Abstract
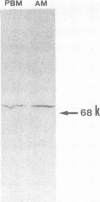
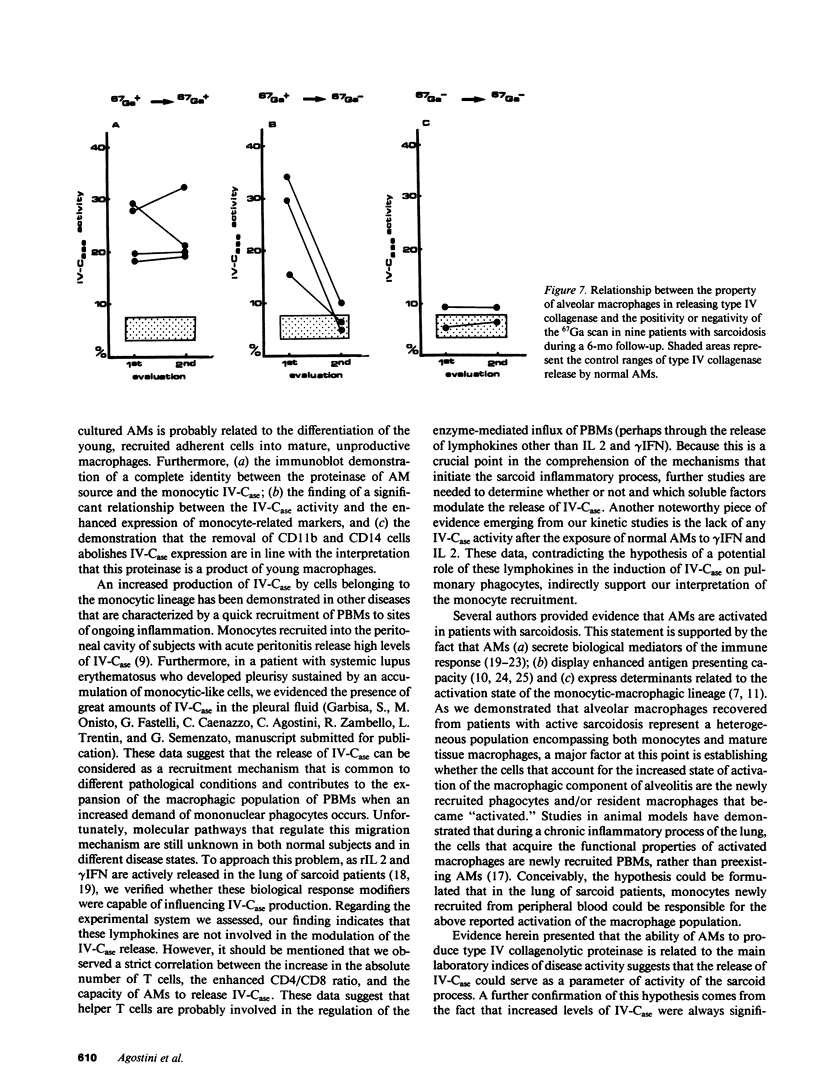
Alveolar macrophages (AMs) recovered from the bronchoalveolar lavage (BAL) of 44 patients with sarcoidosis were evaluated for their ability to release type IV collagenolytic metalloproteinase (IV-Case). This enzyme, which is produced by peripheral blood monocytes (PBMs) but not by tissue macrophages, degrades type IV collagen, the major structural component of vessel wall basement membranes, and helps to promote the migration of PBMs from the blood compartment to peripheral tissues. Our results demonstrated that AMs from patients with active sarcoidosis released significantly increased levels of IV-Case with respect to patients with inactive disease and control subjects. After in vitro culture, sarcoid AMs secreted IV-Case during the first 24 h of collection; after that time, AMs progressively lost their ability to release IV-Case. Exposition of both sarcoid and normal AMs to recombinant IL 2 or gamma IFN did not influence their property to release IV-Case. The immunoblot analysis of IV-Case demonstrated complete identity between IV-Case released by AMs and the degradative enzyme obtained from PBMs. The increased property to release IV-Case was significantly related to the increase of the absolute number of AMs and, in particular, of AMs bearing two determinants that are usually expressed by most PBMs (CD11b and CD14). Selective depletion of CD11b+/CD14+ AMs from the entire macrophagic population was associated with the recovery of the IV-Case activity to normal values. A positive correlation was also found between the increase in the absolute number of lung T cells and the enhanced CD4/CD8 pulmonary ratio. A 6-mo follow-up study indicated a significant association between the positivity for the 67Gallium scan and the increased property of AMs to release IV-Case. Our data are consistent with the hypothesis that a IV-Case mediated influx of peripheral monocytes takes place in the lung of sarcoid patients. Furthermore, the correlation found between the IV-Case release and disease activity suggests that this assay could represent a useful tool in sarcoidosis disease staging.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. S., Sharma O. P., Gacad M. A., Singer F. R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983 Nov;72(5):1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini C., Poletti V., Zambello R., Trentin L., Siviero F., Spiga L., Gritti F., Semenzato G. Phenotypical and functional analysis of bronchoalveolar lavage lymphocytes in patients with HIV infection. Am Rev Respir Dis. 1988 Dec;138(6):1609–1615. doi: 10.1164/ajrccm/138.6.1609. [DOI] [PubMed] [Google Scholar]
- Agostini C., Trentin L., Zambello R., Luca M., Masciarelli M., Cipriani A., Marcer G., Semenzato G. Pulmonary alveolar macrophages in patients with sarcoidosis and hypersensitivity pneumonitis: characterization by monoclonal antibodies. J Clin Immunol. 1987 Jan;7(1):64–70. doi: 10.1007/BF00915427. [DOI] [PubMed] [Google Scholar]
- Bitterman P. B., Saltzman L. E., Adelberg S., Ferrans V. J., Crystal R. G. Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest. 1984 Aug;74(2):460–469. doi: 10.1172/JCI111443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blussé van Oud Alblas A., van der Linden-Schrever B., van Furth R. Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intravenous administration of heat-killed bacillus Calmette-Guérin. J Exp Med. 1981 Aug 1;154(2):235–252. doi: 10.1084/jem.154.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilosi M., Menestrina F., Capelli P., Montagna L., Lestani M., Pizzolo G., Cipriani A., Agostini C., Trentin L., Zambello R. Immunohistochemical analysis of sarcoid granulomas. Evaluation of Ki67+ and interleukin-1+ cells. Am J Pathol. 1988 May;131(2):191–198. [PMC free article] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract. N Engl J Med. 1984 Jan 26;310(4):235–244. doi: 10.1056/NEJM198401263100406. [DOI] [PubMed] [Google Scholar]
- Daniele R. P., Elias J. A., Epstein P. E., Rossman M. D. Bronchoalveolar lavage: role in the pathogenesis, diagnosis, and management of interstitial lung disease. Ann Intern Med. 1985 Jan;102(1):93–108. doi: 10.7326/0003-4819-102-1-93. [DOI] [PubMed] [Google Scholar]
- Fels A. O., Nathan C. F., Cohn Z. A. Hydrogen peroxide release by alveolar macrophages from sarcoid patients and by alveolar macrophages from normals after exposure to recombinant interferons alpha A, beta, and gamma and 1,25-dihydroxyvitamin D3. J Clin Invest. 1987 Aug;80(2):381–386. doi: 10.1172/JCI113083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbisa S., Ballin M., Daga-Gordini D., Fastelli G., Naturale M., Negro A., Semenzato G., Liotta L. A. Transient expression of type IV collagenolytic metalloproteinase by human mononuclear phagocytes. J Biol Chem. 1986 Feb 15;261(5):2369–2375. [PubMed] [Google Scholar]
- Garbisa S., Kniska K., Tryggvason K., Foltz C., Liotta L. A. Quantitation of basement membrane collagen degradation by living tumor cells in vitro. Cancer Lett. 1980 Jun;9(4):359–366. doi: 10.1016/0304-3835(80)90030-0. [DOI] [PubMed] [Google Scholar]
- Hance A. J., Douches S., Winchester R. J., Ferrans V. J., Crystal R. G. Characterization of mononuclear phagocyte subpopulations in the human lung by using monoclonal antibodies: changes in alveolar macrophage phenotype associated with pulmonary sarcoidosis. J Immunol. 1985 Jan;134(1):284–292. [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Young R. C., Jr, Kawanami O., Ferrans V. J., Crystal R. G. Maintenance of granuloma formation in pulmonary sarcoidosis by T lymphocytes within the lung. N Engl J Med. 1980 Mar 13;302(11):594–598. doi: 10.1056/NEJM198003133021102. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W. Release of interleukin-1 by alveolar macrophages of patients with active pulmonary sarcoidosis. Am Rev Respir Dis. 1984 Apr;129(4):569–572. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lea T., Vartdal F., Davies C., Ugelstad J. Magnetic monosized polymer particles for fast and specific fractionation of human mononuclear cells. Scand J Immunol. 1985 Aug;22(2):207–216. doi: 10.1111/j.1365-3083.1985.tb01873.x. [DOI] [PubMed] [Google Scholar]
- Lem V. M., Lipscomb M. F., Weissler J. C., Nunez G., Ball E. J., Stastny P., Toews G. B. Bronchoalveolar cells from sarcoid patients demonstrate enhanced antigen presentation. J Immunol. 1985 Sep;135(3):1766–1771. [PubMed] [Google Scholar]
- Nathan C. F., Murray H. W., Cohn Z. A. The macrophage as an effector cell. N Engl J Med. 1980 Sep 11;303(11):622–626. doi: 10.1056/NEJM198009113031106. [DOI] [PubMed] [Google Scholar]
- Pinkston P., Bitterman P. B., Crystal R. G. Spontaneous release of interleukin-2 by lung T lymphocytes in active pulmonary sarcoidosis. N Engl J Med. 1983 Apr 7;308(14):793–800. doi: 10.1056/NEJM198304073081401. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y. Bronchoalveolar lavage. Am Rev Respir Dis. 1987 Jan;135(1):250–263. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- Robinson B. W., McLemore T. L., Crystal R. G. Gamma interferon is spontaneously released by alveolar macrophages and lung T lymphocytes in patients with pulmonary sarcoidosis. J Clin Invest. 1985 May;75(5):1488–1495. doi: 10.1172/JCI111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenzato G., Agostini C., Zambello R., Trentin L., Chilosi M., Angi M. R., Ossi E., Cipriani A., Pizzolo G. Activated T cells with immunoregulatory functions at different sites of involvement in sarcoidosis. Phenotypic and functional evaluations. Ann N Y Acad Sci. 1986;465:56–73. doi: 10.1111/j.1749-6632.1986.tb18481.x. [DOI] [PubMed] [Google Scholar]
- Velo G. P., Spector W. G. The origin and turnover of alveolar macrophages in experimental pneumonia. J Pathol. 1973 Jan;109(1):7–19. doi: 10.1002/path.1711090103. [DOI] [PubMed] [Google Scholar]
- Venet A., Hance A. J., Saltini C., Robinson B. W., Crystal R. G. Enhanced alveolar macrophage-mediated antigen-induced T-lymphocyte proliferation in sarcoidosis. J Clin Invest. 1985 Jan;75(1):293–301. doi: 10.1172/JCI111688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Maarsseveen A., Alberts C., van der Schoot J., van Royen E., Hens C., Mullink H., de Groot J. Radionuclides in detecting active granuloma formation. Gallium-67 scintigraphy and histopathology with autoradiographic findings. Ann N Y Acad Sci. 1986;465:427–434. doi: 10.1111/j.1749-6632.1986.tb18519.x. [DOI] [PubMed] [Google Scholar]