Abstract
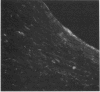
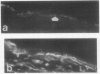
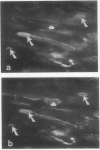
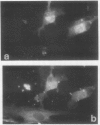
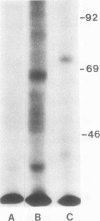
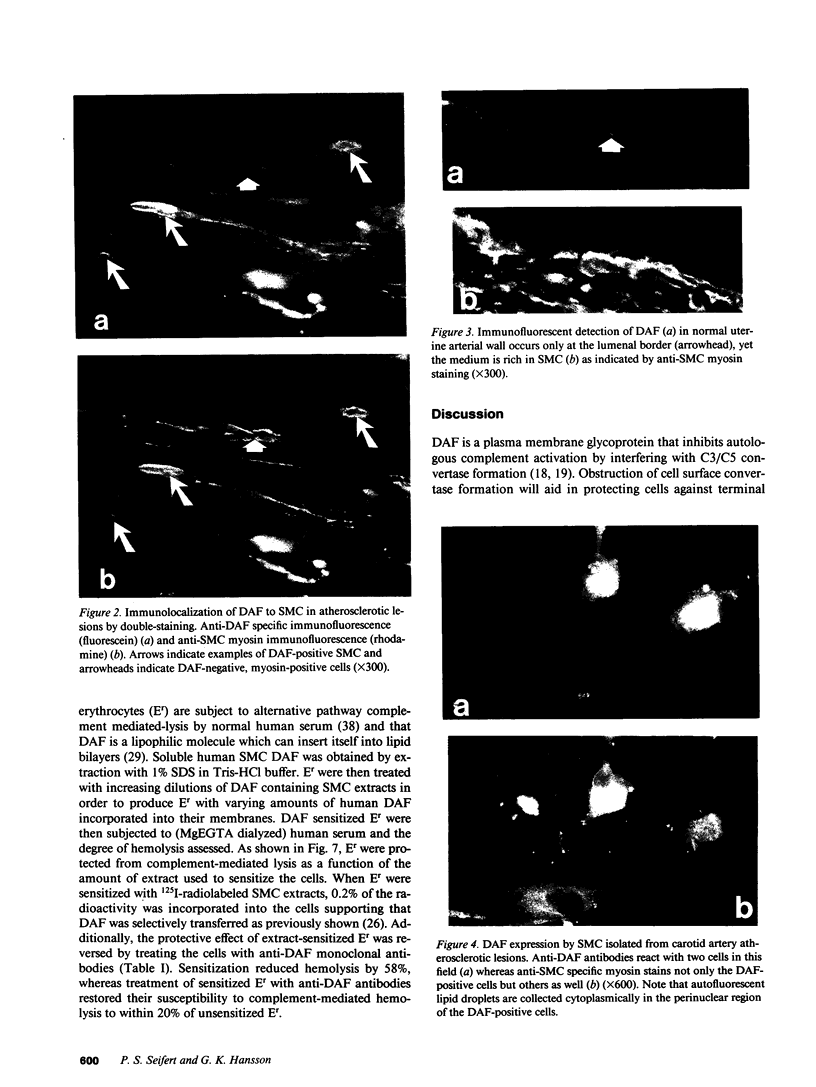
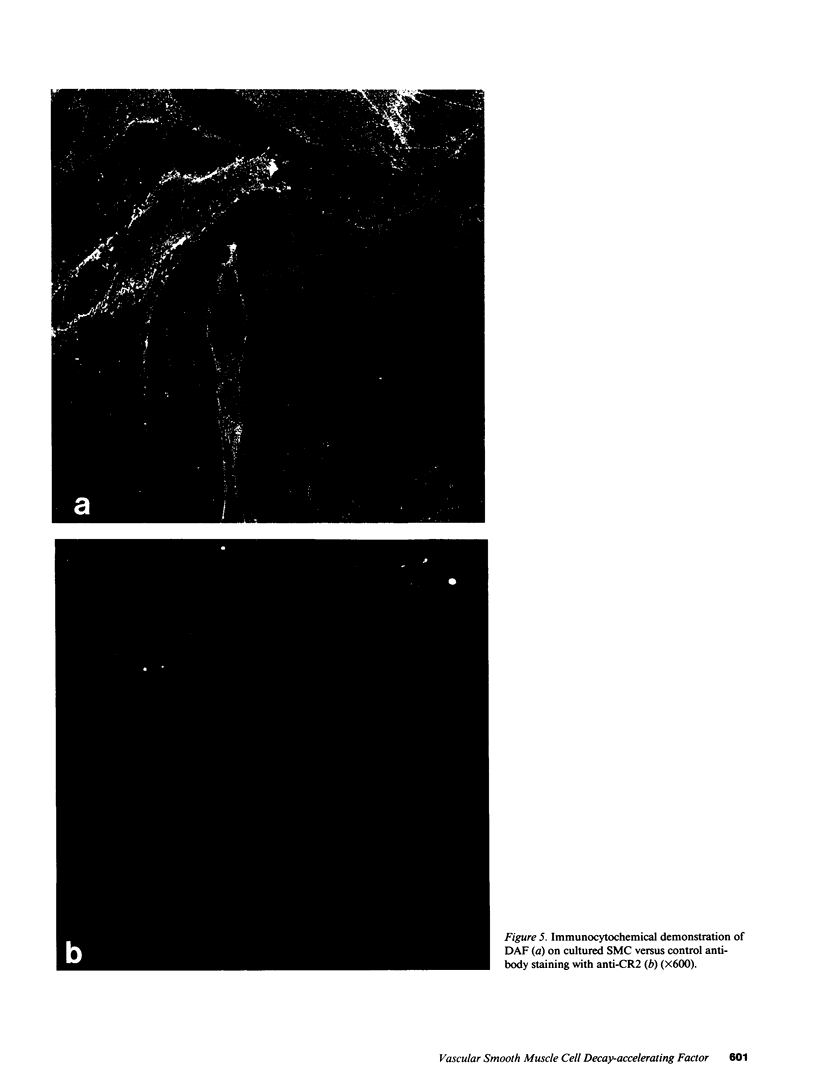
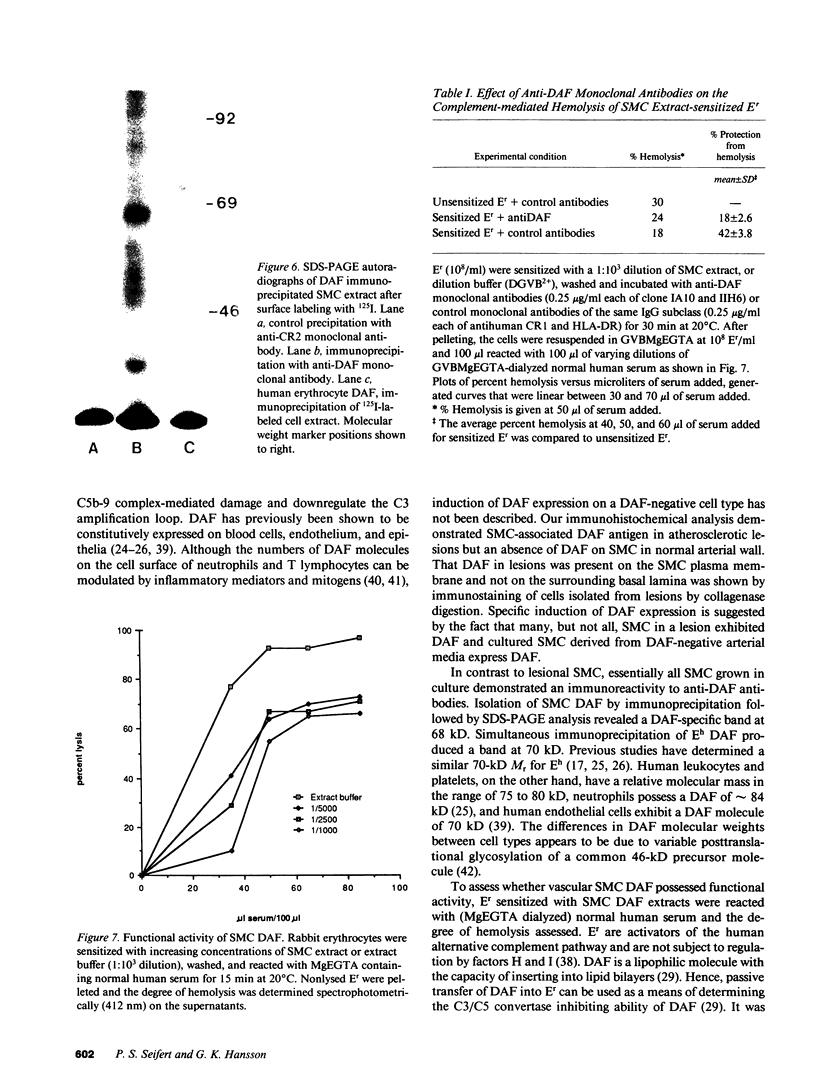
Decay-accelerating factor (DAF) is a constitutively expressed plasma membrane glycoprotein on blood cells and endothelium that inhibits cell surface C3/C5 convertase formation, thus inhibiting complement activation and protecting cells from lysis by the terminal complement components. Using monoclonal anti-DAF antibodies in conjunction with anti-smooth muscle cell (SMC)-specific myosin antibodies, it was found by immunohistochemistry that vascular SMC in advanced human carotid atherosclerotic lesions express DAF antigen. The percentage of DAF-positive SMC ranged from 20 to 60% between different patient samples and SMC DAF expression was limited to SMC in the lesion proper. Normal arterial wall SMC exhibited no DAF-specific immunostaining. Essentially 100% of passaged cultured vascular SMC derived from normal human uterine artery, or from umbilical vein, expressed DAF as assessed by immunocytochemistry. A 68-kD band was observed on SDS-PAGE autoradiograms of DAF-immunoprecipitated radiolabeled cultured SMC extracts. Sensitization of rabbit erythrocytes with DAF-containing SMC extracts conferred protection against complement-mediated hemolysis in normal human serum and the protective effect could be reversed by treatment with anti-DAF antibodies. We conclude that DAF is induced on vascular SMC during atherogenesis and in culture.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asch A. S., Kinoshita T., Jaffe E. A., Nussenzweig V. Decay-accelerating factor is present on cultured human umbilical vein endothelial cells. J Exp Med. 1986 Jan 1;163(1):221–226. doi: 10.1084/jem.163.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Adler S., Yang Y., Couser W. G. Complement activation by heat-killed human kidney cells: formation, activity, and stabilization of cell-bound C3 convertases. J Immunol. 1984 Aug;133(2):877–881. [PubMed] [Google Scholar]
- Berger M., Medof M. E. Increased expression of complement decay-accelerating factor during activation of human neutrophils. J Clin Invest. 1987 Jan;79(1):214–220. doi: 10.1172/JCI112786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocan T. M., Guyton J. R. Human aortic fibrolipid lesions. Progenitor lesions for fibrous plaques, exhibiting early formation of the cholesterol-rich core. Am J Pathol. 1985 Aug;120(2):193–206. [PMC free article] [PubMed] [Google Scholar]
- Cole J. L., Housley G. A., Jr, Dykman T. R., MacDermott R. P., Atkinson J. P. Identification of an additional class of C3-binding membrane proteins of human peripheral blood leukocytes and cell lines. Proc Natl Acad Sci U S A. 1985 Feb;82(3):859–863. doi: 10.1073/pnas.82.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosio F. G., Zager R. A., Sharma H. M. Atheroembolic renal disease causes hypocomplementaemia. Lancet. 1985 Jul 20;2(8447):118–121. doi: 10.1016/s0140-6736(85)90225-9. [DOI] [PubMed] [Google Scholar]
- Davis L. S., Patel S. S., Atkinson J. P., Lipsky P. E. Decay-accelerating factor functions as a signal transducing molecule for human T cells. J Immunol. 1988 Oct 1;141(7):2246–2252. [PubMed] [Google Scholar]
- Fager G., Hansson G. K., Ottosson P., Dahllöf B., Bondjers G. Human arterial smooth muscle cells in culture. Effects of platelet-derived growth factor and heparin on growth in vitro. Exp Cell Res. 1988 Jun;176(2):319–335. doi: 10.1016/0014-4827(88)90334-5. [DOI] [PubMed] [Google Scholar]
- Fearon D. T., Austen K. F. Activation of the alternative complement pathway with rabbit erythrocytes by circumvention of the regulatory action of endogenous control proteins. J Exp Med. 1977 Jul 1;146(1):22–33. doi: 10.1084/jem.146.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fearon D. T. Regulation of the amplification C3 convertase of human complement by an inhibitory protein isolated from human erythrocyte membrane. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5867–5871. doi: 10.1073/pnas.76.11.5867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Inoue T., Ogawa K., Iida K., Tamura N. The mechanism of action of decay-accelerating factor (DAF). DAF inhibits the assembly of C3 convertases by dissociating C2a and Bb. J Exp Med. 1987 Nov 1;166(5):1221–1228. doi: 10.1084/jem.166.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt D. E., Greenberg C. S., Yamada O., Craddock P. R., Jacob H. S. Cholesterol and atheroma lipids activate complement and stimulate granulocytes. A possible mechanism for amplification of ischemic injury in atherosclerotic states. J Lab Clin Med. 1981 Jul;98(1):68–77. [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Kral J. G. Accumulation of IgG and complement factor C3 in human arterial endothelium and atherosclerotic lesions. Acta Pathol Microbiol Immunol Scand A. 1984 Nov;92(6):429–435. doi: 10.1111/j.1699-0463.1984.tb04424.x. [DOI] [PubMed] [Google Scholar]
- Hansson G. K., Lagerstedt E., Bengtsson A., Heideman M. IgG binding to cytoskeletal intermediate filaments activates the complement cascade. Exp Cell Res. 1987 Jun;170(2):338–350. doi: 10.1016/0014-4827(87)90311-9. [DOI] [PubMed] [Google Scholar]
- Iida K., Nussenzweig V. Complement receptor is an inhibitor of the complement cascade. J Exp Med. 1981 May 1;153(5):1138–1150. doi: 10.1084/jem.153.5.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Gabbiani G., Hansson G. K. Expression of class II transplantation antigen on vascular smooth muscle cells in human atherosclerosis. J Clin Invest. 1985 Jul;76(1):125–131. doi: 10.1172/JCI111934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Nussenzweig V. Endogenous association of decay-accelerating factor (DAF) with C4b and C3b on cell membranes. J Immunol. 1986 May 1;136(9):3390–3395. [PubMed] [Google Scholar]
- Kinoshita T., Medof M. E., Silber R., Nussenzweig V. Distribution of decay-accelerating factor in the peripheral blood of normal individuals and patients with paroxysmal nocturnal hemoglobinuria. J Exp Med. 1985 Jul 1;162(1):75–92. doi: 10.1084/jem.162.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacsovics T., Tschopp J., Kress A., Isliker H. Antibody-independent activation of C1, the first component of complement, by cardiolipin. J Immunol. 1985 Oct;135(4):2695–2700. [PubMed] [Google Scholar]
- Larson D. M., Fujiwara K., Alexander R. W., Gimbrone M. A., Jr Heterogeneity of myosin antigenic expression in vascular smooth muscle in vivo. Lab Invest. 1984 Apr;50(4):401–407. [PubMed] [Google Scholar]
- Lublin D. M., Krsek-Staples J., Pangburn M. K., Atkinson J. P. Biosynthesis and glycosylation of the human complement regulatory protein decay-accelerating factor. J Immunol. 1986 Sep 1;137(5):1629–1635. [PubMed] [Google Scholar]
- McManus L. M., Kolb W. P., Crawford M. H., O'Rourke R. A., Grover F. L., Pinckard R. N. Complement localization in ischemic baboon myocardium. Lab Invest. 1983 Apr;48(4):436–447. [PubMed] [Google Scholar]
- Medof M. E., Kinoshita T., Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. J Exp Med. 1984 Nov 1;160(5):1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof M. E., Walter E. I., Rutgers J. L., Knowles D. M., Nussenzweig V. Identification of the complement decay-accelerating factor (DAF) on epithelium and glandular cells and in body fluids. J Exp Med. 1987 Mar 1;165(3):848–864. doi: 10.1084/jem.165.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson-Weller A., Burge J., Fearon D. T., Weller P. F., Austen K. F. Isolation of a human erythrocyte membrane glycoprotein with decay-accelerating activity for C3 convertases of the complement system. J Immunol. 1982 Jul;129(1):184–189. [PubMed] [Google Scholar]
- Nicholson-Weller A., March J. P., Rosen C. E., Spicer D. B., Austen K. F. Surface membrane expression by human blood leukocytes and platelets of decay-accelerating factor, a regulatory protein of the complement system. Blood. 1985 May;65(5):1237–1244. [PubMed] [Google Scholar]
- Niculescu F., Hugo F., Rus H. G., Vlaicu R., Bhakdi S. Quantitative evaluation of the terminal C5b-9 complement complex by ELISA in human atherosclerotic arteries. Clin Exp Immunol. 1987 Aug;69(2):477–483. [PMC free article] [PubMed] [Google Scholar]
- Niculescu F., Rus H. G., Vlaicu R. Immunohistochemical localization of C5b-9, S-protein, C3d and apolipoprotein B in human arterial tissues with atherosclerosis. Atherosclerosis. 1987 May;65(1-2):1–11. doi: 10.1016/0021-9150(87)90002-5. [DOI] [PubMed] [Google Scholar]
- Ross R., Wight T. N., Strandness E., Thiele B. Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol. 1984 Jan;114(1):79–93. [PMC free article] [PubMed] [Google Scholar]
- Rosse W. F. The control of complement activation by the blood cells in paroxysmal nocturnal hemoglobinuria. Blood. 1986 Feb;67(2):268–269. [PubMed] [Google Scholar]
- Schäfer H., Mathey D., Hugo F., Bhakdi S. Deposition of the terminal C5b-9 complement complex in infarcted areas of human myocardium. J Immunol. 1986 Sep 15;137(6):1945–1949. [PubMed] [Google Scholar]
- Seifert P. S., Catalfamo J. L., Dodds W. J. Complement C5a (desArg) generation in serum exposed to damaged aortic endothelium. Exp Mol Pathol. 1988 Apr;48(2):216–225. doi: 10.1016/0014-4800(88)90058-5. [DOI] [PubMed] [Google Scholar]
- Seifert P. S., Kazatchkine M. D. Generation of complement anaphylatoxins and C5b-9 by crystalline cholesterol oxidation derivatives depends on hydroxyl group number and position. Mol Immunol. 1987 Dec;24(12):1303–1308. doi: 10.1016/0161-5890(87)90125-8. [DOI] [PubMed] [Google Scholar]
- Seifert P. S., Kazatchkine M. D. The complement system in atherosclerosis. Atherosclerosis. 1988 Oct;73(2-3):91–104. doi: 10.1016/0021-9150(88)90030-5. [DOI] [PubMed] [Google Scholar]
- Seya T., Turner J. R., Atkinson J. P. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J Exp Med. 1986 Apr 1;163(4):837–855. doi: 10.1084/jem.163.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli O., Ropraz P., Trzeciak A., Benzonana G., Gillessen D., Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: a new probe for smooth muscle differentiation. J Cell Biol. 1986 Dec;103(6 Pt 2):2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small D. M. George Lyman Duff memorial lecture. Progression and regression of atherosclerotic lesions. Insights from lipid physical biochemistry. Arteriosclerosis. 1988 Mar-Apr;8(2):103–129. doi: 10.1161/01.atv.8.2.103. [DOI] [PubMed] [Google Scholar]
- Vlaicu R., Niculescu F., Rus H. G., Cristea A. Immunohistochemical localization of the terminal C5b-9 complement complex in human aortic fibrous plaque. Atherosclerosis. 1985 Nov;57(2-3):163–177. doi: 10.1016/0021-9150(85)90030-9. [DOI] [PubMed] [Google Scholar]
- Vlaicu R., Rus H. G., Niculescu F., Cristea A. Immunoglobulins and complement components in human aortic atherosclerotic intima. Atherosclerosis. 1985 Apr;55(1):35–50. doi: 10.1016/0021-9150(85)90164-9. [DOI] [PubMed] [Google Scholar]
- Wilson J. G., Tedder T. F., Fearon D. T. Characterization of human T lymphocytes that express the C3b receptor. J Immunol. 1983 Aug;131(2):684–689. [PubMed] [Google Scholar]