating that WetA may regulate genes in these cells as well as in conidia [28]. Additionally, accumulation of wetA mRNA requires wetA+ activity during conidiation, suggesting that wetA is autogenously regulated [12,28].
WetA is functionally conserved and required in A. flavus for many aspects of its biology, including spore viability, wall integrity, and stress tolerance. Although the wetA null mutant forms the C2 spore wall layer as found in WT, the ΔwetA C2 layer is hyper-condensed in A. flavus while it fails to condense in A. nidulans, A. fumigatus, and P. digitatum [13,15] (Fig 2B and 2D). The condensation of the C2 layer along with the formation of the C3 and C4 layers are the final stage of conidial maturation, which contributes to the impermeability of the conidial wall [29].
WetA is essential for establishing the heat stress tolerance in Ascomycetes [13,15,17]. However, loss of wetA in different species results in variable degrees of tolerance to oxidative stress and osmotic stress. The A. fumigatus [13], A. flavus (Fig 2H), and F. graminearum [14] wetA deletion mutants are highly sensitive to H2O2. However, the B. bassiana [17] wetA deletion mutants showed WT level tolerance to H2O2 and the P. digitatum [15] wetA deletion mutants even showed enhanced tolerance to H2O2. Loss of wetA causes reduced osmotic stress tolerance in A. fumigatus [13], P. digitatum [15], and B. bassiana [17], but has not in F. graminearum [14]. Somewhat surprisingly, even though the ΔwetA conidial structure and stress tolerance were impaired, the small numbers of intact ΔwetA conidia that could be isolated showed consistant phenotypes as the WT conidia. However, only about 30% of the total ΔwetA conidia remain intact on day 7 after inoculation, while almost 100% of WT conidia remain intact (Fig 2G).
WetA appears to be involved in both the trehalose biosynthetic and degradation pathways in A. flavus (Fig 2F, Table 2 and S7 Table), both of which are required for conidial stress tolerance and viability. The velvet regulator VosA is known as the regulator which couples sporogenesis and trehalose biogenesis, and it activates wetA in A. nidulans [31]. Our data show that WetA also activates vosA in A. flavus (Table 1 and S6 Table), suggesting an inter-dependent activation between the two regulators in conidia. While the loss of wetA does not completely block vosA expression in conidia (S6 Table) and vice versa [31], almost no trehalose can be detected in the ΔwetA (Fig 2F) and ΔvosA [31] conidia, suggesting that both WetA and VosA are required for proper trehalose biosynthesis in conidia. Although WetA is required for trehalose biosynthesis in A. flavus (Fig 2F), A. fumigatus [13], and B. bassiana [17], loss of wetA did not alter the trehalose amount in F. graminearum [14] and P. digitatum [15].
Based on microscopy images, trehalose quantifications, and β-(1,3)-glucan quantifications from our group as well as others, studies proposed that WetA regulates conidial wall integrity and trehalose content [13–15,17,29,58]. Our RNA-seq analyses further expands our understanding of how WetA affects overall conidial wall integrity. More than 75% of the genes assigned to the Cellular Component GO category “fungal-type cell wall” in the A. flavus genome were differentially expressed in the ΔwetA conidia (Fig 4B), resulting in this category being statistically enriched amongst WetA-regulated genes. This suggests that WetA plays a global regulatory role in spore wall integrity. The Aspergillus conidial wall is composed of chitin, β-(1,3)-glucan, α-(1,3)-glucan, melanin, and hydrophobin [61] (Fig 5A). Our transcriptome analyses support the hypothesis that genes involved in the metabolic pathways of the conidial wall components are differentially expressed in the ΔwetA conidia (Table 2 and S7 Table). Taken together, we present a summary of the role of WetA in governing conidial maturation by regulating the metabolic pathways of trehalose and conidial wall components in A. flavus (Fig 5A). However, the WetA-mediated regulatory circuits governing maturation and stress responses of conidia among various fungal species may be genetically re-wired in each fungal species, as is the case for other proteins involved in the regulation of asexual development and secondary metabolism [62].
Our data suggest that WetA is necessary to turn off the conidiation initiation process after the formation of conidiophores. Loss of wetA resulted in greatly enhanced levels of brlA and abaA in A. flavus (Fig 1D, Table 1 and S6 Table) A. fumigatus [13], P. digitatum [15], and B. bassiana [17]. Moreover, our transcriptome analyses indicate that loss of wetA resulted in altered mRNA levels of various regulators of conidiation (Table 1 and S6 Table), implying an upstream regulatory role of WetA in conidia (Fig 5B). Consistent with this, we observed earlier conidiation in the ΔwetA mutant compared to WT in A. flavus (Fig 3H). However, the ΔwetA mutant showed delayed conidiation in A. fumigatus [13], suggesting that WetA-mediated feedback regulation of conidiation has undergone genetic rewiring in Aspergilli.
About 46% of genes positioned in the predicted SMG clusters showed altered mRNA levels in the ΔwetA conidia, including 15 genes predicted to encode polyketide synthases (PKSs) and PKS-like proteins, and 19 genes predicted to encode non-ribosomal polyketide synthases (NRPSs) and NRPS-like proteins (Table 3 and S8 Table), suggesting that WetA affects biosynthesis of several secondary metabolites in conidia. The backbone gene, aflC, and the transcription factor, aflR, of the AF cluster were differentially expressed in the ΔwetA conidia (Table 1 and S8 Table). Although there is no significant difference in AFB amount between WT and ΔwetA conidia (S2 Fig), the loss of wetA reduces the amount of AFB1 and AFB2 in submerged culture (Fig 3F and 3G), suggesting that WetA exerts temporal and spatial regulation of aflatoxin metabolism.
Table 3. DEGs within the secondary metabolite biosynthesis clusters.
| BACKBONE GENES | ||||
| mRNA level decreased in ΔwetA | mRNA level increased in ΔwetA | |||
| CLUSTER | GENE ID | CLUSTER | GENE ID | |
| PKS/PKS-LIKE | 3 | AFLA_127090 | 6 | AFLA_053870 |
| 12 | AFLA_079360 | 10 | AFLA_010000 | |
| 20 | AFLA_116220 | 24 | AFLA_118940 | |
| 21 | AFLA_116890 | 24 | AFLA_118960 | |
| 55 | AFLA_006170 | 50 | AFLA_002900 | |
| 58 | AFLA_137870 | |||
| 59 | AFLA_139410 | |||
| 71 | AFLA_060020 | |||
| 71 | AFLA_060010 | |||
| 74 | AFLA_062820 | |||
| NRPS/NRPS-LIKE | 10 | AFLA_010020 | 11 | AFLA_010620 |
| 10 | AFLA_010010 | 26 | AFLA_119820 | |
| 23 | AFLA_118440 | 27 | AFLA_121520 | |
| 25 | AFLA_119110 | 41 | AFLA_101700 | |
| 35 | AFLA_038600 | 44 | AFLA_064240 | |
| 45 | AFLA_064560 | 46 | AFLA_066720 | |
| 52 | AFLA_004450 | 48 | AFLA_069330 | |
| 54 | AFLA_005440 | 61 | AFLA_023020 | |
| 63 | AFLA_028720 | 69 | AFLA_109430 | |
| 65 | AFLA_105190 | |||
| TRANSCRIPTION FACTORS | ||||
| mRNA level decreased in ΔwetA | mRNA level increased in ΔwetA | |||
| CLUSTER | GENE ID | CLUSTER | GENE ID | |
| 18 | AFLA_087810 | 31 | AFLA_096370 | |
| 31 | AFLA_096330 | 71 | AFLA_059960 | |
| 40 | AFLA_100300 | |||
| 59 | AFLA_139360 | |||
| 63 | AFLA_028760 | |||
| 66 | AFLA_105530 | |||
Loss of wetA results in reduced radial growth and lowered hyphal branching rates (Fig 3A, 3C, 3D and 3E). RNA-seq results showed about 50% of the genes in the GO categories, “hyphal tip” and “site of polarised growth”, exhibited increased mRNA levels in ΔwetA conidia (S3 Table). Put together, WetA may play a regulatory role in hyphal development. It is possible that WetA regulates both hyphal and conidial cell wall assembly and therefore affects both the radial growth rate and branching rate of A. flavus.
We observed differential conidial and hyphal development between light and dark conditions in the ΔwetA mutant (Fig 3A and 3B). Transcriptome analyses revealed that several light-sensor-encoding genes were differentially expressed in the ΔwetA conidia, including fphA, nopA, gprF, and gprR. FphA is a phytochrome that represses sexual development in A. nidulans under red-light induction [63,64]. NopA is a fungal opsin type GPCR that represses conidiation in Neurospora crassa [65,66], but its function in Aspergilli is still vague [49,67]. GprF and GprR repress conidiation under the dark condition [49]. Taken together, WetA may be involved in light-dependent regulatory pathways that affect conidiation and hyphal development.
Our data show that WetA plays multiple roles in governing development (Figs 2 and 3). Transcriptome analyses showed that WetA affects mRNA levels of 160 genes predicted to encode TFs and 9 genes predicted to encode GPCRs (Fig 4C, Table 1 and S10 Table). G-protein signaling governs normal growth, development, and mycotoxin production in filamentous fungi [68]. GPCRs are known to be involved in multiple cell processes, including carbon and nitrogen sensing, aflatoxin repression, germination, quorum sensing, oxylipin sensing, light sensing, and osmotic, acidic pH, ROS, and cell wall stress responses [49]. Taken together, our data suggest that the absence of WetA function results in disturbed expression of TFs and GPCRs leading to downstream pleiotropic effects. As annotation of the A. flavus genome improves to the levels of that of A. nidulans, A. oryzae, A. fumigatus, and A. niger genomes, we may identify additional putative regulators influenced by WetA in conidia.
Transcripts of wetA were not detectable during vegetative growth in A. flavus, as observed in A. nidulans and A. fumigatus. However, loss of wetA caused reduced growth rate, reduced hyphal branching rate, and reduced AFB1 production during vegetative growth (Fig 3). One possible explanation is that the WetA protein is stable and present throughout the vegetative growth phase. This was the case of vosA, where mRNA of vosA is not detectable in vegetative cells, but the VosA protein is present at a high level until conidiation occurs and is still able to repress brlA expression in vegetative cells [31,69]. Another explanation is that WetA regulates several signaling regulatory pathways in conidia, which keep functioning in the regulation of other biological processes during development.
In conclusion, we present a genetic model depicting the molecular mechanisms of WetA-mediated regulation in cellular and chemical development in A. flavus (Fig 5B). WetA affects the pathways of conidial content and conidial wall component metabolism, and further affects conidia viability and stress tolerance. Furthermore, WetA exerts feedback control of conidiation initiation by regulating upstream regulators of asexual development.
Supporting information
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
The RNA-seq dataset has been submitted to the GEO database (Accession number: GSE95711).
Funding Statement
This work was supported by Hatch (WIS01665 and WIS01889) to JHY and the Intelligent Synthetic Biology Center of Global Frontier Projects (2015M3A6A8065838) to SCK and JHY. The sponsors or funders did not play any role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, and in the preparation, review, or approval of the manuscript.
References
- 1.Ebbole DJ. The Conidium. Cellular and Molecular Biology of Filamentous Fungi. American Society of Microbiology; 2010. pp. 577–590. doi: 10.1128/9781555816636.ch36 [Google Scholar]
- 2.Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW. Aspergillus flavus: human pathogen, allergen and mycotoxin producer. Microbiology. 2007;153: 1677–1692. doi: 10.1099/mic.0.2007/007641-0 [DOI] [PubMed] [Google Scholar]
- 3.Payne GA. Process of contamination by aflatoxin-producing fungi and their impact on crops. Mycotoxins Agric food Saf. Marcel Dekker: New York, NY, USA; 1998;9: 279–306. [Google Scholar]
- 4.Kensler TW, Roebuck BD, Wogan GN, Groopman JD. Aflatoxin: A 50-year odyssey of mechanistic and translational toxicology. Toxicol Sci. Oxford University Press; 2011;120: S28–S48. doi: 10.1093/toxsci/kfq283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Udomkun P, Wiredu AN, Nagle M, Müller J, Vanlauwe B, Bandyopadhyay R. Innovative technologies to manage aflatoxins in foods and feeds and the profitability of application–A review. Food Control. 2017;76: 127–138. doi: 10.1016/j.foodcont.2017.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calvo AM, Wilson RA, Bok JW, Keller NP. Relationship between secondary metabolism and fungal development. Microbiol Mol Biol Rev. 2002;66: 447–459. doi: 10.1128/MMBR.66.3.447-459.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hicks JK, Yu J-H, Keller NP, Adams TH. Aspergillus sporulation and mycotoxin production both require inactivation of the FadA Gα protein-dependent signaling pathway. EMBO J. 1997;16: 4916–4923. doi: 10.1093/emboj/16.16.4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J- H, Keller NP. Regulation of secondary metabolism in filamentous fungi. Annu Rev Phytopathol. 2005;43: 437–458. doi: 10.1146/annurev.phyto.43.040204.140214 [DOI] [PubMed] [Google Scholar]
- 9.Adams TH, Wieser JK, Yu J-H. Asexual sporulation in Aspergillus nidulans. Microbiol Mol Biol Rev. 1998;62: 35–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbein AD, Pan YT, Pastuszak I, Carroll D. New insights on trehalose: a multifunctional molecule. Glycobiology. Oxford University Press; 2003;13: 17R–27. doi: 10.1093/glycob/cwg047 [DOI] [PubMed] [Google Scholar]
- 11.Klich M, Pitt J. A laboratory guide to the common Aspergillus species and their teleomorphs. Commonwealth Scientific and Industrial Research Organization, Division of Food Processing; 1988.
- 12.Boylan MT, Mirabito PM, Willett CE, Zimmerman CR, Timberlake WE. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans. Mol Cell Biol. 1987;7: 3113–3118. doi: 10.1128/mcb.7.9.3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tao L, Yu J-H. AbaA and WetA govern distinct stages of Aspergillus fumigatus development. Microbiology. 2011;157: 313–326. doi: 10.1099/mic.0.044271-0 [DOI] [PubMed] [Google Scholar]
- 14.Son H, Kim M-G, Min K, Lim JY, Choi GJ, Kim J-C, et al. WetA is required for conidiogenesis and conidium maturation in the ascomycete fungus Fusarium graminearum. Eukaryot Cell. 2014;13: 87–98. doi: 10.1128/EC.00220-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang M, Sun X, Zhu C, Xu Q, Ruan R, Yu D, et al. PdbrlA, PdabaA and PdwetA control distinct stages of conidiogenesis in Penicillium digitatum. Res Microbiol. 2015;166: 56–65. doi: 10.1016/j.resmic.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 16.Ogawa M, Tokuoka M, Jin FJ, Takahashi T, Koyama Y. Genetic analysis of conidiation regulatory pathways in koji-mold Aspergillus oryzae. Fungal Genet Biol. Elsevier Inc.; 2010;47: 10–18. doi: 10.1016/j.fgb.2009.10.004 [DOI] [PubMed] [Google Scholar]
- 17.Li F, Shi H-Q, Ying S-H, Feng M-G. WetA and VosA are distinct regulators of conidiation capacity, conidial quality, and biological control potential of a fungal insect pathogen. Appl Microbiol Biotechnol. Springer Berlin Heidelberg; 2015;99: 10069–10081. doi: 10.1007/s00253-015-6823-7 [DOI] [PubMed] [Google Scholar]
- 18.Prade RA, Timberlake WE. The Penicillium chrysogenum and Aspergillus nidulans wetA developmental regulatory genes are functionally equivalent. Mol Gen Genet. 1994;244: 539–47. [DOI] [PubMed] [Google Scholar]
- 19.Katoh K, Standley DM. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol Biol Evol. 2013;30: 772–780. doi: 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (* and other methods), Version 4.0 b10. Sinauer Associates, Sunderland Massachusetts. 2002.
- 21.Whelan S, Goldman N. A general empirical model of protein evolution derived from multiple protein families using a maximum-likelihood approach. Mol Biol Evol. 2001;18: 691–9. [DOI] [PubMed] [Google Scholar]
- 22.Piskacek S, Gregor M, Nemethova M, Grabner M, Kovarik P, Piskacek M. Nine-amino-acid transactivation domain: Establishment and prediction utilities. Genomics. 2007;89: 756–768. doi: 10.1016/j.ygeno.2007.02.003 [DOI] [PubMed] [Google Scholar]
- 23.Nguyen Ba AN, Pogoutse A, Provart N, Moses AM. NLStradamus: a simple Hidden Markov Model for nuclear localization signal prediction. BMC Bioinformatics. 2009;10: 202 doi: 10.1186/1471-2105-10-202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas PD, Campbell MJ, Kejariwal A, Mi H, Karlak B, Daverman R, et al. PANTHER: a library of protein families and subfamilies indexed by function. Genome Res. Cold Spring Harbor Laboratory Press; 2003;13: 2129–41. doi: 10.1101/gr.772403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang YC, Timberlake WE. Identification of Aspergillus brlA response elements (BREs) by genetic selection in yeast. Genetics. 1993;133: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrianopoulos A, Timberlake WE. ATTS, a new and conserved DNA binding domain. Plant Cell. American Society of Plant Biologists; 1991;3: 747 doi: 10.1105/tpc.3.8.747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrianopoulos A, Timberlake WE. The Aspergillus nidulans abaA gene encodes a transcriptional activator that acts as a genetic switch to control development. Mol Cell Biol. American Society for Microbiology; 1994;14: 2503–2515. doi: 10.1128/mcb.14.4.2503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall MA, Timberlake WE. Aspergillus nidulans wetA activates spore-specific gene expression. Mol Cell Biol. 1991;11: 55–62. doi: 10.1128/mcb.11.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sewall TC, Mims CW, Timberlake WE. Conidium differentiation in Aspergillus nidulans wild-type and wet-white (wetA) mutant strains. Dev Biol. 1990;138: 499–508. http://dx.doi.org/10.1016/0012-1606(90)90215-5 [DOI] [PubMed] [Google Scholar]
- 30.Mirabito PM, Adams TH, Timberlake WE. Interactions of three sequentially expressed genes control temporal and spatial specificity in Aspergillus development. Cell. 1989;57: 859–868. http://dx.doi.org/10.1016/0092-8674(89)90800-3 [DOI] [PubMed] [Google Scholar]
- 31.Ni M, Yu J-H. A novel regulator couples sporogenesis and trehalose biogenesis in Aspergillus nidulans. PLoS One. Public Library of Science; 2007;2: e970 doi: 10.1371/journal.pone.0000970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Käfer E. Meiotic and mitotic recombination in Aspergillus and its chromosomal aberrations. Adv Genet. 1977;19: 33–131. doi: 10.1016/S0065-2660(08)60245-X [DOI] [PubMed] [Google Scholar]
- 33.Seo J-A, Guan Y, Yu J-H. Suppressor mutations bypass the requirement of fluG for asexual sporulation and sterigmatocystin production in Aspergillus nidulans. Genetics. 2003;165: 1083–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J-H, Hamari Z, Han KH, Seo JA, Reyes-Dominguez Y, Scazzocchio C. Double-joint PCR: a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genet Biol. 2004;41: 973–981. doi: 10.1016/j.fgb.2004.08.001 [DOI] [PubMed] [Google Scholar]
- 35.Szewczyk E, Nayak T, Oakley CE, Edgerton H, Xiong Y, Taheri-Talesh N, et al. Fusion PCR and gene targeting in Aspergillus nidulans. Nat Protoc. 2006;1: 3111–20. doi: 10.1038/nprot.2006.405 [DOI] [PubMed] [Google Scholar]
- 36.Han KH, Seo JA, Yu J-H. Regulators of G-protein signalling in Aspergillus nidulans: RgsA downregulates stress response and stimulates asexual sporulation through attentuation of GanB (Gα) signalling. Mol Microbiol. 2004;53: 529–540. doi: 10.1111/j.1365-2958.2004.04163.x [DOI] [PubMed] [Google Scholar]
- 37.Park H-S, Man Yu, Lee M-K, Maeng PJ, Kim SC, Yu J-H, et al. Velvet-mediated repression of β-glucan synthesis in Aspergillus nidulans spores. Sci Rep. Nature Publishing Group; 2015;5: 10199 doi: 10.1038/srep10199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lilleho jEB, Ciegler A, Hall HH. Fungistatic action of aflatoxin B1. Experientia. Birkhäuser-Verlag; 1967;23: 187–188. doi: 10.1007/BF02136277 [DOI] [PubMed] [Google Scholar]
- 39.Andrews S. FastQC: a quality control tool for high throughput sequence data [Internet]. 2010. Available: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 40.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14: R36 doi: 10.1186/gb-2013-14-4-r36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anders S, Pyl PT, Huber W. HTSeq—a Python framework to work with high-throughput sequencing data. Bioinformatics. Oxford University Press; 2015;31: 166–169. doi: 10.1093/bioinformatics/btu638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15: 550 doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, Lewis S. AmiGO: online access to ontology and annotation data. Bioinformatics. Oxford University Press; 2009;25: 288–289. doi: 10.1093/bioinformatics/btn615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Maere S, Heymans K, Kuiper M. BiNGO: a Cytoscape plugin to assess overrepresentation of Gene Ontology categories in Biological Networks. Bioinformatics. Oxford University Press; 2005;21: 3448–3449. doi: 10.1093/bioinformatics/bti551 [DOI] [PubMed] [Google Scholar]
- 45.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. Cold Spring Harbor Laboratory Press; 2003;13: 2498–504. doi: 10.1101/gr.1239303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene Ontology: tool for the unification of biology. Nat Genet. Nature Publishing Group; 2000;25: 25–29. doi: 10.1038/75556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mi H, Dong Q, Muruganujan A, Gaudet P, Lewis S, Thomas PD. PANTHER version 7: improved phylogenetic trees, orthologs and collaboration with the Gene Ontology Consortium. Nucleic Acids Res. 2010;38: D204–D210. doi: 10.1093/nar/gkp1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grice CM, Bertuzzi M, Bignell EM. Receptor-mediated signaling in Aspergillus fumigatus. Front Microbiol. Frontiers; 2013;4: 26 doi: 10.3389/fmicb.2013.00026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Affeldt KJ, Carrig J, Amare M, Keller NP. Global survey of canonical Aspergillus flavus G protein-coupled receptors. MBio. American Society for Microbiology; 2014;5: e01501–14. doi: 10.1128/mBio.01501-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weber T, Blin K, Duddela S, Krug D, Kim HU, Bruccoleri R, et al. antiSMASH 3.0—a comprehensive resource for the genome mining of biosynthetic gene clusters. Nucleic Acids Res. Oxford University Press; 2015;43: W237–W243. doi: 10.1093/nar/gkv437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inglis DO, Binkley J, Skrzypek MS, Arnaud MB, Cerqueira GC, Shah P, et al. Comprehensive annotation of secondary metabolite biosynthetic genes and gene clusters of Aspergillus nidulans, A. fumigatus, A. niger and A. oryzae. BMC Microbiol. 2013;13: 91 doi: 10.1186/1471-2180-13-91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timberlake WE, Boylan MT, Mirabito PM, Willett CE, Zimmerman CR. Isolation and physical characterization of three essential conidiation genes from Aspergillus nidulans. Antonie Van Leeuwenhoek. 1987;53: 317 doi: 10.1007/BF00400554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desjardins CA, Champion MD, Holder JW, Muszewska A, Goldberg J, Bailão AM, et al. Comparative genomic analysis of human fungal pathogens causing paracoccidioidomycosis. PLoS Genet. 2011;7: e1002345 doi: 10.1371/journal.pgen.1002345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martinelli SD, Clutterbuck AJ. A quantitative survey of conidiation mutants in Aspergillus nidulans. J Gen Microbiol. 1971;69: 261–268. doi: 10.1099/00221287-69-2-261 [DOI] [PubMed] [Google Scholar]
- 55.Adams TH, Boylan MT, Timberlake WE. brlA is necessary and sufficient to direct conidiophore development in Aspergillus nidulans. Cell. 1988;54: 353–362. http://dx.doi.org/10.1016/0092-8674(88)90198-5 [DOI] [PubMed] [Google Scholar]
- 56.Timberlake WE. Translational triggering and feedback fixation in the control of fungal development. Plant Cell. American Society of Plant Biologists; 1993;5: 1453 doi: 10.1105/tpc.5.10.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sewall TC. Cellular effects of misscheduled brlA, abaA, and wetA expression in Aspergillus nidulans. Can J Microbiol. 1994;40: 1035–1042. [DOI] [PubMed] [Google Scholar]
- 58.Clutterbuck AJ. A mutational analysis of conidial development in Aspergillus nidulans. Genetics. 1969;63: 317–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin K- S, Kim YH, Yu J-H. Proteomic analyses reveal the key roles of BrlA and AbaA in biogenesis of gliotoxin in Aspergillus fumigatus. Biochem Biophys Res Commun. 2015;463: 428–433. doi: 10.1016/j.bbrc.2015.05.090 [DOI] [PubMed] [Google Scholar]
- 60.Timberlake WE. Molecular genetics of Aspergillus development. Annu Rev Genet. 1990;24: 5–36. doi: 10.1146/annurev.ge.24.120190.000253 [DOI] [PubMed] [Google Scholar]
- 61.Erwig LP, Gow N AR. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol. Nature Research; 2016;14: 163–176. doi: 10.1038/nrmicro.2015.21 [DOI] [PubMed] [Google Scholar]
- 62.Lind AL, Wisecaver JH, Smith TD, Feng X, Calvo AM, Rokas A. Examining the evolution of the regulatory circuit controlling secondary metabolism and development in the fungal genus Aspergillus. PLOS Genet. 2015;11: e1005096 doi: 10.1371/journal.pgen.1005096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blumenstein A, Vienken K, Tasler R, Purschwitz J, Veith D, Frankenberg-Dinkel N, et al. The Aspergillus nidulans phytochrome FphA represses sexual development in red light. Curr Biol. 2005;15: 1833–1838. doi: 10.1016/j.cub.2005.08.061 [DOI] [PubMed] [Google Scholar]
- 64.Purschwitz J, Muller S, Kastner C, Schoser M, Haas H, Espeso EA, et al. Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Curr Biol. 2008;18: 255–259. doi: 10.1016/j.cub.2008.01.061 [DOI] [PubMed] [Google Scholar]
- 65.Bieszke JA, Braun EL, Bean LE, Kang S, Natvig DO, Borkovich KA. The nop-1 gene of Neurospora crassa encodes a seven transmembrane helix retinal-binding protein homologous to archaeal rhodopsins. Proc Natl Acad Sci U S A. National Academy of Sciences; 1999;96: 8034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bieszke JA, Li L, Borkovich KA. The fungal opsin gene nop-1 is negatively-regulated by a component of the blue light sensing pathway and influences conidiation-specific gene expression in Neurospora crassa. Curr Genet. 2007;52: 149–157. doi: 10.1007/s00294-007-0148-8 [DOI] [PubMed] [Google Scholar]
- 67.Rodriguez-Romero J, Hedtke M, Kastner C, Uller S, Fischer R. Fungi, Hidden in soil or up in the air: Light makes a difference. Annu Rev Microbiol. 2010;64: 585–610. doi: 10.1146/annurev.micro.112408.134000 [DOI] [PubMed] [Google Scholar]
- 68.Li L, Wright SJ, Krystofova S, Park G, Borkovich KA. Heterotrimeric G protein signaling in filamentous fungi. Annu Rev Microbiol. 2007;61: 423–452. doi: 10.1146/annurev.micro.61.080706.093432 [DOI] [PubMed] [Google Scholar]
- 69.Park H- S, Ni M, Jeong KC, Kim YH, Yu J- H. The role, interaction and regulation of the Velvet regulator VelB in Aspergillus nidulans. PLoS One. Public Library of Science; 2012;7: e45935 doi: 10.1371/journal.pone.0045935 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
The RNA-seq dataset has been submitted to the GEO database (Accession number: GSE95711).