Abstract
Introduction
Age-associated muscle strength decline is a major contributing factor to increased late-life functional decline and comorbidity, and is strongly associated with early mortality. Although all parts of the neuromuscular system seem to be affected by aging, dying-back of motor axons likely plays a major role.
Methods
We compared the degeneration in ventral roots and neuromuscular junction denervation in young and aged mice and correlated the findings with strength and electrophysiological measures.
Results
With normal aging, there is little decline in motor axon numbers in the ventral roots, but the neuromuscular junctions show marked partial denervation that is associated with increased jitter on stimulated single fiber electromyography and a decrease in muscle strength.
Conclusions
These findings suggest that dying-back axonal degeneration may be partially responsible for the electrophysiological and strength changes observed with aging.
Keywords: denervation, distal axon, dying-back axonopathy, frailty, neuromuscular junction, SFEMG
Skeletal muscle function declines with aging, and this decline is a major determinant of late-life disability and morbidity and a predictor of overall survival rates in older adults.1–5 Alterations in the peripheral neuromuscular system, including reduced number of motor neurons in the spinal cord,6–9 partial denervation at the neuromuscular junction (NMJ),10,11 and muscle atrophy,12,13 have been observed with aging. However, there has been no unifying explanation on how these phenomena occur in the context of aging. Longitudinal studies of normal aging have shown that muscle weakness starts long before muscle atrophy occurs,14 which suggests that age-related changes in the peripheral nervous system may precede muscle atrophy. This observation is similar to what is seen in amyotrophic lateral sclerosis (ALS), a motor neuron disease, where substantial evidence suggests that synaptic dysfunction at the NMJ precedes the motor neuron degeneration and is likely responsible for muscle weakness in early stages of the disease.15–18 In normal aging, similar observations have been made that greater early changes occur at the NMJ than in motor neurons in the spinal cord.11,16,19,20 Considering that nerve cells undergo distal dying-back axonal degeneration under metabolic stress,21 it is possible that both ALS and normal aging undergo this centripetal pattern of degeneration in the peripheral nervous system. A recent study showed that ALS is a distal axonopathy, demonstrating NMJ denervation as the earliest change in the nervous system of SOD1 knockout mice, an animal model of ALS.22 However, given the significant changes in the central nervous system that occur with aging, it is also possible that motor neurons in the spinal cord initiate the axonal degeneration and eventually cause NMJ denervation in a centripetal fashion. It remains to be examined whether the age-associated muscle weakness is the result of a dying-back or dying-forward process. The demonstration of dying-back or dying-forward axonopathy requires direct comparison between the distal and proximal parts of motor axons, which has not been done in the context of normal aging. In this study, we provide electrophysiological and histological evidence of distal axonal degeneration in age-associated muscle weakness by comparing the most distal (NMJ) and proximal (ventral roots) parts of motor neurons in young (2–4 months) and old (22–25 months) C57BL/6J mice.
METHODS
Animals
Wild-type C57BL/6J mice were divided into 2 age groups, young and old. All the mice in the young group were 2–4 months old, whereas old mice were 22–25 months old. For comparison, there were at least 4–7 mice per group, and all were male. Experiments were performed in accordance with protocols approved by the animal care and use committee at Johns Hopkins University.
Grip Strength Measurement
Grip strength was measured using a Chatillon force measurement device (Ametek, Berwyn, Pennsylvania). The mouse was held by the tail and allowed to grasp the bar on the device. As the experimenter pulled the tail at a 45° angle, the maximum force generated was recorded in kilogram-force (kgF), which was divided by the body weight (grams) of each mouse. Grip strengths (kgF/g) of forelimbs and all limbs were measured separately. All measurements were done 3 times, and the average score was recorded.
Stimulated Single Fiber Electromyography
Stimulated single fiber electromyography (SSFEMG) was performed using a Cadwell electromyography (EMG) unit (Sierra Wave, Kennewick, Washington). The mice were first anesthetized using isoflurane delivered via a nose cone. After anesthesia was induced, a 27-gauge, 25-mm single fiber needle electrode with a platinum–iridium recording surface (Technomed Europe) was inserted into the right gastrocnemius, and a stimulating needle was inserted near the right sciatic nerve. Motor axons were stimulated at a rate of 10 Hz. Other SSFEMG settings included 500 Hz to 10 kHz for the filters, sweep speed range from 1 to 2 ms/div, and gain range from 100 μV to 1 mV. Stimulation intensity was adjusted within a range of 10–30 mA to avoid visible muscle contraction. A single fiber action potential (SFAP) was defined by a potential that arose within 2 ms from stimulation and was >200 μV in amplitude with a rise time of <200 μs.23 The SFAPs were recorded in response to needle stimulation of intramuscular nerves by moving the needle to at least 10 different locations. The average mean consecutive difference (MCD) from the 10 different locations was then calculated.
Immunofluorescent Quantification of NMJs
Soleus muscles were dissected, pinned in mild stretch, and fixed by immersion for 20 minutes in 4% para-formaldehyde with phosphate-buffered saline (PBS; pH 7.4). After rinsing in PBS, muscles were soaked in 15% sucrose with PBS (overnight at 4°C), followed by 30% sucrose with PBS for at least 24 hours. Muscle tissues were embedded in OCT and sectioned with a cryostat (HM 550; Microm GmbH, Walldorf, Germany) into 40-mm sections and placed on glass slides for staining. For a pre-synaptic marker, sections were incubated with βIII-tubulin (1:500; Promega Catalog No. G7121) antibody overnight at 4°C, followed by secondary fluorescein isothiocyanate–labeled goat anti-mouse (1:100; Jackson ImmunoResearch Catalog No. 115-095-205) antibody. Rhodomine bungarotoxin (1:40, ThermoFisher Catalog No. B13423) was used as a postsynaptic marker. Stained sections were examined under a fluorescence and confocal microscope. The Zeiss LSM Image Examiner version 1.0.0.241 (Carl Zeiss, Oberkochen, Germany) was used for imaging analysis.
Ventral Root Nerve Morphometry
Spinal and peripheral nerve roots were exposed, dissected out, and immersion-fixed in 3% buffered glutaraldehyde (pH 7.4) at 4°C for 24 hours. Ventral roots from L5 were resected and transferred into 0.1 mol/L phosphate buffer at 4°C before post-fixation in osmium tetroxide. They were embedded in plastic, sectioned at 1 mm using an Ultracut E microtome (Reichert Technologies, Depew, New Jersey), and stained with toluidine blue (1% toluidine blue in 1% sodium tetraborate). Digital images of the ventral roots were taken using an unbiased sampling method of non-overlapping regions of the whole cross-section of the ventral roots. Total numbers of myelinated axons per cross-section of L5 ventral roots were quantified using ImageJ software (National Institutes of Health, Bethesda, Maryland). The morphometric evaluations were made from both large and small myelinated fibers. Axon interiors were visually identified and manually marked as solid objects. In addition, axon numbers, axon diameters, and myelin thicknesses were calculated. For each sample, at least 200 myelinated axons were measured, and the average was counted as n =1 for analysis.
Statistical Analysis
Unpaired Student t-tests (GraphPad Prism, GraphPad, Inc., San Diego, California) were used to compare grip strength, total axon count per root, axon density, axon diameter, G-ratio, acetylcholine receptor (AChR) occupancy, postsynaptic area, and mean MCDs between young and old mice. P <0.05 was considered statistically significant.
RESULTS
Grip Strength Reduced in Older Mice
Given that distal axonal degeneration most severely affects the distal limbs, we measured the grip strength of the limbs and compared findings between young and old mice. Average grip strengths for both forelimbs and all limbs, normalized by body weight, were significantly reduced in the old mice, compared with the young mice, as shown in Figure 1, reflecting the fact that limb muscle strength declines with age.
FIGURE 1.
Comparison of grip strength between old and young mice. Grip strength of both forelimbs (A) and all limbs (B) are reduced in the old mice (N =5), compared with the young mice (N =4).
NMJ Denervation More Extensive in Older Mice
Considering that the preterminal portion of the axon at the NMJ is the most distal part of the motor axon, dying-back axonal degeneration will result in partial or full retraction of the presynaptic axon from the NMJ. Using Z-stacked confocal images of the NMJs, we compared the occupancy of the postsynaptic endplate region by nerve terminals between young and old mice by calculating the percentage of total presynaptic area over the total postsynaptic area of the NMJs. As seen in Figure 2, postsynaptic motor endplates of old mice were fragmented and disorganized, whereas those of young mice maintained a pretzel-shaped, branched structure in a well-organized fashion (Fig. 2A and B). When quantified, the NMJs in the old mice showed significantly lower occupancy of postsynaptic area by presynaptic terminals, suggesting partial denervation of the NMJs in the old mice (Fig. 2C). In addition, numerous muscle fibers were observed in the old mice exclusively with AChRs scattered diffusely along the entire fiber without forming an NMJ structure with pre-terminal axons, suggesting complete denervation (Fig. 2E and F).24 Morphological differences between young and old NMJs were also examined. The total size of the postsynaptic area was larger in the old mice than in the young mice, which is consistent with earlier observations16,19,20,25 (Fig. 2D).
FIGURE 2.
Partial denervation of NMJs in soleus muscles of young and old mice. Double immunofluorescence staining was performed (green =βIII-tubulin; red =bungarotoxin), and the confocal images were analyzed. (A) NMJ of soleus muscle in a young mouse. Note that postsynaptic motor endplates (yellow arrow) appear well-organized, distinct, and compact, with presynaptic axons covering wide areas of motor endplates. (B) NMJ of soleus muscle in an old mouse. Postsynaptic motor endplates are fragmented and disorganized (yellow arrow). Diameters of the motor endplates are larger, and presynaptic axons do not cover the entire areas of the motor end-plates, suggesting partial denervation (yellow arrow). (C) The percentage coverage of presynaptic axons over the postsynaptic motor endplate (AChR occupancy) was calculated using confocal imaging software; the results show significantly greater coverage in the young mice (N =5, 91.93%) than in the old mice (N =5, 40.97%). (D) Comparison of postsynaptic endplate area. The postsynaptic endplate areas are much greater in the old mice (N =5, average 497.74 μm2 than in the young mice (N =5, average 299.76 μm2. Representative images of completely denervated NMJs in old mice are seen in panels (E) and (F) (green =βIII-tubulin; red =bungarotoxin).
Number of Axons in Lumbar Ventral Roots Comparable in Young and Old Mice
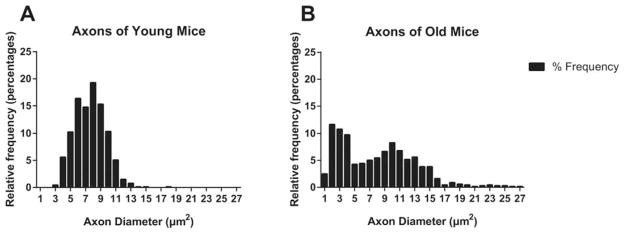
The ventral roots consist of only motor fibers before they merge with sensory fibers at the neural foramina, making them the most proximal portion of the motor axon. Therefore, direct comparison of proximal and distal parts of the motor axons was possible by comparing ventral roots and preterminal axons of intramuscular motor nerves. After harvesting L5 ventral roots from 2–4-month-old and 22–25-month-old mice, we examined cross-sections of the ventral roots and carried out nerve morphometry using ImageJ software. There were no statistical differences in either the number of axons per root or per cross-section of ventral roots between the 2 age groups (Fig. 3A and B). We also compared the diameter of ventral root axons and found that there was no statistical difference between the 2 age groups (Fig. 3C). The G-ratio is a ratio of axon diamater to fiber diameter; it evaluates the relationship between axon diameter and myelin thickness and is an important determinant of conduction velocity, with an optimal G-ratio being 0.6.26 In our study, there was no statistical difference in G-ratio between the 2 age groups, and the median G-ratios were 0.7248 for the young group and 0.7215 for the old group (Fig. 3D). However, it is noteworthy that, in both axon diameter and G-ratio, standard errors were much greater in old mice than in young mice (axon diameter: young 0.2879 vs. old 0.5194; G-ratio: young 0.0061 vs. old 0.0187), whereas the mean values remained statistically not different. As seen in Figure 4, the axon diameter frequency histogram shows a Gaussian distribution of axon diameter in the young mice, whereas the old mice show a bimodal distribution of axon diameter with outliers in both directions. In fact, this greater variability in G-ratio was consistent with our observation under the microscope, which showed numerous thinly myelinated fibers in the axons of old mice (Fig. 3E and F).
FIGURE 3.
Comparison of L5 ventral roots between old and young mice. (A) Total counts of axons of cross-sections of L5 ventral roots are compared between young (N =7) and old (N =6) mice. Note that the standard deviation of the old group is greater than that of the young group, but there is no statistical difference in total axon counts between the 2 groups. (B–D) There were no statistical differences between the young and old groups for axon count per area, axon diameter, or G-ratio. (E) Cross-section of a young L5 ventral root. Note that the ratio of axonal diameter and myelin thickness is constant between different axons, and most axons have similar sizes. (F) Cross-section of an old L5 ventral root. Note the variation in ratio of axonal diameter and myelin thickness (yellow arrows, the axons with larger diameter have thinner myelin), with some axons showing early signs of myelin stripping. However, the average G-ratio between the 2 groups was not statistically different, although the standard deviation was greater in the old group.
FIGURE 4.
Relative frequency histogram of axon diameter in young and old mice. (A) Axon diameters of young mice centered around the mean value (7.62 μm2, composing a Gaussian distribution. (B) Axon diameters of old mice show a bimodal distribution with some outliers toward both larger and smaller diameters.
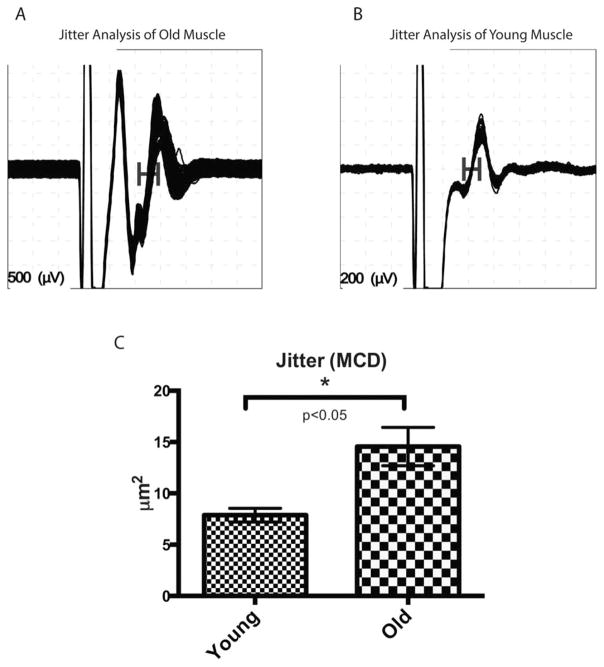
Increased SSFEMG Jitter Values in Older Mice
SSFEMG was performed in the gastrocnemius–sole-us muscle complex under anesthesia, as described in the Methods section. Single fiber EMG jitter values are known to accurately represent the safety factor of neuromuscular transmission, and an increase in jitter suggests abnormal neuromuscular transmission.27 In this study, the mean jitter level was calculated as the MCD from 100 different pairs, and a mean MCD was measured from 10 different sites per animal. Mean MCDs were compared between the 2 age groups; the mean MCD of old mice is significantly higher than that of young mice, which suggests abnormal neuromuscular transmission in an age-dependent manner (Fig. 5A–C). These results are consistent with previous studies that showed age-related, abnormal neuromuscular transmission in intracellular recordings in aged Drosophila and rodent models.28–30
FIGURE 5.
Jitter analysis of SSFEMG. (A) Jitter analysis of old mice. Note the increased jitter (horizontal bar) in the second single fiber action potential. (B) Jitter analysis of young mice. (C) Jitter as represented by MCD (mean consecutive difference). Mean jitter of old mice (N =5) was significantly increased over that of young mice (N =4).
DISCUSSION
In this study we have shown a greater extent of nerve degeneration in distal axons of motor neurons in old mice, as compared with the most proximal portions of ventral roots. This observation suggests that a dying-back axonal process underlies age-associated muscle weakness and provides a unifying explanation of various observations in the neuromuscular system with aging, such as degeneration of NMJs, reduced number of motor neurons, and muscle atrophy. Garcia et al. compared mitochondrial morphology in motor neurons and preterminal axons of young and old rats. They found that preterminal axons in aged animals had a larger number of mitochondria with swollen cristae and many more abnormally large mitochondria, suggesting that abnormal mitochondria cause dying-back axonal degeneration; however, no studies have directly compared proximal and distal portions of motor axons in the context of normal aging.31 Characterizing this neurological process is crucially important for a number of reasons. First, molecular investigation of age- associated muscle weakness becomes possible by focusing on known molecular mechanisms of dying-back axonal degeneration. Second, this approach to studying NMJ change may facilitate the development of novel diagnostic strategies that could detect changes years before the dying-back axonopathy progresses. Third, such a diagnostic strategy approach would eventually facilitate development of preventive or rehabilitative strategies.
The results from NMJ analyses are consistent with previous studies.19,20,25,32 We showed more presynaptic denervation in the soleus muscle of old mice and found that old NMJs have larger postsynaptic area and are more fragmented, as also described in earlier works.19,25 It remains to be determined whether the age-related partial denervation of the presynaptic area is driven by signals from the nerve or muscle side. Regardless, considering that maintaining NMJ structure requires a complex interaction between both nerve and muscle, the partial retraction of presynaptic nerve terminals is likely initiated by both. Another interesting question is whether or not reinnervation occurs as the denervation progresses, although the absence of fiber type grouping in previous studies suggests that attempted reinnervation is not successful in the context of aging.33 It appears that the progression of age-associated axonal degeneration becomes irreversible once it reaches a certain point.
We performed various analyses of ventral roots and found no statistical differences in measurements of axon count per area, axon diameter, or G-ratio between the 2 age groups. Quantitative analyses of ventral root morphology have shown varied results depending on the methodology and animal species,34 and some studies indicated a selectively reduced number of large myelinated fibers in the ventral roots.34 From our specimens, we observed an abundance of thinly myelinated fibers in ventral roots of some of the old mice, which may suggest that some fibers had abnormal conduction of action potentials even in the proximal portion of the motor neuron. However, this was normalized by the presence of thickly myelinated fibers, and there was no significant difference in the G-ratio between the 2 age groups. This contrasts with the dramatic difference of NMJ denervation as described earlier. Considering that ventral roots and NMJs are 2 different parts of the same motor neuron, these results support the premise that dying-back axonal degeneration occurs with aging.
SSFEMG had not been used previously to investigate neuromuscular transmission in aging research, and our study has successfully shown a significant difference in jitter level between young and old mice. However, there have been a few studies using single fiber EMG in humans to examine the dying-back process in motor neuron diseases.35,36 We applied the same concept to rodents in this study. The advantages of using SSFMEG are that: (1) increased jitter is among the most sensitive indicators of neuromuscular transmission defects27; (2) it is an in vivo technique that can be performed when animals are alive; and (3) it is a clinically established technique with normal reference values in both humans and mice.27,37
Specific dysregulation in molecular pathways has been suggested to explain distal axonal degeneration, including metabolic dysregulation, covalent modification, mitochondrial dysfunction, reactive oxygen species, and axonal transport defects.21 Normal aging also accompanies complex changes in various metabolic pathways, and it is likely that the same metabolic changes contribute to age-related distal axonal degeneration. Given the association between neuromuscular function and late-life survival, deciphering the molecular pathways that drive age-related distal axonal degeneration may lead to further understanding of the etiology of sarcopenia and the broader pathophysiological changes that accompany aging.
Acknowledgments
Supported by the K-12 Rehabilitation Medicine Scientist Training Program, the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the National Institutes on Aging (R21-AG025143), and the Johns Hopkins Older Americans Independence Center (P30-AG021334).
Abbreviations
- AChR
acetylcholine receptor
- ALS
amyotrophic lateral sclerosis
- MCD
mean consecutive difference
- NMJ
neuromuscular junction
- PBS
phosphate-buffered saline
- SFAP
single fiber action potential
- SSFEMG
stimulated single fiber electromyography
References
- 1.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 3.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 4.Xue QL, Beamer BA, Chaves PH, Guralnik JM, Fried LP. Heterogeneity in rate of decline in grip, hip, and knee strength and the risk of all-cause mortality: the women’s health and aging study II. J Am Geriatr Soc. 2010;58:2076–2084. doi: 10.1111/j.1532-5415.2010.03154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashizume K, Kanda K. Differential effects of aging on motoneurons and peripheral nerves innervating the hindlimb and forelimb muscles of rats. Neurosci Res. 1995;22:189–196. doi: 10.1016/0168-0102(95)00889-3. [DOI] [PubMed] [Google Scholar]
- 7.Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol (1985) 1993;74:868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- 8.Hashizume K, Kanda K, Burke RE. Medial gastrocnemius motor nucleus in the rat: age-related changes in the number and size of motoneurons. J Comp Neurol. 1988;269:425–430. doi: 10.1002/cne.902690309. [DOI] [PubMed] [Google Scholar]
- 9.Tomlinson BE, Irving D. The numbers of limb motor neurons in the human lumbosacral cord throughout life. J Neurol Sci. 1977;34:213–219. doi: 10.1016/0022-510x(77)90069-7. [DOI] [PubMed] [Google Scholar]
- 10.Wokke JH, Jennekens FG, van den Oord CJ, Veldman H, Smit LM, Leppink GJ. Morphological changes in the human end plate with age. J Neurol Sci. 1990;95:291–310. doi: 10.1016/0022-510x(90)90076-y. [DOI] [PubMed] [Google Scholar]
- 11.Arizono N, Koreto O, Iwai Y, Hidaka T, Takeoka O. Morphometric analysis of human neuromuscular junction in different ages. Acta Pathol Jpn. 1984;34:1243–1249. doi: 10.1111/j.1440-1827.1984.tb00551.x. [DOI] [PubMed] [Google Scholar]
- 12.Rosenberg IH. Sarcopenia: origins and clinical relevance. Clin Geriatr Med. 2011;27:337–339. doi: 10.1016/j.cger.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Walston JD. Sarcopenia in older adults. Curr Opin Rheumatol. 2012;24:623–627. doi: 10.1097/BOR.0b013e328358d59b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt MC, Schwartz AV, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 15.Salameh JS, Brown RH, Jr, Berry JD. Amyotrophic lateral sclerosis: Review. Semin Neurol. 2015;35:469–476. doi: 10.1055/s-0035-1558984. [DOI] [PubMed] [Google Scholar]
- 16.Valdez G, Tapia JC, Lichtman JW, Fox MA, Sanes JR. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS One. 2012;7:e34640. doi: 10.1371/journal.pone.0034640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegedus J, Putman CT, Tyreman N, Gordon T. Preferential motor unit loss in the SOD1 G93A transgenic mouse model of amyotrophic lateral sclerosis. J Physiol. 2008;586:3337–3351. doi: 10.1113/jphysiol.2007.149286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, et al. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci USA. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschenes MR, Hurst TE, Ramser AE, Sherman EG. Presynaptic to postsynaptic relationships of the neuromuscular junction are held constant across age and muscle fiber type. Dev Neurobiol. 2013;73:744–753. doi: 10.1002/dneu.22095. [DOI] [PubMed] [Google Scholar]
- 21.Cashman CR, Hoke A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neurosci Lett. 2015;596:33–50. doi: 10.1016/j.neulet.2015.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, et al. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Meekins GD, Carter GT, Emery MJ, Weiss MD. Axonal degeneration in the trembler-j mouse demonstrated by stimulated single-fiber electromyography. Muscle Nerve. 2007;36:81–86. doi: 10.1002/mus.20786. [DOI] [PubMed] [Google Scholar]
- 24.Steinbach JH. Neuromuscular junctions and alpha-bungarotoxin-binding sites in denervated and contralateral cat skeletal muscles. J Physiol. 1981;313:513–528. doi: 10.1113/jphysiol.1981.sp013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Lee Y, Thompson WJ. Changes in aging mouse neuromuscular junctions are explained by degeneration and regeneration of muscle fiber segments at the synapse. J Neurosci. 2011;31:14910–14919. doi: 10.1523/JNEUROSCI.3590-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomiak T, Hu B. What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS One. 2009;4:e7754. doi: 10.1371/journal.pone.0007754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanders DB, Stalberg EV. AAEM minimonograph #25: Single-fiber electromyography. Muscle Nerve. 1996;19:1069–1083. doi: 10.1002/(SICI)1097-4598(199609)19:9<1069::AID-MUS1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 28.Mahoney RE, Rawson JM, Eaton BA. An age-dependent change in the set point of synaptic homeostasis. J Neurosci. 2014;34:2111–2119. doi: 10.1523/JNEUROSCI.3556-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly SS, Robbins N. Progression of age changes in synaptic transmission at mouse neuromuscular junctions. J Physiol. 1983;343:375–383. doi: 10.1113/jphysiol.1983.sp014898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimune H, Numata T, Chen J, Aoki Y, Wang Y, Starr MP, et al. Active zone protein bassoon co-localizes with presynaptic calcium channel, modifies channel function, and recovers from aging related loss by exercise. PLoS One. 2012;7:e38029. doi: 10.1371/journal.pone.0038029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia ML, Fernandez A, Solas MT. Mitochondria, motor neurons and aging. J Neurol Sci. 2013;330:18–26. doi: 10.1016/j.jns.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS One. 2011;6:e28090. doi: 10.1371/journal.pone.0028090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT. Denervation causes fiber atrophy and myosin heavy chain co-expression in senescent skeletal muscle. PLoS One. 2012;7:e29082. doi: 10.1371/journal.pone.0029082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ansved T, Larsson L. Quantitative and qualitative morphological properties of the soleus motor nerve and the L5 ventral root in young and old rats. relation to the number of soleus muscle fibers. J Neurol Sci. 1990;96:269–282. doi: 10.1016/0022-510x(90)90138-d. [DOI] [PubMed] [Google Scholar]
- 35.Cui LY, Liu MS, Tang XF. Single fiber electromyography in 78 patients with amyotrophic lateral sclerosis. Chin Med J (Engl) 2004;117:1830–1833. [PubMed] [Google Scholar]
- 36.Rodriquez AA, Agre JC, Franke TM. Electromyographic and neuromuscular variables in unstable postpolio subjects, stable postpolio subjects, and control subjects. Arch Phys Med Rehabil. 1997;78:986–991. doi: 10.1016/s0003-9993(97)90062-9. [DOI] [PubMed] [Google Scholar]
- 37.Gooch CL, Mosier DR. Stimulated single fiber electromyography in the mouse: techniques and normative data. Muscle Nerve. 2001;24:941–945. doi: 10.1002/mus.1092. [DOI] [PubMed] [Google Scholar]