Abstract
Paclitaxel, one of the most commonly used cancer chemotherapeutic drugs, effectively extends the progression-free survival of breast, lung, and ovarian cancer patients. However, paclitaxel and other chemotherapy drugs elicit peripheral nerve fiber dysfunction or degeneration that leads to peripheral neuropathy in a large proportion of cancer patients. Patients receiving chemotherapy also often experience changes in mood, including anxiety and depression. These somatic and affective disorders represent major dose-limiting side effects of chemotherapy. Consequently, the present study was designed to develop a preclinical model of paclitaxel-induced negative affective symptoms in order to identify treatment strategies and their underlying mechanisms of action. Intraperitoneal injections of paclitaxel (8 mg/kg) resulted in the development and maintenance of mechanical and cold allodynia. Carboplatin, another cancer chemotherapeutic drug that is often used in combination with paclitaxel, sensitized mice to the nociceptive effects of paclitaxel. Paclitaxel also induced anxiety-like behavior, as assessed in the novelty suppressed feeding and light/dark box tests. In addition, paclitaxel-treated mice displayed depression-like behavior during the forced swim test and an anhedonia-like state in the sucrose preference test. In summary, paclitaxel produced altered behaviors in assays modeling affective states in C57BL/6J male mice, while increases in nociceptive responses were longer in duration. The characterization of this preclinical model of chemotherapy-induced allodynia and affective symptoms, possibly related to neuropathic pain, provides the basis for determining the mechanism(s) underlying severe side effects elicited by paclitaxel, as well as for predicting the efficacy of potential therapeutic interventions.
1. Introduction
Various neoplastic diseases, such as breast, lung, and ovarian cancer, are commonly treated with paclitaxel, a chemotherapeutic drug in the taxane class. The anti-tumor effect of paclitaxel is mediated through its binding to microtubules of the cytoskeleton and enhancement of tubulin polymerization, thereby resulting in cell cycle arrest, and ultimately apoptotic cell death (Jordan and Wilson, 2004). Although paclitaxel effectively increases both progression-free survival and overall survival in cancer patients, it also produces painful sensory and emotional deficits (Dranitsaris et al., 2015; Seretny et al., 2014). Specifically, paclitaxel causes chemotherapy-induced peripheral neuropathy (CIPN), a result of peripheral nerve fiber dysfunction or degeneration, acutely in 59–78% of cancer patients and chronically in 30% of cancer patients (Beijers et al., 2012). CIPN is characterized by sensory symptoms such as numbness, tingling, cold and mechanical allodynia, as well as an overall decrease in quality of life. In addition, cancer patients receiving chemotherapy experience behavioral symptoms including fatigue, anxiety, and depression. For example, approximately 58% of cancer patients suffer from depression, while anxiety is prevalent in approximately 11.5% of the cancer patient population (Massie, 2004; Mehnert et al., 2014). Importantly, patients with comorbidities of depression and anxiety suffer from increased severity of symptoms and experience delayed recovery, which may interfere with positive outcomes (Massie, 2004). In comparison, 34% and 25% of the general population of patients experiencing neuropathic pain report respective feelings of depression and anxiety (Gustorff et al., 2008).
It is clear that there is a critical need to determine the mechanisms underlying these behavioral symptoms elicited by cancer chemotherapy drugs, as well as to identify new targets to prevent or treat these side effects. A necessary requisite to accomplish these aims is to establish relevant preclinical models of chemotherapy-induced side effects. However, to our knowledge there are presently no published preclinical studies that have characterized paclitaxel-induced affective-like behaviors. Thus, the objectives of the current study were to develop a mouse model of paclitaxel-induced side effects. Multiple assessments of nociceptive and affective-related behaviors were performed in mice treated with one cycle of paclitaxel (i.p., every other day for a total of four injections). After determining the dose-response curve and time-course of paclitaxel-induced mechanical and cold allodynia following systemic administration in mice, the impact of paclitaxel was assessed on multiple affective behavioral phenotypes in individual cohorts of mice, such as nest building, anxiety- (light/dark box test, novelty suppressed feeding), depression- (forced swim test), and anhedonia- (sucrose preference test) related behaviors. In addition, studies investigated the nociceptive effect of carboplatin treatment alone and in combination with paclitaxel due to the use of the carboplatin-paclitaxel combination in the clinic.
2. Methods
2.1. Animals
Adult male C57BL/6J mice (8 weeks at beginning of experiments, 20–30 g) were purchased from The Jackson Laboratory (Bar Harbor, ME). A total of 197 mice were used, with 84 used to assess nociceptive effects and 113 used to assess affective-like behaviors. Mice were housed in an AAALAC-accredited facility in groups of four, then individually housed for the duration of the nesting, novelty suppressed feeding (NSF), and sucrose preference assays in order to accurately assess the ability of each individual mouse to nest, and to measure the food or sucrose consumed by each individual mouse. Mice were group-housed for all other behavioral assays. Food and water were available ad libitum, except when under the food restrictions of the NSF assay. The mice in each cage were randomly allocated to different treatment groups. All behavioral testing on animals was performed in a blinded manner; behavioral assays were conducted by an experimenter blinded to the treatment groups. Experiments were performed during the light cycle (7:00 am to 7:00 pm) and were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Animals were euthanized via CO2 asphyxiation, followed by cervical dislocation. Any subjects that showed behavioral disturbances unrelated to chemotherapy-induced pain were excluded from further behavioral testing. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010).
2.2. Drugs
Paclitaxel and carboplatin were purchased from Tocris (Bristol, United Kingdom). Paclitaxel was dissolved in a mixture of 1:1:18 [1 volume ethanol/1 volume Emulphor-620 (Rhone-Poulenc, Inc., Princeton, NJ)/18 volumes distilled water]. Carboplatin was dissolved in 0.9% saline. All injections were administered intraperitoneally (i.p.) in a volume of 1 ml/100 g body weight.
2.3. Induction of CIPN model
In the clinic, low-dose paclitaxel therapy consists of administering 80 mg/m2 intravenously once every week; the duration of treatment is dependent upon disease progression and limiting toxicity (Seidman et al., 2008). To mimic this low-dose regimen, our studies involved i.p. injections of 2, 4, or 8 mg/kg paclitaxel every other day for a total of four injections (1 cycle), resulting in a cumulative human equivalent dose of 28.4–113.5 mg/m2 (Reagan-Shaw et al., 2007). A low-dose regimen (8 mg/kg, 1 cycle) results in long-term mechanical allodynia, which better represents the clinical manifestation of peripheral neuropathy, and allows for affective-related behavioral measures to not be obscured by severe motor deficits and weight loss. When referring to the time at which affective behavioral assays were conducted, “post-paclitaxel injection” refers to the time after the first of four paclitaxel injections.
2.3.1. Immunohistochemistry and quantification of intra-epidermal nerve fibers (IENFs)
The staining procedure was based on a previously described method of Bennett et al., 2011 with modifications. The glabrous skin of the hind paw was excised, placed in freshly prepared 4% paraformaldehyde in 0.1 M PBS (pH 7.4), and stored overnight at 4°C in the same fixative. The samples were embedded in paraffin and sectioned at 25 μm. Sections were deparaffinized, washed with PBS, and incubated at room temperature for 30 min in blocking solution (5% normal goat serum and 0.3% Triton X-100 in PBS). Sections were incubated with a 1:1000 dilution of the primary antibody, PGP9.5 (Fitzgerald - cat# 70R-30722, MA, USA) overnight at 4°C in a humidity chamber. Following PBS washes, sections were incubated for 90 min at room temperature with a 1:250 dilution of goat anti-rabbit IgG (H+L) secondary antibody conjugated with Alexa Fluor® 594 (Life Technologies - cat# A11037, OR, USA). Sections were mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA) and examined using a Zeiss Axio Imager A1 – Fluorescence microscope (Carl Zeiss, AG, Germany). Sections were examined in a blinded fashion under 63× magnification. The IENFs in each section were counted in a blinded fashion and the density of fibers is expressed as fibers/mm. An individual cohort consisting of 6 mice per group was used.
2.3.2. Cycles of paclitaxel
To investigate the impact of paclitaxel treatment on peripheral sensitization following repeated cycles, we used the lowest paclitaxel dose in this study for a total of two cycles. Mice were injected with vehicle or paclitaxel (2 mg/kg) for each cycle. Mechanical thresholds were evaluated between the days of injection and subsequently once per week. The second cycle of treatment began one week after the first cycle. An individual cohort consisting of 6 mice per group was used.
2.3.3. Carboplatin-paclitaxel treatment
In this study, we first investigated if carboplatin, which is often used in combination with paclitaxel for chemotherapeutic intervention, would induce allodynia in mice on its own after systemic administration. To explore the effect of carboplatin on changes in nociceptive behavior, mice were injected with carboplatin (0, 5, or 20 mg/kg) for 1 cycle and tested for 7 days. In a separate experiment, we studied the impact the carboplatin treatment on paclitaxel-induced allodynia using the sequence of carboplatin-paclitaxel administration. Mice were first injected with carboplatin (5 mg/kg, 1 cycle), then another cycle of injections was administered with a low dose of paclitaxel (1 mg/kg). The second cycle of treatment (paclitaxel, 1 mg/kg) began one week following the first cycle (carboplatin, 5 mg/kg). Mechanical thresholds were evaluated between the days of injection. An individual cohort consisting of 6 mice per group was used.
2.4. Assessment of nociceptive behavior
An individual cohort consisting of 6 mice per group was used for the assessment of mechanical and cold allodynia; the mice had a resting period of 24 hours between assays. An additional cohort consisting of 6 mice per group was used for the locomotor activity test to assess potential paclitaxel-induced motor deficits.
2.4.1. Mechanical allodynia evaluation (von Frey test)
Mechanical allodynia thresholds were determined using von Frey filaments according to the method suggested by Chaplan et al. (1994) and as described in our previous report (Bagdas et al., 2015). The mechanical threshold is expressed as log 10 (10 £ force in [mg]).
2.4.2. Cold allodynia evaluation (acetone test)
This test was conducted as previously described (Otrubova et al., 2013), but with slight modifications. Briefly, mice were placed in a Plexiglas cage with mesh metal flooring and allowed to acclimate for 30 min before testing. 10 μl of acetone was projected via air burst from the pipette onto the plantar surface of each hind paw. Time spent licking, lifting, and/or shaking the hind paw was recorded by a stopwatch over the course of 60 s.
2.5. Locomotor activity test
The test was performed as described previously in Bagdas et al. (2015). Briefly, mice were placed into individual Omnitech (Columbus, OH) photocell activity cages (28 × 16.5 cm) containing two banks of eight cells each. Interruptions of the photocell beams, which assess walking and rearing, were then recorded for the next 30 min. Data are expressed as the number of photocell interruptions.
2.6. Assessment of affective behaviors
2.6.1. Nesting procedure
The nesting procedure was adapted as previously described by Negus et al. (2015) with some modifications. Briefly, mice were housed individually in cages containing corn cob bedding and all previous nesting material was removed from the home cage prior to conducting the nesting assay. For each cage, one compressed cotton nestlet was weighed and cut into 6 rectangular pieces of equal size. The mice were then relocated to a quiet, dark room. After an acclimation period of approximately 30 min, the nestlet pieces were then placed on top of the wire cage lid, parallel to the wire and evenly spaced. The mice were allowed 120 min to nest, after which the weight of the nestlet pieces remaining on the cage lid and the nest quality (0–2; 0 = no nest formed, 1 = some nesting activity, 2 = established nest) was recorded. The percentage of animals that did nest, the amount of nesting material acquired (percent weight used), and the ability to participate in innate murine nesting behavior (nest quality) were determined. The nesting assay was conducted with three individual cohorts of mice: one at 1 week (n = 6 per group), one at 2 weeks (n = 6 per group), and another at both 8 and 11 weeks (n = 6 Veh, n = 7 PAC) post-paclitaxel (8 mg/kg, i.p) or vehicle injection. These specific cohorts were used for both the nesting and NSF assays, since nesting is not thought to be a stress-inducing task. The mice had a resting period of one week between assays.
2.6.2. Novelty suppressed feeding (NSF)
The NSF test measures a rodent’s aversion to eating in a novel environment. It assesses stress-induced anxiety by measuring the latency of an animal to approach and eat a familiar food in an aversive environment (Bodnoff et al, 1988). Mice were housed individually in cages with wood-chip bedding and were deprived of food for 24 h. At the end of the deprivation period, the mice were relocated to a quiet, dark room. After an acclimation period of approximately 30 min, the mice were allowed access to an unused, pre-weighed food pellet in a clean test cage containing fresh wood-chip bedding, which was placed directly under a bright light. Each mouse was placed in a corner of the test cage, and a stopwatch was immediately started. The latency to eat (s), defined as the mouse sitting on its haunches and biting the pellet with the use of forepaws, was recorded. The amount of food (g) consumed by the mouse in 5 min was measured, serving as a control for change in appetite as a possible confounding factor. The NSF assay was conducted with two individual cohorts of mice, one at 3 weeks (n = 6 per group) and another at both 9 and 11 weeks (n = 6 Veh, n = 7 PAC) post-paclitaxel (8 mg/kg, i.p.) or vehicle injection. These specific cohorts were used for both the nesting and NSF assays, since nesting is not thought to be a stress-inducing task. The mice had a resting period of one week between assays.
2.6.3. Light/dark box (LBD) test
The light/dark box test is based upon a conflict between the innate aversion to brightly illuminated areas and spontaneous exploratory activity (Crawley and Goodwin, 1980). The test was adapted as previously described (Wilkerson et al., 2016) with minor modifications. Briefly, the LDB apparatus consisted of a small, enclosed dark box (36 × 10 × 34 cm) with a passage way (6 × 6 cm) leading to a larger, light box (36 × 21 × 34 cm). The mice were acclimated to the testing room for 30 min prior to testing. Mice were placed in the light compartment and allowed to explore the apparatus for 5 min. The number of entries into the light compartment and the total time spent (s) in the light compartment were recorded for 5 min by a video monitoring system and measured by ANY-MAZE software (Stoelting Co., Wood Dale, IL). Individual cohorts of mice (n = 6 per group) were tested at 3, 6, and 9 weeks post-paclitaxel (8 mg/kg, i.p.) or vehicle injection.
2.6.4. Forced swim test (FST)
The forced swim test was performed as described previously by Damaj et al. (2004), the common method for assessing depression-like behavior in mice (Bogdanova et al., 2013). Briefly, mice were gently placed into individual glass cylinders (25 × 10 cm) containing 10 cm of water, maintained at 24°C, and left for 6 min. Immobility was recorded (s) during the last 4 min. A mouse was considered to be immobile when floating in an upright position and only making small movements to keep its head above water, but not producing displacements. An individual cohort of mice (n = 6 per group) was tested throughout the FST study at 1, 2, 3, and 4 weeks post-paclitaxel (8 mg/kg, i.p) or vehicle injection.
2.6.5. Sucrose preference
The sucrose preference test is used as a measure of anhedonia-like behavior (Thompson and Grant, 1971). Mice had access to two, 25 ml sipper tubes, one containing normal drinking water and the other containing a 2% sucrose solution. Mice were housed individually, with access to food, water, and 2% sucrose 24 h per day. Mice were acclimated to the cages with sipper tubes for 3 days prior to injection (days 1–3), during which baseline measurements were taken. Paclitaxel (8 mg/kg, i.p.) or vehicle injections started on day 4. Water and sucrose intake were measured on days 1, 2, 3, 4, 5, and 6, as well as on days 10, 11 and 12. The location of both sipper tubes was switched daily to avoid place preference. Sucrose preference was calculated as a percentage of the volume of 2% sucrose consumed over the total fluid intake volume. An individual cohort of mice (n = 8 per group) was tested during the vehicle/paclitaxel treatment.
2.7. Statistical analyses
In the current study, a power analysis calculation was performed with the Lamorte’s Power Calculator (Boston University Research Compliance) to determine the sample size of animals for each group (Charan and Kantharia, 2013). For assessing the nociceptive behaviors, the calculation showed that an n of 5 was required to achieve a power of 90% with an alpha error of 0.05; we used 6 mice per group. For the behavioral assays, the calculations showed that an n of 5 for novelty suppressed feeding, an n of 5 for nesting, an n of 8 for the light/dark box test, an n of 6 for the forced swim test, and an n of 8 for sucrose preference was required to achieve a power of 90% with an alpha error of 0.05; we used 6 to 8 mice per group. The data were analyzed with GraphPad Prism software, version 6 (GraphPad Software, Inc., La Jolla, CA) and are expressed as mean ± SEM. Before conducting statistical analyses, normality and variance tests were performed; normality of residuals was determined by the Shapiro-Wilk test for n > 6 or the Kolmogorov-Smirnov test for n ≤ 6, and equal variance was determined by the F test. Data that did not pass the normality test were analyzed by non-parametric tests, and data that did not have equal variance were analyzed without the assumption of equal standard deviations. Data were normalized to initial vehicle measurements when appropriate. Unpaired t tests were performed to compare behaviors of vehicle- and paclitaxel-treated mice at a single time point. Two-way repeated measure analysis of variance (ANOVA) tests were conducted, and followed by the Bonferroni post hoc test, when behavioral outcomes of vehicle- and paclitaxel-treated mice were being compared over multiple time points. Differences were considered to be significant at P < 0.05.
3. Results
3.1. Paclitaxel induced changes in nociceptive behaviors in mice
Initial experiments determined the effect of paclitaxel on the development of mechanical and cold allodynia as a function of the drug dose. As anticipated, increased nociceptive responses and duration of effects were related to dose of paclitaxel. However, no significant changes in body weight gain or spontaneous activity were observed. As seen in Figures 1A and B, paclitaxel induced both mechanical allodynia [Fdose × time (21, 105) = 9.481, P < 0.0001] and cold allodynia [Fdose × time (9, 45) = 14.76, P < 0.0001] in dose- and time-related manners, respectively. At 8 mg/kg paclitaxel, mechanical allodynia was observed on day 1 post-paclitaxel injection, and this effect was sustained for more than 90 days (data not shown). On the other hand, 2 and 4 mg/kg paclitaxel induced mechanical allodynia beginning on day 3, and the effects did not differ in terms of magnitude or time to recover. With regard to cold allodynia, paclitaxel presented a clear dose-dependent induction on day 8 post-paclitaxel injection. However, mice that received 2 or 4 mg/kg paclitaxel recovered by day 22, whereas the 8 mg/kg group continued to exhibit cold allodynia. In regards to general body condition, even the highest dose of paclitaxel (8 mg/kg) did not significantly alter body weight [Fdose × time (5, 25) = 1.093, P > 0.05; Supplementary Fig. 1A], or motor coordination [Fdose × time (4, 40) = 0.5204, P > 0.05; Supplementary Fig. 1B].
Figure 1.
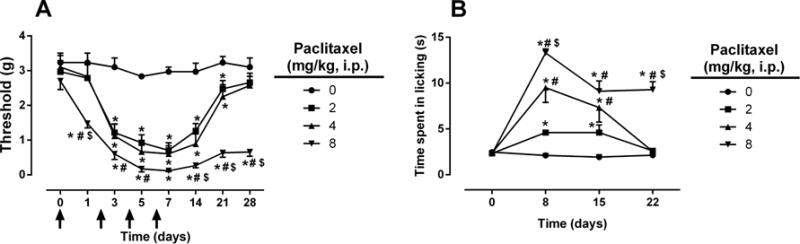
Paclitaxel induces nociceptive behaviors. Paclitaxel doses of 2, 4, and 8 mg/kg (i.p., every other day for a total of 4 injections) induce both mechanical (A) and cold (B) allodynia in a dose and time dependent manner. Arrows indicate vehicle/paclitaxel injections on days 0, 2, 4, and 6. Baseline measurements were taken before vehicle/paclitaxel administration on day 0. The same cohort was tested for both mechanical and cold allodynia; n = 6 per group (data expressed as mean ± SEM). *P < 0.05 vs vehicle; #P < 0.05 vs paclitaxel (2 mg/kg); $P < 0.05 vs paclitaxel (4 mg/kg). BL, baseline.
3.1.1. Paclitaxel decreased the density of intra-epidermal nerve fibers (lENFs)
Because changes in the density of peripheral nerve fibers represent a hallmark of CIPN, we studied the changes in peripheral nerve fiber density following paclitaxel treatment using immunohistochemistry. At 28 days post-paclitaxel injection, mice treated with paclitaxel (8 mg/kg, 1 cycle) demonstrated significant reductions in the density of IENFs when compared to vehicle-treated mice [t = 3.736, df = 10, P < 0.01; Fig. 2A]. Representative immunostained sections of foot pads from vehicle- (Fig. 2B; upper panel) and paclitaxel-treated mice (Fig. 2B; lower panel) show the reduction in IENFs following paclitaxel treatment.
Figure 2.
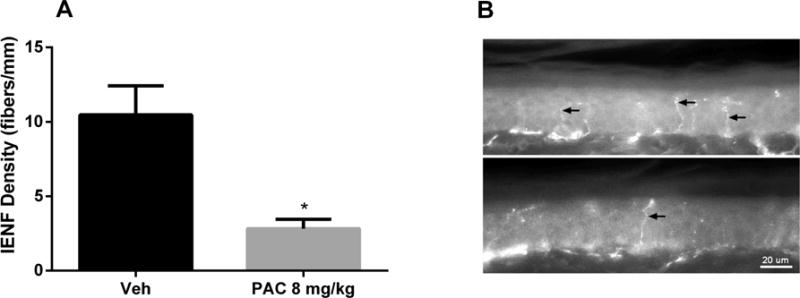
Paclitaxel induces a reduction in intra-epidermal nerve fiber (IENF) density at 28 days post-paclitaxel injection. A) Quantification of IENF density in mice treated with one cycle of paclitaxel (8 mg/kg, i.p., every other day for a total of 4 injections) shows a significant reduction compared to vehicle. One cohort was tested; n = 6 per group (data expressed as mean ± SEM). *P < 0.05 vs vehicle. Veh, vehicle; PAC, paclitaxel. B) Immunostained sections of vehicle-(upper panel) and paclitaxel-treated (lower panel) hind foot pad skin showing the reduction of lENFs (arrows) following paclitaxel treatment. Bar represents 20 microns in both images.
3.1.2. Impact of repeated drug cycles on paclitaxel-induced mechanical allodynia
To investigate the effect of repeated cycles of paclitaxel on mechanical allodynia, mice were injected with two cycles of a low dose of paclitaxel (2 mg/kg). As expected, the first cycle of paclitaxel (2 mg/kg) was capable of inducing mechanical allodynia. Indeed, paclitaxel (2 mg/kg) induced a significant reduction in mechanical threshold that lasted for at least 14 days after the first injection of paclitaxel [Fdose × tme (7, 35) = 8.436, P < 0.0001; Fig 3A]. After a one week wash-out period, mice received another cycle of paclitaxel (2 mg/kg). Surprisingly, the effects of paclitaxel were significantly enhanced in the mice subjected to a second cycle, which was demonstrated by a further decrease in mechanical threshold [Fdose × tme (3, 15) = 48.61, P < 0.0001; Supplementary Fig. 2]. In addition, mice that received a second cycle of paclitaxel treatment (2 mg/kg) displayed a much longer duration of allodynia (Fig. 3B) compared to one cycle of treatment (Fig. 3A) [Fdose × time (13, 65) = 10.97, P < 0.0001; Fig. 3B]. Whereas mice given one cycle recovered by day 21 post-paclitaxel injection, mice given two cycles recovered by day 63 after the first injection of paclitaxel. Calculation of the area under the curve (AUC) threshold for the initial 28 days of both the first and second cycles of paclitaxel treatment revealed significant differences (2.5 fold difference) between cycles [Ftreatment (3, 20) = 60.35, P < 0.0001; Fig. 3C].
Figure 3.
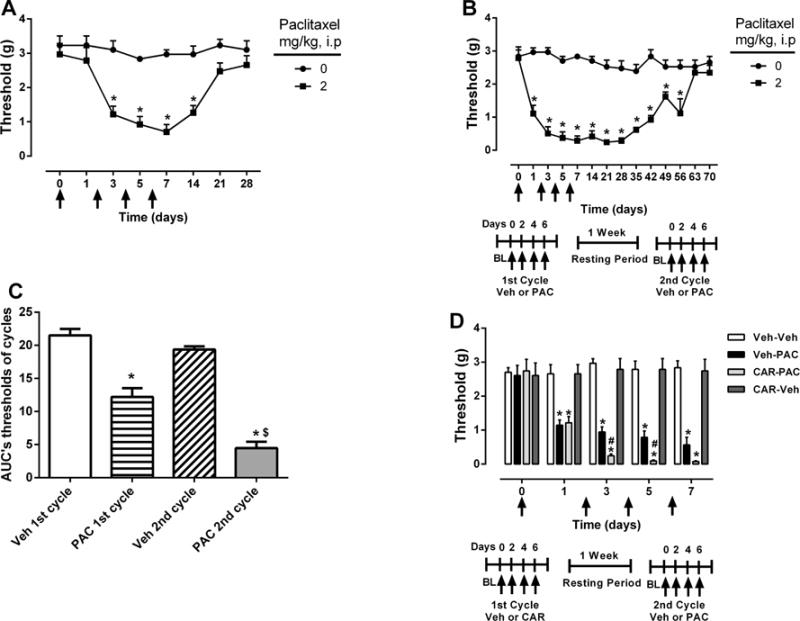
Mice are sensitized to cutaneous stimulation after second cycle of paclitaxel treatment. A) Mice treated with one cycle of paclitaxel (2 mg/kg) or vehicle (i.p., every other day for a total of 4 injections). B) Mice from 3A treated with a second cycle of paclitaxel (2 mg/kg) or vehicle (i.p., every other day for a total of 4 injections). C) AUC mechanical threshold for initial 28 days of first and second cycles of paclitaxel treatment. D) Comparison of mechanical thresholds during the second cycle of treatment between mice treated with carboplatin (5 mg/kg) alone and with carboplatin followed by a low dose of paclitaxel (1 mg/kg). Arrows indicate vehicle/paclitaxel/carboplatin injections on days 0, 2, 4, and 6 of each cycle. Baseline measurements were taken before vehicle/paclitaxel/carboplatin administration on day 0. One cohort was tested; n = 6 per group (data expressed as mean ± SEM). *P < 0.05 vs vehicle; $P < 0.05 vs first cycle of paclitaxel (2 mg/kg), #P < 0.05 vs carboplatin (5 mg/kg). Veh, vehicle; PAC, paclitaxel; CAR, carboplatin.
3.1.3. Paclitaxel induced allodynia following carboplatin treatment
We further investigated the impact of carboplatin treatment on paclitaxel-induced allodynia. Mice given one cycle of carboplatin alone did not demonstrate significant mechanical nociceptive changes. As shown in Supplementary Figure 3, one cycle of 5 or 20 mg/kg carboplatin did not significantly reduce the mechanical threshold [Fdose × time (8, 40) = 0.4526, P > 0.05]. However, in a separate cohort of mice, a low-dose paclitaxel (1 mg/kg) cycle administered one week following the completion of the carboplatin (5 mg/kg) cycle led to a significant reduction of mechanical threshold compared to the vehicle-paclitaxel group [Fdose × tme (12, 60) = 16.65, P < 0.0001; Fig. 3D].
3.2. Paclitaxel induced changes in affective-related behaviors in mice
To assess whether paclitaxel interferes with the natural behavior of mice, a nesting assay was conducted at various time points after paclitaxel treatment was initiated. However, paclitaxel did not interfere with nesting activity [z = 0.856, P > 0.05; z = 1.000, P > 0.05], the quantity of nesting material used [t = 0.08655, df = 10, P > 0.05; t = 0.03402, df = 10, P > 0.05], or nest quality [t = 0.4152, df = 10, P > 0.05; t = 0.2033, df = 10, P > 0.05] at 1 and 2 weeks post-paclitaxel injection, respectively (Fig. 4). Similar results were observed at 8 and 11 weeks post-paclitaxel injection, in which nesting activity was not significantly affected by paclitaxel [z = 0.926, P > 0.05; Fig. 4A]. The use of nesting material [Ftreatment × tme (1,11) = 1.157, P > 0.05] and nest quality [Ftreatment × time (1,11) = 0.0094, P > 0.05] were also not found to be significantly altered (Fig. 4B and C).
Figure 4.
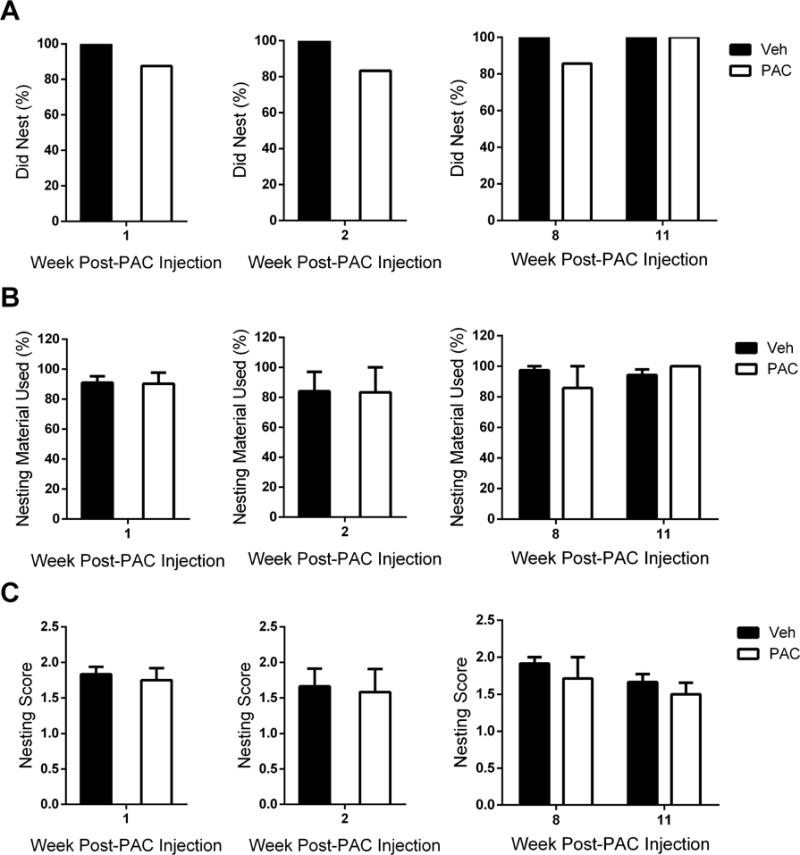
Paclitaxel does not influence the nesting behavior of mice. Mice were allowed 120 minutes to nest at weeks 1, 2, 8, and 11 post-paclitaxel (8 mg/kg, i.p.) or vehicle injection. A) It was determined that mice had participated in nesting activity if at least one nestlet piece had been chewed or pulled into the home cage. A comparison of proportions via z-tests between vehicle- and paclitaxel-treated mice was not significant at any time point. B) The percentage of nesting material used was determined by the following equation: (weight of initial nestlet pieces - weight of remaining nestlet pieces)/weight of initial nestlet pieces. C) The quality of each nest was evaluated on a scale ranging from 0 to 2 (0 = no nest formed, 1 = some nesting activity, 2 = established nest). Individual cohorts were tested at 1 week (n = 6 per group), 2 weeks (n = 6 per group), 8 and 11 weeks (n = 6 Veh, n = 7 PAC) post-paclitaxel (8 mg/kg, i.p.) or vehicle injection; data expressed as mean ± SEM. Post-PAC injection refers to the time following the first of four paclitaxel injections. Veh, vehicle; PAC, paclitaxel.
With regard to affective-related changes, we assessed anxiety-, depression-, and anhedonia-like behaviors at various time points in mice treated with paclitaxel, according to the aforementioned treatment regimen. Alterations in anxiety were assessed utilizing the novelty suppressed feeding (NSF) assay. Paclitaxel significantly increased the latency to eat in a novel environment at 3 and 9 weeks post-paclitaxel injection (Fig. 5A and C). A significant increase in latency to eat occurred at 3 weeks post-paclitaxel treatment [t = 2.224, df = 12, P < 0.05, Fig. 5A]. When comparing latency to eat at weeks 9 and 11 post-paclitaxel treatment, the factor of time was significant [Ftime (1, 23) = 16.20, P < 0.001, Fig. 5C]. In addition, significant differences in latency to eat between vehicle- and paclitaxel-treated mice occurred at 9 weeks post-paclitaxel injection (P < 0.05), which dissipated by week 11, and between paclitaxel-treated mice at weeks 9 and 11 (P < 0.01). The amount of food consumed in the test cage was not impacted by paclitaxel treatment (Fig. 5B and D).
Figure 5.
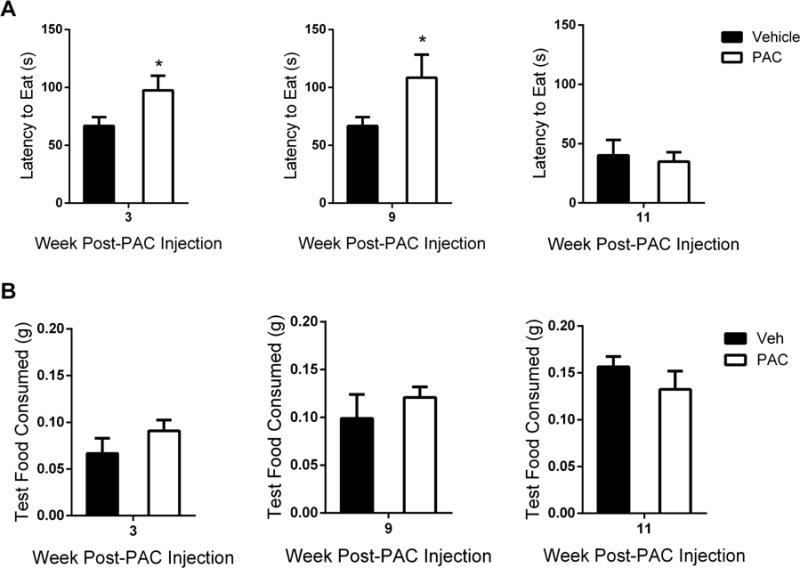
Paclitaxel induces anxiety-like behavior in the novelty suppressed feeding assay. (A) Latency to eat test cage food was determined as the time in seconds from the when the mouse was placed inside the test cage until the mouse sat on its haunches while holding and biting the food pellet. (B) Consumption of test cage food was calculated with the following equation: (initial weight of food pellet - weight of food pellet after 5 min eating period in test cage). Individual cohorts were tested at 3 weeks (n = 6 per group), 9 and 11 weeks (n = 6 Veh, n = 7 PAC) post-paclitaxel (8 mg/kg, i.p.) or vehicle injection; data expressed as mean ± SEM. *P < 0.05 vs vehicle. Post-PAC injection refers to the time following the first of four paclitaxel injections. Veh, vehicle; PAC, paclitaxel.
Paclitaxel was also found to induce anxiety-like behavior in the light/dark box (LDB) test, in which time spent in the light compartment of the LDB apparatus was significantly decreased at 3 weeks [t = 2.277, df = 14, P < 0.05], 6 weeks [t = 2.350, df = 14, P < 0.05], and 9 weeks [t = 2.309, df = 14, P < 0.05] post-paclitaxel treatment (Fig. 6). Importantly, the number of entries into the light compartment was not significantly decreased at any time point for the paclitaxel-treated mice (Table 1), suggesting that the decrease in time spent in the light compartment is not due to motor deficits (Supplementary Fig. 1B).
Figure 6.
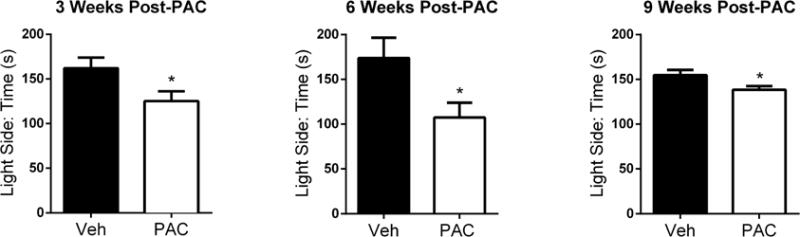
Paclitaxel induces anxiety-like behavior in the light/dark box test. Mice were free to explore both light and dark compartments for 5 min. The study was conducted with individual cohorts of mice (n = 8 per group) at 3, 6, and 9 weeks post-paclitaxel (8 mg/kg, i.p.) or vehicle injection; data expressed as mean ± SEM. *P < 0.05 vs vehicle. Post-PAC injection refers to the time following the first of four paclitaxel injections. Veh, vehicle; PAC, paclitaxel.
Table 1.
Paclitaxel treatment does not interfere with entry into the light compartment of the light/dark box apparatus. Unpaired t tests revealed no significant differences between vehicle- and paclitaxel-treated mice at any time point. One experiment was conducted with individual cohorts of mice (n = 8 per group) at each time point. Post-PAC injection refers to the time following the first of four paclitaxel injections. Data are expressed as mean ± SEM.
| Light Side: Number of Entries | |||
|---|---|---|---|
| 3 Weeks Post-PAC | 6 Weeks Post-PAC | 9 Weeks Post-PAC | |
| Vehicle | 16 ± 1.7 | 15 ± 2.0 | 14 ± 1.7 |
| Paclitaxel | 14 ± 1.9 | 13 ± 1.9 | 12 ± 1.7 |
The mice were then evaluated for depression-like behavior in FST, an experimental paradigm that assesses immobility when placed in a container of water. Within the same cohort of mice, paclitaxel treatment induced an emotional-like deficit during FST [Ftreatment × time (3,15) = 6.200, P < 0.01; Fig. 7]. The time spent immobile during FST was significantly increased at 2 and 3 weeks post paclitaxel-injection (P < 0.01), an effect that dissipated by week 4 (Fig. 7).
Figure 7.
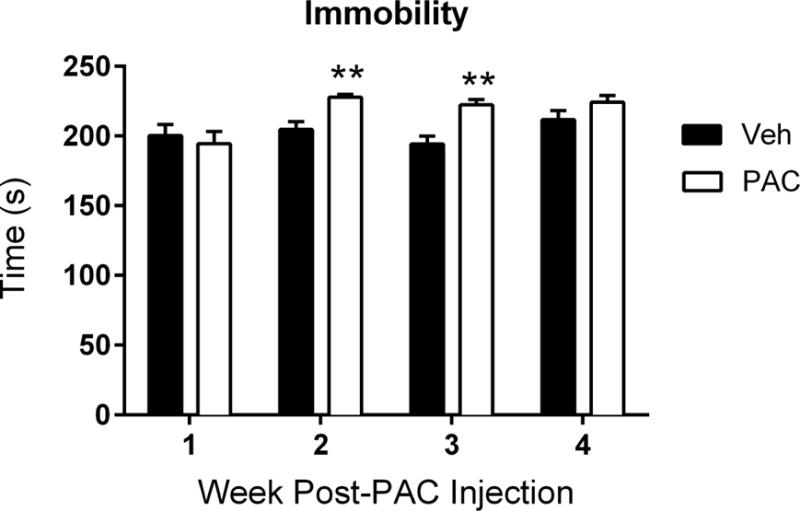
Paclitaxel induces depression-like behavior in the forced swim test. Time represents the number of seconds the mouse was immobile when placed in water; the cut-off time was 240 seconds. The same cohort of mice (n = 6 per group) was tested at weeks 1, 2, 3, and 4 post-paclitaxel (8 mg/kg, i.p.) or vehicle injection; data expressed as mean ± SEM. **P < 0.01 vs vehicle. Post-PAC injection refers to the time following the first of four paclitaxel injections. Veh, vehicle; PAC, paclitaxel.
Lastly, anhedonia-like behavior was assessed using the sucrose preference test. The interaction between paclitaxel treatment and time was significant within the same cohort of mice [Ftreatment × time (8,112) = 9.424, P < 0.0001, Fig. 8]. Paclitaxel produced a significant decrease in sucrose preference during (P < 0.0001) and shortly after (P < 0.01, P < 0.05) completion of the treatment regimen when compared to vehicle-treated mice (Fig. 8). To ensure that the decrease in consumatory behavior was not due to a decrease in overall consumption, we assessed total fluid intake between vehicle- and paclitaxel-treated mice, which was found to not differ significantly between the two groups (Supplementary Fig. 4).
Figure 8.
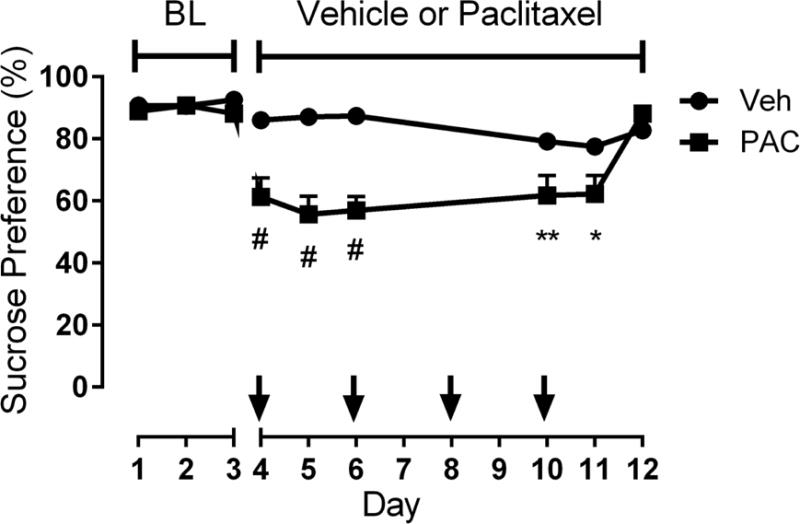
Paclitaxel induces anhedonia-like behavior in the sucrose preference test. Mice were provided with two sipper tubes, one containing normal drinking water and the other containing a 2% sucrose solution, for 24 h per day. Sucrose preference was determined as the percentage of 2% sucrose volume consumed over the total fluid intake volume. Arrows indicate the time of each paclitaxel (8 mg/kg, i.p.) or vehicle injection. The study was conducted with the same cohort of mice (n = 8 per group) during paclitaxel (8 mg/kg, i.p.) or vehicle injections; data expressed as mean ± SEM. *P < 0.05, **P < 0.01, #P < 0.0001 vs vehicle. BL, baseline; Veh, vehicle; PAC, paclitaxel.
4. Discussion
The results of the present study demonstrate that a clinically relevant dosing regimen of paclitaxel given systemically to male C57BL/6J mice causes the induction and long-term maintenance of mechanical and cold allodynia, as well as negative affective-related symptoms, including anxiety- and depression-like behaviors of shorter duration. These changes occurred without significant decreases in body weight or impairment of locomotion following paclitaxel treatment (Supplementary Fig. 1), findings that are in accordance with other studies showing that various doses of paclitaxel do not alter body weight (Boehmerle et al., 2014) or locomotor activity (Deng et al., 2015; Nieto et al., 2008).
Few studies have been performed under similar experimental conditions examining the effect of various doses of paclitaxel on the development of mechanical and cold allodynia, especially during the early period of injection and regarding the magnitude of that allodynia. Our results are consistent with other reports showing that paclitaxel induces both mechanical and cold allodynia in male mice (Deng et al., 2015; Slivicki et al., 2016; Naji-Esfahani et al., 2016). Interestingly, Ward et al. (2011) reported that a cycle of low-dose paclitaxel (1 or 2 mg/kg) elicited a considerably greater magnitude of cold allodynia in female mice than in male mice. Importantly, it has been noted in the clinic that neuropathic pain is more prevelant in women than in men (Fillingim et al., 2009). Therefore, it is possible that sex differences may arise in affective-like behaviors, along with nociceptive behaviors, following paclitaxel treatment.
With regard to morphological changes, our experiments show that 8 mg/kg paclitaxel produces a robust decrease in the density of intra-epidermal nerve fibers (IENFs), which is consistent with the results of Krukowski et al. (2015) that demonstrate significant reductions in IENF density following repeated adminsitrations of paclitaxel in mice. Additional studies in rats have shown a dose-dependent decrease in IENFs following a wide range of paclitaxel doses (0.5 – 32 mg/kg), as well as a correlation between paclitaxel-induced loss of IENFs and allodynia (Bennett et al., 2011; Ko et al., 2014). Also, it is known that the polymodal C and Aδ fibers are retracted following paclitaxel administration (Basbaum et al., 2009; Landowski et al., 2016; Vichaya et al., 2015). Despite the decrease in IENF density, the remaining nociceptive fibers can become hyperactive and/or sensitized due to their release of chemical mediators of inflammation, such as substance P and calcitonin gene-related peptide (CGRP), as well as exposure to pro-inflammatory cytokines released by infiltrating immune cells, such as macrophages (Carozzi et al., 2015).
The present study also revealed that two cycles of 2 mg/kg paclitaxel (cumulative dose of 16 mg/kg) causes mice to exhibit lower mechanical thresholds than mice that received the same cumulative dose following one cycle of 4 mg/kg paclitaxel (Supplementary Fig. 2; Fig. 1A). This finding suggests that sensitization occurs during the first cycle of paclitaxel treatment. The observed sensitization may be due to the accumulation of paclitaxel in the periphery, as detectable concentrations of paclitaxel have been measured in the dorsal root ganglia and the sciatic nerve up to 26 days post-paclitaxel dosing (Wozniak et al., 2016).
In the clinic, paclitaxel has been administered in combination with cisplatin in non-small cell lung cancer patients. The combination produces additional neurotoxicity, and even two cycles of the treatment can result in neuropathy (Arrieta et al., 2010). In an attempt to avoid this toxicity, paclitaxel and carboplatin have been used in combination. Carboplatin is considered to be less neurotoxic than cisplatin and only 4–6% of patients who receive carboplatin may develop peripheral neuropathy (McWhinney et al., 2009). Furthermore, a study in ovarian cancer patients revealed that the carboplatin-paclitaxel treatment induced significantly less peripheral neuropathy than that produced by the cisplatin-paclitaxel treatment (Neijt et al., 2000). Clinical studies have shown that administration of carboplatin before paclitaxel is feasible in patients (Markman et al., 2003; Davidson et al., 2016). Our data show that in contrast to paclitaxel, mice treated with carboplatin (5 or 20 mg/kg) alone failed to show signficant allodynia. However, when a low dose of carboplatin (5 mg/kg) was followed by a low dose of paclitaxel (1 mg/kg), mice develop more severe mechanical allodynia when compared to paclitaxel alone, suggesting that carboplatin sensitized the mice to subsequent paclitaxel treatment. To our knowledge, studies of carboplatin- or carboplatin-paclitaxel-induced mechanical allodynia in mice have not been reported previously.
This work also investigated the affective-related consequences of paclitaxel treatment. Using a paclitaxel regimen that caused a long-lasting allodynia (8 mg/kg, 1 cycle), we observed an increase in the latency to eat during the NSF assay and aversion to the light compartment of the LDB apparatus. These effects in two tests of anxiety suggest that, under the present experimental conditions, paclitaxel induces an anxiety-like state. We also found that paclitaxel-treated mice exhibit increased immobility time during FST and anhedonia-like behavior in the sucrose preference test. The observed decrease in sucrose preference could also indicate that an alteration in taste (dysgeusia), a phenomenon seen in some patients receiving paclitaxel (Turcott et al., 2016), is occurring during paclitaxel treatment; yet, we cannot make that conclusion from a single oral consumption assay. The possible taste alteration may produce decreased appetite, but no significant changes in body weight were detected. Collectively, these results indicate that in addition to peripheral neuropathy signs, paclitaxel induces a deficit in the emotional-like state of the mice. Conversely, paclitaxel did not affect nesting behavior, an assay that has been shown to reflect pain-depressed behavior when lactic acid and complete Freund adjuvant (CFA) are used as noxious stimuli (Negus et al., 2015). The lack of an effect in this assay is consistent with the hypothesis that the value of a habit-like survival task does not alter depending on the motivational state (Rock, et al. 2014). Thus, the necessity of establishing a nest for thermoregulation, fitness, and shelter may overcome the nociceptive and negative affective symptoms of paclitaxel.
To increase our understanding of paclitaxel-induced toxicity, the relationship between nociceptive and affective symptoms needs to be considered, as well as the temporal order in which these side effects develop. Studies have shown that the pathology of a tumor itself can cause emotional disturbances in rodents (Pyter et al., 2009), but our experiments in non-tumor-bearing mice reveal that paclitaxel alone is also capable of inducing anxiety- and depression- like behaviors. At 1 week post-paclitaxel injection, we observed the development of both mechanical and cold allodynia, as well as anhedonia-like behavior (Table 2). Anxiety- and depression-like behaviors arise in the subsequent weeks following paclitaxel treatment. The immediate appearance of nociceptive symptoms is consistent with paclitaxel acting directly on the peripheral nervous system, but there may be a separate central mechanism of the drug. While paclitaxel seems to accumulate in peripheral organs such as the peripheral nervous system, it has been detected in the brain of mice following tail vein injection, even at low concentrations (Gangloff et al., 2005; Kemper et al., 2003), suggesting that it crossed the blood brain barrier. Therefore, the presence of paclitaxel in the central nervous system and/or paclitaxel-induced peripheral neuropathy itself may be causing changes in affective behaviors through neuroinflammation mechanisms and/or an induction of central neurotoxicity. It is also possible that paclitaxel-induced sensitization of immune responses may have played a role in the development of peripheral neuropathy, and perhaps of affective-like behaviors. Indeed, hypersensitivity to stimuli, not only in neuropathic pain but also in inflammatory pain, can be explained by both peripheral and central sensitization of sensory nerve fibers (Fornasari, 2012). In regards to the neuroimmune interface, glial responses have also been shown to play a role in central and peripheral nervous system function during neuropathic pain (Scholz and Woolf, 2007).
Table 2.
Summary of onset and duration of nociceptive, natural, and affective behaviors. Post-PAC injection refers to the time following the first of four paclitaxel injections. NSF, novelty suppressed feeding; LDB, light/dark box; FST, forced swim test; (−), no phenotype; (+), nociceptive/affective behavior; ND, not determined.
| Weeks Post-PAC Injection | |||||||
|---|---|---|---|---|---|---|---|
| Behavior | Assay | 1 | 2–3 | 4–5 | 6–7 | 8–9 | 10–11 |
| Nociceptive | Mechanical Allodynia | + | + | + | + | + | + |
| Cold Allodynia | + | + | ND | ND | ND | ND | |
| Natural | Nesting | − | − | ND | ND | − | − |
| Anxiety-like | NSF | ND | + | ND | ND | + | − |
| LDB | ND | + | ND | + | + | ND | |
| Depression-like | FST | − | + | − | ND | ND | ND |
| Sucrose Preference | + | − | ND | ND | ND | ND | |
The differences between the onset, duration, and resolution of these affective behaviors should also be considered. Although changes in nociceptive behavior, such as mechanical allodynia, occur immediately following paclitaxel administration, there appears to be a delay in the initiation of emotional-like deficits. Clinically, somatic and affective symptoms can occur simultaneously. Breast cancer patients often experience a cluster of symptoms including pain (77%), anxiety (21%), and depression (36%), indicating that they may share a common mechanism (So et al., 2009). Those patients receiving chemotherapy experience the cluster symptoms to a greater degree and are at a higher risk for decreased quality of life.
The time-dependent development of both anxiety- and depression-like behaviors has also been observed in other mouse neuropathic pain models. La Porta et al. (2016) reported ipsilateral mechanical and cold allodynia from day 3 to day 27 post-partial sciatic nerve ligation (PSNL) in Swiss albino male mice, with enhanced anxiety-like behavior in the elevated plus maze from 1 to 3 weeks post-PSNL and increased depressive-like behavior during FST, but only at 3 weeks post-PSNL. Also, a significant decrease in sucrose preference was observed from day 1 to day 20 post-PSNL. Although this study utilized a different model of neuropathic pain, alterations in nociceptive behaviors were also induced immediately and persisted for approximately four weeks. However, we found that anxiety-like behavior can be maintained for 9 weeks following nerve exposure to a noxious stimulus. Consistent findings were made in regards to depression-like behavior, in which increased immobility during FST did not appear until 2–3 weeks. We recognize that repeated testing of the same cohort during FST could be a limitation, however, vehicle-treated mice did not express adaptation to the assay. The development of anhedonia-like behavior was also similar, during which a decrease in sucrose preference was observed the day following PSNL or paclitaxel treatment, but the effect only persisted for 11 days post-paclitaxel injection, whereas PSNL induced this behavior until day 20.
Similarly, using sciatic nerve constriction (SNC) in male C57BL/6J mice, Yalcin, et al. (2011) reported that ipsilateral mechanical allodynia persisted for 90 days, and increased anxiety-like behavior in the light/dark box test was observed at 4, 7, and 8 weeks post-SNC, a time-dependent effect similar to that seen in the present study. Latency to first contact and bite the food pellet during the NSF assay was observed at 5 and 8 weeks post-SNC, an effect that appeared earlier in paclitaxel-induced neuropathic pain. Increased immobility in neuropathic mice was observed at 8 and 9 weeks post-SNC during FST, whereas paclitaxel-induced neuropathic pain caused immobility at 2 and 3 weeks post-paclitaxel injection. The differences and similarities amongst these studies illustrate the importance of establishing a clinically relevant model specific to the type of neuropathic pain of interest in order to best determine the responsible mechanisms. Also, these data suggest that multiple pathways and/or brain regions are involved in the manifestation of affective-related behaviors. Yet it remains plausible that paclitaxel administration and models of nerve injury share common mechanisms for the induction of affective-related behaviors.
In conclusion, this work characterizes a preclinical mouse model of both the nociceptive and negative affective symptoms of paclitaxel treatment, which can be utilized to test the efficacy of potential therapeutics for the treatment of paclitaxel-induced side effects, as well as investigate mechanisms of action. In addition, this study allows for the separate investigation of chemotherapy-induced pain-realted behaviors in a tumor-free environment, which cannot be ethically accomplished in a clinical setting.
Supplementary Material
Supplementary Figure 1. Paclitaxel has no effect on body weight and spontaneous activity in mice. One cycle of paclitaxel (8 mg/kg, i.p., every other day for a total of 4 injections) did not cause significant loss of body weight (A) or alteration in motor coordination (B) compared to vehicle-treated mice. Vehicle/paclitaxel injections were administered on days 0, 2, 4, and 6. Baseline measurements were taken before vehicle/paclitaxel administration on day 0. One cohort was tested (n = 6 per group); data expressed as mean ± SEM.
Supplementary Figure 2. Paclitaxel sensitizes mice to cutaneous stimulation after second cycle. Comparison of mechanical threshold during the initial 28 days of one- or two-cycle paclitaxel (2 mg/kg, i.p.) or vehicle treatment. Arrows indicate vehicle/paclitaxel injections on days 0, 2, 4, and 6. Baseline measurements were taken before vehicle/paclitaxel administration on day 0. One cohort was tested (n = 6 per group); data expressed as mean ± SEM. *P < 0.05 vs vehicle; #P < 0.05 vs first cycle of paclitaxel (2 mg/kg). Veh, vehicle; PAC, paclitaxel.
Supplementary Figure 3. Carboplatin alone does not induce mechanical allodynia. Dose-response curve for mice treated with one cycle of carboplatin at doses of 0, 5, and 20 mg/kg, i.p., every other day for a total of 4 injections. Arrows indicate vehicle/carboplatin injections on days 0, 2, 4, and 6. Baseline measurements were taken before vehicle/carboplatin administration on day 0. One cohort was tested (n = 6 per group); data expressed as mean ± SEM.
Supplementary Figure 4. Paclitaxel treatment does not interfere with total fluid intake. The total fluid intake is the sum of volumes of water and 2% sucrose consumed by each mouse. Arrows indicate the time of each paclitaxel (8 mg/kg, i.p.) or vehicle injection (n = 8 per group); data expressed as mean ± SEM. A two-way ANOVA was performed, followed by the Bonferroni post hoc test; no significant differences between vehicle- and paclitaxel-treated mice were found at any time point. BL, baseline; Veh, vehicle; PAC, paclitaxel.
Highlights.
Paclitaxel induces mechanical and cold allodynia in a dose-dependent manner.
Carboplatin enhances magnitude of paclitaxel-induced mechanical allodynia.
Paclitaxel induces anxiety-, depression-, and anhedonia-like behaviors in mice.
Acknowledgments
This study was supported by NIH grant R01-CA206028 to MID and DAG, and partly supported by pilot funding from the VCU Massey Cancer Center supported, in part, with funding from NIH-NCI Cancer Center Support Grant P30 CA016059.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest
The authors declare no conflicts of interest.
References
- Arrieta O, Michel Ortega RM, Villanueva-Rodriguez G, Serna-Thomé MG, Flores-Estrada D, Diaz-Romero C, Rodríguez CM, Martínez L, Sánchez-Lara K. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer. 2010;10:50. doi: 10.1186/1471-2407-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdas D, AlSharari SD, Freitas K, Tracy M, Damaj MI. The role of alpha5 nicotinic acetylcholine receptors in mouse models of chronic inflammatory and neuropathic pain. BiochemPharmacol. 2015;97:590–600. doi: 10.1016/j.bcp.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Review Cellular and Molecular Mechanisms of Pain. 2009:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijers AJM, Jongen JLM, Vreugdenhil G. Chemotherapy-induced neurotoxicity: The value of neuroprotective strategies. Neth J Med. 2012;70:18–25. [PubMed] [Google Scholar]
- Bennett GJ, Liu GK, Xiao WH, Jin HW, Siau C. Terminal arbor degeneration - a novel lesion produced by the antineoplastic agent paclitaxel. Eur J Neurosci. 2011;33:1667–1676. doi: 10.1111/j.1460-9568.2011.07652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnoff SR, Suranyi-Cadotte B, Aitken DH, Quirion R, Meaney MJ. The effects of chronic antidepressant treatment in an animal model of anxiety. Psychopharmacology (Berl) 1988;95:298–302. doi: 10.1007/BF00181937. [DOI] [PubMed] [Google Scholar]
- Boehmerle W, Huehnchen P, Peruzzaro S, Balkaya M, Endres M. Electrophysiological, behavioral and histological characterization of paclitaxel, cisplatin, vincristine and bortezomib-induced neuropathy in C57Bl/6 mice. Sci Rep. 2014;4:6370. doi: 10.1038/srep06370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanova OV, Kanekar S, Anci KED, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav. 2013;118:227–239. doi: 10.1016/j.physbeh.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carozzi VA, Canta A, Chiorazzi A. Chemotherapy-induced peripheral neuropathy: What do we know about mechanisms? Neurosci Lett. 2015;596:90–107. doi: 10.1016/j.neulet.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Charan J, Kantharia ND. How to calculate sample size in animal studies? J Pharmacol and Pharmacother. 2013;4:303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley J, Goodwin FK. Preliminary report of a simple animal behavior model for the anxiolytic effects of benzodiazepines. Pharmacol Biochem Behav. 1980;13:167–170. doi: 10.1016/0091-3057(80)90067-2. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, Lukas RJ, Martin BR. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Davidson BA, Foote J, Clark LH, Broadwater G, Havrilesky LJ. Tumor grade and chemotherapy response in endometrioid endometrial cancer. Gynecol Oncol Reports. 2016;17:3–6. doi: 10.1016/j.gore.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L, Guindon J, Cornett BL, Makriyannis A, Mackie K, Hohmann AG. Chronic cannabinoid receptor 2 activation reverses paclitaxel neuropathy without tolerance or cannabinoid receptor 1-dependent withdrawal. Biol Psychiatry. 2015;77:475–487. doi: 10.1016/j.biopsych.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranitsaris G, Yu B, King J, Kaura S, Zhang A. Nab-paclitaxel, docetaxel, or solvent-based paclitaxel in metastatic breast cancer: A cost-utility analysis from a Chinese health care perspective. Clin Outcomes Res. 2015;7:249–256. doi: 10.2147/CEOR.S82194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, Gender, and Pain: A Review of Recent Clinical and Experimental Findings. J Pain. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornasari D. Pain mechanisms in patients with chronic pain. Clin Drug Investig. 2012;32:45–52. doi: 10.2165/11630070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gangloff A, Hsueh WA, Kesner AL, Kiesewetter DO, Pio BS, Pegram MD, Beryt M, Townsend A, Czernin J, Phelps ME, Silverman DHS. Estimation of paclitaxel biodistribution and uptake in human-derived xenografts in vivo with (18)F-fluoropaclitaxel. J Nucl Med. 2005;46:1866–1871. doi:46/11/1866 [pii] [PubMed] [Google Scholar]
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, Rieder A. Prevalence of self-reported neuropathic pain and impact on quality of life: A prospective representative survey. Acta Anaesthesiol Scand. 2008;52:132–136. doi: 10.1111/j.1399-6576.2007.01486.x. [DOI] [PubMed] [Google Scholar]
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–265. doi: 10.1038/nr1317. [DOI] [PubMed] [Google Scholar]
- Kemper EM, Van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, Van Tellingen O. Increased penetration of paclitaxel into the brain by inhibition of P-glycoprotein. Clin Cancer Res. 2003;9:2849–2855. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M, Hu M, Hsieh Y, Lan C, Tseng T. Neuropeptides Peptidergic intraepidermal nerve fibers in the skin contribute to the neuropathic pain in paclitaxel-induced peripheral neuropathy. Neuropeptides. 2014;48:109–117. doi: 10.1016/j.npep.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Krukowski K, Nijboer CH, Huo X, Kavelaars A, Heijnen CJ. Prevention of chemotherapy-induced peripheral neuropathy by the small-molecule inhibitor pifithrin-[mu] Pain. 2015;156:2184–2192. doi: 10.1097/j.pain.0000000000000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Porta C, Lara-Mayorga IM, Negrete R, Maldonado R. Effects of pregabalin on the nociceptive, emotional and cognitive manifestations of neuropathic pain in mice. Eur J Pain (United Kingdom) 2016:1–13. doi: 10.1002/ejp.868. [DOI] [PubMed] [Google Scholar]
- Markman M, Elson P, Kulp B, Peterson G, Zanotti K, Webster K, Belinson J. Carboplatin plus paclitaxel combination chemotherapy: Impact of sequence of drug administration on treatment-induced neutropenia. Gynecol Oncol. 2003;91:118–122. doi: 10.1016/S0090-8258(03)00517-1. [DOI] [PubMed] [Google Scholar]
- Massie MJ. Prevalence of depression in patients with cancer. J Natl Cancer Inst Monogr. 2004;10021:57–71. doi: 10.1093/jncimonographs/lgh014. [DOI] [PubMed] [Google Scholar]
- McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehnert A, Brähler E, Faller H, Härter M, Keller M, Schulz H, Wegscheider K, Weis J, Boehncke A, Hund B, Reuter K, Richard M, Sehner S, Sommerfeldt S, Szalai C, Wittchen HU, Koch U. Four-week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol. 2014;32:3540–3546. doi: 10.1200/JCO.2014.56.0086. [DOI] [PubMed] [Google Scholar]
- Naji-Esfahani H, Vaseghi G, Safaeian L, Pilehvarian AA, Abed A, Rafieian-Kopaei M. Gender differences in a mouse model of chemotherapy-induced neuropathic pain. Lab Anim. 2015 doi: 10.1177/0023677215575863. [DOI] [PubMed] [Google Scholar]
- Negus SS, Neddenriep B, Altarifi AA, Carroll FI, Leitl MD, Miller LL. Effects of ketoprofen, morphine, and kappa opioids on pain-related depression of nesting in mice. Pain. 2015;156:1153–1160. doi: 10.1097/j.pain.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijt JP, Engelholm SA, Tuxen MK, Sørensen PG, Hansen M, Sessa C, De Swart CAM, Hirsch FR, Lund B, Van Houwelingen HC. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18:3084–3092. doi: 10.1200/JCO.2000.18.17.3084. [DOI] [PubMed] [Google Scholar]
- Nieto FR, Entrena JM, Cendan CM, Del Pozo E, Vela JM, Baeyens JM. Tetrodotoxin inhibits the development and expression of neuropathic pain induced by paclitaxel in mice. Pain. 2008;137:520–531. doi: 10.1016/j.pain.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Otrubova K, Brown M, McCormick MS, Han GW, O’Neal ST, Cravatt BF, Stevens RC, Lichtman AH, Boger DL. Rational design of fatty acid amide hydrolase inhibitors that act by covalently bonding to two active site residues. J Am Chem Soc. 2013;135:6289–6299. doi: 10.1021/ja4014997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Pineros V, Galang JA, McClintock MK, Prendergast BJ. Peripheral tumors induce depressive-like behaviors and cytokine production and alter hypothalamic-pituitary-adrenal axis regulation. Proc Natl Acad Sci U S A. 2009;106:9069–74. doi: 10.1073/pnas.0811949106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- Rock ML, Karas AZ, Rodriguez KBG, Gallo MS, Pritchett-Corning K, Karas RH, Aronovitz M, Gaskill BN. The time-to-integrate-to-nest test as an indicator of wellbeing in laboratory mice. J Am Assoc Lab Anim Sci. 2014;53:24–8. [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL, Ungaro P, Norton L, Winer E, Hudis C. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, Macleod MR, Colvin LA, Fallon M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155:2461–2470. doi: 10.1016/j.pain.2014.09.020. [DOI] [PubMed] [Google Scholar]
- Slivicki RA, Ali YO, Lu HC, Hohmann AG. Impact of genetic reduction of NMNAT2 on chemotherapy-induced losses in cell viability in vitro and peripheral neuropathy in vivo. PLoS One. 2016;11:e0147620. doi: 10.1371/journal.pone.0147620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So WKW, Marsh G, Ling WM, Leung FY, Lo JCK, Yeung M, Li GKH. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol Nurs Forum. 2009;36:E205–14. doi: 10.1188/09.ONF.E205-E214. [DOI] [PubMed] [Google Scholar]
- Thompson RD, Grant CV. Automated preference testing apparatus for rating palatability of foods. J Exp Anal Behav. 1971;15:215–220. doi: 10.1901/jeab.1971.15-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcott JG, Juarez-Hernandez E, De la Torre-Vallejo M, Sanchez-Lara K, Luvian-Morales J, Arrieta O. Value: Changes in the detection and recognition thresholds of three basic tastes in lung cancer patients receiving cisplatin and paclitaxel and its association with nutritional and quality of life parameters. Nutr Cancer. 2016;68:241–249. doi: 10.1080/01635581.2016.1144075. [DOI] [PubMed] [Google Scholar]
- Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, Heijnen CJ, Walker AK. Mechanisms of chemotherapy-induced behavioral toxicities. 2015;9:1–17. doi: 10.3389/fnins201500131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SJ, Ramirez MD, Neelakantan H, Walker EA. Cannabidiol prevents the development of cold and mechanical allodynia in paclitaxel-treated female c57bl6 mice. Anesth Analg. 2011;113:947–950. doi: 10.1213/ANE.0b013e3182283486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson JL, Ghosh S, Bagdas D, Mason BL, Crowe MS, Hsu KL, Wise LE, Kinsey SG, Damaj MI, Cravatt BF, Lichtman AH. Diacylglycerol lipase β inhibition reverses nociceptive behaviour in mouse models of inflammatory and neuropathic pain. Br J Pharmacol. 2016;173:1678–1692. doi: 10.1111/bph.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak KM, Vornov JJ, Wu Y, Nomoto K, Littlefield BA, DesJardins C, Yu Y, Lai G, Reyderman L, Wong N, Slusher BS. Sustained accumulation of microtubule-binding chemotherapy drugs in the peripheral nervous system: correlations with time course and neurotoxic severity. Cancer Res. 2016;76:3332–3339. doi: 10.1158/0008-5472.CAN-15-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcin I, Bohren Y, Waltisperger E, Sage-Ciocca D, Yin JC, Freund-Mercier MJ, Barrot M. A time-dependent history of mood disorders in a murine model of neuropathic pain. Biol Psychiatry. 2011;70:946–953. doi: 10.1016/j.biopsych.2011.07.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Paclitaxel has no effect on body weight and spontaneous activity in mice. One cycle of paclitaxel (8 mg/kg, i.p., every other day for a total of 4 injections) did not cause significant loss of body weight (A) or alteration in motor coordination (B) compared to vehicle-treated mice. Vehicle/paclitaxel injections were administered on days 0, 2, 4, and 6. Baseline measurements were taken before vehicle/paclitaxel administration on day 0. One cohort was tested (n = 6 per group); data expressed as mean ± SEM.
Supplementary Figure 2. Paclitaxel sensitizes mice to cutaneous stimulation after second cycle. Comparison of mechanical threshold during the initial 28 days of one- or two-cycle paclitaxel (2 mg/kg, i.p.) or vehicle treatment. Arrows indicate vehicle/paclitaxel injections on days 0, 2, 4, and 6. Baseline measurements were taken before vehicle/paclitaxel administration on day 0. One cohort was tested (n = 6 per group); data expressed as mean ± SEM. *P < 0.05 vs vehicle; #P < 0.05 vs first cycle of paclitaxel (2 mg/kg). Veh, vehicle; PAC, paclitaxel.
Supplementary Figure 3. Carboplatin alone does not induce mechanical allodynia. Dose-response curve for mice treated with one cycle of carboplatin at doses of 0, 5, and 20 mg/kg, i.p., every other day for a total of 4 injections. Arrows indicate vehicle/carboplatin injections on days 0, 2, 4, and 6. Baseline measurements were taken before vehicle/carboplatin administration on day 0. One cohort was tested (n = 6 per group); data expressed as mean ± SEM.
Supplementary Figure 4. Paclitaxel treatment does not interfere with total fluid intake. The total fluid intake is the sum of volumes of water and 2% sucrose consumed by each mouse. Arrows indicate the time of each paclitaxel (8 mg/kg, i.p.) or vehicle injection (n = 8 per group); data expressed as mean ± SEM. A two-way ANOVA was performed, followed by the Bonferroni post hoc test; no significant differences between vehicle- and paclitaxel-treated mice were found at any time point. BL, baseline; Veh, vehicle; PAC, paclitaxel.


