Abstract
Nanodelivery systems usually improve the biodistribution of drugs, leading to reduced side effects and enhanced therapeutic efficacy. However, only a small portion of the injected nanoparticle dose accumulates in pathological tissue. Challenges in drug delivery arise due to a multitude of transport obstacles in the body, including the endothelium, the extracellular matrix, and the cell membrane. In general, nanoparticles are designed to overcome only a few biological barriers, making them inadequate for localized drug delivery. Accordingly, a multifunctional and multicomponent systems are required to effectively address a wide variety of transport obstacles. A suitable approach to obtain high levels of multifunctionality is to bring together the nanoscale with the microscale, resulting in post-nano strategies for drug delivery. This review discusses several such post-nano approaches, with an emphasis on the multistage vector (MSV) platform. The MSV consists of three components on different spatial scales, each intended to address biological barriers that exist in a specific compartment in the body. The first stage vector is a microparticle that is designed to navigate in the vascular compartment. The second stage vector consists of nanoparticles that are released from the microparticle into the tissue interstitium, where they address biological barriers in extracellular and intracellular compartments. The final component of the system is a small molecule therapeutic agent. A new generation of microparticle-based strategies with expanded applications has recently been developed, including injectable nanoparticle generators and silicon particles for immunotherapy. Notably, the advantage of incorporating microstructures in drug delivery vehicles is apparent from the observation that superior functionality only appears on the microscale, highlighting the inherent functional limitations of nanostructures.
Graphical Abstract
This review discusses several post-nano solutions for drug delivery based on porous silicon microparticles. DOPC, dioleoylphosphatidylcholine; PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid); siRNA, small interfering RNA.
Introduction
In past decades, several nanodrugs have entered the market and thousands are currently being evaluated in the preclinical setting.1, 2 The main advantage of nano-sized carriers is improved transport properties. Accordingly, nanoparticles usually exhibit improved biodistribution profiles compared to small molecules or antibodies.3, 4 Nevertheless, a recent literature review of drug delivery studies revealed that the median tumor accumulation of nanoparticles was 0.7% of the administered dose.5 Challenges in drug delivery arise due to biological barriers, such as degradation, clearance, the vascular wall, the extracellular matrix, and the cell membrane.6 Therefore, successful drug delivery is dependent on overcoming a multitude of transport obstacles in the body. Despite the development of several nanodelivery strategies to overcome specific biological barriers, nanomedicine has failed to concurrently tackle multiple transport obstacles. This failure could explain why nano-based drugs have been less promising than initially expected.7 Moreover, transport barriers vary from patient to patient, from lesion to lesion, and change throughout the course of the disease, further complicating the drug delivery process. Accordingly, there is a need to implement a drug delivery approach that addresses transport from a more comprehensive perspective. Although nanotechnology has served a critical role in overcoming individual transport problems, this technology may not be sufficient to undertake the wide-ranging challenges of drug delivery. To adequately address the complexity of transport in the body, systems that are able to incorporate a large number of components and functions is necessary.8 One approach to obtain a multifunctional and multipronged platform for drug delivery is to use innovative solutions that bring together the nanoscale with the microscale. Indeed, the term post-nano does not imply the cessation of the use of nano-based systems; rather, it indicates the integration of these systems with microstructures. The term can be equated with the post-genomic era, where knowledge gained from genome sequencing is being combined with proteomics, metabolomics, and bioinformatics. In this regard, knowledge gained from decades of nanotechnology research can be integrated with the microscale to develop drug delivery vehicles with superior functionality. In this review, post-nano strategies for drug transport are discussed with particular emphasis on the multistage vector (MSV) platform, which is a porous-silicon carrier that incorporates both nano and microstructures. Canham was the first to propose the use of porous silicon for implantable biomedical applications.9 Silicon particles for systemic injection were first disclosed in the patent “Therapeutic microdevices and methods of making and using same”.10 Porous silicon displays several advantages, including a large surface area, tunable characteristics, biodegradability, and lack of toxicity.11 Previously, several studies have utilized porous silicon nanoparticles as injectable drug delivery systems.12–15 However, this review focuses specifically on multistage porous silicon microvectors.
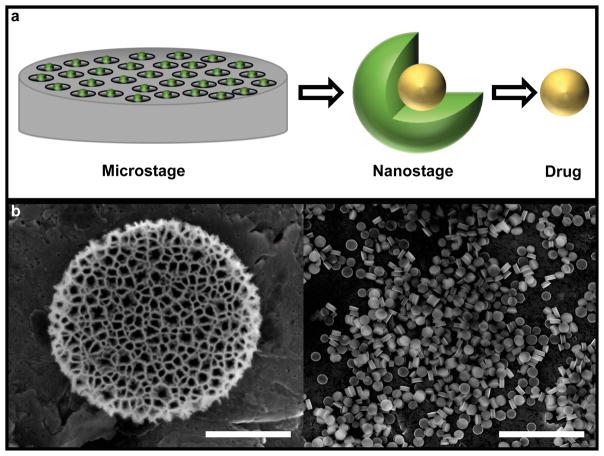
The MSV is a drug carrier composed of three components that are active at different stages in the drug delivery process (Fig. 1a). The first stage vector is a disc-shaped porous silicon microparticle (Fig. 1b) that displays optimal blood flow dynamics and preferentially adheres to tumor vasculature.16, 17 Indeed, red blood cells tend to push discoidal microparticles towards the vessel wall, giving them an opportunity to interact with endothelial cells.18 In fact, it was shown that discoidal particles marginate against the vascular wall to a greater extent than spherical and hemispherical particles of equal weight.19 Mathematical modeling and experimental studies have also demonstrated that disc-shaped shaped microparticles are superior to spherical or rod-shaped particles in regards to establishing interactions with the endothelium.20–23 Notably, discoidal microparticles also exhibit preferential accumulation in tumor vasculature due to low shear rates, which reduce the probability of particle dislodgement from the vessel wall.18, 24 The microparticle component of the MSV is designed to navigate in the circulatory system and does not typically enter the tissue interstitium. On the contrary, the second stage vector, which consists of nanoparticles loaded within the pores of the silicon microparticle, is designed to enter the tissue interstitium upon degradation of the silicon material (Fig. 2).25 Notably, the degradation of porous silicon is increased in the tumor microenvironment26 due to high levels of reactive oxygen species.27 Depending on the application, different types of nanoparticles can be loaded into the pores of the microparticle, including liposomes,28, 29 polymeric nanoparticles,30–32 micelles,33 and gold nanoparticles.34 Since the first stage vector rapidly adheres to inflamed vasculature, the second stage nanoparticles are released in close vicinity to the vascular wall, thus, enabling them to gain rapid access to fenestrations in inflamed endothelium. In the tissue interstitium, the nanoparticles navigate through the extracellular matrix and usually facilitate cellular internalization of the third stage vector, which consists of a therapeutic agent.
Figure 1.
The multistage vector (MSV) a) Schematic representation of the MSV, which is composed of three components of different spatial scales. The first stage vector is a biodegradable porous silicon microparticle that can be loaded with second stage nanoparticles, which encapsulate third stage therapeutic agents. The microstage, nanostage, and drug component are sequentially utilized to overcome biological barriers in the body. b) Scanning electron microscopy (SEM) images of MSVs. Scale bar, 500 nm (left), 10 μm (right).
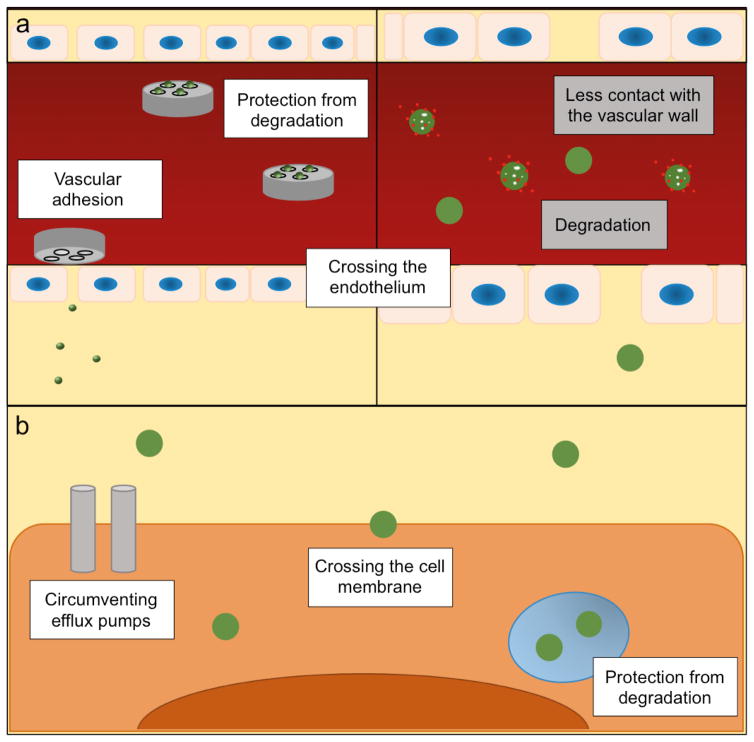
Figure 2.
Biological barriers in drug delivery. a) The MSV (left panel) and a nanodelivery system (right panel) in the circulatory system. The MSV adheres to the vascular wall, while the nanodelivery system displays less contact with the endothelium. The microparticle-component of the MSV protects nanoparticles from degradation in the circulation. The MSV forms vascular depots that gradually release nanoparticles, which can enter the interstitium through fenestrations in the vascular wall. The left and right panels have not been drawn to the same scale. b) Nanoparticles in the intracellular environment. Nanoparticles are able to cross the cell membrane, protect the payload from intracellular degradation, and circumvent drug efflux pumps.
The therapeutic agent in the MSV can also be freely selected based on the intended application. Previous studies have employed a wide range of third stage vectors including micro RNAs (miRNAs),35 small interfering RNA (siRNAs),28, 30, 32, 35, 36 chemotherapeutic agents,31, 33 antibiotics,37 nonsteroidal anti-inflammatory drugs,38 natural compounds,38 and proteins.39 The vast variety of nanoparticles and therapeutic agents that are compatible with the MSV makes this platform a highly versatile drug delivery system that can be used for several applications. The versatility of this system is further increased through the selection of porous silicon microparticles that display optimal size and shape parameters for a specific condition. The geometry and porosity of the MSV can be precisely tailored using electrochemical etching and photolithography. For a detailed overview on MSV fabrication and functionalization, please refer to a review by Wolfram et al.25 MSVs with various diameters (0.5 – 2.6 μm) and heights (0.2 – 0.7 μm) display different biodistribution profiles.24 For instance, while particles with ~1 μm diameters are optimal for accumulation in breast cancer tumors,24 those with a diameter exceeding 2 μm display increased deposition in tumors in the lungs.40
Various animal studies have assessed the potential toxicity of intravenously injected silicon particles by examining organ histology and measuring markers of acute, subacute, and immunotoxicity.28, 32, 35, 36, 41, 42 None of these studies have found any indications of toxicity associated with therapeutically relevant doses of silicon microparticles (~5–100 mg/kg for mice), suggesting that this platform is safe for use as a drug delivery system. Additionally, silicon is a naturally occurring element in living organisms, and humans have several grams of silicon distributed throughout the body, primarily in the bones, nails, and kidneys.43
Previously, we published a review that focuses on the structure, design, and general application of the MSV.25 The aim of this review is to give an in-depth overview of a new generation of MSV-based strategies that display additional functional properties. For instance, an innovative design approach permits loading of different nanodrugs in the same silicon particle, enabling the use of combination therapies. Other examples of post-nano platforms include MSV-based systems that utilize biomimetic coatings or stimuli-responsive release strategies to overcome transport barriers and improve drug delivery. In addition, porous silicon microparticles have proven effective as both immune adjuvants and delivery vehicles for antigens, highlighting the promising use of this platform for immunotherapy. This review will discuss various MSV-based post-nano solutions for improving the biodistribution, safety, and therapeutic efficacy of drugs.
MSV for combination therapy
A major advantage of the MSV is that it enables codelivery of different therapeutic agents. While conventional nanodelivery systems are capable of encapsulating multiple drugs, the MSV permits the concurrent use of different types of nanocarriers that are optimized to deliver a specific cargo. For example, Mi et al. utilized the MSV for codelivery of siRNA- containing liposomes and docetaxel-containing polymeric nanoparticles.40 Previous studies have demonstrated that 1,2-dioleoyl-sn-phosphatidylcholine (DOCP) liposomes44, 45 and poly(lactic-co-glycolic acid) (PLGA)/PEG nanoparticles46, 47 are suitable for siRNA and docetaxel delivery, respectively. Therefore, these nanoparticles were selected as second stage carriers for MSV delivery. Notably, successful codelivery of therapeutic agents introduces opportunities for developing efficient combination therapies, which are of critical importance in the treatment of late-stage malignancies that exhibit drug resistance. For instance, since the most common mutation in metastatic melanoma is the BRAF V600E mutation, which is associated with a poor prognosis,48 clinical trials that combine chemotherapy and BRAF inhibitors are currently underway for the treatment of this disease.49 However, the therapeutic agents in these trials are separately administered, making it challenging to obtain an optimal therapeutic ratio at the target site.
In the MSV study, the performance of combination therapy with docetaxel and anti-BRAF siRNA was evaluated. Since the most common site of metastasis in melanoma is the lungs,50 the MSV codelivery strategy was further optimized to achieve high accumulation of particles in lung tissue.40 Specifically, the dimensions of the first stage vector were adjusted to 2.6 μm × 0.7 μm (diameter × height) to provide preferential accumulation of particles in lung capillaries. In a mouse model, encapsulation of liposomes in the MSV led to a tenfold increase in drug accumulation in metastatic nodules compared to liposomal delivery alone. Therapeutic efficacy studies revealed that codelivery of docetaxel and anti-BRAF siRNA in the MSV dramatically increased survival in comparison to concurrent administration of siRNA liposomes and docetaxel nanoparticles. Specifically, ~88% of mice were alive in the MSV group after 80 days, while the corresponding value was ~24% in the nanoparticle group. Moreover, MSV monotherapy was less efficient at reducing the tumor burden and prolonging survival than MSV combination therapy. The proposed mechanism for synergy was normalization of phosphorylated extracellular signal-regulated kinase (p-ERK) levels and reduction of mitogen activated protein kinase (MEK) levels.
In conclusion, the MSV enables the efficient use of various types of combination therapy, while simultaneously providing an opportunity to design the first stage vector to exhibit tropism for specific vasculature networks.
Stimuli-responsive MSV
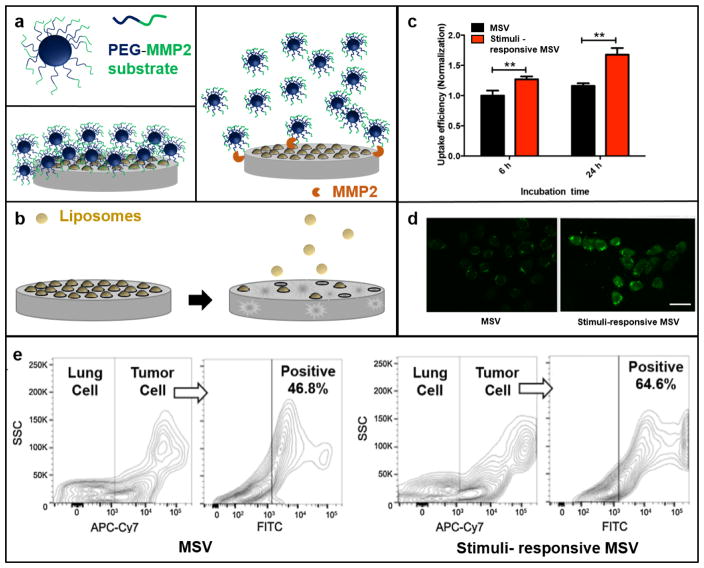
The MSV can be further functionalized to display additional transport-enhancing properties. For instance, a stimuli-responsive MSV was developed to release nanoparticles in the tumor microenvironment.51 Specifically, the MSVs were conjugated with polymeric nanoparticles through a peptide substrate linker responsive to matrix metalloproteinase-2 (MMP2) (Fig. 3a,b), which is an enzyme that plays a fundamental role in tumor invasion and metastasis by degrading components of the extracellular matrix.52 The performance of the enzyme-responsive MSV was evaluated in melanoma cells and in a mouse model of melanoma lung metastasis. A375 melanoma cells were used in this study, since they were shown to express high levels of MMP2 in vitro and in vivo.51 The results demonstrated that in the presence or absence of MMP2, 80% and 40% of nanoparticles were released after 6 h, respectively. Consequently, the levels of nanoparticle internalization were also increased in the MMP2 group (Fig. 3c,d). Similarly, intravenous administration of the stimuli-responsive MSV resulted in higher levels of drug accumulation in cancer cells compared to delivery with the regular MSV (Fig. 3e).
Figure 3.
The stimuli-responsive MSV. a) A peptide substrate for matrix metalloproteinase-2 (MMP2) was conjugated to poly(lactic-co-glycolic acid) (PLGA)-polyethylene glycol (PEG) nanoparticles. The nanoparticles were then conjugated to the surface of the MSV. In the presence of MPP2, the polymeric nanoparticles were released from the silicon microparticle. b) After disassociation of the polymeric nanoparticles from the MSV, gradual degradation of the silicon material leads to the release of a second set of nanoparticles loaded within the pores of the MSV. c, d) Cellular uptake of the MSV and the stimuli-responsive MSV in the presence of MMP2. Scale bar, 20 μm. e) Uptake of MSVs in melanoma lung metastases quantified with flow cytometry. Cancer cells, APC-Cy7; particles, FITC. Partially reproduced from 51 with permission. SSC, side scatter.
Several other stimuli-responsive delivery systems have also been developed, including pH-sensitive and redox potential-sensitive systems.53 However, a major limitation of these strategies is that drug release occurs in the extracellular matrix where the stimuli triggers are located. A critical distinction between nanodelivery systems and the MSV is that the former is designed to release small molecules, while the latter releases nanoparticles. Consequently, the enzyme-responsive MSV overcomes the challenges of extracellular aggregation and degradation of drugs, since therapeutic agents are loaded inside nanocarriers. Moreover, the nanoparticles aid in the intracellular uptake of the third stage cargo. Taken together, the incorporation of microstructures in stimuli-responsive drug delivery systems provides a means of overcoming several biological barriers, while simultaneously regulating drug release kinetics.
Biomimetic MSV
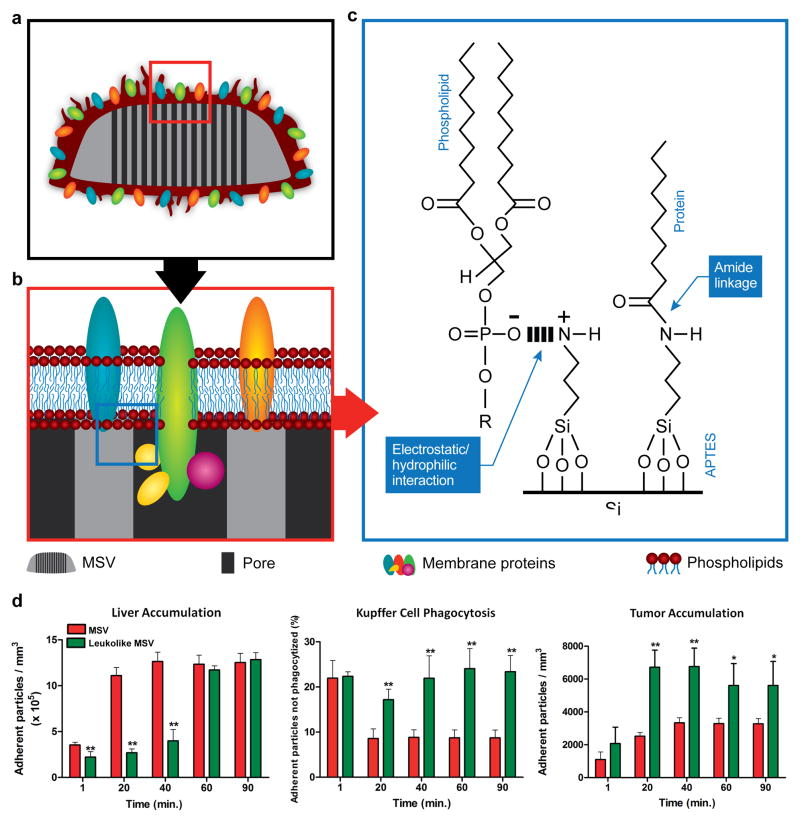
Biomimetic strategies for addressing transport obstacles in the body have recently gained popularity.54 Such strategies include the utilization of viral components,55 self-peptides,56 and membrane coatings.57 For instance, the leukolike MSV is a MSV particle covered with cell membrane fragments from leukocytes (Fig. 4a–c). This cell type is an attractive membrane donor, since it displays enhanced binding to inflamed endothelium.57 Cell membranes from leukocytes were isolated by ultracentrifugation and reconstituted as proteolipid patches that were retained on the MSV surface through electrostatic and hydrophobic interactions between the anionic patches and the cationic surface of the MSV. MSVs coated with leukocyte membranes displayed a zeta potential that was very similar to that of leukocytes (leukolike MSV: – 26 mV; leukocytes: – 31.16 mV).57 In regards to the shelf life of the leukolike MSV, it has been shown that lyophilized proteolipid patches stored at −20° or −80° remain stable for at least one month.58 Prior to treatment, these patches can rapidly and easily be assembled with MSVs. Notably, the leukolike surface was able to protect MSVs from opsonization and decrease particle uptake by phagocytic cells. Specifically, the coated MSVs displayed a 75% decrease in phagocytic uptake compared to uncoated MSVs.57 In addition, the leukolike MSV exhibited longer circulation times and improved tumor accumulation compared to regular MSVs (Fig. 4d).57 In particular, leukolike MSVs displayed a twofold decrease in liver uptake and a twofold increase in tumor accumulation 40 min post-injection.57
Figure 4.
Schematic of the leukolike MSV. a) The MSV was coated with cell membrane patches from leukocyte cells. b) Membrane proteins were successfully transferred onto the MSV. c) Schematic illustrating the chemical interactions between silicon and proteins/phospholipids. d) Time-dependent liver accumulation, Kupffer cell phagocytosis, and tumor accumulation of the MSV and leukolike MSV in mice. Reproduced from 57 with permission. APTES, (3-Aminopropyl)triethoxysilane.
Proteins embedded in the cell membranes of leukocytes play a crucial role in many cellular processes, including cell communication with endothelial cells. It has been demonstrated that the leukocyte coating on the MSV surface retained a protein content that enabled particles to form functional interactions with the endothelium.59 In particular, it was reported that over 300 different proteins were successfully transferred to the leukolike MSV.59 Flow cytometry studies were used to confirm that specific proteins involved in endothelial adhesion and transendothelial migration, such as macrophage-1 antigen (MAC-1) and lymphocyte function-associated antigen 1 (LFA-1), were present on the surface of the MSV.59 In order to assess the transport of leukolike MSVs across an endothelial layer, a transwell chamber assay was utilized. An inflammatory environment was simulated by the addition of tumor necrosis factor (TNF-α). The results revealed that 30% of non-coated MSVs were able to traverse the endothelium, while the corresponding value was 70% for leukolike MSVs.57 Additionally, transendothelial electrical resistance measurements demonstrated that leukolike MSVs increased the permeability of the endothelial cell layer.57 The anticancer activity of the leukolike MSV was also evaluated using a transwell setup, in which endothelial cells were grown in the upper chamber and cancer cells in the lower chamber. Doxorubicin-loaded leukolike MSVs applied to the upper chamber caused a 57% reduction in cancer cell viability, while free doxorubicin and doxorubicin nanoparticles only had a modest effect on the viability of cancer cells (10–20% reduction).57
Additional studies were performed to evaluate the importance of the cell membrane source. Specifically, in vitro and in vivo experiments were conducted to compare the performance of MSVs coated with syngeneic or xenogeneic leukocyte membranes. Syngeneic leukolike MSVs were prepared by coating particles with murine macrophage membranes (mMSV), while xenogeneic leukolike MSVs had a human T lymphocyte coating (hMSV).60 Cell culture experiments revealed that although mMSVs and hMSVs both interacted with murine macrophages, only the former was able to avoid cellular internalization. Furthermore, in vivo results demonstrated that mMSVs exhibited delayed liver accumulation and increased circulation time compared to the hMSVs.60 These results indicate that syngeneic membrane components are necessary for effective avoidance of the mononuclear phagocyte system, an effect that is most likely due to interspecies differences in self-tolerance proteins.
Microparticle platform for immunotherapy
Immunotherapy is the prevention or treatment of a pathological condition through modulation of the host immune system. This form of therapy relies on the presence of disease-specific antigens. Immunity against an antigen is initiated through cellular processing of the antigen in antigen-presenting cells (APCs), such as dendritic cells (DCs).61 APCs present antigens to naive T cells that are activated to become effector T cells. For therapeutic purposes, various steps in the antigen recognition cascade can be modulated in order to improve immunity against antigens. The most common agents used for immunotherapy are small molecules, proteins, and peptides.62–64 Nevertheless, such agents usually display limited immunogenicity due to degradation and can in certain cases cause systemic toxicity.65 The use of nanoparticles or cells for immunotherapy provides unique solutions for treating disease. For instance, in 2010, the Food and Drug Administration (FDA) in the United States approved the first cancer vaccine based on DCs.66 This treatment is an example of an ex vivo immunotherapy strategy based on the reinjection of antigen-pulsed DCs into cancer patients. In addition to cell therapy, nanoparticles can be used as delivery vehicles for antigens.67, 68 Nanodelivery strategies tend to improve antigen-presentation on APCs, since nanoparticles exhibit different internalization and intracellular processing pathways compared to small molecules and proteins.
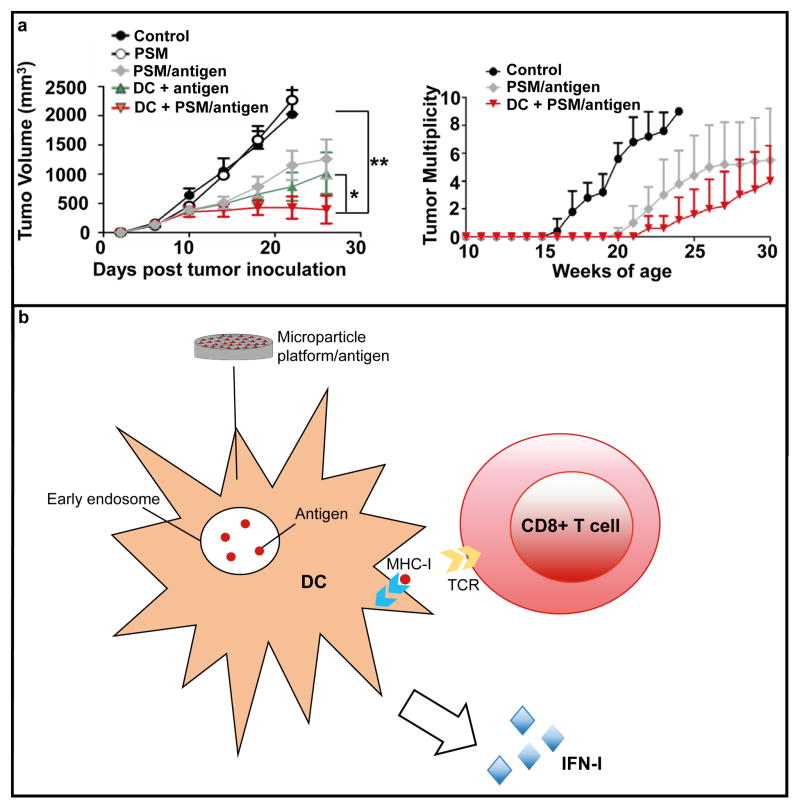
In addition to nanoparticles, microparticles have recently been used to improve immunotherapy. For example, the use of porous silicon microparticles as drug delivery vehicles and adjuvants for cancer vaccines was recently demonstrated. Specifically, a human epidermal growth factor receptor 2 (HER2) peptide antigen was encapsulated in liposomes that were loaded into the pores of silicon particles.69 Although porous silicon microparticles have previously been used for the delivery of HER2 antibodies intended to suppress HER2 signaling,70 the aforementioned study employed a HER2 peptide as an antigen to evoke an immune response against cancer cells that overexpress this receptor. The antigen-containing particles were used to prime DCs that were injected into mice bearing tumors expressing HER2. The results from the study revealed that DCs were more effective at suppressing tumor growth when they were primed with antigen-containing porous silicon microparticles as opposed to a soluble HER2 antigen (Fig. 5a). It was also demonstrated that the observed anticancer activity was HER2 specific, since the use of other antigens did not have an effect on tumor growth. Analysis of the tumor microenvironment showed that injection of microparticle-treated DCs caused a 2–3 fold increase in the cytotoxic T cell marker granzyme and the Th1 cytokine interferon gamma (IFN-γ) compared to antigen priming without the microparticle.69 Comparison of the two priming strategies also demonstrated that the tumor levels of type I interferon (IFN-I) were substantially higher in the microparticle group. Notably, the expression of IFN-I in tumor tissue has been correlated with a favorable outcome in cancer patients.71
Figure 5.
Porous silicon microparticles (PSM) for immunotherapy. a) Inhibition of tumor growth (primary breast cancer) in control mice and mice treated with PSM, antigen-loaded PSM (PSM/antigen), dendritic cells (DC) primed with antigen (DC + antigen), or DC primed with PSM-antigen. Reproduced from 69 with permission. b) Schematic illustrating the mechanism by which PSM elicits an immune response. PSM enhances antigen cross presentation in dendritic cells (DCs) by endosomal delivery of antigens and by activating type I interferon (IFN-I) responses. APTES, (3-Aminopropyl)triethoxysilane. MHC-I, major histocompatibility complex-I; TCR, T cell receptor.
To establish which immune cells were responsible for suppressing tumor growth in response to DC therapy, CD4 T cells, CD8 T cells, or macrophages were depleted prior to treatment. The results indicated that depletion of CD8 T cells rendered the treatment ineffective, while the absence of CD4 T cells and macrophages did not affect the therapeutic outcome.69 In accordance with these results, the highest intratumoral levels of HER2-specific CD8 T cells were detected in mice receiving treatment with microparticle-primed DC cells. The superior performance of the microparticle priming strategy was attributed to increased and prolonged cross-presentation, which occurred due to endosomal delivery of the antigen and induction of type I interferon (IFN-I) expression in DCs. Specifically, phagocytosis of the silicon microparticle resulted in activation of TIR-domain-containing adapter-inducing interferon-β (TRIF) and mitochondrial antiviral-signaling protein (MAVS) signaling pathways. TRIF and MAVS have previously been identified as mediators of microbial and tumor DNA/RNA-induced IFN-I responses.72 It is possible that rearrangement of membrane structures during particle internalization plays a crucial role in silicon microparticle-induced IFN-I responses. However, further studies are necessary to elucidate the exact mechanism by which discoidal silicon microparticles promote IFN-I signaling. It is worth noting that the microparticle serves a dual purpose as an antigen delivery system and an immune adjuvant, making this platform unique compared to most nanoparticle-based therapeutics for immunotherapy (Fig. 5b).
In addition to evaluating the anticancer activity of DC therapy, mice were also directly injected with antigen-containing microparticles. Interestingly, injection of the microparticles resulted in a similar reduction in tumor burden as administration of DCs primed with a soluble antigen.69 These results suggest that the microparticle system could be a promising tool for both cell-based and particle-based immunotherapy.
Injectable nanoparticle generator
An alternative approach to loading microparticles with nanoparticles is to trigger the formation of nanoparticles inside microstructures. Recently, we designed a drug delivery system that serves as a nanoparticle generator in aqueous solutions (Fig. 6a).73 Specifically, a porous silicon particle was conjugated to poly(L-glutamic acid) that was covalently linked to a chemotherapeutic agent.73 Exposure to aqueous media triggered the formation of polymeric nanoparticles with dimensions that were determined by the pore size of the silicon material. As the silicon material gradually degraded, newly formed nanoparticles were released into the surrounding area. The polymer and chemotherapeutic agent utilized in this study were attached through a pH-sensitive cleavable linker, which triggered the intracellular release of doxorubicin in acidic endosomes.73 This cleavage process ensured that doxorubicin accumulated in intracellular regions that were out of reach from drug efflux pumps.
Figure 6.
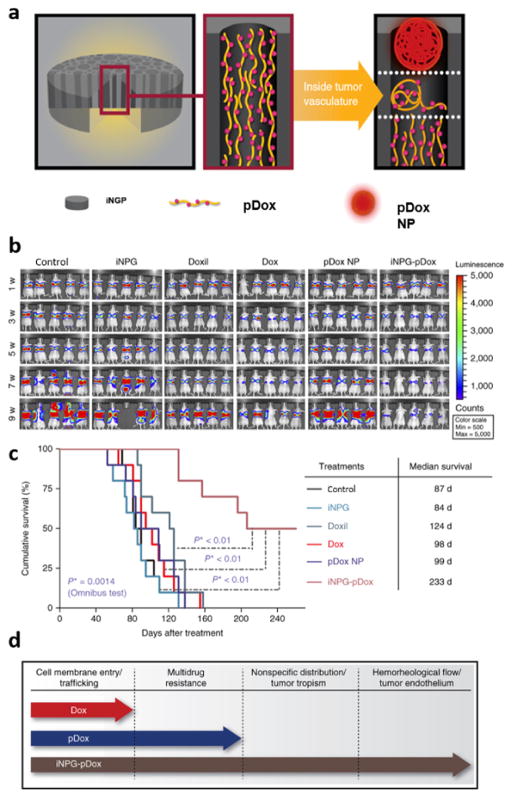
The injectable nanoparticle generator (iNPG). a) Schematic representation of the iNPG. Polymeric doxorubicin (pDox) is loaded inside the pores of the silicon particle. Exposure to an aqueous environment, such as tumor vasculature, triggers the generation and release of polymeric doxorubicin nanoparticles that can enter the tissue interstitium through fenestrations in the vascular wall. b) Bioluminescence images of metastatic breast cancer tumors in the lungs of control mice and mice treated with the empty iNPG, Doxil, Dox, pDox nanoparticles (NP), and iNPG-pDox. c) Survival of mice. d) Diagram showing the biological barriers that Dox, pDox, and iNPG-pDOX are able to overcome. Reproduced from 73 with permission.
The performance of the injectable nanoparticle generator (iNPG) was evaluated in a metastatic triple negative breast cancer (TNBC) mouse model. This type of cancer is characterized by cells that do not express estrogen receptor (ER), progesterone receptor (PR), or HER-2 genes. Consequently, TNBC is unresponsive to endocrine therapy and targeted agents,74, 75 which reduces the treatment options available for this disease. Additionally, TNBC displays aggressive characteristics76 and is usually associated with a poor prognosis.77, 78 In particular, patients with TNBC are susceptible to the development of metastases in the liver, lungs, and brain.76, 79 Since one of the most widely used therapeutic agents for the treatment of metastatic breast cancer is doxorubicin, the porous silicon microparticle was conjugated with a polymeric version of this drug. The dimensions of the first stage vector were also optimized to achieve high levels of lung accumulation in order to treat mice with metastatic lung nodules. Specifically, the height and diameter of the discoidal particles were 700 nm and 2.5 μm, respectively. In tumor-bearing mice, particles with these dimensions displayed a sevenfold increase in lung accumulation compared to free doxorubicin.73 Notably, high concentrations of the drug were still detectable in the lungs seven days post-injection.
The safety and therapeutic efficacy of the iNPG was compared to that of doxorubicin and Doxil, a nanoparticle formulation of the drug. The Food and Drug Administration (FDA) in the United States granted approval for Doxil primarily on the basis of reduced cardiotoxicity,80 which is a common side effect of doxorubicin. The nanoparticle formulation or doxorubicin accumulates to a lesser extent in the heart, thereby, lowering the risk of cardiac side effects arising from the generation of drug-induced reactive oxygen species.81, 82 Since cardiotoxicity can lead to the suspension of therapy, Doxil is in many cases a more favorable alternative to conventional therapy. In a mouse model of multidrug resistant metastatic breast cancer, treatment with doxorubicin had no effect on tumor growth, while treatment with the iNPG led to a substantial reduction in tumor burden (Fig. 6b).73 The results from the study revealed that the median survival of the control, doxorubicin, Doxil, and iNPG groups were 87 days, 98 days, 124 days, and 233 days, respectively (Fig. 6c).73 This observation provides evidence that the polymeric nanoparticle version of doxorubicin released from the iNPG is able to circumvent drug efflux pumps. In addition to evaluating the anticancer activity of various doxorubicin treatments, safety assessments were also performed. Mice that received the iNPG were able to tolerate a doxorubicin dose of 24 mg/kg, while mice receiving Doxil at this dose did not survive. Furthermore, at a doxorubicin dose of 12 mg/kg, markers of cardiotoxicity were substantially lower in the iNPG group compared to the Doxil group. These results are encouraging, since Doxil is the clinical formulation of doxorubicin that displays the most favorable safety profile.
In essence, the iNPG combines different strategies for improving drug delivery. Specifically, geometrical targeting is utilized to obtain high accumulation of drugs in lung tumors, while pH-sensitive intracellular release of doxorubicin from nanoparticles is exploited to circumvent efflux pumps (Fig. 6d).
Conclusion
Challenges in drug delivery arise due to transport barriers in the body. Accordingly, a prerequisite for obtaining a favorable therapeutic index is to address biological obstacles. In this context, a new way to approach cancer treatment is to view the disease as a state of dysregulated mass transport. In fact, cancer-associated transport abnormalities occur on all spatial scales, ranging from atoms to entire organisms. For instance, metastasis can be seen as a dysregulation in the dynamics between cancer cells and the surrounding environment. These concepts lay the basis for the field of transport oncophysics, which strives to take advantage of physics in order to understand transport abnormalities in cancer.8 In particular, the field serves as a means to simplify the distinguishing features of cancer.83 For example, while there are thousands of molecular pathways and mutations that can induce malignancy, these alterations tend to cause similar changes in transport phenomena. Such phenomena can be exploited for therapeutic purposes by designing drug delivery systems that exhibit favorable interactions with biological environments that display transport abnormalities. In this sense, the MSV is an ideal example of a drug delivery system that is designed based on the principles of transport oncophysics. Specifically, the MSV takes advantage of several transport abnormalities in tumors, including reduced shear rates and vascular fenestrations. Notably, the MSV would not be able to exploit both of these transport phenomena, unless it included a microparticle and nanoparticle component. While the microparticle preferentially adheres to tumor vasculature due to reduced shear rates, the nanoparticles selectively enter the tumor interstitium due to large fenestrations in the vascular wall. Moreover, redox potential-sensitive degradation of the MSV causes accelerated nanoparticle release in the tumor microenvironment. These examples serve to illustrate that post-nano strategies for drug delivery are capable of addressing more transport barriers compared to nano-based carriers.
In addition to serving as an example of a post-nano drug delivery system, the MSV is also an optimal platform for personalized medicine. Although there are several common transport abnormalities in cancer, specific transport attributes, such as the quantity and size of vascular fenestrations, differ based on the individual, the type of cancer, the stage of the disease, and the therapeutic regime. Therefore, drug delivery systems can be further optimized based on specific variations of common transport abnormalities. In the case of the MSV, the dimensions, surface functionalization, and porosity of the first stage vector can be fine-tuned to be compatible with the transport characteristics of individual lesions. Additionally, second stage nanoparticles can be freely selected based on the transport phenotype of the disease.
In this review, a new era of post-nano strategies for drug delivery have been presented. For instance, the MSV enables preferential and concurrent delivery of chemotherapy and gene silencing agents to metastatic lung nodules in mice, leading to substantial improvements in survival compared to treatment with nanodelivery vehicles. Moreover, the micron-sized platform permits incorporation of several functional elements, as illustrated by a study in which the MSV was designed to release nanoparticles in response to enzymes in the tumor microenvironment. Additionally, the microscale dimensions of the MSV are ideal for biomimetic strategies. For instance, MSVs with leukocyte membrane coatings exhibited transport properties that mimic those of leukocytes, leading to decreased liver accumulation and increased tumor accumulation. Moreover, porous silicon microparticles can also be utilized as a combined adjuvant and delivery system for antigens in order to improve immunotherapy. An additional example of a post-nano strategy for drug delivery is the injectable nanoparticle generator that was designed to sequentially produce and release cargo in response to specific environmental triggers. This platform displayed superior therapeutic efficacy and safety compared to corresponding small molecule and nanoparticle-based drugs.
In summary, the systems presented in this review serve as examples of post-nano strategies for the treatment of disease. As illustrated above, the micronsized dimensions of the platforms permit them to be further modified to address a wide variety of transport challenges. In fact, life can only be sustained on the microscale, indicating that substantially more can be achieved with microparticles compared to nanoparticles. Namely, although viruses fall within the nano-size range, they would be unable to replicate in the absence of micron-sized host organisms, suggesting that superior functionality reveal at micro levels. In conclusion, the MSV is a testament to the feasibility of utilizing sequential functional components of different spatial scales, starting from the microscale, an approach that could lead to a paradigm shift in drug delivery.
Supplementary Material
Acknowledgments
This work was supported by the Houston Methodist Research Institute. Partial support was acquired from: the Ernest Cockrell Jr. Distinguished Endowed Chair (M.F.), the US Department of Defense (W81XWH-09-1-0212, W81XWH-12-1-0414) (M.F.), the National Institutes of Health (U54CA143837, U54CA151668) (M.F.), Nylands nation Finland (J.W.), Victoriastiftelsen Finland (J.W.), and the Cancer Prevention Research Institute of Texas (RP121071) (M.F. and H.S.).
References
- 1.Wolfram J, Zhu M, Yang Y, Shen J, Gentile E, Paolino D, Fresta M, Nie G, Chen C, Shen H, Ferrari M, Zhao Y. Curr Drug Targets. 2015;16:1671–1681. doi: 10.2174/1389450115666140804124808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suri K, Wolfram J, Shen H, Ferrari M. In: Novel Formulations for Biologics, Vaccines, and Cancer Therapy. 3. Singh M, Salnikova M, editors. I. Elsevier; 2016. pp. 41–58. [Google Scholar]
- 3.Li KC, Pandit SD, Guccione S, Bednarski MD. Biomed Microdevices. 2004;6:113–116. doi: 10.1023/b:bmmd.0000031747.05317.81. [DOI] [PubMed] [Google Scholar]
- 4.Chau Y, Dang NM, Tan FE, Langer R. J Pharm Sci. 2006;95:542–551. doi: 10.1002/jps.20548. [DOI] [PubMed] [Google Scholar]
- 5.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Nature Reviews Materials. 2016;1:16036. [Google Scholar]
- 6.Blanco E, Shen H, Ferrari M. Nat Biotechnol. 2015;33:941–951. doi: 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bae YH, Park K. J Control Release. 2011;153:198–205. doi: 10.1016/j.jconrel.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari M. Trends Biotechnol. 2010;28:181–188. doi: 10.1016/j.tibtech.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canham LT. Adv Mater. 1995;7:1033–1037. [Google Scholar]
- 10.Ferrari M. WO1996041236. WO Pat. issued 1996, with priority date June 7, 1995.
- 11.Coffer JL, Whitehead MA, Nagesha DK, Mukherjee P, Akkaraju G, Totolici M, Saffie RS, Canham LT. Phys Status Solidi A. 2005;202:1451–1455. [Google Scholar]
- 12.Wang CF, Sarparanta MP, Mäkilä EM, Hyvönen ML, Laakkonen PM, Salonen JJ, Hirvonen JT, Airaksinen AJ, Santos HA. Biomaterials. 2015;48:108–118. doi: 10.1016/j.biomaterials.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Kang J, Joo J, Kwon EJ, Skalak M, Hussain S, She ZG, Ruoslahti E, Bhatia SN, Sailor MJ. Adv Mater. 2016 doi: 10.1002/adma.201600634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu W, Thapa R, Liu D, Nissinen T, Granroth S, Narvanen A, Suvanto M, Santos HA, Lehto VP. Mol Pharm. 2015;12:4038–4047. doi: 10.1021/acs.molpharmaceut.5b00473. [DOI] [PubMed] [Google Scholar]
- 15.Wang CF, Makila EM, Kaasalainen MH, Hagstrom MV, Salonen JJ, Hirvonen JT, Santos HA. Acta Biomater. 2015;16:206–214. doi: 10.1016/j.actbio.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Maxwell MJ, Dopheide SM, Turner SJ, Jackson SP. Arterioscler Thromb Vasc Biol. 2006;26:663–669. doi: 10.1161/01.ATV.0000201931.16535.e1. [DOI] [PubMed] [Google Scholar]
- 17.Kuwahara M, Sugimoto M, Tsuji S, Matsui H, Mizuno T, Miyata S, Yoshioka A. Arterioscler Thromb Vasc Biol. 2002;22:329–334. doi: 10.1161/hq0202.104122. [DOI] [PubMed] [Google Scholar]
- 18.Lee TR, Choi M, Kopacz AM, Yun SH, Liu WK, Decuzzi P. Sci Rep. 2013;3:2079. doi: 10.1038/srep02079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gentile F, Chiappini C, Fine D, Bhavane RC, Peluccio MS, Cheng MM, Liu X, Ferrari M, Decuzzi P. J Biomech. 2008;41:2312–2318. doi: 10.1016/j.jbiomech.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 20.van de Ven AL, Kim P, Haley O, Fakhoury JR, Adriani G, Schmulen J, Moloney P, Hussain F, Ferrari M, Liu X, Yun SH, Decuzzi P. J Control Release. 2012;158:148–155. doi: 10.1016/j.jconrel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SY, Ferrari M, Decuzzi P. Nanotechnology. 2009;20:495101. doi: 10.1088/0957-4484/20/49/495101. [DOI] [PubMed] [Google Scholar]
- 22.Decuzzi P, Ferrari M. Biomaterials. 2006;27:5307–5314. doi: 10.1016/j.biomaterials.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 23.Adriani G, de Tullio MD, Ferrari M, Hussain F, Pascazio G, Liu X, Decuzzi P. Biomaterials. 2012;33:5504–5513. doi: 10.1016/j.biomaterials.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Godin B, Chiappini C, Srinivasan S, Alexander JF, Yokoi K, Ferrari M, Decuzzi P, Liu X. Adv Funct Mater. 2012;22:4225–4235. doi: 10.1002/adfm.201200869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wolfram J, Shen H, Ferrari M. J Control Release. 2015;219:406–415. doi: 10.1016/j.jconrel.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzur-Balter A, Shatsberg Z, Beckerman M, Segal E, Artzi N. Nat Commun. 2015;6:6208. doi: 10.1038/ncomms7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liou GY, Storz P. Free Radic Res. 2010;44:479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka T, Mangala LS, Vivas-Mejia PE, Nieves-Alicea R, Mann AP, Mora E, Han HD, Shahzad MM, Liu X, Bhavane R, Gu J, Fakhoury JR, Chiappini C, Lu C, Matsuo K, Godin B, Stone RL, Nick AM, Lopez-Berestein G, Sood AK, Ferrari M. Cancer Res. 2010;70:3687–3696. doi: 10.1158/0008-5472.CAN-09-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen H, Rodriguez-Aguayo C, Xu R, Gonzalez-Villasana V, Mai J, Huang Y, Zhang G, Guo X, Bai L, Qin G, Deng X, Li Q, Erm DR, Aslan B, Liu X, Sakamoto J, Chavez-Reyes A, Han HD, Sood AK, Ferrari M, Lopez-Berestein G. Clin Cancer Res. 2013;19:1806–1815. doi: 10.1158/1078-0432.CCR-12-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shen J, Wu X, Lee Y, Wolfram J, Yang Z, Mao ZW, Ferrari M, Shen H. J Vis Exp. 2015;15:52075. doi: 10.3791/52075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blanco E, Sangai T, Hsiao A, Ferrati S, Bai L, Liu X, Meric-Bernstam F, Ferrari M. Cancer Lett. 2013;334:245–252. doi: 10.1016/j.canlet.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 32.Zhang M, Xu R, Xia X, Yang Y, Gu J, Qin G, Liu X, Ferrari M, Shen H. Biomaterials. 2014;35:423–431. doi: 10.1016/j.biomaterials.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martinez JO, Evangelopoulos M, Bhavane R, Acciardo S, Salvatore F, Liu X, Ferrari M, Tasciotti E. Curr Drug Targets. 2015;16:1582–1590. doi: 10.2174/1389450115666141015113914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen H, You J, Zhang G, Ziemys A, Li Q, Bai L, Deng X, Erm DR, Liu X, Li C, Ferrari M. Adv Healthc Mater. 2012;1:84–89. doi: 10.1002/adhm.201100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen J, Xu R, Mai J, Kim HC, Guo X, Qin G, Yang Y, Wolfram J, Mu C, Xia X, Gu J, Liu X, Mao ZW, Ferrari M, Shen H. ACS Nano. 2013;7:9867–9880. doi: 10.1021/nn4035316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu R, Huang Y, Mai J, Zhang G, Guo X, Xia X, Koay EJ, Qin G, Erm DR, Li Q, Liu X, Ferrari M, Shen H. Small. 2013;9:1799–1808. doi: 10.1002/smll.201201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yazdi IK, Murphy MB, Loo C, Liu X, Ferrari M, Weiner BK, Tasciotti E. J Tissue Eng. 2014;5 doi: 10.1177/2041731414536573. 2041731414536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scavo MP, Gentile E, Wolfram J, Gu J, Barone M, Evangelopoulos M, Martinez JO, Liu X, Celia C, Tasciotti E, Vilar E, Shen H. Colloids Surf B Biointerfaces. 2015;136:694–703. doi: 10.1016/j.colsurfb.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Scott B, Shen J, Nizzero S, Boom K, Persano S, Mi Y, Liu X, Zhao Y, Blanco E, Shen H, Ferrari M, Wolfram J. Pharmacol Res. 2016;111:413–421. doi: 10.1016/j.phrs.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mi Y, Mu C, Wolfram J, Deng Z, Hu TY, Liu X, Blanco E, Shen H, Ferrari M. Adv Healthc Mater. 2016;5:936–946. doi: 10.1002/adhm.201500910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mai J, Huang Y, Mu C, Zhang G, Xu R, Guo X, Xia X, Volk DE, Lokesh GL, Thiviyanathan V, Gorenstein DG, Liu X, Ferrari M, Shen H. J Control Release. 2014;187:22–29. doi: 10.1016/j.jconrel.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanaka T, Godin B, Bhavane R, Nieves-Alicea R, Gu J, Liu X, Chiappini C, Fakhoury JR, Amra S, Ewing A, Li Q, Fidler IJ, Ferrari M. Int J Pharm. 2010;402:190–197. doi: 10.1016/j.ijpharm.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jugdaohsingh R. J Nutr Health Aging. 2007;11:99–110. [PMC free article] [PubMed] [Google Scholar]
- 44.Merritt WM, Lin YG, Spannuth WA, Fletcher MS, Kamat AA, Han LY, Landen CN, Jennings N, De Geest K, Langley RR, Villares G, Sanguino A, Lutgendorf SK, Lopez-Berestein G, Bar-Eli MM, Sood AK. J Natl Cancer Inst. 2008;100:359–372. doi: 10.1093/jnci/djn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landen CN, Chavez-Reyes A, Bucana C, Schmandt R, Deavers MT, Lopez-Berestein G, Sood AK. Cancer Res. 2005;65:6910–6918. doi: 10.1158/0008-5472.CAN-05-0530. [DOI] [PubMed] [Google Scholar]
- 46.Noori Koopaei M, Khoshayand MR, Mostafavi SH, Amini M, Khorramizadeh MR, Jeddi Tehrani M, Atyabi F, Dinarvand R. Iran J Pharm Res. 2014;13:819–833. [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng J, Teply BA, Sherifi I, Sung J, Luther G, Gu FX, Levy-Nissenbaum E, Radovic-Moreno AF, Langer R, Farokhzad OC. Biomaterials. 2007;28:869–876. doi: 10.1016/j.biomaterials.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, Ewing R, Floyd Y, Gray K, Hall S, Hawes R, Hughes J, Kosmidou V, Menzies A, Mould C, Parker A, Stevens C, Watt S, Hooper S, Wilson R, Jayatilake H, Gusterson BA, Cooper C, Shipley J, Hargrave D, Pritchard-Jones K, Maitland N, Chenevix-Trench G, Riggins GJ, Bigner DD, Palmieri G, Cossu A, Flanagan A, Nicholson A, Ho JW, Leung SY, Yuen ST, Weber BL, Seigler HF, Darrow TL, Paterson H, Marais R, Marshall CJ, Wooster R, Stratton MR, Futreal PA. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 49.Gupta A, Love S, Schuh A, Shanyinde M, Larkin JM, Plummer R, Nathan PD, Danson S, Ottensmeier CH, Lorigan P, Collins L, Wise A, Asher R, Lisle R, Middleton MR. Ann Oncol. 2014;25:968–974. doi: 10.1093/annonc/mdu054. [DOI] [PubMed] [Google Scholar]
- 50.Jang S, Atkins MB. Lancet Oncol. 2013;14:e60–69. doi: 10.1016/S1470-2045(12)70539-9. [DOI] [PubMed] [Google Scholar]
- 51.Mi Y, Wolfram J, Mu C, Liu X, Blanco E, Shen H, Ferrari M. Pharmacol Res. 113:92–99. doi: 10.1016/j.phrs.2016.08.024. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jezierska A, Motyl T. Med Sci Monit. 2009;15:RA32–40. [PubMed] [Google Scholar]
- 53.Li H, Yu SS, Miteva M, Nelson CE, Werfel T, Giorgio TD, Duvall CL. Adv Funct Mater. 2013;23:3040–3052. doi: 10.1002/adfm.201202215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Nat Rev Drug Discov. 2011;10:521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 55.Manchester M, Singh P. Adv Drug Deliv Rev. 2006;58:1505–1522. doi: 10.1016/j.addr.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez PL, Harada T, Christian DA, Pantano DA, Tsai RK, Discher DE. Science. 2013;339:971–975. doi: 10.1126/science.1229568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parodi A, Quattrocchi N, van de Ven AL, Chiappini C, Evangelopoulos M, Martinez JO, Brown BS, Khaled SZ, Yazdi IK, Enzo MV, Isenhart L, Ferrari M, Tasciotti E. Nat Nanotechnol. 2013;8:61–68. doi: 10.1038/nnano.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Molinaro R, Corbo C, Martinez JO, Taraballi F, Evangelopoulos M, Minardi S, Yazdi IK, Zhao P, De Rosa E, Sherman MB, De Vita A, Toledano Furman NE, Wang X, Parodi A, Tasciotti E. Nat Mater. 2016;15:1037–1046. doi: 10.1038/nmat4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Corbo C, Parodi A, Evangelopoulos M, Engler DA, Matsunami RK, Engler AC, Molinaro R, Scaria S, Salvatore F, Tasciotti E. Curr Drug Targets. 2015;16:1540–1547. doi: 10.2174/1389450115666141109211413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Evangelopoulos M, Parodi A, Martinez JO, Yazdi IK, Cevenini A, van de Ven AL, Quattrocchi N, Boada C, Taghipour N, Corbo C, Brown BS, Scaria S, Liu X, Ferrari M, Tasciotti E. Biomaterials. 2016;82:168–177. doi: 10.1016/j.biomaterials.2015.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banchereau J, Steinman RM. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 62.Pardoll DM. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gokhale AS, Satyanarayanajois S. Immunotherapy. 2014;6:755–774. doi: 10.2217/imt.14.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miller MJ, Foy KC, Kaumaya PT. Discov Med. 2013;15:166–176. [PubMed] [Google Scholar]
- 65.Gangadhar TC, Vonderheide RH. Nat Rev Clin Oncol. 2014;11:91–99. doi: 10.1038/nrclinonc.2013.245. [DOI] [PubMed] [Google Scholar]
- 66.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF Investigators IS. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 67.Goldberg MS. Cell. 2015;161:201–204. doi: 10.1016/j.cell.2015.03.037. [DOI] [PubMed] [Google Scholar]
- 68.Moon JJ, Huang B, Irvine DJ. Adv Mater. 2012;24:3724–3746. doi: 10.1002/adma.201200446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xia X, Mai J, Xu R, Perez JE, Guevara ML, Shen Q, Mu C, Tung HY, Corry DB, Evans SE, Liu X, Ferrari M, Zhang Z, Li XC, Wang RF, Shen H. Cell Rep. 2015;11:957–966. doi: 10.1016/j.celrep.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fenollosa R, Garcia-Rico E, Alvarez S, Alvarez R, Yu X, Rodriguez I, Carregal-Romero S, Villanueva C, Garcia-Algar M, Rivera-Gil P, de Lera AR, Parak WJ, Meseguer F, Alvarez-Puebla RA. J Nanobiotechnology. 2014;12:35. doi: 10.1186/s12951-014-0035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zitvogel L, Galluzzi L, Kepp O, Smyth MJ, Kroemer G. Nat Rev Immunol. 2015;15:405–414. doi: 10.1038/nri3845. [DOI] [PubMed] [Google Scholar]
- 72.Prinz M, Knobeloch KP. Front Immunol. 2012;3:67. doi: 10.3389/fimmu.2012.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu R, Zhang G, Mai J, Deng X, Segura-Ibarra V, Wu S, Shen J, Liu H, Hu Z, Chen L, Huang Y, Koay E, Liu J, Ensor JE, Blanco E, Liu X, Ferrari M, Shen H. Nat Biotechnol. 2016;34:414–418. doi: 10.1038/nbt.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Arnedos M, Bihan C, Delaloge S, Andre F. Ther Adv Med Oncol. 2012;4:195–210. doi: 10.1177/1758834012444711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Ruijter TC, Veeck J, de Hoon JP, van Engeland M, Tjan-Heijnen VC. J Cancer Res Clin Oncol. 2011;137:183–192. doi: 10.1007/s00432-010-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Anders CK, Carey LA. Clin Breast Cancer. 2009;9(Suppl 2):S73–81. doi: 10.3816/CBC.2009.s.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L. Nat Rev Clin Oncol. 2016 doi: 10.1038/nrclinonc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ovcaricek T, Frkovic SG, Matos E, Mozina B, Borstnar S. Radiol Oncol. 2011;45:46–52. doi: 10.2478/v10019-010-0054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pogoda K, Niwińska A, Murawska M, Pieńkowski T. Med Oncol. 2013;30:388. doi: 10.1007/s12032-012-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barenholz Y. J Control Release. 2012;160:117–134. doi: 10.1016/j.jconrel.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 81.Deavall DG, Martin EA, Horner JM, Roberts R. J Toxicol. 2012;2012:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. J Mol Cell Cardiol. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Michor F, Liphardt J, Ferrari M, Widom J. Nat Rev Cancer. 2011;11:657–670. doi: 10.1038/nrc3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.