Abstract
Discovering how to improve survival and establishing clinical reference points for children diagnosed with endemic Burkitt lymphoma (eBL) in resource-constrained settings has recaptured international attention. Using multivariate analyses, we evaluated 428 children with eBL in Kenya for age, gender, tumor stage, nutritional status, hemoglobin, lactate dehydrogenase (LDH), Epstein-Barr virus (EBV) and Plasmodium falciparum prior to induction of chemotherapy (cyclophosphamide, vincristine, methotrexate, and doxorubicin) to identify predictive and prognostic biomarkers of survival. During this ten year prospective study period, 22% died in-hospital and 78% completed six-courses of chemotherapy. Of those, 16% relapsed or died later; 31% achieved event-free-survival; and 31% were lost to follow-up; the overall one-year survival was 45%. After adjusting for co-variates, low hemoglobin (<8g/dL) and high LDH (>400 mU/ml) were associated with increased risk of death (adjusted Hazard Ratio (aHR)=1.57 [0.97 to 2.41]) and aHR=1.84, [0.91 to 3.69], respectively). Anemic children with malaria were 3.55 times more likely to die [1.10 to 11.44] compared to patients without anemia or malarial infection. EBV load did not differ by tumor stage nor was it associated with survival. System-level factors can also contribute to poor outcomes. Children were more likely to die when inadvertently overdosed by more than 115% of the correct dose of cyclophosphamide (aHR=1.43 [0.84 to 2.43]), or doxorubicin (aHR=1.25, [0.66 to 2.35]), compared to those receiving accurate doses of the respective agent in this setting. This study codifies risk factors associated with poor outcomes for eBL patients in Africa and provides a benchmark by which to assess improvements in survival for new chemotherapeutic approaches.
Introduction
Endemic Burkitt lymphoma (eBL) accounts for approximately half of all cancers, and 90% of lymphomas, diagnosed among children in Equatorial Africa.1,2 Early infection with both Epstein-Barr virus (EBV) and Plasmodium falciparum malaria are thought to be co-factors in the etiology of eBL (reviewed3). Incidence rates of eBL vary by region in Africa such that children living in malaria holoendemic areas experience higher rates of eBL compared to children from lower transmission areas.4,5 BL is one of the most aggressive human tumors and is characterized by deregulation of the c-MYC oncogene in B-cells.6,7 Although over 90% of B-cell lymphomas diagnosed in developed countries will achieve complete remission with intensive chemotherapy and supportive care,8 estimated survival rates for eBL diagnosed in Africa are considerably lower. Recent studies carried out in Uganda and Malawi reported survival rates approaching 50-70%,2,9,10 whereas other studies report long-term survival rates as low as 33%.11,12 Poor outcomes among children with eBL have been attributed to many factors, including delays in the diagnosis and initiation of treatment, abandonment of care, treatment-related toxicities, and scarcity of chemotherapy and other supplies for comprehensive supportive care. In recent years, varying combinations and doses of cytotoxic agents have been investigated with the goal of identifying regimens with maximal therapeutic effect and minimal toxicity in resource-constrained settings.13 However, a consensus has yet to be reached as to which regimen is optimal and which biomarkers are most informative for eBL.
The observational study presented here includes data collected from children diagnosed with eBL from 2003 to 2011 at Jaramogi Oginga Odinga Teaching and Referral Hospital (JOORTH) in Kisumu, Kenya. The hospital protocol included a 2 year out-patient follow-up period. The survival rate of children treated with conventional combination chemotherapy was examined including children who unintentionally received higher or lower than recommended dosages. In addition, the utility of other clinical variables on predicting survival is evaluated, including age, gender, tumor stage, nutritional status, serum hemoglobin and lactate dehydrogenase (LDH) levels, Epstein-Barr virus (EBV) copy number, and Plasmodium falciparum infection status. Findings from this study provide a ten year picture of treatment and survival for Kenyan children diagnosed with eBL and establishes a baseline by which therapeutic strategies can be compared within future clinical trials in Africa.
Methods
Study population and ethical approvals
This observational study prospectively enrolled 609 children aged <15 years old admitted to JOORTH, the regional cancer referral hospital in western Kenya, between March 2003 and September 2011 for suspected eBL.4 Children were excluded if another cancer was ultimately diagnosed (n=28); if guardians withdrew them prematurely from hospital (n=5), or if they were transferred to another hospital (n=1). The catchment area for JOOTRH spanned western Kenya and displayed the expected geographic overlap with malaria transmission (Figure 1).3 Written informed consent was obtained from parent prior to child's enrollment. Ethical approvals were obtained from the University of Massachusetts Medical School, the Kenya Medical Research Institute and JOOTRH.
Figure 1. Spatial Distribution and catchment area in western Kenya of pediatric Burkitt lymphoma patients admitted to Jaramogi Oginga Odinga Teaching and Referral Hospital in Kisumu, Kenya.
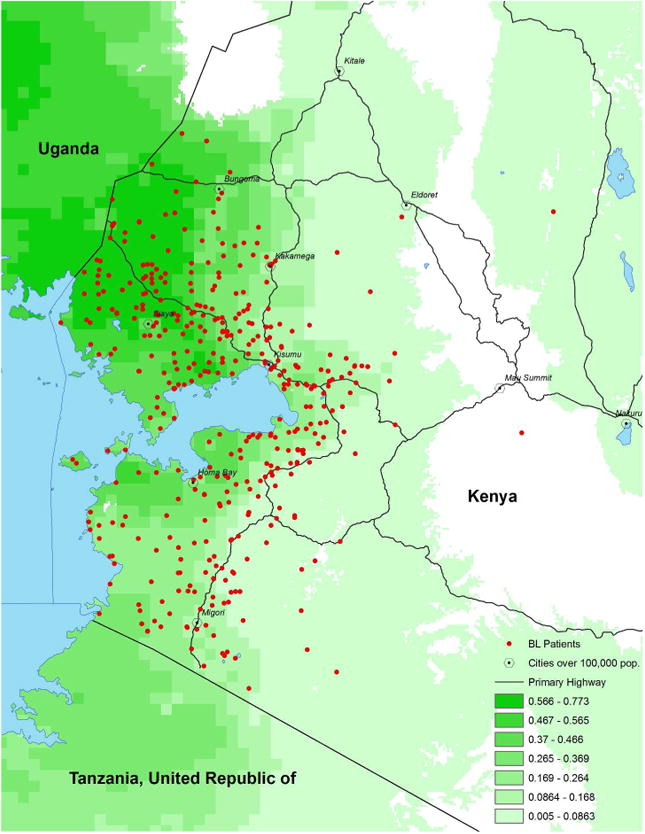
Map of western Kenya shows Plasmodium falciparum malaria transmission intensity (shaded by increasing intensity in green) and home location (red spots) of pediatric Burkitt lymphoma patients admitted to Jaramogi Oginga Odinga Teaching and Referral Hospital in Kisumu, Kenya, from 2003 to 2011.
Cytopathological diagnosis and staging
Two independent pathologists confirmed diagnosis by cytopathology using fine needle aspiration (FNA) slide review and May-Grunewald Giemsa staining.14 Tumor sites were determined by ultrasound or plain radiography. Central nervous system involvement was evaluated by lumbar puncture for cerebral spinal fluid cytology. Tumors were staged according to St. Jude/Murphy Staging for childhood non-Hodgkin lymphoma15 which is considered prognostic for eBL.16 Of note, bone marrow aspiration was not routinely carried out unless the child had profound cytopenia.
Regimen
Chemotherapy was administered as specified by the JOOTRH Burkitt lymphoma Protocol (Supplemental Materials). In brief, this regimen included induction-consolidation at weekly intervals over six weeks of intravenous cyclophosphamide (1200mg/m2) and vincristine (1.5mg/m2); doxorubicin (60mg/m2) on days 1 and 22; 4 weekly interval doses of intrathecal (IT) methotrexate (7.5mg/m2); and a tapering dose of oral prednisone. After hospital discharge, surviving children received monthly maintenance therapy (300 mg/m2 cyclophosphamide and 1.5mg/m2 vincristine) for the next 24 months as out-patients.
Laboratory tests
Complete blood counts were measured prior to each chemotherapy administration. Total white blood cell concentration greater than 1.0×103 cells/mcl and platelet levels greater than 100×103/mcl were required to qualify for chemotherapy. Blood counts below these levels resulted in delayed chemotherapy until the following week provided levels improved. Blood transfusions were given if hemoglobin levels were less than 5g/dL. Serum electrolytes, renal and liver function, and blood clotting profile were not routinely assessed unless clinically indicated. P. falciparum infection was determined by microscopy using World Health Organization standards.17 Malaria was treated according to Kenyan National Ministry of Health guidelines. Serum LDH was measured as a surrogate of tumor burden.18 Peripheral blood EBV titers were measured as previously described by quantitative PCR.19
Clinical and demographic data
Height and weight was recorded in the medical records upon admission and drug treatment plan once diagnosis of cancer was confirmed. Body Surface Area (BSA) and chemotherapy drug dosages were calculated by the hospital medical officer.20 Clinical data were abstracted from medical records by trained research personnel. For children who completed in-hospital treatment but failed to attend outpatient clinic, attempts were made by a case manager to contact parents to ascertain child's wellbeing and invite them for continued outpatient assessment with transport reimbursement paid by the ongoing research project.
During data cleaning, inconsistencies between admission height and weight and those recorded on the drug treatment plan were resolved by returning to the original medical records. Therefore, to confirm the accuracy of BSA calculations used to measure chemotherapy dose, we re-calculated BSA for each patient using the Mosteller equation20 and verified height and weight as recorded in each patient's medical record. We therefore defined chemotherapy dosage status by determining prescribed doses based on verified BSA compared to dose of drugs actually received. Children who inadvertently received >115% of the recommended dose were classified as overdosed, while children who received <85% were classified as underdosed. Children who received doses within 15% of that recommended for their BSA were classified as correctly dosed.
Nutritional anthropometric status was retrospectively derived for each child by calculating a standardized BMI using the WHO 2007 SPSS Macro Package.21 Any child below two standard deviations (i.e. Z-score<-2) from median weight-for-height was considered malnourished.
Statistical analysis
The case deletion method was used to account for incomplete record information. Comparability of children contributing only to the survival analysis (N=428) and those of all children enrolled in the study (N=575) was ascertained. Skewed LDH and EBV distributions were analyzed using the non-parametric Kolmogorov-Smirnov test.
Two survival timepoints were calculated: 1) short-term, in-hospital survival measured in days from the date of hospital admission to the date of discharge or death; and 2) long-term survival measured in days from the date of hospital admission to the date of death, date of last known contact, date of last treatment or documented relapse when date of death was unavailable. Children discharged alive but subsequently lost to follow-up were censored at the date of discharge. We chose to evaluate both in-hospital and long-term survival rates for two reasons. The first was to determine if clinically actionable risk factors (i.e. EBV load, anemia, malaria, nutritional status) were predictive of immediate death (within the first 2-3 months after diagnosis) in contrast to risk factors that might contribute to residual effects (i.e. drug dosing, EBV load) and death after successful completion of 6 doses of induction-consolidation chemotherapy.
Kaplan-Meier curves were fitted to calculate crude median survival times; sub-groups were compared with the Mantel-Cox log-rank test. Cox Proportional Hazards models were constructed to further investigate the relation between outcomes of interest and time to death, adjusting for potential confounders age, gender, nutritional status and tumor stage, generating estimates for adjusted Hazard Ratios (aHR) and their associated 95% Confidence Intervals (CI). Likelihood Ratio (LR) tests were used to assess the appropriateness of the proportional hazards assumption for all predictors simultaneously, using a time-dependent covariate constructed around dosage variables. All analyses were conducted using SPSS v. 22 (Armonk, NY).
Results
Kenyan children (n=575) with cytopathologically confirmed eBL were eligible to participate in this study but were excluded if they were HIV positive (n=6), had incomplete anthropomorphic data, were missing documentation of date of death or discharge, or died before treatment could be initiated (Figure 2). Demographic and baseline clinical data are shown in Table 1. Of note, 19.7% met criteria for malnutrition, 14.8% had hemoglobin <8g/dL, and 17.9% had Plasmodium falciparum infections at time of admission.
Figure 2. Summary of eligibility and outcomes.
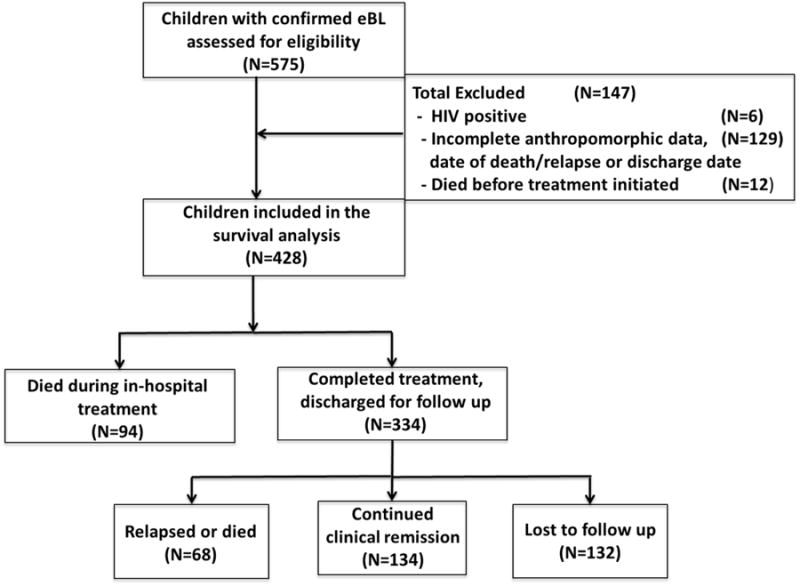
Children diagnosed (n=575) with endemic Burkitt lymphoma admitted to Jaramogi Oginga Odinga Teaching and Referral Hospital in Kisumu, Kenya, 2003-2011. Exclusion was primarily due to incomplete maintenance of paper medical records.
Table 1. Baseline characteristics upon admission of children treated for Burkitt lymphoma at Jaramogi Oginga Odinga Teaching and Referral Hospital in Kisumu, Kenya, 2003-2011.
| Characteristic | N (%) | Mean/Median [min-max Range] |
|---|---|---|
| Age at Admission (years)1 | 569 | 7.5 [0.8-14.4] |
| Body Surface Area (m2, recorded)2 | 0.8 [0.1-1.6] | |
| Gender3 | ||
| Male | 348 (61.2) | |
| Female | 221 (38.8) | |
| Tumor Stage4 | ||
| I | 249 (43.8) | |
| II | 17 (3.0) | |
| III | 289 (50.8) | |
| IV | 14 (2.5) | |
| Hemoglobin5, 6 | ||
| Mean level (g/dL) | 10.0 [3.1-17.4] | |
| Children with low hemoglobin (<8 g/dL) | 54 (14.8) | |
| Nutritional Status7 | ||
| BMI SDS | -0.6 [-4.8-4.3] | |
| Malnourished (BMI SDS <-2) | 97 (19.7) | |
| Malaria infection status8 | ||
| Children with smear-positive P. falciparum parasitemia | 99 (17.9) | |
| Mean geometric parasite density | 61 [1-888] | |
| EBV9 | ||
| Children with detectable EBV | 122 (89.7) | |
| Median (copies/μg DNA) | 8,732 [21-382,833] | |
| Lactate dehydrogenase (LDH)10 | ||
| Median (mU/ml) | 512 [30-2,930] | |
| Percent with elevated LDH (>400 mU/ml)6 | 82 (59.9) |
Data from 569 children diagnosed with eBL exclusive of 6 who were HIV positive
BSA available for 566 children
Documentation of gender available for 569 children
Tumor staging available for 569 children
Hemoglobin measurements at admission available for 385 children
Reference ranges for normal LDH and hemoglobin values available in Supplemental Materials
Weight and height data available for 553 children
569 children evaluated for blood stage Plasmodium falciparum parasitemia
EBV measurements available for 136 children
LDH measurements available for 137 children
Survival analyses included 428 children with complete chemotherapy data (Figure 2). There were no significant differences in demographic or baseline clinical characteristics between the 141 (non-HIV infected) children excluded and those contributing to the survival analyses. However, most of the 129 children excluded due to incomplete outcome data were those enrolled during the first few years of the study. This was prior to implementing transport reimbursement and reminder cell phone calls for monthly out-patient appointments. Of the 428 children with eBL, 22% died in-hospital during the induction-consolidation phase of therapy and the remaining 78% completed the prescribed six-courses of chemotherapy and were subsequently discharged for outpatient maintenance chemotherapy. In-hospital survival rates did not vary dramatically over the 8-year study period, however adherence to follow-up increased roughly two-fold after appointment reminders and transport reimbursements were implemented.
The median hospital stay for the overall study population was 57 days (range 1-183). Following discharge, 16% of the children relapsed or died (median overall survival 164 days, range 8-1,189); 31% were alive and in clinical remission at last known contact (median overall survival 559 days, range 13-2,659); and 31% were lost to follow up (i.e. last contact was day of discharge from hospital).
Of 428 children, 88% received correctly prescribed cyclophosphamide doses based on their BSA, while 8% were underdosed and 3% were overdosed. Similar numbers were found for vincristine and doxorubicin administration. Overdosing was more common for methotrexate, with 16% of children overdosed and 6% underdosed. (Figure 3, Supplemental material Table 1). For cyclophosphamide and vincristine, dosage status was concordant in 94% of the children. Dosage status was concordant across all four agents in 76% of the children, with 73% accurately dosed and 3% underdosed. No children were overdosed with all four agents.
Figure 3. Chemotherapy doses stratified by in-hospital survival status.
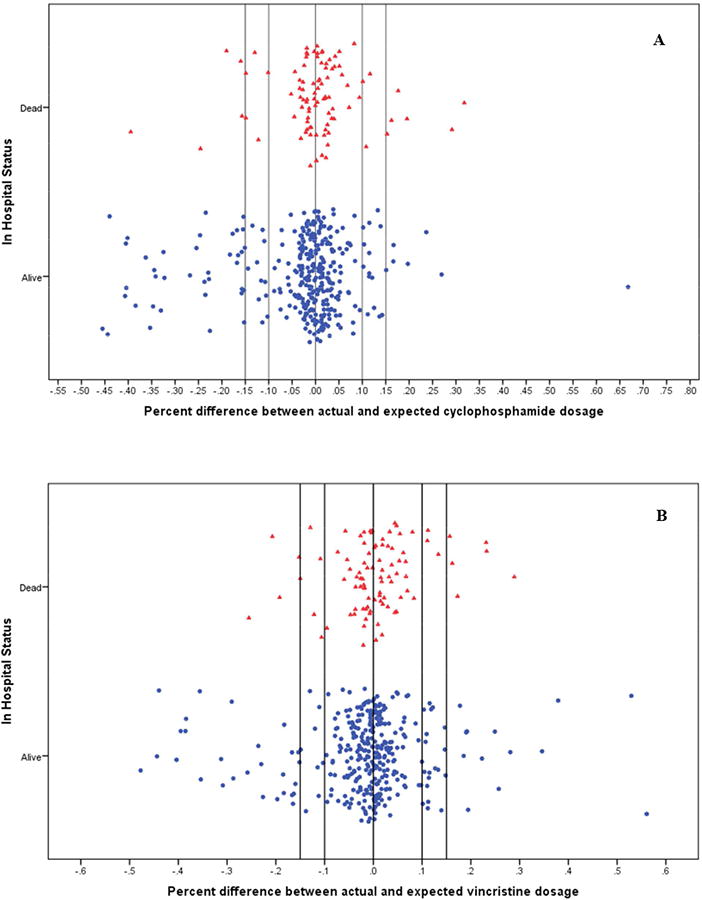
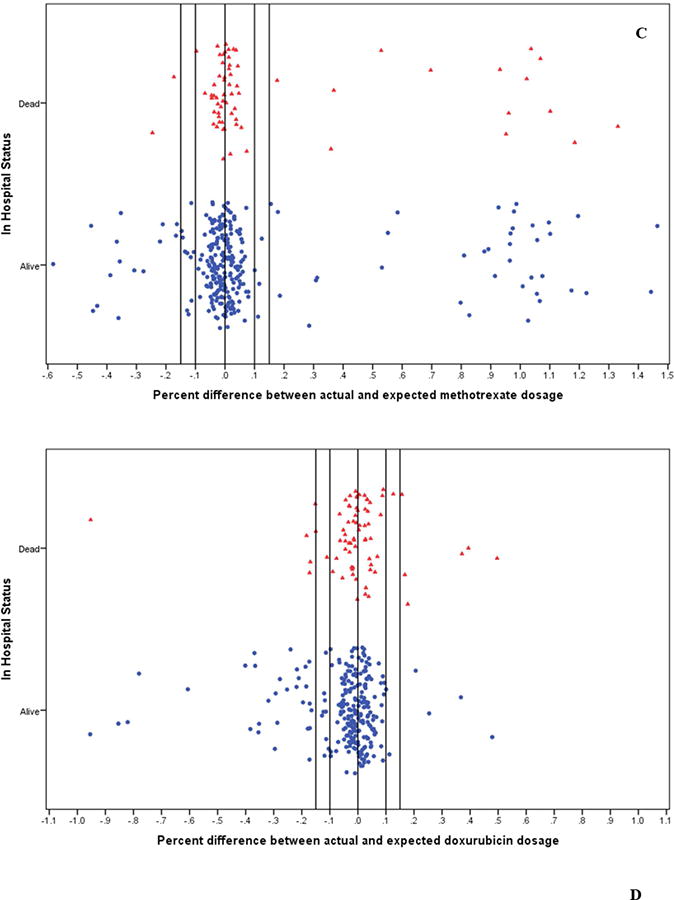
Induction-consolidation was scheduled at six weekly intervals of intravenous (A) cyclophosphamide (1200mg/m2) and (B) vincristine (1.5mg/m2); 4 weekly interval doses of intrathecal (C) methotrexate (7.5mg/m2); and (D) doxorubicin (60mg/m2) on days 1 and 22. Chemotherapy dosage status was defined by determining recommended doses based on retrospectively re-calculated BSA and comparing to dose of drugs received. Children who inadvertently received >115% of the recommended dose for a chemotherapy drug based on their re-calculated BSA were classified as overdosed, while children who received <85% of the recommended dose were classified as underdosed. Children who received doses within 15% of that recommended for their BSA were classified as correctly dosed.
Assessment of risk factors and long-term survival
During the data cleaning and analysis we noticed an unintentional, random miscalculation of dosages for a small yet noteworthy number of children (Figure 3, Supplemental material Table 2). This was a random occurrence over the 8 years of treatment data analyzed and did not cluster by month or year of enrollment (tested for each drug, data not shown). Within the context of the current debate as to the ‘best’ treatment regimen for pediatric BL in resource-constrained settings, with proponents for high-dose, short-course versus low-dose, metronomic therapies, we first analyzed our data to determine if there was an impact on survival associated with high-versus-low dose using the same drug regimen. Using multivariate HR adjusted for age, gender, nutritional status and tumor stage, we found children who were inadvertently overdosed with cyclophosphamide had a suggestive increased risk of death compared to children who received the correct dosing (aHR=1.43, [0.84 to 2.43]; Figure 4A). Children overdosed with doxorubicin were at a slightly increased risk of death or relapse (aHR=1.25, [0.66 to 2.35]; Figure 4D). In contrast, children underdosed with either of these two drugs, cyclophosphamide or doxorubicin (Figures 4A and 4D), had similar survival rates as compared to children receiving correct doses of the respective agent. There were no appreciable differences in eBL survival by dosage for vincristine or methotrexate (Figures 4B and 4C). LR tests for the assumption of proportional hazards yielded overall p values varying between 0.121 and 0.247, suggesting the assumption was met. (Supplemental Table 2).
Figure 4. Cumulative survival of children with endemic Burkitt lymphoma according to chemotherapy drug dosage status.
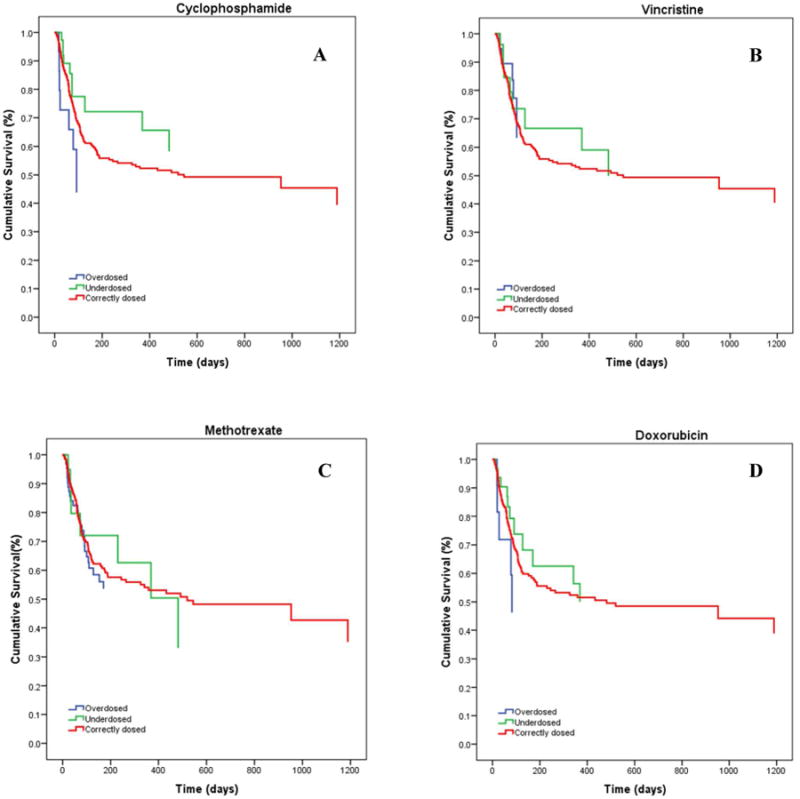
(A) Children who were inadvertently overdosed with cyclophosphamide were at increased risk of death compared to children who received correct dosing of cyclophosphamide (aHR=1.43, [0.84 to 2.43]). (D) Children overdosed with doxorubicin were at a slight increased risk of death or relapse (aHR: 1.25, [0.66 to 2.35]). In contrast, children underdosed with either of these two drugs, cyclophosphamide or doxorubicin, had similar survival rates as compared to children receiving correct doses of the respective agent. There were no appreciable differences in eBL survival by dosage status for (B) vincristine or (C) methotrexate. LR tests for the assumption of proportional hazards yielded overall p values varying between 0.121 and 0.247.
The majority of children (N=318) were treated with all four chemotherapeutic drugs; the remaining 110 were treated with three of the four agents, cyclophosphamide, vincristine and methotrexate, due to intermittent shortages and expense of doxorubicin. Survival rates of these two subgroups were compared in a secondary analysis. Median survival for the “three-drug” group was 545 days (range 3-2073) and similar to the median survival for the “four-drug” group was 433 days (range 8-2467; p=0.94). A multivariable model adjusting for age, gender, nutritional status and tumor stage showed an aHR=1.04 [0.67 to 1.62] comparing the “three-drug to the “four-drug” regimen demonstrating no added survival benefit when using doxorubicin in combination with the other three drugs. There were no differences in tumor stage or other covariates measured for the children who received doxorubicin versus those who did not, as this was the result of random drug shortages and independent of patient status.
The subsequent analyses included only children receiving the appropriate drug doses. After adjusting for age, gender, and nutritional status, children diagnosed with tumors at stage II or higher were more likely to die compared to children diagnosed with stage I disease (aHR=1.56, [1.10 to 2.21]).
We explored several models examining the effects of baseline hemoglobin levels and malaria infection on long-term survival. After adjusting for age, gender, tumor stage and nutritional status, baseline serum hemoglobin was found to be a predictor of survival, with admission levels <8g/dL associated with a 57% increased risk of death or relapse, as compared to children with levels ≥8g/dL (aHR=1.57 [0.97 to 2.41]). Interestingly, this association persisted after further adjustment for malarial parasitemia, but was attenuated when models included the interaction effects of these two variables (aHR=1.25, [0.75 to 2.08]). Of note, this model showed a significant interaction between baseline hemoglobin and malaria status, as anemic children infected with malaria were 3.55 times less likely to survive [1.10 to 11.44] as compared to those without anemia or malaria. Similar models were explored using hemoglobin at week 3; however, this measure was not shown to be a predictor of survival and interaction with malarial status was not observed.
We further explored associations between peripheral blood EBV titers (N=80), serum LDH levels (N=77), and tumor stage with survival. As expected, children with advanced tumor staging had higher serum levels of LDH compared to children with stage I tumors (p=0.064). In multivariable models, elevated LDH (>400 mU/ml) was associated with increased risk of death or relapse (HR=1.84, [0.91 to 3.69], Figure 5A). We did not observe significant differences in EBV load (copies/μg DNA) by tumor stage (p=0.583), nor was EBV load found to be associated with long-term survival (Figure 5B). Children with EBV loads >2000 and between >200 to 2000 copies/μg human DNA had aHR=0.85 [0.34 to 2.16] and aHR=0.95 [0.33 to 2.73] respectively, as compared to children with EBV loads between 0 to 200 copies/μg DNA (Figure 5C).
Figure 5. Association between lactate dehydrogenase and Epstein-Barr virus levels and overall risk of death among children with Burkitt lymphoma, Kisumu, Kenya.
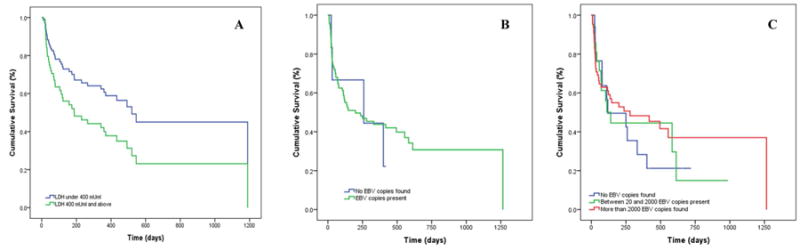
In multivariate models adjusted for age, gender, nutritional status and tumor stage, (A) elevated serum LDH (>400 mU/ml) was associated with increased risk of death or relapse (aHR=1.84 [0.91 to 3.69]). In contrast, (B) EBV load (copies/μg DNA) was not associated with tumor stage (p=0.583) and was not found to be associated with long-term survival. Even when categorizing viral loads, (C) children with EBV viral loads of >2000 and between >200 - 2000 copies/μg DNA were 0.85 [0.34 to 2.16] and 0.95 [0.33 to 2.73] times less likely to die or suffer a relapse, respectively, as compared to children with EBV viral loads between 0 – 200 copies/μg DNA.
In-hospital survival
Additional analyses restricted to the in-hospital period observed an increased association between overdosing with cyclophosphamide and doxorubicin and survival. Children were over two times more likely to die in-hospital when overdosed with cyclophosphamide (aHR=2.14 [0.83 to 5.49], or doxorubicin (aHR=2.02, [0.71 to 5.77], as compared to those receiving accurate doses of the respective agent. In contrast, overdosing with vincristine or methotrexate, or underdosing on any of the four agents, was not found to be an important predictor of in-hospital survival.
Other factors found to be associated with early death included tumor stage and nutritional status. Children with advanced tumor stage (II or higher) were 1.81 times more likely to die in-hospital as compared to those with stage I tumors [1.14 to 2.90] after adjusting for age, gender and nutritional status. Similarly, in multivariable analyses, children who were malnourished were 1.48 times more likely to die in-hospital as compared to those who were not [0.94 to 2.33]. Both effect sizes for tumor stage and nutrition persisted in separate models restricted to children accurately dosed with cyclophosphamide, as well as doxorubicin. There was also no difference in in-hospital survival between those who were treated with the “three-drug” as compared to the “four-drug” regimen (aHR=1.11, 95% CI: 0.85 to 1.90).
Discussion
This study establishes a historical reference point spanning 10 years (enrollment from 2003 to 2011 with 2 year follow-up) for children diagnosed with eBL in western Kenya. In this study, the overall survival rate was 45% (134/296). Of those who completed induction-consolidation, 16% relapsed or died within the first year after diagnosis. There was a significant lost to follow-up (31%) even when transportation and medical costs were provided. This demonstrates the need for creative retention strategies such as patient advocacy and case management as components of any clinical trial in Africa to minimize attrition. Studies of abandonment of childhood cancer treatment in Kenya have identified barriers in addition to financial constraints, namely lack of parents' psychosocial support networks and training for healthcare providers to improve communication skills related specifically to cancer22,23. Our study also highlights the need to mitigate system-level errors by implement training of medical staff and pharmacists for the delivery of complex multi-agent cancer-directed therapy and computer-based quality control measures. Our analysis of administered treatment suggests that children who received a higher dose of cyclophosphamide experienced inferior survival. Additionally, the combination of anemia and malaria was among the strongest predictors of poor prognosis and highlights the need to integrate anti-malarials and blood transfusions within eBL treatment regimens.
The demographics in our study closely resembles those reported in recent studies in similar settings.8,11,24–29 Independent of chemotherapy dosing, having more advanced disease was associated with worse survival, as previously demonstrated.25,30 Tumor stage at diagnosis, however, was slightly different in our study population compared to other recent studies, with the majority presenting with abdominal tumors.8,24–26,29,31 This difference could be due to improved ascertainment of abdominal tumors or earlier recognition of cancer as a diagnosis. While incomplete medical charts and more comprehensive tumor staging methods were a limitation, the long term follow-up data available suggest that eBL outcomes at JOOTRH compare with recent studies complied from similar resource-limited settings.13
There have been several prior efforts to define epidemiological, clinical, and biochemical prognostic biomarkers for eBL. Magrath and colleagues reported a retrospective analysis of 42 children with eBL in Uganda and found tumor burden, related surrogate measures (uric acid, LDH), and antibodies against early antigen of EBV (anti-EA) were associated with worse outcomes.32 A more recent analysis of 180 eBL children in Ghana also found that tumor stage and LDH were associated with survival, in addition to serum creatinine.33 Similarly, our study showed that elevated LDH was associated with poor prognosis. Elevated LDH levels in eBL and other malignancies reflect increased cellular proliferation, which correlates with tumor burden.
Interestingly, our study showed no association between EBV load at baseline and risk of death or relapse over long-term follow up, despite EBV being recognized as a key risk factor for developing eBL. Prior studies that examined the role of EBV as a prognostic indicator in eBL have focused on patterns of viral gene expression.2 Few of these have evaluated the utility of a quantitative measure of EBV load as a predictor of clinical outcomes. Previous research conducted in NK/T-cell lymphoma has shown that high EBV loads suggest an adverse prognosis.34 Other studies have found that plasma EBV DNA levels may be a marker for both tumor load and therapeutic response in a variety of EBV-associated lymphoid malignancies, including BL, and may be useful for monitoring treatment progress.35–37 Our findings contrast with this earlier research, suggesting that EBV may play a role in facilitating tumorigenesis but may become a bystander when c-myc translocation appropriates EBV-infected B cells.
Limited data exists regarding the effects of nutritional status on outcomes among children with eBL. In our study, we observed an increased risk of in-hospital death among malnourished children; however, over long-term follow up, nutritional status was non-prognostic, perhaps due to the hospital nutrition program. These findings support earlier research led by Israels and colleagues that investigated the relationship between nutritional status, neutropenic episodes and death among Malawian children undergoing treatment for eBL.38 In their study, malnutrition was associated with increased frequency, severity and duration of neutropenia, in addition to higher rates of treatment delays and treatment-related mortality. Collectively, these findings suggest that malnutrition may diminish tolerance to chemotherapy, thereby increasing the risk of infectious complications as well as worse survival outcomes.
The intermittent availability of doxorubicin within the study period offered the opportunity to compare survival outcomes among children treated with cyclophosphamide, vincristine and IT methotrexate and those treated with all four agents. Although numbers were limited, our analyses suggest that these two treatment regimens were comparable and the addition of doxorubicin does not confer a survival benefit. We did not design the study to capture various causes of death. Paradoxically, an alternative interpretation is that supportive care plays a role independent of which chemotherapeutic regimen is used and that basic improvements in patient care might go far toward improving survival for these children.
The risks of administering high-intensity treatment in settings with limited supportive care have been well characterized throughout the literature.13 To our knowledge, however, treatment practices, including cytotoxic dosing patterns, have been largely unexplored in previous research on eBL. One study evaluating a modified version of the LMB protocol in Malawi examined the clinical course among all treatment-related deaths, and found that sub-optimal supportive care was likely implicated in 6 out of 10 deaths.27 Identified issues included mismanaged antibiotics (delayed, inappropriate dosing, or wrong route) and continued treatment despite severe toxicity. Although data on supportive care were limited in our study, our findings emphasize the importance of administering correct dosages, particularly for cyclophosphamide, as this agent remains a mainstay of eBL chemotherapeutic regimens and has been administered as a monotherapy in some settings.10,25,26
There were several limitations to this study aside from sample size and loss to follow-up. Even though eBL is the main pediatric cancer within this population, pathologic diagnosis by FNA is not ideal. Based on our molecular sub-set analysis (unpublished data) it is possible that ∼10% of cases could have been misclassified as eBL. This further supports the need for proper diagnosis and telepathology for consultation in Africa. There were no metrics to assess chemo-toxicity or classify extent of disseminated disease. Bone marrow aspiration was not routinely performed, therefore a subset of patients may have been inadvertently under-staged. The prevalence of malnutrition is likely higher than reported, as using weight-for-height to assess wasting has limited sensitivity, particularly for those with large abdominal tumors.39 The lack of standardized provision of supportive care and prophylaxis for tumor lysis syndrome (i.e. intravenous fluids, allopurinol and furosemide) may have contributed to early mortality. The scope of this study would have been strengthened with additional long-term data on cause-specific morbidity and mortality.
In summary, the challenges of early eBL diagnosis are many, and have previously been explored among this cohort.40 Many of the challenges related to cancer treatment stem from the delicate balance of pursuing dose intensities that can achieve high response rates while minimizing cytotoxic-related complications, particularly within the context of limited supportive care and a high prevalence of co-morbid conditions. This study establishes a foundation upon which to build a resource-appropriate eBL treatment regimen in order to achieve higher survival rates than currently reported.
What's new?
Endemic Burkitt lymphoma (eBL) is the most common pediatric cancer diagnosed in Equatoria Africa. B-cell lymphomas have the potential for complete remission with intensive chemotherapy and supportive care, however estimated survival rates for eBL patients in Africa are significantly lower compared to those in developed countries. Therefore a risk-stratification algorithm appropriate for these patients is needed. To compound the clinical picture, Epstein-Barr virus (EBV) and Plasmodium falciparum malaria are co-factors in eBL risk. This study shows that malaria, anemia and high-dose cyclophosphamide contribute to poor outcomes for children diagnosed with eBL. In contrast, EBV load does not appear to be a prognostic or predictive biomarker.
Acknowledgments
We thank the children and their families for their participation and the study staff at Jaramogi Oginga Odinga Teaching and Referral Hospital for their assistance with this study. We thank the UMMS-KEMRI staff, namely Dorine Omenha, John Oyombe, Pamella Omollo and Dorothy Osendo and the late Joseph N'jenga. We thank Valerie Valant, Marie-Michele Sainvil, Solange Bayard and Hannah Hoerner for help with clinical data verification. We also thank the Director, Kenya Medical Research Institute for approving this manuscript for publication. This study was supported by the US National Institutes of Health, National Cancer Institute (K08 AI51565, R01 CA134051, R01 CA189806) and The Thrasher Research Fund (02833-7).
Footnotes
Conflict of interest statement: None declared
Contributor Information
Geoffrey Buckle, Molecular Medicine, University of Massachusetts Medical School, 55 Lake Avenue North, Worcester, MA 01655.
Louise Maranda, Quantitative Health Sciences, University of Massachusetts Medical School, 55 Lake Avenue North, ASC6.1063, Worcester, MA 01655.
Jodi Skiles, Pediatrics, Hemotology/Oncology, Indiana University School of Medicine, Full address: 705 Riley Hospital Drive, RI 2629, Indianapolis, IN 46202.
John Michael Ong'echa, Center for Global, Health Research Kenya Medical Research Institute, P. O. Box 1578-40100, Kisumu, Kenya.
Joslyn Foley, Molecular Medicine, University of Massachusetts Medical School, 373 Plantation St. Biotech 2, Suite 318, Worcester, MA 01605.
Mara Epstein, Quantitative Health Sciences, University of Massachusetts Medical School, 365 Plantation St. Biotech 1, Suite 100, Worcester, MA 01605.
Terry A. Vik, Pediatrics, Hemotology/Oncology, Indiana University School of Medicine, Full address: 705 Riley Hospital Drive, ROC 4340, Indianapolis, IN 46202.
Andrew Schroeder, Direct Relief, 27 S. La Patera Ln., Goleta, CA 93117.
Jennifer Lemberger, Direct Relief, 27 S. La Patera Ln., Goleta, CA 93117.
Alan Rosmarin, Medicine, Hematology/Oncology, University of Massachusetts Medical School, 55 Lake Avenue North, H8-533, Worcester, MA 01655.
Scot C. Remick, Physician Leader, Oncology and Senior Scientist, Maine Medical Center and Maine Medical Center Research Institute, Portland, ME 04074
Jeffrey A. Bailey, Medicine, Bioinformatics, University of Massachusetts Medical School, 55 Lake Avenue North, ASC4.1077, Worcester, MA 01655.
John Vulule, Center for Global Health Research, Kenya Medical Research Institute, P. O. Box 1578-40100, Kisumu, Kenya.
Juliana A. Otieno, Jaramogi Oginga Odinga Teaching and Referral Hospital, Kenya Ministry of Health, P.O. Box 849-40100, Kisumu, Kenya.
Ann M. Moormann, Molecular Medicine, University of Massachusetts Medical School, 373 Plantation Street, Biotech 2, Suite 318, Worcester MA, 01605 USA.
References
- 1.Parkin DM, Sohier R, O'Conor GT. IARC Sci Publ. 60. 1985. Geographic distribution of Burkitt's lymphoma; pp. 155–164. [PubMed] [Google Scholar]
- 2.Molyneux EM, Rochford R, Griffin B, et al. Burkitt's lymphoma. Lancet. 2012;379(9822):1234–1244. doi: 10.1016/S0140-6736(11)61177-X. [DOI] [PubMed] [Google Scholar]
- 3.Moormann AM, Snider CJ, Chelimo K. The company malaria keeps: how co-infection with Epstein-Barr virus leads to endemic Burkitt lymphoma. Curr Opin Infect Dis. 2011;24(5):435–441. doi: 10.1097/QCO.0b013e328349ac4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rainey JJ, Omenah D, Sumba PO, Moormann AM, Rochford R, Wilson ML. Spatial lustering of endemic Burkitt's lymphoma in high-risk regions of Kenya. Int J Cancer. 2007;120(1):121–127. doi: 10.1002/ijc.22179. [DOI] [PubMed] [Google Scholar]
- 5.Burkitt D. A “tumour safari” in East and Central Africa. Br J Cancer. 1962;16:379–386. doi: 10.1038/bjc.1962.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnston JM, Yu MT, Carroll WL. c-myc hypermutation is ongoing in endemic, but not all Burkitt's lymphoma. Blood. 1991;78(9):2419–2425. [PubMed] [Google Scholar]
- 7.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982;79:7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hesseling P, Broadhead R, Mansvelt E, et al. The 2000 Burkitt lymphoma trial in Malawi. Pediatr Blood Cancer. 2005;44(3):245–250. doi: 10.1002/pbc.20254. [DOI] [PubMed] [Google Scholar]
- 9.Orem J, Maganda A, Mbidde EK, Weiderpass E. Clinical characteristics and outcome of children with Burkitt lymphoma in Uganda according to HIV infection. Pediatr Blood Cancer. 2009;52:455–458. doi: 10.1002/pbc.21769. [DOI] [PubMed] [Google Scholar]
- 10.Kazembe P, Hesseling PB, Griffin BE, Lampert I, Wessels G. Long term survival of children with Burkitt lymphoma in Malawi after cyclophosphamide monotherapy. Med Pediatr Oncol. 2003;40(1):23–25. doi: 10.1002/mpo.10190. [DOI] [PubMed] [Google Scholar]
- 11.Ngoma T, Adde M, Durosinmi M, et al. Treatment of Burkitt lymphoma in equatorial Africa using a simple three-drug combination followed by a salvage regimen for patients with persistent or recurrent disease. Br J Haematol. 2012;158(6):749–762. doi: 10.1111/j.1365-2141.2012.09236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesseling P, Israels T. Practical recommendations for the management of children with endemic Burkitt lymphoma (BL) in a resource-limited setting. Pediatr blood …. 2013 Nov;:1–6. doi: 10.1002/pbc. [DOI] [PubMed] [Google Scholar]
- 13.Moormann AM, Skiles JL, Otieno JA, Buckle GC, Vik TA. Optimal management of endemic Burkitt lymphoma : a holistic approach mindful of limited resources. 2014:91–99. [Google Scholar]
- 14.Mulama DH, Bailey JA, Foley J, et al. Sickle cell trait is not associated with endemic Burkitt lymphoma: an ethnicity and malaria endemicity-matched case-control study suggests factors controlling EBV may serve as a predictive biomarker for this pediatric cancer. Int J Cancer. 2014;134(3):645–653. doi: 10.1002/ijc.28378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy SB, Fairclough DL, Hutchison RE, Berard CW. Non-Hodgkin's lymphomas of childhood: an analysis of the histology, staging, and response to treatment of 338 cases at a single institution. J Clin Oncol. 1989;7(2):186–193. doi: 10.1200/JCO.1989.7.2.186. [DOI] [PubMed] [Google Scholar]
- 16.Lister TA, Crowther D, Sutcliffe SB, et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol. 1989;7(11):1630–1636. doi: 10.1200/JCO.1989.7.11.1630. [DOI] [PubMed] [Google Scholar]
- 17.Parasitological Confirmation of Malaria Diagnosis. Geneva. 2009 [Google Scholar]
- 18.Asito AS, Piriou E, Odada PS, et al. Elevated anti-Zta IgG levels and EBV viral load are associated with site of tumor presentation in endemic Burkitt's lymphoma patients: a case control study. Infect Agent Cancer. 2010;5:13. doi: 10.1186/1750-9378-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moormann AM, Chelimo K, Sumba OP, et al. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005;191(8):1233–1238. doi: 10.1086/428910. [DOI] [PubMed] [Google Scholar]
- 20.Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317(17):1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski RJ, Ogden CL, Guo SS, et al. CDC Growth Charts for the United States: methods and development. Vital Health Stat 11. 2000;2002;(246):1–190. [PubMed] [Google Scholar]
- 22.Mostert S, Njuguna F, Langat SC, et al. Two overlooked contributors to abandonment of childhood cancer treatment in Kenya: Parents' social network and experiences with hospital retention policies. Psychooncology. 2014;23(6):700–707. doi: 10.1002/pon.3571. [DOI] [PubMed] [Google Scholar]
- 23.Njuguna F, Mostert S, Slot a, et al. Abandonment of childhood cancer treatment in Western Kenya. Arch Dis Child. 2014:609–614. doi: 10.1136/archdischild-2013-305052. [DOI] [PubMed] [Google Scholar]
- 24.Kazembe P, Hesseling PB, Griffin BE, Lampert I, Wessels G. Long term survival of children with burkitt lymphoma in Malawi after cyclophosphamide monotherapy. Med Pediatr Oncol. 2003;40:23–25. doi: 10.1002/mpo.10190. [DOI] [PubMed] [Google Scholar]
- 25.Traoré F, Coze C, Atteby JJ, et al. Cyclophosphamide monotherapy in children with Burkitt lymphoma: a study from the French-African Pediatric Oncology Group (GFAOP) Pediatr Blood Cancer. 2011;56(1):70–76. doi: 10.1002/pbc.22746. [DOI] [PubMed] [Google Scholar]
- 26.Hesseling P, Molyneux E, Kamiza S, Israels T, Broadhead R. Endemic Burkitt lymphoma: a 28-day treatment schedule with cyclophosphamide and intrathecal methotrexate. Ann Trop Paediatr. 2009;29(1):29–34. doi: 10.1179/146532809X402006. [DOI] [PubMed] [Google Scholar]
- 27.Hesseling PB, Broadhead R, Molyneux E, et al. Malawi pilot study of Burkitt lymphoma treatment. Med Pediatr Oncol. 2003;41(6):532–540. doi: 10.1002/mpo.10322. [DOI] [PubMed] [Google Scholar]
- 28.Davidson A, Desai F, Hendricks M, et al. The evolving management of Burkitt's lymphoma at Red Cross Children′s Hospital. 2006;96(9):950–954. [PubMed] [Google Scholar]
- 29.Harif M, Barsaoui S, Benchekroun S, et al. Treatment of B-Cell Lymphoma With LMB Modified Protocols in Africa — Report of the French-African Pediatric Oncology Group (GFAOP) 2008:1138–1142. doi: 10.1002/pbc. April 2007. [DOI] [PubMed] [Google Scholar]
- 30.Olweny CLM, Katongole-mbidde E, Otim D, Lwanga SK, Ziegler JL, Magrath IT. Long-term experience with Burkitt's lymphoma in Uganda. 1980;266:261–266. doi: 10.1002/ijc.2910260302. [DOI] [PubMed] [Google Scholar]
- 31.Koffi GK, Tolo A, Nanho DC, et al. Results of treatment with CMA, a low intermediate regimen, in endemic Burkitt lymphomas in sub-Saharian Africa: Experience of Côte d'Ivoire. Int J Hematol. 2010;91:838–843. doi: 10.1007/s12185-010-0591-z. [DOI] [PubMed] [Google Scholar]
- 32.Magrath I, Lee Y, Anderson T, Henle W. Prognostic factors in Burkitt's lymphoma: Importance of total tumor burden. Cancer. 1980 doi: 10.1002/1097-0142(19800315)45:6<1507::aid-cncr2820450634>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 33.Arthur FKN, Owusu L, Yeboah FA, Rettig T, Osei-Akoto A. Prognostic significance of biochemical markers in African Burkitt's lymphoma. Clin Transl Oncol. 2011;13:731–736. doi: 10.1007/s12094-011-0724-8. [DOI] [PubMed] [Google Scholar]
- 34.Hsieh PP, Tung CL, Chan ABW, et al. EBV viral load in tumor tissue is an important prognostic indicator for nasal NK/T-cell lymphoma. Am J Clin Pathol. 2007;128(4):579–584. doi: 10.1309/MN4Y8HLQWKD9NB5E. [DOI] [PubMed] [Google Scholar]
- 35.Lei KI, Chan LY, Chan WY, Johnson PJ, Lo YM. Quantitative analysis of circulating cell-free Epstein-Barr virus (EBV) DNA levels in patients. Br J Haematol. 2000;111(1):239–246. doi: 10.1046/j.1365-2141.2000.02344.x. [DOI] [PubMed] [Google Scholar]
- 36.Solassol J, Kreuzer KA, Lass U, Schmidt CA. Epstein-Barr Virus DNA Quantitation Assessed by a Real-Time Polymerase Chain Reaction in a Case of Burkitt's Lymphoma. Leukemia & lymphoma. 2001;41:669–673. doi: 10.3109/10428190109060358. [DOI] [PubMed] [Google Scholar]
- 37.Lei KI, Chan LY, Chan WY, Johnson PJ, Lo YM. Circulating cell-free Epstein-Barr virus DNA levels in patients with EBV-associated lymphoid malignancies. Ann N Y Acad Sci. 2001;945:80–83. doi: 10.1046/j.1365-2141.2000.02344.x. [DOI] [PubMed] [Google Scholar]
- 38.Israels T, van der Wetering MD, Hesseling PB, van Geloven N, Caron H, Molyneux E. Malnutrition and neutropenia in children treated for Burkitt lymphoma in Malawi. Pediatr blood …. 2009 Mar;:47–52. doi: 10.1002/pbc. [DOI] [PubMed] [Google Scholar]
- 39.Israëls T, Chirambo C, Caron HN, Molyneux EM. Nutritional status at admission of children with cancer in Malawi. Pediatr Blood Cancer. 2008;51:626–628. doi: 10.1002/pbc.21697. [DOI] [PubMed] [Google Scholar]
- 40.Buckle GC, Collins JP, Sumba PO, et al. Factors influencing time to diagnosis and initiation of treatment of endemic Burkitt Lymphoma among children in Uganda and western Kenya: a cross-sectional survey. Infect Agent Cancer. 2013;8(1):36. doi: 10.1186/1750-9378-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]


