Abstract
Owing to the devastation caused by tuberculosis along with the unsatisfactory performance of the Bacillus Calmette–Guérin (BCG) vaccine, a more efficient vaccine than BCG is required for the global control of tuberculosis. A number of studies have demonstrated an essential role of biotin biosynthesis in the growth and survival of several microorganisms, including mycobacteria, through deletion of the genes involved in de novo biotin biosynthesis. In this study, we demonstrate that a bioA mutant of Mycobacterium tuberculosis (MtbΔbioA) is highly attenuated in the guinea pig model of tuberculosis when administered aerogenically as well as intradermally. Immunization with MtbΔbioA conferred significant protection in guinea pigs against an aerosol challenge with virulent M. tuberculosis, when compared with the unvaccinated animals. Booster immunization with MtbΔbioA offered no advantage over a single immunization. These experiments demonstrate the vaccinogenic potential of the attenuated M. tuberculosis bioA mutant against tuberculosis.
Introduction
The vaccine Bacillus Calmette–Guérin (BCG), an attenuated strain of Mycobacterium bovis, has been widely administered since 1921. BCG protects children against severe forms of tuberculosis (TB) but demonstrates highly variable efficacy (0–80%) against adult pulmonary tuberculosis in different field trials with poor efficacy in high TB burden countries, in general [1–5]. There is a clear need for a new effective TB vaccine and a number of candidate vaccines based on several different approaches are currently being evaluated in clinical trials [6]. However, one of the most advanced TB vaccine candidate that attempted to boost the efficacy of BCG was unable to induce any additional efficacy over BCG in a recent human clinical trial [7]. One promising approach of vaccine development against TB is the generation of attenuated Mycobacterium tuberculosis mutant strains. Vaccine strains based on attenuated M. tuberculosis mutants harness the benefits of close antigenic similarity with the disease-causing organism. A study comparing BCG and M. tuberculosis substrains has shown that a large number of T-cell epitopes are missing from BCG substrains but are present in all five M. tuberculosis strains employed for the comparative analysis [8]. Technological advancements have accelerated site-specific genetic modifications in mycobacteria. In addition, identification of virulence factors as well as essential biosynthetic pathways of M. tuberculosis has enabled the generation of rationally attenuated M. tuberculosis mutant strains. Several M. tuberculosis mutant strains have been shown to be safe and attenuated while immunization with these strains have resulted in the generation of varying degree of protection against TB in animal models when compared with the protection afforded by BCG [9–16]. Though several attenuated M. tuberculosis mutant based vaccine strains have been generated, MTBVAC (M. tuberculosis mutant with deletions in the genes phoP and fadD26) is the first and the only live attenuated M. tuberculosis vaccine strain that is being assessed in human clinical trials [17]. Thus, further efforts are needed towards the development of novel attenuated M. tuberculosis strains and evaluation of their efficacy against tuberculosis.
Biotin is central to cell metabolism in both prokaryotes and eukaryotes. It is an essential cofactor for various enzymes catalyzing the transfer of CO2 during carboxylation, decarboxylation and transcarboxylation reactions. Bacteria and plants can synthesize biotin de novo but mammals do not contain the enzymes required for biotin synthesis and depend on its supplementation through diet or gut microbiota [18–20]. BioA (7,8-diaminopelargonic acid synthase) catalyzes the conversion of KAPA (7-keto-8-aminopelargonic acid) to DAPA (7,8-diaminopelargonic acid) in the biotin biosynthesis pathway [21]. The precursor for biotin biosynthesis is a pimeloyl moiety that may be obtained from different sources but the final four enzymatic steps are conserved between all biotin-synthesizing organisms [19, 22]. Since biotin biosynthetic pathway is unique to bacteria and plants, it has been an important target for developing inhibitors against M. tuberculosis [23–25]. Several evidences support the essentiality of biotin biosynthesis for the in vitro as well as in vivo survival of mycobacteria. Firstly, the bioA gene mutant of M. smegmatis was found to be auxotrophic in nature and depended on the de novo biosynthesis of biotin for optimal growth and survival in the stationary phase even in the presence of biotin in the media [26]. Secondly, a hallmark study employing transposon mutagenesis identified genes of biotin biosynthetic pathway (bioF, bioA and bioB) to be essential for the survival of M. tuberculosis in mice which suggested that M. tuberculosis does not have access to exogenous biotin in vivo [27]. Thirdly, an M. marinum biotin auxotroph was shown to be growth defective in vitro in macrophages as well as severely attenuated in vivo in a zebrafish model of tuberculosis [28]. Importantly, transporters that facilitate the uptake of exogenous biotin have not been identified in mycobacteria but biotin, when supplied in copious amounts in the growth media, seem to enter the bacilli by passive diffusion or by yet unidentified mechanism [19, 29]. The importance of BioA was substantiated in a recent study by Park et al., wherein bioA gene mutant of M. tuberculosis was constructed and this M. tuberculosisΔbioA strain was found to be auxotrophic for biotin [30]. Further, Park et al. evaluated the biotin biosynthetic pathway as a drug target against M. tuberculosis and demonstrated that BioA was essential for the establishment of infection as well as for the persistence of M. tuberculosis in mice [30]. However, certain features of tuberculosis in mice are quite distinct from humans and may have significant implications on vaccine evaluation and pathological studies. Most commonly used mice strains, C57BL/6 and BALB/c are inherently resistant to M. tuberculosis infection and do not develop severe pathology as seen in human TB [31]. Certain CD1 isoforms known to play a noteworthy role in immune response against TB are not found in mice but are present in guinea pigs [32, 33]. Also, granulomas in mice are not hypoxic and do not caseate unlike in human TB [34, 35]. Moreover, there are studies where vaccines exhibiting promise in the murine model failed when tested in guinea pigs [36–38]. Manifestation of TB in guinea pigs and humans bear a large number of similarities [39]. Guinea pigs are vulnerable to infection with a few inhaled M. tuberculosis bacilli and demonstrate many aspects of human TB such as caseating and hypoxic granulomas, pulmonary lesions of both primary and hematogenous origin, presence of Langhans multinucleated giant cells, robust delayed-type hyper-sensitivity (DTH) response and dissemination of infection [40–43]. Guinea pigs do not develop latency, rather they develop chronic progressive disease and lung tissue damage leading to weight loss and death, making this model ideal for studying TB associated pathology [40, 44, 45]. Guinea pig model is the gold standard for TB vaccine evaluation. Owing to the susceptibility to M. tuberculosis infection and consistent efficacy demonstrated following BCG vaccination, guinea pig model is accepted as a more stringent model for efficacy testing than mice.
In this study, we constructed a bioA (Rv1568) mutant of M. tuberculosis (MtbΔbioA) and demonstrated its attenuation in the highly susceptible guinea pig model of tuberculosis following administration via the aerosol as well as intradermal route. Subsequently, the MtbΔbioA strain was evaluated in single and booster immunization protocol for its ability to protect against virulent M. tuberculosis challenge in the guinea pig model of experimental tuberculosis.
Materials and methods
Bacterial strains and culture conditions
Bacterial strains and plasmids employed in this study are listed in Table 1. Mycobacterial strains were grown in Middlebrook (MB) 7H9 media (BD Difco) supplemented with 0.5% glycerol, 1X ADC (albumin-dextrose-catalase, BD Difco) and 0.05% Tween 80 or on MB7H11 agar (BD Difco) supplemented with 1X OADC (oleic acid-albumin-dextrose-catalase, BD Difco) and 0.5% glycerol. Cultures were grown at 37°C with constant shaking at 200 rpm. Agar plates were incubated at 37°C for 3–4 weeks. For infection and vaccination purposes, mycobacterial cultures in mid-log phase were resuspended in phosphate-buffered saline (PBS) and frozen in aliquots, until further use. The colony-forming units of the PBS stocks were determined by plating appropriate dilutions in duplicates on supplemented MB7H11 agar. For modified 7H9, 0.5 g ammonium sulfate, 0.5 g L-glutamic acid, 0.1 g sodium citrate, 1 mg pyridoxine, 2.5 g disodium phosphate, 1.0 g monopotassium phosphate, 0.04 g ferric ammonium citrate, 0.05 g magnesium sulfate, 0.5 mg calcium chloride, 1 mg zinc sulfate, 1 mg copper sulfate were added per litre of media and supplemented with 0.2% glycerol, 1X ADC (BD Difco) and 0.05% Tween 80.
Table 1. Bacterial strains, plasmids and primers employed in this study.
| Strains | Description | Reference |
| E.coli XL-1 Blue | endA1 gyrA96 (nalR) thi-1 recA1 relA1 lac glnV44 F' [::Tn10 proAB + lacIq Δ (lacZ) M15] hsdR17 (rK- mK+) | Stratagene, Heidelberg, Germany |
| E.coli HB101 | F-(gpt-proA) 62 leuB6 glnV44 ara-14 galK2 lacY1 (mcrC-mrr) rpsL20 (Strr) xyl-5 mtl-1 recA13 | Life Technologies, CA, USA |
| M. tuberculosis H37Rv | Virulent strain of M. tuberculosis | Dr. J. S. Tyagi, AIIMS, New Delhi, India |
| M. tuberculosis H37Rv/pJV53 | M. tuberculosis H37Rv recombineering strain harbouring pJV53 and expressing recombineering proteins gp60 and gp61 | [46] |
| MtbΔbioA | M. tuberculosis H37Rv bioA mutant | This study |
| MtbΔbioA.comp | M. tuberculosis H37Rv bioA mutant complemented with full length bioA gene in extrachromosomal plasmid | This study |
| M. tuberculosis Erdman | Virulent strain of M. tuberculosis | Dr. J. S. Tyagi, AIIMS, New Delhi, India |
| BCG Danish | Vaccine strain against tuberculosis | BCG laboratories, Chennai, India |
| Plasmids | Description | Reference |
| pJV53 | Mycobacterial- E. coli shuttle vector encoding recombineering proteins gp60 and gp61 | [47] |
| pYUB854 | Mycobacterial cosmid vector with hygromycin resistance gene cassette flanked by resolvase sites and multiple cloning sites | [48] |
| pYUBΔbioA | pYUB854 with hygromycin resistance cassette flanked with bioA amplicons I and II | This study |
| pSD5.hsp65 | Mycobacterial- E. coli shuttle expression vector containing hsp65 promoter | [49, 50] |
| pVR1 | A pSD5 derivative containing the chloramphenicol resistance gene under mycobacterial trrn promoter | [51] |
| pVR1.bioA | pVR1 carrying the full length mycobacterial bioA gene under hsp65 promoter | This study |
| Primers | Sequence | Reference |
| BioA-AI-F | 5' -gatatcactagtatgccaatagcgttcgctga- 3' | This study |
| BioA-AI-R | 5' -agtactagatcttttaaagcggtccaccaggagctcat- 3' | This study |
| BioA-AII-F | 5' -agtactggtacctttaaatccatcggcgtcatcgaa- 3' | This study |
| BioA-AII-R | 5' -gatatccttaaggcgtcaccacaaccc- 3' | This study |
| F-bioA | 5' -gattatcatatgggatccatggctgcggcgactggc- 3' | This study |
| R-bioA | 5' -cttatatcctcgagtcatggcagtgagcctac- 3' | This study |
Escherichia coli strains HB101 (Life Technologies) and XL-1 Blue (Stratagene) were used to clone specific DNA segments into pYUB854 and pVR1 plasmids, respectively (Table 1). E. coli cells were grown in Luria Bertani (LB) broth at 37°C with constant shaking at 200 rpm. When required, kanamycin (25 μg/ml), chloramphenicol (30 μg/ml) and hygromycin (50 μg/ml for mycobacteria and 150 μg/ml for E. coli) were added to the growth media.
Disruption of the bioA gene in M. tuberculosis
For the generation of MtbΔbioA mutant, internal 916 bp segment of the bioA gene was deleted in the genome of M. tuberculosis H37Rv and was replaced with the hygromycin resistance cassette by employing a linear allelic exchange substrate (AES). To generate the linear ΔbioA::hyg AES, two set of primers were designed to amplify amplicon I and amplicon II (Table 1). The 729 bp amplicon I consisted of 200 bp of 5′ proximal end of the bioA gene and 529 bp of immediate upstream region of the bioA gene. The 648 bp amplicon II comprised of 198 bp of 3′ distal end of the bioA gene and 450 bp of the region immediately downstream of the bioA gene. BioA is encoded by the first gene of an operon consisting of three downstream genes (bioF1, bioD and Rv1571). Hence, the amplicons were designed such that homologous recombination would result in deletion of internal 916 bp segment of the bioA gene leaving behind ~200 bp region at the 5′ and 3′ end of the bioA gene along with its upstream and downstream regions intact. Moreover, the hygromycin resistance cassette was inserted in reverse orientation to the bioA gene. The amplicon I and amplicon II were PCR amplified and cloned into the vector pYUB854 at BglII/SpeI and KpnI/AflII restriction sites, respectively, flanking the hygromycin resistance cassette to generate pYUBΔbioA (Table 1). Subsequently, the 3.4 kb linear AES (ΔbioA::hyg AES) was excised out of the vector pYUBΔbioA by AflII/SpeI restriction digestion and electroporated into M. tuberculosis H37Rv/pJV53 as described earlier [47] to generate the bioA mutant of M. tuberculosis.
Construction of complemented strain of MtbΔbioA
The pJV53 recombineering plasmid employed for the generation of the mutant was removed from the MtbΔbioA strain prior to the complementation. For this, MtbΔbioA was cultured in MB7H9 media in the absence of kanamycin (selection marker for pJV53) and appropriate dilutions of tertiary culture were plated on MB7H11 agar containing hygromycin. Individual colonies obtained after the incubation of agar plates for 3–4 weeks at 37°C were screened for the loss of pJV53 by patching on MB7H11 agar plates with or without kanamycin (S1 Fig). In this study, 80% of the colonies screened showed kanamycin sensitive phenotype and thus demonstrated the loss of pJV53 from MtbΔbioA.
For complementation of MtbΔbioA strain, 1.3 kb full-length bioA gene from mycobacteria was cloned downstream to hsp65 promoter in the vector pSD5.hsp65 resulting in the generation of pSD5.hsp65-bioA (Table 1). The hsp65-bioA segment was further subcloned into the XbaI and MluI sites of the mycobacterial shuttle vector pVR1, to generate pVR1.bioA (Table 1). The plasmid pVR1.bioA was subsequently electroporated into MtbΔbioA strain to generate the complemented strain, MtbΔbioA.comp. The transformants were selected on MB7H11 agar plates supplemented with 30 μg/ml chloramphenicol followed by incubation at 37°C for 3–4 weeks. For immunoblot analysis, 10 μg cell lysate of M. tuberculosis H37Rv, MtbΔbioA mutant and MtbΔbioA.comp strains were subjected to SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) in 12.5% polyacrylamide gel. Immunoblotting was performed using anti-BioA rabbit polyclonal antiserum (kindly provided by Dr. Dirk Schnappinger and Dr. Sabine Ehrt, Weill Cornell Medical College, New York) [30] at a dilution of 1:2000 followed by addition of goat anti-rabbit secondary antibody conjugated to horseradish peroxidise (Jackson Immuno Research Inc.). Blot was detected with 1 mg/ml of 3’,3’–diaminobenzidine in phosphate buffered saline containing 0.3% H2O2.
Experimental animals
Female guinea pigs (Dunkin-Hartley) of 200–300 g were procured from Disease Free Small Animal House Facility, Lala Lajpat Rai University, Hisar, India. The animals were housed in individually ventilated cages under standardized conditions in a Biosafety level III facility at University of Delhi South Campus (UDSC), New Delhi, India and were provided with food and water ad libitum. Animals were allowed to acclimate, following which they were randomized to different experimental groups.
Ethics statement
All guinea pig experiments included in this study were reviewed and approved by the Institutional Animal Ethics Committee of University of Delhi South Campus, New Delhi, India (Ref. No. 9/IAEC/AKT/Biochem/UDSC/29.08.2014 and Ref No. 26/IAEC/AKT/Biochem/UDSC/17.8.2015). All animals were routinely cared for according to the guidelines of CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), India and all efforts were made to ameliorate animal suffering. Guinea pigs were intradermally vaccinated by injecting 100 μl of suspension and were euthanized by CO2 asphyxiation, whenever required, during daytime in the Biosafety level III facility. No animals died prior to the experimental timepoints.
Evaluation of attenuation and in vivo growth kinetics of MtbΔbioA in guinea pigs
To evaluate the extent of attenuation of the MtbΔbioA strain, guinea pigs in groups of six were infected via aerosols with ~10–30 bacilli of M. tuberculosis H37Rv or MtbΔbioA or MtbΔbioA.comp in an aerosol chamber (A4224—full body inhalation exposure system, Glas-Col Inc., USA). Animals were euthanized by CO2 asphyxiation at 3, 6 and 12 weeks post infection. For enumeration of bacillary load, specific portions of lungs and spleen were aseptically removed, weighed and homogenized separately in saline. Appropriate dilutions were plated in duplicates on MB7H11 agar plates and colonies were enumerated following incubation at 37°C for 3–4 weeks. The bacillary load was expressed as log10 CFU/organ. Visual scoring of gross lesions for the lungs, spleen and liver was carried out based on tissue involvement, areas of inflammation, number and size of tubercles and the extent of necrosis. The scores are graded from 1–4 based on the modified Mitchison scoring system [52]. For histopathological evaluation, specific portion of lungs and liver were removed and fixed in 10% buffered formalin. 5 μm thick sections of paraffin embedded tissues were stained with haematoxylin-eosin (H&E). The sections were examined for the granulomas and graded by a certified pathologist (who was blinded to the experimental groups) according to the criteria described previously by Lasco et al [53]. Briefly, granuloma with necrosis was given a score of 5, granuloma without necrosis was given a score of 2.5 and granuloma with fibrous tissue was given a score of 1. All granulomas in each section were scored and the scores were added up to obtain a total granuloma score of lungs or liver of each animal. In addition, sections were semi-quantitatively assessed for percentage of the section occupied by granuloma and this was expressed as granuloma fraction.
To evaluate in vivo growth kinetics and attenuation of MtbΔbioA following intradermal administration, animals in the groups of five were intradermally injected with 1 x 106 CFU of either M. tuberculosis, BCG or MtbΔbioA in 100 μl of saline. Animals were euthanized by CO2 asphyxiation at 1, 3 and 6 weeks post inoculation. Bacillary enumeration, gross pathological evaluation and histopathological evaluation were carried out as described in the previous section. The bacillary load was expressed as log10 CFU/organ.
Evaluation of protective efficacy of MtbΔbioA against M. tuberculosis infection
Groups of guinea pigs (n = 6) were intradermally immunized with either (i) 1 x 106 CFU of BCG (Danish strain) in 100 μl of saline or (ii) 1 x 106 CFU of MtbΔbioA in 100 μl of saline or (iii) two doses of 1 x 106 CFU of MtbΔbioA administered at 6-week interval (ΔbioA/ΔbioA). Control group (sham-immunized) guinea pigs received 100 μl of saline intradermally. Guinea pigs were challenged with aerosolized virulent M. tuberculosis Erdman strain (~50 bacilli in the lungs per animal) at 12 weeks post primary immunization and were euthanized by CO2 asphyxiation at 4 weeks post challenge. Organs were subjected to bacterial enumeration, gross pathological and histopathological evaluation as described in the previous section. Samples from the BCG-immunized animals were plated on MB-7H11 agar containing 2 μg/ml of thiophene-2-carboxylic acid hydrazide (Sigma) to inhibit the growth of residual BCG, if any.
Statistical analysis
For comparison between the groups for the determination of bacillary load in the lungs or spleen and organ weight of experimental guinea pigs, unpaired t-test (two-tailed) or one-way analysis of variance (ANOVA) with the Tukey's multiple comparison test was employed, wherever appropriate. For comparison between the groups for the analyses of total granuloma score, Mann-Whitney test (two-tailed) was employed. Nonparametric Kruskal-Wallis test followed by the Dunn's multiple comparison test was employed for comparison between the groups of animals for the analysis of the gross pathological damage. Differences were considered significant when p <0.05. Prism software (GraphPad Software Inc., CA) was employed for generation of graphs and statistical analysis.
Results
Disruption of the bioA gene in M. tuberculosis and characterization of the mutant
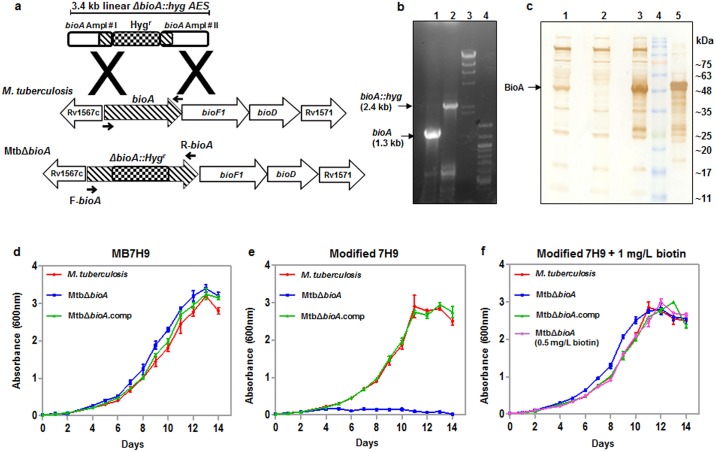
To generate the MtbΔbioA mutant, a 3.4 kb linear ΔbioA::hyg AES was employed as described in the methods section. Disruption of the bioA gene in M. tuberculosis was confirmed by polymerase chain reaction (PCR) analysis by using bioA gene specific primers (Fig 1a and 1b, Table 1). A 1.3 kb PCR product was observed in the case of M. tuberculosis (Fig 1b). However, a 2.4 kb PCR product was observed in the case of MtbΔbioA which corresponds with the replacement of 916 bp internal segment of the bioA gene with the ~ 2.0 kb hygromycin resistance cassette (Fig 1b). Further, M. tuberculosis, MtbΔbioA and complement strain (MtbΔbioA.comp) were subjected to immunoblot analysis with anti-BioA rabbit polyclonal antiserum (Fig 1c). In the M. tuberculosis cell lysate, BioA was detected as a ~48 kDa band which was absent from the cell lysate of MtbΔbioA mutant (Fig 1c). Restoration of the ~48 kDa band was observed in the cell lysate of MtbΔbioA.comp strain confirming the complementation of MtbΔbioA. The results of PCR and immunoblot analysis confirm the disruption of the bioA gene in MtbΔbioA.
Fig 1. Confirmation of disruption of the bioA gene in M. tuberculosis.
(a) Diagrammatic representation of the bioA locus in M. tuberculosis and MtbΔbioA. The bioA gene is the first gene of an operon consisting of three downstream genes bioF1, bioD and Rv1571. The figure depicts the disruption of the bioA gene in MtbΔbioA by homologous recombination. Arrows show the location of the bioA gene specific primers F-bioA and R-bioA employed for the confirmation of disruption of the bioA gene by PCR. (b) PCR based confirmation of disruption of bioA gene in MtbΔbioA. A 1.3 kb PCR amplification product was obtained with the M. tuberculosis genomic DNA as template (lane 1) and 2.4 kb PCR amplification product was obtained with MtbΔbioA genomic DNA as template (lane 2). λHindIII DNA molecular mass marker and 100 bp ladder were loaded in lanes 3 and 4, respectively. (c) Confirmation of disruption of bioA in MtbΔbioA by immunoblot analysis. 10 μg of cell lysate of M. tuberculosis (lane 1), MtbΔbioA (lane 2) and MtbΔbioA.comp (lane 3) strains were separated on a 12.5% polyacrylamide gel and immunoblot analysis was carried out with anti-BioA polyclonal antiserum. BioA (~48 kDa band) was detected in the cell lysate of the M. tuberculosis and MtbΔbioA.comp. The disruption of bioA in MtbΔbioA was confirmed by the absence of the ~48 kDa band in lane 2. Protein molecular weight marker and 150 ng of purified BioA were loaded in lanes 4 and 5, respectively. (d, e, f) Influence of disruption of the bioA gene on the growth of M. tuberculosis in culture media. M. tuberculosis, MtbΔbioA and MtbΔbioA.comp were cultured separately in (d) MB7H9 medium, (e) modified 7H9 medium (prepared without biotin) and (f) modified 7H9 medium supplemented with 1 mg/L of biotin. Growth at 37°C/200 rpm was monitored for 14 days by measuring the absorbance at 600 nm. MtbΔbioA failed to grow in modified 7H9 medium and the growth was restored to wild type levels when modified 7H9 was supplemented with 1 mg/L or 0.5 mg/L of biotin. Two independent growth analysis experiments were carried out in duplicates and the values of absorbance are represented as the mean (±SE).
Next, we compared the growth of M. tuberculosis, MtbΔbioA and MtbΔbioA.comp in MB7H9 media (which contains 0.5 mg/L of biotin) as well as in 7H9 media prepared without biotin (modified 7H9). We did not observe any difference in the growth of M. tuberculosis, MtbΔbioA and MtbΔbioA.comp in MB7H9 medium (Fig 1d). However, MtbΔbioA failed to grow in modified 7H9 media (Fig 1e). The growth of MtbΔbioA was completely restored when modified 7H9 media was supplemented with 1 mg/L or 0.5 mg/L of biotin (Fig 1f). MtbΔbioA.comp strain demonstrated similar growth kinetics as M. tuberculosis in all the three culture conditions. These results demonstrate that the disruption of the bioA gene in M. tuberculosis renders the pathogen auxotrophic for biotin that can be complemented with the wild-type copy of the bioA gene.
Biotin auxotroph of M. tuberculosis is highly attenuated in guinea pigs
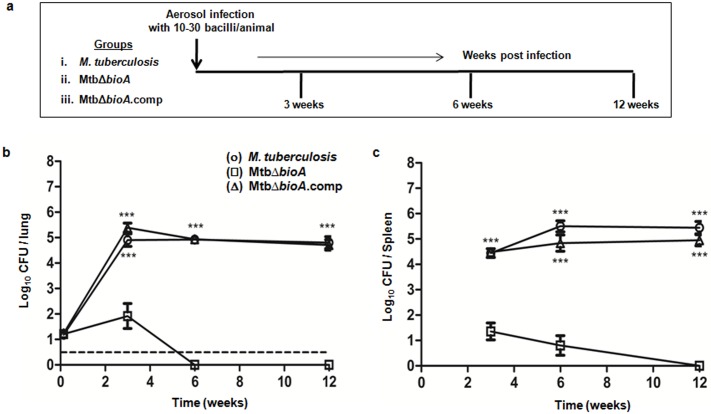
In order to evaluate the effect of disruption of the bioA gene on the virulence of M. tuberculosis, guinea pigs were infected with M. tuberculosis, MtbΔbioA or MtbΔbioA.comp strains via the aerosol route (Fig 2a). M. tuberculosis infected animals demonstrated an increase in the bacterial burden during the initial phase of infection (Fig 2b and 2c). At 3 weeks post infection, the M. tuberculosis infected animals exhibited a high bacillary load of 4.90 log10 CFU in the lungs and 4.44 log10 CFU in the spleen (Fig 2b and 2c). Thereafter, the bacterial load remained steady in the lungs of M. tuberculosis infected animals. In the spleen, the bacillary load of M. tuberculosis stabilized after 6 weeks. Relative to M. tuberculosis infection, MtbΔbioA infected animals showed significantly reduced ability to multiply in guinea pig organs (Fig 2b and 2c). At 3 weeks post infection, MtbΔbioA infected animals exhibited around 103 fold less bacillary load in the lungs and spleen, when compared with the bacillary load in M. tuberculosis infected animals (Fig 2b and 2c). Thereafter, the bacterial numbers in the lungs rapidly declined and no bacilli were recovered from the lungs of the animals infected with MtbΔbioA at 6 weeks post infection (Fig 2b). In the spleen, MtbΔbioA persisted in low numbers at 6 weeks and was gradually eliminated by 12 weeks post infection (Fig 2c). Thus, MtbΔbioA exhibits an attenuated phenotype in guinea pigs. The bacterial burden observed in the lungs and spleen of animals infected with the MtbΔbioA.comp strain was comparable to that of M. tuberculosis infected animals at all the timepoints screened. This demonstrated that the attenuation observed for the MtbΔbioA strain was attributable to the disruption of the bioA gene.
Fig 2. Influence of disruption of the bioA gene on the attenuation of M. tuberculosis in guinea pigs.
(a) Experimental protocol for evaluating the influence of disruption of the bioA gene on the pathogenicity of M. tuberculosis in guinea pigs infected via aerosol. The figure shows the bacillary load in the (b) lungs and (c) spleen of guinea pigs at 3, 6 and 12 weeks post infection with M. tuberculosis (○), MtbΔbioA (□) or MtbΔbioA.comp (△) strains. Three animals per group were euthanized to determine the retained dose in the lungs following aerosol infection. Each data point at 3, 6, and 12 weeks represents the mean (±SE) of the log10 CFU/organ obtained with six guinea pigs per experimental group. Dotted line depicts the limit of detection for pulmonary bacillary load, meaning thereby that at 6 and 12 weeks post infection no bacilli were obtained from the total homogenate of half lung of MtbΔbioA infected animals. No bacilli were obtained from the complete spleen homogenates of MtbΔbioA infected animals at 12 weeks post infection. *** p <0.001 [unpaired t-test (two-tailed)] when compared with MtbΔbioA infected animals.
bioA mutant of M. tuberculosis is unable to cause tuberculous pathology in guinea pigs
At 3 weeks post aerosol infection, M. tuberculosis or MtbΔbioA.comp infected animals exhibited moderate gross pathological damage of lungs and spleen with occasionally visible large tubercles (S2a Fig). Lungs of few of the animals infected with MtbΔbioA showed scanty small sized lesions. Spleen and liver of animals infected with MtbΔbioA showed minimal involvement with negligible gross pathological damage. At 6 and 12 weeks post infection, both M. tuberculosis and MtbΔbioA.comp infected animals exhibited progressively worsening pulmonary as well as extra-pulmonary gross pathological damage (S2b and S2c Fig). However, at these timepoints, animals infected with MtbΔbioA showed minimal involvement of lungs, spleen and liver with significantly less score when compared with the organs of M. tuberculosis or MtbΔbioA.comp infected animals (S2b and S2c Fig). The comparison of mean weights of lungs and spleen of infected guinea pigs, as a measure of disease severity, demonstrated that MtbΔbioA infected animals had significantly less mean spleen weight when compared with M. tuberculosis and MtbΔbioA.comp infected animals at all the timepoints screened (S2d Fig). In addition, relative to M. tuberculosis and MtbΔbioA.comp infected animals, a significant reduction in the mean lung weight of guinea pigs infected with MtbΔbioA was observed by 12 weeks post infection (S2d Fig).
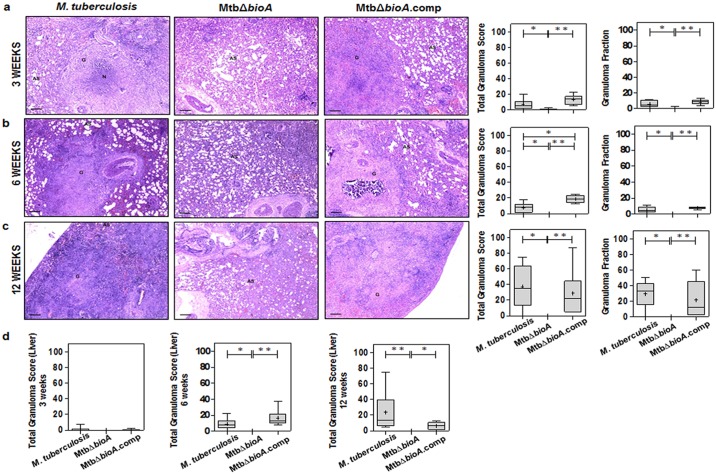
Histopathological evaluation of lung tissues demonstrated that M. tuberculosis and MtbΔbioA.comp infected animals showed progressively worsening granulomatous pathology in the lungs with granulomas composed of epithelioid cells and lymphocytes with occasional central necrosis (Fig 3a). MtbΔbioA infected animals demonstrated significantly less total granuloma score of the lungs when compared to M. tuberculosis and MtbΔbioA.comp infected animals at all the timepoints screened (Fig 3). At 3 weeks post infection, five out of six animals infected with MtbΔbioA demonstrated minimal histopathological changes in lung parenchyma with scattered lymphocytic infiltration but without granulomatous lesions compared to infection with M. tuberculosis and MtbΔbioA.comp, where granulomatous pathology was observed along with few necrotic granulomas (Fig 3a). No histopathological evidence of granuloma formation were observed at 6 and 12 weeks post infection with MtbΔbioA (Fig 3b and 3c). Upon histopathological evaluation of the liver at 3, 6 and 12 weeks post infection, MtbΔbioA infected animals did not show any evidence of granulomatous pathology in the liver while M. tuberculosis and MtbΔbioA.comp infected animals showed progressive pathological damage of the hepatic tissue (Fig 3d). Taken together, the gross and histopathological evaluation of organs from MtbΔbioA infected animals indicates that the bioA mutant of M. tuberculosis is unable to cause pathological damage in guinea pig organs.
Fig 3. Histopathology of guinea pig tissues post aerosol infection with M. tuberculosis, MtbΔbioA or MtbΔbioA.comp strains.
The figure depicts representative 40x magnification photomicrographs of formalin fixed, paraffin embedded and haematoxylin-eosin (H&E) stained 5 μm sections of lung tissue of guinea pigs aerogenically infected with M. tuberculosis, MtbΔbioA or MtbΔbioA.comp and euthanized at (a) 3 weeks, (b) 6 weeks and (c) 12 weeks post infection. AS, G and N denote alveolar spaces, granuloma and necrosis, respectively. The graphical representation of the total granuloma score and granuloma fraction are shown alongside by box plot of all six animals per group (the mean is represented by ‘+’, median value is denoted by horizontal line, box represents the inter quartile range and the minimum and maximum value is denoted by whiskers). (d) Total granuloma score for liver sections of infected animals (n = 6 animals per group) at 3, 6 and 12 weeks post infection. MtbΔbioA infected animals showed negligible pulmonary as well as hepatic granulomatous pathological damage. M. tuberculosis and MtbΔbioA.comp infected animals displayed increasing number of granulomas in the lungs as well as liver. The scale bars depict 200 μm. *p <0.05 and **p <0.01 (Mann-Whitney test, two-tailed).
Difference in persistence of MtbΔbioA and BCG following intradermal administration
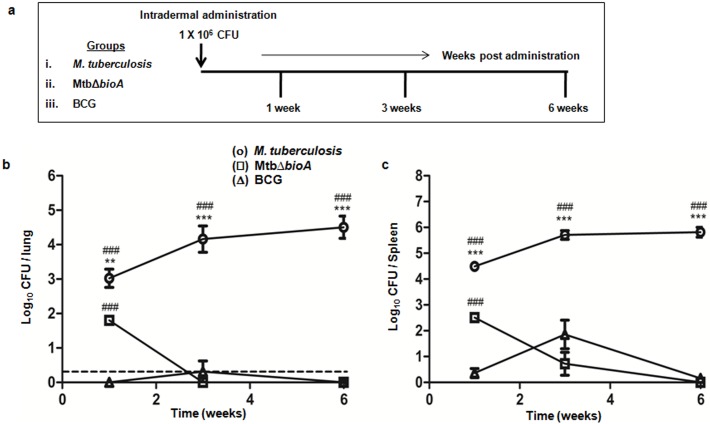
Prior to the evaluation of vaccine efficacy, in vivo growth kinetics and attenuation of MtbΔbioA was analyzed following intradermal administration in guinea pigs and was compared with M. tuberculosis and BCG (Fig 4a). Animals injected with M. tuberculosis exhibited an increase in the bacillary load in the lungs and spleen from 1 week to 3 weeks post inoculation after which the bacillary load stabilized (Fig 4b and 4c). However, relative to M. tuberculosis inoculated animals, MtbΔbioA inoculated animals exhibited significantly reduced bacillary load [by 1.220 log10 CFU in the lungs (p = 0.0024) and by 1.985 log10 CFU in the spleen (p <0.0001)] as early as 1 week post injection (Fig 4b and 4c). The bacterial burden of MtbΔbioA declined rapidly in both lungs and spleen. No bacilli could be recovered from the lungs and spleen of all of the MtbΔbioA inoculated animals at 3 and 6 weeks post inoculation, respectively (Fig 4b and 4c). Animals inoculated with BCG showed the lowest bacillary load at 1 week post inoculation with no bacilli in the lungs and 0.36 log10 CFU in the spleen (Fig 4b and 4c). Subsequently, the bacillary load of BCG showed slight increase from 1 to 3 weeks post inoculation. The increase in bacillary load was more pronounced in the spleen (Fig 4c). Thereafter, the bacterial load declined and only one out of five animals inoculated with BCG showed 0.77 log10 CFU in the spleen while no bacilli were recovered from the lungs by 6 weeks post inoculation (Fig 4b and 4c). Thus, even though significantly higher (p <0.0001) bacillary load of MtbΔbioA was observed in the organs when compared with BCG at 1 week post inoculation, the bacillary numbers of MtbΔbioA rapidly declined to levels below that of BCG and were undetectable by 6 weeks. However, BCG showed some persistence in the spleen (Fig 4c). Histopathologically, at 3 and 6 weeks post intradermal administration, M. tuberculosis infected animals exhibited pathological damage of the lungs with granulomas composed of epithelioid cells and lymphocytes without any necrosis (Fig 5b and 5c). However, no evidence of granulomatous lesion were seen in the lungs of the animals inoculated with MtbΔbioA or BCG at any of the timepoints screened (Fig 5).
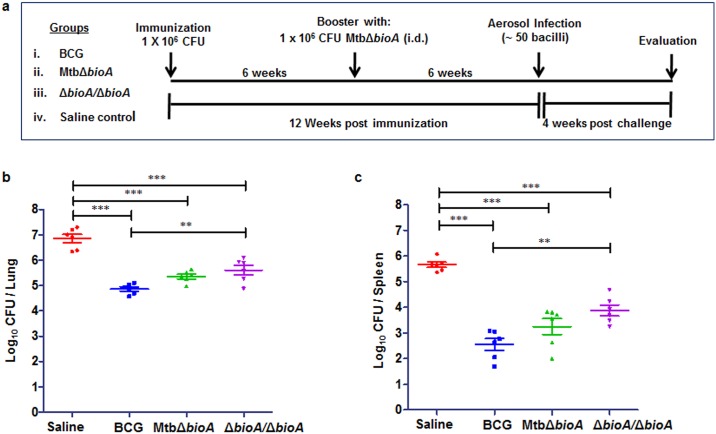
Fig 4. In vivo growth kinetics of MtbΔbioA in guinea pigs following intradermal administration.
(a) Experimental protocol for the evaluation of in vivo growth kinetics and attenuation of MtbΔbioA in guinea pigs upon intradermal administration. The figure shows the bacillary load in the (b) lungs and (c) spleen of guinea pigs at 1, 3 and 6 weeks post inoculation with M. tuberculosis (○), MtbΔbioA (□) or BCG (△). Each data point represents the mean (±SE) of the log10 CFU/organ obtained with five guinea pigs per experimental group per timepoint. Dotted line depicts the limit of detection for pulmonary bacillary load, meaning thereby that no bacterial colony was obtained from the total homogenate of half lung of all the MtbΔbioA inoculated animals as well as in some of the BCG inoculated animals at 3 and 6 weeks post administration. No bacterial colony was obtained from the total spleen homogenates of MtbΔbioA inoculated animals at 6 weeks post administration. **p <0.01 and ***p <0.001 [unpaired t-test (two-tailed)] when compared with animals injected with MtbΔbioA. ###p <0.001 [unpaired t-test (two-tailed)] when compared with animals injected with BCG.
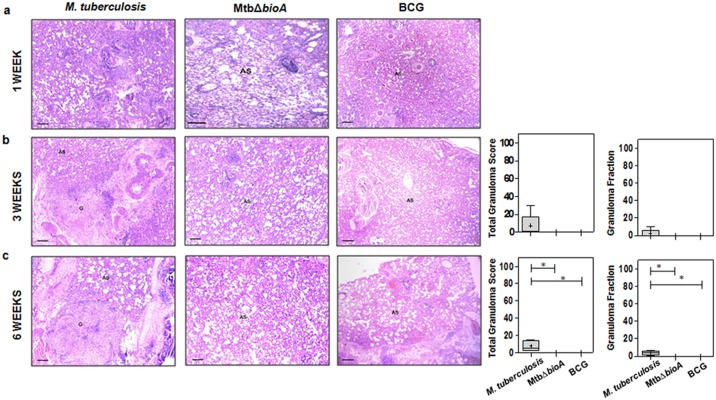
Fig 5. Histopathological analysis of guinea pig lungs post intradermal administration of M. tuberculosis, MtbΔbioA or BCG.
The figure depicts representative 40x magnification photomicrographs of formalin fixed, paraffin embedded and haematoxylin-eosin (H&E) stained 5 μm sections of lung tissue of guinea pigs intradermally inoculated with M. tuberculosis, MtbΔbioA or BCG and euthanized at (a) 1 week, (b) 3 weeks and (c) 6 weeks post inoculation. AS denotes alveolar spaces and G denotes granuloma. The graphical representation of the total granuloma score and granuloma fraction are shown alongside by box plot of 5 animals per group (the mean is represented by ‘+’, median value is denoted by horizontal line, box represents the inter quartile range and the minimum and maximum value is denoted by whiskers). While animals inoculated with M. tuberculosis showed increasing pathological damage, the animals infected with MtbΔbioA or BCG continued to exhibit no granulomatous pathology. The scale bars depict 200 μm. *p <0.05 (Mann-Whitney test, two-tailed).
Vaccination of guinea pigs with MtbΔbioA limits both pulmonary as well as extra-pulmonary multiplication of virulent M. tuberculosis
Having assessed the attenuation of the MtbΔbioA strain in guinea pigs via the aerosol as well as the intradermal route, we next evaluated the ability of this strain to induce protection against virulent M. tuberculosis challenge in guinea pig model of experimental TB. As shown in Fig 6a, guinea pigs were vaccinated intradermally (i.d.) with (i) BCG or (ii) MtbΔbioA. In addition, we also evaluated the effect of boosting MtbΔbioA with a second dose administered at 6-week interval (ΔbioA/ΔbioA). Guinea pigs were challenged with aerosolized virulent M. tuberculosis Erdman strain at 12 weeks post primary immunization and protective efficacy was evaluated four weeks post challenge. The sham-immunized guinea pigs showed the maximum infection with a bacillary load of 6.85 log10 CFU in the lungs and 5.66 log10 CFU in the spleen (Fig 6b and 6c). All the vaccinated groups demonstrated significant reduction in the bacillary load in the lungs and spleen, relative to bacterial burden in sham-immunized controls (Fig 6b and 6c). Vaccination with BCG resulted in the highest reduction in the bacterial load in the lungs (by 1.99 log10 CFU, p <0.0001) and in the spleen (by 3.114 log10 CFU, p <0.0001), when compared with sham-immunized controls. MtbΔbioA vaccinated animals exhibited efficient control over the multiplication of virulent M. tuberculosis in the lungs and significantly reduced the bacillary burden by 1.504 log10 CFU (p <0.0001) when compared with sham-immunized animals (Fig 6b). Additionally, vaccination with MtbΔbioA effectively controlled the dissemination of the challenge strain to the spleen resulting in significant reduction in bacillary burden (by 2.41 log10 CFU, p <0.0001) in the spleen when compared with the sham-immunized animals (Fig 6c). Importantly, even though vaccination with MtbΔbioA imparted protection that was less when compared with the protection imparted by BCG in both lungs and spleen, the difference was not statistically significant (Fig 6b and 6c). When animals were vaccinated with two doses of MtbΔbioA (ΔbioA/ΔbioA), the protection imparted was lower though not statistically different relative to single immunization (Fig 6b and 6c). Moreover, the protection imparted by ΔbioA/ΔbioA vaccine regimen was significantly poor when compared with BCG vaccinated animals in lungs as well as spleen (Fig 6b and 6c). Thus, our observations indicate that single administration of MtbΔbioA is able to efficiently control the multiplication of challenge strain at the primary site of infection as well as exhibit significant control on the hematogenous spread of virulent M. tuberculosis.
Fig 6. Evaluation of protective efficacy of MtbΔbioA in guinea pig model of experimental tuberculosis.
(a) Experimental protocol for assessing the protective efficacy of MtbΔbioA against infection with virulent M. tuberculosis in guinea pigs. Guinea pigs (n = 6) were vaccinated with BCG or a single dose of MtbΔbioA or two doses of MtbΔbioA administered at 6-week interval (ΔbioA/ ΔbioA). Vaccinated guinea pigs were challenged via the aerosol route with ~50 bacilli of virulent M. tuberculosis at 12 weeks from primary immunization and euthanized at 4 weeks post challenge. Sham-immunized animals were taken as control. (b, c) Enumeration of bacillary load in the lungs and spleen of vaccinated guinea pigs at 4 weeks post challenge. In both lungs and spleen, MtbΔbioA imparted significant protection over sham-immunized controls. Booster dose with MtbΔbioA failed to confer any improvement in protective efficacy imparted by single dose of MtbΔbioA. Each data point represents the log10 CFU/ organ for an individual animal and the bar depicts mean (±SE) for each group. **p <0.01 and ***p <0.001 (one-way ANOVA followed by Tukey’s multiple comparison test).
Vaccination with MtbΔbioA protects against pathological damage
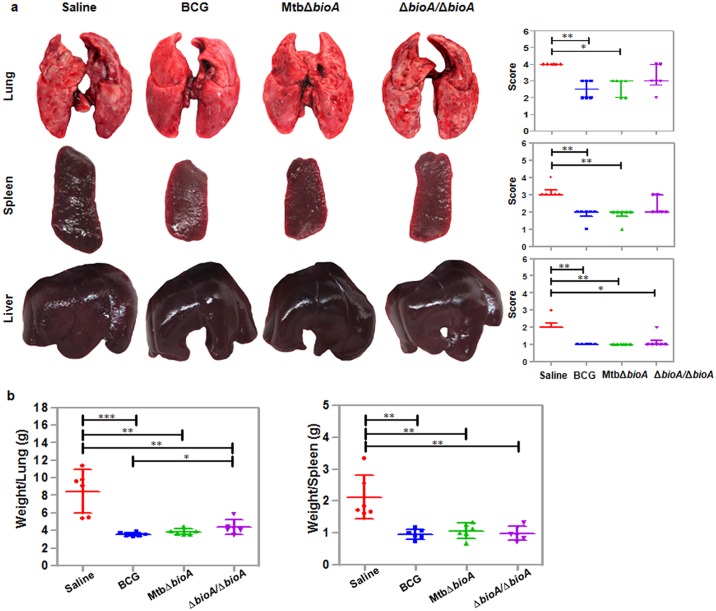
In addition to the bacillary load, pathological damage is an important read out for pronouncing the efficacy of a candidate vaccine. Upon gross pathological evaluation, the maximum damage was noted in sham-immunized animals with numerous small as well as large necrotic tubercles spread throughout the lungs (Fig 7a). Extensive extra-pulmonary damage was seen in the spleen and liver of sham-immunized animals with numerous small tubercles present throughout the organs and marked splenomegaly. BCG vaccinated animals showed significant reduction in pathological damage of lungs, spleen and liver when compared with sham-immunized animals (Fig 7a). Lungs of BCG vaccinated animals demonstrated occasional large and countable number of small tubercles while spleen appeared normal in size with a moderate number of small tubercles (Fig 7a). Importantly, MtbΔbioA vaccinated animals also showed significant reduction in gross pathological damage of lungs, spleen and liver when compared with the sham-immunized animals (Fig 7a). Lungs of MtbΔbioA vaccinated animals showed a countable number of discrete small and large sized tubercles and scanty coalescent tubercles. Gross pathological scores of spleens of MtbΔbioA vaccinated animals were similar to BCG vaccinated animals. Animals belonging to the ΔbioA/ΔbioA group showed maximum pathology among all the vaccinated groups of animals with numerous small and large tubercles in the lungs and moderate involvement of spleen with occasional large tubercles. Animals belonging to all the vaccinated groups showed minimal hepatic damage. The increase in weight of lungs and spleen is frequently associated with severity of disease in infected guinea pigs [54]. The mean organ (lungs and spleen) weights of guinea pigs belonging to all the three vaccinated groups were significantly lower than the mean organ weight of sham-immunized guinea pigs (Fig 7b). BCG and MtbΔbioA vaccinated animals demonstrated comparable mean lung weights. However, the mean lung weight of ΔbioA/ΔbioA vaccinated animals was significantly higher (p = 0.0367) as compared to BCG vaccinated animals (Fig 7b). The mean spleen weights of guinea pigs belonging to all the vaccinated groups were comparable (Fig 7b).
Fig 7. Evaluation of the gross pathological damage of the organs of vaccinated guinea pigs at 4 weeks post challenge.
(a) The figure depicts representative photographs of lungs, spleen and liver of guinea pigs belonging to different groups of vaccination at 4 weeks post challenge. Graphical representation of gross pathological scores assigned to the lungs, spleen and liver of guinea pigs euthanized at 4 weeks post challenge is represented alongside. Each data point represents the score assigned to an individual animal. The bar depicts median (± interquartile range) for each group. * p <0.05 and **p <0.01 (Kruskal-Wallis test followed by the Dunn's multiple comparison test). (b) The graphical representation of organ (lungs and spleen) weights of guinea pigs at 4 weeks post challenge. The bar depicts mean (± SD) for each group. * p <0.05, **p <0.01 and ***p <0.001 [unpaired (two-tailed) t-test].
Histopathological evaluation and granuloma scoring of the lungs and liver tissue of the experimental animals demonstrated that the organs from sham-immunized animals had the maximum total granuloma score with a large number of necrotic granulomas (Fig 8). Significant reduction in the total granuloma score was observed in the lungs of the animals belonging to BCG, MtbΔbioA and ΔbioA/ΔbioA vaccinated group when compared with the sham-immunized animals, indicating reduction in pulmonary granulomatous pathology (Fig 8b). The least total granuloma score of lungs was observed for the BCG vaccinated animals (Fig 8b). MtbΔbioA vaccinated animals exhibited significantly higher (p = 0.0173) average total granuloma score when compared with the animals vaccinated with BCG (Fig 8b). Importantly, however, no necrotic granulomas were seen in the lung sections of any of the animals vaccinated with MtbΔbioA, which was in contrast to that observed for BCG vaccinated animals and animals vaccinated with two doses of MtbΔbioA (Fig 8c). Evaluation of histopathological damage of the liver sections demonstrated that the maximum pathological damage of liver occurred in the sham-immunized animals (Fig 8d). BCG as well as MtbΔbioA vaccinated animals showed comparable hepatic total granuloma score which was significantly reduced (p = 0.005) when compared with sham-immunized animals (Fig 8d). ΔbioA/ΔbioA vaccinated animals showed marginally higher total granuloma score of the liver sections when compared with BCG or MtbΔbioA vaccinated animals.
Fig 8. Histopathological analysis of vaccinated animals at 4 weeks post challenge.
(a) The figure depicts representative 40x magnification photomicrographs of formalin fixed, paraffin embedded and haematoxylin-eosin (H&E) stained 5 μm sections of lung tissue of guinea pigs (n = 6) vaccinated with BCG, MtbΔbioA, two doses of MtbΔbioA (ΔbioA/ΔbioA) or saline and euthanized at 4 weeks post challenge. The scale bars depict 200 μm. AS and G denote alveolar spaces and granuloma, respectively. (b) The graphical representation of the total granuloma score of the lung sections is shown by box plot of 6 animals per group (the mean is represented by ‘+’, median value is denoted by horizontal line, box represents the inter quartile range and the minimum and maximum value is denoted by whiskers). *p <0.05 and **p <0.01 (Mann-Whitney test, two tailed). (c) The figure depicts the graphical representation of the number of granulomas that were scored in lung section from each animal in a group. The black region depicts the number of necrotic granulomas out of the total number of granulomas seen in lung section from each animal. (d) The graphical representation of the total granuloma score of the liver sections of 6 animals per group is shown by box plot (the mean is represented by ‘+’, median value is denoted by horizontal line, box represents the inter quartile range and the minimum and maximum value is denoted by whiskers). **p <0.01 (Mann-Whitney test, two tailed).
Moreover, in order to confirm that the vaccine strain does not get reactivated upon virulent M. tuberculosis infection and contribute towards the bacillary load observed at 4 weeks post challenge, all the lung and spleen homogenates from MtbΔbioA and ΔbioA/ΔbioA vaccinated groups were plated on agar media containing hygromycin. No bacilli were seen in any of the plates following incubation at 37°C for 3–4 weeks, thus confirming that reactivation of vaccine strain does not occur.
Discussion
Novel TB vaccines based on attenuated M. tuberculosis strains follow the Pasteurian approach where the vaccine strain is a modified form of the pathogen minus its disease-causing ability. Attenuated M. tuberculosis strains have shown promise as potential BCG replacement vaccines. In this study, we show that biotin biosynthesis is essential for the growth of M. tuberculosis in vitro as well as in guinea pig tissues. Further, we provide evidence that the attenuated biotin auxotroph of M. tuberculosis has the potential to generate protection against virulent M. tuberculosis challenge.
We generated a bioA gene mutant of M. tuberculosis and demonstrated its inability to grow in biotin deficient modified 7H9 media. The complementation of MtbΔbioA with the full-length bioA gene restored its ability to grow in the absence of external biotin supplementation. The MtbΔbioA strain was rapidly eliminated from the lungs and spleens of guinea pigs irrespective of the route of administration. Following aerosol infection with MtbΔbioA, no bacilli were recovered from the lungs at 6 weeks post infection and from the spleen at 12 weeks post infection. Limited survival of the biotin auxotroph in the host tissue was concomitantly associated with negligible granulomatous pathology. Additionally, following the intradermal administration of MtbΔbioA in guinea pigs, the bacillary numbers rapidly declined and were undetectable in the lungs by 3 weeks and in spleen by 6 weeks post inoculation. The in vivo growth kinetics of MtbΔbioA following intradermal administration was different from that observed for the standard TB vaccine strain, BCG. Importantly, however, no evidence of granulomatous pathology was observed for MtbΔbioA as well as BCG at any of the timepoints screened. These results indicate that the auxotrophic mutant MtbΔbioA either doesn’t have access to biotin available from the host or it is insufficient to support the growth of the mutant, thereby rendering it attenuated for growth and virulence in guinea pigs. Our attenuation studies in guinea pigs corroborate with earlier observations of attenuated phenotypes of M. smegmatis, M. marinum and M. tuberculosis bioA gene mutants in mice or zebrafish [26–28, 30]. Though mice and guinea pig both have been extensively used for virulence as well as vaccine evaluation studies against tuberculosis, various aspects of the disease are represented to varied levels in the different animal models of experimental tuberculosis [44]. Thus, the in vivo kinetics and attenuation of a strain may vary depending on the animal model of evaluation such as an attenuated M. tuberculosis mutant in SigH (MtbΔsigH) was shown to have contrasting replication capability in mice and primates [55, 56]. Our findings reconfirm the auxotrophic nature of MtbΔbioA mutant and are in agreement with the conclusions drawn by Park et al. who reported the inability of a bioA gene mutant of M. tuberculosis to grow in Sauton’s liquid media and also demonstrated the dependence of M. tuberculosis on biotin biosynthesis for its growth and survival following aerosol infection of mice [30]. Further detailed study of the biodistribution of the MtbΔbioA strain to organs such as lymph nodes, liver, kidney and brain (in addition to lungs and spleen) would help generate knowledge required for appropriate downstream development of this strain.
Importantly, since MtbΔbioA persists in the host tissue during the early phase of infection, especially in the spleen, we evaluated its ability to generate protection against virulent M. tuberculosis challenge. Although preliminary, our short-term vaccine evaluation study in guinea pigs demonstrated that the attenuated biotin auxotroph of M. tuberculosis has the potential to impart protection. MtbΔbioA vaccinated animals demonstrated significant reduction in the bacillary load in the lungs and spleen, when compared with sham-immunized animals. Vaccination with MtbΔbioA could not reduce the bacterial burden to the same extent as in animals vaccinated with BCG. However, the difference was not statistically significant. Importantly, as opposed to the other vaccinated groups, none of the animals vaccinated with a single dose of MtbΔbioA showed necrotic granulomas. Maximum necrosis was observed in sham-immunized animals. Necrotic granulomas have been associated with immunopathogenesis and necrosis can damage airway lining leading to bacterial transmission [57]. Thus, vaccination with a single dose of MtbΔbioA demonstrated the ability to protect against pathological damage. However, administration of booster dose of MtbΔbioA resulted in off-putting effects on the protection afforded by a single dose of MtbΔbioA. Similar observations have been noted in other studies wherein the repeat administration of mycobacteria resulted in the abrogation of protection [14, 58–60]. The explanation for such an observation seems to be derived from Koch phenomenon, wherein repeat exposure to mycobacteria or its antigens result in a tissue damaging heightened inflammatory response [58, 61].
Several studies have compared the protective capacities of live attenuated M. tuberculosis mutants with BCG and have demonstrated varying degrees of success. Vaccine candidates such as MTBVAC, showed significantly superior protection when compared with BCG in short-term evaluation in mice and was found to be safe and immunogenic in healthy adults volunteers [9, 17, 62, 63]. Vaccination with M. tuberculosis phoP mutant strain, the prototype for MTBVAC, resulted in protection similar to BCG in mice and guinea pig in short term studies while demonstrating significantly better protection than BCG in guinea pig survival assay. [11]. Recently, mucosal vaccination with a sigH mutant of M. tuberculosis was shown to generate high levels of protection in rhesus macaques, when compared with BCG [64]. On the other hand, certain attenuated vaccine constructs such as a M. tuberculosisΔpanCD, M. tuberculosisΔlysA, M. tuberculosisΔlysApanCD and M. tuberculosisΔleuDpanCD were found to confer protection comparable to BCG [12, 14, 15, 65]. Interestingly, correlation of in vivo persistence of an attenuated M. tuberculosis strain with its ability to impart protection remains largely unknown. M. tuberculosisΔpanCD mutant persists for months in vivo and the persistence of the strain is thought to contribute towards the generation of protection comparable to BCG [15]. On the other hand, a mutant like M. tuberculosisΔlysApanCD shows rapid decline in bacillary numbers in vivo but generates protection comparable to BCG [65]. For such highly attenuated strains the initial interaction of the vaccine antigens with the immune system appears to be important for generating appropriate response and conferring protection [65]. Our data on the MtbΔbioA strain highlight the vaccinogenic potential of highly attenuated M. tuberculosis strains against virulent M. tuberculosis challenge.
MtbΔbioA provides no greater protection than BCG in the short-term vaccine efficacy study in guinea pigs employed in this work. Short-term protective efficacy studies are considered discriminative, predictive of survival and enable quick screening of active/inactive strains especially when compared with unvaccinated animals [66]. However, more stringent and appropriately powered survival study would be critical to determine the efficacy of the MtbΔbioA strain as compared to BCG. Importantly, inspite of a short period of persistence in guinea pig tissue, MtbΔbioA demonstrated the ability to protect against virulent M. tuberculosis Erdman challenge. These results encourage further studies on the MtbΔbioA mutant. Potential strategies may include mucosal vaccination with MtbΔbioA. Recently, mucosal vaccination with MtbΔsigH was shown to induce strong central memory responses in the lungs resulting in the generation of significant protection from a lethal challenge in rhesus macaques [67]. Further, MtbΔbioA strain can be modified by overexpressing immunodominant antigens that might boost the immune response. In addition, it is recommended by the regulatory authorities that there should be at least two independent mutations for live attenuated M. tuberculosis based vaccines in order to minimize the probability of reversion to wild type phenotype [68]. MtbΔbioA strain can be further modified by introducing additional mutations to enhance its safety profile. Our efforts in this direction are in progress. Moreover, the protection imparted by MtbΔbioA may be enhanced by combining the attenuating biotin auxotrophy with mutations that impact pathways involved in immunomodulation. Inactivating the secA2 gene in M. tuberculosis has been shown to enhance T cell priming [10]. Combining highly attenuating lysine auxotrophy with secA2 mutation (M. tuberculosisΔsecA2ΔlysA) has been shown to result in a safe and immunogenic vaccine that imparts significantly higher protection when compared with BCG [69]. In this study, the MtbΔbioA strain has been shown to be protective against challenge with M. tuberculosis Erdman strain. The possibility of strain-specific response cannot be ruled out and it would be worthwhile to test its potential against challenge with clinical M. tuberculosis strains. Additionally, some of the promising attenuated M. tuberculosis mutants, such as MTBVAC and MtbΔsigH are derived from clinical isolates of M. tuberculosis and have been shown to be safe, immunogenic and efficacious [9, 56]. Thus, it would be interesting to employ biotin auxotrophy to construct attenuated strains of clinical M. tuberculosis isolates and evaluate their vaccine efficacy against TB. Nevertheless, this work highlights the potential of biotin auxotrophy as a promising starting point for the development of novel attenuated strains as vaccines against TB.
Supporting information
(a) Figure depicts the pictorial representation of the protocol of subsequent sub-culturing employed for the curing of plasmid pJV53 from MtbΔbioA. MtbΔbioA was sub-cultured in MB7H9 media in the absence of kanamycin and appropriate dilutions of tertiary culture were plated on MB7H11 agar without kanamycin and incubated at 37°C for 3–4 weeks. (b) Screening of colonies for the loss of pJV53. Individual colonies obtained on the agar plates were screened for the loss of pJV53 by patching on MB7H11 agar plates with or without kanamycin.
(TIF)
The figure depicts representative photographs of lungs, spleen and liver of guinea pigs following aerosol infection with M. tuberculosis, MtbΔbioA or MtbΔbioA.comp strains at (a) 3 weeks, (b) 6 weeks and (c)12 weeks post infection. Graphical representation of gross pathological scores assigned to the lungs, spleen and liver of each guinea pig at a particular time point is represented alongside. Each data point represents the score assigned to an individual animal. The bar depicts median(±interquartile range) for each group. *p <0.05 and **p <0.01 (Kruskal-Wallis test followed by Dunn's multiple comparison test). (d) The graphical representation of the organ (lungs and spleen) weights of guinea pigs at 3, 6 and 12 weeks post aerosol infection. Each data point represents the organ weight of an individual animal. The bar depicts mean (± SD) for each group. * p <0.05 and **p <0.01 [unpaired (two-tailed) t-test].
(TIF)
Acknowledgments
We thank Dr. Graham F. Hatfull and Dr. Julia C van Kessel, University of Pittsburgh, Pennsylvania, United States of America for providing reagents for the recombineering method. We thank Dr. Dirk Schnappinger and Dr. Sabine Ehrt, Weill Cornell Medical College, New York, United States of America for providing us with anti-BioA antibodies. We thank Dr. Ashok Mukherjee for helping with histopathological analysis. DBT-supported Distributed Information Sub-Centre, Department of Biochemistry, University of Delhi South Campus, New Delhi, is acknowledged for providing computational facilities. We acknowledge DBT-supported DNA sequencing Facility, Department of Biochemistry, University of Delhi South Campus, New Delhi. We are thankful to Tannupriya Gosain for helping with the guinea pig experiments. Technical assistance provided by Priti Singh is highly acknowledged. Lab attendants Bahadur Singh, Devender and Jeevan are acknowledged for their assistance. We acknowledge Dr. Garima Khare, Dr. Priyanka Chauhan, Dr, Shingar Sharma and Akshay Rohilla for critical reading of the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by financial grant from Department of Biotechnology, Ministry of Science and Technology, Government of India (Grant Number – BT/01/COE/05/06-II). RK and PN are grateful to Council of Scientific and Industrial Research (CSIR), India, for research fellowships. SM is grateful to Department of Biotechnology, Government of India for research fellowship. SS is grateful to Indian Council of Medical Research (ICMR), India for research fellowship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rodrigues LC, Diwan VK, Wheeler JG. Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis. Int J Epidemiol. 1993;22(6):1154–8. Epub 1993/12/01. . [DOI] [PubMed] [Google Scholar]
- 2.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, et al. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271(9):698–702. . [PubMed] [Google Scholar]
- 3.Fine PE. Variation in protection by BCG: implications of and for heterologous immunity. Lancet. 1995;346(8986):1339–45. . [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Doherty TM. The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat Rev Microbiol. 2005;3(8):656–62. doi: 10.1038/nrmicro1211 . [DOI] [PubMed] [Google Scholar]
- 5.Aronson JD, Aronson CF, Taylor HC. A twenty-year appraisal of BCG vaccination in the control of tuberculosis. AMA Arch Intern Med. 1958;101(5):881–93. . [DOI] [PubMed] [Google Scholar]
- 6.Ginsberg AM, Ruhwald M, Mearns H, McShane H. TB vaccines in clinical development. Tuberculosis (Edinb). 2016;99 Suppl 1:S16–20. Epub 2016/07/30. doi: 10.1016/j.tube.2016.05.013 . [DOI] [PubMed] [Google Scholar]
- 7.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet. 2013;381(9871):1021–8. doi: 10.1016/S0140-6736(13)60177-4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Zhang Y, Zheng H, Pan Y, Liu H, Du P, et al. Genome sequencing and analysis of BCG vaccine strains. PLoS One. 2013;8(8):e71243 doi: 10.1371/journal.pone.0071243 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arbues A, Aguilo JI, Gonzalo-Asensio J, Marinova D, Uranga S, Puentes E, et al. Construction, characterization and preclinical evaluation of MTBVAC, the first live-attenuated M. tuberculosis-based vaccine to enter clinical trials. Vaccine. 2013;31(42):4867–73. doi: 10.1016/j.vaccine.2013.07.051 . [DOI] [PubMed] [Google Scholar]
- 10.Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM, Chen B, et al. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest. 2007;117(8):2279–88. doi: 10.1172/JCI31947 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin C, Williams A, Hernandez-Pando R, Cardona PJ, Gormley E, Bordat Y, et al. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006;24(17):3408–19. doi: 10.1016/j.vaccine.2006.03.017 . [DOI] [PubMed] [Google Scholar]
- 12.Pavelka MS Jr., Chen B, Kelley CL, Collins FM, Jacobs Jr WR Jr. Vaccine efficacy of a lysine auxotroph of Mycobacterium tuberculosis. Infect Immun. 2003;71(7):4190–2. doi: 10.1128/IAI.71.7.4190-4192.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sambandamurthy VK, Derrick SC, Hsu T, Chen B, Larsen MH, Jalapathy KV, et al. Mycobacterium tuberculosis DeltaRD1 DeltapanCD: a safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine. 2006;24(37–39):6309–20. doi: 10.1016/j.vaccine.2006.05.097 . [DOI] [PubMed] [Google Scholar]
- 14.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR Jr., Bloom BR, et al. Protection elicited by a double leucine and pantothenate auxotroph of Mycobacterium tuberculosis in guinea pigs. Infect Immun. 2004;72(5):3031–7. doi: 10.1128/IAI.72.5.3031-3037.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambandamurthy VK, Wang X, Chen B, Russell RG, Derrick S, Collins FM, et al. A pantothenate auxotroph of Mycobacterium tuberculosis is highly attenuated and protects mice against tuberculosis. Nat Med. 2002;8(10):1171–4. doi: 10.1038/nm765 . [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Jeon BY, Bardarov S, Chen M, Morris SL, Jacobs WR Jr. Protection elicited by two glutamine auxotrophs of Mycobacterium tuberculosis and in vivo growth phenotypes of the four unique glutamine synthetase mutants in a murine model. Infect Immun. 2006;74(11):6491–5. doi: 10.1128/IAI.00531-06 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spertini F, Audran R, Chakour R, Karoui O, Steiner-Monard V, Thierry AC, et al. Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. Lancet Respir Med. 2015;3(12):953–62. doi: 10.1016/S2213-2600(15)00435-X . [DOI] [PubMed] [Google Scholar]
- 18.Dey S, Lane JM, Lee RE, Rubin EJ, Sacchettini JC. Structural characterization of the Mycobacterium tuberculosis biotin biosynthesis enzymes 7,8-diaminopelargonic acid synthase and dethiobiotin synthetase. Biochemistry. 2010;49(31):6746–60. doi: 10.1021/bi902097j . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salaemae W, Azhar A, Booker GW, Polyak SW. Biotin biosynthesis in Mycobacterium tuberculosis: physiology, biochemistry and molecular intervention. Protein Cell. 2011;2(9):691–5. doi: 10.1007/s13238-011-1100-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Said HM. Cell and molecular aspects of human intestinal biotin absorption. J Nutr. 2009;139(1):158–62. doi: 10.3945/jn.108.092023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoner GL, Eisenberg MA. Purification and properties of 7, 8-diaminopelargonic acid aminotransferase. J Biol Chem. 1975;250(11):4029–36. . [PubMed] [Google Scholar]
- 22.Schneider G, Lindqvist Y. Structural enzymology of biotin biosynthesis. FEBS Lett. 2001;495(1–2):7–11. . [DOI] [PubMed] [Google Scholar]
- 23.Dai R, Wilson DJ, Geders TW, Aldrich CC, Finzel BC. Inhibition of Mycobacterium tuberculosis transaminase BioA by aryl hydrazines and hydrazides. Chembiochem. 2014;15(4):575–86. doi: 10.1002/cbic.201300748 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann S, Colliandre L, Labesse G, Ploux O. Inhibition of 7,8-diaminopelargonic acid aminotransferase from Mycobacterium tuberculosis by chiral and achiral anologs of its substrate: biological implications. Biochimie. 2009;91(7):826–34. doi: 10.1016/j.biochi.2009.03.019 . [DOI] [PubMed] [Google Scholar]
- 25.Mann S, Marquet A, Ploux O. Inhibition of 7,8-diaminopelargonic acid aminotransferase by amiclenomycin and analogues. Biochem Soc Trans. 2005;33(Pt 4):802–5. doi: 10.1042/BST0330802 . [DOI] [PubMed] [Google Scholar]
- 26.Keer J, Smeulders MJ, Gray KM, Williams HD. Mutants of Mycobacterium smegmatis impaired in stationary-phase survival. Microbiology. 2000;146 (Pt 9):2209–17. doi: 10.1099/00221287-146-9-2209 . [DOI] [PubMed] [Google Scholar]
- 27.Sassetti CM, Rubin EJ. Genetic requirements for mycobacterial survival during infection. Proc Natl Acad Sci U S A. 2003;100(22):12989–94. doi: 10.1073/pnas.2134250100 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu J, Niu C, Wang D, Li M, Teo W, Sun G, et al. MMAR_2770, a new enzyme involved in biotin biosynthesis, is essential for the growth of Mycobacterium marinum in macrophages and zebrafish. Microbes Infect. 2011;13(1):33–41. doi: 10.1016/j.micinf.2010.08.010 . [DOI] [PubMed] [Google Scholar]
- 29.Rodionov DA, Mironov AA, Gelfand MS. Conservation of the biotin regulon and the BirA regulatory signal in Eubacteria and Archaea. Genome Res. 2002;12(10):1507–16. doi: 10.1101/gr.314502 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woong Park S, Klotzsche M, Wilson DJ, Boshoff HI, Eoh H, Manjunatha U, et al. Evaluating the sensitivity of Mycobacterium tuberculosis to biotin deprivation using regulated gene expression. PLoS Pathog. 2011;7(9):e1002264 doi: 10.1371/journal.ppat.1002264 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medina E, North RJ. Resistance ranking of some common inbred mouse strains to Mycobacterium tuberculosis and relationship to major histocompatibility complex haplotype and Nramp1 genotype. Immunology. 1998;93(2):270–4. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dascher CC, Hiromatsu K, Naylor JW, Brauer PP, Brown KA, Storey JR, et al. Conservation of a CD1 multigene family in the guinea pig. J Immunol. 1999;163(10):5478–88. . [PubMed] [Google Scholar]
- 33.Dascher CC, Brenner MB. Evolutionary constraints on CD1 structure: insights from comparative genomic analysis. Trends Immunol. 2003;24(8):412–8. . [DOI] [PubMed] [Google Scholar]
- 34.Rhoades ER, Frank AA, Orme IM. Progression of chronic pulmonary tuberculosis in mice aerogenically infected with virulent Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78(1):57–66. . [DOI] [PubMed] [Google Scholar]
- 35.Tsai MC, Chakravarty S, Zhu G, Xu J, Tanaka K, Koch C, et al. Characterization of the tuberculous granuloma in murine and human lungs: cellular composition and relative tissue oxygen tension. Cell Microbiol. 2006;8(2):218–32. doi: 10.1111/j.1462-5822.2005.00612.x . [DOI] [PubMed] [Google Scholar]
- 36.Williams A, Hatch GJ, Clark SO, Gooch KE, Hatch KA, Hall GA, et al. Evaluation of vaccines in the EU TB Vaccine Cluster using a guinea pig aerosol infection model of tuberculosis. Tuberculosis (Edinb). 2005;85(1–2):29–38. doi: 10.1016/j.tube.2004.09.009 . [DOI] [PubMed] [Google Scholar]
- 37.Smith DA, Parish T, Stoker NG, Bancroft GJ. Characterization of auxotrophic mutants of Mycobacterium tuberculosis and their potential as vaccine candidates. Infect Immun. 2001;69(2):1142–50. doi: 10.1128/IAI.69.2.1142-1150.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baldwin SL, D'Souza C, Roberts AD, Kelly BP, Frank AA, Lui MA, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infect Immun. 1998;66(6):2951–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta UD, Katoch VM. Animal models of tuberculosis. Tuberculosis (Edinb). 2005;85(5–6):277–93. doi: 10.1016/j.tube.2005.08.008 . [DOI] [PubMed] [Google Scholar]
- 40.Smith DW, McMurray DN, Wiegeshaus EH, Grover AA, Harding GE. Host-parasite relationships in experimental airborne tuberculosis. IV. Early events in the course of infection in vaccinated and nonvaccinated guinea pigs. Am Rev Respir Dis. 1970;102(6):937–49. doi: 10.1164/arrd.1970.102.6.937 . [DOI] [PubMed] [Google Scholar]
- 41.Smith DW, Harding GE. Animal model of human disease. Pulmonary tuberculosis. Animal model: Experimental airborne tuberculosis in the guinea pig. Am J Pathol. 1977;89(1):273–6. . [PMC free article] [PubMed] [Google Scholar]
- 42.Ordway D, Palanisamy G, Henao-Tamayo M, Smith EE, Shanley C, Orme IM, et al. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. J Immunol. 2007;179(4):2532–41. . [DOI] [PubMed] [Google Scholar]
- 43.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, et al. Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76(6):2333–40. doi: 10.1128/IAI.01515-07 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McMurray DN. Disease model: pulmonary tuberculosis. Trends Mol Med. 2001;7(3):135–7. Epub 2001/04/05. . [DOI] [PubMed] [Google Scholar]
- 45.Padilla-Carlin DJ, McMurray DN, Hickey AJ. The guinea pig as a model of infectious diseases. Comp Med. 2008;58(4):324–40. . [PMC free article] [PubMed] [Google Scholar]
- 46.Reddy PV, Puri RV, Khera A, Tyagi AK. Iron storage proteins are essential for the survival and pathogenesis of Mycobacterium tuberculosis in THP-1 macrophages and the guinea pig model of infection. J Bacteriol. 2012;194(3):567–75. doi: 10.1128/JB.05553-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Kessel JC, Hatfull GF. Recombineering in Mycobacterium tuberculosis. Nat Methods. 2007;4(2):147–52. doi: 10.1038/nmeth996 . [DOI] [PubMed] [Google Scholar]
- 48.Bardarov S, Bardarov Jr S Jr., Pavelka Jr MS Jr., Sambandamurthy V, Larsen M, Tufariello J, et al. Specialized transduction: an efficient method for generating marked and unmarked targeted gene disruptions in Mycobacterium tuberculosis, M. bovis BCG and M. smegmatis. Microbiology. 2002;148(Pt 10):3007–17. doi: 10.1099/00221287-148-10-3007 . [DOI] [PubMed] [Google Scholar]
- 49.DasGupta SK, Jain S, Kaushal D, Tyagi AK. Expression systems for study of mycobacterial gene regulation and development of recombinant BCG vaccines. Biochem Biophys Res Commun. 1998;246(3):797–804. Epub 1998/06/10. doi: 10.1006/bbrc.1998.8724 . [DOI] [PubMed] [Google Scholar]
- 50.Dhar N, Rao V, Tyagi AK. Recombinant BCG approach for development of vaccines: cloning and expression of immunodominant antigens of M. tuberculosis. FEMS Microbiol Lett. 2000;190(2):309–16. Epub 2000/10/18. . [DOI] [PubMed] [Google Scholar]
- 51.Reddy PV, Puri RV, Chauhan P, Kar R, Rohilla A, Khera A, et al. Disruption of mycobactin biosynthesis leads to attenuation of Mycobacterium tuberculosis for growth and virulence. J Infect Dis. 2013;208(8):1255–65. doi: 10.1093/infdis/jit250 . [DOI] [PubMed] [Google Scholar]
- 52.Jain R, Dey B, Dhar N, Rao V, Singh R, Gupta UD, et al. Enhanced and enduring protection against tuberculosis by recombinant BCG-Ag85C and its association with modulation of cytokine profile in lung. PLoS One. 2008;3(12):e3869 doi: 10.1371/journal.pone.0003869 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lasco TM, Cassone L, Kamohara H, Yoshimura T, McMurray DN. Evaluating the role of tumor necrosis factor-alpha in experimental pulmonary tuberculosis in the guinea pig. Tuberculosis (Edinb). 2005;85(4):245–58. doi: 10.1016/j.tube.2005.01.001 . [DOI] [PubMed] [Google Scholar]
- 54.Klinkenberg LG, Lee JH, Bishai WR, Karakousis PC. The stringent response is required for full virulence of Mycobacterium tuberculosis in guinea pigs. J Infect Dis. 2010;202(9):1397–404. Epub 2010/09/25. doi: 10.1086/656524 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mehra S, Golden NA, Stuckey K, Didier PJ, Doyle LA, Russell-Lodrigue KE, et al. The Mycobacterium tuberculosis stress response factor SigH is required for bacterial burden as well as immunopathology in primate lungs. J Infect Dis. 2012;205(8):1203–13. Epub 2012/03/10. doi: 10.1093/infdis/jis102 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaushal D, Schroeder BG, Tyagi S, Yoshimatsu T, Scott C, Ko C, et al. Reduced immunopathology and mortality despite tissue persistence in a Mycobacterium tuberculosis mutant lacking alternative sigma factor, SigH. Proc Natl Acad Sci U S A. 2002;99(12):8330–5. Epub 2002/06/13. doi: 10.1073/pnas.102055799 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F. Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol. 2009;10(9):943–8. doi: 10.1038/ni.1781 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moreira AL, Tsenova L, Aman MH, Bekker LG, Freeman S, Mangaliso B, et al. Mycobacterial antigens exacerbate disease manifestations in Mycobacterium tuberculosis-infected mice. Infect Immun. 2002;70(4):2100–7. doi: 10.1128/IAI.70.4.2100-2107.2002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Basaraba RJ, Izzo AA, Brandt L, Orme IM. Decreased survival of guinea pigs infected with Mycobacterium tuberculosis after multiple BCG vaccinations. Vaccine. 2006;24(3):280–6. doi: 10.1016/j.vaccine.2005.07.103 . [DOI] [PubMed] [Google Scholar]
- 60.Taylor JL, Turner OC, Basaraba RJ, Belisle JT, Huygen K, Orme IM. Pulmonary necrosis resulting from DNA vaccination against tuberculosis. Infect Immun. 2003;71(4):2192–8. doi: 10.1128/IAI.71.4.2192-2198.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Koch R. A Further Communication on a Remedy for Tuberculosis. Br Med J. 1891;1(1568):125–7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geldmacher C, Hatherill M. First-in-human trial of a live-attenuated Mycobacterium tuberculosis vaccine. Lancet Respir Med. 2015;3(12):906–7. Epub 2015/11/26. doi: 10.1016/S2213-2600(15)00474-9 . [DOI] [PubMed] [Google Scholar]
- 63.Aguilo N, Uranga S, Marinova D, Monzon M, Badiola J, Martin C. MTBVAC vaccine is safe, immunogenic and confers protective efficacy against Mycobacterium tuberculosis in newborn mice. Tuberculosis (Edinb). 2016;96:71–4. Epub 2016/01/21. doi: 10.1016/j.tube.2015.10.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaushal D, Foreman TW, Gautam US, Alvarez X, Adekambi T, Rangel-Moreno J, et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun. 6:8533 Epub 2015/10/16. doi: 10.1038/ncomms9533 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sambandamurthy VK, Derrick SC, Jalapathy KV, Chen B, Russell RG, Morris SL, et al. Long-term protection against tuberculosis following vaccination with a severely attenuated double lysine and pantothenate auxotroph of Mycobacterium tuberculosis. Infect Immun. 2005;73(2):1196–203. doi: 10.1128/IAI.73.2.1196-1203.2005 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Williams A, Hall Y, Orme IM. Evaluation of new vaccines for tuberculosis in the guinea pig model. Tuberculosis (Edinb). 2009;89(6):389–97. Epub 2009/10/10. doi: 10.1016/j.tube.2009.08.004 . [DOI] [PubMed] [Google Scholar]
- 67.Kaushal D, Foreman TW, Gautam US, Alvarez X, Adekambi T, Rangel-Moreno J, et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun. 2015;6:8533 doi: 10.1038/ncomms9533 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kamath AT, Fruth U, Brennan MJ, Dobbelaer R, Hubrechts P, Ho MM, et al. New live mycobacterial vaccines: the Geneva consensus on essential steps towards clinical development. Vaccine. 2005;23(29):3753–61. doi: 10.1016/j.vaccine.2005.03.001 . [DOI] [PubMed] [Google Scholar]
- 69.Hinchey J, Jeon BY, Alley H, Chen B, Goldberg M, Derrick S, et al. Lysine auxotrophy combined with deletion of the SecA2 gene results in a safe and highly immunogenic candidate live attenuated vaccine for tuberculosis. PLoS One. 2011;6(1):e15857 Epub 2011/01/26. doi: 10.1371/journal.pone.0015857 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a) Figure depicts the pictorial representation of the protocol of subsequent sub-culturing employed for the curing of plasmid pJV53 from MtbΔbioA. MtbΔbioA was sub-cultured in MB7H9 media in the absence of kanamycin and appropriate dilutions of tertiary culture were plated on MB7H11 agar without kanamycin and incubated at 37°C for 3–4 weeks. (b) Screening of colonies for the loss of pJV53. Individual colonies obtained on the agar plates were screened for the loss of pJV53 by patching on MB7H11 agar plates with or without kanamycin.
(TIF)
The figure depicts representative photographs of lungs, spleen and liver of guinea pigs following aerosol infection with M. tuberculosis, MtbΔbioA or MtbΔbioA.comp strains at (a) 3 weeks, (b) 6 weeks and (c)12 weeks post infection. Graphical representation of gross pathological scores assigned to the lungs, spleen and liver of each guinea pig at a particular time point is represented alongside. Each data point represents the score assigned to an individual animal. The bar depicts median(±interquartile range) for each group. *p <0.05 and **p <0.01 (Kruskal-Wallis test followed by Dunn's multiple comparison test). (d) The graphical representation of the organ (lungs and spleen) weights of guinea pigs at 3, 6 and 12 weeks post aerosol infection. Each data point represents the organ weight of an individual animal. The bar depicts mean (± SD) for each group. * p <0.05 and **p <0.01 [unpaired (two-tailed) t-test].
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.